Introduction
Hepatocellular carcinoma (HCC) is the fifth most
frequently diagnosed cancer worldwide, and commonly leads to
cancer-related mortality (1). The
molecular mechanisms involved in HCC carcinogenesis are a focus of
extensive investigation. The cell signaling effects of mutagens on
specific HCC oncogenes and tumor-suppressor genes have been
reported.
Interferon regulatory factor-1 (IRF-1) was
identified as an IFN-inducible master transcription factor that
plays important roles in immunity and oncogenesis (2). IRF-1 has been identified as a
tumor-suppressor gene through regulation of the cell cycle and
apoptosis in addition to its function in immunomodulation and
antiviral response (3–8).
Our previous study found that interferon-γ (IFNγ)
induced autophagy in HCC cells through IRF-1 (9). Additionally, aberrant expression of
IRF-1 has been found in many malignant tumors including melanoma,
leukemia, gastric and breast cancer, and esophageal squamous cell
carcinoma (10). Carcinogenesis
signaling in HCV-mediated HCC was found to be related to
suppression of IRF-1, and downregulation of IRF-1 was found to
predict a poor prognosis in HCC (11,12).
However, the molecular mechanisms of IRF-1-mediated suppression of
HCC growth are not well defined.
MicroRNAs (miRNAs) are small non-coding RNA
molecules of 20–30 nucleotides which specifically recognize and
suppress particular mRNAs at the post-transcriptional level by
exerting a translational blockade or causing degradation of mRNAs
(13). This regulation is involved
in fundamental cellular processes, including cell cycle,
differentiation, metabolism, as well as carcinogenesis and tumor
progression (14). Furthermore,
miRNAs are frequently observed to be dysregulated in HCC (15,16).
Recently, a study found that 193 miRNAs were differentially
expressed in an HCC cell line compared to normal liver cells
(17). microRNA-23a (miR-23a),
located in the miR-23a/24/27a cluster, has been shown to be
upregulated in HCC, and can suppress apoptotic activities in HCC
cells (15,16,18,19).
IFNγ stimulation of melanoma cells showed that miR-23a is inversely
associated with IRF-1 (20).
Additionally, research has demonstrated that miR-23a targets IRF-1
to suppress the apoptosis of gastric cancer cells and facilitates
the replication of herpes simplex virus type 1 in HeLa cells
(21,22).
In the present study, we showed that IRF-1
expression was decreased in primary human HCC tumors and human HCC
cell lines. The expression of IRF-1 induced by IFNγ in hepatocytes
and HCC cells demonstrated that miR-23a is inversely correlated to
IRF-1. We identified an miR-23a binding site in the 3′-untranslated
region (3-UTR) of the human IRF-1 gene and showed that miR-23a
decreased the post-transcriptional expression of IRF-1. These
findings suggest that the targeting of IRF-1 by miR-23a may be a
molecular basis for IRF-1 downregulation in HCC and provide new
insight into the regulation of HCC by miRNAs.
Materials and methods
Acquisition of human tissue
specimens
Seven paired HCC and adjacent liver tissues were
obtained from patients who underwent hepatectomy at the Liver
Cancer Center of the University of Pittsburgh School of Medicine
(Pittsburgh, PA, USA). All human tissues were acquired in
accordance with the University of Pittsburgh Institutional Review
Board (IRB) approved protocol.
Cell lines
The primary human hepatocytes (hHCs) were isolated
at the University of Pittsburgh as part of the NIH-funded Liver
Tissue and Cell Distribution System. The hepatocytes were used
immediately following receipt and cultured in Williams' medium E
(Lonza, Walkersville, MD, USA) with 5% newborn calf serum. The
human HCC cell lines Huh-7 and HepG2 and colon cancer cell line
HCT116 were purchased from the American Type Culture Collection
(ATCC; Rockville, MD, USA). Huh-7 and HepG2 cells were cultured in
Dulbecco's modified Eagle's medium (DMEM) (Lonza), while HCT116
cells were cultured with McCoy's 5A medium (Gibco/Life
Technologies, Grand Island, NY, USA), containing 10%
heat-inactivated fetal bovine serum (FBS) (Clontech, Mountain View,
CA, USA), 100 U/ml penicillin, 100 µg/ml streptomycin, 15
mmol/l HEPES and 200 mmol/l L-glutamine. All cells were incubated
at 37°C in a humidified incubator containing 5% CO2.
Quantitative reverse
transcriptase-polymerase chain reaction (qRT-PCR)
Total RNA was extracted with TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) according to the manufacturer's
protocol. Then, 1 µg of total RNA from each sample was
reverse transcribed to single-stranded cDNA with RNA to cDNA
EcoDry™ Premix (Clontech). One microliter of cDNA was diluted
50-fold with nuclease-free water and used as a template for the
following qRT-PCR. The IRF-1 mRNA expression was quantified using
the IRF-1 primer, as well as the SYBR-Green PCR Master Mix with the
StepOne Plus Real-Time PCR system (both from Applied Biosystems,
Foster City, CA, USA). The IRF-1 primers were: 5′-ACCCTGGCTAGA
GATGCAGA-3′ (forward), and 5′-GCTTTGTATCGGCCTGTGTG-3′ (reverse);
GAPDH primers were: 5′-GGGAAGCTTGTCATCAATGG-3′ (forward), and
5′-CATCGCCCCACTTGATTTTG-3′ (reverse). The qPCR cycling conditions
used were as follows: 95°C for 10 min, 95°C for 15 sec, 60°C for 1
min, 95°C for 15 sec and 60°C for 1 min. Exponential amplification
had been confirmed up to 40 cycles of the amplification. The
relative gene expression levels were calculated using the
2−ΔΔCt method. All primers were purchased from
Invitrogen.
miR-23a expression was determined by quantitative
RT-PCR using TaqMan miRNA assays according to the manufacturer's
protocol. Reverse transcription reactions were prepared using the
TaqMan MicroRNA Reverse Transcription kit (Applied Biosystems,
Foster City, CA, USA). Each 15 µl multiplex reaction
contained 10 ng total RNA as template. Prior to real-time PCR, the
multiplex RT-reactions were diluted with 100 µl
nuclease-free water. The diluted RT-products were mixed with TaqMan
Universal PCR Master Mix, without UNG (Applied Biosystems). U6
snRNA was used for normalization. miR-23a and U6 snRNA primers were
purchased from Applied Biosystems. The qPCR cycling conditions used
were as follows: 50°C for 2 min, 95°C for 10 min, 95°C for 15 sec
and 60°C for 1 min. The relative gene expression levels were
calculated using the 2−ΔΔct method.
Western blotting
Whole protein was extracted with cell lysis buffer
(Cell Signaling Technology, Danvers, MA, USA). Nuclear protein was
extracted as previously described (23). A total of 20 µg of nuclear
protein was electrophoresed on 10% SDS-polyacrylamide gels and
transferred to polyvinylidene difluoide membranes. After blocking
with 5% non-fat milk at room temperature for 1 h, the membranes
were incubated with a 1:1,000 dilution of anti-IRF-1 or lamin A/C
(Cell Signaling Technology) antibodies overnight, respectively.
Lamin A/C was used as a loading control. Then, the membranes were
washed with Tris-buffered saline and Tween-20 (TBST) for three
times, and incubated with a 1:10,000 dilution of goat anti-rabbit
secondary antibody for 1 h, and developed onto X-ray film using
chemiluminescent reagent.
Cell infection
The adenovirus of the miR-23a inhibitor
(admiRa-has-miR-23a-Off Virus) and its negative control (NC)
(admiR-23a-Off Negative Control Virus) were purchased from Applied
Biological Materials (Richmond, BC, Canada), and were amplified by
the Vector Core Facility at the University of Pittsburgh. Huh-7 and
HepG2 cells were infected for 48 h with either the ad-NC adenovirus
or the ad-miR-23a inhibitor. After 48 h of infection, the cells
were harvested, and then total RNA and nuclear protein were
extracted to determine the expression of IRF-1.
Immunofluorescent staining
Immunofluorescent staining was performed according
to our previous study (9). Huh-7
cells were cultured on coverslips, fixed with 2% paraformaldehyde
in phosphate-buffered saline (PBS) for 15 min, permeabilized with
0.1% Triton X-100 and 10% FBS in PBS for 30 min at room
temperature, and incubated with the primary IRF-1 antibodies (Cell
Signaling Technology) for 1 h, which was diluted in a 1:150 ratio.
Next, Alexa Fluor 488 anti-rabbit IgG antibody (1:500; Invitrogen)
was applied for 1 h at room temperature. After washing with PBS,
the slides were stained with 4′,6-diamidine-2′-phenylindole
dihydrochloride (DAPI) and mounted, and then observed with a
Olympus Fluoview FV1000 II microscope (Olympus, Tokyo, Japan).
Plasmid construct
pMIR-IRF-1-3′UTR plasmid was subcloned into the
multicloning site of the retroviral vector. The 528-bp of the human
IRF-1 3′UTR sequence containing the miR-23a binding site was
amplified by PCR from cDNA of the HCC cell line Huh-7 and inserted
into the cloning site to construct pMIR-IRF-1-3′UTR. The primers
were: 5′-AAA ACTAGTAGTGTCTGGCTTTTTCCTCTGA-3′ (forward) and
5′-TTTAAGCTTATGACATTTCCAATTTTAA-3′ (reverse). The recombinant
plasmid was re-cut with endonuclease HindIII and SacI
and sequenced and compared with BLAST for confirmation.
Transfection
The mirVana™ miRNA mimics and inhibitor of
hsa-miR-23a-3p were transfected in cells in 6-well plates using
Lipofectamine 2000 (Invitrogen) for 48 h according to the
manufacturer's protocol. miR-23a-3p mimics and inhibitors were
purchased from Ambion (Life Technologies, Grand Island, NY, USA).
The procedure was followed according to the manufacturer's
protocol. For the luciferase assay, the pMIR-IRF-1-3′UTR plasmid
was transfected into cells in 12-well plates using Lipofectamine
3000 (Invitrogen) for 48 h.
Luciferase assay
The pMIR-REPORT™ miRNA expression reporter vector
system (Applied Biosystems) was used to evaluate miRNA regulation
and the β-gal reporter control plasmid was used to normalize the
transfection efficiency. HCT116 and HepG2 cells were cultured in a
12-well plate and transfected with 200 ng β-gal combined with 500
ng of the pMIR-REPORT empty vector or pMIR-IRF-1-3′UTR plasmid.
Furthermore, the cells were co-transfected with the
pMIR-IRF-1-3′UTR plasmid and 50 pmol of the miR-23a mimic,
inhibitor and its NC (Ambion), respectively. miRNA NC was used to
normalize the total volume for transfection. Serum-free medium was
replaced with growth medium after 6 h. Relative luciferase and
β-galactosidase activities were measured with the reporter lysis
buffer and luciferase substrate (Promega, Madison, WI, USA). The
cells were lysed 48 h after transfection. The relative luciferase
unit (RLU) was measured using the Dual-Luciferase Report Assay
(BioTek, Winooski, VT, USA).
Statistical analysis
Statistical analysis was performed using SPSS for
Windows version 19.0 (SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± SD. Student's t-test was used for raw data
analysis and a value of p<0.05 was accepted as statistically
significant.
Results
IRF-1 expression is repressed in HCC
tumors and HCC cell lines
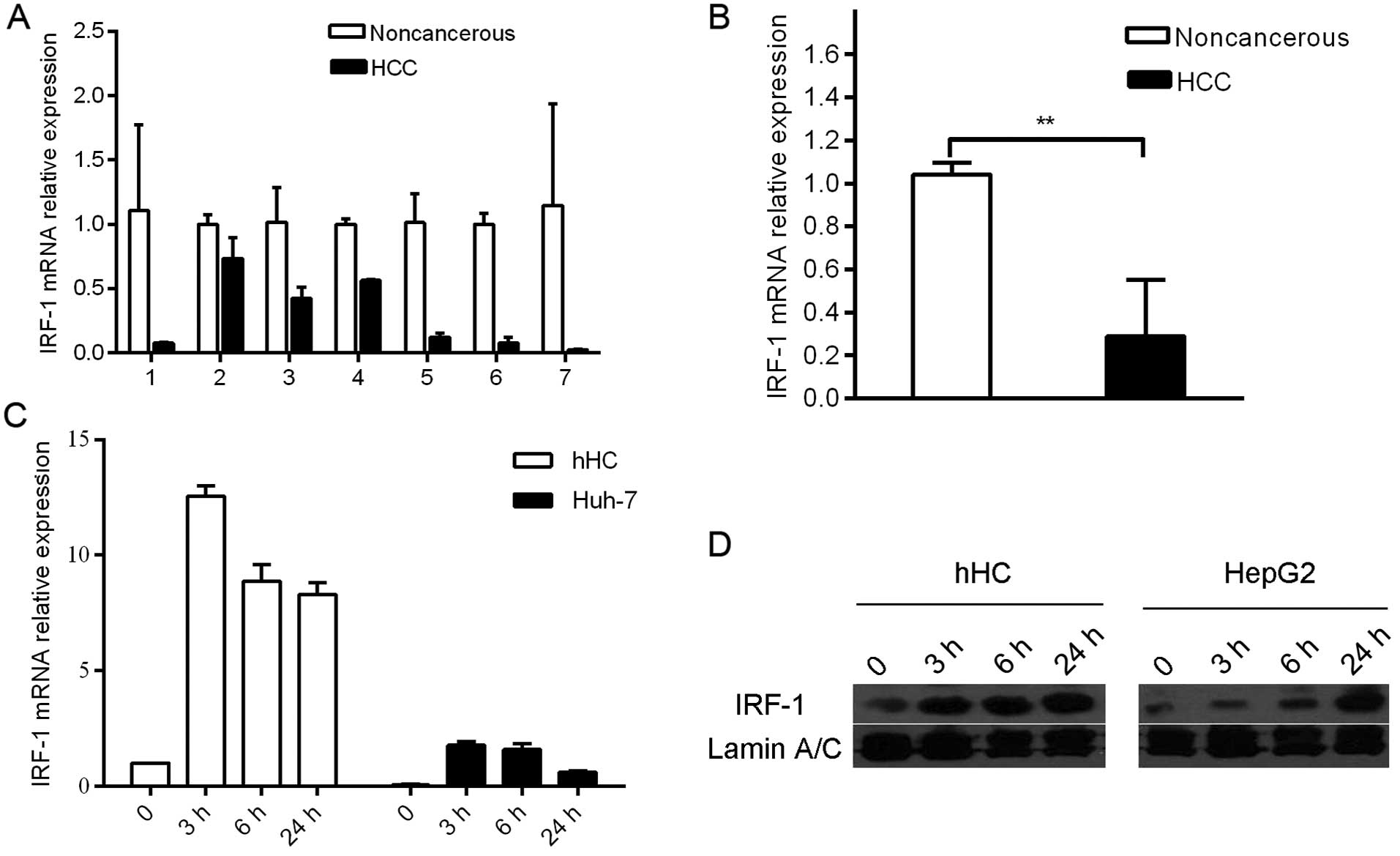
In the in vivo studies, IRF-1 mRNA expression
was down-regulated in 7 of the 7 human HCC tumor tissues compared
to that in the adjacent background liver (Fig. 1A and B). In the in vitro
studies, expression levels of IFNγ-stimulated IRF-1 mRNA and
protein were compared in the human hepatocye (hHC) cultures and HCC
(Huh-7 and HepG2) cell lines. IRF-1 mRNA and protein was induced by
IFNγ in a time-dependent manner; however, the magnitude of
induction was markedly less in the HCC tumor cells compared to that
in the primary hHCs (Fig. 1C and
D).
miR-23a expression is inversely
correlated with IRF-1 mRNA in the HCC cell lines induced by
IFNγ
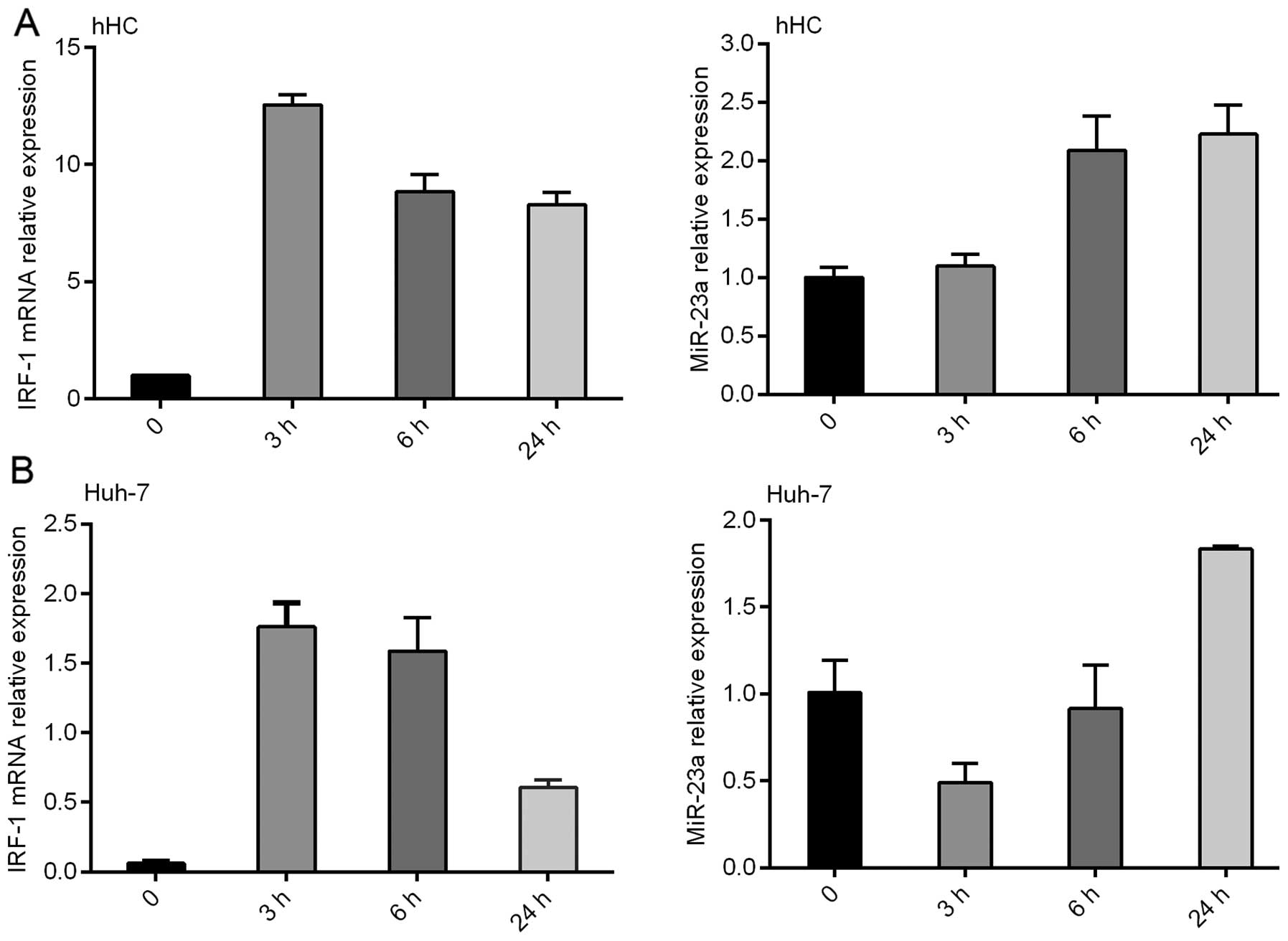
IFNγ induced IRF-1 mRNA expression in the primary
hHCs and Huh-7 HCC cells in a time-dependent manner, with a peak
IRF-1 mRNA level observed at 3 h, which was decreased by 24 h
(Fig. 2). Notably, miR-23a
expression was also increased by IFNγ, however the induction peaked
at 24 h and was inversely correlated with IRF-1 mRNA induction.
miR-23a downregulates expression of
IRF-1
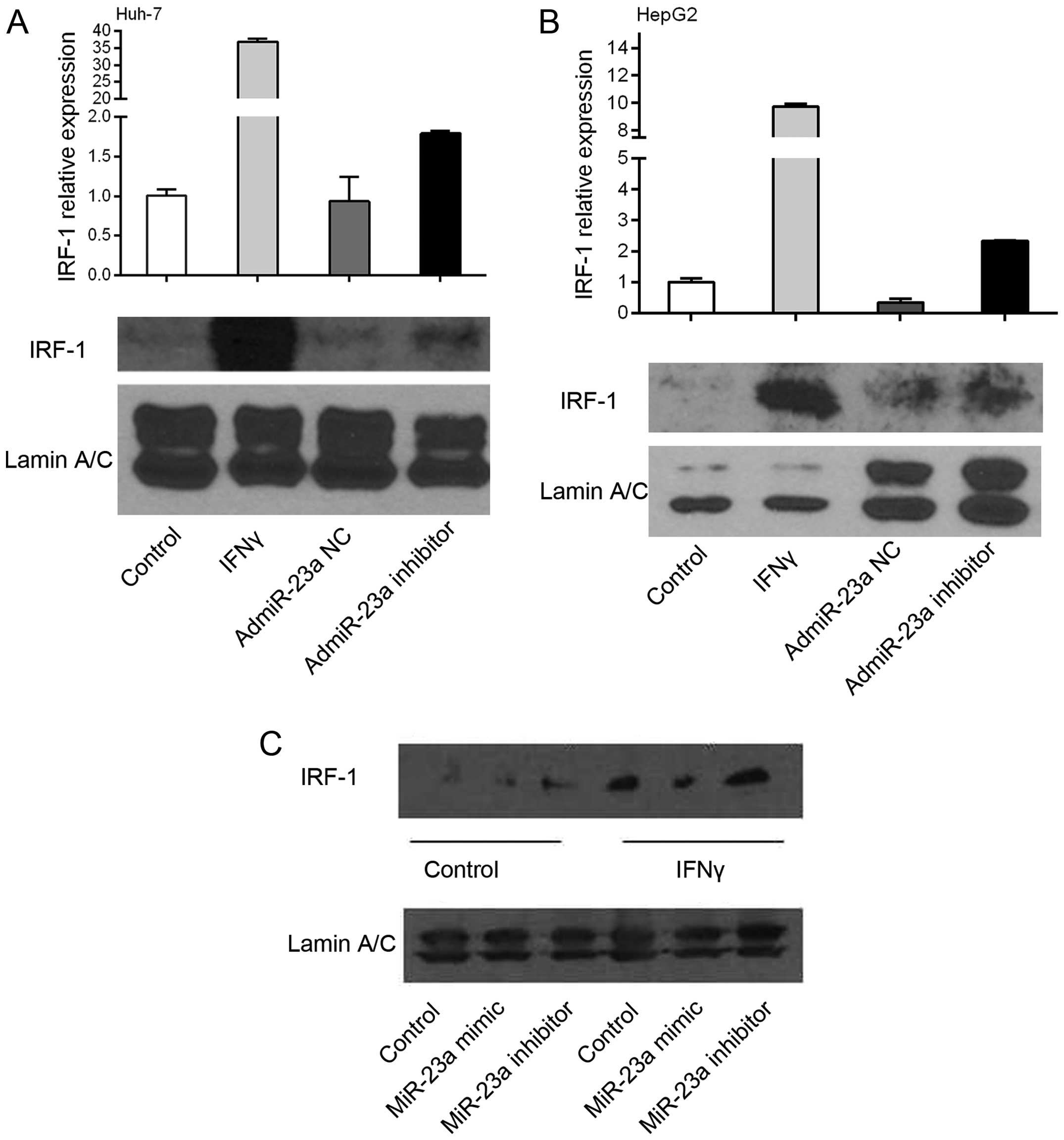
To determine a cause/effect relationship between
miR-23a and IRF-1 expression, human HCC Huh-7 and HepG2 cells were
infected with the adenovirus overexpressing the miR-23a (admiR-23a)
inhibitor or NC. The miR-23a inhibitor increased basal IRF-1 mRNA
levels 2 to 3-fold as determined by real-time PCR, while the NC had
no effect (Fig. 3A and B). These
findings suggest that endogenous miR-23a suppresses basal IRF-1
mRNA levels in tumor cells, since the inhibitor increased basal
IRF-1 mRNA. As expected, IFNγ markedly induced IRF-1 mRNA
expression, however addition of the miR-23a inhibitor did not
further increase the IRF-1 mRNA (data not shown). Basal IRF-1
nuclear protein levels in the HepG2 cells were increased by the
miR-23a inhibitor. In contrast, miR-23a mimic decreased
IFNγ-induced IRF-1 nuclear protein levels, while the miR-23a
inhibitor had no significant effect compared to IFNγ alone
(Fig. 3C).
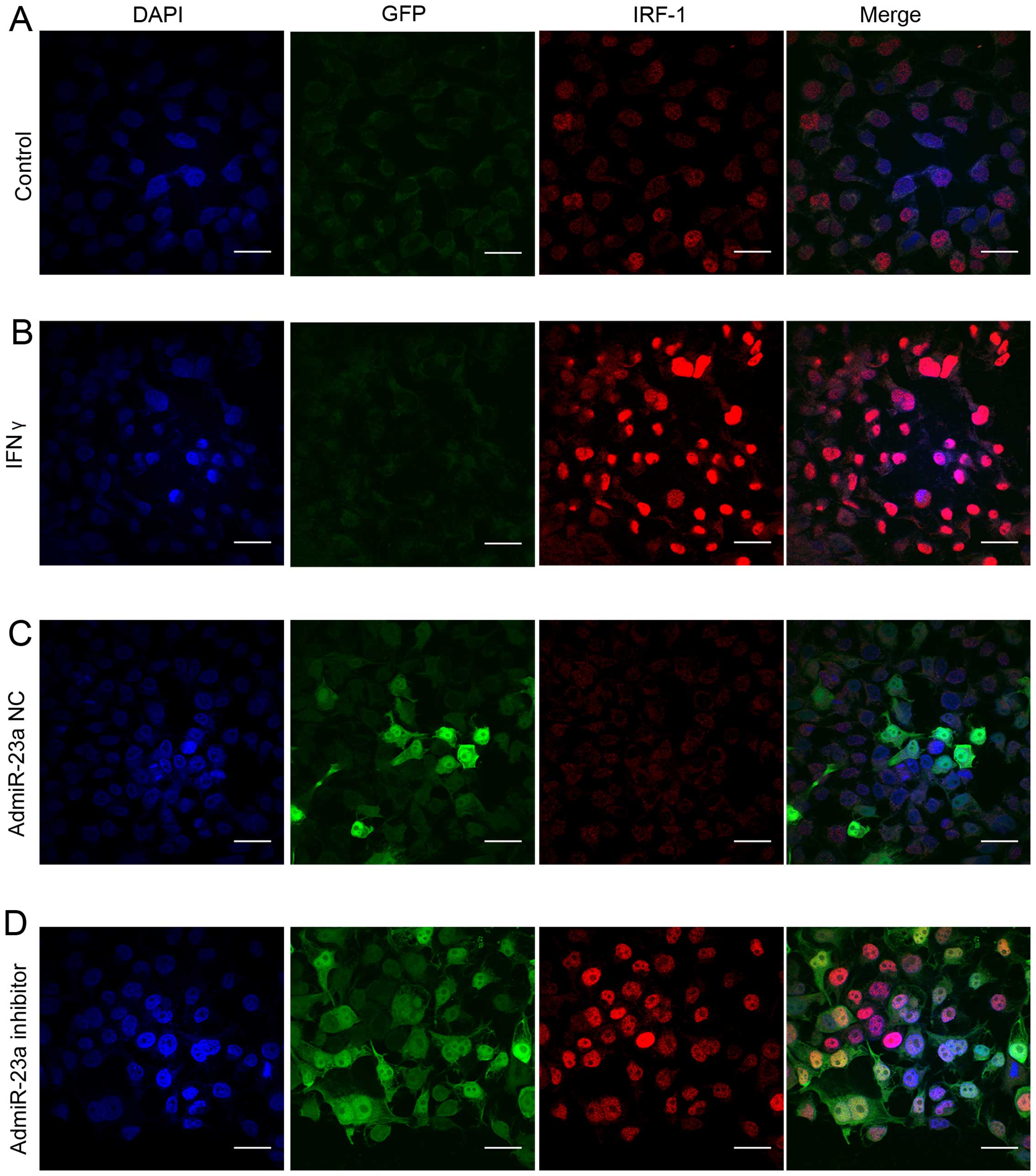
To observe the expression of IRF-1 nuclear protein
in the HCC cells, confocal immunofluorescent staining was performed
in human Huh-7 cells. Only minimal basal IRF-1 nuclear protein was
observed in resting Huh-7 cells (Fig.
4A). As expected, IFNγ strongly induced IRF-1 nuclear protein
staining (Fig. 4B). Infection with
the admiR-23a inhibitor increased basal IRF-1 nuclear protein
expression (Fig. 4D), while the NC
had no effect (Fig. 4C).
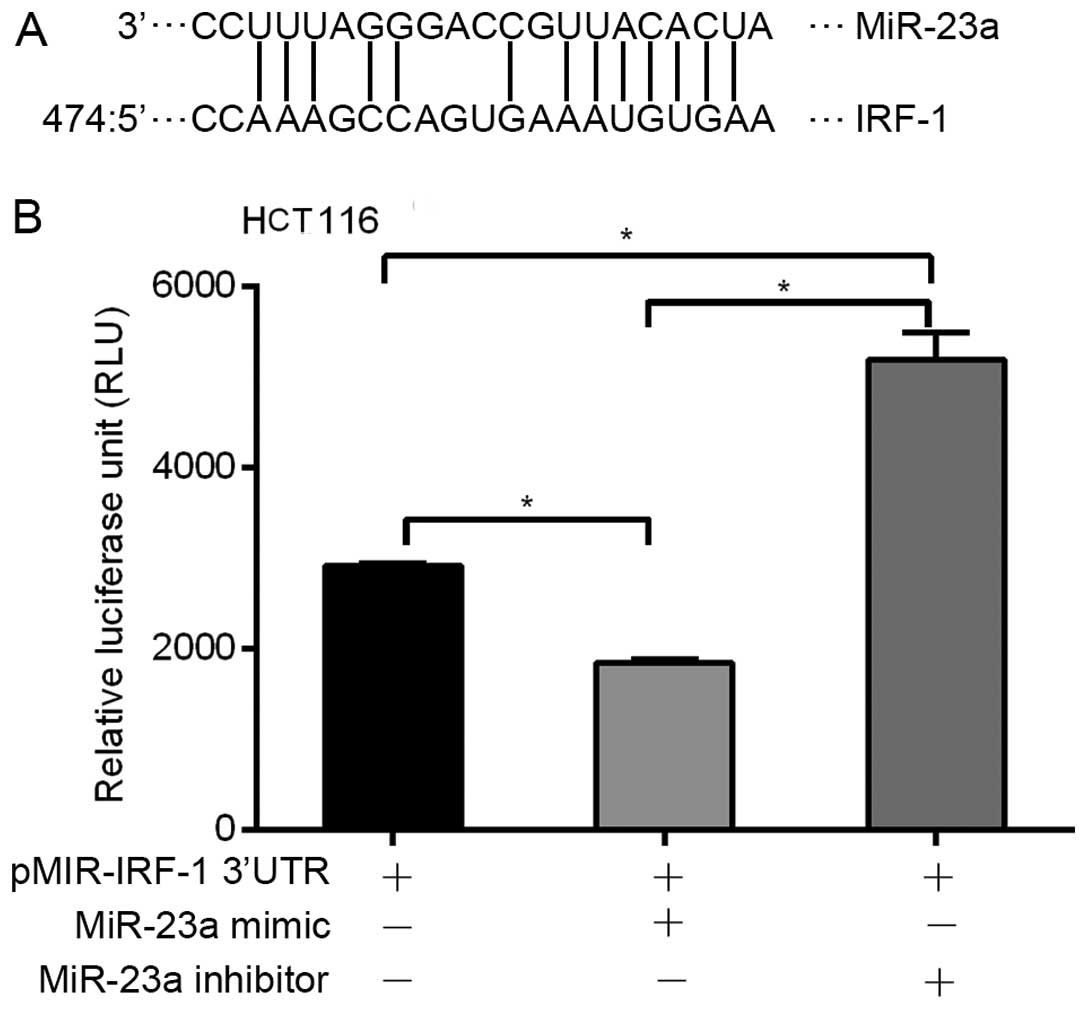
miR-23a targets the specific binding site
in the IRF-1 mRNA 3′UTR
miRNAs are known to modulate gene expression by
binding to specific miRNA sequences in the 3′UTR of target genes.
Therefore, we used MicroInspector software to identify putative
miR-23a binding sequence(s) in the 3′UTR of the human IRF-1 mRNA.
An miR-23a binding sequence match was identified at nucleotide 474
in the 528-base pair (bp) IRF-1 3′UTR (Fig. 5A). To show a functional role for
this binding site, the 528-bp human IRF-1 3′UTR was cloned into a
luciferase reporter plasmid pMIR-IRF-1 3′-UTR and transfected into
human HCT116 cells. Addition of the miR-23a mimic significantly
decreased basal luciferase reporter activity, while addition of the
miR-23a inhibitor significantly increased basal reporter activity
(Fig. 5B). Transfection with the
β-galactosidase expression plasmid was used to control for
transfection efficiency (data not shown). These results suggest
that miR-23a binds to the human IRF-1 3′UTR region, and suppresses
post-transcriptional activity.
Discussion
IRF-1 functions as a tumor-suppressor gene to
inhibit the oncogenesis and progression of malignant tumors through
regulation of apoptosis and the cell cycle. DNA damage-inducing
cell apoptosis relies on the cooperation of IRF-1 and p53 pathways
(24). Additionally, IRF-1 binds to
distinct sites in the promoter with upregulation of p53 upregulated
modulator of apoptosis (PUMA) to activate apoptosis (7). In a previous study, we identified that
IFNγ induced autophagy in HCC cells through IRF-1 (9). In the present study, we investigated
the mechanism by which IRF-1 is repressed in HCC. The major and
novel findings were as follows. i) IRF-1 expression was
downregulated in the resected human HCC tumors compared to that
observed in the background liver. ii) miR-23a downregulates the
expression of IRF-1 in HCC cells; and iii) the IRF-1
3′-untranslated region (3′UTR) has a miR-23a binding site that
binds miR-23a and decreases post-transcriptional activity. These
findings suggest that miR-23a targeting IRF-1 may be the molecular
basis for IRF-1 down-regulation in HCC and provide new insight into
the regulation of HCC by miRNAs.
IRF-1 regulates cell apoptosis induced by DNA
damage, which is dependent on cell type and differentiation
(7,25). IFNγ induces apoptosis in breast
cancer cells through IRF-1 resulting in caspase cleavage and
reduced expression of the apoptosis-suppressor gene survivin
independent of p53, in addition to cell cycle arrest dependent on
upregulation of p21 (6,26). The expression of IRF-1 has been
shown to be reduced in several types of malignant tumors and we
observed that the basal level of IRF-1 was suppressed in
IFNγ-induced HCC cell lines as well as in HCC tissues when compared
to that in adjacent non-cancerous tissues, which may account for
the oncogenesis and progression of HCC.
Several possible mechanisms have been reported to
account for the inability of IRF-1 to exert a tumor-suppressor
effect. The genetic alteration of the IRF-1 genotype with loss of
heterozygosity in leukemia or myelodysplastic syndrome (MDS) as
well as loss of one IRF-1 allele in esophageal and gastric cancers
has been reported (10,25). Additionally, inactivation of IRF-1
has been demonstrated in various types of cancers. Upregulation of
nucleophosmin to inhibit the function of IRF-1 was observed in
myeloid leukemia cells (27). A low
level of IRF-1 mRNA was discovered in breast cancer and HCC
(10,25).
In the present study, we investigated the role of
the specific miR-23a in the post-transcriptional regulation of
IRF-1. Previous studies demonstrated increased expression of
miR-23a in HCC along with anti-apoptotic function and subsequent
promotion of tumor proliferation (18,19).
In addition, miR-23a was believed to play a role in gluconeogenesis
in HCC, which is involved in the process of liver tumorigenesis and
correlates with the prognosis of HCC (28). Furthermore, miR-23a was noted to
play a role in regulating tumor cell response to chemotherapeutic
agents (29).
We observed that miR-23a and IRF-1 mRNA are
inversely expressed in human hepatocytes (hHCs) and HCC cell lines
induced by IFNγ. The miR-23a inhibitor increased basal expression
of IRF-1 in the HCC cells, while a specific miR-23a mimic
suppressed IFNγ-induced IRF-1 expression. These findings add new
important information to the complex signaling pathways in
inflammation-associated carcinogenesis.
Previously studies have shown that miRNAs regulate
hundreds of genes via inducing mRNA degradation or prohibiting gene
translation by direct binding to the 3′UTR of target mRNAs
(29–31). Indeed we identified an miR-23a
binding site in the 3′UTR of the human IRF-1 mRNA, and showed that
this 3′UTR was responsive to the miR-23a mimic and inhibitor in the
reporter assays. These findings are consistent with a recent study
showing that miR-23a targets IRF-1 and modulates cell proliferation
and paclitaxel-induced apoptosis in gastric cancer (21). Moreover, miR-23b was shown to have
an important role in promoting avian leukosis virus subgroup J
(ALV-J) replication by binding the IRF-1-specific sequence
(32). Another group showed that
IFNγ could induce miR-29b by recruiting IRF-1 to binding sites in
the miR-29b promoter in colorectal cancer (31). In a related study, IRF-1 was found
to regulate miR-203 transcription by binding to the miR-203
promoter in cervical cancer (33).
In summary, these results demonstrated that IRF-1
expression is downregulated in resected human HCC tumors compared
to background liver. miR-23a inhibition increases basal expression
of IRF-1, and the finding of a functional miR-23a binding site in
the 3′UTR of the human IRF-1 gene suggests that miR-23a mediates
post-transcriptional suppression of IRF-1. Taken together, these
findings are consistent with the notion that the targeting of IRF-1
by endogenous miR-23a may be the molecular basis for IRF-1
downregulation in HCC. These findings also provide new insight into
the regulation of HCC by miRNAs during inflammatory conditions.
Acknowledgments
The present study was supported by the NIH HHSN2762
01200017C Liver Tissue and Cell Distribution System (LTCDS)
contract (D.A.G.), and the NIH grant 1S10OD019973-01 from the
Center for Biologic Imaging at the University of Pittsburgh. We
thank Nicole Martik-Hays and Kimberly Ferrero for isolating the
hHCs.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tamura T, Yanai H, Savitsky D and
Taniguchi T: The IRF family transcription factors in immunity and
oncogenesis. Annu Rev Immunol. 26:535–584. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Connett JM, Hunt SR, Hickerson SM, Wu SJ
and Doherty GM: Localization of IFN-gamma-activated Stat1 and IFN
regulatory factors 1 and 2 in breast cancer cells. J Interferon
Cytokine Res. 23:621–630. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yim JH, Ro SH, Lowney JK, Wu SJ, Connett J
and Doherty GM: The role of interferon regulatory factor-1 and
interferon regulatory factor-2 in IFN-gamma growth inhibition of
human breast carcinoma cell lines. J Interferon Cytokine Res.
23:501–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim PK, Armstrong M, Liu Y, Yan P, Bucher
B, Zuckerbraun BS, Gambotto A, Billiar TR and Yim JH: IRF-1
expression induces apoptosis and inhibits tumor growth in mouse
mammary cancer cells in vitro and in vivo. Oncogene. 23:1125–1135.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stang MT, Armstrong MJ, Watson GA, Sung
KY, Liu Y, Ren B and Yim JH: Interferon regulatory factor-1-induced
apoptosis mediated by a ligand-independent fas-associated death
domain pathway in breast cancer cells. Oncogene. 26:6420–6430.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao J, Senthil M, Ren B, Yan J, Xing Q, Yu
J, Zhang L and Yim JH: IRF-1 transcriptionally upregulates PUMA,
which mediates the mitochondrial apoptotic pathway in IRF-1-induced
apoptosis in cancer cells. Cell Death Differ. 17:699–709. 2010.
View Article : Google Scholar
|
|
8
|
Armstrong MJ, Stang MT, Liu Y, Yan J,
Pizzoferrato E and Yim JH: IRF-1 inhibits NF-κB activity,
suppresses TRAF2 and cIAP1 and induces breast cancer cell specific
growth inhibition. Cancer Biol Ther. 16:1029–1041. 2015. View Article : Google Scholar :
|
|
9
|
Li P, Du Q, Cao Z, Guo Z, Evankovich J,
Yan W, Chang Y, Shao L, Stolz DB, Tsung A, et al: Interferon-γ
induces autophagy with growth inhibition and cell death in human
hepatocellular carcinoma (HCC) cells through interferon-regulatory
factor-1 (IRF-1). Cancer Lett. 314:213–222. 2012. View Article : Google Scholar
|
|
10
|
Yanai H, Negishi H and Taniguchi T: The
IRF family of transcription factors: Inception, impact and
implications in oncogenesis. Oncoimmunology. 1:1376–1386. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Esmat G, El-Bendary M, Zakarya S, Ela MA
and Zalata K: Role of Helicobacter pylori in patients with
HCV-related chronic hepatitis and cirrhosis with or without
hepatocellular carcinoma: Possible association with disease
progression. J Viral Hepat. 19:473–479. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yi Y, Wu H, Gao Q, He HW, Li YW, Cai XY,
Wang JX, Zhou J, Cheng YF, Jin JJ, et al: Interferon regulatory
factor (IRF)-1 and IRF-2 are associated with prognosis and tumor
invasion in HCC. Ann Surg Oncol. 20:267–276. 2013. View Article : Google Scholar
|
|
13
|
Carthew RW and Sontheimer EJ: Origins and
mechanisms of miRNAs and siRNAs. Cell. 136:642–655. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lujambio A and Lowe SW: The microcosmos of
cancer. Nature. 482:347–355. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Otsuka M, Kishikawa T, Yoshikawa T, Ohno
M, Takata A, Shibata C and Koike K: The role of microRNAs in
hepato-carcinogenesis: Current knowledge and future prospects. J
Gastroenterol. 49:173–184. 2014. View Article : Google Scholar
|
|
16
|
D'Anzeo M, Faloppi L, Scartozzi M,
Giampieri R, Bianconi M, Del Prete M, Silvestris N and Cascinu S:
The role of micro-RNAs in hepatocellular carcinoma: From molecular
biology to treatment. Molecules. 19:6393–6406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bai Y, Xue Y, Xie X, Yu T, Zhu Y, Ge Q and
Lu Z: The RNA expression signature of the HepG2 cell line as
determined by the integrated analysis of miRNA and mRNA expression
profiles. Gene. 548:91–100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He XX, Kuang SZ, Liao JZ, Xu CR, Chang Y,
Wu YL, Gong J, Tian DA, Guo AY and Lin JS: The regulation of
microRNA expression by DNA methylation in hepatocellular carcinoma.
Mol Biosyst. 11:532–539. 2015. View Article : Google Scholar
|
|
19
|
Huang S, He X, Ding J, Liang L, Zhao Y,
Zhang Z, Yao X, Pan Z, Zhang P, Li J, et al: Upregulation of
miR-23a~27a~24 decreases transforming growth factor-beta-induced
tumor-suppressive activities in human hepatocellular carcinoma
cells. Int J Cancer. 123:972–978. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nazarov PV, Reinsbach SE, Muller A, Nicot
N, Philippidou D, Vallar L and Kreis S: Interplay of microRNAs,
transcription factors and target genes: Linking dynamic expression
changes to function. Nucleic Acids Res. 41:2817–2831. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu X, Ru J, Zhang J, Zhu LH, Liu M, Li X
and Tang H: miR-23a targets interferon regulatory factor 1 and
modulates cellular proliferation and paclitaxel-induced apoptosis
in gastric adenocarcinoma cells. PLoS One. 8:e647072013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ru J, Sun H, Fan H, Wang C, Li Y, Liu M
and Tang H: MiR-23a facilitates the replication of HSV-1 through
the suppression of interferon regulatory factor 1. PLoS One.
9:e1140212014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shao L, Guo Z and Geller DA:
Transcriptional suppression of cytokine-induced iNOS gene
expression by IL-13 through IRF-1/ISRE signaling. Biochem Biophys
Res Commun. 362:582–586. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tanaka N, Ishihara M, Lamphier MS, Nozawa
H, Matsuyama T, Mak TW, Aizawa S, Tokino T, Oren M and Taniguchi T:
Cooperation of the tumour suppressors IRF-1 and p53 in response to
DNA damage. Nature. 382:816–818. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen FF, Jiang G, Xu K and Zheng JN:
Function and mechanism by which interferon regulatory factor-1
inhibits oncogenesis. Oncol Lett. 5:417–423. 2013.PubMed/NCBI
|
|
26
|
Armstrong MJ, Stang MT, Liu Y, Gao J, Ren
B, Zuckerbraun BS, Mahidhara RS, Xing Q, Pizzoferrato E and Yim JH:
Interferon regulatory factor 1 (IRF-1) induces
p21WAF1/CIP1 dependent cell cycle arrest and
p21WAF1/CIP1 independent modulation of survivin in
cancer cells. Cancer Lett. 319:56–65. 2012. View Article : Google Scholar
|
|
27
|
Kondo T, Minamino N, Nagamura-Inoue T,
Matsumoto M, Taniguchi T and Tanaka N: Identification and
characterization of nucleophosmin/B23/numatrin which binds the
anti-oncogenic transcription factor IRF-1 and manifests oncogenic
activity. Oncogene. 15:1275–1281. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang B, Hsu SH, Frankel W, Ghoshal K and
Jacob ST: Stat3-mediated activation of microRNA-23a suppresses
gluconeogenesis in hepatocellular carcinoma by down-regulating
glucose-6-phosphatase and peroxisome proliferator-activated
receptor gamma, coactivator 1 alpha. Hepatology. 56:186–197. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang N, Zhu M, Tsao SW, Man K, Zhang Z and
Feng Y: MiR-23a-mediated inhibition of topoisomerase 1 expression
potentiates cell response to etoposide in human hepatocellular
carcinoma. Mol Cancer. 12:1192013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhong X, Coukos G and Zhang L: miRNAs in
human cancer. Methods Mol Biol. 822:295–306. 2012. View Article : Google Scholar
|
|
31
|
Yuan L, Zhou C, Lu Y, Hong M, Zhang Z,
Zhang Z, Chang Y, Zhang C and Li X: IFN-γ-mediated IRF1/miR-29b
feedback loop suppresses colorectal cancer cell growth and
metastasis by repressing IGF1. Cancer Lett. 359:136–147. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Z, Chen B, Feng M, Ouyang H, Zheng M,
Ye Q, Nie Q and Zhang X: MicroRNA-23b promotes avian leukosis virus
subgroup J (ALV-J) replication by targeting IRF1. Sci Rep.
5:102942015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mao L, Zhang Y, Mo W, Yu Y and Lu H:
BANF1is downregulated by IRF1-regulated microRNA-203 in cervical
cancer. PLoS One. 10:e01170352015. View Article : Google Scholar
|