Introduction
Breast cancer is the most commonly diagnosed
malignancy and the leading cause of death from cancer among women
and the incidence rate is higher in young women in comparison to
older ones (1,2). One breast cancer subtype,
triple-negative breast cancer (TNBC) accounts for ~15–20% of breast
cancer cases and occurs more commonly in younger women (3,4). TNBCs
are highly aggressive and often have a poor prognosis when compared
to other breast cancer subtypes, including luminal and HER2-type
breast cancers (5,6). Due to the lack of ER, PR, and HER2
expression, TNBCs are insensitive to anti-hormonal and
HER2-targeted therapies (7).
Although sensitive to chemotherapy, TNBCs have an intrinsic
aggressive clinical course associated with high proliferation, high
histologic grade, earlier time to recurrence, and higher risk of
distant recurrence (5). Thus, many
researchers have attempted to discover promising therapeutic
targets for the treatment of TNBC breast cancer patients.
Stanniocalcins (STCs) are secreted glycoprotein
hormones and are involved in calcium/phosphate homeostasis and cell
development (8,9). STCs consist of STC-1 and -2 and are
broadly expressed in multiple mammalian tissues including the
ovary, prostate, and kidney (8,10,11).
In particular, STC-1, a 56-kDa homodimeric glycoprotein hormone, is
highly expressed in a variety of cancers such as breast, ovarian,
and colorectal cancers (12–15).
STC-1 is involved in various biological events such as cell
proliferation, apoptosis, and inflammation (14,16).
Furthermore, STC-1 triggers tumor angiogenesis via upregulation of
vascular endothelial growth factor (VEGF) in gastric cancer cells
(17). Abnormal STC-1 expression is
generally correlated with tumorigenesis and poor clinical outcomes
in ovarian and colorectal cancers (10,14,18).
However, little is understood about the clinical significance of
STC-1 expression and the intracellular signaling events in breast
cancer.
Ninety percent of all cancer-related deaths are
caused by tumor metastasis. Metastasis is a process which includes
excessive tumor cell proliferation, degradation of the surrounding
stroma microenvironment [extracellular matrix (ECM)], migration,
and invasion (19,20). In this multiple-step process, the
biological activity of matrix metalloproteinases (MMPs), a large
family of ECM-degrading enzymes, facilitates tumor cell growth,
migration, and angiogenesis through cleavage of ECM components
(21). The expression of MMPs in
tumors is primarily regulated at the transcriptional level in
response to growth factors and cytokines (22,23).
Enhancement of MMP-1 expression by TNF-α is mediated by coordinate
activation of c-Jun N-terminal protein kinase (JNK) and p38 MAPK
pathways (24). In particular, the
JNK/c-Jun signaling pathway accelerates cell migration and invasion
and promotes metastasis in breast cancer cells (25–28).
In contrast, Fyn-related kinase (FRK) was found to reduce cell
migration and invasion via inhibiting the JNK/c-Jun signaling
pathway in glioma cells (28).
The aim of this study was to investigate the
clinical significance of STC-1 in basal-type breast cancer as well
as the regulatory mechanism of STC-1-induced cell invasion.
Consequentially, elevated STC-1 expression was associated with poor
prognosis in basal-type breast cancer patients. The levels of STC-1
mRNA and protein expression were also significantly increased in
the TNBC cells. Furthermore, recombinant human STC-1 treatment
augmented the invasiveness of the TNBC cells while STC-1 siRNA
overexpression suppressed the invasiveness of the TNBC cells. In
addition, we found for the first time that STC-1 upregulated MMP-9
expression through the JNK/c-Jun-dependent signaling pathway.
STC-1-induced MMP-9 expression was associated with the invasiveness
of the TNBC cells. Therefore, we demonstrated that aberrant STC-1
expression enhanced the invasiveness of the TNBC cells through
JNK/c-Jun-dependent MMP-9 induction. STC-1 may be a promising
therapeutic target for the treatment of TNBC patients.
Materials and methods
Reagents
Cell culture media and antibiotics were purchased
from Life Technologies (Rockville, MD, USA). Fetal bovine serum
(FBS) was purchased from Hyclone (Logan, UT, USA).
Anti-phospho-JNK, c-Jun, ERK, p38, and STC-1 antibodies were
purchased from Epitomics (Burlingame, CA, USA). Anti-total-JNK,
ERK, p38, and β-actin antibodies were purchased from AbFrontier
(Seoul, Korea). Secondary horseradish peroxidase (HRP)-conjugated
antibodies were purchased from Santa Cruz Biotechnology (Santa
Cruz, CA, USA). Recombinant human STC-1 was purchased from ProSpec
(ProSpec HOR-259; Israel). SP600125 was purchased from Calbiochem
(San Diego, CA, USA).
Cell cultures and drug treatment
MCF7, Hs578T, MDA-MB-231, and MDA-MB-468 breast
cancer cells were cultured in Dulbecco's modified Eagle's medium
(DMEM) supplemented with 10% FBS, 100 IU/ml penicillin, and 100
µg/ml streptomycin. BT474 and SKBR3 breast cancer cells were
cultured in RPMI-1640 supplemented with 10% FBS, 100 IU/ml
penicillin, and 100 µg/ml streptomycin. Cells were grown in
a humidified atmosphere with 5% CO2 at 37°C. In the drug
treatment experiment, after serum starvation for 24 h, the cells
were pre-treated with SP600125 for 1 h, and then treated with
recombinant human STC-1 for 24 h.
Analysis of public database
Expression data were downloaded from a public
database [Kaplan-Meier plotter database (http://kmplot.com/breast)]. The clinical value of
STC-1 was analyzed by Kaplan-Meier survival plots in basal-type
breast cancer patients. The hazard ratio with 95% confidence
intervals and log-rank P-values were calculated.
Real-time polymerase chain reaction
(PCR)
Total RNA was extracted from the cells using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA), according to the
manufacturer's protocol. Isolated RNA samples were then used for
RT-PCR. Samples of total RNA (1 µg) were reverse-transcribed
into cDNA in 20-µl reaction volumes using a first-strand
cDNA synthesis kit for RT-PCR, according to the manufacturer's
instructions (MBI Fermentas, Hanover, MD, USA). Gene expression
levels were quantified by real-time PCR using a SensiMix SYBR kit
(Bioline, Ltd., London, UK) and 100 ng of cDNA per reaction. The
primer sequences used for this analysis were as follows: human
STC-1 (forward, 5′-CACACCCACGAGCTGACTTC-3′ and reverse,
5′-TCTCCCTGGTTATGCACTCTC-3′) and ACTB as an internal control
(forward, 5′-TCACCATTGGCAATGAGCGGTT-3′ and reverse,
5′-AGTTTCGTGGATGCCACAGGACT-3′). An annealing temperature of 60°C
was used for all of the primers. PCRs were performed in a standard
384-well plate format with an ABI 7900HT real-time PCR detection
system (Applied Biosystems, Foster City, CA, USA). For data
analysis, the raw threshold cycle (Ct) value was first normalized
to the housekeeping gene for each sample to obtain a ΔCt. The
normalized ΔCt was then calibrated to the control cell samples
resulting in a ΔΔCt.
Western blotting
Cell lysates were prepared for detection of STC-1,
β-actin, anti-total and phospho-JNK, c-Jun, ERK, p38 expression.
Equal proteins (50 µg) were boiled for 5 min in Laemmli
sample buffer and then electrophoresed on 10% sodium dodecyl
sulfate-polyacrylamide (SDS-PAGE) gels. Separated proteins were
transferred to polyvinylidene fluoride (PVDF) membranes, and the
membranes were blocked with 10% skim milk in Tris-buffered saline
(TBS) containing 0.01% Tween-20 (TBST) for 15 min. The blots were
washed three times in TBST and then incubated with STC-1, β-actin,
anti-total and phospho-JNK, c-Jun, ERK, p38 antibodies in TBST
buffer at 4°C overnight. The blots were washed three times in TBST
and subsequently incubated with secondary HRP-conjugated antibodies
(1:2,000 dilution) in TBST buffer. After a 1-h incubation at room
temperature (RT), the blots were washed three times in TBST. ECL™
Prime reagent (GE Healthcare, Buckinghamshire, UK) was used for
development.
Cell invasion assay
Twenty-four-well Boyden chambers with
Matrigel-coated filters (8-µm pore size) were purchased from
Becton-Dickinson (San Diego, CA, USA). Hs578T and MDA-MB-231 breast
cancer cells transfected with scrambled and STC-1 siRNA were tested
for invasion at 48 h. The cells were resuspended in culture media
(5×104 cells/well) and were seeded into the
Matrigel-coated upper compartment of the invasion chambers. Fresh
culture media with 5% FBS were added to the lower compartment of
the invasion chamber. In addition, Hs578T cells were resuspended in
culture media and then seeded into the Matrigel-coated upper
compartment of the invasion chambers in the presence or absence of
50 ng/ml STC-1 and/or 10 µM SP600125. Fresh culture media
with 5% FBS were added to the lower compartment of the invasion
chamber. After 24 h (Hs578T cells) or 48 h (MDA-MB-231 cells) of
incubation, the cells on the upper side of the filter were removed
using cotton swabs. Invaded cells through the Matrigel were located
on the underside of the filter. The underside of the filter was
fixed in 100% methanol, washed in 1X PBS, and stained using
hematoxylin and eosin (H&E). These cells were analyzed using
Aperio ScanScope XT (Aperio Technologies, Vista, CA, USA).
STC-1 siRNA transfection
Scrambled and STC-1 siRNAs were purchased from
Bioneer (Daejeon, Korea). Hs578T and MDA-MB-231 TNBC cells were
seeded in a 6-well plate. Cell transfection was performed using
Lipofectamine 2000 (Invitrogen) according to the manufacturer's
protocol. The cells were maintained in culture media without FBS
and antibiotics for 24 h while Lipofectamine transfection, and then
further incubated in fresh culture media with 10% FBS for 24 h.
Enzyme-linked immunosorbent assay
ELISA assay was performed on culture media (200
µl) collected from Hs578T and MDA-MB-231 TNBC cells.
Secreted protein levels of STC-1 were measured using an ELISA kit
(R&D Systems, Minneapolis, MN, USA), according to the
manufacturer's instructions. Secreted protein levels were analyzed
at a wavelength of 450 nm on a spectrometer (SpectraMax 190;
Molecular Devices, Sunnyvale, CA, USA).
Zymography
Samples (100 µl) were resuspended in loading
buffer and run on a 10% SDS-PAGE gel that contained 0.5 mg/ml
gelatin without prior denaturation. After electrophoresis, the gels
were washed to remove SDS and incubated for 30 min at RT in a
renaturing buffer. The gels were incubated for 48 h at 37°C in a
developing buffer. Next, the gels were subsequently stained with
Coomassie Brilliant Blue G-250, destained in 30% methanol, and
flooded with 10% acetic acid to detect gelatinase secretion.
Statistical analysis
Statistical significance was determined using a
Student's t-test. The results are presented as means ± SEM. All
quoted P-values were two-tailed and differences were considered
statistically significant when P<0.05. Statistical analysis was
performed using Microsoft Excel.
Results
Elevated STC-1 expression is associated
with poor prognosis in basal-type breast cancer patients
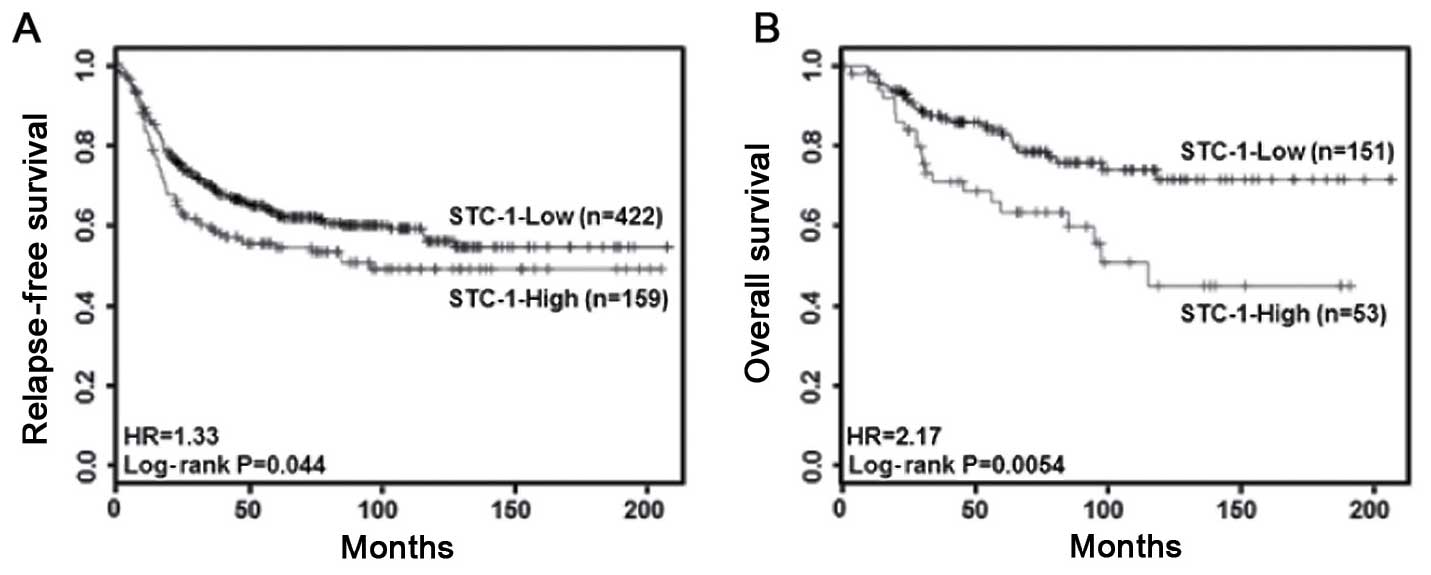
Clinically, to investigate the correlation between
STC-1 expression and the prognosis of breast cancer patients, we
analyzed the clinical value of STC-1 in breast cancer patients
using a Kaplan-Meier plotter database (http://kmplot.com/breast). As shown in Fig. 1A and B, we found that breast cancer
patients with a high level of STC-1 presented with poor prognosis.
In the basal-type breast cancer patients, patients with high
expression of STC-1 showed poorer relapse-free (P=0.044, Fig. 1A) and overall survival (P=0.0054,
Fig. 1B) than did patients with low
expression. However, the level of STC-1 expression did not affect
the relapse-free and overall survival of the luminal- and HER2-type
breast cancer patients (data not shown). Therefore, we demonstrated
that aberrant STC-1 expression is associated with poor clinical
outcomes in basal-type breast cancer patients.
The levels of STC-1 expression in breast
cancer cells
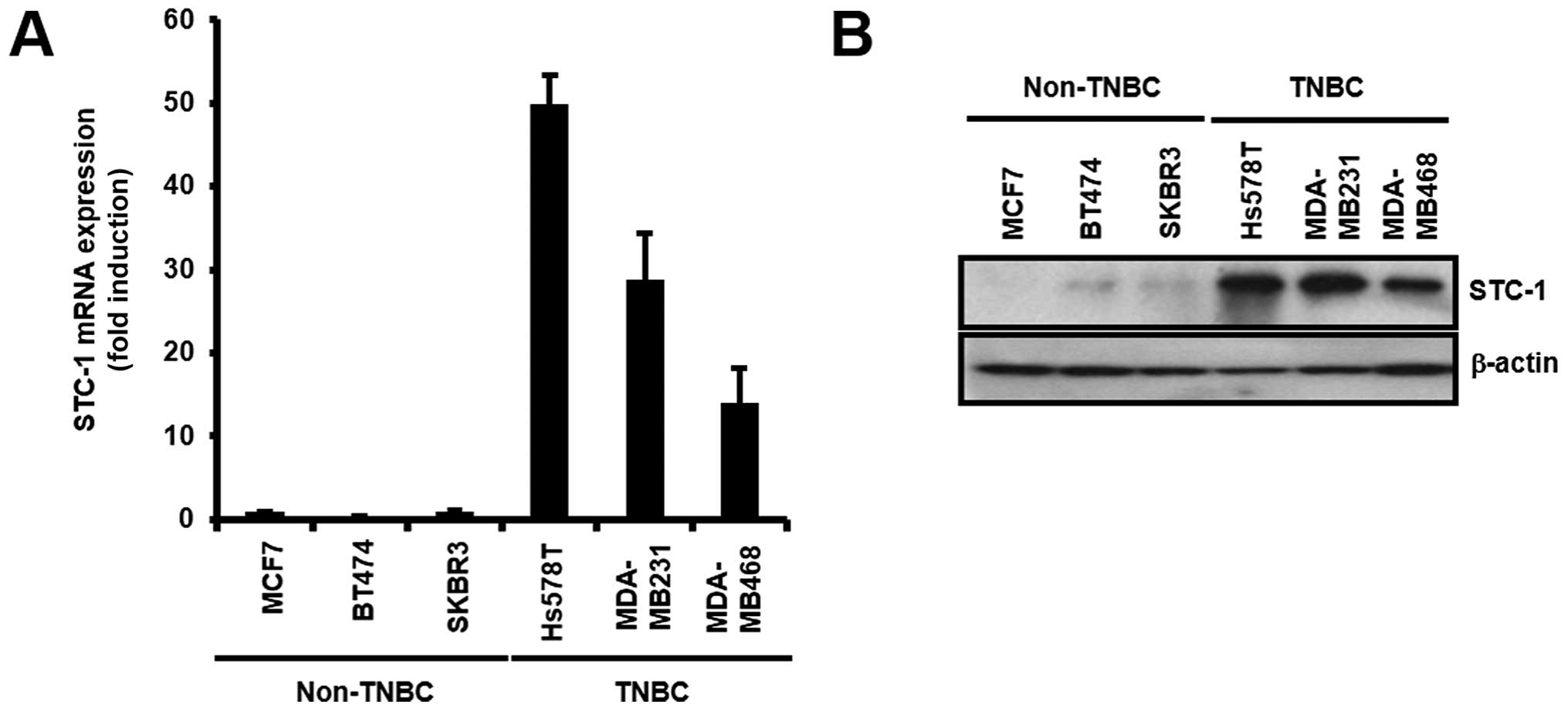
Next, we measured the levels of STC-1 mRNA and
protein expression in various breast cancer cell lines. Our results
showed that the levels of STC-1 expression were higher in the TNBC
cells than these levels in the non-TNBC cells (Fig. 2A and B). The levels of STC-1 mRNA
expression were significantly increased in the TNBC cells when
compared with levels in the non-TNBC cells (Fig. 2A). The levels of STC-1 mRNA
expression were significantly increased by 103.16±9.51-fold (Hs578T
cells), 58.45±1.71-fold (MDA-MB-231 cells), and 27.88±3.94-fold
(MDA-MB-468 cells) of the control level (MCF7 cells) (Fig. 2A). The STC-1 mRNA level of the
Hs578T cells was the highest among the TNBC cells (Fig. 2A). Under the same condition, STC-1
protein levels were also increased in the TNBC cells (Fig. 2B). Based on these results, we
demonstrated that the levels of STC-1 expression were the highest
in the TNBC cells among all the breast cancer cell types.
Induction of STC-1 expression enhances
the invasiveness of TNBC cells
To verify the effect of STC-1 on TNBC cells, Hs578T
and MDA-MB-231 TNBC cells were treated with 50 ng/ml STC-1 for 24
and 48 h, respectively. As shown in Fig. 3A, recombinant human STC-1
significantly increased the invasiveness of the TNBC cells. The
rates of cell invasion in response to STC-1 treatment were
increased by 180.38 and 231.53%, compared to the vehicle-treated
Hs578T and MDA-MB-231 cells, respectively (Fig. 3A). In contrast, we investigated
whether STC-1 siRNA overexpression suppresses the invasion ability
of TNBC cells. Hs578T and MDA-MB-231 cells were transfected with
scrambled or STC-1 siRNA for 48 h, respectively. We observed that
the levels of STC-1 mRNA and secreted protein were decreased by
STC-1 siRNA overexpression in the Hs578T and MDA-MB-231 TNBC cells.
The levels of STC-1 mRNA expression were decreased to
0.58±0.07-fold and 0.39±0.06-fold of the control level in the STC-1
siRNA overexpressed cells, respectively (Fig. 3B, left). Under the same conditions,
the levels of STC-1 secreted protein were decreased to
0.49±0.04-fold and 0.23±0.003-fold of the control level in the
STC-1 siRNA overexpressed cells, respectively (Fig. 3B, right). In addition, the
invasiveness of TNBC cells was completely suppressed by STC-1 siRNA
overexpression (Fig. 3C). The rates
of cell invasion by STC-1 silencing were decreased to 56.96 and
58.16% of the control Hs578T and MDA-MB-231 cells, respectively
(Fig. 3C). Based on these results,
the levels of STC-1 expression were directly associated with the
invasive ability of the TNBC cells.
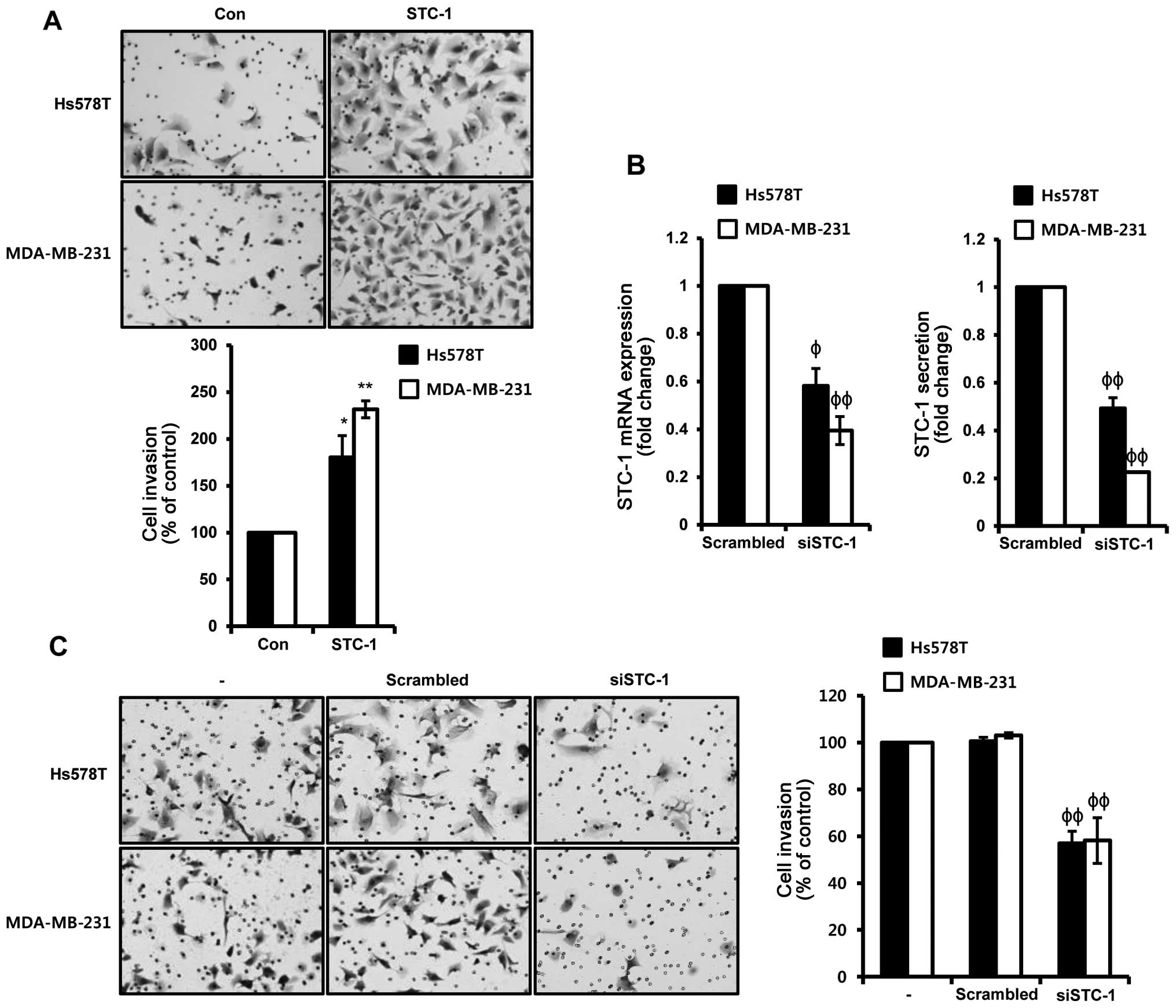 | Figure 3Induction of STC-1 expression enhances
the invasiveness of TNBC cells. (A) After serum starvation for 24
h, Hs578T and MDA-MB-231 TNBC cells were treated with 50 ng/ml
STC-1 for 24 and 48 h, respectively. Invasiveness of TNBC cells was
analyzed by Boyden chamber assay, as described in 'Materials and
methods'. (B and C) Hs578T and MDA-MB-231 TNBC cells were
transfected with scrambled and STC-1 siRNA for 48 h, respectively.
(B) The levels of STC-1 mRNA and protein secretion were analyzed by
real-time PCR (left) and ELISA (right), respectively, in scrambled
and STC-1 siRNA overexpressed cells. (C) After transfection for 48
h, invasiveness of scrambled and STC-1 siRNA overexpressed cells
was analyzed by Boyden chamber assay, as described in ῾Materials
and methods᾽. These results are representative of three independent
experiments. The values shown are the means ± SEM.
*P<0.05, **P<0.01 vs. control,
ϕP<0.05, ϕϕP<0.01 vs. scrambled siRNA
overexpressed cells. |
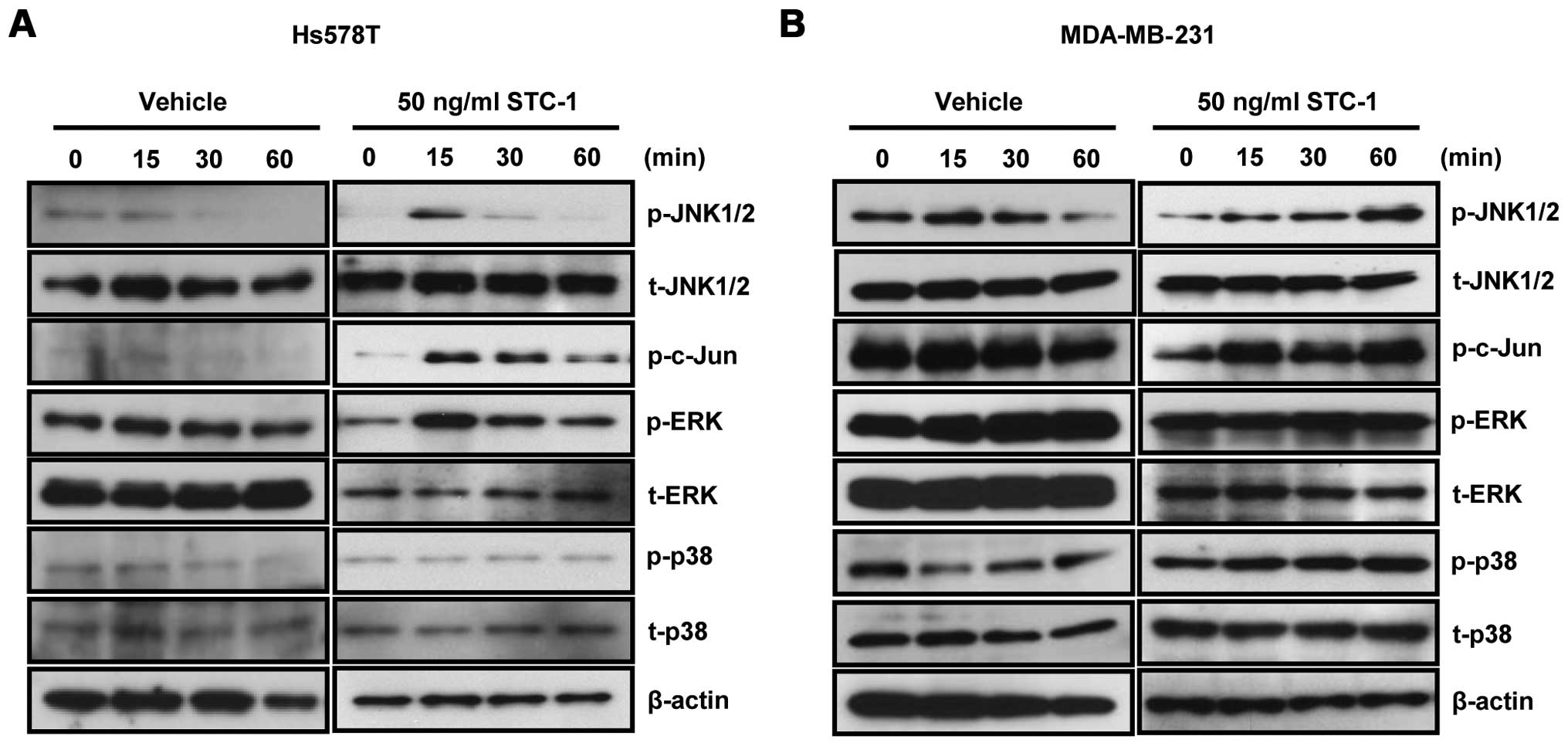
STC-1 activates the phosphorylation of
JNK/c-Jun in TNBC cells
Next, we investigated how JNK/c-Jun regulates the
invasiveness of TNBC cells in response to STC-1. After recombinant
human STC-1 treatment for the indicated time, whole cell lysates
were harvested for detecting the activities of signaling molecules
in the Hs578T and MDA-MB-231 TNBC cells. Our results showed that
exogenous STC-1 increased the phosphorylation of JNK and c-Jun in
both the Hs578T (Fig. 4A) and
MDA-MB-231 (Fig. 4B) TNBC cells.
The phosphorylation of JNK and c-Jun reached a maximum level 15 min
after STC-1 treatment in the Hs578T cells (Fig. 4A). We also observed that the
STC-1-induced phosphorylation of JNK and c-Jun was still activated
after 60 min in the MDA-MB-231 cells (Fig. 4B). The phosphorylation of ERK was
activated by STC-1 in the Hs578T cells, whereas the phosphorylation
of ERK was not activated by STC-1 in the MDA-MB-231 cells (Fig. 4A and B). The phosphorylation of p38
did not change in response to STC-1 treatment in both the Hs578T
and MDA-MB-231 cells (Fig. 4A and
B). Therefore, we demonstrated that STC-1-induced JNK/c-Jun
pathway activation may play an important role in the invasiveness
of TNBC cells.
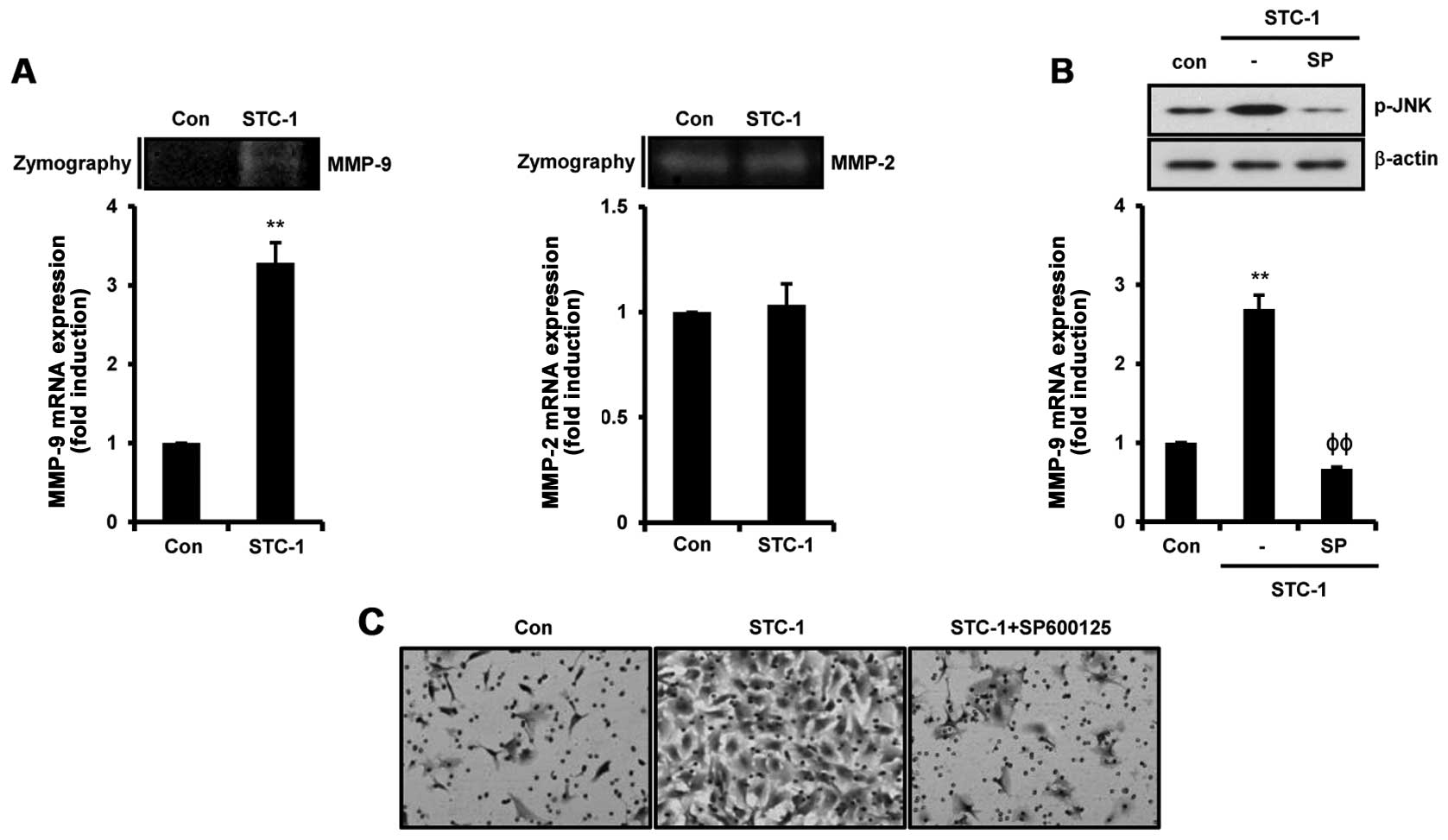
STC-1-induced cell invasion is suppressed
by SP600125 in Hs578T TNBC cells
To ascertain the alteration of invasion-related
genes such as MMPs by STC-1, we treated Hs578T TNBC cells with 50
ng/ml STC-1. As shown in Fig. 5A,
STC-1 stimulated the levels of MMP-9 expression but not MMP-2
expression. The levels of MMP-9 mRNA expression were increased by
3.28±0.26-fold from the control level following STC-1 treatment
(Fig. 5A). Under the same
condition, STC-1 induced MMP-9 protein expression in the Hs578T
TNBC cells (Fig. 5A). Next, we
pre-treated Hs578T TNBC cells with SP600125 for 1 h and then the
cells were treated with 50 ng/ml STC-1. As expected, we observed
that SP600125 completely suppressed STC-1-induced JNK
phosphorylation in the Hs578T TNBC cells (Fig. 5B). STC-1-induced MMP-9 mRNA
expression was decreased to 0.67±0.02-fold of the control level by
SP600125 (Fig. 5B). Furthermore, we
investigated whether the JNK/c-Jun signaling pathway is associated
with STC-1-induced cell invasion in the Hs578T TNBC cells. The
induction of cell invasiveness by STC-1 was also significantly
decreased by SP600125 (Fig. 5C).
Based on these results, we demonstrated that JNK/c-Jun signaling
activation by STC-1 triggers cell invasion through upregulation of
MMP-9.
Discussion
STC-1 is highly expressed in a variety of cancers
and has prognostic significance in colorectal and gastric cancers
(15,29). Abnormal STC-1 expression is
associated with poor prognosis in colorectal, esophageal, and
gastric cancer patients (15,29,30).
However, to date, the clinical significance of STC-1 expression in
breast cancer has not been well established. In this study, we
investigated the correlation between STC-1 expression and survival
of basal-type breast cancer patients as well as the regulatory
mechanism of STC-1 in cell invasion. Consistent with previous
reports, our results showed that high expression of STC-1 directly
influences relapse-free and overall survival of basal-type breast
cancer patients. However, the level of STC-1 expression had no
relevance in regards to the survival of luminal- and HER2-type
breast cancer patients (data not shown). Therefore, we demonstrated
that the STC-1 expression level may be a promising prognostic
marker in basal-type breast cancer patients.
STC-1 is involved with various biological events
such as cell proliferation, migration, invasion, apoptosis, and
inflammation in colon, gastric, and cervical cancer (14,16,31,32).
In a recent study, we reported that the motility of TNBC cells such
as invasion and migration was higher than these abilities in
non-TNBC cells through TGF-β1 and -β2 expression (33). Here, we also observed that the
levels of STC-1 mRNA and protein expression were significantly
increased in the TNBC cells compared with these levels in the
non-TNBC cells. Furthermore, our results showed that the
invasiveness of TNBC cells was significantly increased by
recombinant human STC-1 treatment. In contrast, STC-1 siRNA
overexpression suppressed the invasiveness of the TNBC cells. Based
on these reports, we demonstrated that STC-1 expression was
directly associated with cell invasion in a variety of tumor cells
including breast cancer cells.
Abnormal activities of intracellular signaling
molecules such as mitogen-activated protein kinases (MAPKs) in
response to extracellular stimuli regulate a variety of cellular
activities including cell proliferation, invasion, and
transformation (34). Activated
MAPKs phosphorylate various substrate proteins including
transcription factors such as c-Jun, ATF2, and p53 (34,35).
Activation of the JNK/c-Jun signaling pathway is involved with cell
invasion and metastasis in ovarian and glioma cancer (27,28).
Heterotrimeric G protein, G12, also enhanced cell invasion through
the activation of JNK in breast cancer cells (25). Consistent with these reports, we
found that STC-1 activated the phosphorylation of JNK and c-Jun in
the TNBC cells. In addition, STC-1-induced cell invasion was
completely suppressed by a specific JNK inhibitor, SP600125.
Therefore, we demonstrated that the STC-1-induced cell invasion was
regulated through the JNK/c-Jun-dependent signaling pathway in the
TNBC cells.
The aim of this study was to investigate the
clinical significance of STC-1 and the regulatory mechanism of
STC-1-induced TNBC cell invasion. Aberrant STC-1 expression was
associated with poor prognosis in basal-type breast cancer
patients. The levels of STC-1 expression and the cell invasiveness
were significantly increase in the TNBC cells when compared with
that noted in the non-TNBC cells. On the other hand, cell
invasiveness of TNBC was decreased by STC-1 siRNA overexpression.
Furthermore, STC-1 induced the phosphorylation of JNK and c-Jun in
the Hs578T and MDA-MB-231 TNBC cells and then augmented the
invasive capacity of the TNBC cells. STC-1-induced cell invasion
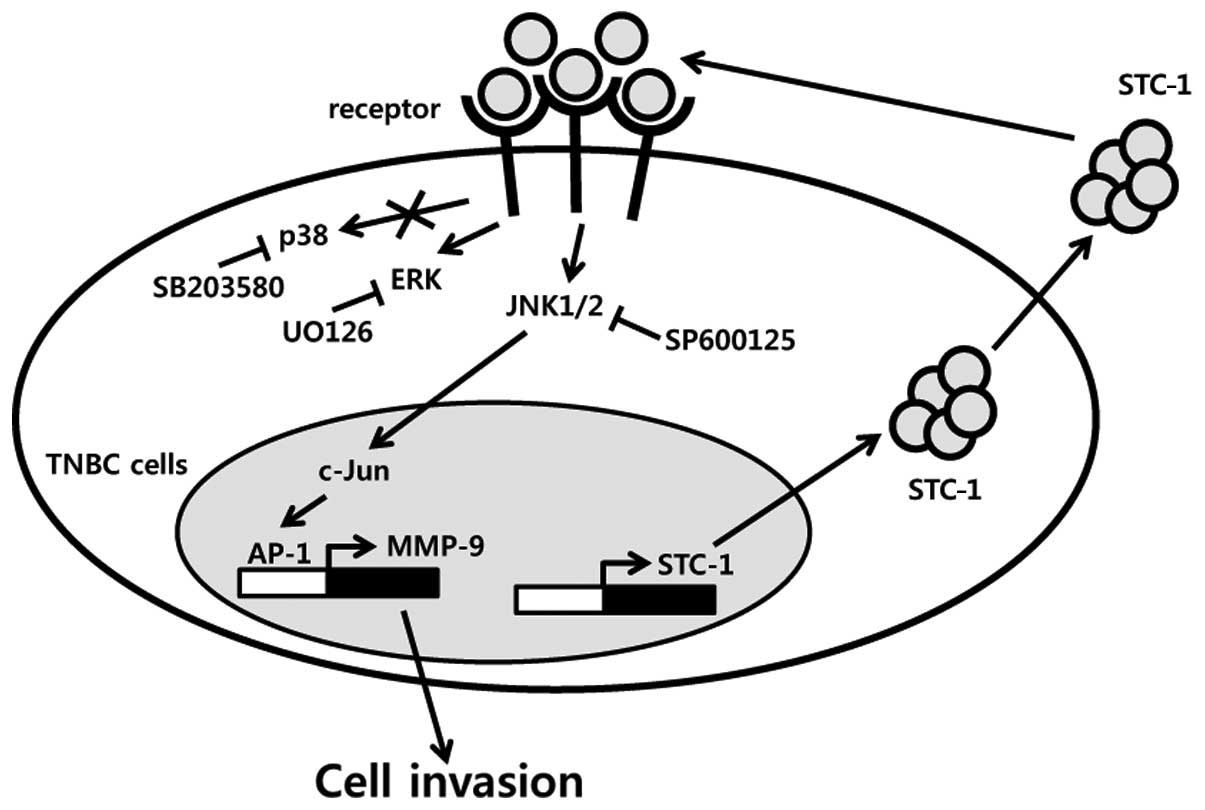
was suppressed by SP600125 in the TNBC cells. As shown in Fig. 6, we demonstrated that STC-1 enhances
the levels of MMP-9 expression through the JNK/c-Jun-dependent
signaling pathway and then triggers cell invasion. Thus, we suggest
that STC-1 may be a promising therapeutic target for the treatment
of patients with TNBC tumors.
Acknowledgments
This research was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education
(2016R1D1A1B01010508).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leclère B, Molinié F, Trétarre B, Stracci
F, Daubisse-Marliac L and Colonna M; GRELL Working Group: Trends in
incidence of breast cancer among women under 40 in seven European
countries: A GRELL cooperative study. Cancer Epidemiol. 37:544–549.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Curtis C, Shah SP, Chin SF, Turashvili G,
Rueda OM, Dunning MJ, Speed D, Lynch AG, Samarajiwa S, Yuan Y, et
al METABRIC Group: The genomic and transcriptomic architecture of
2,000 breast tumours reveals novel subgroups. Nature. 486:346–352.
2012.PubMed/NCBI
|
|
4
|
Lehmann BD, Bauer JA, Chen X, Sanders ME,
Chakravarthy AB, Shyr Y and Pietenpol JA: Identification of human
triple-negative breast cancer subtypes and preclinical models for
selection of targeted therapies. J Clin Invest. 121:2750–2767.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stevens KN, Vachon CM and Couch FJ:
Genetic susceptibility to triple-negative breast cancer. Cancer
Res. 73:2025–2030. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cleator S, Heller W and Coombes RC:
Triple-negative breast cancer: Therapeutic options. Lancet Oncol.
8:235–244. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang AC, Jellinek DA and Reddel RR:
Mammalian stanniocalcins and cancer. Endocr Relat Cancer.
10:359–373. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoshiko Y and Aubin JE: Stanniocalcin 1 as
a pleiotropic factor in mammals. Peptides. 25:1663–1669. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yeung BH, Law AY and Wong CK: Evolution
and roles of stanniocalcin. Mol Cell Endocrinol. 349:272–280. 2012.
View Article : Google Scholar
|
|
11
|
Varghese R, Wong CK, Deol H, Wagner GF and
DiMattia GE: Comparative analysis of mammalian stanniocalcin genes.
Endocrinology. 139:4714–4725. 1998.PubMed/NCBI
|
|
12
|
Fujiwara Y, Sugita Y, Nakamori S, Miyamoto
A, Shiozaki K, Nagano H, Sakon M and Monden M: Assessment of
Stanniocalcin-1 mRNA as a molecular marker for micrometastases of
various human cancers. Int J Oncol. 16:799–804. 2000.PubMed/NCBI
|
|
13
|
Joensuu K, Heikkilä P and Andersson LC:
Tumor dormancy: Elevated expression of stanniocalcins in late
relapsing breast cancer. Cancer Lett. 265:76–83. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu G, Yang G, Chang B, Mercado-Uribe I,
Huang M, Zheng J, Bast RC, Lin SH and Liu J: Stanniocalcin 1 and
ovarian tumorigenesis. J Natl Cancer Inst. 102:812–827. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tamura S, Oshima T, Yoshihara K, Kanazawa
A, Yamada T, Inagaki D, Sato T, Yamamoto N, Shiozawa M, Morinaga S,
et al: Clinical significance of STC1 gene expression in patients
with colorectal cancer. Anticancer Res. 31:325–329. 2011.PubMed/NCBI
|
|
16
|
Block GJ, Ohkouchi S, Fung F, Frenkel J,
Gregory C, Pochampally R, DiMattia G, Sullivan DE and Prockop DJ:
Multipotent stromal cells are activated to reduce apoptosis in part
by upregulation and secretion of stanniocalcin-1. Stem Cells.
27:670–681. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He LF, Wang TT, Gao QY, Zhao GF, Huang YH,
Yu LK and Hou YY: Stanniocalcin-1 promotes tumor angiogenesis
through up-regulation of VEGF in gastric cancer cells. J Biomed
Sci. 18:392011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Du YZ, Gu XH, Li L and Gao F: The
diagnostic value of circulating stanniocalcin-1 mRNA in non-small
cell lung cancer. J Surg Oncol. 104:836–840. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: New insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liedtke C, Mazouni C, Hess KR, André F,
Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B,
Green M, et al: Response to neoadjuvant therapy and long-term
survival in patients with triple-negative breast cancer. J Clin
Oncol. 26:1275–1281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Egeblad M and Werb Z: New functions for
the matrix metal-loproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim S, Choi JH, Lim HI, Lee SK, Kim WW,
Cho S, Kim JS, Kim JH, Choe JH, Nam SJ, et al: EGF-induced MMP-9
expression is mediated by the JAK3/ERK pathway, but not by the
JAK3/STAT-3 pathway in a SKBR3 breast cancer cell line. Cell
Signal. 21:892–898. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han J, Bae SY, Oh SJ, Lee J, Lee JH, Lee
HC, Lee SK, Kil WH, Kim SW, Nam SJ, et al: Zerumbone suppresses
IL-1β-induced cell migration and invasion by inhibiting IL-8 and
MMP-3 expression in human triple-negative breast cancer cells.
Phytother Res. 28:1654–1660. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Westermarck J, Holmström T, Ahonen M,
Eriksson JE and Kähäri VM: Enhancement of fibroblast collagenase-1
(MMP-1) gene expression by tumor promoter okadaic acid is mediated
by stress-activated protein kinases Jun N-terminal kinase and p38.
Matrix Biol. 17:547–557. 1998. View Article : Google Scholar
|
|
25
|
Juneja J, Cushman I and Casey PJ: G12
signaling through c-Jun NH2-terminal kinase promotes breast cancer
cell invasion. PLoS One. 6:e260852011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bachmeier BE, Albini A, Vené R, Benelli R,
Noonan D, Weigert C, Weiler C, Lichtinghagen R, Jochum M and
Nerlich AG: Cell density-dependent regulation of matrix
metalloproteinase and TIMP expression in differently tumorigenic
breast cancer cell lines. Exp Cell Res. 305:83–98. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang MC, Chen CA, Chen PJ, Chiang YC,
Chen YL, Mao TL, Lin HW, Lin Chiang WH and Cheng WF: Mesothelin
enhances invasion of ovarian cancer by inducing MMP-7 through
MAPK/ERK and JNK pathways. Biochem J. 442:293–302. 2012. View Article : Google Scholar
|
|
28
|
Zhou X, Hua L, Zhang W, Zhu M, Shi Q, Li
F, Zhang L, Song C and Yu R: FRK controls migration and invasion of
human glioma cells by regulating JNK/c-Jun signaling. J Neurooncol.
110:9–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fang Z, Tian Z, Luo K, Song H and Yi J:
Clinical significance of stanniocalcin expression in tissue and
serum of gastric cancer patients. Chin J Cancer Res. 26:602–610.
2014.PubMed/NCBI
|
|
30
|
Shirakawa M, Fujiwara Y, Sugita Y, Moon
JH, Takiguchi S, Nakajima K, Miyata H, Yamasaki M, Mori M and Doki
Y: Assessment of stanniocalcin-1 as a prognostic marker in human
esophageal squamous cell carcinoma. Oncol Rep. 27:940–946.
2012.
|
|
31
|
Peña C, Céspedes MV, Lindh MB, Kiflemariam
S, Mezheyeuski A, Edqvist PH, Hägglöf C, Birgisson H, Bojmar L,
Jirström K, et al: STC1 expression by cancer-associated fibroblasts
drives metastasis of colorectal cancer. Cancer Res. 73:1287–1297.
2013. View Article : Google Scholar
|
|
32
|
Guo F, Li Y, Wang J, Li Y, Li Y and Li G:
Stanniocalcin1 (STC1) inhibits cell proliferation and invasion of
cervical cancer cells. PLoS One. 8:e539892013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim S, Lee J, Jeon M, Nam SJ and Lee JE:
Elevated TGF-β1 and -β2 expression accelerates the epithelial to
mesenchymal transition in triple-negative breast cancer cells.
Cytokine. 75:151–158. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Davis RJ: Signal transduction by the JNK
group of MAP kinases. Cell. 103:239–252. 2000. View Article : Google Scholar : PubMed/NCBI
|