Introduction
Prostate cancer is the second leading cause of
cancer-related mortality in males, and the incidence has been
rising rapidly worldwide, including the incidence in low-risk
populations (1,2). The etiological factors for prostate
cancer include genetic changes, sex hormones, diet and environment
(3,4). Based on prostate cancer pathogenesis
and characteristics, various advanced strategies have been applied
in clinical practice. However, deleterious side effects frequently
occur and make current strategies ineffective against stage T3
prostate cancer (5). Therefore,
exploring effective management strategies and identifying
therapeutic targets are urgently needed for the treatment of
prostate cancer.
Myosins are motor proteins that move along
cytoskeletal filaments by using energy derived from ATP (6,7).
Myosins constitute a superfamily of more than 18 known members
(8). Myosin VI (MYO6) is a
member of the unconventional myosin protein, which moves towards
the minus ends of polarized actin filaments in the opposite
direction to all other myosins. Previous studies indicate that it
can promote cancer-related cell migration and cellular functions
(9–11). For instance, the in vitro
migration and colony formation were impaired in LNCap human
prostate cancer cells after MYO6 knockdown (12). The cell spreading and migration of
high-grade ovarian carcinoma cells were impeded by knockdown of
MYO6 (13). The
overexpression of cancer-specific MYO6 has been shown
primarily restricted in human prostate and breast cancers (12). In addition, MYO6 was shown to
regulate protein secretion in prostate cancer cells (14).
To explore the relationship between MYO6 and
prostate cancer, the association among MYO6 expression
profiles with clinical and pathological features of prostate cancer
was analyzed. Then, lentivirus-based shRNA was used to efficiently
knock down the expression of MYO6 in prostate cancer DU145
and PU-3 cells. We aimed to investigate its possible function to
impact growth in prostate cancer cells in vitro.
Materials and methods
Immunohistochemistry
A total of 148 cases of prostate cancer tissues used
in this research were biopsy samples obtained from Fuzhou General
Hospital of the Nanjing Military Command (Fuzhou, China).
Immunohistochemistry was carried out as previously described
(15) using Histostain-Plus 3rd Gen
IHC Detection kit (85-9073; Invitrogen). Following fixation with
100% acetone and quenching of endogenous peroxidase, the samples
were blocked with 2% normal goat serum (dilution 1:300, M0691;
Sigma). Then the samples were incubated with mouse anti-MYO6 mouse
(dilution 1:50, SC-50461; Santa Cruz Biotechnology), washed with
PBS and incubated with biotinylated goat anti-mouse IgG secondary
antibody. Finally, the staining intensity was scored visually and
recorded as follows: − for negative, −+ for slightly positive, +
for moderately positive and ++ for strongly positive
immunoreactivity.
Cell culture
The prostate cancer cell lines DU145 and PC-3 and
human embryonic kidney cell line 293 (HEK293) were purchased from
the Cell Bank of the Chinese Academy of Science (Shanghai, China).
DU145 cells were cultured in Ham's F-12 (#11765-054; Gibco-BRL)
medium containing 10% fetal bovine serum (FBS, #S1810; Biowest) and
1% non-essential amino acids (NEAA). PC-3 cells were plated in
Ham's F-12 supplemented with 10% FBS. HEK293T cells were grown in
Dulbecco's modified Eagle's medium (DMEM, SH30243.01; Hyclone) with
10% FBS. Cells were maintained in a humidified atmosphere with air
containing 5% CO2 at 37°C.
Lentiviral plasmid construction
Three segments of MYO6 (NCBI accession no.
NM_004999) targeted by shRNA were designed by siRNA-designing
software. The sequences of the shRNA targets were
5′-GTGAATCCAGAGATAAGTTTACTCGAGTAAACTTATCTCTGGATTCACTTTTT-3′ for
MYO6 shRNA s1 and
5′-CCAGATTTAACCATTCCATAACTCGAGTTATGGAATGGTTAAATCTGGTTTTTT-3′ for
MYO6 shRNA s2. The control shRNA sequence was
5′-GCGGAGGGTTTGAAAGAATATCTCGAGATATTCTTTCAAACCCTCCGCTTTTTT-3′. Three
nucleotide sequences were cloned into the pFH-L lentiviral vector
(Shanghai Hollybio, China), respectively. For lentivirus packaging,
the HEK293T cells were transfected with pFH-L-MYO6 shRNA s1,
pFH-L-MYO6 shRNA s2, or control shRNA with virion-packaging
elements (pVSVG-I and pCMVΔR8.92; Shanghai Hollybio) using
Lipofectamine 2000 (Invitrogen) to generate three groups shMYO6
(S1), shMYO6 (S2) and shCon. Forty-eight hours later, the
supernatant was collected and the lentiviral vector particles were
harvested by centrifugation (4,000 x g, 10 min at 4°C). Then, the
particles were filtered through a 45-µm filter and the viral
concentrate was collected by filtrate centrifuged for 20 min at
4,000 × g at 4°C.
Quantitative real-time PCR (qRT-PCR)
analysis
Total RNA was extracted from cells using TRIzol
reagent (Gibco-BRL). Primers for MYO6 (forward,
AATCACTGGCTCACATGCAG and reverse, AATGCGAGGTTTGTGTCTCC) and
actin (forward, GTGGACATCCGCAAAGAC and reverse,
AAAGGGTGTAACGCAACTA) were designed to evaluate mRNA expression of
MYO6 on BioRad Connet Real-Time PCR platform (Bio-Rad,
Hercules, CA, USA). Each 20 µl reaction contained 2X SYBR
Premix Ex Taq 10 µl, forward and reverse primers (2.5
µM) 0.8 µl, cDNA5 µl, and ddH2O 4.2
µl. The qPCR procedures were as followed: initial
denaturation for 1 min at 95°C, denaturation for 5 sec at 95°C, and
annealing for 40 cycles at 60°C. Relative expression of MYO6
mRNA was calculated by using the 2−ΔΔCt method.
Western blot assay
Lentivirus-transduced cells were washed twice with
ice-cold PBS and lysed in 2X SDS sample buffer (10 mM EDTA, 4% SDS,
10% glycine in 100 mM Tris-HCl buffer, pH 6.8) at 4°C. Cell
proteins were separated by SDS-PAGE and transferred to
polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA).
The membranes were blocked by 4% nonfat dry milk and incubated with
mouse anti-MYO6 (1:1,000 dilution, cat #M0691; Sigma), rabbit
anti-Akt3 (1:500 dilution, 21641-1-AP; Proteintech), rabbit
anti-PARP (1:1,000 dilution, #9542; Cell Signaling Technology),
rabbit anti-GAPDH (1:100,000 dilution, 10494-1-AP) and mouse
anti-β-actin (1:2,000 dilution, 60008-1-1g) (both from
Proteintech). After washing in PBS with 0.05% Tween-20, the
membranes were incubated with horseradish peroxidase
(HRP)-conjugated goat anti-mouse (1:5,000 dilution, SC-2005) and
HRP-conjugated goat anti-rabbit (1:5,000 dilution, SC-2054) (both
from Santa Cruz Biotechnology), respectively. MYO6 protein was
visualized by enhanced chemiluminescence (ECL; Amersham) according
to the manufacturer's protocol.
MTT assay
Tetrazolium (MTT) colorimetric assay was used to
measure cell viability and activity. DU145 and PC-3 cells were
plated at a concentration of 3,000 cells/dish and 2,000 cells/dish
in 96-well plates. Afterwards, DU145 and PC-3 cells were infected
by the lentivirus encoding MYO6 shRNA or the control shRNA
for 96 h. Then, 20 µl MTT solution (stock solution 5 mg/ml
PBS) was added to each well and incubated for 5 h at 37°C. The
MTT-containing medium was then removed, and 150 µl DMSO was
added to each tube. The optical density at 595 nm was detected
using a microplate reader (Bio-Rad).
Colony-forming capability analysis
DU145 and PC-3 cells transfected with shMYO6 (S1)
and shCon were grown in 6-well plates at a density of 500
cells/well and incubated for 8 and 9 days, respectively. The medium
was replaced every 2–3 days until 8–9 days in culture of the DU145
and PC-3 cells, and then the cells were washed in PBS before being
fixed in 4% paraformadehyde for 10 min. After treatment, the cells
were stained with crystal violet (0.5% crystal violet in 20%
methanol) for 20 min. A fluorescence microscope (Zeiss) was used to
detect cell colonies. The result showed that more than 50 cells
were counted per colony.
Analysis of the cell cycle distribution
of prostate DU145 cells
To investigate cell cycle distribution, flow
cytometry of propidium iodide (PI) staining was carried out. After
infection with shMYO6 (S1) or shCon for 5 days, the DU145 cells
were then adjusted to a concentration of 2×106
cells/dish in 6-cm dishes and cultured for 40 h at 37°C. After
being washed with ice-cold PBS, the cells were fixed with 1 ml of
70% cold alcohol and kept at 4°C for 20 min. The supernatant was
discarded by centrifugation, resuspended in a mixture of 1 ml PI
(10 µg/ml) and DNase-free RNase (20 µg/ml) and
incubated for 20 min. The cell cycle progression was analyzed by
flow cytometry (FACSCalibur; Becton Dickinson) after the sample was
filtered through a 50-µm nylon mesh.
Detection of intracellular signaling
To stimultaneously detect 18 vital and
well-characterized signaling molecules, the cell lystates were
analyzed by PathScan® Intracellular Signaling Array kit
according to the manufacturer's instructions. After infection with
shMYO6 (S1) or shCon for 5 days, the DU145 cells were rinsed twice
with ice-cold 1X PBS and immediately dissolved in 1X cell lysis
buffer. Array Blocking Buffer was then added to each sample and
blocked for 20 min. An equal volume of lysate was placed in each
samples and incubated for 2 h at room temperature. Before
incubation with HRP-linked streptavidin, each reaction was
incubated with detective antibody mixture for 1 h at room
temperature. The slides were exposed to film for 25 sec after being
developed with LumiGLO/Peroxide reagent (Cell Signaling
Technology).
Statistical analysis
Statistical analysis was carried out using SPSS 13.0
software. Each experiment was performed at least three times, and
the results are presented as mean ± SD. Student's t-test was used
to detect the significance of the differences (P<0.05) between
the experimental and control groups.
Results
Expression of MYO6 in prostate cancer and
normal prostate tissues
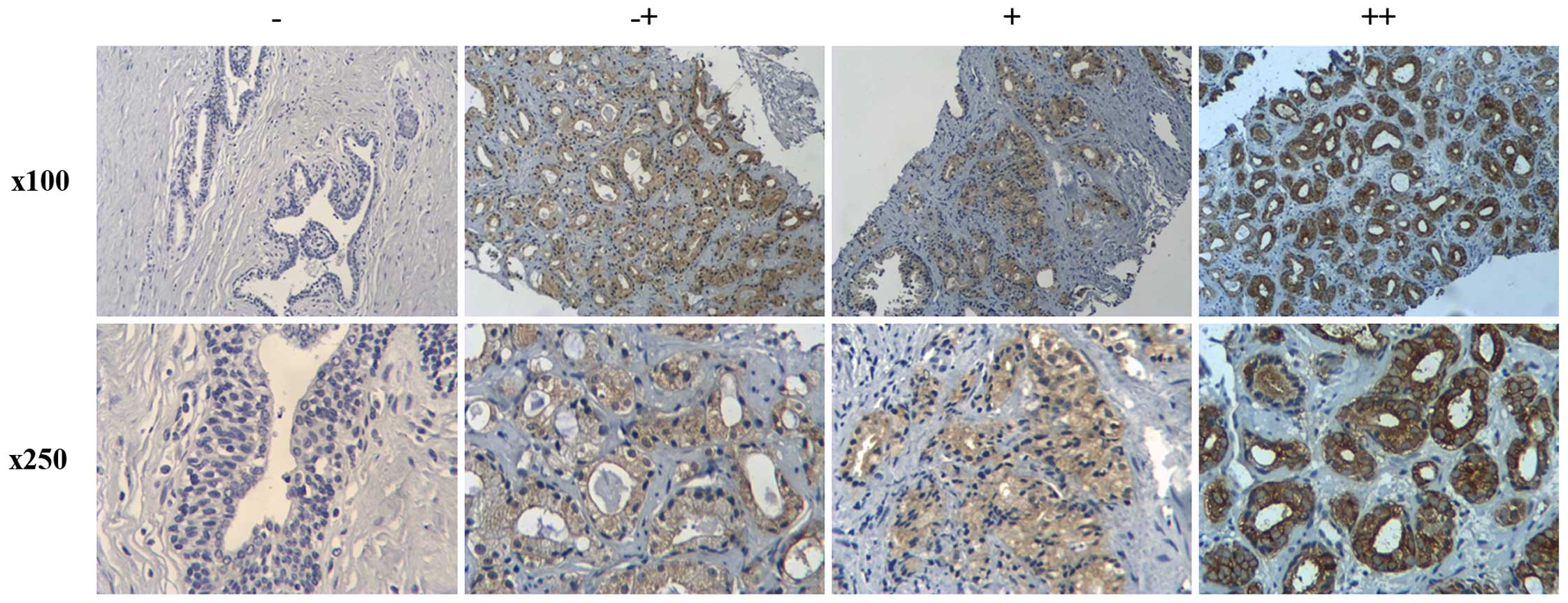
Immunohistochemistry was used to clarify the
expression of MYO6 in prostate cancer. Representative images of
four degrees of MYO6 expression intensity are shown in Fig. 1. The association between MYO6
expression and the clinicopathologic parameters are shown in
Table I. Higher expression of MYO6
was found to be significantly related with Gleason score
(P<0.01). However, there was no significantly difference between
MYO6 expression and patient age.
 | Table IExpression of MYO6 is
positively correlated with pathological grade in the prostate
cancer tissues (n=148). |
Table I
Expression of MYO6 is
positively correlated with pathological grade in the prostate
cancer tissues (n=148).
| Clinical pathologic
parameters | Total | Expression of MYO6
| P-value |
|---|
| − | −~+ | + | ++ |
|---|
| Age (years) | | | | | | 0.2876 |
| ≤70 | 58 | 7 | 11 | 33 | 7 | |
| >70 | 90 | 8 | 17 | 61 | 4 | |
| Gleason score | | | | | | 0.0059 |
| I (2–4) | 10 | 1 | 5 | 4 | 0 | |
| II (5–6) | 25 | 6 | 7 | 10 | 2 | |
| III (7–10) | 113 | 8 | 16 | 80 | 9 | |
Expression of MYO6 is significantly
suppressed in prostate cancer cells after infection with shMYO6
(S1)
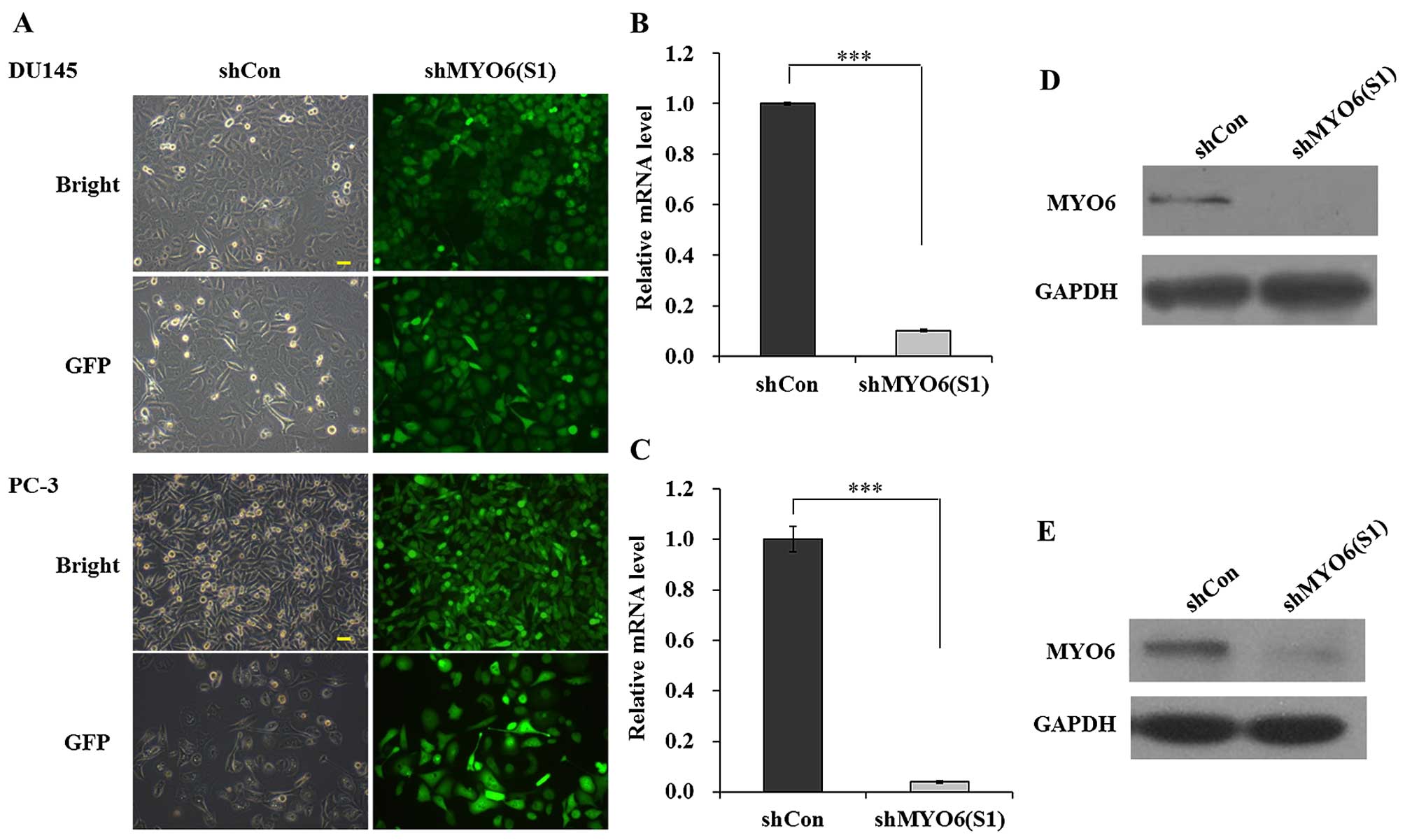
To study the potential relationship between
MYO6 levels and prostate cancer risk, the expression of MYO6
was knocked down in DU145 and PC-3 cells using lentiviral-mediated
RNA interference. As shown in Fig.
2A, most of the cells presented GFP-positive signals suggesting
satisfactory infection efficacy for shMYO6 (S1). Then the knockdown
efficacy was further determined in the DU145 and PC-3 cells using
qRT-PCR and western blot analysis. Levels of MYO6 mRNA were found
to be much lower in the DU145 and PC-3 cells after infection with
shMYO6 (S1) than that in the shCon-transfected cells (Fig. 2B and C, P<0.001). A further
examination of MYO6 protein expression was performed in the DU145
and PC-3 cells following shMYO6 (S1) and shCon infection. Western
blot analysis also showed that MYO6 protein was reduced following
MYO6 knockdown (Fig. 2D and
E). These results suggest that shMYO6 (S1) significantly
downregulated MYO6 expression in the DU145 and PC-3
cells.
Knockdown of MYO6 by shMYO6 (S1) inhibits
the proliferation of prostate cancer cells
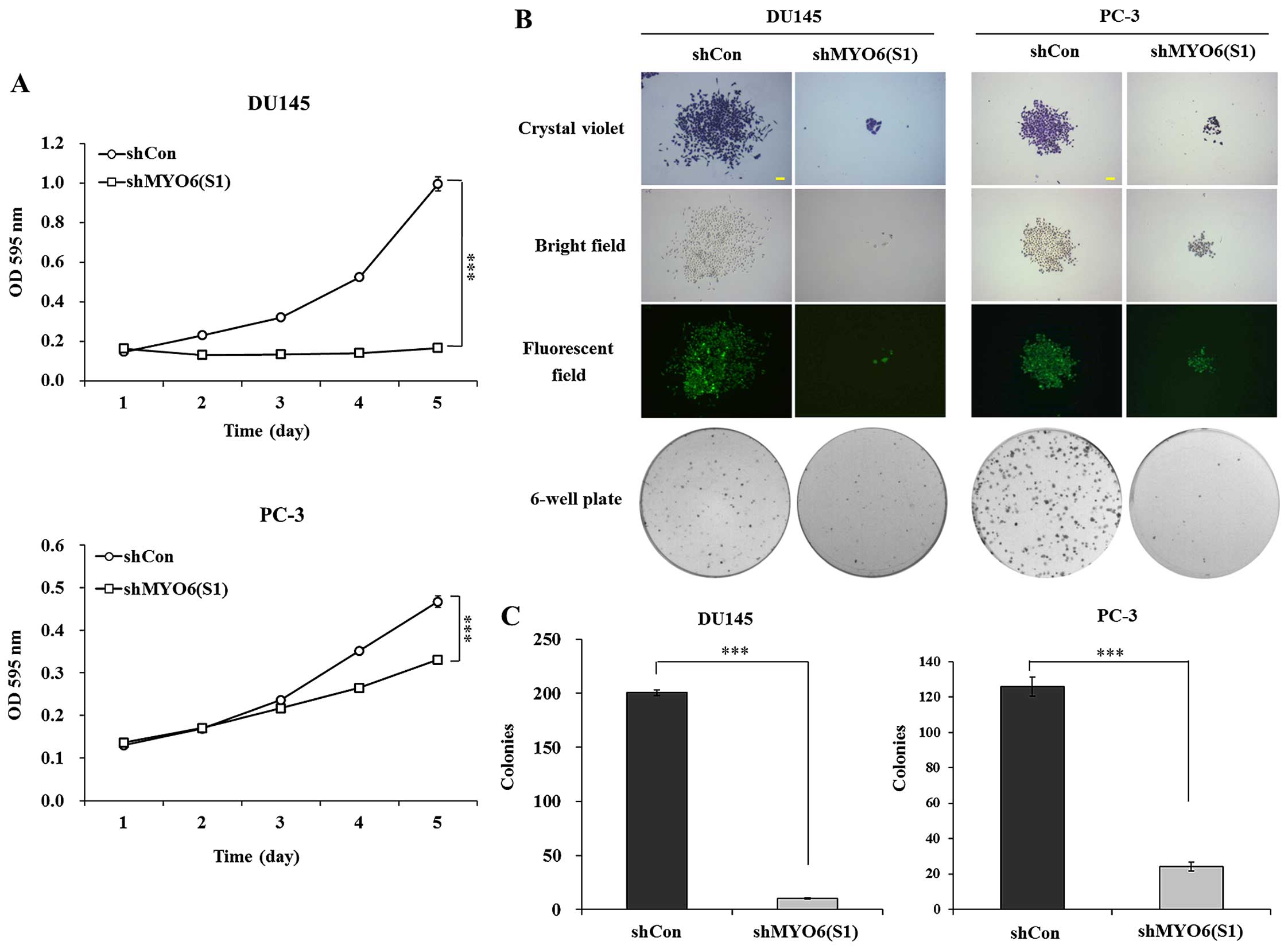
An MTT assay was used to determine the effect of
MYO6 knockdown on cell proliferation. As shown in Fig. 3A, the growth curve of the shMYO6
(S1)-treated cells started to decrease from day 2, compared with
the shCon-treatedcells in the DU145 and PC-3 cells. The decline
reached 83.3% (P<0.001) and 19.8% (P<0.001) on day 5 in the
DU145 and PC-3 cells, respectively, compared with the shCon-treated
cells. These data indicate that shMYO6 (S1)-mediated MYO6 knockdown
obviously suppressed the proliferation of the DU145 and PC-3
cells.
Then, the long-term effect of MYO6 silencing
on cell proliferation was determined by colony formation assay. As
shown in Fig. 3B, there were fewer
and smaller colonies in the shMYO6 (S1)-treated cells than those in
the shCon-treated cells. Moreover, statistical analysis further
confirmed that the number of colonies that formed in the cells was
significantly decreased in the shMYO6 (S1)-treated cells (Fig. 3C, P<0.001). The results showed
that MYO6 knockdown mediated by shMYO6 (S1) markedly
inhibited the cell proliferation of the DU145 and PC-3 cells.
Knockdown of MYO6 by shMYO6 (S2)
suppresses the proliferation of prostate cancer cells
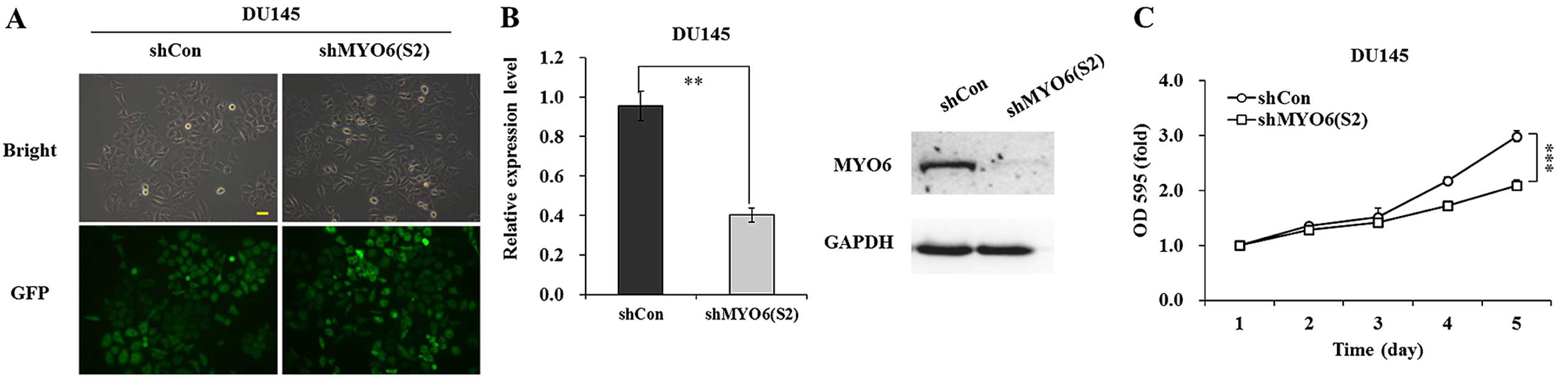
The knockdown efficiency of MYO6 by the other
recombinant lentivirus shMYO6 (S2) was determined in DU145 cells.
After four days of infection, more than 90% of the DU145 cells
strongly expressed GFP fluorescence (Fig. 4A). In addition, the mRNA and protein
levels of MYO6 were significantly downregulated in the shMYO6
(S2)-treated DU145 cells (Fig. 4B).
MTT assay showed that cell viability was significantly decreased in
the shMYO6 (S2)-treated DU145 cells (P<0.001), compared with the
cell viability of the shCon-treated cells (Fig. 4C).
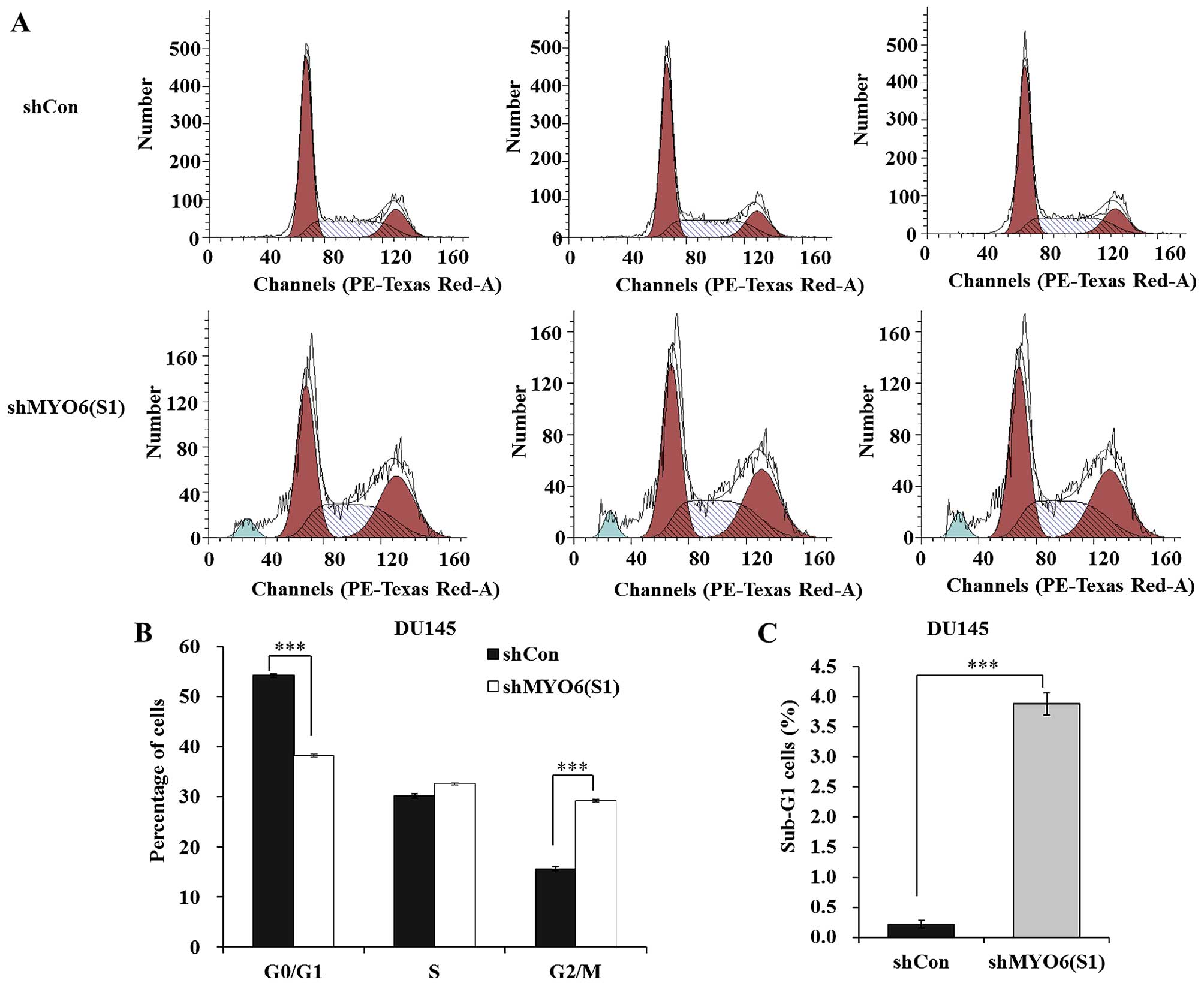
Knockdown of MYO6 arrests DU145 cells at
the G2/M phase and sub-G1 phase
In order to investigate the mechanisms underlying
the growth suppressive effect of MYO6 knockdown, the cell
cycle distribution of DU145 cells was analyzed using flow
cytometric analysis (Fig. 5A). As
shown in Fig. 5B, the percentage of
cells in G0/G1 was significantly decreased whereas the percentage
of cells in the G2/M phase was markedly increased in the shMYO6
(S1)-treated cells compared with those in the shCon-treated cells
(P<0.001). Notably, more cells were accumulated in sub-G1 phase,
representing early apoptosis in the shMYO6 (S1)-treated cells
compared with the number of cells in the shCon-treated cells
(Fig. 5C, P<0.001). These data
suggest that MYO6 knockdown suppresses prostate cancer cell
growth via blockade of cell cycle progression.
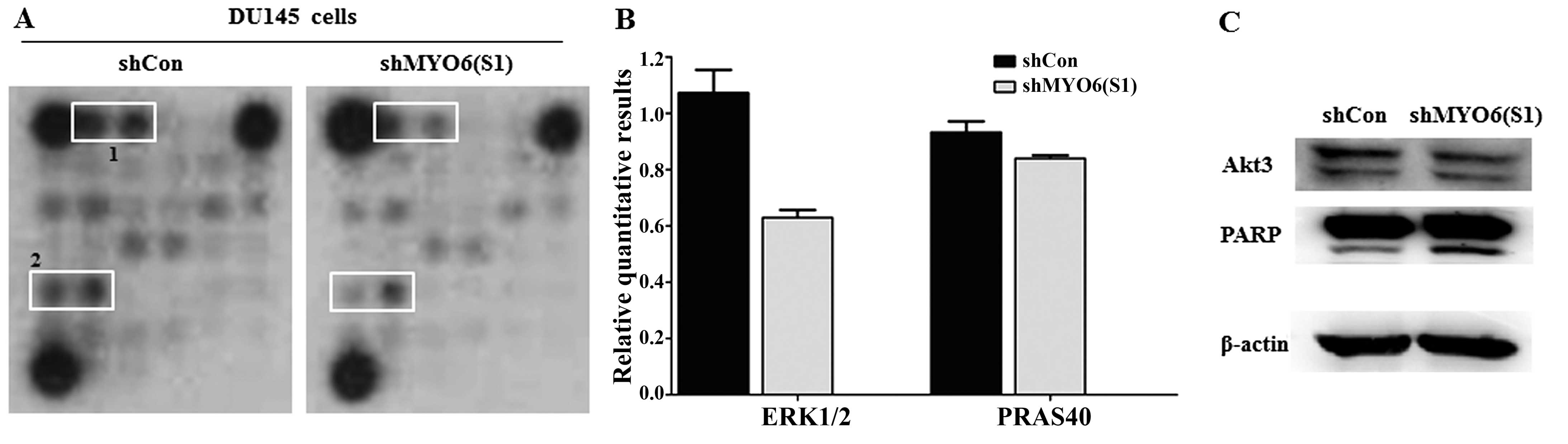
MYO6 knockdown inhibits ERK1/2, AKT3,
PRAS40 and PARP activation
To further explore the molecular mechanisms
underlying MYO6-mediated prostate cancer cell growth,
PathScan® Intracellular Signaling Array kit was used to
detect the modifications of signaling molecules in the shMYO6
(S1)-treated DU145. As shown in Fig.
6A, the phosphorylated levels of ERK1/2 (Thr202/Tyr204) and
PRAS (Thr246) were downregulated in the shMYO6 (S1)-treated cells
compared with levels in the shCon-treated cells. Moreover, the
expression of AKT3, as a downstream effector molecule of ERK-1/2
and PRAS40, was slightly downregulated in the shMYO6 (S1)-treated
cells. Apoptosis marker PARP presented higher expression in the
shMYO6 (S1)-treated cells, as determined by western blot analysis.
These results indicate that MYO6 knockdown inhibited the
growth of prostate cancer cells via blockade of ERK1/2, AKT3,
PRAS40 and activation of PARP.
Discussion
Prostate cancer is one of the most heterogeneous
cancers histologically and clinically (16). MYO6 is related to actin motor and
participates in intracellular vesicle trafficking and transport
(17,18). The present study aimed to explore a
potential link between MYO6 and prostate cancer. Our results
showed that higher expression of MYO6 was found to be
significantly related with Gleason score, which indicates that
MYO6 is associated with the development of prostate cancer.
To further explore the biological function of MYO6 in
prostate cancer, the expression of MYO6 was specifically
knocked down in two prostate cancer cell lines DU145 and PC-3.
Decreased MYO6 expression by two shRNAs both impaired cell
proliferation and colony formation. Moreover, the DU145 cells were
arrested at the G2/M and sub-G1 phases in response to MYO6
knockdown. Wang et al previously also observed inhibited
cell proliferation and impaired colony formation, as well as G2/M
and sub-G1 phase arrest in breast cancer cells after MYO6
silencing (19).
To reveal the molecular mechanisms underlying
MYO6-mediated prostate cancer cell proliferation, various signaling
molecules involved in cell growth and survival in DU145 cells after
MYO6 knockdown were investigated. ERK1/2 is a member of the
mitogen-activated protein kinase superfamily, and its
phosphorylation through the Ras-Raf-MEK-ERK (or ERK pathway)
signaling network, can regulate cell motility, invasiveness, and
apoptosis (20,21). The Ras-Raf-MEK-ERK pathway is
frequently active in cancer through upstream signaling molecule
activation to promote human tumor+ development (22,23).
In the present study, the ERK1/2 phosphorylation was decreased in
prostate cancer cells by MYO6 knockdown. This indicates that
the ERK1/2 is a downstream target of MYO6 and the
Ras-Raf-MEK-ERK pathway may be suppressed by knockdown of
MYO6.
PRAS40 has been shown to be overexpressed in breast
and lung cancer cells, indicating that it plays an important role
in cancer growth (24). PRAS40 is
also a critical downstream protein of the Akt3 signaling cascade
and the elevation of PRAS40 phosphorylation facilitates melanoma
tumor cell growth (25,26). A previous report also demonstrated
that knockdown of PRAS40 and Akt3 protein levels corresponded to
increased levels of cleaved caspase-3, which is a marker of
apoptosis (25). PARP is one of the
most used diagnostic tools for the detection of apoptosis in cells
(27). As a specificity substrate,
when PARP is cut by the cleavage of caspases, apoptosis will be
induced. The present research found that more cells accumulated in
the sub-G1 phase. Furthermore, western blot data revealed that PARP
was activated and AKT3 was suppressed by MYO6 knockdown.
Furthermore, in OVCA429 and DOV13 cells, reduced Akt3 activity was
found to lead to marked accumulation of cells in the G2-M phase
(28). Notably, the increased
PRAS40 phosphorylation paralleled increased Akt3 activity during
melanoma cancer development (25).
In this investigation, G2/M arrest was observed in the prostate
cancer cells following MYO6 knockdown. This result suggested
that Akt3 activity was decreased due to deregulated phosphorylation
of PRAS40, which led to prostate cancer cell arrest at the G2/M
phase.
In conclusion, we firstly identified that
MYO6 plays an important role in prostate cancer cell growth.
It would be important to confirm the oncogenic function of
MYO6 in prostate cancer in vivo. Collectively,
MYO6 could be considered as a potential therapeutic target
for the treatment of prostate cancer.
Acknowledgments
This work was supported by the National Natural
Science Foundation of China (nos. 81272247 and 81372751).
References
|
1
|
Varambally S, Dhanasekaran SM, Zhou M,
Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt
RG, Otte AP, et al: The polycomb group protein EZH2 is involved in
progression of prostate cancer. Nature. 419:624–629. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hsing AW, Tsao L and Devesa SS:
International trends and patterns of prostate cancer incidence and
mortality. Int J Cancer. 85:60–67. 2000. View Article : Google Scholar
|
|
3
|
Wolk A, Andersson SO and Bergström R:
Prospective study of sex hormone levels and risk of prostate
cancer. J Natl Cancer Inst. 89:8201997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Visakorpi T, Kallioniemi AH, Syvänen AC,
Hyytinen ER, Karhu R, Tammela T, Isola JJ and Kallioniemi OP:
Genetic changes in primary and recurrent prostate cancer by
comparative genomic hybridization. Cancer Res. 55:342–347.
1995.PubMed/NCBI
|
|
5
|
Lu W, Singh AK, Khan SA, Senapati D, Yu H
and Ray PC: Gold nano-popcorn-based targeted diagnosis, nanotherapy
treatment, and in situ monitoring of photothermal therapy response
of prostate cancer cells using surface-enhanced Raman spectroscopy.
J Am Chem Soc. 132:18103–18114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jung EJ, Liu G, Zhou W and Chen X: Myosin
VI is a mediator of the p53-dependent cell survival pathway. Mol
Cell Biol. 26:2175–2186. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vale RD: Switches, latches, and
amplifiers: Common themes of G proteins and molecular motors. J
Cell Biol. 135:291–302. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Homma K, Yoshimura M, Saito J, Ikebe R and
Ikebe M: The core of the motor domain determines the direction of
myosin movement. Nature. 412:831–834. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Buss F, Spudich G and Kendrick-Jones J:
Myosin VI: Cellular functions and motor properties. Annu Rev Cell
Dev Biol. 20:649–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Szczyrba J, Löprich E, Wach S, Jung V,
Unteregger G, Barth S, Grobholz R, Wieland W, Stöhr R, Hartmann A,
et al: The microRNA profile of prostate carcinoma obtained by deep
sequencing. Mol Cancer Res. 8:529–538. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morille M, Passirani C, Vonarbourg A,
Clavreul A and Benoit JP: Progress in developing cationic vectors
for non-viral systemic gene therapy against cancer. Biomaterials.
29:3477–3496. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dunn TA, Chen S, Faith DA, Hicks JL, Platz
EA, Chen Y, Ewing CM, Sauvageot J, Isaacs WB, De Marzo AM, et al: A
novel role of myosin VI in human prostate cancer. Am J Pathol.
169:1843–1854. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshida H, Cheng W, Hung J, Montell D,
Geisbrecht E, Rosen D, Liu J and Naora H: Lessons from border cell
migration in the Drosophila ovary: A role for myosin VI in
dissemination of human ovarian cancer. Proc Natl Acad Sci USA.
101:8144–8149. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Puri C, Chibalina MV, Arden SD, Kruppa AJ,
Kendrick-Jones J and Buss F: Overexpression of myosin VI in
prostate cancer cells enhances PSA and VEGF secretion, but has no
effect on endocytosis. Oncogene. 29:188–200. 2010. View Article : Google Scholar :
|
|
15
|
Fernandes BF, Odashiro AN, Saraiva VS,
Logan P, Antecka E and Burnier MN Jr: Immunohistochemical
expression of melan-A and tyrosinase in uveal melanoma. J Carcinog.
6:62007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Singh D, Febbo PG, Ross K, Jackson DG,
Manola J, Ladd C, Tamayo P, Renshaw AA, D'Amico AV, Richie JP, et
al: Gene expression correlates of clinical prostate cancer
behavior. Cancer Cell. 1:203–209. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Demichelis F, Setlur SR, Beroukhim R,
Perner S, Korbel JO, Lafargue CJ, Pflueger D, Pina C, Hofer MD,
Sboner A, et al: Distinct genomic aberrations associated with ERG
rearranged prostate cancer. Genes Chromosomes Cancer. 48:366–380.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Santos JI, Teixeira AL, Dias F, Gomes M,
Nogueira A, Assis J and Medeiros R: Restoring TGFβ1 pathway-related
microRNAs: Possible impact in metastatic prostate cancer
development. Tumour Biol. 35:6245–6253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang H, Wang B, Zhu W and Yang Z:
Lentivirus-mediated knockdown of myosin VI inhibits cell
proliferation of breast cancer cell. Cancer Biother Radiopharm.
30:330–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mebratu Y and Tesfaigzi Y: How ERK1/2
activation controls cell proliferation and cell death: Is
subcellular localization the answer? Cell Cycle. 8:1168–1175. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shin SY, Rath O, Choo SM, Fee F, McFerran
B, Kolch W and Cho KH: Positive- and negative-feedback regulations
coordinate the dynamic behavior of the Ras-Raf-MEK-ERK signal
transduction pathway. J Cell Sci. 122:425–435. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Franklin RA, Montalto G, Cervello M, Libra M, Candido S,
Malaponte G, et al Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascade
inhibitors: How mutations can result in therapy resistance and how
to overcome resistance. Oncotarget. 3:1068–1111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Armin Z, Czernilofsky AP, Gy?Rgy K, Julja
S, Heinz S and Jakob T: Signaling through RAS-RAF-MEK-ERK: From
basics to bedside. Curr Med Chem. 14:601–623. 2007. View Article : Google Scholar
|
|
24
|
Huang B and Porter G: Expression of
proline-rich Akt-substrate PRAS40 in cell survival pathway and
carcinogenesis. Acta Pharmacol Sin. 26:1253–1258. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Madhunapantula SV, Sharma A and Robertson
GP: PRAS40 deregulates apoptosis in malignant melanoma. Cancer Res.
67:3626–3636. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sharma A, Sharma AK, Madhunapantula SV,
Desai D, Huh SJ, Mosca P, Amin S and Robertson GP: Targeting Akt3
signaling in malignant melanoma using isoselenocyanates. Clin
Cancer Res. 15:1674–1685. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bressenot A, Marchal S, Bezdetnaya L,
Garrier J, Guillemin F and Plenat F: Assessment of apoptosis by
immunohistochemistry to active caspase-3, active caspase-7, or
cleaved PARP in monolayer cells and spheroid and subcutaneous
xenografts of human carcinoma. J Histochem Cytochem. 57:289–300.
2009. View Article : Google Scholar :
|
|
28
|
Cristiano BE, Chan JC, Hannan KM, Lundie
NA, Marmy-Conus NJ, Campbell IG, Phillips WA, Robbie M, Hannan RD
and Pearson RB: A specific role for AKT3 in the genesis of ovarian
cancer through modulation of G(2)-M phase transition. Cancer Res.
66:11718–11725. 2006. View Article : Google Scholar : PubMed/NCBI
|