Introduction
Lung cancer remains a major cause of human
mortality. Treatment of non-small cell lung cancer (NSCLC) is being
improved by a better understanding of the molecular mechanisms
involved in tumor initiation and progression, mainly in
adenocarcinoma. The discovery of EGFR activating mutations
and ALK rearrangements in a subset of NSCLC has led to major
changes in the therapeutic strategy. Anti-EGFR and anti-ALK
therapies achieve disease regression and improvement in survival in
some patients (1,2). As a consequence, detection of
ALK rearrangements, present in ~3–5% of NSCLC, has become
mandatory to screen for patients who may benefit anti-ALK targeted
therapy. Searching for an ALK-rearrangement using the Vysis
LSI ALK Dual Color Break Apart fluorescence in situ
hybridization (FISH) probe (Abbott Molecular, Rungis, France) is
the Food and Drug Administration (FDA)-approved molecular test and
is considered as the 'gold-standard'. ALK-rearranged NSCLC
are defined as tumors with 15% or more nuclei with rearranged
signals (first count in 50 nuclei and if considered as equivocal,
i.e., 5–25 positive cells among these 50 first nuclei, the count
must include 50 additional tumor nuclei) (3,4). In
addition to FISH testing, many studies have suggested the use of
ALK immunohistochemistry (IHC) and RT-PCR to detect
ALK-rearranged cancers especially under the European
guidelines. Although most of the studies have reported a close
correlation between FISH and IHC, many of them, including the
largest ones, have reported some discordance between both
techniques (5–21). These discordances are all relevant
as ALK FISH+IHC− and ALK
FISH−IHC+ patients may respond to anti-ALK
therapy (6,10,15).
Additional methods such as next generation sequencing and real-time
polymerase chain reaction have been proposed as complementary or
even replacement techniques for ALK screening. IHC with
different antibodies or FISH with other probes or brightfield
combined IHC-in situ hybridization were also proposed to
improve the diagnostic accuracy (8,15,19,22–26).
Nevertheless, discrepant cases are still described. Recently, some
authors introduced the concept of 'borderline' ALK-positive
because of ALK FISH percentages of rearranged nuclei close
to the threshold of 15% and of 'ALK-equivocal' tumors to
describe tumors with challenging ALK FISH and/or ALK IHC
analysis results, and/or discrepancies between FISH and IHC
(10,15). Some, but not all, of these
'ambiguous' ALK tumors respond to crizotinib treatment, all
the more if they also strongly express c-MET, another potential
target of crizotinib (6,10,15).
ALK screening strategy is still debated to maximize
ALK-rearranged NSCLC detection and to minimize ALK
false positivity. Ambiguous ALK-rearranged tumors represent
a major diagnostic and therapeutic challenge.
In this study, we report our experience in
ALK rearrangement screening in lung adenocarcinoma using the
FDA-approved FISH probe and IHC. Clinical outcomes of the
crizotinib-treated patients were also reported. This study
identifies and describes the issues concerning ambiguous
ALK-rearranged tumors.
Materials and methods
Cases studied
We included all the ALK-rearranged
adenocarcinoma cases identified by FISH and diagnosed at the
University Hospital Morvan cancer molecular genetics platform from
January 2010 to December 2014 for which sufficient tumor material
was available to perform IHC analyses. These specimens (primary
tumors and metastases) were formalin-fixed and paraffin embedded
(FFPE). ALK analyses were conducted as part of the
diagnostic work-up for the therapeutic management of patients with
advanced stages of NSCLC according the French National Cancer
Institute guidelines, together with EGFR and KRAS
mutation screening. c-MET and complementary anti-ALK IHC analyses
with different antibodies were also performed on samples with
sufficient amount of tumor cells. The present study was conducted
following our national and institutional guidelines. All samples
were included in a registered tumour tissue collection and the
present study was conducted in compliance with the Helsinki
Declaration and after approval by our Institutional Review Board
(CHRU Brest, CPP n° DC-2008-214). Response to crizotinib treatment
was provided by the oncologists in charge of the therapeutic
management of the ALK-positive patients. Therapy response
was quoted by the oncologists assuming the clinical follow-up of
the patients using clinical and radiological criteria, as used in
other ALK-NSCLC dedicated studies.
ALK fluorescent in situ hybridization
(FISH)
Tissue sections 3-µm thick were laid on
SuperFrost® Plus slides. After deparaffinization, the
slides were pre-treated with Dako Histology FISH Accessory kit
(Dako, Glostrup, Denmark) following the manufacturer's
instructions. Slides were washed in distilled water and dehydrated
in increasing concentrations of alcohol (70, 90 and 100%) and
air-dried at room temperature. Ten microliters of the Vysis LSI
ALK Dual Color Break Apart Rearrangement Probe was placed
onto the tissue sections. Slides were denaturated at 73°C for 5 min
and then hybridized at 37°C for 16 h on a Dako Hybridizer.
Following hybridization, the slides were washed with buffer,
counter-colored with 4′,6-diamidino-2-phenylindole (DAPI) solution
and coverslipped. They were then read using an epifluorescence
microscope (Zeiss, Le Pecq, France) connected to a CCD camera and
software for analyzing fluorescent signals (ISIS software;
MetaSystems, Altlussheim, Germany).
At least 50 tumor nuclei (and, if required, 100
tumor nuclei following the FISH test guidelines) were assessed for
each case considering the following criteria: ALK FISH was
considered positive (i.e., ALK-rearranged) if there was a
split between the orange (3′-end) and the green (5′-end) signals
(i.e., orange and green signals being two or more signals apart) or
an isolated single orange signal in ≥15% of tumor nuclei. We also
noted the mean ALK copy number in tumor nuclei (counting
both fused ALK and single 3′ALK signals).
ALK and c-MET immunohistochemistry
First line IHC was performed using the monoclonal
antibody anti-ALK p80 (clone 5A4; CliniSciences, Nanterre, France)
at a dilution of 1:25. Immunohistochemistry was performed on
Ventana Benchmark XT® automated slide preparation system
using OptiView DAB IHC Detection kit (both from Roche Diagnostics,
Meylan, France). This IHC has successfully obtained European and
French external quality controls. Briefly, IHC was performed on
3-µm thick tissue sections. OptiView® DAB IHC
Detection kit was used according to Ventana staining procedure
including pre-treatment with cell conditioner 1 for 92 min,
followed by incubation with diluted antibody at 37°C for 1 h.
Antibody incubation and signal amplification steps were followed by
counterstaining with one drop of hematoxylin for 20 min and one
drop of bluing reagent for 4 min. Subsequently, the slides were
removed from the immunostainer, washed in water with dishwashing
detergent, and mounted. Immunostaining was scored as negative
(score 0), or as positive with faint staining (score 1+), moderate
(score 2+) or intense (score 3+) staining of the tumor cells.
Samples with a sufficient amount of tumors cells
were analyzed with additional IHC using the same IHC protocol with
two other anti-ALK antibodies (clone D5F3, prediluted, Ventana,
Roche Diagnostics; clone 1A4, 1:100, Origene, Rockville, MD, USA)
and with anti-c-MET antibody (clone SP44, prediluted, Ventana,
Roche Diagnostics) following the manufacturer's instructions.
Results
Cases included
Fifty-five ALK FISH-positive tumors from 55
patients, including 24 treated with crizotinib, were included in
our study. Data concerning these 55 tumors and patients, including
their response to crizotinib, are summarized in Table I. The 55 patients consisted of 30
men and 25 women with a mean age of 61 years (range, 28 to 88
years). Thirty-seven patients had a history of present or past
smoking and 14 were never-smokers (no data for 4 patients). In
addition to ALK FISH positivity, a KRAS mutation was
identified in 7 tumors and a EGFRL858R in another. Complete
response to crizotinib was observed only in one patient (case 10).
A partial response (i.e., tumor regression or stable disease) was
noted in 16 patients. The disease continued to progress despite
crizotinib treatment in the other 7 patients.
 | Table ISummary of the 55 ALK-positive
cases in fluorescent in situ hybridization. |
Table I
Summary of the 55 ALK-positive
cases in fluorescent in situ hybridization.
| Case | Gender | Age | Smoker | KRAS | EGFR | c-MET IHC | ALK FISH
nuclei positive (%) | Main ALK
FISH positive pattern | ALK IHC (5A4
clone) | ALK IHC (D5F3
clone) | ALK IHC (1A4
clone) | Response to
crizotinib |
|---|
| 1 | M | 74 | Yes | – | – | 0 | 80 | SRS | 3+ | 3+ | 3+ | Progression |
| 2 | M | 54 | No | – | – | NC | 75 | SRS | 3+ | NC | NC | Partial
response |
| 3 | F | 62 | No | – | – | 2+ | 70 | SS | 3+ | 3+ | 3+ | Partial
response |
| 4 | M | 40 | No | – | – | 2+ | 65 | SRS | 3+ | 3+ | NC | Partial
response |
| 5 | M | 51 | No | – | – | 3+ | 31 | SS | 3+ | 3+ | 3+ | Partial
response |
| 6 | F | 65 | Yes | – | – | 3+ | 95 | SRS | 3+ | 3+ | NC | No crizotinib |
| 7 | M | 64 | Yes | – | – | NC | 40 | SRS | 2+ | 3+ | 3+ | No crizotinib |
| 8 | M | 74 | Yes | – | – | 2+ | 72 | SS | 2+ | NC | NC | Stable disease |
| 9 | F | 32 | Yes | – | – | NC | 30 | SRS | 3+ | 3+ | 3+ | Progression |
| 10 | F | 61 | Yes | – | – | 3+ | 50 | SS | 2+ | 3+ | 3+ | Complete
response |
| 11 | F | 74 | No | – | – | 3+ | 80 | SS | 2+ | 2+ | 3+ | Partial
response |
| 12 | F | 35 | No | – | – | 2+ | 80 | SS | 2+ | 3+ | 3+ | Partial
response |
| 13 | F | 57 | Yes | – | – | 2+ | 70 | SS | 2+ | NC | NC | Partial
response |
| 14 | F | 48 | No | – | – | NC | 45 | SRS | 2+ | NC | NC | Partial
response |
| 15 | F | 67 | No | – | – | 2+ | 16 (B) | SS | 2+ | 3+ | 3+ | Partial
response |
| 16 | F | 65 | Yes | – | – | 1+ | 80 | SRS | 2+ | NC | NC | No crizotinib |
| 17 | F | 60 | ND | – | – | 2+ | 70 | SS | 2+ | 3+ | 2+ | No crizotinib |
| 18 | F | 70 | Yes | – | – | NC | 70 | SS | 2+ | 3+ | 2+ | No crizotinib |
| 19 | M | 36 | Yes | – | – | NC | 64 | SRS | 2+ | 3+ | 2+ | No crizotinib |
| 20 | M | 77 | No | – | – | NC | 63 | SS | 2+ | 3+ | NC | No crizotinib |
| 21 | F | 61 | Yes | – | – | NC | 40 | SS | 2+ | 3+ | NC | No crizotinib |
| 22 | M | 75 | No | – | – | NC | 35 | SS | 2+ | 2+ | NC | No crizotinib |
| 23 | F | 28 | Yes | – | – | 1+ | 30 | SS | 2+ | 3+ | 3+ | No crizotinib |
| 24 | M | 61 | No | – | – | NC | 15 (B) | SS | 2+ | 3+ | NC | No crizotinib |
| 25 | M | 70 | Yes | NC | NC | 3+ | 15 (B) | SS | 2+ | NC | NC | No crizotinib |
| 26 | M | 60 | Yes | NC | NC | NC | 30 | SS | 1+ | NC | NC | Partial
response |
| 27 | F | 80 | No | – | – | NC | 30 | SS | 1+ | NC | NC | Partial
response |
| 28 | M | 55 | Yes | – | – | NC | 99 | SRS | 1+ | 2+ | 2+ | No crizotinib |
| 29 | M | 65 | Yes | – | – | NC | 25 | SRS | 1+ | NC | NC | No crizotinib |
| 30 | F | 78 | Yes | – | – | NC | 20 (B) | SS | 1+ | 1+ | NC | No crizotinib |
| 31 | M | 60 | Yes | NC | NC | 2+ | 20 (B) | SS | 1+ | NC | NC | No crizotinib |
| 32 (A) | F | 62 | Yes | G12S | – | NC | 20 (B) | SS | 0 | NC | NC | Stable disease |
| 33 (A) | M | 58 | Yes | G13C | – | 1+ | 30 | SS | 0 | 0 | 0 | Progression |
| 34 (A) | M | 49 | Yes | NC | NC | NC | 25 | SS | 0 | NC | NC | Progression |
| 35 (A) | M | 74 | Yes | – | – | 3+ | 17 (B) | SRS | 0 | 0 | 1+ | Progression |
| 36 (A) | F | 51 | Yes | G12V | – | 2+ | 16 (B) | SRS | 0 | NC | 1+ | Progression |
| 37 (A) | M | 73 | Yes | – | – | 2+ | 25 | SS | 0 | 0 | 0 | Partial
response |
| 38 (A) | F | 64 | No | NC | NC | NC | 20(B) | SS | 0 | NC | NC | Partial
response |
| 39 (A) | M | 75 | ND | – | – | NC | 80 | SS | 0 | 0 | 1+ | No crizotinib |
| 40 (A) | M | 63 | Yes | – | – | NC | 60 | SS | 0 | 0 | 1+ | No crizotinib |
| 41 (A) | M | 54 | Yes | – | – | NC | 47 | SS | 0 | NC | NC | No crizotinib |
| 42 (A) | M | 58 | Yes | – | – | NC | 40 | SRS | 0 | NC | NC | No crizotinib |
| 43 (A) | M | 53 | Yes | – | – | NC | 30 | SS | 0 | 0 | 0 | No crizotinib |
| 44 (A) | F | 80 | Yes | G12C | – | 3+ | 30 | SS | 0 | 0 | NC | No crizotinib |
| 45 (A) | M | 58 | Yes | – | – | NC | 25 | SS | 0 | 0 | NC | No crizotinib |
| 46 (A) | F | 43 | Yes | – | – | NC | 20 (B) | SS | 0 | 0 | 1+ | No crizotinib |
| 47 (A) | F | 78 | No | – | L858R | 3+ | 20 (B) | SRS | 0 | NC | NC | No crizotinib |
| 48 (A) | M | 65 | ND | – | – | NC | 18 (B) | SS | 0 | NC | NC | No crizotinib |
| 49 (A) | M | 78 | ND | – | – | NC | 18 (B) | SS | 0 | 0 | NC | No crizotinib |
| 50 (A) | M | 88 | Yes | G12D | – | NC | 17 (B) | SRS | 0 | NC | NC | No crizotinib |
| 51 (A) | M | 61 | Yes | – | – | NC | 17 (B) | SS | 0 | 0 | 1+ | No crizotinib |
| 52 (A) | F | 58 | Yes | – | – | NC | 16 (B) | SS | 0 | 0 | 1+ | No crizotinib |
| 53 (A) | F | 57 | Yes | – | – | NC | 40 | SS | NC | NC | NC | Partial
response |
| 54 (A) | F | 50 | Yes | G12V | – | 0 | 17 (B) | SS | NC | NC | NC | Progression |
| 55 (A) | M | 45 | Yes | G13D | – | NC | 25 | SS | NC | NC | NC | No crizotinib |
ALK fluorescent in situ
hydridization
Table II summarizes
the main FISH results. The mean percentage of positive nuclei per
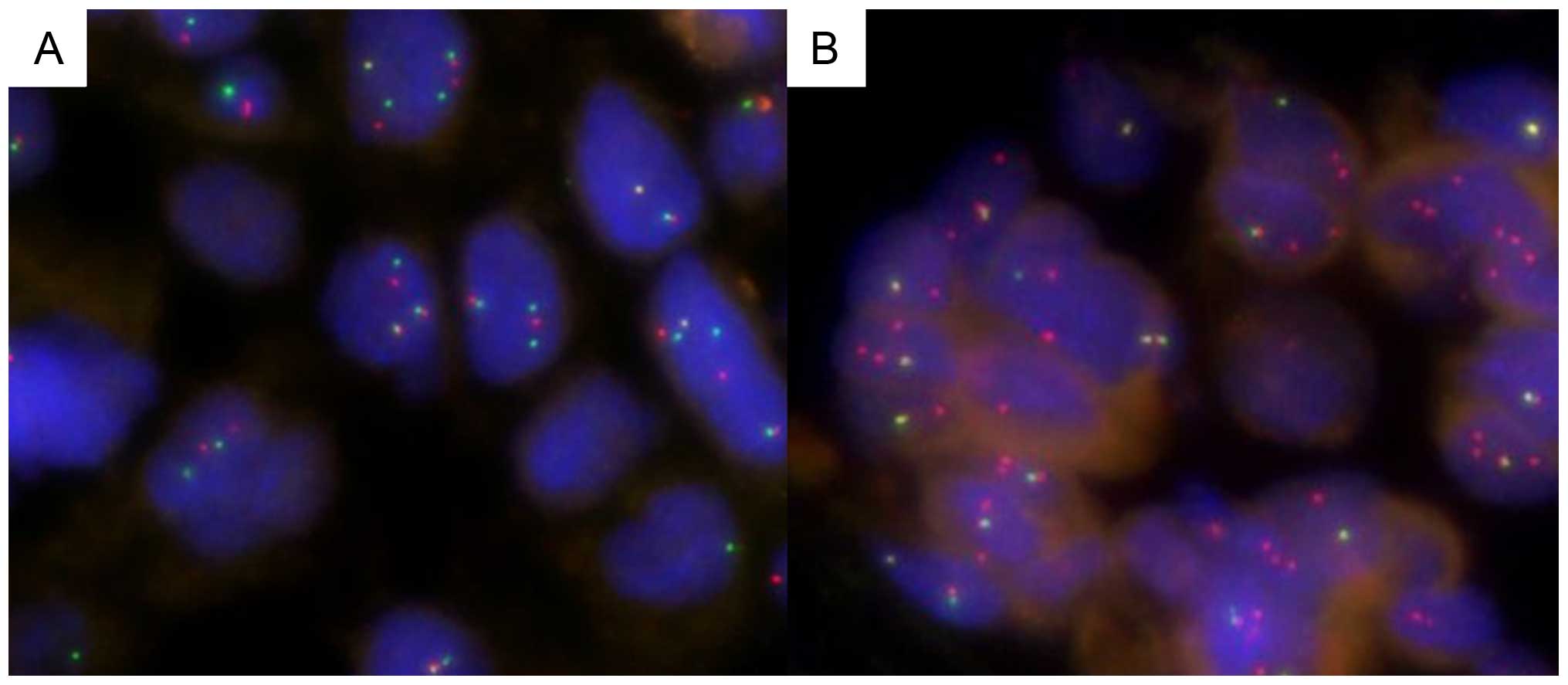
tumor was 41.4% (from 15 to 99%). Split and isolated 3′ signals
co-existed in most of the tumors (mostly split signal in 39 tumors
and isolated 3′ signal in 16 samples). The percentage of positive
nuclei were between 15 and 20% in 17 tumors. Only one (1/55-1.8%)
tumor presented a high ALK copy number (i.e., >6 ALK copy
numbers per nucleus). Fig. 1
presents examples of ALK FISH positive patterns.
 | Table IISummary of the ALK FISH patterns and
correlation with immunohistochemistry (5A4 clone) and response to
anti-ALK targeted therapy. |
Table II
Summary of the ALK FISH patterns and
correlation with immunohistochemistry (5A4 clone) and response to
anti-ALK targeted therapy.
| Number of
cases | Main ALK
rearrangement signals
| Mean ALK
copy no. per nucleus
|
|---|
| Split | Single 3′ | <6 | >6 |
|---|
| ALK
FISH+IHC+ Response+ | 13 | 10 | 3 | 13 | 0 |
| ALK
FISH+IHC+ Response− | 2 | 0 | 2 | 2 | 0 |
| ALK
FISH+IHC+ Not treated | 16 | 10 | 6 | 16 | 0 |
| ALK
FISH+IHC− Response+ | 3 | 3 | 0 | 3 | 0 |
| ALK
FISH+IHC− Response− | 4 | 2 | 2 | 4 | 0 |
| ALK
FISH+IHC− Not treated | 14 | 11 | 3 | 13 | 1a |
| ALK
FISH+IHC NC Response+ | 1 | 1 | 0 | 1 | 0 |
| ALK
FISH+IHC NC Response− | 1 | 1 | 0 | 1 | 0 |
| ALK
FISH+IHC NC Not treated | 1 | 1 | 0 | 1 | 0 |
| Total | 56 | 39 | 17 | 55 | 1 |
ALK immunohistochemistry
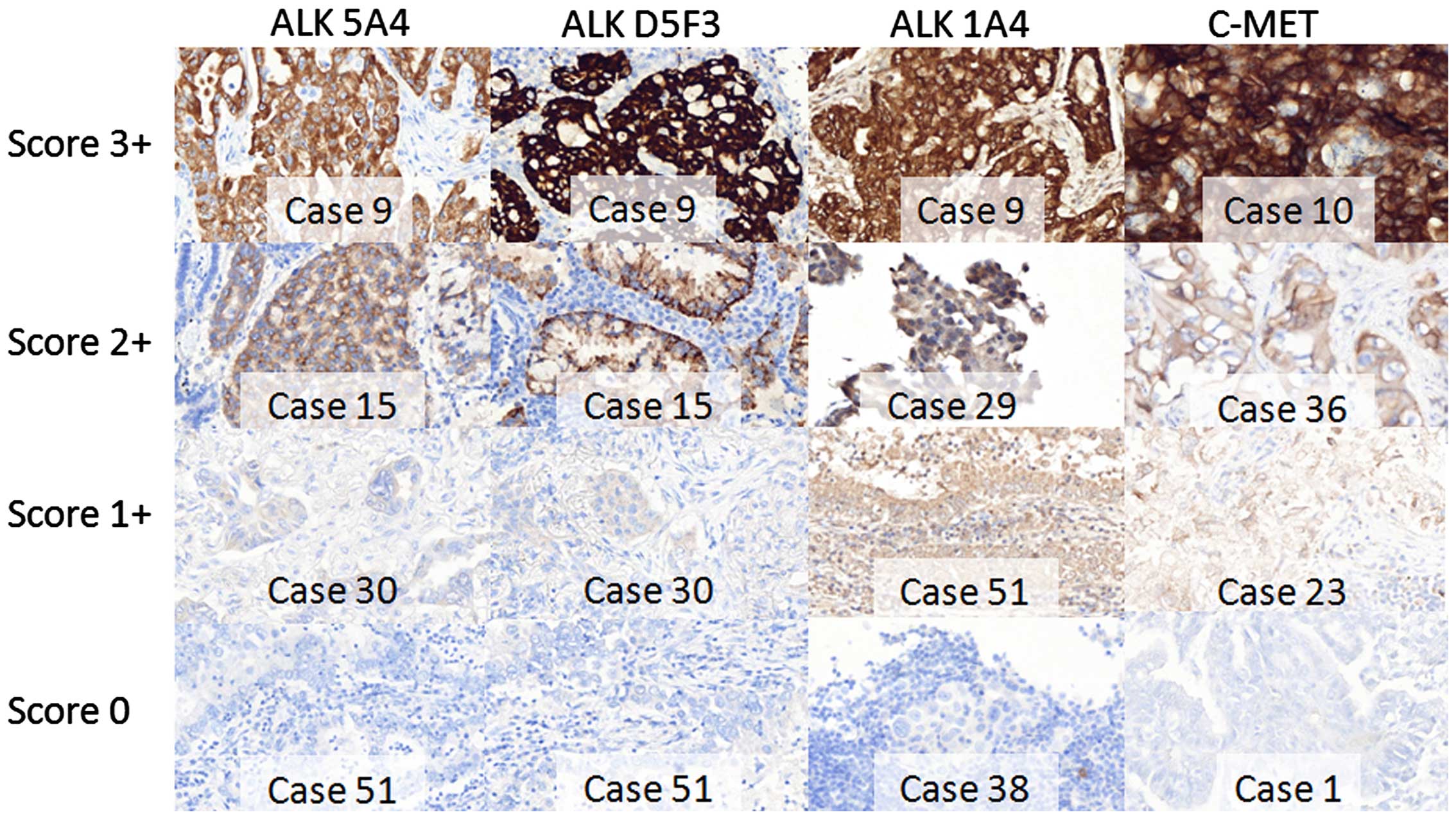
ALK IHC using clone 5A4 was non-contributive in
three cases (Table I). Twenty-one
tumors (38.2%) were immunonegative. Thirty-one tumors were
considered ALK positive (56.3%) with a 3+ staining in 7/31 cases, a
2+ staining in 18/31 cases and a 1+ staining in 6/31 cases.
Additional IHC using clones D5F3 and 1A4 was contributive for only
33 and 24 cases, respectively, because of progressive cell
depletion in small biopsies. IHC with clone D5F3 was positive in
21/33 (63.6%) tumors with the higher rate of strong 3+ staining
intensity in 17/33 (51.5%) tumors. IHC with clone 1A4 was positive
in 21/24 (87.5%) tumors. Twelve and three tumors remained
immunonegative with clones D5F3 and 1A4, respectively. Table III summarizes the results of ALK
IHC with different antibodies and examples of staining are shown in
Fig. 2.
 | Table IIISummary of the results of ALK
immunohistochemistry analyses. |
Table III
Summary of the results of ALK
immunohistochemistry analyses.
| ALK antibody | Insufficient
material | Negative (−) | Score 1+ | Score 2+ | Score 3+ | No of positive
cases/total (%) |
|---|
| Clone 5A4 | 3 | 21 | 6 | 18 | 7 | 31/52 (59.6) |
| Clone D5F3 | 22 | 12 | 1 | 3 | 17 | 21/33 (63.6) |
| Clone 1A4 | 31 | 3 | 7 | 4 | 10 | 21/24 (87.5) |
c-MET expression
c-MET IHC was performed in only 23/55 tumors because
of cell depletion. Among these 23 samples, 18 samples were
considered positive (i.e., 2+ or 3+ staining intensity) and 5
samples were considered negative (i.e., 1+ or 0 staining). Indeed,
in our daily practice, we also screen every NSCLC patient for c-MET
expression but not for c-MET amplification which is only performed
when a clinician requires this information for inclusion in a
c-MET-related specific treatment trial. Therefore, c-MET FISH was
not performed in these ALK-positive NSCLC cases.
Correlation between response to
crizotinib, FISH and IHC results
Table II summarizes
the distribution of patients according to their response to
crizotinib and ALK FISH and IHC results. Thirty-one patients had a
concordant FISH+IHC+ status. Of note, two of
the 15 treated ALK FISH+IHC+ patients
did not respond to crizotinib (cases 1 and 9). Three of the 21
ALK FISH+IHC− patients had a response
to anti-ALK therapy. A partial response was observed in case 37
with 25% of positive tumor nuclei also expressing a 2+ staining
with c-MET IHC. A stable disease was observed in case 32 having a
20% ALK FISH rearranged status and a KRAS G12S mutation. A
partial response was observed in case 38 with 20% ALK FISH
rearranged nuclei without contributive c-MET IHC and EGFR
and KRAS mutational analyses.
Discussion
ALK-rearranged NSCLC are classically reported to be
adenocarcinomas involving young and never-smoker patients,
characterized by mucinous and cribriform histopathological features
and the absence of association to EGFR or KRAS
mutations (27–31). Most of these ALK-rearranged NSCLC
respond to crizotinib (32). A
strong correlation between the FISH-rearranged status of the tumor
and the expression of the ALK protein detected by IHC was reported
in many studies (5–12,14–21,30).
In addition, the mean copy number of the ALK gene in
ALK-rearranged tumor is admitted to be usually low, with
<6 ALK copies per nucleus, in contrast to tumors lacking
ALK rearrangement in which high ALK copy gain is
frequent (33).
We classified the ALK-rearranged tumors in
our study into two groups. The first group included
ALK-rearranged tumors by both FISH and IHC positivity
(FISH+IHC+), without high ALK copy
gain and no EGFR and KRAS mutation. The second group,
designated as ambiguous ALK-positive, contained those
ALK positive tumors that did not correspond to these
criteria (in fact mainly FISH+IHC−cases).
Seventeen tumors, so-called borderline tumors, had a percentage of
rearranged nuclei ≤20% and were included in the first or second
group. Most of these 'borderline' tumors were
FISH+IHC− but some were
FISH+IHC+ (Table
I) (10,15).
Ambiguous ALK phenotype is presented by
tumors being positive for only FISH or IHC. Some large studies have
pointed out a significant rate of discrepancies between FISH and
IHC (6,11,12).
In our study, 24 tumors could be considered as ALK
'ambiguous'-positive tumors because they were IHC negative or
non-contributive. Four of the 9 patients among these 24 cases
treated with crizotinib showed a response. In a large French study,
only 53.3% (80/150) of ALK-positive tumors were
FISH+IHC+ and 24% (36/150) were
FISH+IHC−; 19 tumors were
FISH−IHC+ and 15 FISH non-contributive IHC
(6). Crizotinib-responders are
reported among FISH+IHC− and
FISH−IHC+ cases, pointing out that combining
FISH and IHC is important to minimize the risk of
ALK-testing false-negativity. Indeed, examples of
crizotinib-responders are reported even in patients with
rearrangement rates as high as 60% by FISH although they are
IHC− (6).
Confrontation of ALK status with EGFR
and KRAS mutational status speaks in favor of an accurate
screening strategy. In the study by Cabillic et al on 3,244
NSCLC, 8 (5.3%) and 14 (9.3%) of the 150 ALK-positive tumors
were also mutated for EGFR and KRAS, respectively
(6). Another French study reported
a 7% rate (11/150) of ALK FISH+IHC−
tumors mutated for EGFR and KRAS genes (11). In our opinion, even if the concept
of mutually exclusive mutations/rearrangements concerning
ALK, EGFR and KRAS is widely accepted, the
challenging cases of double mutants justify parallel analyses of
these three genes instead of a multistep algorithm that would lead
to analyze ALK only in EGFR and KRAS wild-type
tumors. Moreover, ALK inhibitors are reported to be effective in
patients with co-alterations in ALK and EGFR
(34).
Tumors having a percentage of ALK-rearranged
nuclei between 15 and 20%, in the so-called 'borderline' or
'equivocal' grey-zone, face a particular analytic issue. In our
study, 17 tumors could be considered as borderline tumors. Three of
the 6 patients treated with crizotinib showed a response. A study
by Camidge et al on 13 ALK-positive patients among 73
patients was concordant with the threshold of 15% FISH-rearranged
nuclei to consider a tumor as ALK-rearranged or not
(35). In this study, the lowest
percentage of rearranged nuclei in the so-called
ALK-positive tumors was ~22% and the highest percentage in
the ALK-negative tumors was ~10%. No tumor had a percentage
of rearranged nuclei between 10 and 20%. Of note, up to 11% of
rearranged nuclei were encountered within non-tumor areas (35). More recently, many studies reported
tumors within this 'grey-zone' from 10 to 20% of rearranged tumor
nuclei, with various combinations of discordance between FISH and
IHC results. These studies also discussed the interest of using
different FISH probes and anti-ALK antibodies (6,10,11,15).
Detection of potential ALK-rearranged tumors that could benefit
anti-ALK therapy beyond the threshold of 15% remains a challenging
issue that justifies a systematic use of anti-ALK IHC complementary
to ALK FISH to detect ALK
FISH−IHC+ cases (17,18).
Indeed, a few cases with a rate as low as 5% of ALK-positive
nuclei associated with IHC positivity are reported to respond to
crizotinib therapy (11,15). As this grey-zone is really close to
the percentage of ALK-rearranged nuclei observed in
non-tumor tissue, one can hypothesize that some of these tumors
with rearranged nuclei from 15 to 20% could be technical
false-positive results. Nevertheless, crizotinib-responders were
reported in these grey-zone borderline tumors supporting the
biological significance of the FISH positivity (6,10,15).
Some authors hypothesized that a high ALK copy number,
and/or a c-MET expression in these ALK 'borderline' tumors
could explain the response or absence of response of the patients
to anti-ALK therapy. However, the biological relevance of these two
additional molecular defects is still not clearly demonstrated
(10). Intra-tumor heterogeneity
was proposed to have implications in the detection of
ALK-rearrangements (7,36). A
combination of multiple FISH analyses with different probes was
also proposed to allow enhancement of the detection of ALK
rearrangements in borderline and ambiguous tumors (15). In our study, most of the ALK
borderline tumors within this grey-zone were also ambiguous
FISH+IHC− tumors. Even if the IHC negative
feature could be corrected using different antibodies in some
samples, cell depletion can prevent efficient comparison of
antibodies, as in our study. We tested the three supplementary
antibodies in only half of the cases. Nevertheless, 7 samples
initially considered FISH+IHC− were weakly
positive (1+) for at least one additional antibody.
Furthermore, cell depletion in small biopsies can
hamper the carrying out of EGFR and KRAS molecular
analyses, and in the near future from analyzing other oncogenes
such as ROS1. The diagnostic strategy must take into account
the problem of tiny biopsies, in concomitant molecular and IHC
analyses. Tissue handling, processing and sectioning must be
optimal to minimize tumor wastage (4).
To conclude, it is crucial to be aware of the
therapeutic implications despite discordances between FISH and IHC
in ALK ambiguous and borderline positive tumors. These
lesions - with diagnostic and therapeutic issues because of
potential response to anti-ALK targeted therapies - must be studied
further to facilitate the diagnosis of ALK-rearranged tumors
in an intent-to-treat strategy. Additional FISH analyses with
bacterial artificial chromosome clones or reverse
transcriptase-polymerase chain reaction targeting already known
ALK fusion partners could be helpful to solve the issue of
borderline and/or ambiguous ALK-positive tumors.
In the meantime, the issue remains partially
unsolved. Nevertheless, our data clearly emphasize that, besides
using different FISH probes to solve certain ambiguous cases, using
different IHC could also help to elucidate some of the
first-appearing discrepant data. Still, some discrepant cases
remain unsolved and the prediction of a response or progression
following crizotinib treatment in these challenging cases remains
difficult. Clinicians and pathologists must be aware of these
potential issues to reach a personalized diagnostic strategy in the
era of personalized medicine. New sampling and additional FISH and
IHC analyses are parts of this personalized diagnostic
strategy.
Acknowledgments
This study was supported by the 'Omnium group'. The
authors wish to thank Mrs. Stéphanie Bouvier, Ms. Sandrine Duigou
and the Brest Biobank for their technical assistance in this
study.
References
|
1
|
Kwak EL, Bang YJ, Camidge DR, Shaw AT,
Solomon B, Maki RG, Ou SH, Dezube BJ, Jänne PA, Costa DB, et al:
Anaplastic lymphoma kinase inhibition in non-small-cell lung
cancer. N Engl J Med. 363:1693–1703. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Soda M, Choi YL, Enomoto M, Takada S,
Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K,
Hatanaka H, et al: Identification of the transforming EML4-ALK
fusion gene in non-small-cell lung cancer. Nature. 448:561–566.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lindeman NI, Cagle PT, Beasley MB, Chitale
DA, Dacic S, Giaccone G, Jenkins RB, Kwiatkowski DJ, Saldivar JS,
Squire J, et al: Molecular testing guideline for selection of lung
cancer patients for EGFR and ALK tyrosine kinase inhibitors:
Guideline from the College of American Pathologists, International
Association for the Study of Lung Cancer, and Association for
Molecular Pathology. Arch Pathol Lab Med. 137:828–860. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thunnissen E, Bubendorf L, Dietel M,
Elmberger G, Kerr K, Lopez-Rios F, Moch H, Olszewski W, Pauwels P,
Penault-Llorca F, et al: EML4-ALK testing in non-small cell
carcinomas of the lung: A review with recommendations. Virchows
Arch. 461:245–257. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alì G, Proietti A, Pelliccioni S, Niccoli
C, Lupi C, Sensi E, Giannini R, Borrelli N, Menghi M, Chella A, et
al: ALK rearrangement in a large series of consecutive non-small
cell lung cancers: Comparison between a new immunohistochemical
approach and fluorescence in situ hybridization for the screening
of patients eligible for crizotinib treatment. Arch Pathol Lab Med.
138:1449–1458. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cabillic F, Gros A, Dugay F, Begueret H,
Mesturoux L, Chiforeanu DC, Dufrenot L, Jauffret V, Dachary D,
Corre R, et al: Parallel FISH and immunohistochemical studies of
ALK status in 3244 non-small-cell lung cancers reveal major
discordances. J Thorac Oncol. 9:295–306. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Conklin CM, Craddock KJ, Have C, Laskin J,
Couture C and Ionescu DN: Immunohistochemistry is a reliable
screening tool for identification of ALK rearrangement in
non-small-cell lung carcinoma and is antibody dependent. J Thorac
Oncol. 8:45–51. 2013. View Article : Google Scholar
|
|
8
|
Hofman P, Ilie M, Hofman V, Roux S, Valent
A, Bernheim A, Alifano M, Leroy-Ladurie F, Vaylet F, Rouquette I,
et al: Immunohistochemistry to identify EGFR mutations or ALK
rearrangements in patients with lung adenocarcinoma. Ann Oncol.
23:1738–1743. 2012. View Article : Google Scholar
|
|
9
|
Hutarew G, Hauser-Kronberger C, Strasser
F, Llenos IC and Dietze O: Immunohistochemistry as a screening tool
for ALK rearrangement in NSCLC: Evaluation of five different ALK
antibody clones and ALK FISH. Histopathology. 65:398–407. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ilie MI, Bence C, Hofman V, Long-Mira E,
Butori C, Bouhlel L, Lalvée S, Mouroux J, Poudenx M, Otto J, et al:
Discrepancies between FISH and immunohistochemistry for assessment
of the ALK status are associated with ALK 'borderline'-positive
rearrangements or a high copy number: A potential major issue for
anti-ALK therapeutic strategies. Ann Oncol. 26:238–244. 2015.
View Article : Google Scholar
|
|
11
|
Lantuejoul S, Rouquette I, Blons H, Le
Stang N, Ilie M, Begueret H, Grégoire V, Hofman P, Gros A, Garcia
S, et al: French multicentric validation of ALK rearrangement
diagnostic in 547 lung adenocarcinomas. Eur Respir J. 46:207–218.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McLeer-Florin A, Moro-Sibilot D, Melis A,
Salameire D, Lefebvre C, Ceccaldi F, de Fraipont F, Brambilla E and
Lantuejoul S: Dual IHC and FISH testing for ALK gene rearrangement
in lung adenocarcinomas in a routine practice: A French study. J
Thorac Oncol. 7:348–354. 2012. View Article : Google Scholar
|
|
13
|
Paik JH, Choe G, Kim H, Choe JY, Lee HJ,
Lee CT, Lee JS, Jheon S and Chung JH: Screening of anaplastic
lymphoma kinase rearrangement by immunohistochemistry in non-small
cell lung cancer: Correlation with fluorescence in situ
hybridization. J Thorac Oncol. 6:466–472. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Savic S, Diebold J, Zimmermann AK, Jochum
W, Baschiera B, Grieshaber S, Tornillo L, Bisig B, Kerr K and
Bubendorf L: Screening for ALK in non-small cell lung carcinomas:
5A4 and D5F3 antibodies perform equally well, but combined use with
FISH is recommended. Lung Cancer. 89:104–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Selinger C, Cooper W, Lum T, McNeil C,
Morey A, Waring P, Amanuel B, Millward M, Peverall J, Van Vliet C,
et al: Equivocal ALK fluorescence in-situ hybridization (FISH)
cases may benefit from ancillary ALK FISH probe testing.
Histopathology. 67:654–663. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Selinger CI, Rogers TM, Russell PA,
O'Toole S, Yip P, Wright GM, Wainer Z, Horvath LG, Boyer M,
McCaughan B, et al: Testing for ALK rearrangement in lung
adenocarcinoma: A multicenter comparison of immunohistochemistry
and fluorescent in situ hybridization. Mod Pathol. 26:1545–1553.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sholl LM, Aisner DL, Varella-Garcia M,
Berry LD, Dias-Santagata D, Wistuba II, Chen H, Fujimoto J, Kugler
K, Franklin WA, et al LCMC Investigators: Multi-institutional
oncogenic driver mutation analysis in lung adenocarcinoma: the lung
cancer mutation consortium experience. J Thorac Oncol. 10:768–777.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sullivan HC, Fisher KE, Hoffa AL, Wang J,
Saxe D, Siddiqui MT and Cohen C: The role of immunohistochemical
analysis in the evaluation of EML4-ALK gene rearrangement in lung
cancer. Appl Immunohistochem Mol Morphol. 23:239–244. 2015.
View Article : Google Scholar
|
|
19
|
Teixidó C, Karachaliou N, Peg V,
Gimenez-Capitan A and Rosell R: Concordance of IHC, FISH and RT-PCR
for EML4-ALK rearrangements. Transl Lung Cancer Res. 3:70–74.
2014.
|
|
20
|
Wynes MW, Sholl LM, Dietel M, Schuuring E,
Tsao MS, Yatabe Y, Tubbs RR and Hirsch FR: An international
interpretation study using the ALK IHC antibody D5F3 and a
sensitive detection kit demonstrates high concordance between ALK
IHC and ALK FISH and between evaluators. J Thorac Oncol. 9:631–638.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zwaenepoel K, Van Dongen A, Lambin S, Weyn
C and Pauwels P: Detection of ALK expression in non-small-cell lung
cancer with ALK gene rearrangements - comparison of multiple
immunohistochemical methods. Histopathology. 65:539–548. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gruber K, Horn H, Kalla J, Fritz P,
Rosenwald A, Kohlhäufl M, Friedel G, Schwab M, Ott G and Kalla C:
Detection of rearrangements and transcriptional up-regulation of
ALK in FFPE lung cancer specimens using a novel, sensitive,
quantitative reverse transcription polymerase chain reaction assay.
J Thorac Oncol. 9:307–315. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gruber K, Kohlhäufl M, Friedel G, Ott G
and Kalla C: A novel, highly sensitive ALK antibody 1A4 facilitates
effective screening for ALK rearrangements in lung adenocarcinomas
by standard immunohistochemistry. J Thorac Oncol. 10:713–716. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim H, Yoo SB, Choe JY, Paik JH, Xu X,
Nitta H, Zhang W, Grogan TM, Lee CT, Jheon S, et al: Detection of
ALK gene rearrangement in non-small cell lung cancer: A comparison
of fluorescence in situ hybridization and chromogenic in situ
hybridization with correlation of ALK protein expression. J Thorac
Oncol. 6:1359–1366. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nitta H, Tsuta K, Yoshida A, Ho SN, Kelly
BD, Murata LB, Kosmeder J, White K, Ehser S, Towne P, et al: New
methods for ALK status diagnosis in non-small-cell lung cancer: An
improved ALK immunohistochemical assay and a new, Brightfield, dual
ALK IHC-in situ hybridization assay. J Thorac Oncol. 8:1019–1031.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pekar-Zlotin M, Hirsch FR, Soussan-Gutman
L, Ilouze M, Dvir A, Boyle T, Wynes M, Miller VA, Lipson D, Palmer
GA, et al: Fluorescence in situ hybridization,
immunohistochemistry, and next-generation sequencing for detection
of EML4-ALK rearrangement in lung cancer. Oncologist. 20:316–322.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gainor JF, Varghese AM, Ou SH, Kabraji S,
Awad MM, Katayama R, Pawlak A, Mino-Kenudson M, Yeap BY, Riely GJ,
et al: ALK rearrangements are mutually exclusive with mutations in
EGFR or KRAS: An analysis of 1,683 patients with non-small cell
lung cancer. Clin Cancer Res. 19:4273–4281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jokoji R, Yamasaki T, Minami S, Komuta K,
Sakamaki Y, Takeuchi K and Tsujimoto M: Combination of
morphological feature analysis and immunohistochemistry is useful
for screening of EML4-ALK-positive lung adenocarcinoma. J Clin
Pathol. 63:1066–1070. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Just PA, Cazes A, Audebourg A, Cessot A,
Pallier K, Danel C, Vacher-Lavenu MC, Laurent-Puig P, Terris B and
Blons H: Histologic subtypes, immunohistochemistry, FISH or
molecular screening for the accurate diagnosis of ALK-rearrangement
in lung cancer: A comprehensive study of Caucasian non-smokers.
Lung Cancer. 76:309–315. 2012. View Article : Google Scholar
|
|
30
|
Paik JH, Choi CM, Kim H, Jang SJ, Choe G,
Kim DK, Kim HJ, Yoon H, Lee CT, Jheon S, et al: Clinicopathologic
implication of ALK rearrangement in surgically resected lung
cancer: A proposal of diagnostic algorithm for ALK-rearranged
adenocarcinoma. Lung Cancer. 76:403–409. 2012. View Article : Google Scholar
|
|
31
|
Shaw AT, Yeap BY, Mino-Kenudson M,
Digumarthy SR, Costa DB, Heist RS, Solomon B, Stubbs H, Admane S,
McDermott U, et al: Clinical features and outcome of patients with
non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol.
27:4247–4253. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa
K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, et al
PROFILE 1014 Investigators: First-line crizotinib versus
chemotherapy in ALK-positive lung cancer. N Engl J Med.
371:2167–2177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Salido M, Pijuan L, Martínez-Avilés L,
Galván AB, Cañadas I, Rovira A, Zanui M, Martínez A, Longarón R,
Sole F, et al: Increased ALK gene copy number and amplification are
frequent in non-small cell lung cancer. J Thorac Oncol. 6:21–27.
2011. View Article : Google Scholar
|
|
34
|
Won JK, Keam B, Koh J, Cho HJ, Jeon YK,
Kim TM, Lee SH, Lee DS, Kim DW and Chung DH: Concomitant ALK
translocation and EGFR mutation in lung cancer: A comparison of
direct sequencing and sensitive assays and the impact on
responsiveness to tyrosine kinase inhibitor. Ann Oncol. 26:348–354.
2015. View Article : Google Scholar
|
|
35
|
Camidge DR, Kono SA, Flacco A, Tan AC,
Doebele RC, Zhou Q, Crino L, Franklin WA and Varella-Garcia M:
Optimizing the detection of lung cancer patients harboring
anaplastic lymphoma kinase (ALK) gene rearrangements potentially
suitable for ALK inhibitor treatment. Clin Cancer Res.
16:5581–5590. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Abe H, Kawahara A, Azuma K, Taira T,
Takase Y, Fukumitsu C, Murata K, Yamaguchi T, Akiba J, Ishii H, et
al: Heterogeneity of anaplastic lymphoma kinase gene rearrangement
in non-small-cell lung carcinomas: A comparative study between
small biopsy and excision samples. J Thorac Oncol. 10:800–805.
2015. View Article : Google Scholar : PubMed/NCBI
|