Introduction
A process of generation of new blood vessels has
been proved to be necessary for sustained tumor growth and cancer
progression. Inhibiting angiogenesis pathway has long been a
significant hope for the development of novel, effective and target
orientated antitumor agents arresting the tumor proliferation and
metastasis (1).
Nitric oxide (NO) is a free radical involved in
physiological as well as in pathophysiological processes, and
synthesized from the conversion of L-arginine to citrulline by
three distinct forms of NO synthase (NOS): endothelial NOS (eNOS),
inducible NOS and neuronal NOS in different cell types and tissues
(2).
One of the many physiological functions of NO is as
an important modulator of endothelial function pertaining to
angiogenesis (3). NO has been shown
to stimulate and inhibit the proliferation, migration and
differentiation of endothelial cells in vitro and
angiogenesis in vivo (4). In
tumor biology, NO was demonstrated to promote either tumor invasion
and angiogenesis or tumor regression (5), chiefly increasing DNA synthesis, cell
proliferation and migration of endothelial cells to promote tumor
angiogenesis (6).
Specifically, eNOS was shown to modulate
cancer-related events (angiogenesis, apoptosis, cell cycle,
invasion and metastasis) and genetic studies showed that eNOS gene
polymorphisms are associated with the development of multiple types
of cancers (7,8). The present study found a positive
correlation between the expression of nitric oxide synthase (NOS)
and tumor progression; treatment with inhibitors of NOS,
NG-methyl-l-arginine (NMMA) and NG-nitro-l-arginine methyl ester
(L-NAME), had antitumor and antimetastatic effects that were partly
attributed to reduced tumor cell invasiveness (9). The above suggests that eNOS-derived NO
signaling is one of the key factors in regulating endothelial
function and inducing tumor angiogenesis.
Tetrahydrobiopterin (BH4) is an essential cofactor
required for the activity of eNOS. Suboptimal concentrations of BH4
in the endothelium reduce the biosynthesis of NO, and
preferentially produces superoxide (10) and contributes to angiogenesis
regulation (11). In vivo,
de novo BH4 biosynthesis is regulated by the ratelimiting
enzyme guanosine triphosphate cyclohydrolase I (GTPCH; EC
3.5.4.16), which converts GTP to dihydroneopterin triphosphate. BH4
is produced through further steps, which are catalyzed by
6-pyruvoyl tetrahydropterin synthase and sepiapterin reductase
(12). Recent evidence suggests an
important role for BH4 synthesis in angiogenesis by the activation
of eNOS for NO production, which is maintained by a
phosphatidylinositol 3-kinase (PI3K)/Akt positive feedback loop
through effects on wild-type Ras in endothelial cells (13). Theoretically, BH4 biosynthesis has
been directly associated with activation of Akt and eNOS
phosphorylation in inducing angiogenesis (14). However, impacts of downregulation of
endogenous BH4 by GTPCH pathway on angiogenesis and the mechanism
is not known in hepatocellular carcinoma (HCC).
In the present study, we investigated the effects of
downregulation of BH4 by 2,4-diamino-6-hydroxypyrimidine (DAHP) on
anti-angiogenesis and explored the mechanism via a BALB/c-nu mouse
HCC xenograft model. On the basis of our results, we propose that
downregulation of BH4 could be a promising novel therapeutic
strategy, and GTPCH could be a new target of the treatment for
HCC.
Materials and methods
Reagents
6R-5,6,7,8-Tetrahydrobiopterin dihydrochloride (BH4)
and DAHP were purchased from Sigma-Aldrich (Shanghai, China), and
dissolved in phosphate-buffered saline (PBS).
Cell culture
Primary human umbilical vein endothelial cells
(HUVECs) were purchased from American Type Culture Collection
(ATCC; Manassas, VA, USA) and grown in endothelial cell growth
medium-2 (EGM-2) with bullet kit at 37°C in 5% CO2.
HUVECs were used at passages 4–10. Before experiments, the cells
were deprived of fetal bovine serum (FBS) for 3–4 h for addition of
reagents. All samples were determined in triplicate.
HepG-2 cells purchased from the Institute of Yunnan
Provincial Tumors (Kunming, China) and grown in RPMI-1640 with 10%
FBS and with bullet kit at 37°C in 5% CO2 in our
laboratory. All samples were determined in triplicate.
Tubulogenesis
Ninety-six-well culture plates were coated with 50
µl/well of growth factor-reduced Matrigel (BD Biosciences,
Shanghai, China). HUVECs (1×105/well) were incubated
with 2% FBS for 24 h. Then, the cells were incubated with 30
µg/well of DAHP for 48 h. The number of loops was counted in
images captured at a magnification of ×20.
Animals
BALB/c-nu mice used in the present study were
obtained from Vital River Laboratory Animal Technology Co. Ltd.
(Beijing, China) and were maintained on a standard 12 h:12 h
light-dark cycle with free access to food and water. Male mice,
aged 4–6 weeks and weighing 18–22 g were used in the experiments.
All of the animal procedures were in accordance with the
institutional guidelines of Kunming Medical University.
Animal procedures and experimental
groups
In the axillary region, mice received subcutaneous
implants of 1×107 HepG2 cells. Cells (107)
suspended in 100 µl of PBS (BD Biosciences) were
subcutaneously injected into flanks of BALB/c-nu mice and resultant
tumors measured twice weekly. Mice were treated with 80 mg/kg
(i.p.) DAHP once a day for two weeks once tumors reached a volume
of 100 mm3 as previously described (15). Equal volumes of saline were injected
in control mice. The long and short diameter of tumor was,
respectively, recorded as A and B two weeks after the treatment,
and then the volume of the tumor was calculated via the formula V =
0.5 × A × B2.
Histopathology
Samples were respectively harvested after a period
of time. The tumor were fixed using 4% formaldehyde and were
embedded using paraffin then serially sectioned (5 mm) in
toto. Every third slide was stained with hematoxylin and eosin
(H&E) for histomorphometric analyses. One or two images/slide
were captured with a microscope (Olympus, Japan) at a magnification
of ×200 and at a resolution of 640×480 pixels.
Immunohistochemistry for CD31
Paraffin-embedded tissue blocks from formalin-fixed
tumor samples were sectioned, dewaxed and rehydrated following
standard protocols. Sections were incubated for 2 h at room
temperature with the anti-CD31 monoclonal antibody (Proteintech,
Wuhan, China), followed by incubations with saturating amounts of
biotin-labeled secondary antibody and streptavidin-peroxidase for
20 min each. After incubation in a solution containing 0.06 mM
diaminobenzidine (Dako) and 2 mM hydrogen peroxide in 0.05% PBS (pH
7.6) for 5 min, slides were washed, dehydrated with alcohol and
xylene, and mounted with coverslips. Sections were examined with a
microscope (Olympus) and images were acquired at a magnification of
×400. For quantification, the product of the proportion of positive
cells in quartiles (0–4) and the staining intensity (0, no
staining; 1, weak; 2, moderate; and 3, strong) was calculated,
yielding a total immunostaining score ranging from 0 to 12.
Western blotting
Thirty micrograms of cytosolic protein was
electrophoresed on 12% acrylamide sodium dodecyl sulfate gels and
was transferred to nitrocellulose membranes (Schleicher &
Schuell, Keene, NH, USA). To block non-specific binding, 5% non-fat
dry milk in PBS-Tween (0.1%) was added to the membrane for 1 h at
room temperature. Membranes were washed in PBS-Tween then incubated
overnight with mouse mAbs for eNOS, p-eNOS, AktT, p-AkT and GTPCH
(dilution 1:1,000; Santa Cruz Biotechnology, Shanghai, China), and
a rabbit anti-mouse polyclonal antibody for mouse β-actin (dilution
1:100; Kangwei Shiji Biotechnology, Peking, China) followed by
incubation with a secondary antibody for 1 h. After repeat washings
with PBS-Tween, membranes were developed using the SuperSignal
detection system (Pierce Chemical, Rockford, IL, USA) and were
exposed to film. Quantification of the western blot analyses for
eNOS, p-eNOS, Akt, p-Akt and GTPCH was performed using gel analysis
software.
Quantitative real-time RT-PCR for K-ras
mRNA
Total RNA was extracted from frozen tumor tissue by
homogenization in 1 ml of TRIzol solution (Sigma-Aldrich). The
tumor was incubated for 5 min in 1 ml of TRIzol, and the residual
tissue was removed. Total tissue RNA (1 µg), dissolved in
RNase-free water was used in RT-PCR (Maxima® QuantiTect
SYBR-Green RT-PCR kit; Fermentas, Shanghai, China) using the
following primers for various K-ras mRNA transcripts (5′-3′): mouse
K-ras forward, TGCAAACTGTCAGCTTTATCTCAA and reverse,
CTTCATTATCCTGCTTCCCATC; mouse β-actin forward,
CGTGCGTGACATCAAAGAGAAG and reverse, CCAAGAAGGAAGGCTGGAAAA.
Quantitative fluorescent real-time RT-PCR analysis was performed to
compare relative quantities of mRNA in mouse tumor tissue using the
ABI PRISM 7300 system (Applied Biosystems Inc., Foster City, CA,
USA). Samples were processed in triplicate, with a reverse
transcriptase (RT) negative control reaction for each sample. For
K-ras RT-PCR analysis, standards were prepared using 10-fold
dilutions of BALB/c-nu/nu mouse tumor total RNA. Quantification was
performed using DataAssist™ v3.0 software (Applied Biosystems Inc.)
to generate standard curves, expressing relative quantities of PCR
products in the experimental samples in arbitrary units relative to
the standard curve. Mean values were calculated from triplicate
samples to produce 1. Results from at least six animals per group
were pooled to produce the mean and SEM.
NO content
Tumor tissue nitrite and nitrate levels were
determined using a commercially available kit (Beyotime
Biotechnology, Shanghai, China).
Biopterin measurements
Total biopterin (BH4, BH2 and biopterin) and BH4
(BH4=total biopterin, BH2-biopterin) levels were measured using
high-performance liquid chromatography (HPLC) analysis with
fluorescent detection after differential iodine oxidation of tissue
extracts in either acidic or alkaline conditions as previously
described (16).
Statistical analysis
The data are expressed as the mean ± SEM.
Statistical significance of differences between means was assessed
using independent sample t-test. SPSS for Windows version 18.0
(SPSS, Inc., Chicago, IL, USA) was used to complete all of the
analyses, and a P-value of <0.05 was considered to indicate a
statistically significant result.
Results
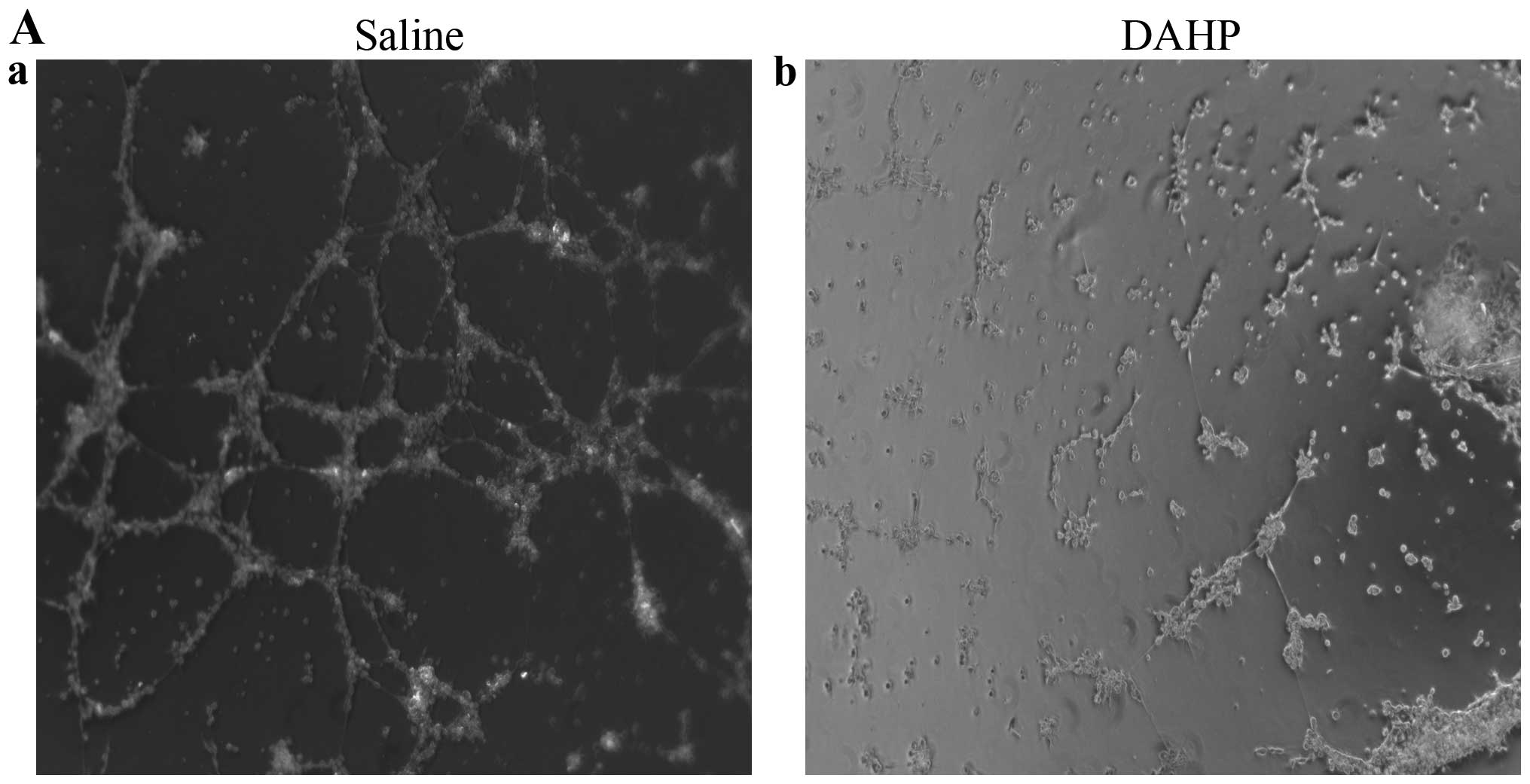
Effects of the administration of DAHP on
tubulogenesis of HUVEC in vitro
We firstly determined the effects of the
administration of DAHP on tubule formation of HUVEC in
vitro. The relative tubule length was significantly less in the
DAHP group (0.99±0.03) than in the saline group (0.26±0.03;
P<0.01) (Fig. 1). This confirms
that the administered DAHP inhibits tubule formation of HUVEC in
vitro.
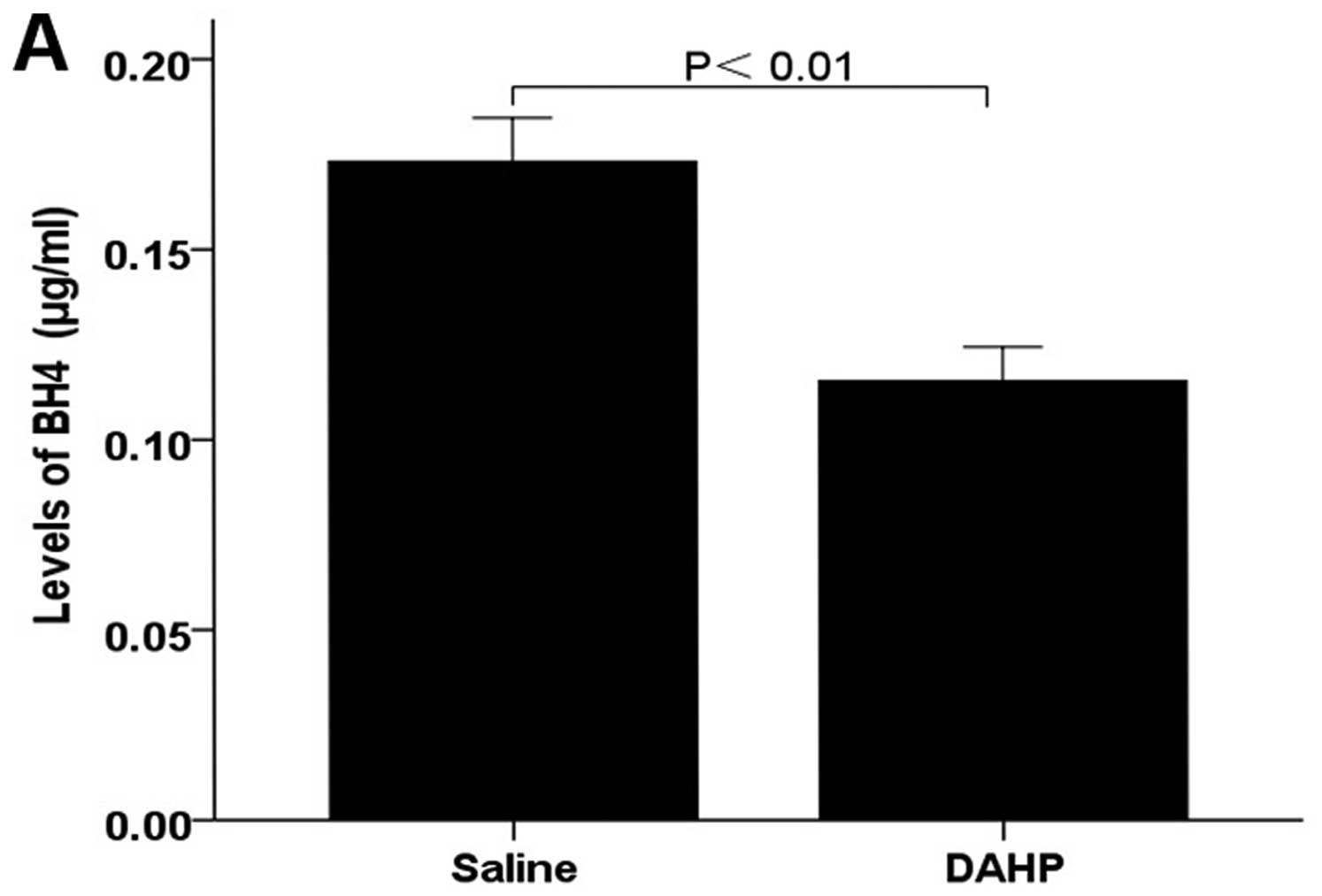
Effects of the administration of DAHP on
BH4 levels in the tumor tissue
We next determined whether the administration of
DAHP resulted in downregulation of BH4 in tumor tissues. Total
tumor biopterin and BH4 tissue levels were assessed by HPLC. Values
are expressed as mean ± SEM (n=6 animals/group) in µg/ml.
BH4 levels was significantly lower in the DAHP group (BH4:
0.12±0.01) than in the saline group (BH4:0.17±0.01; P<0.01)
(Fig. 2A). This confirms that the
administered DAHP decreases BH4 levels of the tumor tissue.
Effects of the administration of DAHP on
NO production
NO content was assessed using tumor tissue
nitrite/nitrate levels as indirect parameters. Nitrite/nitrate
levels in the tumor tissue were determined by the Greiss reaction.
Nitrite/nitrate levels in the tumor tissue were significantly
decreased by ~2-fold in the DAHP group (nitrite/nitrate:
11.70±0.62) compared with that observed in the saline group
(nitrite/nitrate: 24.77±0.54; P<0.01) (Fig. 2B). These findings suggest that
treatment with DAHP decreases NO synthesis of the tumor tissue.
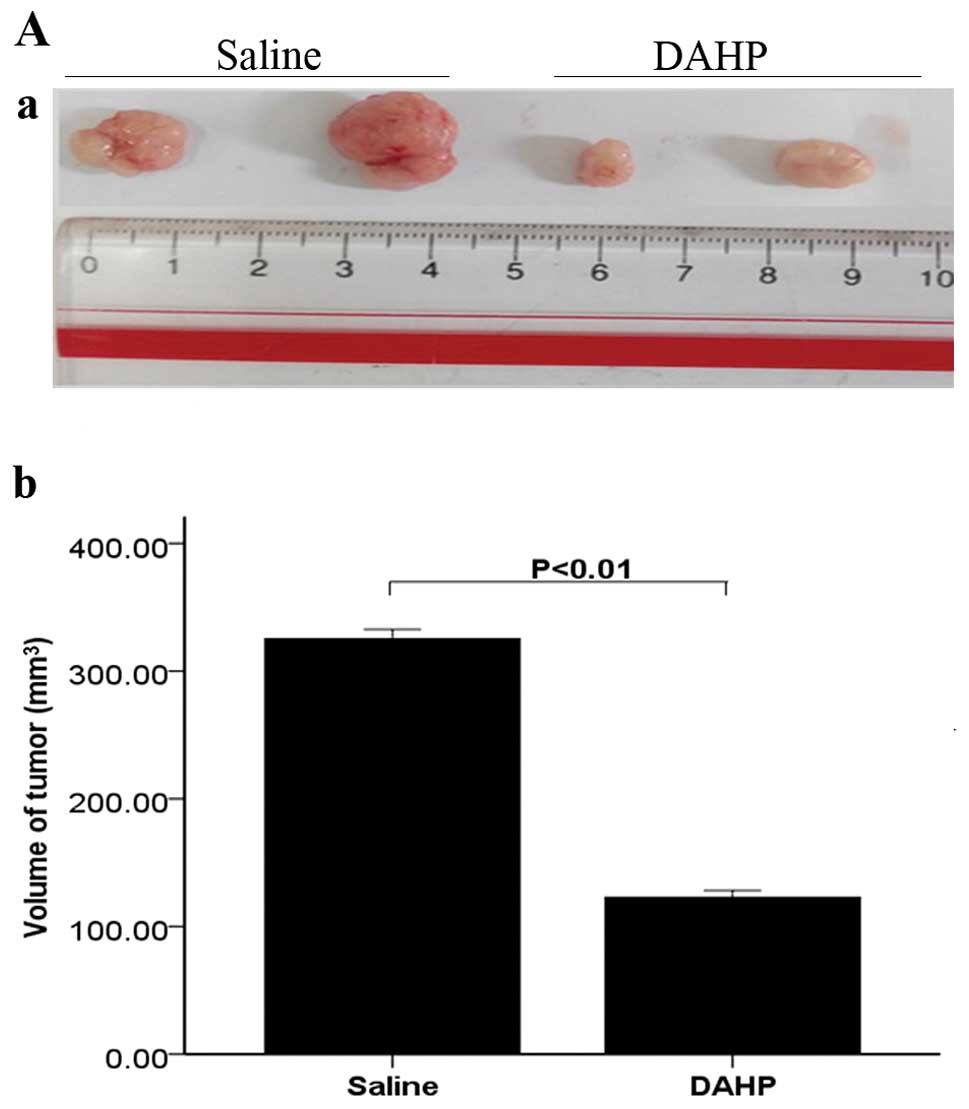
Morphology and histopathology
The tumor volume was significantly lower in the DAHP
group (122.87±5.39) than in the saline group (325.45±7.25;
P<0.01) (Fig. 3A). Histological
findings determined by H&E staining were quantified using Image
Pro Plus software. Treatment with DAHP (449.20±43.46) led to a
significantly decreased number of cancer cells compared,
respectively, with the saline group (1,171.20±51.67; P<0.01)
(Fig. 3B). These findings suggest
that treatment with DAHP inhibits growth of tumors in HCC.
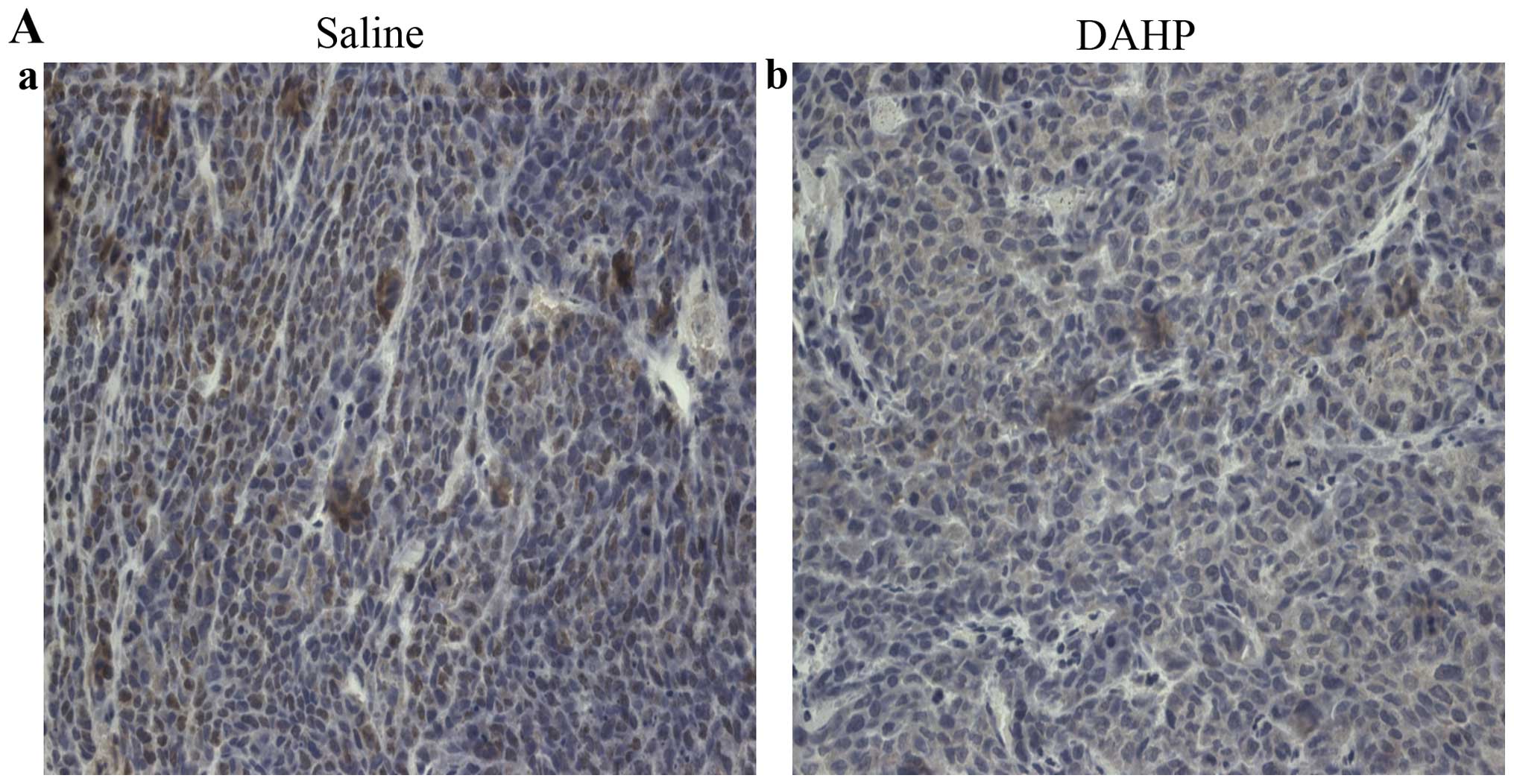
Effects of the administration of DAHP on
the expression of CD31 protein
Tumor angiogenesis was assessed by CD31
immunostaining. CD31 in the tumor tissue was significantly lower in
the DAHP group (1.40±0.11) compared with that observed in the
saline group (1.94±0.15; P<0.01) (Fig. 4). These findings suggest that
treatment with DAHP inhibits tumor angiogenesis.
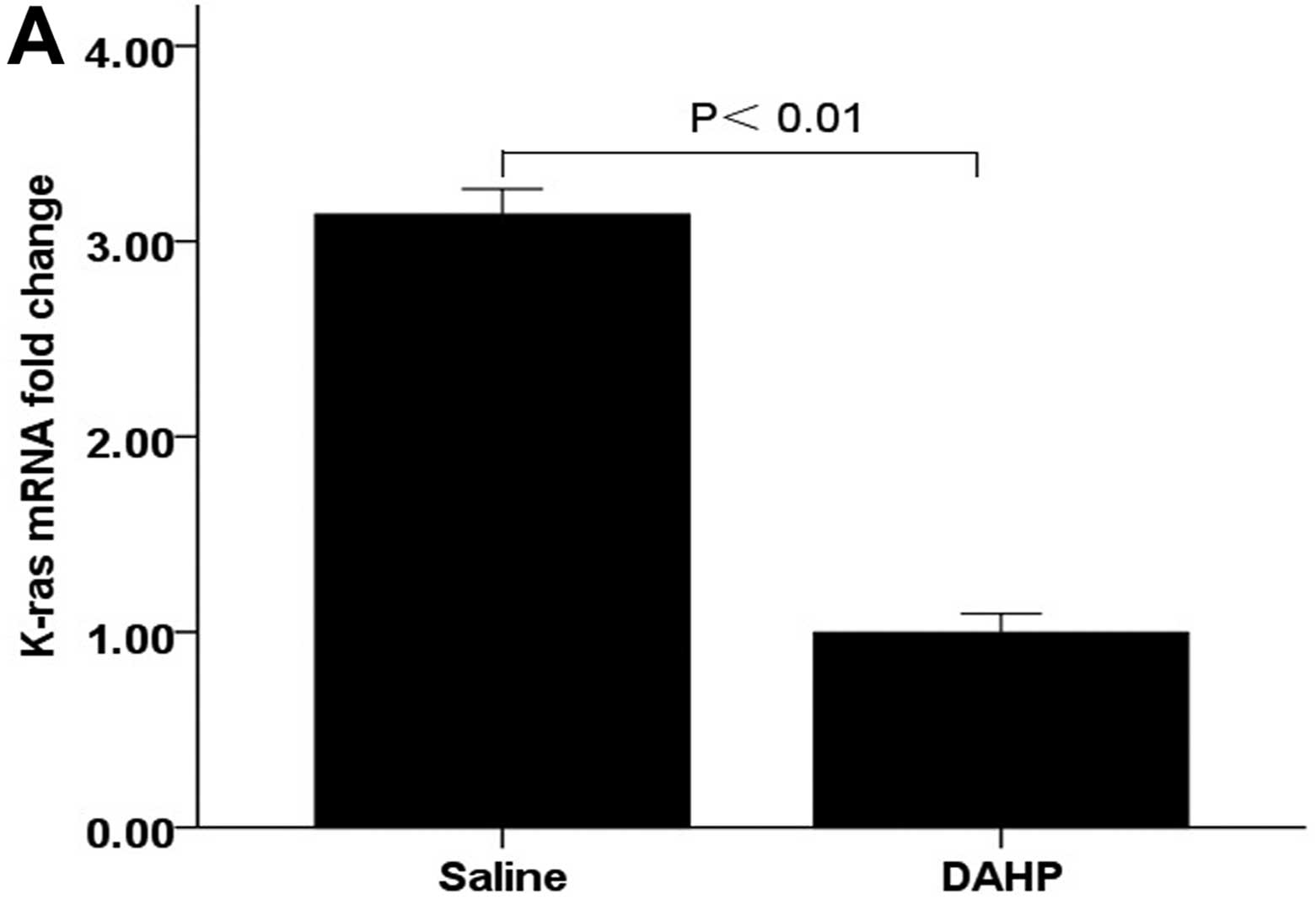
Effects of the administration of DAHP on
the activation of wild-type Ras protein
We next invetigated the effects of downregulation of
BH4 levels on K-ras mRNA by comparing with the saline control.
K-ras mRNA in the tumor tissue was significantly lower in the DAHP
group (0.99±0.10) compared with the saline group (3.14±0.13;
P<0.01) (Fig. 5A). These
findings suggest that treatment with DAHP decreases K-ras mRNA of
the tumor.
Effects of the administration of DAHP on
GTPCH expression in angiogenesis
We investigated the effects of DAHP specifically on
the expression of GTPCH by comparing saline-treated mice. GTPCH in
the tumor tissue was significantly lower in the DAHP group
(0.78±0.04) compared with the saline group (0.86±0.06; P<0.05)
(Fig. 5B). These findings suggest
that treatment with DAHP decreases the expression of GTPCH, with a
corresponding decrease in the BH4 levels.
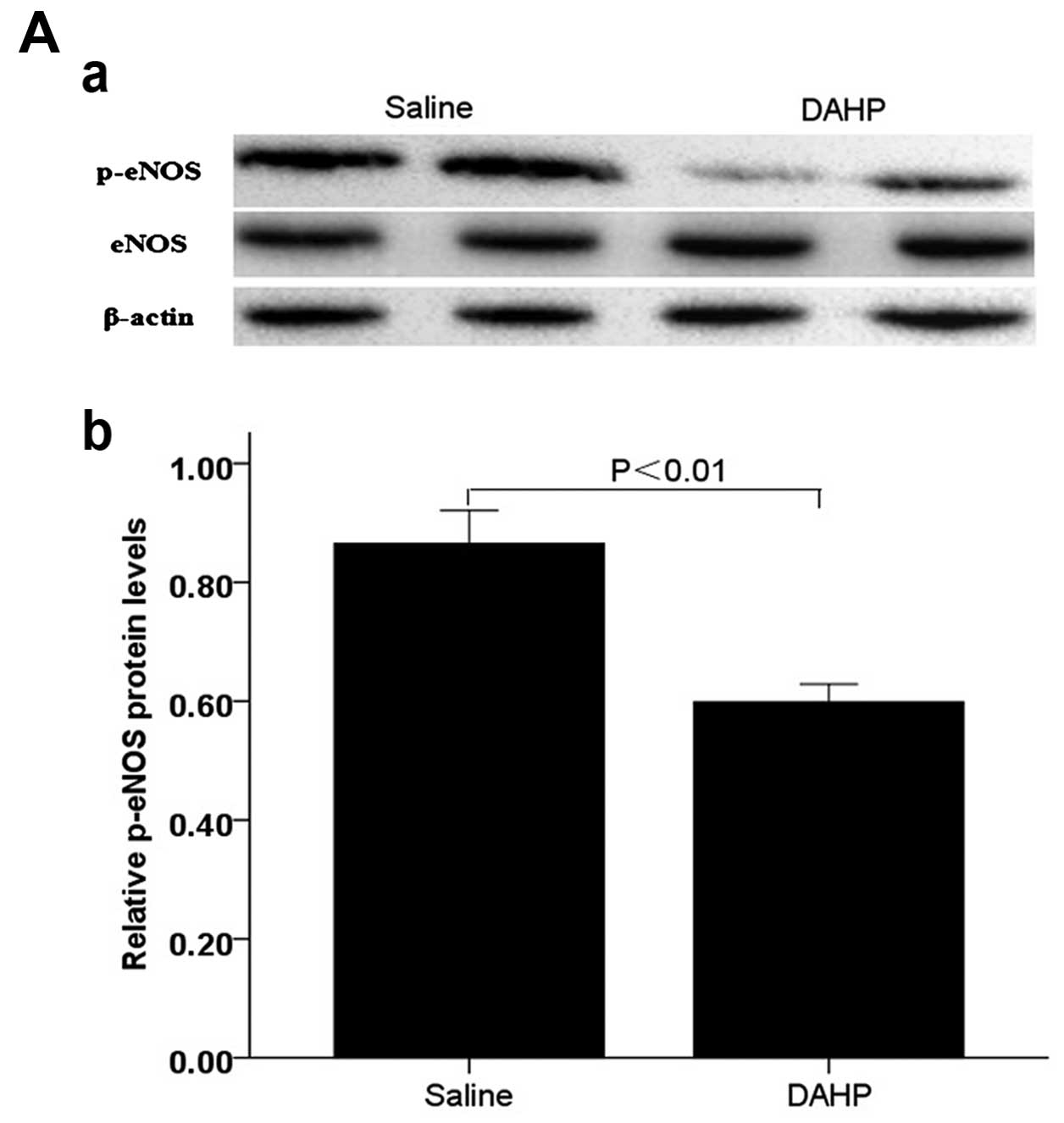
Effects of the administration of DAHP on
phosphorylation of eNOS and Akt via PI3K pathway
We next investigated the effects of downregulation
of BH4 levels specifically on phosphorylation of eNOS and Akt via
PI3K pathway by comparing saline-treated mice. Expression of p-eNOS
(Ser1177) and p-Akt (Ser473) in the tumor
tissue were significantly lower in the administration with DAHP
group compared with that observed in the saline group (p-eNOS:
0.60±0.03 vs. saline: 0.87±0.06; P<0.01; p-Akt: 0.67±0.03 vs.
saline: 0.91±0.05; P<0.01) (Fig.
6).
These results showed that DAHP inhibits
PI3K-dependent phosphorylation of Akt/eNOS, with a corresponding
decrease in the angiogenesis markers such as CD31. Moreover, the
consequent descension of NO is paralleled by inhibition of
intracellular wild-type Ras and PI3K signaling.
Discussion
There are many treatment options for patients with
early-stage hepatocellular carcinoma (HCC), but the treatment
options in advanced-stage HCC are limited, and the survival rate is
dismal. HCC is a highly vascular tumor, its growth is dependent on
the formation of new blood vessels (17). Anti-angiogenic therapies with
sorafenib were the first systemic therapy to demonstrate improved
survival in patients with advanced-stage HCC (18). This important development in the
treatment of HCC raises hope, but cannot meet the needs of the
patients. Thus, novel therapeutic approaches are desperately
needed.
As previously described, multiple Ras family members
directly bind to and activate the p110 catalytic subunit of the
class I PI3Ks (19). Ras driven
tumors exploit the functions of PI3K pathways in mitosis,
apoptosis, motility, proliferation and differentiation. PI3K and
Akt may regulate tumor angiogenesis by the induction of NOS
(20). Tumor angiogenesis is
regulated by the tumor microenvironment composed of tumor, vascular
endothelial and stromal cells. In addition to cancer cells, the
microvascular endothelial cells recruited by the tumor are
important for cancer development (21,22).
PI3K/Akt pathway also controls the tumor microenvironment,
including endothelial cells (23).
PI3K can regulate endothelial migration, proliferation and survival
through the effect of its downstream targets such as NOS, to
regulate tumor angiogenesis (24).
Our data suggested that inhibiting BH4 synthesis can decrease K-ras
mRNA, and activation of phosphorylation of eNOS and Akt, and
inhibited the produces of NO. This is corresponding to the
descending levels of angiogenesis markers such as CD31.
Previous studies have shown that increasing BH4
synthesis can promote endothelial cell proliferation, migration and
tubule formation in cultures in vitro (25). Moreover, the present study
demonstrates also that BH4 synthesis via either the pterin salvage
or the de novo pathway induces angiogenesis in tumor
xenografts. These effects correlated with increases in eNOS
produced NO that depended, in turn, on the activation of wild-type
Ras and its downstream PI3K/Akt effectors (26). BH4 synthesis increases
phosphorylation of eNOS at Ser1177 and Akt phosphorylation resulted
in eNOS-derived NO rather than superoxide (27,28).
Theoretically, downregulation of BH4 synthesis could inhibit tumor
angiogenesis, and GTPCH would be a therapeutic target of
anti-angiogenesis for HCC. Our data suggested that downregulation
BH4 synthesis via DAHP can inhibit K-ras mRNA and activation of
phosphorylation of eNOS and Akt.
In the present study, we showed that in DAHP-treated
tumors, BH4 and NO levels remain lower compared with the saline
controls, and CD31 are lower than in controls. We suggested that
there were inhibitory effects of DAHP on tumor angiogenesis,
dependent on downregulation of BH4 synthesis. We found that DAHP
downregulates eNOS and Akt protein expression, corresponding to
decreased eNOS phosphorylation at Ser1177 and Akt phosphorylation.
It is well-known that DAHP is recognized as a specific competitive
inhibitor of GTPCH as it has structural similarity to GTP (29). It also acts indirectly on GTPCH by
directly binding to a GTPCH feedback regulatory protein to
negatively inhibit GTPCH activity (30). Our data suggested that DAHP
downregulates GTPCH protein expression, with a corresponding
decrease of the BH4 levels and contents of NO. Indeed, in the
present study, decreased CD31 indicated that tumor angiogenesis was
inhibited in HCC.
Taken together, our data suggested that DAHP,
recognized as a specific competitive inhibitor of GTPCH, can
decrease tumor BH4 and NO by the inhibition of the wild-type
Ras-PI3K/Akt pathway, and then inhibiting angiogenesis. Therefore,
we now give proof-of-principle that strategies targeting BH4
synthetic pathways may be a rational way to inhibit angiogenesis,
and to control potential progression of HCC. Clearly, our
recognition of DAHP in anti-angiogenesis in HCC is incomplete. In
the future, we must investigate the precise mechanisms responsible
for these findings to develop the most suitable treatment strategy
for HCC.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81201910). We
thank Yao Qian for technical assistance in the experiments.
References
|
1
|
Gacche RN and Meshram RJ: Targeting tumor
micro-environment for design and development of novel
anti-angiogenic agents arresting tumor growth. Prog Biophys Mol
Biol. 113:333–354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stuehr DJ: Mammalian nitric oxide
synthases. Biochim Biophys Acta. 1411:217–230. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marinos RS, Zhang W, Wu G, Kelly KA and
Meininger CJ: Tetrahydrobiopterin levels regulate endothelial cell
proliferation. Am J Physiol Heart Circ Physiol. 281:H482–H489.
2001.PubMed/NCBI
|
|
4
|
Ridnour LA, Isenberg JS, Espey MG, Thomas
DD, Roberts DD and Wink DA: Nitric oxide regulates angiogenesis
through a functional switch involving thrombospondin-1. Proc Natl
Acad Sci USA. 102:13147–13152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Burke AJ, Sullivan FJ, Giles FJ and Glynn
SA: The yin and yang of nitric oxide in cancer progression.
Carcinogenesis. 34:503–512. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fukumura D, Kashiwagi S and Jain RK: The
role of nitric oxide in tumour progression. Nat Rev Cancer.
6:521–534. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ying L and Hofseth LJ: An emerging role
for endothelial nitric oxide synthase in chronic inflammation and
cancer. Cancer Res. 67:1407–1410. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barbieri A, Palma G, Rosati A, Giudice A,
Falco A, Petrillo A, Petrillo M, Bimonte S, Di Benedetto M,
Esposito G, et al: Role of endothelial nitric oxide synthase (eNOS)
in chronic stress-promoted tumour growth. J Cell Mol Med.
16:920–926. 2012. View Article : Google Scholar
|
|
9
|
Jadeski LC and Lala PK: Nitric oxide
synthase inhibition by NG-nitro-L-arginine methyl ester
inhibits tumor-induced angiogenesis in mammary tumors. Am J Pathol.
155:1381–1390. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Katusic ZS, d'Uscio LV and Nath KA:
Vascular protection by tetrahydrobiopterin: Progress and
therapeutic prospects. Trends Pharmacol Sci. 30:48–54. 2009.
View Article : Google Scholar :
|
|
11
|
Lowndes SA, Sheldon HV, Cai S, Taylor JM
and Harris AL: Copper chelator ATN-224 inhibits endothelial
function by multiple mechanisms. Microvasc Res. 77:314–326. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thöny B, Auerbach G and Blau N:
Tetrahydrobiopterin biosynthesis, regeneration and functions.
Biochem J. 347:1–16. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen L, Zeng X, Wang J, Briggs SS, O'Neill
E, Li J, Leek R, Kerr DJ, Harris AL and Cai S: Roles of
tetrahydrobiopterin in promoting tumor angiogenesis. Am J Pathol.
177:2671–2680. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sugiyama T, Levy BD and Michel T:
Tetrahydrobiopterin recycling, a key determinant of endothelial
nitric-oxide synthase-dependent signaling pathways in cultured
vascular endothelial cells. J Biol Chem. 284:12691–12700. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lampson BL, Kendall SD, Ancrile BB,
Morrison MM, Shealy MJ, Barrientos KS, Crowe MS, Kashatus DF, White
RR, Gurley SB, et al: Targeting eNOS in pancreatic cancer. Cancer
Res. 72:4472–4482. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alp NJ, Mussa S, Khoo J, Cai S, Guzik T,
Jefferson A, Goh N, Rockett KA and Channon KM:
Tetrahydrobiopterin-dependent preservation of nitric oxide-mediated
endothelial function in diabetes by targeted transgenic
GTP-cyclohydrolase I overexpression. J Clin Invest. 112:725–735.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sampat KR and O'Neil B: Antiangiogenic
therapies for advanced hepatocellular carcinoma. Oncologist.
18:430–438. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu AX, Duda DG, Sahani DV and Jain RK:
HCC and angiogenesis: Possible targets and future directions. Nat
Rev Clin Oncol. 8:292–301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rodriguez-Viciana P and Downward J: Ras
activation of phosphatidylinositol 3-kinase and Akt. Methods
Enzymol. 333:37–44. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Engelman JA, Chen L, Tan X, Crosby K,
Guimaraes AR, Upadhyay R, Maira M, McNamara K, Perera SA, Song Y,
et al: Effective use of PI3K and MEK inhibitors to treat mutant
Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med.
14:1351–1356. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stoeltzing O, Meric-Bernstam F and Ellis
LM: Intracellular signaling in tumor and endothelial cells: The
expected and, yet again, the unexpected. Cancer Cell. 10:89–91.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Phung TL, Ziv K, Dabydeen D, Eyiah-Mensah
G, Riveros M, Perruzzi C, Sun J, Monahan-Earley RA, Shiojima I,
Nagy JA, et al: Pathological angiogenesis is induced by sustained
Akt signaling and inhibited by rapamycin. Cancer Cell. 10:159–170.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng H, Dai T, Zhou B, Zhu J, Huang H,
Wang M and Fu G: SDF-1alpha/CXCR4 decreases endothelial progenitor
cells apoptosis under serum deprivation by PI3K/Akt/eNOS pathway.
Atherosclerosis. 201:36–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Michaelis M, Michaelis R, Suhan T, Schmidt
H, Mohamed A, Doerr HW and Cinatl J Jr: Ribavirin inhibits
angiogenesis by tetrahydrobiopterin depletion. FASEB J. 21:81–87.
2007. View Article : Google Scholar
|
|
26
|
Dimmeler S, Fleming I, Fisslthaler B,
Hermann C, Busse R and Zeiher AM: Activation of nitric oxide
synthase in endothelial cells by Akt-dependent phosphorylation.
Nature. 399:601–605. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Du YH, Guan YY, Alp NJ, Channon KM and
Chen AF: Endothelium-specific GTP cyclohydrolase I overexpression
attenuates blood pressure progression in salt-sensitive low-renin
hypertension. Circulation. 117:1045–1054. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ceylan-Isik AF, Guo KK, Carlson EC,
Privratsky JR, Liao SJ, Cai L, Chen AF and Ren J: Metallothionein
abrogates GTP cyclohydrolase I inhibition-induced cardiac
contractile and morphological defects: Role of mitochondrial
biogenesis. Hypertension. 53:1023–1031. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kolinsky MA and Gross SS: The mechanism of
potent GTP cyclohydrolase I inhibition by
2,4-diamino-6-hydroxypyrimidine: Requirement of the GTP
cyclohydrolase I feedback regulatory protein. J Biol Chem.
279:40677–40682. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xie L, Smith JA and Gross SS: GTP
cyclohydrolase I inhibition by the prototypic inhibitor
2,4-diamino-6-hydroxypyrimidine. Mechanisms and unanticipated role
of GTP cyclohydrolase I feedback regulatory protein. J Biol Chem.
273:21091–21098. 1998. View Article : Google Scholar : PubMed/NCBI
|