Introduction
Colorectal cancer (CRC) is one of the most prevalent
cancers worldwide and the third ranked fatal malignancy in the
United States (1,2). Although there has been progress in
diagnosis, surgery, combined chemotherapy and targeted therapy
(3,4), CRC remains a significant adverse
influence on human health with the 5-year survival of only 65%
(5). KRAS has long been known as
the most frequently mutated gene in nearly all types of cancers.
Particularly, approximately 40% of the CRCs harbor a mutation in
KRAS (6). Mutations in codons 12
and 13 in exon 2 accounts for 90% of all KRAS mutations, and KRAS
gene mutation test has been used as a predictor clinically to
determine the lack of efficacy on targeting agents such as
cetuximab and panitumumab (7–10).
Although still controversial, several studies have demonstrated
that KRAS mutation in colorectal carcinoma is correlated to
unfavorable survival and enhanced tumor aggressiveness (11,12).
However, recent therapeutic targeting of RAS in CRC has proven
intractable, due to frequent mutation in KRAS (13). Therefore, searching for new
therapeutic targets within the RAS signaling network is necessary
and strongly warranted.
GATA binding protein 2 (GATA2), a key member of zinc
finger transcription factors family, is identified as a critical
regulator of growth, differentiation and survival of hematopoietic
stem cells (14–16). Increasing evidence has shown that
GATA2 expression is correlated with hematologic pathophysiologies
and the proliferation and progression of solid tumors (17–23).
It was reported that overexpression of GATA2 would contribute to
the development of breast cancer through negatively regulating the
transcription of phosphatase and tensin homologue (PTEN) (20). As for prostate cancer, upregulated
GATA2 expression has been reported to be correlated with tumor
progression and GATA2 has been suggested to be a pioneer factor in
the regulation of androgen receptor related genes (21,22).
Kumar et al demonstrated that RAS-pathway
mutant non-small cell lung cancer (NSCLC) cells depended on GATA2
for viability and regulation of the GATA2-related signaling
pathways remarkably provoking regression of NSCLC (24). Further studies proved that delivery
of GATA2 siRNA with selected carrier downregulated GATA2 expression
and caused pronounced synthetic lethal effect of NSCLC in
vivo (25). However, the
association between KRAS mutation and GATA2 expression and its
prognostic value in CRC remains unexplored.
In this study, we performed immunohistochemistry to
examine GATA2 expression and dideoxy sequencing to detect KRAS
mutation in the same patients in a CRC cohort. We demonstrated that
GATA2 was a prospective indicator for poor prognosis in KRAS mutant
CRC patients and GATA2-related pathways may be potential new
therapeutic targets for KRAS mutant CRC patients.
Materials and methods
Ethics statement
All procedures performed in studies involving human
participants were in accordance with the Ethical Standards of the
Research Ethics Committee of Peking University Cancer Hospital and
Institute (Beijing, China) (no. 2012071710) and with the 1964
Helsinki Declaration and its later amendments or comparable ethical
standards.
Patients and tissue specimens
This retrospective study included a total of 236
patients who were diagnosed with CRC and then received primary
tumor resection at the Department of Gastrointestinal Surgery IV,
Peking University Cancer Hospital and Institute from 2005 to 2012.
All the CRC tissues for immunochemistry analysis were obtained from
surgically removed tumors and routinely embedded in paraffin. The
clinicopathological characteristics and tumor stages were estimated
in accordance with the American Joint Committee on Cancer (AJCC)
classification guidelines. Postoperative follow-up results were
available for all the 236 patients who have been followed up until
March 2015. For the utility of these clinical samples for research,
prior written informed consent of the patients and approval from
the Research Ethics Committee of Peking University Cancer Hospital
and Institute, (Beijing, China) were obtained.
KRAS mutation detection
Formalin-fixed, paraffin-embedded (FFPE) samples
with ≥50% tumor cells were collected from patients mentioned above.
After extracting genome DNA from the FFPE samples, fragments
contained codons 12 and 13 in KRAS exon 2 were amplified by PCR
(KRAS forward, 5′-GGTACTGGTGGAGTATTTGATAG-3′ and reverse,
5′-TGGTCCTGCACCAGTAATATG-3′). LA Taq polymerase was used to perform
the PCR. The product size was 248 bp. The reaction mixtures
recommended by the manufacturers were used. The initial
denaturation step was 5 min at 94°C. The thermal profile was 45
cycles of 94°C for 30 sec, 56°C for 30 sec and 72°C for 20 sec. The
final elongation was 10 min at 72°C. The PCR products were
separated on 1% agarose gel electrophoresis and then sequenced
using the same forward primer by Invitrogen 3730XL genetic
analyzer. The sequencing results were analyzed with Chromas
software under the condition of signal/noise >98% (26).
Immunohistochemistry
Paraffin-embedded tumor tissue blocks were cut
4-µm thick and then baked overnight at 72°C. Then they were
deparaffinized with xylene twice and rehydrated with graded
ethanol. The slides were heated in antigen retrieval buffer
containing 0.01 M sodium citrate-hydrochloric acid (pH 6.0) for 10
min in a high pressure apparatus at 150°C. After having been cooled
in room temperature for 1 h, the slides were treated with 3%
hydrogen peroxide to inhibit endogenous peroxidase activity. After
blocking nonspecific binding with goat serum, the sections were
incubated with a rabbit polyclonal antibody against GATA2 (1:200;
Santa Cruz Biotechnology) at 4°C overnight, followed by incubation
with second antibody from the EnVision™ kit (Dako Cytomation) at
room temperature for 30 min. The visualization signal was developed
with diaminobenzidine (Sigma). Sections were counterstained with
hematoxylin.
Evaluation of GATA2
immunohistochemistry
GATA2 staining scores were determined independently
by two pathologists previously uninformed about the
histopathological features and patient information to minimize
subjectivity. Proportion of positive cells and staining intensity
of immunohistochemistry were considered in the scoring process. The
scores for GATA2 staining were determined according to the
following standard (27): −, no
staining or <10% positive cells; +, 10–20% weakly to moderately
positive cells; ++, 10–20% intensively positive cells or 20–50%
weakly positive cells; and +++, 20–50% positive cells with moderate
to strong reactivity or >50% positive cells. All disagreements
on results between the two pathologists were resolved after joint
review and finally consensus was achieved for all the results.
Statistical analysis
Statistical analysis was carried out using SPSS
software 17.0. The Pearson's χ2 verified the correlation
between either KRAS gene status or GATA2 expression and
clinicopathological parameters. Kaplan-Meier method was used to
estimate overall survival curve and differences between groups were
compared by the log-rank test. Univariate and multivariate Cox
proportional hazard regression models were used to evaluate the
predictors for overall survival. A p-value of <0.05 was
considered to be statistically significant.
Results
Types of KRAS mutation
Among the 236 enrolled cases, 64 (27.1%) were found
to harbor mutations in the KRAS gene. As shown in Table I, the KRAS mutations were
distributed between codon 12 [51/64 (79.7%)] and codon 13 [13/64
(20.3%)]. A total of seven types of mutation in exon 2 were
detected. The G>A transitions at nucleotides, namely C12GAT and
C12AGT in codon 12 as well as C13GAC in codon 13, were the most
frequent mutations in this cohort (70.3%).
 | Table IDistribution of various KRAS mutant
patterns. |
Table I
Distribution of various KRAS mutant
patterns.
| KRAS status | No. of cases (%) |
|---|
| Wild | 172 (72.9) |
| C12GAT | 31 (13.1) |
| C13GAC | 12 (5.1) |
| C12GTT | 12 (5.1) |
| C12TGT | 5 (2.1) |
| C12AGT | 2 (0.9) |
| C12GCT | 1 (0.4) |
| C13TGC | 1 (0.4) |
Association between KRAS mutation or
GATA2 expression and clinicopathological features
All 236 patients with CRC were included in this
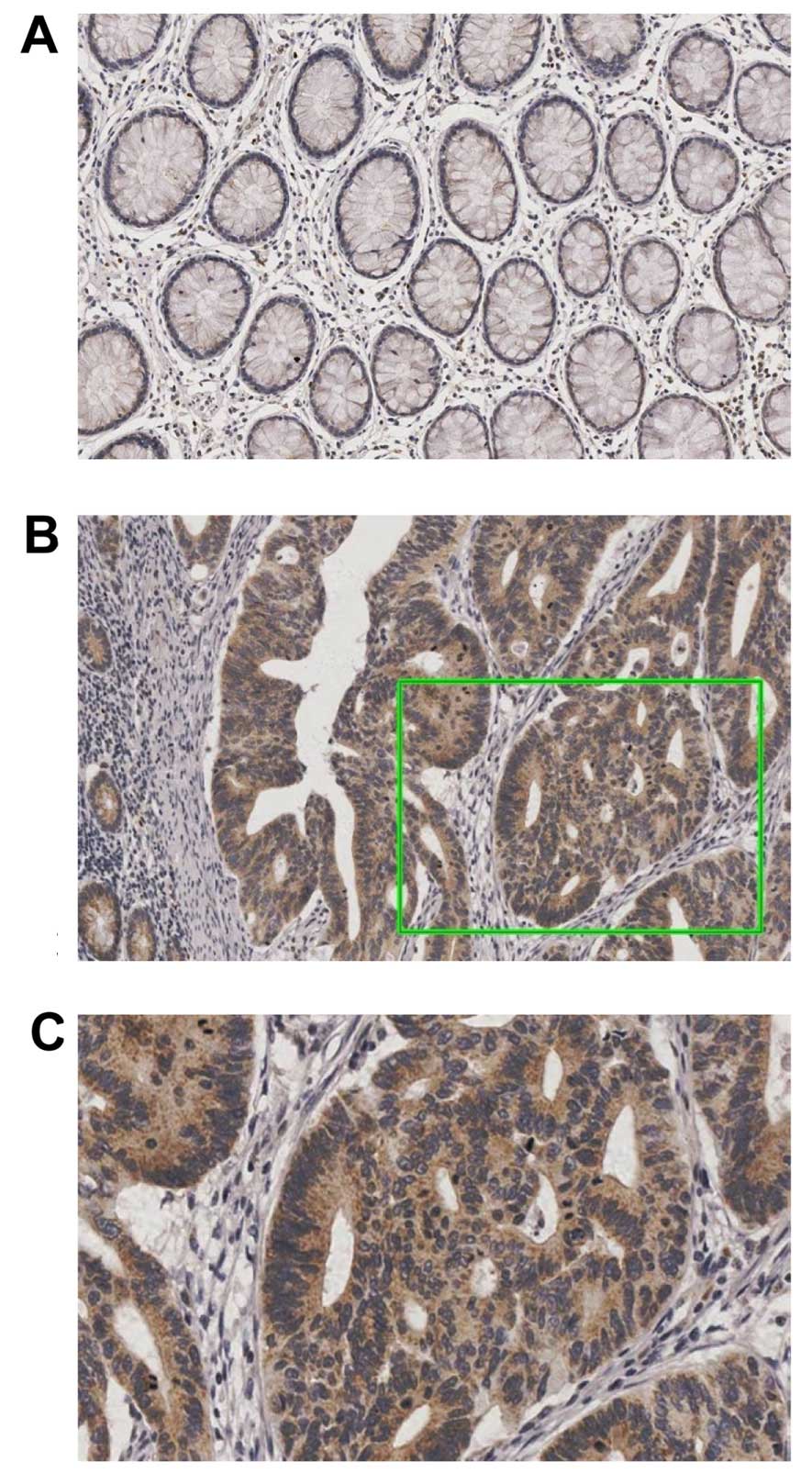
analysis. GATA2 expression was examined in all of the 236 CRC
patients (Fig. 1). Table II shows the correlation between
either KRAS mutation or GATA2 expression and various
clinicopathological features. Statistical analysis indicated that
KRAS mutation was significantly correlated with gender (P=0.011),
tumor location (P=0.024) and overall survival (P=0.012).
Nevertheless, no associations were found between KRAS mutation and
age, tumor size, depth of invasion, lymph node metastasis, TNM
stage, histological type, and tumor differentiation (P>0.05). On
the other hand, GATA2 expression was significantly linked with age
(P=0.012), depth of invasion (P=0.002), lymph node metastasis
(P=0.018), TNM stage (P=0.001) and overall survival (P=0.001). No
correlation was observed between the level of GATA2 expression and
gender, tumor location, tumor size, histological type, and tumor
differentiation (P>0.05). However, KRAS mutation was not
significantly associated with GATA2 expression (P=0.766).
 | Table IICorrelations between KRAS mutation or
GATA2 expression and clinicopathological features in colorectal
cancer patients. |
Table II
Correlations between KRAS mutation or
GATA2 expression and clinicopathological features in colorectal
cancer patients.
| Variables | Cases | KRAS mutation
| P-value | GATA2 expression
| P-value |
|---|
| Wild-type | Mutant | Low | High |
|---|
| Age (years) |
| <60 | 140 | 106 | 34 | 0.237 | 57 | 83 | 0.012 |
| ≥60 | 96 | 66 | 30 | | 24 | 72 | |
| Gender |
| Female | 94 | 60 | 34 | 0.011 | 35 | 59 | 0.443 |
| Male | 142 | 112 | 30 | | 46 | 96 | |
| Tumor location |
| Colon | 134 | 90 | 44 | 0.024 | 44 | 90 | 0.582 |
| Rectum | 102 | 82 | 20 | | 37 | 65 | |
| Tumor size
(cm) |
| ≤4 | 142 | 108 | 34 | 0.178 | 49 | 93 | 0.941 |
| >4 | 94 | 64 | 30 | | 32 | 62 | |
| Depth of
invasion |
| T1/T2 | 35 | 30 | 5 | 0.064 | 20 | 15 | 0.002 |
| T3/T4 | 201 | 142 | 59 | | 61 | 140 | |
| Lymph node
metastasis |
| Negative | 73 | 53 | 20 | 0.949 | 33 | 40 | 0.018 |
| Positive | 163 | 119 | 44 | | 48 | 115 | |
| TNM stage |
| I/II | 54 | 39 | 15 | 0.901 | 29 | 25 | 0.001 |
| III/IV | 182 | 133 | 49 | | 52 | 130 | |
| Histological
type |
|
Adenocarcinoma | 213 | 151 | 62 | 0.110 | 74 | 139 | 0.893 |
| Mucinous | 14 | 13 | 1 | | 4 | 10 | |
| Others | 9 | 8 | 1 | | 3 | 6 | |
| Tumor
differentiation |
| Well | 2 | 1 | 1 | 0.467 | 0 | 2 | 0.259 |
| Moderate | 160 | 116 | 44 | | 50 | 110 | |
| Poor | 53 | 37 | 16 | | 21 | 32 | |
| Unknown | 21 | 18 | 3 | | 10 | 11 | |
| Survival |
| Alive | 138 | 109 | 29 | 0.012 | 59 | 79 | 0.001 |
| Dead | 98 | 63 | 35 | | 22 | 76 | |
Correlation between KRAS mutation or
GATA2 expression and overall survival
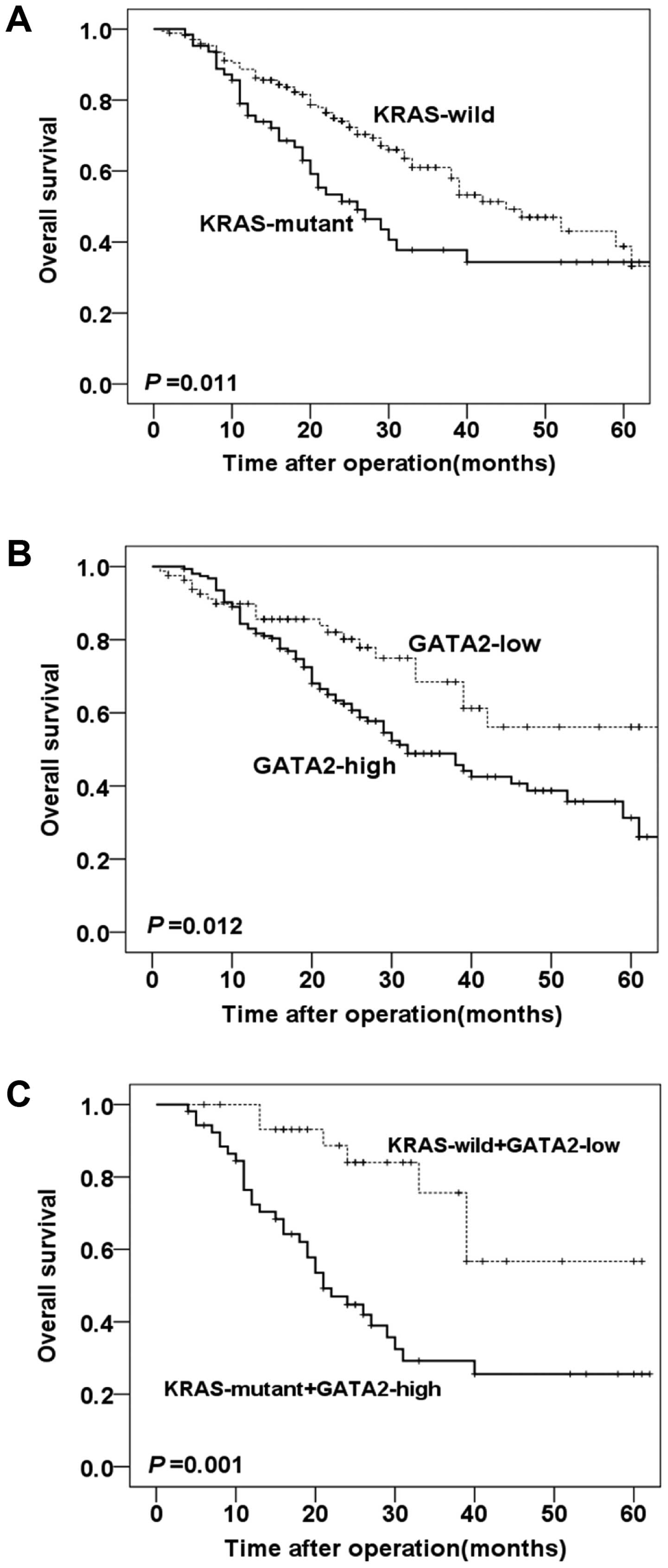
Kaplan-Meier survival curves with log-rank test were
performed to evaluate the association between KRAS mutation or
GATA2 expression level and prognosis. The results revealed that
KRAS mutation in CRC was significantly associated with worse
overall survival (P=0.011, Fig.
2A). Additionally, GATA2 high expression was significantly
correlated with shorter overall survival compared with GATA2 low
expression group (P=0.012, Fig.
2B).
For further analysis, the CRC patients enrolled were
separated into different groups according to KRAS status (wild-type
and mutant) and GATA2 expression (low and high). The Kaplan-Meier
curves in Fig. 2C demonstrated that
KRAS mutant/GATA2 high cancers had poor (P=0.001, Fig. 2C) long-term clinical outcomes in
comparison with KRAS wild/GATA2 low cancers.
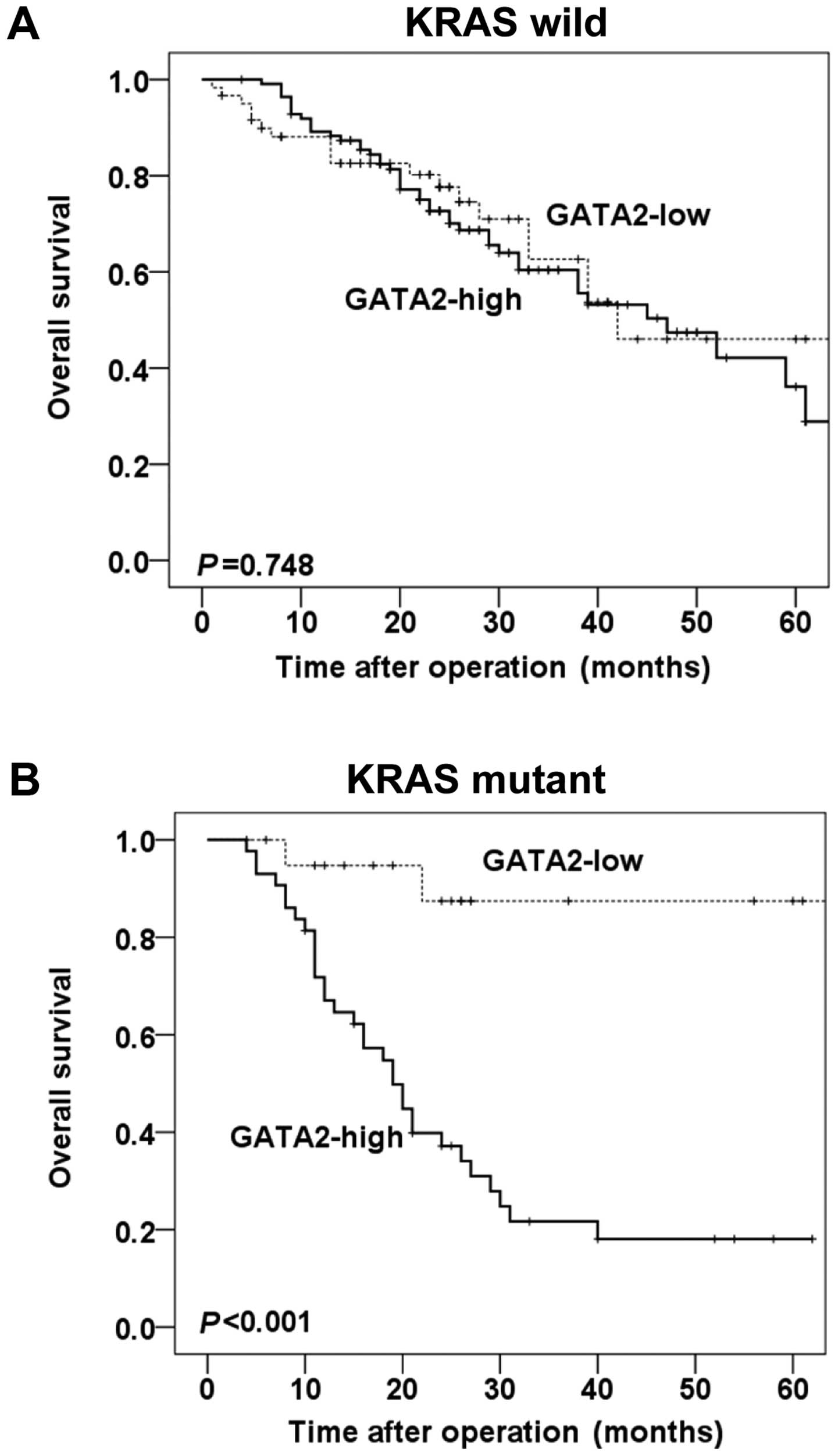
Stratified analysis by KRAS mutation (KRAS wild-type
and KRAS mutant type) showed that in the wild-type KRAS subgroup,
no statistical significance was observed between the levels of
GATA2 expression and overall survival of CRC patients (P=0.748,
Fig. 3A). However, in the mutant
KRAS subgroup, patients with high expression of GATA2 experienced
significantly shorter overall survival, compared with those with
low expression of GATA2 (P<0.001, Fig. 3B).
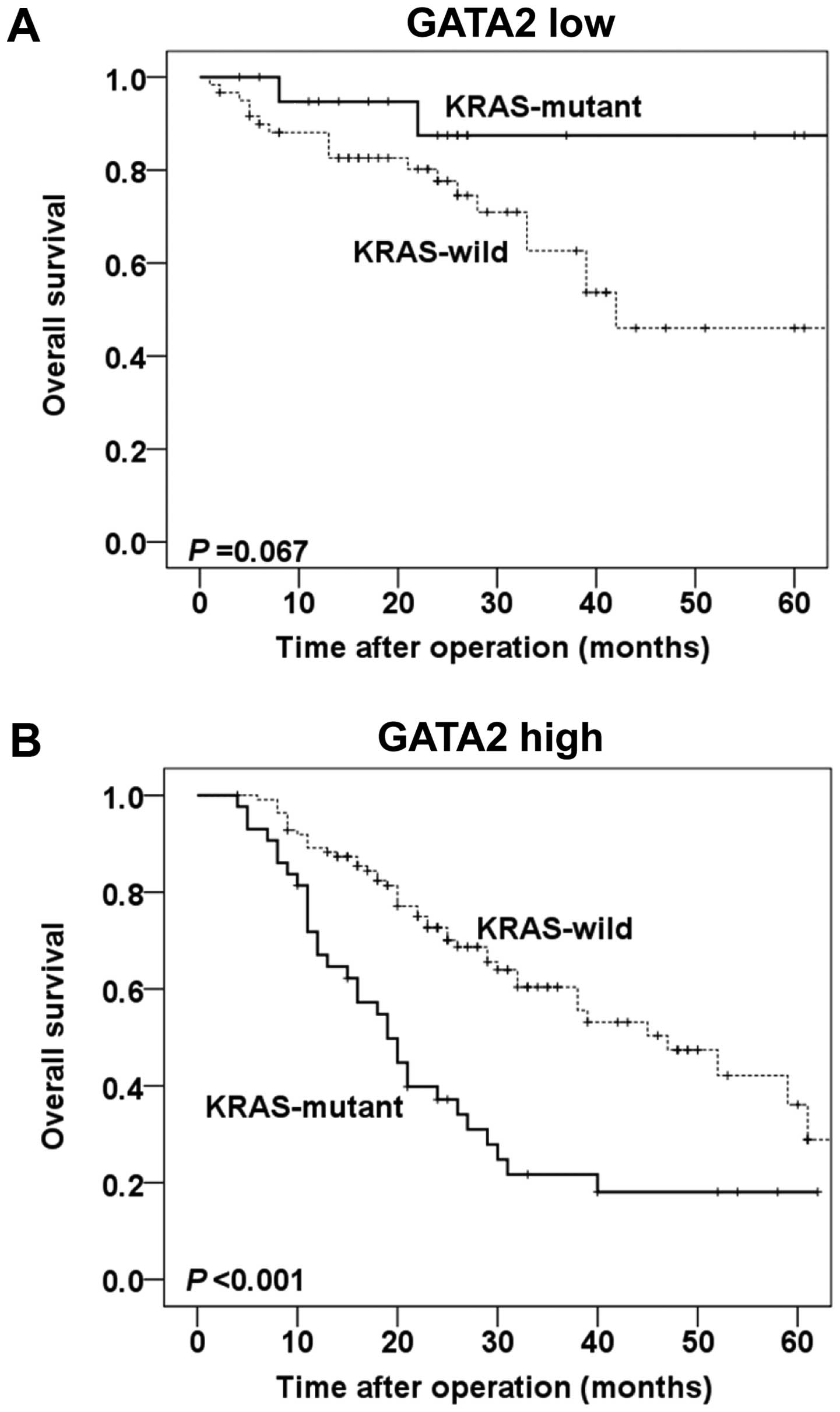
Furthermore, the prognostic value of KRAS gene
status in different GATA2 protein expression levels were also
evaluated. In patients with low GATA2 expression, only a borderline
significance was observed in overall survival between KRAS
wild-type and KRAS mutant groups (P=0.067, Fig. 4A). Whereas in patients with high
GATA2 expression, mutant KRAS was significantly correlated with
poor overall survival, compared with wild-type KRAS (P=0.020,
Fig. 4B).
Cox proportional hazard regression
analysis demonstrated GATA2 as an independent prognostic
factor
In the univariate analysis, patients with KRAS
mutation or GATA-high carcinoma tended to have a shorter overall
survival (HR 1.704; 95% CI 1.123–2.583; P=0.012, HR 1.818; 95% CI
1.129–2.927; P=0.014, respectively, Table III) than those with KRAS wild-gene
type or GATA2-low tumors. Gender, tumor location, tumor size, depth
of tumor invasion and TNM stage also showed significant
associations with overall survival in univariate analysis (HR
1.639; 95% CI 1.102–2.437; P=0.015, HR 1.663; 95% CI 1.097–2.521;
P=0.017, HR 1.586; 95% CI 1.066–2.359; P=0.023, HR 2.575; 95% CI
1.193–5.559; P=0.016, HR 3.447; 95% CI 1.832–6.488; P<0.001,
respectively, Table III). In the
multivariate analysis, the potential risk factors mentioned above
were included into the Cox proportional hazard regression model in
order to avoid covariation. The results showed that GATA2 high
expression, advanced TNM stage (III/IV) and female patients were
retained as independent prognostic factors for poor prognosis (HR
1.645; 95% CI 1.004–2.696; P=0.048, HR 3.058; 95% CI 1.594–5.865;
P=0.001, HR 1.519; 95% CI 1.010–2.283; P=0.045, Table III).
 | Table IIIUnivariate and multivariate analysis
of KRAS status and GATA2 expression with respect to overall
survival. |
Table III
Univariate and multivariate analysis
of KRAS status and GATA2 expression with respect to overall
survival.
| Variables | Univariate
| Multivariate
|
|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (≥60 vs. <60
years) | 1.386 | 0.933–2.060 | 0.106 | | | |
| Gender (female vs.
male) | 1.639 | 1.102–2.437 | 0.015 | 1.519 | 1.010–2.283 | 0.045 |
| Tumor location
(colon vs. rectum) | 1.663 | 1.097–2.521 | 0.017 | 1.508 | 0.973–2.335 | 0.066 |
| Tumor size (>4
vs. ≤4 cm) | 1.586 | 1.066–2.359 | 0.023 | 1.368 | 0.902–2.075 | 0.140 |
| Depth of invasion
(T3/T4 vs. T1/T2) | 2.575 | 1.193–5.559 | 0.016 | 1.165 | 0.505–2.691 | 0.720 |
| TNM stage (III/IV
vs. I/II) | 3.447 | 1.832–6.488 | <0.001 | 3.058 | 1.594–5.865 | 0.001 |
| Histological type
(mucinous vs. adenocarcinoma) | 0.578 | 0.183–1.826 | 0.350 | | | |
| Tumor
differentiation (poor vs. well/moderate) | 1.467 | 0.930–2.315 | 0.099 | | | |
| KRAS mutation
(mutant vs. wild) | 1.704 | 1.123–2.583 | 0.012 | 1.499 | 0.974–2.305 | 0.065 |
| GATA2 expression
(high vs. low) | 1.818 | 1.129–2.927 | 0.014 | 1.645 | 1.004–2.696 | 0.048 |
Discussion
In this study, we analyzed the influence of KRAS
mutation and GATA2 expression on the overall survival based on a
cohort of CRC patients. Our data demonstrated that although there
was no significant correlation between KRAS mutation and expression
of GATA2, high level of GATA2 expression and KRAS mutation were
significantly associated with unfavorable prognosis in CRC
patients, compared to those bearing GATA2-low and wild-type KRAS
tumors, respectively. Moreover, we determined that combined KRAS
mutation and overexpression of GATA2 led to more adverse overall
survival possible independent predictor for poor overall survival
in CRC patients. The results in all showed that high levels of
GATA2 expression predicted significant adverse clinical outcomes
when KRAS mutation occurred in the same patient, suggesting GATA2
protein may be prone to tumorigenesis of KRAS mutant CRC.
The role of GATA2 in hematopoietic malignant
disorders has been well elucidated (15,19).
In contrast, only a few studies in gastrointestinal malignancies
involving GATA2 transcription factor have been done (28). Previous studies suggested that GATA2
expression had influence on the clinical outcomes in patients with
several solid carcinomas, such as breast, prostate, colorectal,
renal and hepatocellular carcinoma (20–23,29,30).
High levels of GATA2 expression predict tumor recurrence,
metastasis, or poor survival except for renal and hepatic cancer
(29,30). Moreover, the role of GATA2 in lung
cancer remains controversial. A recent study demonstrated that
GATA2 levels were indispensable for survival of KRAS mutant NSCLC
cells (24). Co-inhibition of
GATA2-regulated proteasome and Rho-signaling pathway significantly
suppressed the proliferation of lung tumor cells with KRAS
mutation. These results indicated that application of novel
strategy targeting GATA2 related pathways may benefit patients with
RAS pathway mutated NSCLC. On the contrary, Tessema et al
reported that GATA2 was not requisite for the survival of lung
cancer patients with KRAS mutation due to epigenetic repression
(31). Therefore, it is critical to
determine the association between KRAS mutation and GATA2
expression, and verify their clinical significance in CRC patients.
Consistent with previous studies (23,24),
our findings showed that GATA2 played a crucial role in the
long-term outcomes of CRC patients with KRAS mutation, which raised
the possibility that GATA2 protein may influence carcinogenesis and
proliferation of KRAS mutant CRC cells. Accordingly, GATA2 could be
a potential target for the treatment of CRC patients with KRAS
mutation, which indicates resistance to EGFR-targeting antibodies,
including cetuximab and panitumumab. However, GATA2 is
traditionally considered to be undruggable (32). Thus, therapies against
GATA2-regulated pathways may become an alternative strategy for the
treatment of CRC patients with KRAS mutation.
In this cohort, as expected, TNM stage was
identified as an independent prognostic factor to predict patient
clinical outcomes (HR 3.058; 95% CI 1.594–5.865; P=0.001, Table III). Additionally, female gender
was observed as an adverse factor in our study (HR 1.519; 95% CI
1.010–2.283; P=0.045, Table III),
which was in accordance with previous studies (33).
However, potential limitations still exist in this
study. To validate the role of GATA2 expression in CRC patients
harboring KRAS mutation, replication cohorts containing detailed
clinical data are required. In addition, further functional studies
are needed to understand the role of GATA2 and KRAS in CRC.
In conclusion, we demonstrated that elevated GATA2
expression correlated with poor overall survival in CRC patients.
Furthermore, GATA2 overexpression in combination with KRAS mutation
is significantly associated with poor prognosis. Our results
suggested that GATA2 is a promising predictor to identify
individuals with worse long-term clinical outcomes, especially in
KRAS mutant CRC patients. Also, we consider GATA2-related pathways
as potential targets for the development of novel therapies for
KRAS mutant CRC.
Acknowledgments
We thank Dr Bin Dong for histopathological
diagnosis. This study was supported by the National Natural Science
Foundation of China (no. 81272766 and 81450028), the National High
Technology Research and Development Program of China (863 Program,
no. 2014AA020603), Beijing Natural Science Foundation (no.
7162039). Beijing Municipal Administration of Hospitals Clinical
Medicine Development of Special Funding Support (no. XM201309),
Peking University (PKU) 985 Special Funding for Collaborative
Research with PKU Hospitals (to X.S. and Fan Bai).
Abbreviations:
|
CRC
|
colorectal cancer
|
|
GATA2
|
GATA2-binding protein 2
|
|
EGFR
|
epidermal growth factor receptor
|
|
NSCLC
|
non-small cell lung cancer
|
|
DFS
|
disease-free survival
|
|
PTEN
|
phosphatase and tensin homologue
|
|
FFPE
|
formalin-fixed, paraffin-embedded
|
|
PCR
|
polymerase chain reaction
|
References
|
1
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ahmed S, Johnson K, Ahmed O and Iqbal N:
Advances in the management of colorectal cancer: From biology to
treatment. Int J Colorectal Dis. 29:1031–1042. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Rosa M, Pace U, Rega D, Costabile V,
Duraturo F, Izzo P and Delrio P: Genetics, diagnosis and management
of colorectal cancer (Review). Oncol Rep. 34:1087–1096.
2015.PubMed/NCBI
|
|
5
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Macedo MP, Andrade LB, Coudry R, Crespo R,
Gomes M, Lisboa BC, Aguiar S Jr, Soares FA, Carraro DM and Cunha
IW: Multiple mutations in the Kras gene in colorectal cancer:
Review of the literature with two case reports. Int J Colorectal
Dis. 26:1241–1248. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karapetis CS, Khambata-Ford S, Jonker DJ,
O'Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD,
Robitaille S, et al: K-ras mutations and benefit from cetuximab in
advanced colorectal cancer. N Engl J Med. 359:1757–1765. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Amado RG, Wolf M, Peeters M, Van Cutsem E,
Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, et
al: Wild-type KRAS is required for panitumumab efficacy in patients
with metastatic colorectal cancer. J Clin Oncol. 26:1626–1634.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
De Roock W, De Vriendt V, Normanno N,
Ciardiello F and Tejpar S: KRAS, BRAF, PIK3CA, and PTEN mutations:
Implications for targeted therapies in metastatic colorectal
cancer. Lancet Oncol. 12:594–603. 2011. View Article : Google Scholar
|
|
10
|
Inoue Y, Saigusa S, Iwata T, Okugawa Y,
Toiyama Y, Tanaka K, Uchida K, Mohri Y and Kusunoki M: The
prognostic value of KRAS mutations in patients with colorectal
cancer. Oncol Rep. 28:1579–1584. 2012.PubMed/NCBI
|
|
11
|
Arrington AK, Heinrich EL, Lee W, Duldulao
M, Patel S, Sanchez J, Garcia-Aguilar J and Kim J: Prognostic and
predictive roles of KRAS mutation in colorectal cancer. Int J Mol
Sci. 13:12153–12168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kadowaki S, Kakuta M, Takahashi S,
Takahashi A, Arai Y, Nishimura Y, Yatsuoka T, Ooki A, Yamaguchi K,
Matsuo K, et al: Prognostic value of KRAS and BRAF mutations in
curatively resected colorectal cancer. World J Gastroenterol.
21:1275–1283. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sameen S, Barbuti R, Milazzo P, Cerone A,
Del Re M and Danesi R: Mathematical modeling of drug resistance due
to KRAS mutation in colorectal cancer. J Theor Biol. 389:263–273.
2016. View Article : Google Scholar
|
|
14
|
Collin M, Dickinson R and Bigley V:
Haematopoietic and immune defects associated with GATA2 mutation.
Br J Haematol. 169:173–187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsai FY and Orkin SH: Transcription factor
GATA-2 is required for proliferation/survival of early
hematopoietic cells and mast cell formation, but not for erythroid
and myeloid terminal differentiation. Blood. 89:3636–3643.
1997.PubMed/NCBI
|
|
16
|
Ohmori S, Moriguchi T, Noguchi Y, Ikeda M,
Kobayashi K, Tomaru N, Ishijima Y, Ohneda O, Yamamoto M and Ohneda
K: GATA2 is critical for the maintenance of cellular identity in
differentiated mast cells derived from mouse bone marrow. Blood.
125:3306–3315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nandakumar SK, Johnson K, Throm SL,
Pestina TI, Neale G and Persons DA: Low-level GATA2 overexpression
promotes myeloid progenitor self-renewal and blocks lymphoid
differentiation in mice. Exp Hematol. 43:565–577. e1–10. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hsu AP, McReynolds LJ and Holland SM:
GATA2 deficiency. Curr Opin Allergy Clin Immunol. 15:104–109. 2015.
View Article : Google Scholar :
|
|
19
|
Vicente C, Conchillo A, García-Sánchez MA
and Odero MD: The role of the GATA2 transcription factor in normal
and malignant hematopoiesis. Crit Rev Oncol Hematol. 82:1–17. 2012.
View Article : Google Scholar
|
|
20
|
Wang Y, He X, Ngeow J and Eng C: GATA2
negatively regulates PTEN by preventing nuclear translocation of
androgen receptor and by androgen-independent suppression of PTEN
transcription in breast cancer. Hum Mol Genet. 21:569–576. 2012.
View Article : Google Scholar
|
|
21
|
Vidal SJ, Rodriguez-Bravo V, Quinn SA,
Rodriguez-Barrueco R, Lujambio A, Williams E, Sun X, de la
Iglesia-Vicente J, Lee A, Readhead B, et al: A targetable
GATA2-IGF2 axis confers aggressiveness in lethal prostate cancer.
Cancer Cell. 27:223–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu D, Sunkel B, Chen Z, Liu X, Ye Z, Li Q,
Grenade C, Ke J, Zhang C, Chen H, et al: Three-tiered role of the
pioneer factor GATA2 in promoting androgen-dependent gene
expression in prostate cancer. Nucleic Acids Res. 42:3607–3622.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen L, Jiang B, Wang Z, Liu M, Ma Y, Yang
H, Xing J, Zhang C, Yao Z, Zhang N, et al: Expression and
prognostic significance of GATA-binding protein 2 in colorectal
cancer. Med Oncol. 30:4982013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kumar MS, Hancock DC, Molina-Arcas M,
Steckel M, East P, Diefenbacher M, Armenteros-Monterroso E,
Lassailly F, Matthews N, Nye E, et al: The GATA2 transcriptional
network is requisite for RAS oncogene-driven non-small cell lung
cancer. Cell. 149:642–655. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shen S, Mao CQ, Yang XZ, Du XJ, Liu Y, Zhu
YH and Wang J: Cationic lipid-assisted polymeric nanoparticle
mediated GATA2 siRNA delivery for synthetic lethal therapy of KRAS
mutant non-small-cell lung carcinoma. Mol Pharm. 11:2612–2622.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao J, Li YY, Sun PN and Shen L:
Comparative analysis of dideoxy sequencing, the KRAS StripAssay and
pyrosequencing for detection of KRAS mutation. World J
Gastroenterol. 16:4858–4864. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xing X, Peng L, Qu L, Ren T, Dong B, Su X
and Shou C: Prognostic value of PRL-3 overexpression in early
stages of colonic cancer. Histopathology. 54:309–318. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ayanbule F, Belaguli NS and Berger DH:
GATA factors in gastrointestinal malignancy. World J Surg.
35:1757–1765. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peters I, Dubrowinskaja N, Tezval H,
Kramer MW, von Klot CA, Hennenlotter J, Stenzl A, Scherer R, Kuczyk
MA and Serth J: Decreased mRNA expression of GATA1 and GATA2 is
associated with tumor aggressiveness and poor outcome in clear cell
renal cell carcinoma. Target Oncol. 10:267–275. 2015. View Article : Google Scholar
|
|
30
|
Li YW, Wang JX, Yin X, Qiu SJ, Wu H, Liao
R, Yi Y, Xiao YS, Zhou J, Zhang BH, et al: Decreased expression of
GATA2 promoted proliferation, migration and invasion of HepG2 in
vitro and correlated with poor prognosis of hepatocellular
carcinoma. PLoS One. 9:e875052014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tessema M, Yingling CM, Snider AM, Do K,
Juri DE, Picchi MA, Zhang X, Liu Y, Leng S, Tellez CS, et al: GATA2
is epigenetically repressed in human and mouse lung tumors and is
not requisite for survival of KRAS mutant lung cancer. J Thorac
Oncol. 9:784–793. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Darnell JE Jr: Transcription factors as
targets for cancer therapy. Nat Rev Cancer. 2:740–749. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim SE, Paik HY, Yoon H, Lee JE, Kim N and
Sung MK: Sex- and gender-specific disparities in colorectal cancer
risk. World J Gastroenterol. 21:5167–5175. 2015. View Article : Google Scholar : PubMed/NCBI
|