Introduction
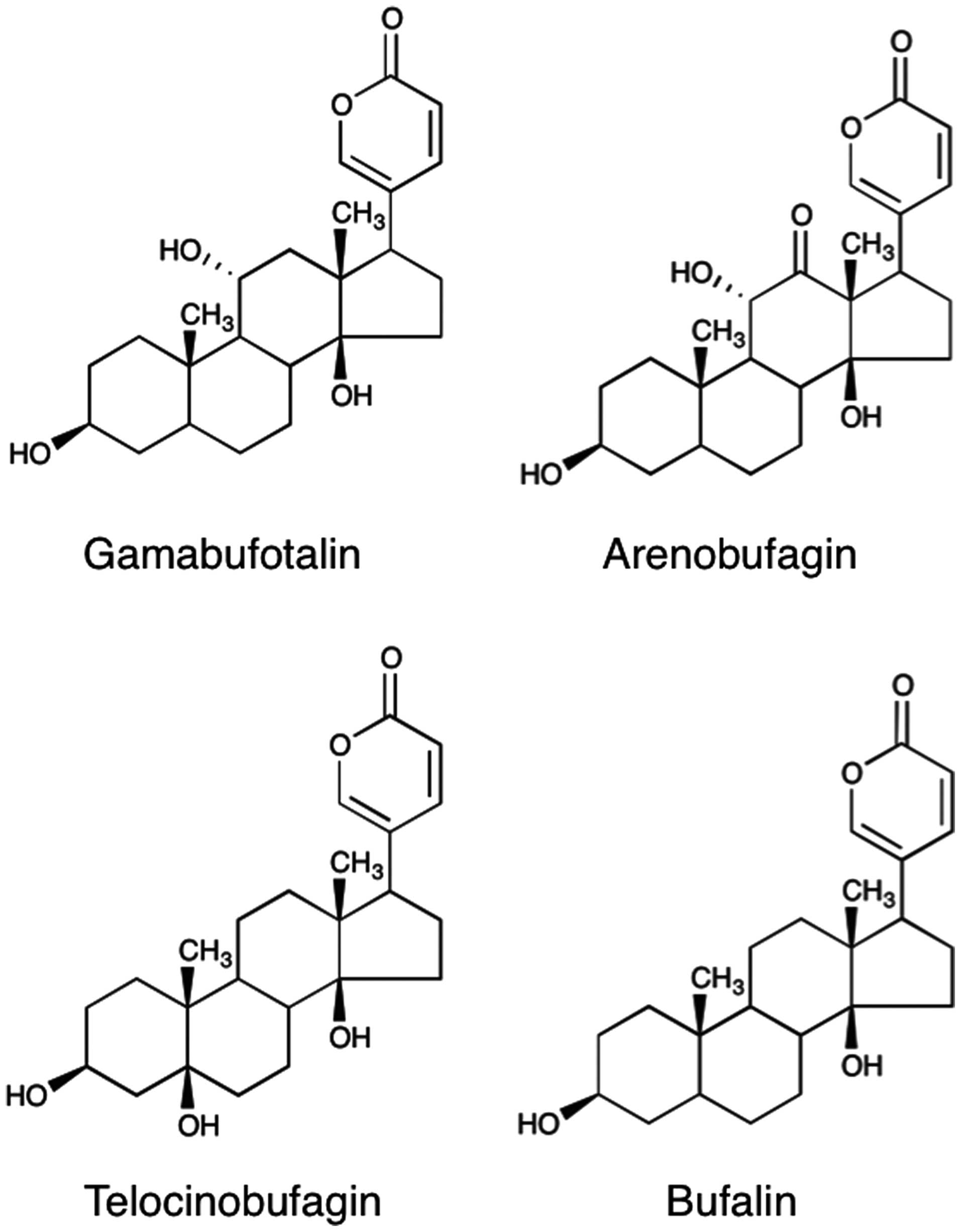
Bufadienolides are the major effective constituents
of cinobufacini (Huachansu), a well-known traditional Chinese
medicine (TCM) that comes from dried toad venom (Chan Su) from the
skin glands of Bufo gargarizans or Bufo
melanostictus, and cinobufacini has been used to treat patients
with various types of cancers such as hepatoma, gallbladder
carcinoma and lung cancer in China (1–3). We
previously identified 10 bufadienolide compounds in cinobufacini
and further suggested that some of them may be used as the quality
control markers for cinobufacini (4,5). Among
these bufadienolide compounds, bufalin seems to be one of the
well-studied active compounds as to its cytocidal effects against a
wide spectrum of cancer cell lines and the mechanisms underlying
its cytotoxicity (6–9). Despite being key active bufadienolide
compounds, the in vitro activities of gamabufotalin and
arenobufagin in human cancer cells, particularly in intractable
cancer cells such as glioblastoma and pancreatic cancer have not
yet been well evaluated.
Immunosuppression has been widely recognized in
cancer patients due to immune tolerance induced by malignant tumor
cells and/or undesirable side-effects of many types of
chemotherapeutic drugs (10–14).
In this regard, CD4+CD25+Foxp3+
regulatory T (Treg) cells have received considerable attention due
to their immunosuppressive properties in vitro and in
vivo (13–16). Furthermore, accumulating evidence
has shown an increased number and function of Treg cells in
patients with solid tumors and hematologic malignancies, suggesting
its critical role in limiting antitumor immune response and
promoting immunological ignorance of cancer cells (10,13,16).
Therefore, immune enhancement therapy by attenuating the number and
function of Treg cells has become a new attractive field that could
be exploited to yield clinical benefit; indeed, depletion or
attenuation of Treg cells has been attempted in tumor therapy
(13,15). Notably, the positive
immunomodulatory effects of medicinal botanicals including TCM have
been recognized in both Western countries and China (17–20).
Cinobufacini has been an important TCM in China and
other Asian countries for centuries, and is currently widely used
as an injection in China. Concerning its antitumor activities, an
in vitro/in vivo study using C3H/HeN mice has
demonstrated that a water-soluble and non-dialyzable fraction of
Chan Su possesses immunomodulatory effects (21). Furthermore, Deng et al
reported that cinobufagin and telocinobufagin, two important
constituents of Chan Su, significantly stimulate the activation of
immunocytes prepared from mice, suggesting that these compounds can
boost the host immune system (22,23).
Despite this, the effects of active bufadienolide compounds on the
alterations of the CD4+ T and Treg cell populations in
peripheral blood mononuclear cells (PBMCs) prepared from healthy
volunteers, has not yet been investigated.
In the present study, the effects of four active
bufadienolide compounds, gamabufotalin, arenobufagin,
telocinobufagin and bufalin, were investigated by focusing on the
growth inhibition in two intractable cancer cells, a human
glioblastoma cell line U-87 and a pancreatic cancer cell line
SW1990. Furthermore, lactate dehydrogenase (LDH) assay was
conducted to explore whether cell membrane damage occurred in these
tumor cells after treatment with gamabufotalin. Human PBMCs were
also used to clarify whether these bufadienolide compounds possess
a selective cytotoxic activity against tumor cells. Of most
importance, the influence of gamabufotalin, endowed with a
relatively high cytotoxic effect against cancer cells and a
relatively low cytotoxic effect on PBMCs among the four active
compounds tested in the present study, on the number of
CD4+ T and Treg cells in mitogen-activated PBMCs were
further evaluated.
Materials and methods
Materials
Four active bufadienolide compounds (Fig. 1), gamabufotalin, arenobufagin,
telocinobufagin and bufalin, were purchased from Baoji Herbest
Bio-Tech Co., Ltd. (Baoji, Shanxi, China). RPMI-1640 medium and
2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbony]-2H-tetrazolium
hydroxide (XTT) were purchased from Sigma-Aldrich (St. Louis, MO,
USA). Concanavalin A (ConA), a conventionally known T-cell mitogen,
was obtained from Seikagaku Kogyo Co., Ltd. (Tokyo, Japan).
Dulbecco's modified Eagle's medium (DMEM) and phenazine
methosulfate (PMS) were purchased from Wako Pure Chemical
Industries (Osaka, Japan). Fetal bovine serum (FBS) was obtained
from Nichirei Biosciences (Tokyo, Japan).
Cell lines and culture conditions
U-87, a human glioblastoma cell line; and SW1990, a
human pancreatic cancer cell line, were obtained from the American
Type Culture Collection (ATCC; Manassas, VA, USA). PBMCs were
isolated from three healthy volunteers (32±9 years of age) using
Mono-Poly resolving medium (DS Pharma Biomedical Co., Ltd., Osaka,
Japan) according to a method previously described with slight
modifications (24,25). Briefly, 3.5 ml of heparinized blood
was loaded on 3 ml of Mono-Poly resolving medium. After
centrifugation at 400 × g for 20 min at room temperature, the
opaque interface containing PBMCs was transferred to a clean
centrifuge tube and washed once with phosphate-buffered saline
(PBS). The two types of cancer cells and PBMCs were cultured in
DMEM and RPMI-1640 medium, respectively, supplemented with 10%
heat-inactivated FBS and antibiotics [100 U/ml of penicillin and
100 µg/ml of streptomycin (Wako Pure Chemical Industries)]
at 37°C in a humidified atmosphere (5% CO2 in air). The
present study was approved by the IRB Committee of Tokyo University
of Pharmacy and Life Sciences. An informed consent was obtained
from all healthy volunteers.
Cell viability assay
For cell viability assay, U-87 and SW1990 cells were
seeded into 96-well plates at a density of 1×104
cells/well in 0.1 ml cell culture medium and allowed to cultivate
for one night, followed by the treatment with various
concentrations of each drug. In contrast, the cell density of PBMCs
was adjusted to 1×106 cells/ml, and 186 µl of the
cell suspension was inoculated into 96-well plates, followed by the
addition of 10 µl of ConA (0.1 mg/ml in PBS) at final
concentrations of 5.0 µg/ml. Subsequently, 4 µl of
each drug solution was added to give final indicated
concentrations. After treatment with each drug for 48 h, cell
viability was determined by the XTT dye-reduction assay according
to the method previously described with slight modifications
(26). The relative cell viability
was expressed as the ratio of the absorbance at 450 nm of each
treatment group against those of the corresponding untreated
control group. Data are shown as means ± SD from three independent
experiments. The IC50 values of each drug for all three
cell types were calculated using GraphPad Prism 5 software.
LDH assay
After treatment with the indicated concentrations of
gamabufotalin for 24 h as described in the previous section of the
cell viability assay, LDH leakage from both the U-87 and SW1990
cells was measured using a LDH cytotoxicity detection kit (Wako
Pure Chemical Industry, Osaka, Japan) according to the method
previously described with slight modifications (26). Culture medium served as the negative
control (NC). Culture supernatants (S) were collected by
centrifugation at 450 × g for 5 min at 4°C and stored at −80°C
until use. Cultured cells without treatment were lysed in the
culture medium containing 0.2% Tween-20, and the cell lysate after
centrifugation at 12,000 × g for 5 min at 4°C was used as the
non-damaged positive control (PC). Furthermore, in order to avoid
an influence of Tween-20, culture medium containing 0.2% Tween-20
served as the NC for PC and was referred to as NCT. Samples were
diluted 16-fold with PBS, then 50 µl was loaded into wells
of a 96-well plate. LDH activities were determined by adding 50
µl of ̔reaction reagent̓ from the kit, followed by
incubation at room temperature for 30 min. The reaction was stopped
by the addition of 100 µl of ̔stopping solution̓ provided
with the kit, and the absorbance at 560 nm was measured with a
microplate reader (EMax® Plus, Molecular Devices, CA,
USA). Cell damage was calculated as a percentage of LDH leakage
from damaged cells using the following formula: LDH leakage (%) =
(S-NC)/(PC-NCT) × 100. Data are presented as the means and SD from
three independent experiments.
Analysis of CD4+ T cells and
Treg
After treatment with 8 and 16 ng/ml of gamabufotalin
for 72 and 96 h, respectively, alterations of CD4+ T
cells and Treg cells population in mitogen-activated PBMCs were
analyzed by staining of cells with specific antibodies using flow
cytometry performed on a FACSCalibur™ II (BD Biosciences, Mountain
View, CA, USA) according to the method previously described with
slight modifications (27,28). Briefly, after treatment for the
indicated time periods, ~1×106 cells were collected and
washed once with PBS (pH 7.4) (Gibco®; Thermo Fisher
Scientific, Waltham, MA, USA), followed by the addition of 10
µl of monoclonal mouse anti-human CD4 PerCP-Cy5.5-conjugated
antibody and 10 µl of monoclonal mouse anti-human CD25
PE-conjugated antibody (BD Biosciences), respectively. To exclude
the amount of non-specific binding, 10 µl of
PerCP-Cy5.5-conjugated and 10 µl of PE-conjugated mouse IgG1
κ isotype control (BD Biosciences) were used and evaluated as
background, respectively. After the incubation for 20 min in the
dark at 37°C, the cells were washed with PBS and resuspended in
diluted Foxp3 buffer A (BD Biosciences), followed by the incubation
for 10 min in the dark at room temperature. Then, the cells were
washed once with PBS and resuspended in 0.2 ml of Foxp3 buffer C
composed of 49 parts Foxp3 buffer A and 1 part Foxp3 buffer B (BD
Biosciences), and incubated for 30 min in the dark at room
temperature. After the incubation, the cells were washed once with
PBS and 10 µl of mouse anti-human Foxp3 Alexa
Fluor® 488-conjugated antibody or 10 µl of Alexa
Fluor® 488-conjugated mouse IgG1 isotype control to
re-suspend the pellet was added, and incubation for 30 min in the
dark at 37°C followed. After washing once with PBS, the cells were
resuspended in 0.4 ml staining buffer [0.4% (v/v) formaldehyde
neutral buffer solution in PBS (pH 7.4); Nacalai Tesque, Inc.,
Kyoto, Japan] and analyzed by flow cytometry, and then the data
were further analyzed using CellQuest software (BD Biosciences).
CD4+ T cells in the lymphocyte fraction were gated, and
the percentages of CD4+ T and Treg cells in the
CD4+ T cells were calculated, respectively.
Statistical analysis
Experiments were independently repeated three times,
and the results are presented as the mean ± standard deviation (SD)
of three assays. Statistical analysis was conducted using one-way
ANOVA followed by the Tukey's or Dunnett's post hoc test methods. A
P<0.05 was considered to indicate a statistically significant
result.
Results
Cytotoxic effects of bufadienolide
compounds against the U-87 and SW1990 cells
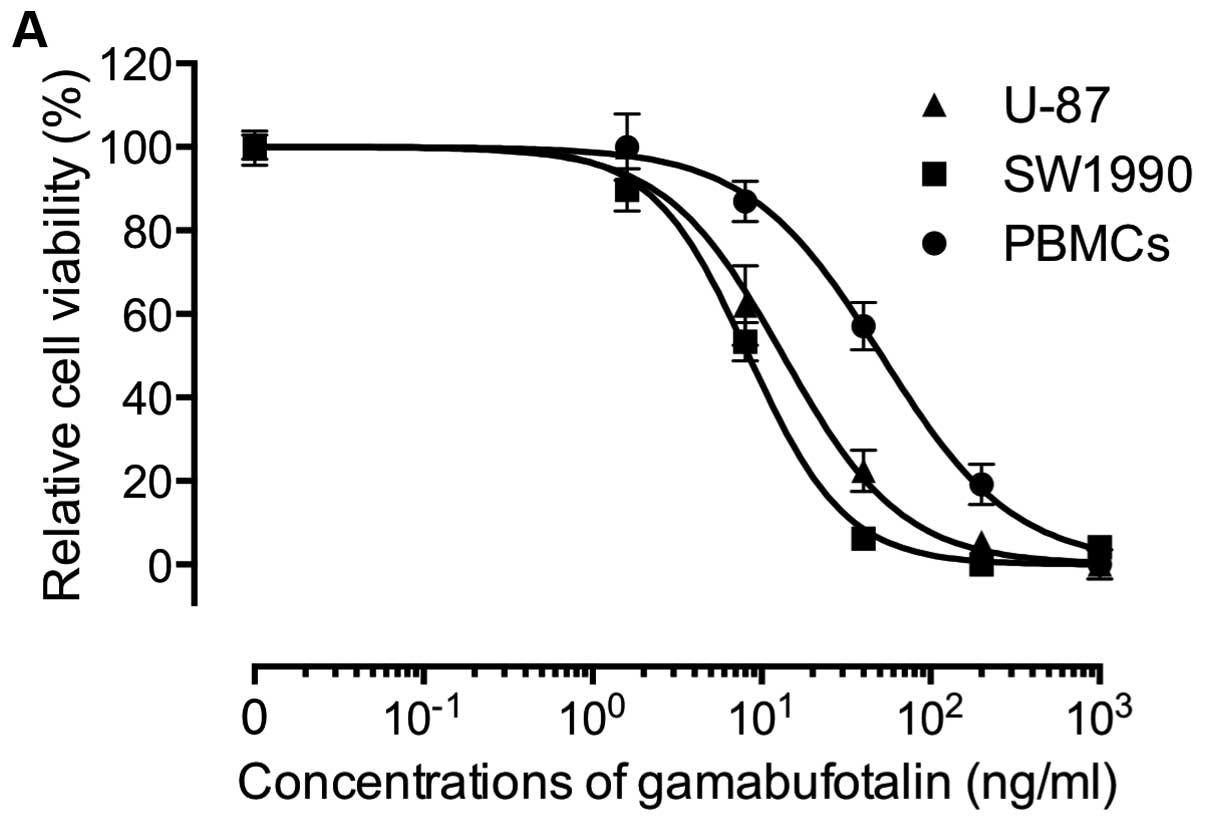
Gamabufotalin and arenobufagin exhibited
dose-dependent cytotoxic effects on both the U-87 and SW1990 cells
after treatment with various concentrations of each drug (1.6, 8,
40, 200 and 1,000 ng/ml) for 48 h, and the IC50 values
of gamabufotalin were 16.8±6.5 and 8.1±1.5 ng/ml in the U-87 and
SW1990 cells, respectively (Fig.
2A). Moreover, similar IC50 values of arenobufagin
were obtained, which were 10.3±3.3 and 9.9±2.2 ng/ml in the U-87
and SW1990 cells, respectively (Fig.
2B). In comparison, a mild cytotoxic effect was observed in
both cancer cell lines treated with telocinobufagin, whereas a
potent cytotoxic effect was observed in the bufalin-treated cancer
cell lines (data not shown). Notably, an ~3- to 5-fold increase in
the IC50 values of gamabufotalin was obtained in the
PBMCs (IC50=44.1±2.4 ng/ml) as compared to both cancer
cell lines, although a clear cytotoxic effect was also observed in
the PBMCs when the concentration of gamabu-fotalin increased up to
40 ng/ml (Figs. 2A and 3). Similarly, the IC50 values
of arenobufagin were 3–4 times higher in the PBMCs
(IC50=36.5±15.8 ng/ml) than those in the two cancer cell
lines (Figs. 2B and 3). Moreover, the IC50 values of
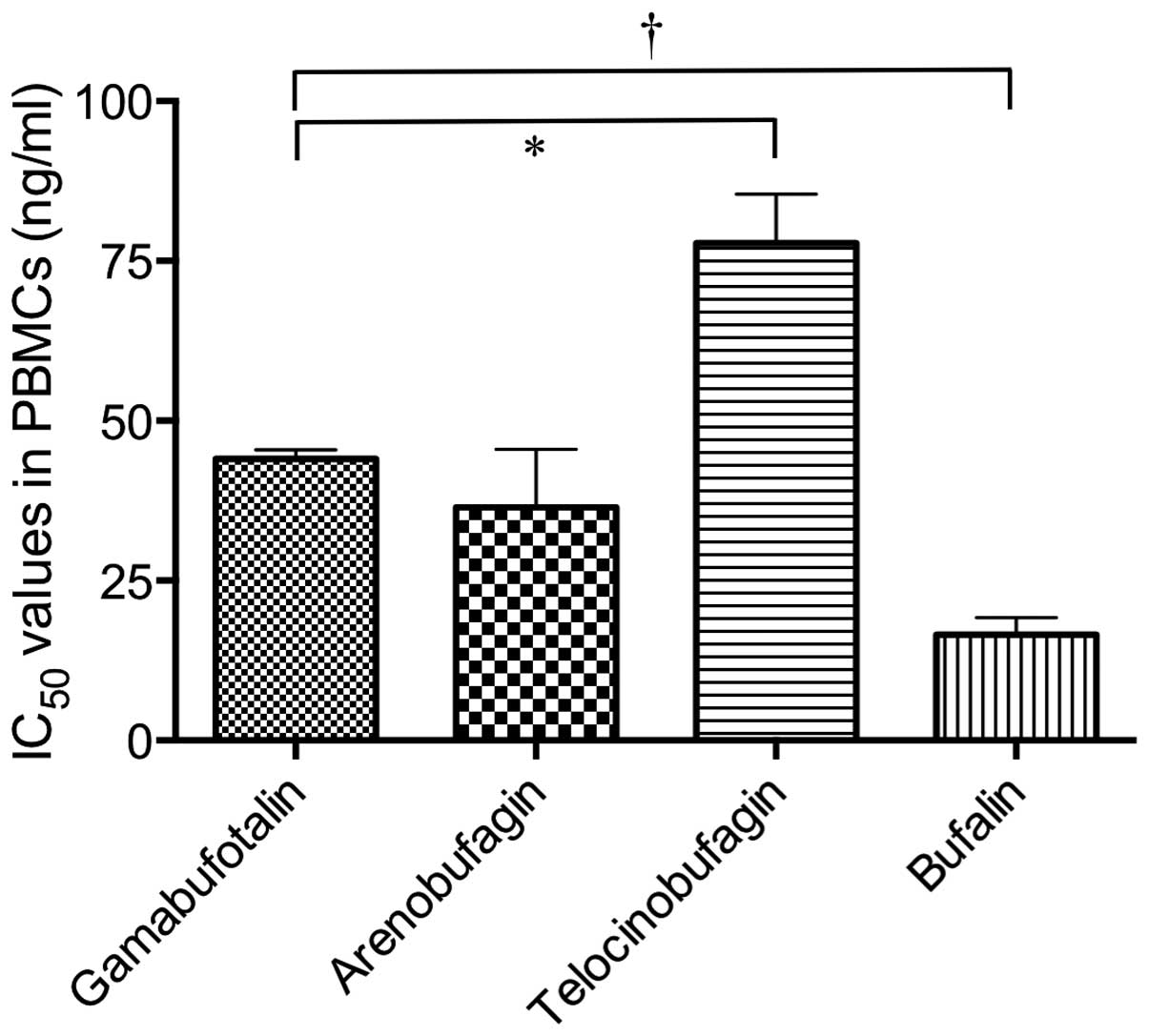
telocinobufagin and bufalin were 77.8±13.3 and 16.5±4.7 ng/ml in
PBMCs, indicating that the rank order for the cytotoxicity of the
four bufadienolide compounds against PBMCs was: bufalin >
arenobufagin > gamabufotalin > telocinobufagin (Fig. 3). These results showed that
gamabufotalin exerted not only a relatively high cytotoxic effect
against cancer cells, but also a relatively low cytotoxic effect on
normal PBMCs among the four active bufadienolide compounds.
Therefore, the effects of gamabufotalin on the alterations of the
CD4+ T and Treg cell populations in mitogen-activated
PBMCs were further evaluated.
Gamabufotalin-mediated LDH leakage in
both the U-87 and SW1990 cells
The release of LDH provides an accurate measure of
the cell membrane integrity and cell viability (24,26).
After treatment with various concentrations of gamabufotalin (1.6,
8, 40, 200 and 1,000 ng/ml) for 24 h, LDH leakage analysis was thus
performed to examine whether gamabufotalin treatment affects cell
membrane integrity. A dose-dependent LDH leakage was observed in
both cancer cell lines (Fig. Furthermore, statistically significant
increments in the LDH leakage were observed at concentrations
starting from 40 and 8 ng/ml of gamabufotalin in the U-87 and
SW1990 cells, respectively, indicating that the sensitivity of the
SW1990 cells to gamabufotalin appeared to be higher than that of
the U-87 cells.
Downregulation of the population of Treg
cells by gamabufotalin
The populations of CD4+ T and Treg cells
in mitogen-activated PBMCs were analyzed by flow cytometry. In this
experiment, 8 and 16 ng/ml of gamabufotalin were selected since
these two concentrations are very close to its IC50
values in both cancer cell lines, and that the concentration of 8
ng/ml was almost non-toxic to PBMCs; the growth inhibition rate by
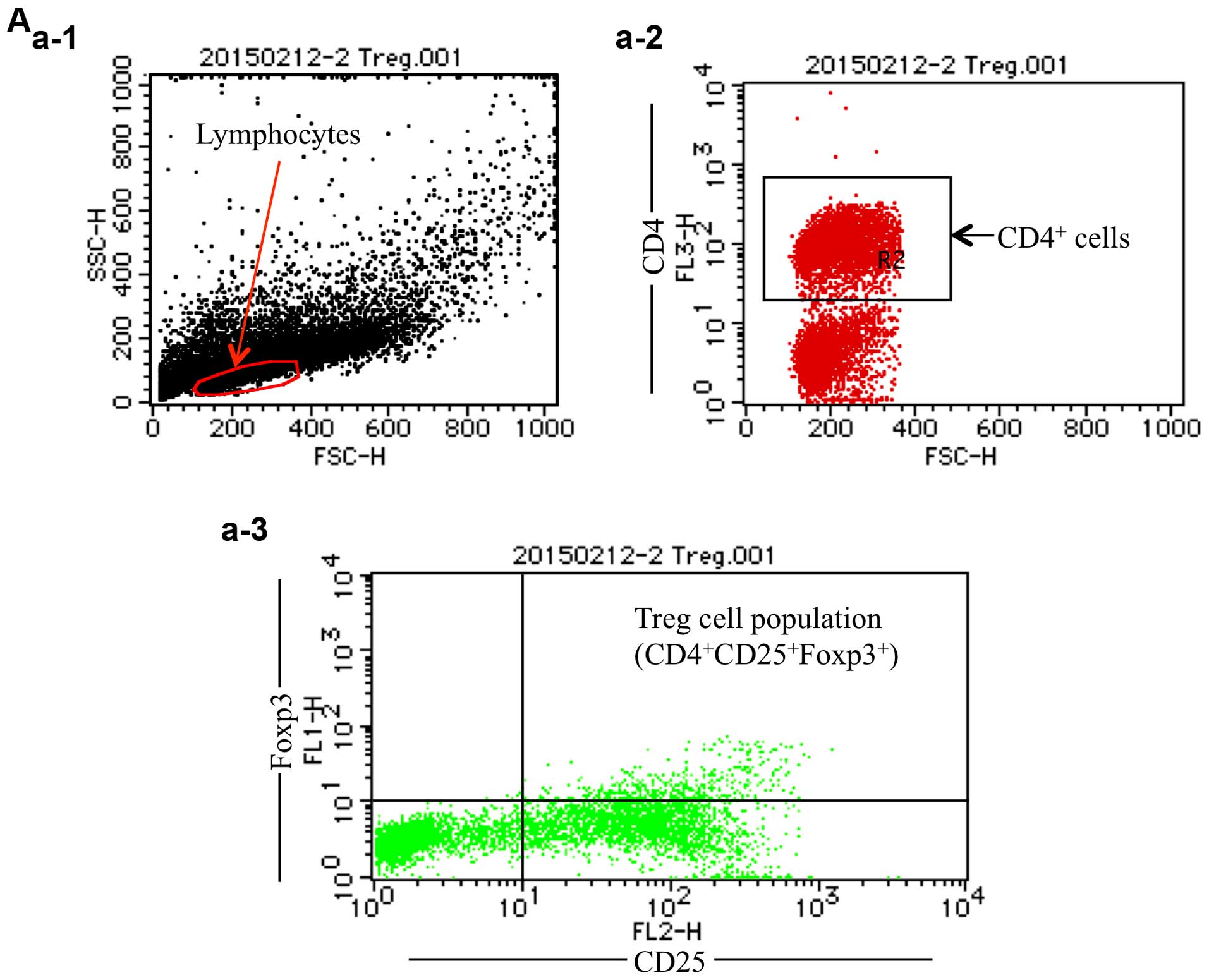
the agent at 16 ng/ml was <20% in PBMCs. Using CellQuest
software, CD4+ T cells in the lymphocyte fraction were
gated, and the percentages of Treg cells in the CD4+ T
cell fraction were further calculated (Fig. 5A). Flow cytometric analysis showed
that almost no alteration in the percentages of CD4+ T
cells in PBMCs after treatment with either 8 or 16 ng/ml
gamabufotalin for 72 and 96 h, respectively, was observed (Fig. 5B and C). Notably, a significant
downregulation of the percentages of Treg cells was observed in the
CD4+ T cells of PBMCs when treated with either 8 or 16
ng/ml gamabufotalin for 72 h, respectively, and the downregulation
continued up to 96 h after treatment with 16 ng/ml gamabufotalin
(Fig. 5D and E).
Discussion
In the present study, we demonstrated that active
bufadienolide compounds including gamabufotalin and arenobufagin
exhibited dose-dependent cytotoxic effects on the U-87 as well as
the SW1990 cells. Notably, among the four active bufadienolide
compounds tested in the present study, the IC50 values
for gamabufotalin and arenobufagin in the PBMCs were much higher
than those in the two different cancer cell lines, whereas similar
phenomena were not observed for telocinobufagin and bufalin. In
fact, gamabufotalin was recently demonstrated to inhibit the cell
growth of non-small cell lung cancer (NSCLC) cell lines such as
A549, H1299 and H322, and have only minimal effects on a human
normal embryonic lung fibroblast cell line (29). These results thus suggest that
bufadienolide compounds such as gamabufotalin and arenobufagin
possess selective cytotoxic activity against tumor cells rather
than normal cells. In addition, a pilot study aimed to evaluate the
efficacy of cinobufacini in patients with advanced cancer
demonstrated that no dose-limiting toxicities were observed with
the use of cinobufacini at doses up to 8 times higher than the
usual therapeutic dose in China, suggesting that cinobufacini has
effective anticancer activity with low toxicity and few
side-effects (3). Our results,
thus, possibly provide supportive evidence for its safety. To the
best of our knowledge, this is the first study to show the
cytotoxicity of gamabufotalin and arenobufagin against glioblastoma
and/or pancreatic cancer cells, although the effects of other
bufadienolide compounds with similar structure such as
gamabufotalin rhamnoside and arenobufagin diacetate on the U373
glioblastoma cell line has been recently investigated (30).
Growth inhibition and apoptosis induction have been
implicated in the antitumor activity of cinobufacini and its main
active bufadienolide compounds (30–32).
Indeed, bufalin is one of the well-studied active compounds with
respect to its in vitro activity against a wide spectrum of
cancer cell lines including leukemia, breast, prostate, gastric and
liver cancer (6), and has been
demonstrated to induce apoptosis of these cancer cells via
modulation of several signaling pathways such as activation of the
intrinsic mitochondrial apoptosis pathway and Fas, downregulation
of the PI3K/Akt or MEK/ERK pathway and activation of the MAPK
cascade including JNK (6–8). Despite being key active bufadienolide
compounds (33,34), the antitumor activity of
gamabufotalin and arenobufagin, and their underlying molecular
mechanisms have not yet been well determined. Until quite recently,
arenobufagin has been demonstrated to induce apoptosis and
autophagy through inhibition of the PI3K/Akt/mTOR pathway, and
intercalate with DNA leading to G2 cell cycle arrest in
human hepatocellular carcinoma cells (35,36).
Moreover, gamabufotalin has recently been demonstrated to induce
apoptosis in NSCLC cell lines A549 and H1299, and suppress vascular
endothelial growth factor (VEGF)-induced anti-apoptosis of human
umbilical vein endothelial cells via the mitochondrial pathway
(29,37). In contrast, the present study found
no evidence of DNA fragmentation and morphological changes such as
nuclear condensation in both the U-87 and the SW1990 cells (data
not shown), indicating no involvement of apoptosis induction in
these cells following treatment with gamabufotalin and
arenobufagin, respectively. Notably, a clear dose-dependent LDH
release was observed in both cancer cell lines after treatment with
gamabufotalin. It is well known that the release of LDH provides an
accurate measure of the cell membrane integrity, and represents a
hallmark of necrosis (38).
Collectively, our results suggested that gamabufotalin and
arenobufagin-induced cell death in both cancer cell lines was
predominantly associated with a necrosis-like phenotype.
Understandably, further investigation is needed to elucidate
details of the mechanism underlying the cell death induction.
Furthermore, whether induction of apoptosis or necrosis occurs
after treatment with these bufadienolide compounds may be dependent
on different cell types.
Since immunosuppression as a result of tumor-induced
tolerance and the side-effects of chemotherapeutic drugs is widely
recognized in cancer patients (10–14),
immune enhancement therapy has become a new attractive field that
could be exploited to yield clinical benefit. There is now
accumulating evidence that Treg cell population is actively
involved in the negative control of a variety of physiological and
pathological immune responses including tumor immunity, and its
increased number and function are considered to be important for
limiting antitumor immune responses and promoting immunological
ignorance of cancer cells (10,13,15).
Furthermore, increasing evidence supports the existence of elevated
numbers of Treg cells in patients with solid tumors as well as
hematologic malignancies, and Treg cell depletion has been
attempted in tumor therapy (10,13,15,16).
In this regard, we demonstrated for the first time that treatment
with as little as 8 ng/ml of gamabufotalin, even an almost nontoxic
concentration to PBMCs, efficiently downregulated the percentages
of Treg cells without impacting the percentages of CD4+
T cells. These results thus suggested that gamabufotalin not only
exhibited a cytocidal effect on tumor cells, but also was capable
of enhancing antitumor immunity by impeding Treg cell expansion and
function. Notably, cinobufagin and telocinobufagin, two important
constituents of Chan Su have been demonstrated to significantly
stimulate the proliferation of mouse splenocytes, and markedly
enhance mouse peritoneal macrophage activation as well as the
percentage of CD4+CD8+ cells (22,23).
The two reagents also increased the levels of several Th1 cytokines
including interferon-γ and tumor necrosis factor-α, while they
decreased the levels of Th2 cytokine interleukin-4, resulting in
the increase in the ratio of Th1/Th2 (22,23).
Taking these previous results and our observations into account, we
thus suggest that gamabufotalin, similar to cinobufagin and
telocinobufagin, may also be developed as a novel immunotherapeutic
agent to treat cancer such as glioblastoma and/or pancreatic
cancer, although there remains urgent need to characterize the
function of Treg cells in gamabufotalin-treated PBMCs by evaluating
alteration in the levels of Th1/Th2 cytokines, and transforming
growth factor-β, known cytokines secreted from Treg cells and also
implicated in the induction of Treg cells (39).
As mentioned above, there is a growing concern about
immunosuppression in tumor-bearing hosts treated with clinical
anticancer drugs (10,11,14).
In parallel, we also demonstrated that As2O3
and As2S2, two different arsenic derivatives
with a remarkable efficacy in the treatment of leukemia and
myelodysplastic syndrome (40–43),
decreased cell viability and proliferation, while they increased
the percentages of Treg cells in PBMCs activated by ConA (27,28).
Since combination therapy is a frequently used method in the
clinical therapy of cancer to improve anticancer effects and reduce
toxicities (44), we thus
hypothesize that gamabufotalin may serve as a promising candidate,
as an adjuvant therapeutic reagent by manipulating Treg cells to
enhance the treatment efficacy of arsenic derivatives and lessen
their side-effects. The efforts to evaluate the in vivo
efficacy of the combination in tumor xenografts in our laboratory
are ongoing.
In conclusion, we demonstrated for the first time
that gamabufotalin and arenobufagin showed selective cytotoxic
effects against tumor cells, but minimal effects on PBMCs,
indicating the possibility of using these active compounds for
intractable cancers such as glioblastoma and pancreatic cancer.
More importantly, we also demonstrated that almost non-toxic
gamabufotalin concentrations to PBMCs efficiently downregulated the
percentages of Treg cells, suggesting that gamabufotalin may
provide therapeutic benefit to patients by not only exhibiting
cytocidal effect against tumor cells but also by enhancing
antitumor immunity.
References
|
1
|
Chen Z, Zhai XF, Su YH, Wan XY, Li J, Xie
JM and Gao B: Clinical observation of cinobufacini injection used
to treat moderate and advanced primary liver cancer. Zhong Xi Yi
Jie He Xue Bao. 1:184–186. 2003.In Chinese. View Article : Google Scholar
|
|
2
|
Qin TJ, Zhao XH, Yun J, Zhang LX, Ruan ZP
and Pan BR: Efficacy and safety of gemcitabine-oxaliplatin combined
with huachansu in patients with advanced gallbladder carcinoma.
World J Gastroenterol. 14:5210–5216. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meng Z, Yang P, Shen Y, Bei W, Zhang Y, Ge
Y, Newman RA, Cohen L, Liu L, Thornton B, et al: Pilot study of
huachansu in patients with hepatocellular carcinoma, nonsmall-cell
lung cancer, or pancreatic cancer. Cancer. 115:5309–5318. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu JQ, Si N, Yang J, Zhao HY, Bian BL and
Wang HJ: Identification of bufadienolides profiling in cinobufacino
by HPLC-DAD-FT-ICR-MS method. Yao Xue Xue Bao. 49:244–248. 2014.In
Chinese. PubMed/NCBI
|
|
5
|
Wu X, Zhao H, Wang H, Gao B, Yang J, Si N
and Bian B: Simultaneous determination of eight bufadienolides in
cinobufacini injection by HPLC coupled with triple quadrupole mass
spectrometry. J Sep Sci. 35:1893–1898. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yin PH, Liu X, Qiu YY, Cai JF, Qin JM, Zhu
HR and Li Q: Anti-tumor activity and apoptosis-regulation
mechanisms of bufalin in various cancers: New hope for cancer
patients. Asian Pac J Cancer Prev. 13:5339–5343. 2012. View Article : Google Scholar
|
|
7
|
Zhu Z, Sun H, Ma G, Wang Z, Li E and Liu Y
and Liu Y: Bufalin induces lung cancer cell apoptosis via the
inhibition of PI3K/Akt pathway. Int J Mol Sci. 13:2025–2035. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Watabe M, Kawazoe N, Masuda Y, Nakajo S
and Nakaya K: Bcl-2 protein inhibits bufalin-induced apoptosis
through inhibition of mitogen-activated protein kinase activation
in human leukemia U937 cells. Cancer Res. 57:3097–3100.
1997.PubMed/NCBI
|
|
9
|
Zhu Z, Li E and Liu Y, Gao Y, Sun H, Wang
Y, Wang Z, Liu X, Wang Q and Liu Y: Bufalin induces the apoptosis
of acute promyelocytic leukemia cells via the downregulation of
survivin expression. Acta Haematol. 128:144–150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sakaguchi S: Naturally arising
Foxp3-expressing CD25+CD4+ regulatory T cells
in immunological tolerance to self and non-self. Nat Immunol.
6:345–352. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Adachi W, Sugenoya A, Horigome N,
Takahashi C, Iida F and Nakayama J: The antitumor activity and
immunosuppressive effects of 5-fluorouracil suppositories in rectal
cancer patients. Surg Today. 22:221–225. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dunn GP, Bruce AT, Ikeda H, Old LJ and
Schreiber RD: Cancer immunoediting: From immunosurveillance to
tumor escape. Nat Immunol. 3:991–998. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Facciabene A, Motz GT and Coukos G:
T-regulatory cells: Key players in tumor immune escape and
angiogenesis. Cancer Res. 72:2162–2171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fadul CE, Fisher JL, Gui J, Hampton TH,
Côté AL and Ernstoff MS: Immune modulation effects of concomitant
temo-zolomide and radiation therapy on peripheral blood mononuclear
cells in patients with glioblastoma multiforme. Neuro Oncol.
13:393–400. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cao Q, Wang L, Du F, Sheng H, Zhang Y, Wu
J, Shen B, Shen T, Zhang J, Li D, et al: Downregulation of
CD4+CD25+ regulatory T cells may underlie
enhanced Th1 immunity caused by immunization with activated
autologous T cells. Cell Res. 17:627–637. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maruyama T, Kono K, Mizukami Y, Kawaguchi
Y, Mimura K, Watanabe M, Izawa S and Fujii H: Distribution of Th17
cells and FoxP3(+) regulatory T cells in tumor-infiltrating
lymphocytes, tumor-draining lymph nodes and peripheral blood
lymphocytes in patients with gastric cancer. Cancer Sci.
101:1947–1954. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cassileth B, Yeung KS and Gubili J: Herbs
and other botanicals in cancer patient care. Curr Treat Options
Oncol. 9:109–116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim SH, Jun CD, Suk K, Choi BJ, Lim H,
Park S, Lee SH, Shin HY, Kim DK and Shin TY: Gallic acid inhibits
histamine release and pro-inflammatory cytokine production in mast
cells. Toxicol Sci. 91:123–131. 2006. View Article : Google Scholar
|
|
19
|
Scimone A and Scimone A: US sees green in
herbal supplements. Chem Mark Rep. 254:FR3–FR4. 1998.
|
|
20
|
Wu CA, Wu JJ, Tsai MJ and Chen RY:
Immunomodulatory effects of a traditional Chinese medicine,
Chi-Shie-Shuan-Bu-An-Shen-Tang, on BALB/c mice. J Ethnopharmacol.
113:300–305. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shimizu Y, Inoue E and Ito C: Effect of
the water-soluble and non-dialyzable fraction isolated from Senso
(Chan Su) on lymphocyte proliferation and natural killer activity
in C3H mice. Biol Pharm Bull. 27:256–260. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cao Y, Song Y, An N, Zeng S, Wang D, Yu L,
Zhu T, Zhang T, Cui J, Zhou C, et al: The effects of
telocinobufagin isolated from Chan Su on the activation and
cytokine secretion of immunocytes in vitro. Fundam Clin Pharmacol.
23:457–464. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang XL, Zhao GH, Zhang J, Shi QY, Guo WX,
Tian XL, Qiu JZ, Yin LZ, Deng XM and Song Y: Immunomodulatory
effects of cinobufagin isolated from Chan Su on activation and
cytokines secretion of immunocyte in vitro. J Asian Nat Prod Res.
13:383–392. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kon A, Yuan B, Hanazawa T, Kikuchi H, Sato
M, Furutani R, Takagi N and Toyoda H: Contribution of membrane
progesterone receptor α to the induction of progesterone-mediated
apoptosis associated with mitochondrial membrane disruption and
caspase cascade activation in Jurkat cell lines. Oncol Rep.
30:1965–1970. 2013.PubMed/NCBI
|
|
25
|
Kikuchi H, Yuan B, Nishimura Y, Imai M,
Furutani R, Kamoi S, Seno M, Fukushima S, Hazama S, Hirobe C, et
al: Cytotoxicity of Vitex agnuscastus fruit extract and its major
component, casticin, correlates with differentiation status in
leukemia cell lines. Int J Oncol. 43:1976–1984. 2013.PubMed/NCBI
|
|
26
|
Yoshino Y, Yuan B, Kaise T, Takeichi M,
Tanaka S, Hirano T, Kroetz DL and Toyoda H: Contribution of
aquaporin 9 and multidrug resistance-associated protein 2 to
differential sensitivity to arsenite between primary cultured
chorion and amnion cells prepared from human fetal membranes.
Toxicol Appl Pharmacol. 257:198–208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song MM, Fang S, Tanaka S, Sugiyama K,
Kiyomi A, Kato R, Onda K, Yuan B, Takagi N, Hu X, et al: Effects of
arsenic disulfide on proliferation, cytokine production, and
frequencies of CD4(+), CD8(+), and regulatory T cells in
mitogen-activated human peripheral blood mononuclear cells. Int
Immunopharmacol. 29:832–838. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tohyama N, Tanaka S, Onda K, Sugiyama K
and Hirano T: Influence of anticancer agents on cell survival,
proliferation, and CD4+CD25+Foxp3+
regulatory T cell-frequency in human peripheral-blood mononuclear
cells activated by T cell-mitogen. Int Immunopharmacol. 15:160–166.
2013. View Article : Google Scholar
|
|
29
|
Yu Z, Guo W, Ma X, Zhang B, Dong P, Huang
L, Wang X, Wang C, Huo X, Yu W, et al: Gamabufotalin, a
bufadienolide compound from toad venom, suppresses COX-2 expression
through targeting IKKβ/NF-κB signaling pathway in lung cancer
cells. Mol Cancer. 13:2032014. View Article : Google Scholar
|
|
30
|
Moreno Y, Banuls L, Urban E, Gelbcke M,
Dufrasne F, Kopp B, Kiss R and Zehl M: Structure-activity
relationship analysis of bufadienolide-induced in vitro growth
inhibitory effects on mouse and human cancer cells. J Nat Prod.
76:1078–1084. 2013. View Article : Google Scholar
|
|
31
|
Qi F, Li A, Inagaki Y, Kokudo N, Tamura S,
Nakata M and Tang W: Antitumor activity of extracts and compounds
from the skin of the toad Bufo bufo gargarizans Cantor. Int
Immunopharmacol. 11:342–349. 2011. View Article : Google Scholar
|
|
32
|
Qi F, Li A, Zhao L, Xu H, Inagaki Y, Wang
D, Cui X, Gao B, Kokudo N, Nakata M, et al: Cinobufacini, an
aqueous extract from Bufo bufo gargarizans Cantor, induces
apoptosis through a mitochondria-mediated pathway in human
hepatocellular carcinoma cells. J Ethnopharmacol. 128:654–661.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moreno Y, Banuls L, Katz A, Miklos W,
Cimmino A, Tal DM, Ainbinder E, Zehl M, Urban E, Evidente A, Kopp
B, et al: Hellebrin and its aglycone form hellebrigenin display
similar in vitro growth inhibitory effects in cancer cells and
binding profiles to the alpha subunits of the
Na+/K+-ATPase. Mol Cancer. 12:332013.
View Article : Google Scholar
|
|
34
|
Tian HY, Wang L, Zhang XQ, Zhang DM, Wang
Y, Liu JS, Jiang RW and Ye WC: New bufadienolides and
C23 steroids from the venom of Bufo bufo gargarizans.
Steroids. 75:884–890. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang DM, Liu JS, Deng LJ, Chen MF, Yiu A,
Cao HH, Tian HY, Fung KP, Kurihara H, Pan JX, et al: Arenobufagin,
a natural bufadienolide from toad venom, induces apoptosis and
autophagy in human hepatocellular carcinoma cells through
inhibition of PI3K/Akt/mTOR pathway. Carcinogenesis. 34:1331–1342.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Deng LJ, Peng QL, Wang LH, Xu J, Liu JS,
Li YJ, Zhuo ZJ, Bai LL, Hu LP, Chen WM, et al: Arenobufagin
intercalates with DNA leading to G2 cell cycle arrest
via ATM/ATR pathway. Oncotarget. 6:34258–34275. 2015.PubMed/NCBI
|
|
37
|
Tang N, Shi L, Yu Z, Dong P, Wang C, Huo
X, Zhang B, Huang S, Deng S, Liu K, et al: Gamabufotalin, a major
derivative of bufadienolide, inhibits VEGF-induced angiogenesis by
suppressing VEGFR-2 signaling pathway. Oncotarget. 7:3533–3547.
2016.
|
|
38
|
Fink SL and Cookson BT: Apoptosis,
pyroptosis, and necrosis: Mechanistic description of dead and dying
eukaryotic cells. Infect Immun. 73:1907–1916. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Peterson RA: Regulatory T-cells: Diverse
phenotypes integral to immune homeostasis and suppression. Toxicol
Pathol. 40:186–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Iriyama N, Yoshino Y, Yuan B, Horikoshi A,
Hirabayashi Y, Hatta Y, Toyoda H and Takeuchi J: Speciation of
arsenic trioxide metabolites in peripheral blood and bone marrow
from an acute promyelocytic leukemia patient. J Hematol Oncol.
5:12012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Iriyama N, Yuan B, Yoshino Y, Hatta Y,
Horikoshi A, Aizawa S, Takeuchi J and Toyoda H: Aquaporin 9, a
promising predictor for the cytocidal effects of arsenic trioxide
in acute promyelocytic leukemia cell lines and primary blasts.
Oncol Rep. 29:2362–2368. 2013.PubMed/NCBI
|
|
42
|
Hu XM, Tanaka S, Onda K, Yuan B, Toyoda H,
Ma R, Liu F and Hirano T: Arsenic disulfide induced apoptosis and
concurrently promoted erythroid differentiation in
cytokine-dependent myelodysplastic syndrome-progressed leukemia
cell line F-36p with complex karyotype including monosomy 7. Chin J
Integr Med. 20:387–393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hu XM, Yuan B, Tanaka S, Zhou Q, Onda K,
Toyoda H and Hirano T: Involvement of oxidative stress associated
with glutathione depletion and p38 mitogen-activated protein kinase
activation in arsenic disulfide-induced differentiation in HL-60
cells. Leuk Lymphoma. 55:392–404. 2014. View Article : Google Scholar
|
|
44
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG): Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 365:1687–1717. 2005.
View Article : Google Scholar : PubMed/NCBI
|