Introduction
Breast cancer is the most frequently diagnosed
malignancy and the leading cause of cancer death among females
worldwide, accounting for 25% of all cancer cases and 15% of the
total cancer deaths in 2012 (1).
According to the American Cancer Society, about 231,840 new cases
of invasive breast cancer and approximately 60,290 new cases of
carcinoma in situ would occur in the US in 2015 (2). To relieve the huge burden of cancer
therapy and promote women's health, there is an urgent need to
discover new drugs to treat breast cancer.
Natural products, such as thymoquinone, wogonin,
naphtho, and quercetin, have been shown to possess different
anticancer functions, such as the induction of cell cycle arrest,
suppression of tumor angiogenesis, and inhibition of cell migration
or invasion (3). Platycodin D (PD),
a triterpenoid saponin derived from the roots of Platycodin
grandiflorum, has been reported to possess a wide rangeof
health benefits, such as anti-atherosclerotic (4), anti-inflammatory (5,6),
hypocholesterolemic, and anti-obesity effects (7), as well as spermicidal and
contraceptive activity (8). Among
the various health benefits of PD, anticancer effects have been
noted in several cancer cell lines, including lung cancer cells
(9), hepatocellular carcinoma cells
(10), gastric cancer cells
(11), prostate cancer cells
(12), and leukemia cells (13,14).
PD has also been reported to inhibit the viability of human breast
cancer cells in vitro (15–17).
The mouse double minute-2 (mdm2) gene, which
was discovered as an oncogene in 1991 (18), has been reported to be amplified in
more than 40 different types of malignancies. As reported by
Rayburn et al (19), the
protein encoded by mdm2, MDM2, is associated with cancer
development, progression, the resistance to chemotherapy, and can
serve as a prognostic marker (20).
The tumor suppressor p53 acts as a transcription factor regulating
genes involved in DNA repair, cell cycle arrest and apoptosis. The
protein function of wild-type p53 gene may be lost because of
overexpression of MDM2/MDMX. The inhibition of MDM2-p53 interaction
presents an appealing therapeutic strategy for the treatment of
cancer (21). As is well known, the
p53 gene is mutated in ~50% malignant tumors (22). There are distinct functional
differences between mutant and wild-type p53. Mutant p53 loses its
tumor suppressive activity and may actually function as an oncogene
(23), which is widely expressed on
the MDA-MB-231 human breast cancer cell line (23). The highly metastatic MDA-MB-231
cells are special cells in that they are triple-negative breast
cancer cells lacking the expression of the estrogen receptor,
progesterone receptor, and human epidermal growth factor receptor
2. These cells are insensitive to many types of chemotherapy and
radiation, mimicking the clinical picture, where there are fewer or
no effective treatment options compared to non-triple-negative
disease. Since PD has been shown to exert anticancer effects in
many types of malignant cells, we wondered whether PD exerts its
functions in inhibiting tumor growth by inhibiting MDM2 and mutant
p53 in MDA-MB-231 cells.
In the present study, we first evaluated the effects
of PD on the viability, proliferation, and cell cycle of MDA-MB-231
human breast cancer cells. MDA-MB-231 xenograft tumors were
subsequently established to assess the functions of PD in
vivo. We also detected the expression of G0/G1 phase-related
proteins, as well as MDM2, MDMX, mutant p53, p21, and p27 in
vitro and in vivo to aid in our understanding of the
mechanism by which PD exerts these effects. We believe that the
present data support further investigations into the application of
PD as a safe and effective natural compound for the treatment of
triple-negative breast cancer.
Materials and methods
Chemicals and reagents
Compound PD (purity: >98%) was purchased from
Chengdu MustBio-Technology Co., Ltd. (Chengdu, China). The
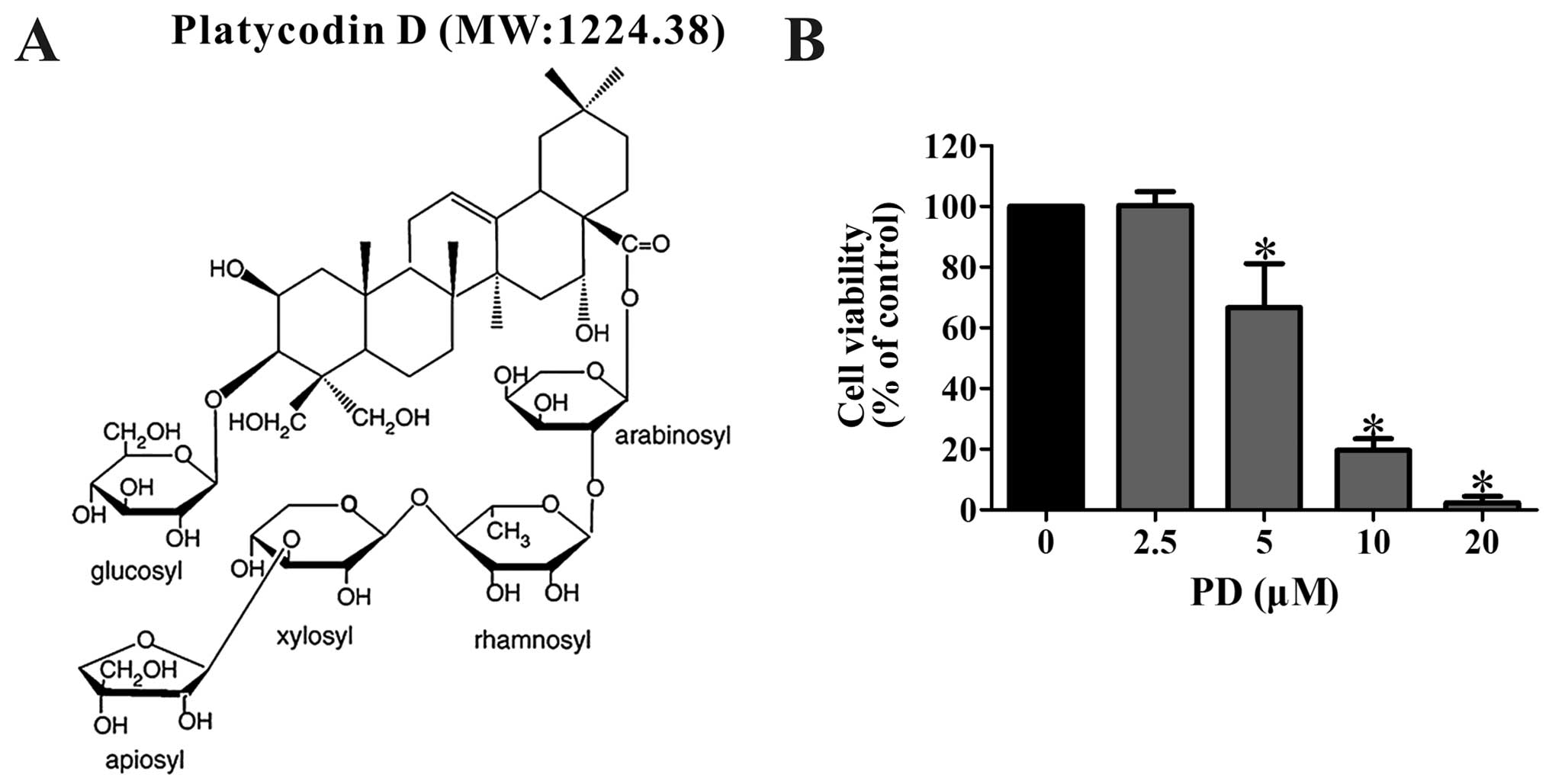
structure and molecular weight of PD were shown in Fig. 1A. RPMI-1640, fetal bovine serum
(FBS), trypsin, and phosphate-buffered saline (PBS) were obtained
from Hyclone (Carlsbad, CA, USA). Dimethyl sulfoxide (DMSO) and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra-zolium bromide (MTT)
were procured from Sigma-Aldrich (St. Louis, MO, USA). The BrdU
Cell Proliferation Assay kit was purchased from Cell Signaling
Technology Inc. (Danvers, MA, USA). The antibodies against human
CDK2, CDK4, CDK6, and Cyclin E used in the western blot analyses
were obtained from Boster Biotechnology (Wuhan, China). The
anti-Ki67 antibody was from Abcam (Burlingame, CA, USA). The mouse
anti-Flag antibody (F-3165) was from Sigma-Aldrich. The anti-human
MDM2 antibody was from Calbiochem (Billerica, MA, USA), while the
anti-p27 antibody was procured from Cell Signaling Technology, Inc.
The MDMX, p21 and p53 antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). The GAPDH antibody was
obtained from Good Here Biotechnology Co., Ltd. (Hangzhou, China)
and the secondary antibodies were all purchased from ZSGB-BIO
(Beijing, China).
The secondary antibody for immunocytochemistry was
obtained from Amyjet Scientific Inc. (Wuhan, China). The MDM2 siRNA
and negative control siRNA were obtained from Guangzhou RiboBio
Co., Ltd. (Guangzhou, China). The liposome transfection reagent,
Lipofectamine 2000, was purchased from Invitrogen Corporation
(Waltham, MA, USA).
Cell lines and cell culture
The highly metastatic human breast cancer cell line,
MDA-MB-231, was obtained from the Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China), and cells were
cultured in RPMI-1640 containing 10% FBS. The cells were maintained
in a humidified incubator at 37°C containing 5% CO2 and
were passaged at a 1:2 dilution upon confluence every two to three
days.
Cell viability assay
The MTT assay was used to assess the impact of PD
treatment on the viability of MDA-MB-231 cells. To determine
whether PD had a concentration-dependent effect on the cell
viability, we designed a concentration gradient from 0 to 20
µM of PD. The cells were seeded into 96-well plates at a
density of 1×104 cells per well. After being cultured in
an incubator overnight, the cells were treated with various
concentrations of PD (0, 2.5, 5, 10 or 20 µM) for 48 h then
5 mg/ml of MTT solution was added into each well. After the cells
were incubated with the MTT solution for 4 h, DMSO was used to
dissolve the formazan crystals, and the absorbance was read at 570
nm using a Bio-Tek Synergy HT multifunction plate reader (BioTek,
Winooski, VT, USA). Three independent experiments were conducted to
determine the IC50 value of PD.
Immunocytochemistry
MDA-MB-231 cells were exposed to 1/1,000 DMSO or 20
µM PD for 48 h to observe the expression of the
proliferation marker, Ki67. After being exposed to DMSO or PD, the
cells were fixed with 4% paraformaldehyde for 30 min, incubated
sequentially with 0.3% Triton X-100 for 1 h, goat serum for 30 min,
and an anti-Ki67 (dilution ratio 1:1,000) antibody overnight at
4°C. After that, the sections were rinsed with PBS, incubated with
a Cy3-conjugated AffiniPure Donkey Anti-Rabbit IgG (H+L) secondary
antibody, and stained with DAPI solution. Finally, the cells were
observed and photographed under a fluorescence microscope.
Cell proliferation assay
To evaluate the effects of PD on the proliferation
of MDA-MB-231 cells, we performed the BrdU Cell Proliferation
Assay. Cells (1×104 cells per well) were seeded into
96-well plates and treated with PD at concentrations of 0, 2.5, 5,
10, or 20 µM for 48 h. The cells were then exposed to BrdU
label for another 10 h, fixed for 30 min, and incubated with an
anti-BrdU antibody for 1 h at room temperature, then treated
according to the manufacturer's instructions. Finally, the
absorbance was read at dual wavelengths of 450 and 540 nm by a
Bio-Tek Synergy HT multifunction plate reader (BioTek).
Cell cycle analysis
To assess the effects of PD on the cell cycle of
MDA-MB-231 cells, we performed a cell cycle analysis using flow
cytometry. The cells were seeded into 50 ml culture bottles at a
density of 1–2×106 cells per bottle and were treated
with different concentrations of PD (0, 2.5, 5, 10, 20 µM).
After being incubated with the various concentrations of PD for 48
h, we collected the cells by centrifugation, washed them twice with
cold PBS, and then fixed them with 75% ethanol overnight at 4°C.
The next day, the cells were resuspended with 100 µg/ml
RNAase and 50 µg/ml PI staining solution. The DNA contents
were detected by a FACSCaliber flow cytometer (BD Biosciences, San
Jose, CA, USA).
Western blot analysis
MDA-MB-231 cells were exposed to various
concentrations of PD (0, 5, 10 and 20 µM) for 24 h. The
cells were then lysed with RIPA buffer containing 1% PMSF. Equal
amounts of protein were subjected to 8-12% SDS-PAGE. After being
electrophoresed for 2 h, the separated proteins were transferred to
PVDF membranes which were blocked in 5% skim milk for 4 h at 37°C,
and then were incubated with specific antibodies overnight at 4°C
with gentle shaking. After being washed with TBST solution for 40
min, the membranes were incubated with goat anti-mouse/rabbit
horseradish peroxidase-conjugated secondary antibodies for 2 h at
37°C, followed by exposure of the membranes to film in a
darkroom.
Transfection experiments using an MDM2
plasmid
The pcDNA3-Flag-MDM2 plasmid was provided by Dr
Ruiwen Zhang, Texas Tech University Health Sciences Center
(Amarillo, TX, USA). MDA-MB-231 cells were trans-fected with 3
µg pcDNA3-Flag-MDM2 plasmid for 5–7 h and were then treated
with PD (20 µM) for 24 h. The procedure was carried out as
previously described (12). Western
blot analysis was performed to assess the expression of various
proteins.
Transient transfection of MDM2 siRNA
MDA-MB-231 cells were transfected with MDM2 siRNA at
a final concentration of 100 nM or with 50 nM of a negative control
siRNA for 5–7 h. Then, 20 µM of PD was added to the
MDM2-silenced cells for 24 h. Finally, a western blot analysis was
performed to detect the expression of MDM2-related proteins. To
verify whether PD inhibited highly metastatic MDA-MB-231 breast
cancer growth by targeting the MDM2 oncogene, we performed the MTT
and BrdU assays on cells transfected with MDM2 siRNA or an MDM2
expression plasmid.
Tumor xenograft study
Female athymic BALB/C nude (nu/nu) mice (4–6 weeks
old) were procured from the Medical Experimental Animal Center of
the Third Military Medical University. The animal care and
experimental procedures were performed with the approval of the
Animal Ethics Committee of the Third Military Medical University.
MDA-MB-231 cells were collected and resuspended in serum-free
RPMI-1640 medium and were mixed with Matrigel (BD Biosciences,
Bedford, MA, USA) at a 3:1 ratio. Each mouse was subcutaneously
injected with 5×106 cells per mouse in the right
subaxillary area to establish a MDA-MB-231 breast cancer xenograft
model. Six days after the injection of the cells, the animals
bearing human breast cancer xenograft tumors were randomly divided
into a control group, 1 mg/kg PD group and 2.5 mg/kg PD group. PD
was dissolved in PEG400:Saline:Ethanol (400:300:200, v/v/v) and was
administered via i.p. injection. All mice were treated five days
per week for four weeks. The body weight and tumor size of each
mouse was monitored every three days by an electronic scale and
Vernier calipers by two different observers. We measured two
perpendicular diameters of the xenograft tumors: the longer
dimension was recorded as 'a' and the shorter dimension was
recorded as 'b'. We converted the two measured values into the
tumor weight using the formula: (a×b2)/2. The mice were
sacrificed after being treated for four weeks. The xenograft tumors
were carefully removed, weighed, photographed, and frozen at −80°C
until used for western blot analysis.
Statistical analysis
Graphic illustrations of the results were obtained
using the Graphpad Prism 5.0 software program (GraphPad Software,
San Diego, CA, USA). SPSS 17.0 was used to perform the statistical
analysis. Measured data were expressed as the means ± standard
deviations (SD) and the statistical significance of differences
between groups was assessed using a one-way ANOVA when the data
were normally distributed and had homogeneous variance. A value of
p<0.05 was considered to be statistically significant.
Results
PD inhibits the growth of MDA-MB-231
breast cancer cells in vitro
We performed three independent MTT assays to assess
the viability of MDA-MB-231 cells following PD treatment. The cells
were treated with various concentrations of PD (0, 2.5, 5, 10, and
20 µM) for 48 h then the survival percentages of the cells
were measured. As shown in Fig. 1B,
these results indicated that the IC50 value for PD was
7.77±1.86 µM and PD significantly decreased the viability of
the MDA-MB-231 cells in a concentration-dependent manner.
PD decreases the proliferation of the
MDA-MB-231 breast cancer cells in vitro
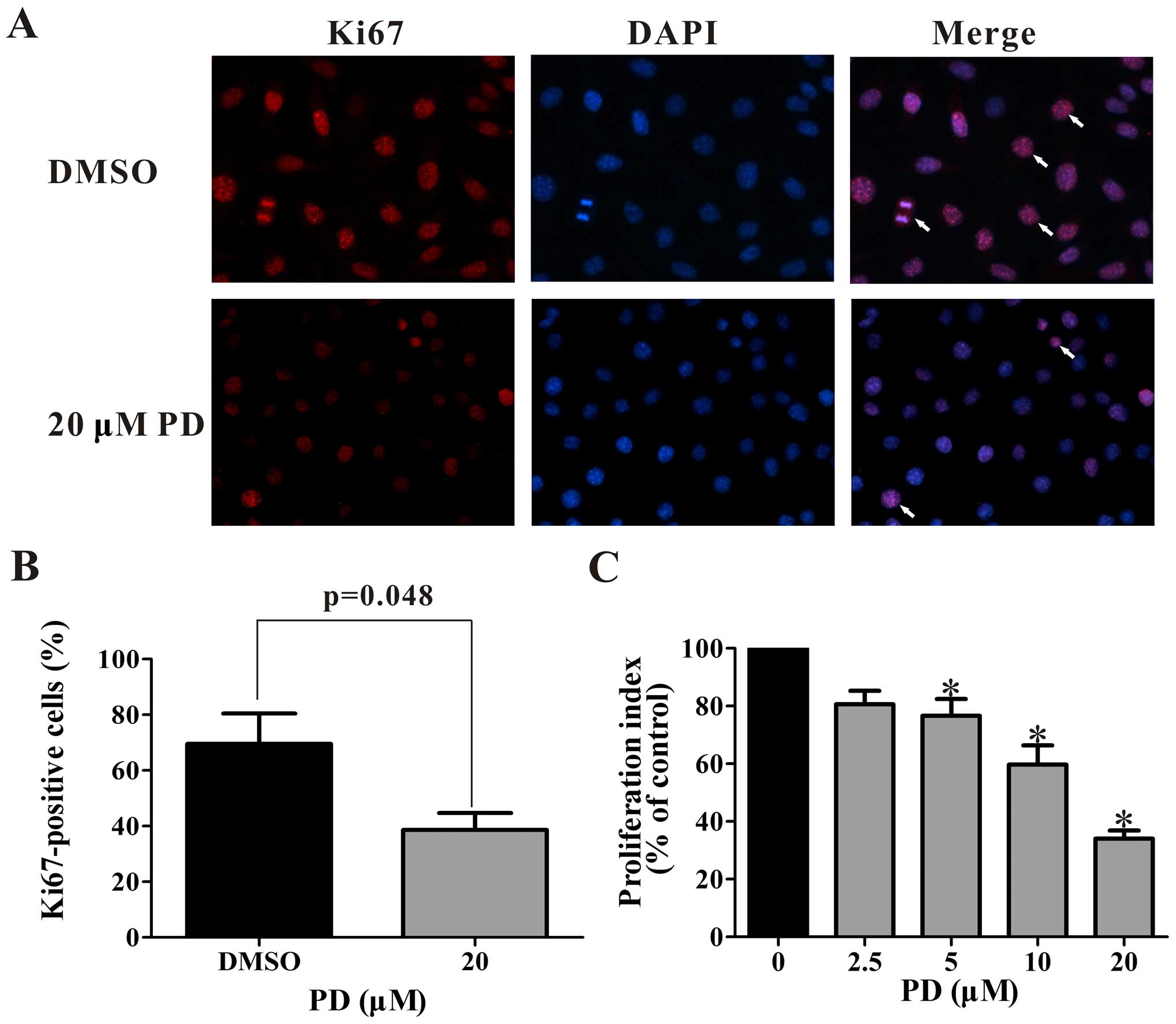
Cell proliferation was detected by
immunocytochemistry and BrdU cell proliferation assay. The
expression of the proliferation marker Ki67, is shown in Fig. 2A and B with immunocytochemistry
method. The proportion of proliferating cells was reduced from
69.5% to 38.6% by treatment with 20 µM PD (Fig. 2B). The BrdU cell proliferation assay
indicated that PD inhibits the proliferation of MDA-MB-231 cells in
a concentration-dependent manner (Fig.
2C). All concentrations of PD over 5 µM significantly
decreased the cell proliferation. In the cells treated with the 20
µM concentration of PD, the proliferation index decreased to
34±0.05% of that in the control group.
PD induces cell cycle arrest in the G0/G1
phase in MDA-MB-231 cells
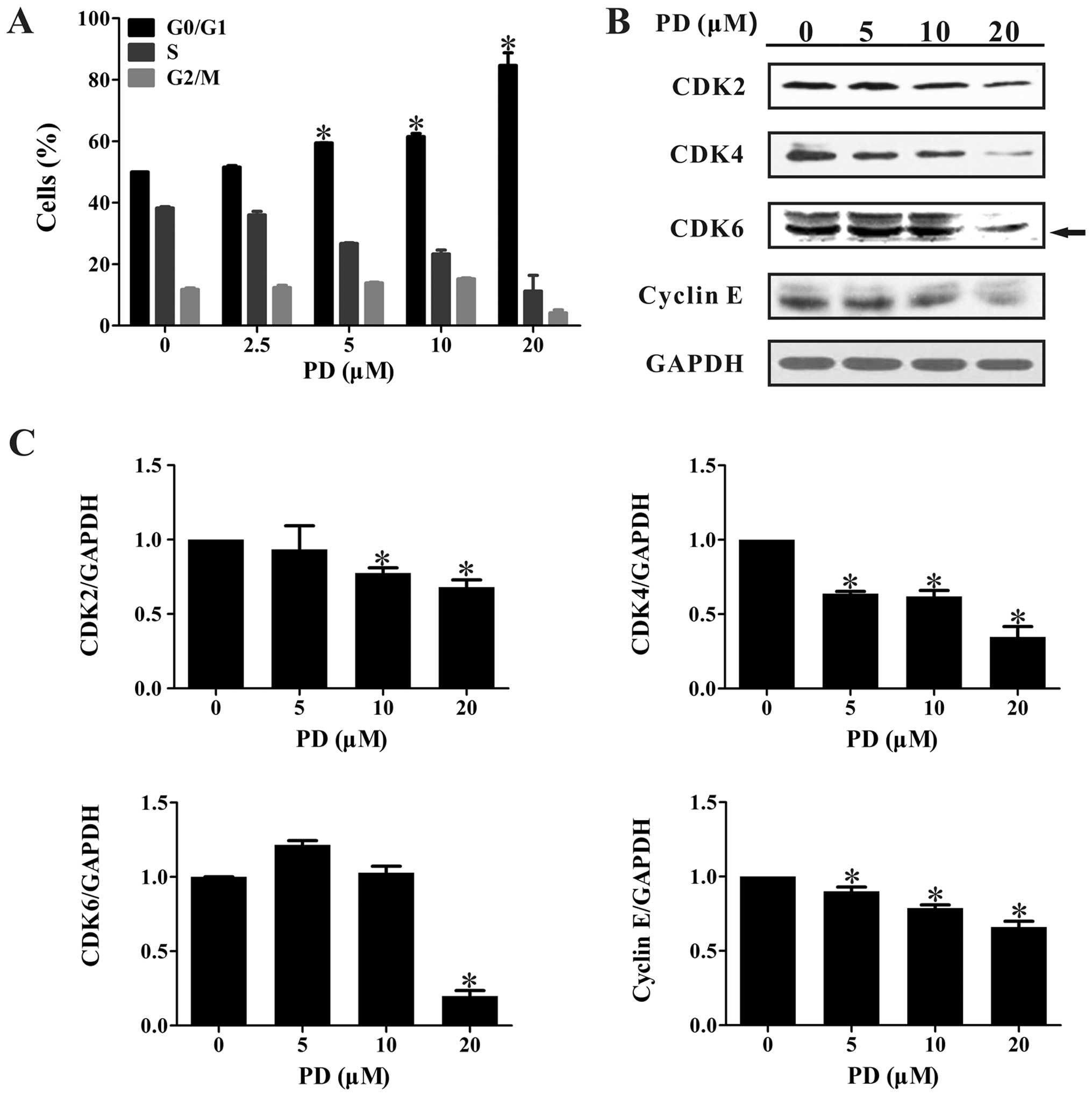
After treatment with various concentrations of PD
for 48 h, the DNA contents of the cells were detected by flow
cytometry. As shown in Fig. 3A, the
majority of the MDA-MB-231 cells were blocked at the G0/G1 phase
following PD treatment, and this occurred in a
concentration-dependent manner. Treatment with 20 µM PD
significantly arrested the cell cycle progression, with 84.67±4.13%
of cells found in the G0/G1 phases after a 48 h treatment.
We also performed western blot assays to detect the
expression of G0/G1 phase-related proteins. As illustrated in
Fig. 3B and C, PD significantly
decreased the expression levels of CDK2, CDK4, CDK6, and Cyclin
E.
PD changes the expression levels of MDM2,
MDMX, mutant p53, p21 and p27
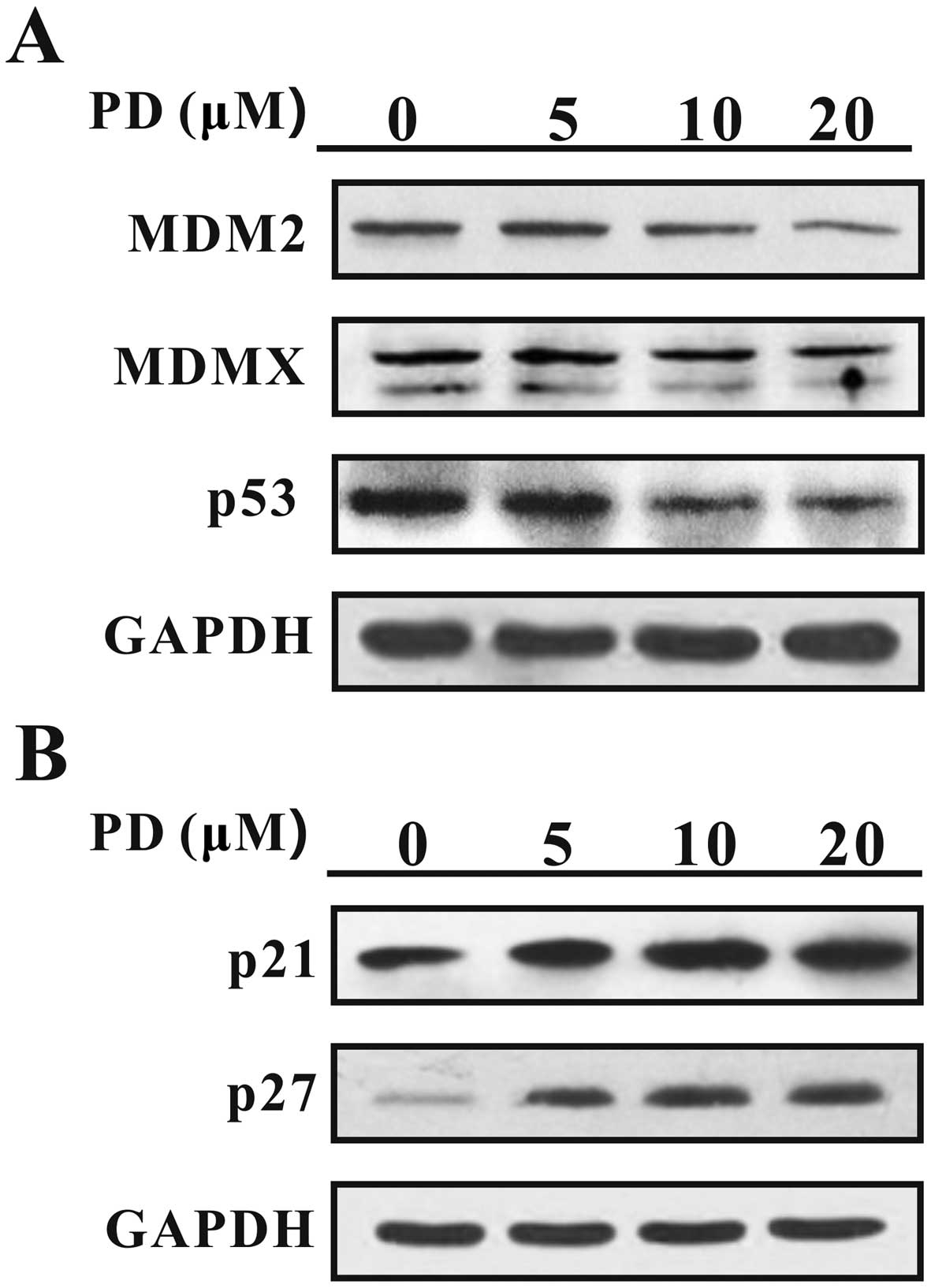
After the cells were treated with PD for 24 h, the
expression levels of MDM2, MDMX, mutant p53, p21 and p27 were
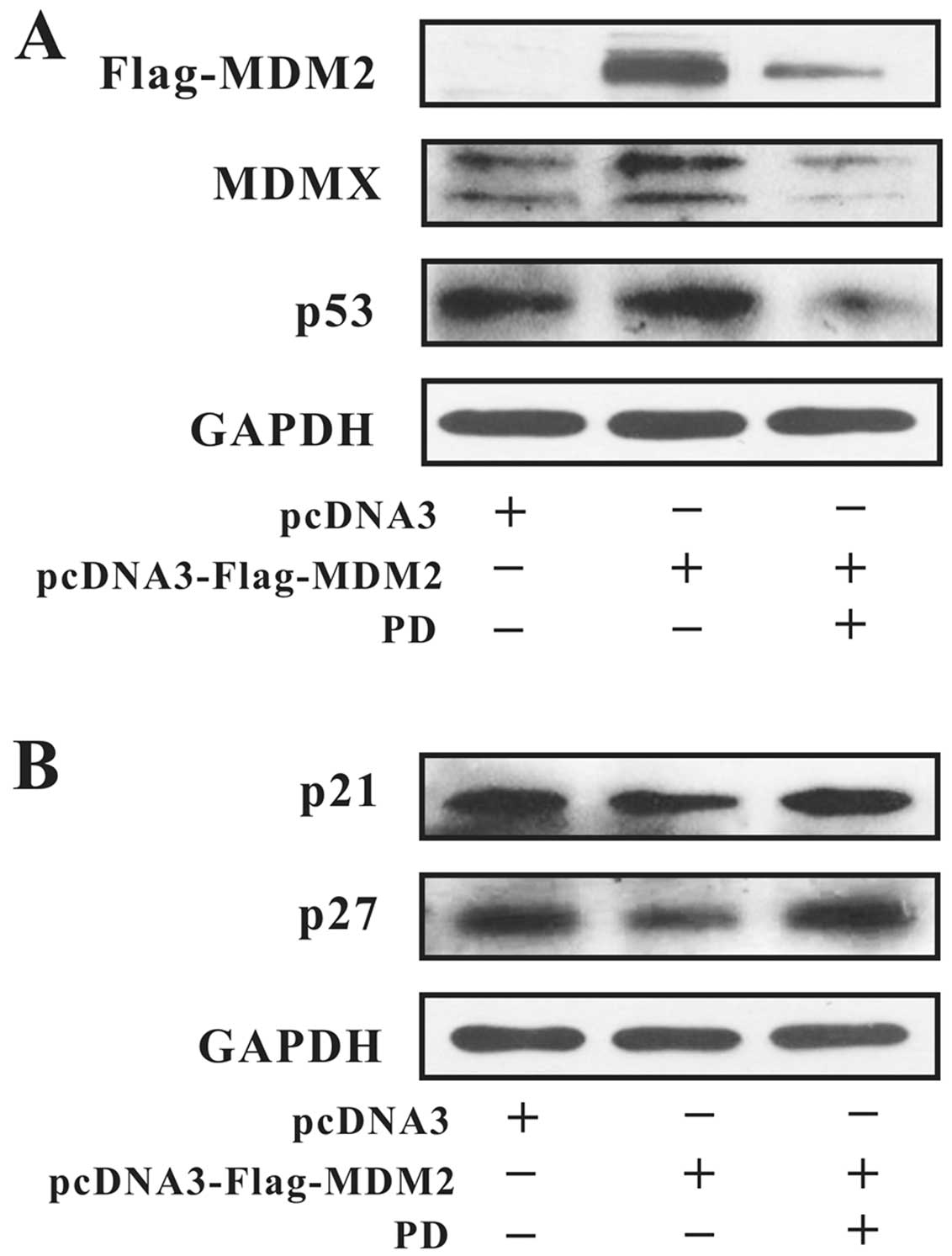
analyzed by western blotting. As shown in Fig. 4A, PD downregulated the expression of
MDM2, MDMX and mutant p53. In contrast, PD upregulated the
expression levels of p21 and p27 (Fig.
4B), which are proteins downstream of MDM2 and MDMX, which
function as cancer suppressors.
To further understand the expression of MDM2-related
proteins, we performed a transfection experiment. A
pcDNA3-Flag-MDM2 plasmid or MDM2 siRNA was used to control the MDM2
expression. As shown in Fig. 5A,
the expression of MDM2 was downregulated after treatment with 20
µM of PD for 24 h. The upregulation of MDMX and mutant p53
were also inhibited by treatment with PD. The changes in p21 and
p27 expression were the opposite of those of MDM2 (Fig. 5B).
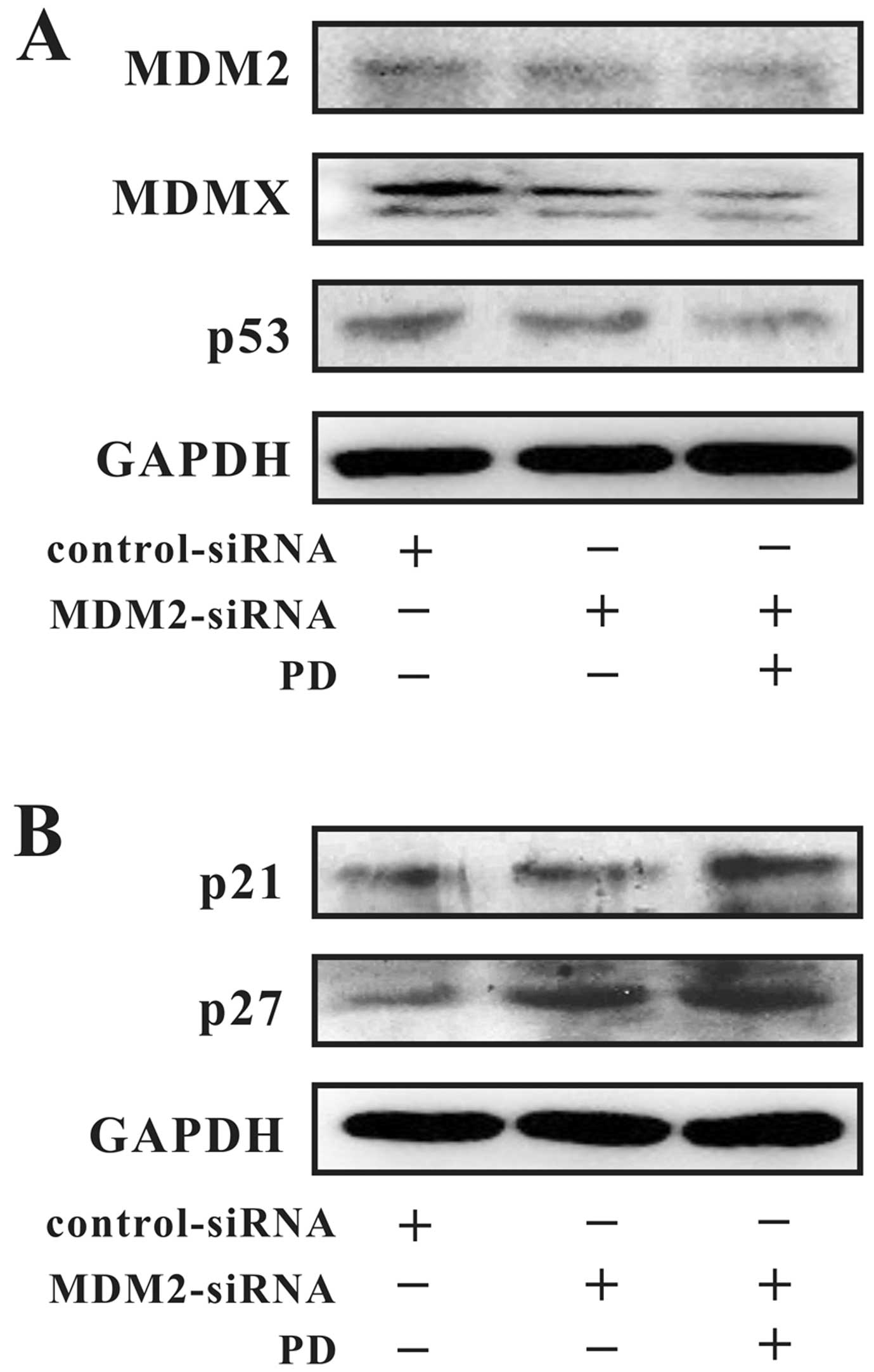
As illustrated in Fig.
6, MDM2 was silenced by MDM2 siRNA and further downregulated by
PD. The expression levels of MDMX and mutant p53 were decreased
along with the MDM2 expression level, but the p21 and p27 proteins
were upregulated.
PD inhibits MDA-MB-231 cell growth by
targeting the MDM2 oncogene
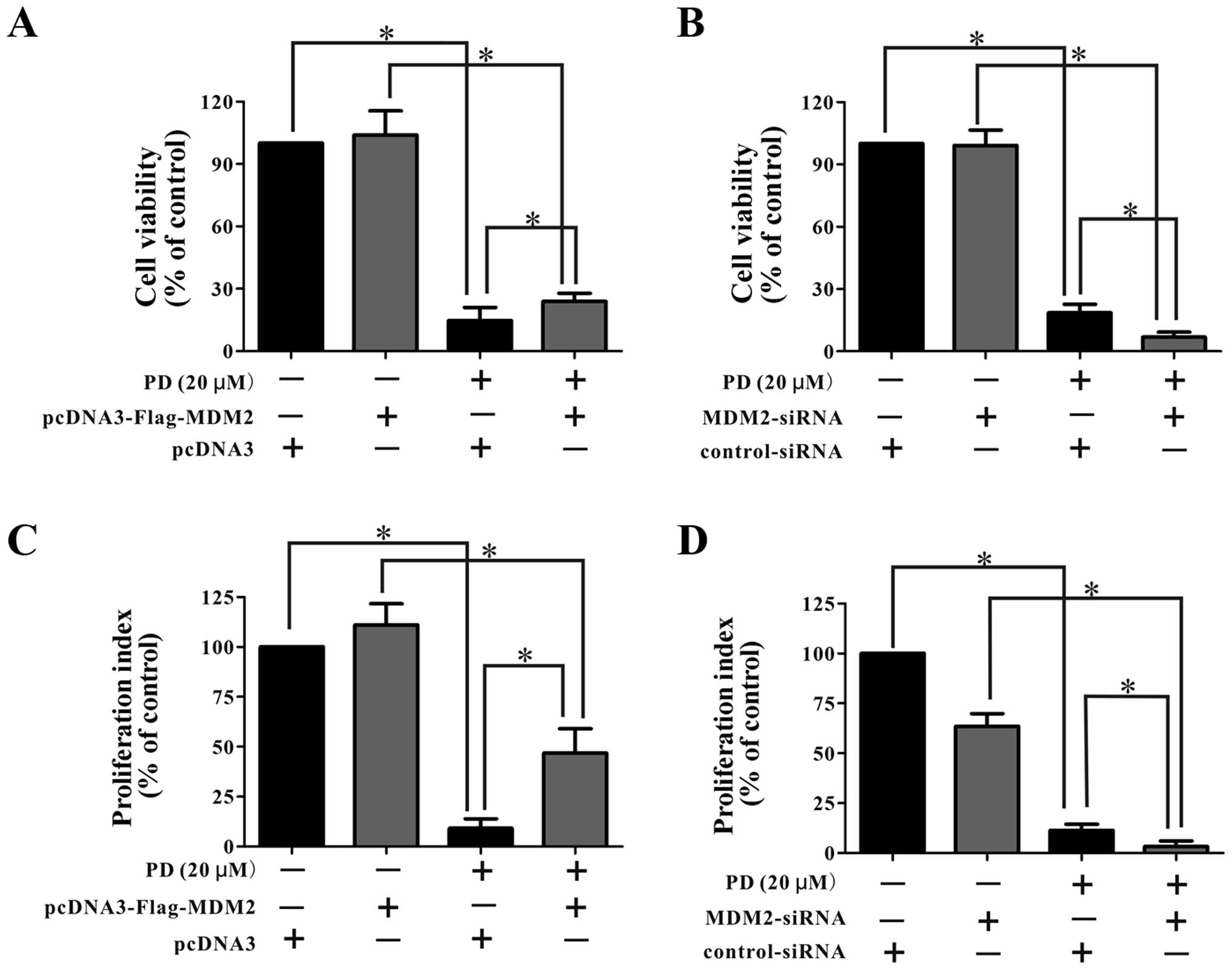
As shown in Fig. 7A and
B, the cells transfected with the pcDNA3-Flag-MDM2 plasmid
showed a similar sensitivity to PD as the parental cells. In
contrast, the cells transfected with siRNA against MDM2 showed
greater inhibition of the cell viability following treatment with
PD than did the parental cells. Similarly, as shown in Fig. 7C and D, the cells transfected with
the pcDNA3-Flag-MDM2 plasmid were less sensitive to treatment with
PD than were the parental cells, while the cells transfected with
siRNA-MDM2 showed a greater decrease in proliferation following
treatment with PD than did the parental cells.
Together, these findings indicate that PD affected
the MDM2, and the effects are related to its anti-proliferative and
anti-growth effects on the cells.
PD inhibits the growth of MDA-MB-231
xenograft tumors
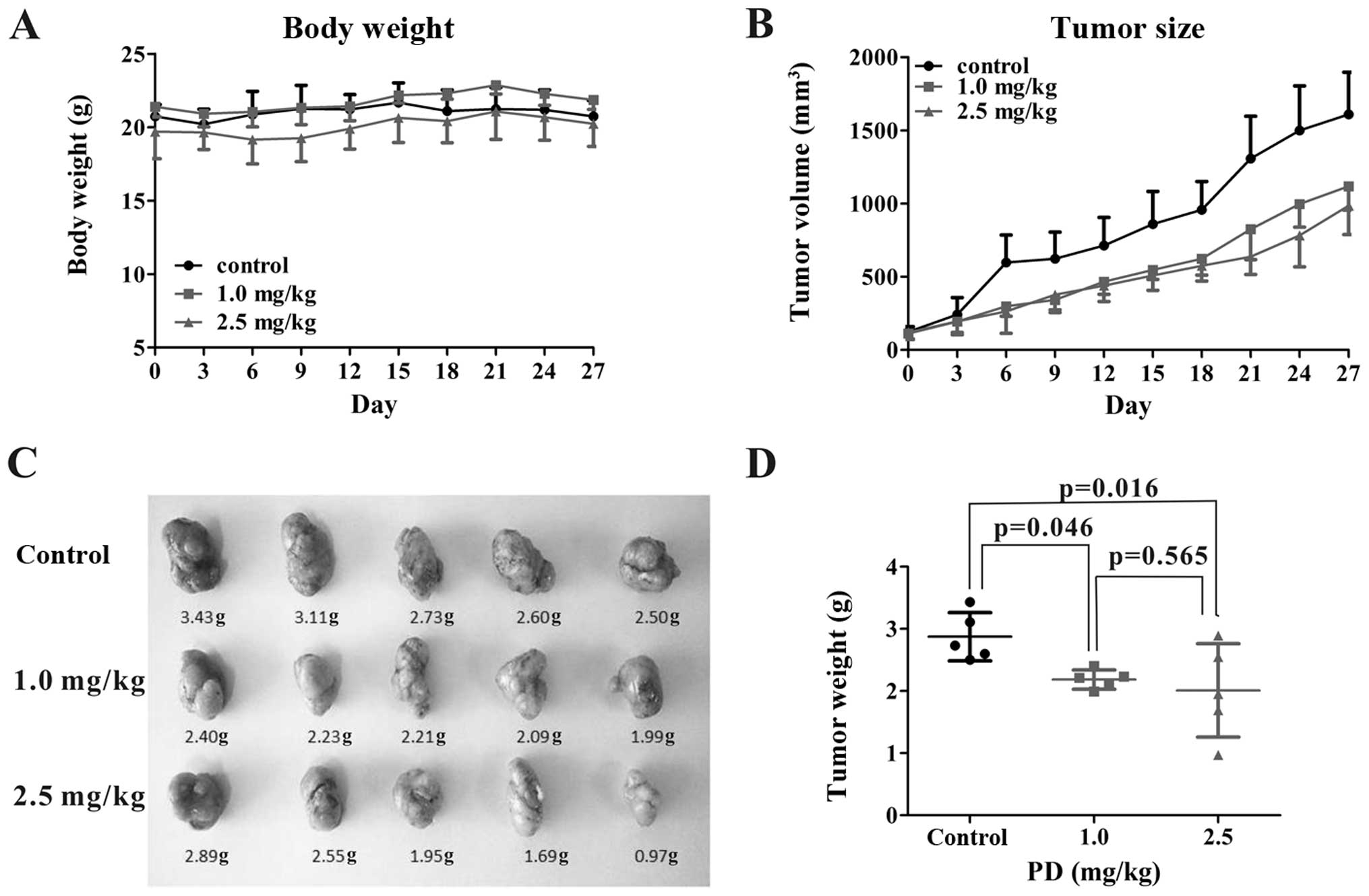
All xenograft tumor-bearing mice were randomly
divided into a control group, 1 mg/kg PD group and 2.5 mg/kg PD
group so that all three groups had a similar xenograft tumor size.
Each mouse was treated five days a week for four weeks. The body
weight and tumor size were analyzed every three days to estimate
the effects of different doses of PD (Fig. 8A and B). As shown in Fig. 8C and D, there were significant
differences in the tumor weight among the three treatment groups.
PD (1 mg/kg and 2.5 mg/kg) inhibited the tumor growth by 24% and
30% compared to the control on day 27 (p<0.05). However, there
were no significant differences in the body weight change among the
three treatment groups (Fig. 8A).
These results indicated that PD administration significantly
inhibited the growth of xenograft tumors even at a dose of 1 mg/kg
body weight. PD treatment did not lead to any significant change in
the body weights of the mice up to 2.5 mg/kg, suggesting that PD
does not exert any major toxicity at this dose.
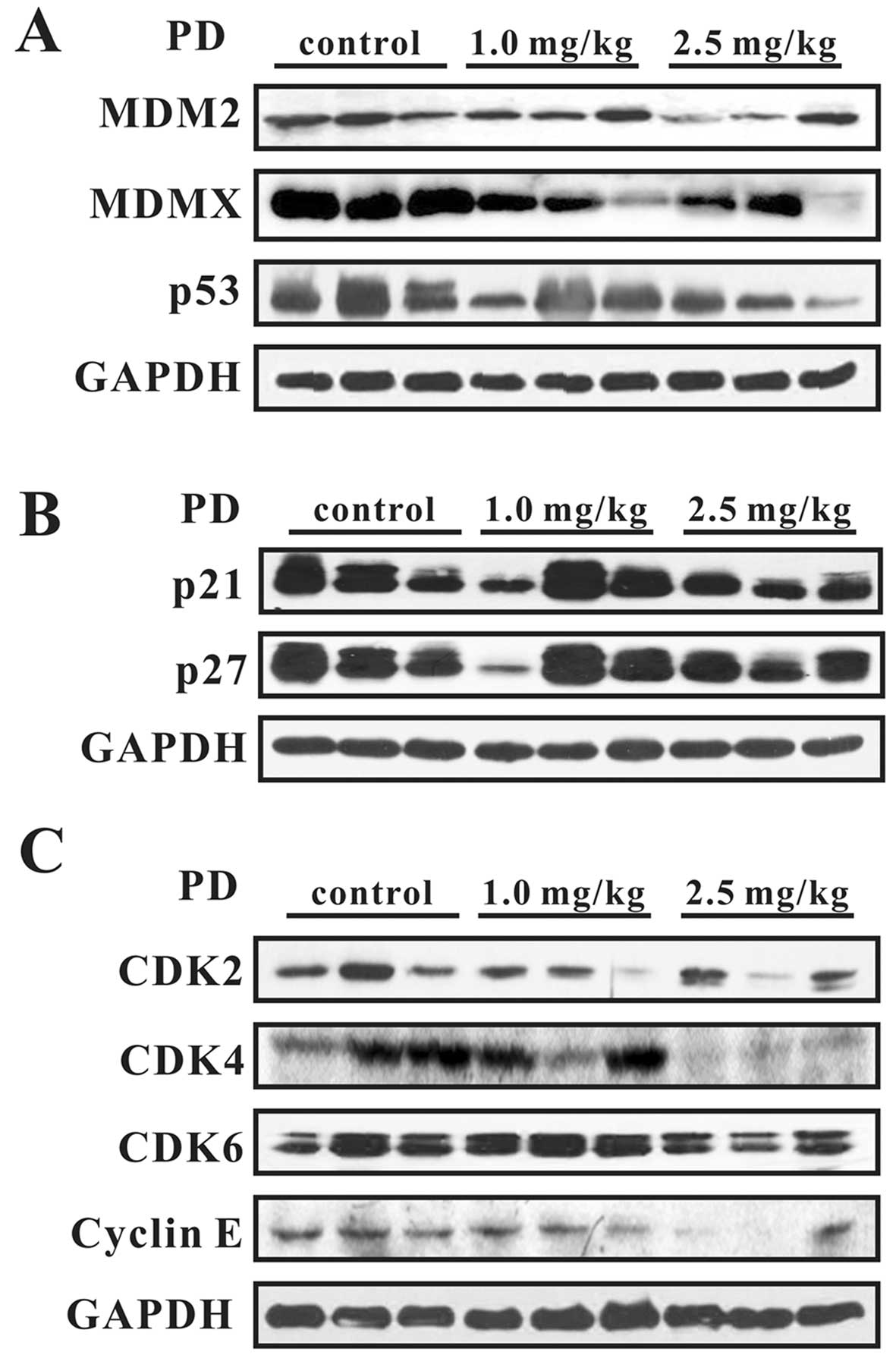
We further examined the expression levels of various
proteins in vivo. As shown in Fig. 9, PD decreased the expression of
MDM2, MDMX, and mutant p53 and increased the expression of p21 and
p27. The expression of G0/G1 phase-related proteins, including
CDK2, CDK4, CDK6, and Cyclin E, was downregulated. These results
were consistent with the in vitro findings.
Discussion
Natural products have been receiving increasing
attention because of their diversity of anticancer effects and
relative safety. As the most abundant triterpenoid saponin derived
from the roots of Platycodin grandiflorum, Platycodin D
(PD), has been reported to show a wide range of health benefits,
such as anti-atherosclerotic (4),
anti-inflammatory (5,6), hypocholesterolemic and anti-obesity
effects (7). Previous studies have
shown that PD has obvious anticancer activity against different
human malignant cancer cells both in vitro (10,11,13,14,16,17)
and in vivo (9,12,15).
Breast cancer is a major public health issue for
women worldwide. A previous study reported that PD inhibited the
growth, migration and invasion of MDA-MB-231 cells by suppressing
the EGFR-mediated Akt and MAPK pathways (15). Furthermore, PD blocked breast
cancer-induced bone loss by suppressing osteoclastogenesis
(24). It was also found to enhance
the anti-proliferative effects of doxorubicin in MCF-7 and
MDA-MB-231 breast cancer cells (25). The combination of PD and osthole
inhibited the proliferation and invasion of MDA-MB-231 and 4T1
human breast cancer cells (17). We
performed the present studies to better understand the potential
mechanisms by which PD decreases the viability of MDA-MB-231
cells.
Our present findings showed that PD decreased the
viability of MDA-MB-231 cells and inhibited their proliferation in
a concentration-dependent manner. The IC50 value of PD
for inhibiting cell growth was less than 10 µM, and
treatment of cells with 20 µM PD decreased the proliferation
index to 34±0.05% of the rate observed in the control group. These
anti-proliferative effects were consistent with those reported in
previous studies (10,12,15,26).
Furthermore, we detected the expression of Ki67 in MDA-MB-231
cells, which is a classic marker of cell proliferation. We observed
that Ki67 expression was significantly reduced by PD, which was
also consistent with the previous finding that PD could inhibit the
expression of Ki67 in MDA-MB-231 xenograft tumors (15).
The results of the cell cycle analysis indicated
that PD induced concentration-dependent cell cycle arrest in the
G0/G1 phase. It is interesting that PD may have different effects
on the cell cycle in different cell lines. As we reported
previously, PD induced cell cycle arrest in the G0/G1 phase in the
DU145 and LnCaP prostate cancer cell lines, but induced arrest in
the G2/M phase in the PC3 prostate cancer cell line (12).
We further explored the antitumor effects and
potential mechanism of action of PD in MDA-MB-231 cells in
vitro and in vivo. MDA-MB-231 cells are triple-negative
(ER/PR/HER2-neg), providing a model of a more difficult-to-treat
type of breast cancer. A previous in vitro study on PD in
MDA-MB-231 cells reported that PD inhibited the growth of cells via
suppression of the EGFR-mediated Akt and MAPK pathways (15). In our study, we focused on the
effects of PD on the expression of mutant p53 and MDM2/MDMX in
MDA-MB-231 cells. The tumor suppressor p53, a classic transcription
factor, plays an important role in regulating many key cellular
processes. When DNA is damaged, p53 regulates a large number of
target genes related to cell cycle arrest, apoptosis, senescence,
autophagy and metabolism (27,28).
However, p53 is mutated in the majority of human cancers, resulting
in a prevention or reversal of its functions (29–31).
It has been reported that mutated p53 contributes to various steps
of tumorigenesis (32). It has been
reported that 60–88% of advanced breast cancers harbor mutant p53.
In a previous study, Kim et al found that the levels of
mutant p53 could be decreased in a concentration- and
time-dependent manner after treatment with a ginsenoside, Rg3, in
MDA-MB-231 cells (33). Our present
data showed that PD inhibited MDA-MB-231 cell viability by
targeting mutant p53, which suggests that PD represents a safe and
effective natural compound that can be used for the treatment of
advanced breast cancer.
MDM2 is a well-known oncogene. The p53-MDM2-MDMX
loop has been reported as a target for cancer therapy (34). MDM2 and MDMX are important negative
regulators of p53 that have been shown to function collaboratively
(35). In cancer patients with
tumors harboring mutant p53 or without p53 expression, including
breast cancer patients, MDM2 overexpression is still found to be
involved in cancer growth and metastasis (36). The MDA-MB-231 cells have mutant p53
(37,38), which works as a driving oncogene
(39). Therefore, we detected the
expression levels of the MDM2, MDMX and mutant p53 proteins in the
present study. The results of the western blot analyses indicated
that PD can downregulate MDM2, MDMX and mutant p53 and upregulate
the expression of p21 and p27. The results of the MTT and BrdU
assays in the MDM2 siRNA- and MDM2 plasmid-transfected cells
indicated that PD inhibited MDA-MB-231 cell growth by targeting the
MDM2 oncogene. Based on these findings, we believe that the effects
of PD are at least partially p53-independent.
We further explored the antitumor activity of PD
using the MDA-MB-231 xenograft tumor model. We found that PD
decreased the growth of the xenograft tumors even at the 1 mg/kg
dose, which was much lower than the dose used in a previous study
of the effects of PD on MDA-MB-231 xenograft tumors (15). We also checked the protein
expression of mutant p53, MDM2, MDMX, p21 and p27 in the xenograft
tumor tissues, and found that all of the differences in expression
between control and treated animals corresponded with those noted
in the in vitro study.
In conclusion, our present study results indicate
that PD can inhibit the growth of MDA-MB-231 human breast cancer
cells and tumors, and these effects may be at least partially
mediated via its targeting of the MDM2 oncogene. PD decreased the
expression levels of MDM2, MDMX, and mutant p53, and upregulated
the expression of proteins downstream of MDM2 (p21 and p27) to
induce cell cycle arrest, inhibit cell proliferation, and promote
cell death.
It is a limitation to draw our conclusion based on
the effects on only one cell line (MDA-MB-231 cells). Thefore,
detailed mechanisms by which PD inhibits the growth of human
triple-negative breast cancer cells will need to be elucidated or
confirmed in future studies. However, our findings and those of
previous studies suggest that PD may represent a potential
anticancer agent for use against advanced breast cancer.
Acknowledgments
This work was supported by an NSFC grant from the
National Natural Science Foundation of China (no. 81171991) to
Hong-Xia Xu.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ward EM, DeSantis CE, Lin CC, Kramer JL,
Jemal A, Kohler B, Brawley OW and Gansler T: Cancer statistics:
Breast cancer in situ. CA Cancer J Clin. 65:481–495. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Safdari Y, Khalili M, Ebrahimzadeh MA,
Yazdani Y and Farajnia S: Natural inhibitors of PI3K/AKT signaling
in breast cancer: Emphasis on newly-discovered molecular mechanisms
of action. Pharmacol Res. 93:1–10. 2015. View Article : Google Scholar
|
|
4
|
Wu J, Yang G, Zhu W, Wen W, Zhang F, Yuan
J and An L: Anti-atherosclerotic activity of platycodin D derived
from roots of Platycodon grandiflorum in human endothelial cells.
Biol Pharm Bull. 35:1216–1221. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chung JW, Noh EJ, Zhao HL, Sim JS, Ha YW,
Shin EM, Lee EB, Cheong CS and Kim YS: Anti-inflammatory activity
of prosapogenin methyl ester of platycodin D via nuclear
factor-kappaB pathway inhibition. Biol Pharm Bull. 31:2114–2120.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang T, Yang S, Du J, Jinfu Y and Shumin
W: Platycodin D attenuates airway inflammation in a mouse model of
allergic asthma by regulation NF-κB pathway. Inflammation.
38:1221–1228. 2015. View Article : Google Scholar
|
|
7
|
Zhao HL, Harding SV, Marinangeli CP, Kim
YS and Jones PJ: Hypocholesterolemic and anti-obesity effects of
saponins from Platycodon grandiflorum in hamsters fed atherogenic
diets. J Food Sci. 73:H195–H200. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu Z, Wang L, Zhou R, Qiu Y, Yang L, Zhang
C, Cai M, Mi M and Xu H: Evaluation of the spermicidal and
contraceptive activity of Platycodin D, a Saponin from Platycodon
grandiflorum. PLoS One. 8:e820682013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park JC, Lee YJ, Choi HY, Shin YK, Kim JD
and Ku SK: In vivo and in vitro antitumor effects of platycodin d,
a saponin purified from platycodi radix on the h520 lung cancer
cell. Evid Based Complement Alternat Med. 2014:4786532014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li T, Xu WS, Wu GS, Chen XP, Wang YT and
Lu JJ: Platycodin D induces apoptosis, and inhibits adhesion,
migration and invasion in HepG2 hepatocellular carcinoma cells.
Asian Pac J Cancer Prev. 15:1745–1749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chun J, Joo EJ, Kang M and Kim YS:
Platycodin D induces anoikis and caspase-mediated apoptosis via p38
MAPK in AGS human gastric cancer cells. J Cell Biochem.
114:456–470. 2013. View Article : Google Scholar
|
|
12
|
Zhou R, Lu Z, Liu K, Guo J, Liu J, Zhou Y,
Yang J, Mi M and Xu H: Platycodin D induces tumor growth arrest by
activating FOXO3a expression in prostate cancer in vitro and in
vivo. Curr Cancer Drug Targets. 14:860–871. 2015. View Article : Google Scholar
|
|
13
|
Kim MO, Moon DO, Choi YH, Shin DY, Kang
HS, Choi BT, Lee JD, Li W and Kim GY: Platycodin D induces
apoptosis and decreases telomerase activity in human leukemia
cells. Cancer Lett. 261:98–107. 2008. View Article : Google Scholar
|
|
14
|
Kim MO, Moon DO, Choi YH, Lee JD, Kim ND
and Kim GY: Platycodin D induces mitotic arrest in vitro, leading
to endoredu-plication, inhibition of proliferation and apoptosis in
leukemia cells. Int J Cancer. 122:2674–2681. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chun J and Kim YS: Platycodin D inhibits
migration, invasion, and growth of MDA-MB-231 human breast cancer
cells via suppression of EGFR-mediated Akt and MAPK pathways. Chem
Biol Interact. 205:212–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu JS and Kim AK: Platycodin D induces
apoptosis in MCF-7 human breast cancer cells. J Med Food.
13:298–305. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ye Y, Han X, Guo B, Sun Z and Liu S:
Combination treatment with platycodin D and osthole inhibits cell
proliferation and invasion in mammary carcinoma cell lines. Environ
Toxicol Pharmacol. 36:115–124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fakharzadeh SS, Trusko SP and George DL:
Tumorigenic potential associated with enhanced expression of a gene
that is amplified in a mouse tumor cell line. EMBO J. 10:1565–1569.
1991.PubMed/NCBI
|
|
19
|
Rayburn ER, Ezell SJ and Zhang R: Recent
advances in validating MDM2 as a cancer target. Anticancer Agents
Med Chem. 9:882–903. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Onel K and Cordon-Cardo C: MDM2 and
prognosis. Mol Cancer Res. 2:1–8. 2004.PubMed/NCBI
|
|
21
|
Nag S, Qin J, Srivenugopal KS, Wang M and
Zhang R: The MDM2-p53 pathway revisited. J Biomed Res. 27:254–271.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brosh R and Rotter V: When mutants gain
new powers: News from the mutant p53 field. Nat Rev Cancer.
9:701–713. 2009.PubMed/NCBI
|
|
23
|
Epstein CB, Attiyeh EF, Hobson DA, Silver
AL, Broach JR and Levine AJ: p53 mutations isolated in yeast based
on loss of transcription factor activity: Similarities and
differences from p53 mutations detected in human tumors. Oncogene.
16:2115–2122. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee SK, Park KK, Kim HJ, Kim KR, Kang EJ,
Kim YL, Yoon H, Kim YS and Chung WY: Platycodin D blocks breast
cancer-induced bone destruction by inhibiting osteoclastogenesis
and the growth of breast cancer cells. Cell Physiol Biochem.
36:1809–1820. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang ZH, Li T, Gao HW, Sun W, Chen XP,
Wang YT and Lu JJ: Platycodin D from Platycodonis Radix enhances
the anti-proliferative effects of doxorubicin on breast cancer
MCF-7 and MDA-MB-231 cells. Chin Med. 9:162014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chun J, Ha IJ and Kim YS:
Antiproliferative and apoptotic activities of triterpenoid saponins
from the roots of Platycodon grandiflorum and their
structure-activity relationships. Planta Med. 79:639–645. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Riley T, Sontag E, Chen P and Levine A:
Transcriptional control of human p53-regulated genes. Nat Rev Mol
Cell Biol. 9:402–412. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hao Q and Cho WC: Battle against cancer:
An everlasting saga of p53. Int J Mol Sci. 15:22109–22127. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Muller PA and Vousden KH: p53 mutations in
cancer. Nat Cell Biol. 15:2–8. 2013. View Article : Google Scholar
|
|
30
|
Hollstein M, Sidransky D, Vogelstein B and
Harris CC: p53 mutations in human cancers. Science. 253:49–53.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Freed-Pastor WA and Prives C: Mutant p53:
One name, many proteins. Genes Dev. 26:1268–1286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rivlin N, Brosh R, Oren M and Rotter V:
Mutations in the p53 tumor suppressor gene: Important milestones at
the various steps of tumorigenesis. Genes Cancer. 2:466–474. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim BM, Kim DH, Park JH, Surh YJ and Na
HK: Ginsenoside Rg3 inhibits constitutive activation of NF-κB
signaling in human breast cancer (MDA-MB-231) cells: ERK and Akt as
potential upstream targets. J Cancer Prev. 19:23–30. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Q, Zeng SX and Lu H: Targeting
p53-MDM2-MDMX loop for cancer therapy. Subcell Biochem. 85:281–319.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pei D, Zhang Y and Zheng J: Regulation of
p53: A collaboration between Mdm2 and Mdmx. Oncotarget. 3:228–235.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qin JJ, Wang W, Voruganti S, Wang H, Zhang
WD and Zhang R: Identification of a new class of natural product
MDM2 inhibitor: In vitro and in vivo anti-breast cancer activities
and target validation. Oncotarget. 6:2623–2640. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Olivier M, Eeles R, Hollstein M, Khan MA,
Harris CC and Hainaut P: The IARC TP53 database: New online
mutation analysis and recommendations to users. Hum Mutat.
19:607–614. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Katayose D, Gudas J, Nguyen H, Srivastava
S, Cowan KH and Seth P: Cytotoxic effects of adenovirus-mediated
wild-type p53 protein expression in normal and tumor mammary
epithelial cells. Clin Cancer Res. 1:889–897. 1995.PubMed/NCBI
|
|
39
|
Walerych D, Napoli M, Collavin L and Del
Sal G: The rebel angel: Mutant p53 as the driving oncogene in
breast cancer. Carcinogenesis. 33:2007–2017. 2012. View Article : Google Scholar : PubMed/NCBI
|