Introduction
Glioma is a malignant brain tumor lacking effective
therapeutic strategies. Underlying biological pathogenic mechanisms
need to be explored to supply new targets for glioma treatment.
Gliomas are classified into four grades according to the World
Health Organization (WHO) (1).
Glioblastoma (GB) belonging to grade IV is the most malignant
glioma with a poor prognosis. Therefore in order to develop novel
therapeutic methods for GB, it is important to elucidate the
fundamental molecular mechanisms leading to glioma formation.
Transforming growth factor-β (TGF-β) is a
multi-functional cytokine regulating cell growth and
differentiation (2). TGF-β promotes
tumor growth in several ways, including regulating
epithelial-to-mesenchymal transition, changing immune response,
stimulating angiogenesis and promoting metastasis (3–6). TGF-β
signaling pathway has been identified to participate in glioma
progression by mediating cell proliferation and invasion (7,8). TGF-β
is overexpressed in glioma tissues in human patients compared with
normal tissues, which suggests TGF-β plays a critical role. In
glioma, TGF-β1 produced by infiltrating microglia enhances glioma
invasion (8). In addition, TGF-β1
prohibits tumor growth by upregulating miRNAs. It is reported that
TGF-β regulates miRNAs including miR-200, miR-205, miR-182, miR-21
and miR-31 (9–11). In TGF-β signaling pathway, SMAD2,
SMAD3, SMAD4 and SMAD7 are critical proteins (12). Alterations of SMAD proteins are
contributed to induce tumor cell proliferation (13). These proteins can be regulated by
many factors. Recently, microRNAs (miRNAs) were reported to be
involved in regulating SMAD proteins (14–16).
miRNAs are usually short non-coding and
single-stranded RNA containing 18–25 nucleotides, which regulate
expressions of many proteins. miRNAs suppress gene expression by
binding to target mRNAs at 3′ untranslated regions (3′UTRs). A
confirmed miRNA target in multiple genes. By this mechanism, miRNAs
are related to cell proliferation, invasion, differentiation and
apoptosis in tumors. Thus, dysfunction of miRNAs is involved in
various types of human cancers including glioma, and miRNAs can act
as both oncogenes and tumor inhibitors during carcinogenesis.
miR-205 is often disordered in various solid tumors.
miR-205 is overexpressed in non-small cell lung cancer and ovarian
cancer cells, promoting tumor progression and chemoresistance
(17–20). It can target the tumor suppressor
gene phosphatase and tensin homolog (PTEN), CYR61 and CTGF,
SH2-containing phosphoinositide 5-phosphatase 2 (SHIP2), then,
promoting tumor growth (21,22).
Markedly, miR-205 functions as a tumor inhibitor by targeting
oncogenes. miR-205 decreases breast cancer cells proliferation by
suppressing ErbB3 and VEGF-A, inhibits melanoma growth by targeting
E2F1, hampers renal carcinomas by degrading Src protein (23–25).
However, the effect of miR-205 on TGF-β1 signaling pathway in
glioma is not understood.
Expression and function of miR-195 in tumor
development is not confirmed. miR-195 level is reported to be
decreased in solid tumors and increased in adenomas (26), which suggests that miR-195 reveals
growth promotion or growth resistance in cancers. The roles of
miR-195 in glioma need to be elucidated by TGF-β1 stimulation.
Accordingly, it is pivotal to explore the biological functions and
molecular mechanisms of miR-205 and miR-195 in the environment with
high level of TGF-β1 to find a novel therapy for glioma.
Materials and methods
Ethics statement
All experiments were approved by the Ethics
Committee of Wuhan University. Glioma cancer tissues were obtained
from Inner Mongolia Autonomous Region People's Hospital, China.
Tissues from patients after surgical procedures were sent for
pathology diagnosis, and were then collected in TRIzol (Ambion,
Austin, TX, USA). Each patient voluntarily participated in the
present study. Non-cancer volunteers were patients without glioma,
but with other brain disease and needed pathological diagnosis.
Cells and reagents
Human GBM cell line U87 was purchased from the
American Type Culture Collection (ATCC; Manassas, VA, USA) (no.
HTB-14™) and was cultured in Dulbecco's modified Eagle's medium
(DMEM) supplemented with 10% of fetal bovine serum (FBS; Gibco,
Grand Island, NY, USA) at 37°C in a humidified chamber. Primers for
quantitative real-time PCR (qRT-PCR) were synthesized by Invitrogen
(Waltham, MA, USA). miR-205 and miR-195 mimics were purchased from
Ambion. Enzyme-linked immunosorbent assay (ELISA) kit of human
TGF-β1 was obtained from BioLegend (San Diego, CA, USA). Anti-SMAD2
mAb, anti-SMAD3 mAb, anti-SMAD4 mAb and anti-SMAD7 mAb were
purchased from Santa Cruz (Santa Cruz, CA, USA). SYBR-Green was
purchased from Toyobo (Satte City, Saitama, Japan). CellTiter
96® AQueous Non-Radioactive Cell Proliferation Assay kit
was obtained from Promega (Madison, WI, USA). TGF-β1 and its
inhibitor LY364947 were obtained from Sigma (St. Louis, MO,
USA).
Transfection
U87 cell line was used for transfection.
Oligonucleotides miR-205 or miR-195 mimics (miR-205 or miR-195
mimics), miRNA mimic negative control (mimic NC), miR-205 inhibitor
or miR-195 inhibitor, inhibitor negative control (inhibitor NC),
were all purchased from Ambion. Cells (2×105) were
cultured in 6-well plates for 24 h, and were then transfected with
100 nM miR-205 and miR-195 mimics, miR-205 and miR-195 inhibitors,
or corresponding controls using the jetPEI transfection kit
(Polyplus Transfection, New York, NY, USA) according to the
manufacturer's instructions. Transfection efficiency was determined
by qRT-PCR.
qRT-PCR
Total RNA was extracted as previously described
(27). Briefly, RNA was isolated
from tissues using TRIzol according to the manufacturer's
instructions. For quantification of miRNA, RNA samples were first
reverse-transcribed with miRNA-specific primers using TaqMan miRNA
reverse transcription kit and qRT-PCR was performed to detect
miR-205 and miR-195 using TaqMan microRNA assay kit and StepOne
Plus Real-Time PCR system (both from (Applied Biosystems, Grand
Island, NY, USA). U6 was used as internal control. The primers were
as follows: miR-205 forward, 5′-CTTGTCCTTCATTCCACCGGA-3′ and
reverse, 5′-TGCCGCCTGAACTTCACTCC-3′ (28); miR-195 forward,
5-GGGGAGCCAAAAGGGTCATCATCT-3′ and reverse,
5′-GAGGGGCCATCCACAGTCTTCT-3′ (29);
U6 forward, CTCGCTTCGGCAGCACA and reverse, AACGCTTCACGA ATTTGCGT
(27). Data were analyzed using the
2−ΔΔCt method.
To detect the effects of TGF-β1 on miR-205 and
miR-195, U87 cells were cultured in 6-well plates and treated with
0, 1, 2, 4 and 8 ng/ml of TGF-β1 for 12 h. mRNA levels of miR-205
and miR-195 were determined using qRT-PCR.
ELISA
TGF-β1 secreted into peripheral blood was detected
using a TGF-β1 ELISA kit (Invitrogen). The serum of glioma patients
and non-cancer volunteers were collected. TGF-β1 concentration was
assayed with ELISA kit following the manufacturer's instructions.
Absorbance detection was performed at 450 nm using a microplate
reader (Bio-Rad, Hercules, CA, USA).
Western blot analysis
Total cellular lysates were prepared with RIPA
buffer containing a 2.5% protease inhibitor cocktail, according to
the manual. Concentration of protein was quantified by a Bradford
assay. Then, 20 µg of the protein samples were separated by
SDS-PAGE (10%) and transferred onto polyvinylidene fluoride (PVDF)
membrane. SMAD proteins were detected by western blotting using
anti-SMAD2 mAb (1:1,000), anti-SMAD3 mAb (1:1,000), anti-SMAD4 mAb
(1:2,000) and anti-SMAD7 mAb (1:1,000), respectively. Horseradish
peroxidase-conjugated goat anti-mouse IgG (Pierce, Rockford, IL,
USA) was used as a secondary antibody. ECL detection systems (UVP,
Upland, CA, USA) were used for detection.
Luciferase activity assay
For pGL3-SMAD2-3′UTR and pGL3-SMAD7-3′UTR plasmid
construction, the SMAD2-3′UTR containing miR-205 binding site and
SMAD7-3′UTR containing miR-195 binding site were amplified and
cloned into dual-reporter vector pGL3. The PCR primers were
designed according to DNA sequence in Gene Bank. KpnI and
XhoI were introduced in the primers for inserting pGL3
vector. 3′UTR of SMAD2 (Gene Bank no. NM_005901.5) were as follows:
P1, 5′-ATGCGGTACCAGCTTCACCAATCAAG-3′ and P2,
5′-GGTGCTCGAGATAACTACTACTGTTA-3′; 3′UTR of SMAD7 (Gene Bank no.
NM_001190821.1) P1, 5′-AATAGGTACCACCGCGTGCGGAGGGGA-3′ and P2,
5′-GGTACTCGAGATACACTGTGTTTGGT-3′.
The pGL3-SMAD3-3′UTR and pGL3-SMAD4-3′UTR plasmids
which contained full length of SMAD3 and SMAD4 3′UTR were purchased
from iGene Biotechnology, Co. (Shanghai, China). For miR-205
binding detection, U87 cells were transiently co-transfected with
pGL3-SMADs-3′UTR and either miR-205 mimics/inhibitor or negative
control. For miR-195 binding detection, U87 cells were transiently
co-transfected with pGL3-SMADs-3′UTR and either miR-195
mimics/inhibitor or negative control. pGL3-basic vector was used as
a negative control. After 12 h, a dual-luciferase reporter assay
system was used to measure luciferase activity according to the
manufacturer's instructions (Promega).
Co-immunoprecipitation (Co-IP)
assays
Co-IP assays were performed using the Co-IP kit
(Thermo Scientific, Waltham, MA, USA) according to the
manufacturer′s protocol. U87 cells were transfected with miR-205
mimic, miR-205 mimic NC, miR-205 inhibitor, miR-205 inhibitor NC,
or miR-195 mimic, miR-195 mimic NC, miR-195 inhibitor and miR-195
inhibitor NC, respectively. Total cellular protein extractions from
the cells were immunoprecipitated with anti-SMAD4 mAb. Anti-p-SMAD2
mAb was used as the detecting antibody by immunoblotting. The
samples were analyzed using the western blotting procedures as
described above.
Colony formation assay
Colony formation assay was used to study the effects
of miR-205 and miR-195 on cell proliferation. After miRNA mimics,
inhibitors and corresponding control transfection, the cells were
seeded at a density of 20 cells/well into a 6-well plate and were
cultured for 7 days in DMEM with 10% FBS at 37°C with 5%
CO2. Colonies that formed were fixed with 4% of
paraformaldehyde for 5 min at room temperature and then stained
with 0.1% crystal violet for 30 sec. The number of colonies was
counted. All the experiments were repeated in triplicate.
Cell invasion assay
Cell migration and invasion assays were performed in
24-well plates using BioCoat™ chambers with 8-µm pore size
(BD Biosciences, San Diego, CA, USA). Forty-eight hours
post-transfection, 5×104 of U87 cells in 500 µl
of serum-free medium were transferred into the upper chamber that
were coated with Matrigel™ matrix for the invasion assays. Complete
medium (500 µl) with 10% FBS was used as a chemoattractant
in the lower chamber. After 48 h of incubation, the cells in the
upper chamber were removed, while the invaded cells were fixed with
4% of paraformaldehyde, stained with 0.1% crystal violet and
counted. All the experiments were repeated in triplicate.
Cell viability detection
To investigate the effects of miR-205 and miR-195 on
glioma cell proliferation, U87 cells were transfected with miRNAs
and controls and kept on culturing for 72 h. Viability of
transfected U87 cells was determined using CellTiter 96®
AQueous Non-Radioactive Cell Proliferation Assay kit according to
the manufacturer's instructions. Absorbance at optical density (OD)
490 nm was determined using a Thermo Scientific Multiskan GO full
wavelength microplate reader.
TGF-β1 has different roles in different cell growth.
To further detect the effect of TGF-β1 on U87 cell proliferation,
invasion and viability, TGF-β1 combined with or without its
inhibitor LY364947 was used to treat U87 cells, the cell
proliferation, invasion and viability were determined according to
the previous protocol.
Results
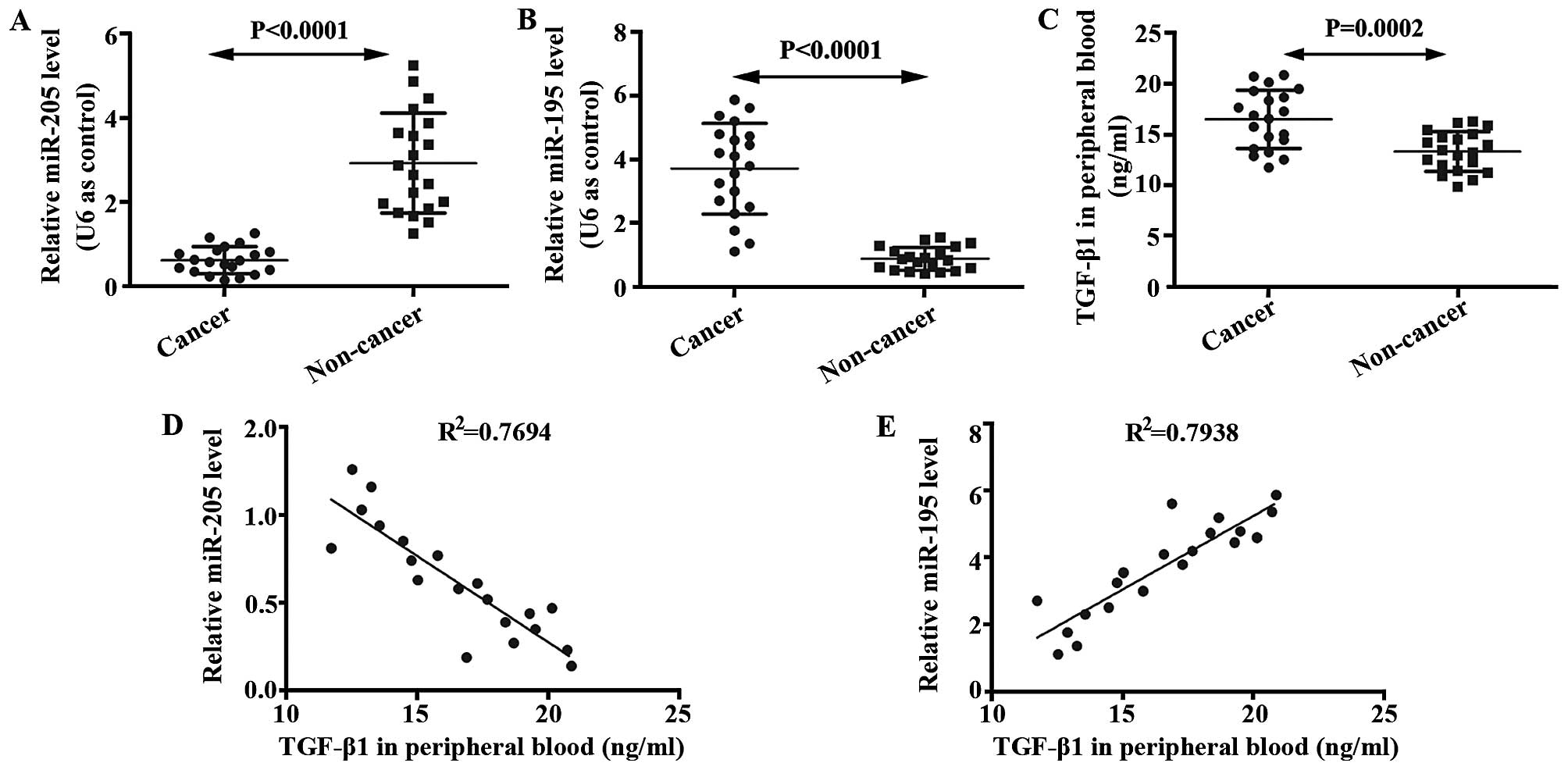
TGF-β1 is negatively correlated with
miR-205, but positively correlated with miR-195 in patients with
brain glioma
We determined expressions of miR-205 and miR-195 in
glioma brain and non-cancer tissues using qRT-PCR. mRNA level of
miR-205 in glioma tissue was lower than that in the non-cancer
tissue (Fig. 1A). In addition, mRNA
level of miR-195 in glioma tissue was higher than that in the
non-cancer tissue (Fig. 1B). ELISA
was performed to analyze levels of TGF-β1 in the serum of glioma
patients and non-cancer volunteers. It was shown that TGF-β1 was
enhanced in glioma patients compared with non-cancer volunteers
(Fig. 1C). The relationships
between miR-205 and TGF-β1, between miR-195 and TGF-β1, were
studied by correlation analysis. As shown in Fig. 1D and E, TGF-β1 is negatively
correlated with miR-205, but positively correlated with miR-195 in
patients with brain glioma.
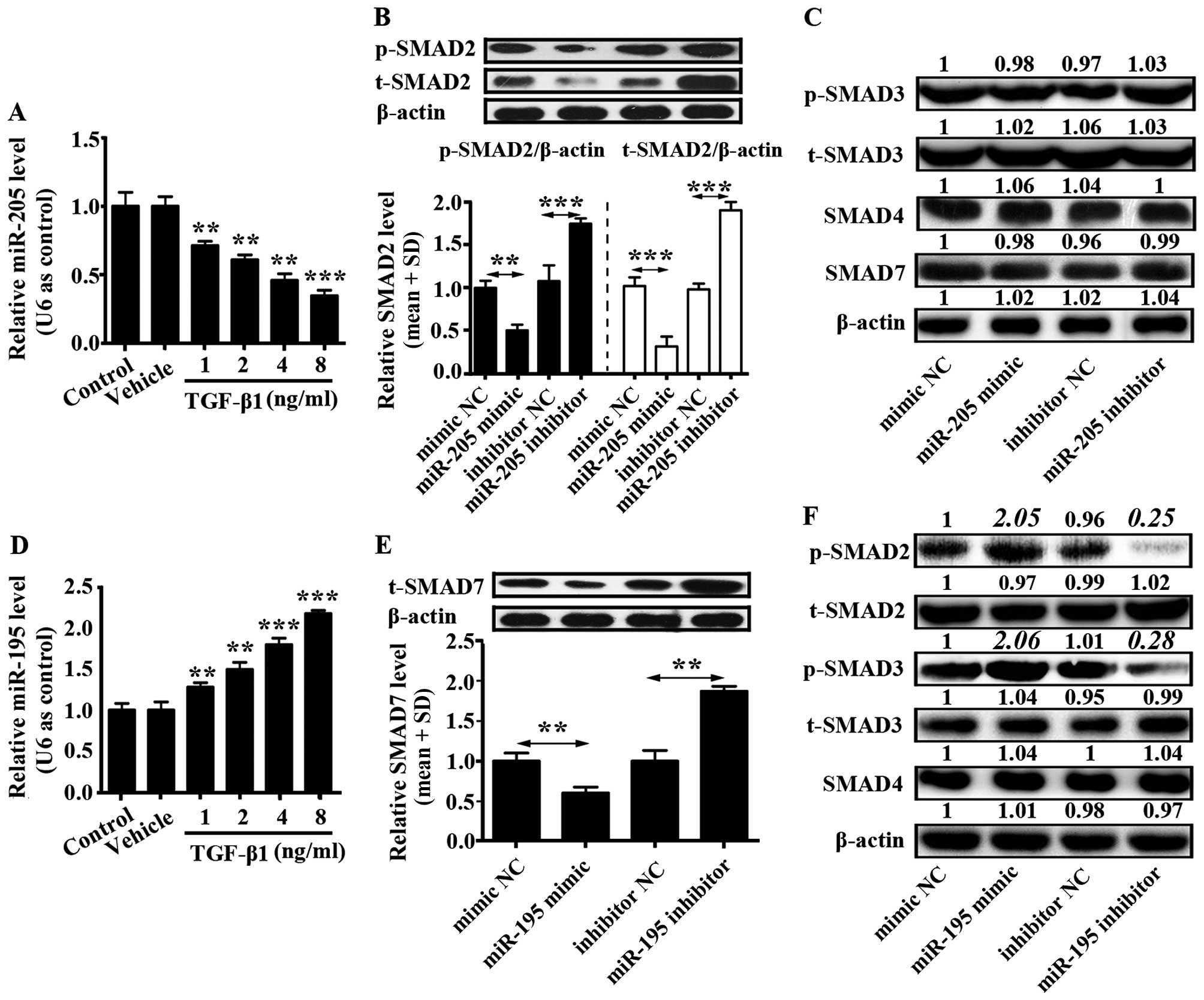
TGF-β1 inhibits miR-205 expression and
promotes miR-195 expression in U87 cells
U87 cells (2×105) were seeded into a
6-well plate and cultured in complete DMEM for 12 h. Then,
different concentrations of TGF-β1 (1, 2, 4 and 8 ng/ml) were added
into U87 cells. Control group was only U87 cells and to vehicle
group was added phosphate-buffered saline (PBS) into the U87 cells.
The mRNA level of miR-205 was downregulated by TGF-β1 in a
concentration-dependent manner, while the mRNA level of miR-195 was
upregulated by TGF-β1 in a concentration-dependent manner (Fig. 2A and D). In addition, effects of
miR-205 and miR-195 on TGF-β1 signaling pathway were separately
examined. U87 cells were transfected with miR-205 and miR-195
mimics, miR-205 and miR-195 inhibitors, or corresponding controls,
and western blotting was used to detect the protein level of SMAD2,
phosphorylated SMAD2 (p-SMAD2), SMAD3, phosphorylated SMAD3
(p-SMAD3), SMAD4 and SMAD7.
It was demonstrated that miR-205 prohibited SMAD2
and phosphorylation of SMAD2 expression, but did not affect levels
of SMAD3, p-SMAD3, SMAD4 and SMAD7 (Fig. 2B and C). miR-205 inhibitor increased
expression of SMAD2 and phosphorylation of SMAD2 (Fig. 2B). Otherwise, miR-195 inhibited the
expression of SMAD7 which reduced the expression of p-SMAD2 and
p-SMAD3. However, there was no influence on the expression of
t-SMAD2, t-SMAD3 and SMAD4 (Fig. 2E and
F). In addition, miR-195 inhibitor enhanced expression of SMAD7
in U87 cells (Fig. 2E). These
findings indicated that TGF-β1 signaling pathway could be activated
by miR-195 inhibiting SMAD7 expression, and this pathway could be
suppressed by miR-205 inhibiting SMAD2 and phosphorylation of SMAD2
in glioma cells.
SMAD2 is a target of miR-205, and SMAD7
is a target of miR-195 in glioma
miR-205 and miR-195 were predicted to act with 3′UTR
of SMAD2 and 3′UTR of SMAD7, respectively using TargetScan software
(Fig. 3A and B). Luciferase
activity assay was performed to verify the functions of miR-205 and
miR-195 on SMAD2 and SMAD7. We found that miR-205 enormously
decreased the luciferase activity of SMAD2 3′UTR in U87 cells
(Fig. 3C). In addition, there was
no effect on luciferase activity of 3′UTR of SMAD3, SMAD4 and SMAD7
(Fig. 3E). Moreover, high
expression of miR-205 significantly inhibited the heteromer
formation of SMAD2 and SMAD4 (Fig. 3G
and I). This result hinted that miR-205 suppressed TGF-β1
signal pathway in glioma directly by inhibiting the heteromers
formation of SMAD2 and SMAD4.
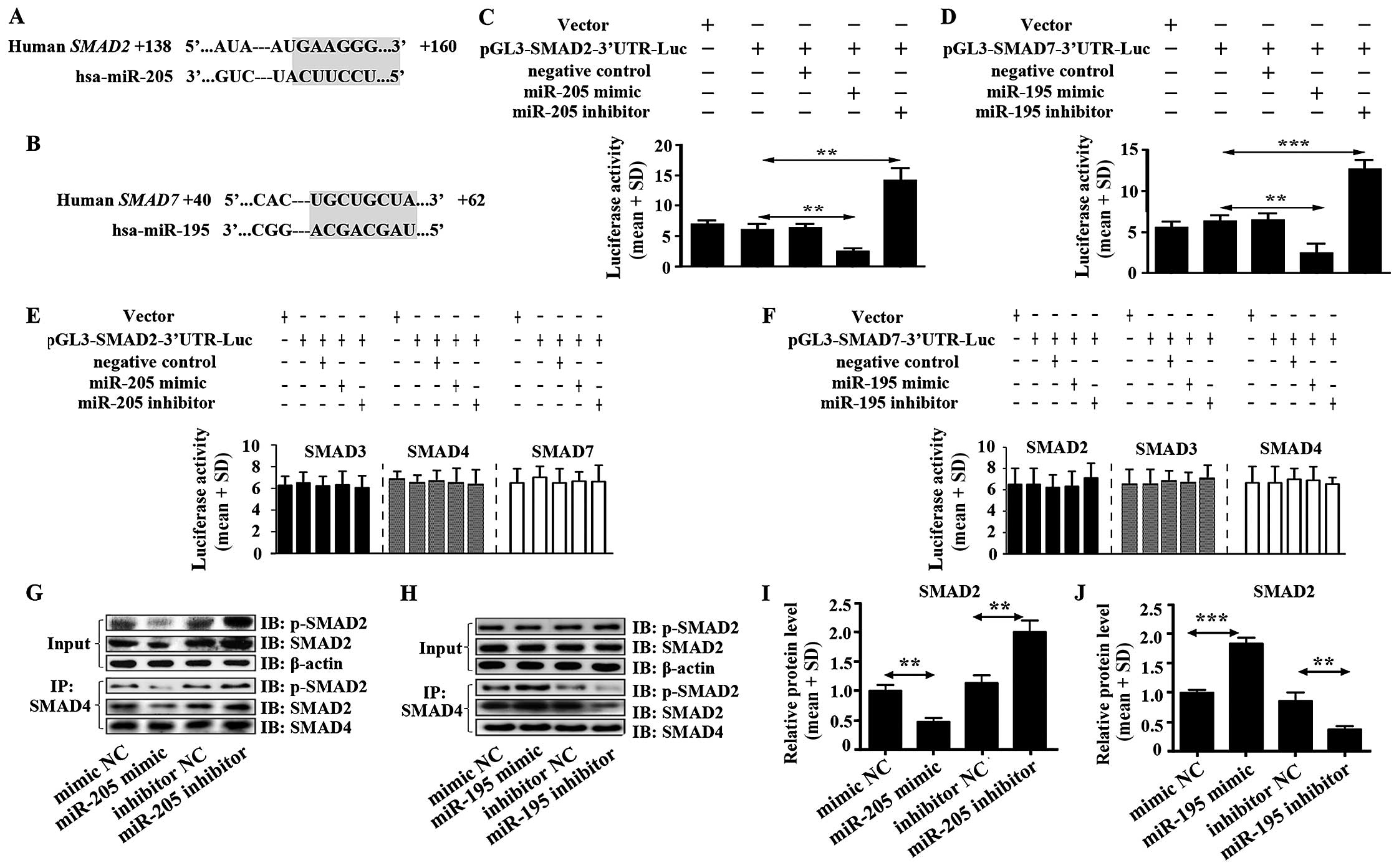 | Figure 3miR-205 targets SMAD2 and miR-195
targets SMAD7. (A) miR-205 was predicted to target 3′UTR of SMAD2
using TargetScan software. (B) miR-195 was predicted to target
3′UTR of SMAD7 using TargetScan software. (C) The luciferase
activity in U87 cells was measured after co-transfection with the
indicated SMAD2 3′UTR plasmid and miR-205 mimic, miR-205 inhibitor
or corresponding negative control for 12 h. (D) The luciferase
activity of U87 cells was measured after co-transfection with the
indicated SMAD7 3′UTR plasmid and miR-195 mimic, miR-195 inhibitor
or corresponding negative control for 12 h. (E) Effect of miR-205
mimic, miR-205 inhibitor on SMAD3, 4, 7-3′UTR luciferase activity
in U87 cells. (F) Influence of miR-195 mimic, miR-195 inhibitor on
SMAD2, 3, 4-3′UTR luciferase activity in U87 cells. (G) Heteromer
formation of p-SMAD2 and SMAD4 was detected by Co-IP in U87 cells
transfected with miR-205 mimic, miR-205 inhibitor or corresponding
negative controls. (I) Heteromers of p-SMAD2 and SMAD4 relative
expression in miR-205 transfected cells was normalized based on
input according to the immunoblotting. (H) Heteromer formation of
p-SMAD2 and SMAD4 was detected by Co-IP in U87 cells transfected
with miR-195 mimic, miR-195 inhibitor or corresponding negative
controls. (J) Heteromers of p-SMAD2 and SMAD4 relative expression
in miR-195 transfected cells was normalized based on input
according to immunoblotting. All data shown are the means ± SD of
three independent experiments; *p<0.05,
**p<0.01. |
miR-195 inhibited extremely the luciferase activity
of SMAD7 3′UTR in U87 cells (Fig.
3D). It was also shown that there was no function of miR-195 on
SMAD2, SMAD3 and SMAD4 (Fig. 3F).
High expression of miR-195 did not suppress heteromer formation of
SMAD2 and SMAD4 but increased heteromer formation (Fig. 3H and J), which suggested that
miR-195 could not inhibit TGF-β1 signal pathway but stimulate the
pathway by promoting heteromer formation of SMAD2 and SMAD4 in
glioma. Mechanism of promoting heteromer formation of SMAD2 and
SMAD4 was contributed to miR-195 inhibiting SMAD7 expression
(Fig. 2D).
We also evaluated impact of the miR-205 and miR-195
blockade. The luciferase activity assay showed that miR-205 and
miR-195 inhibitors enhanced luciferase activity of SMAD2-3′UTR and
SMAD7-3′UTR, respectively, in U87 cells. miR-205 inhibitor but not
miR-195 inhibitor significantly increased the heteromer formation
of SMAD2 and SMAD4 (Fig. 3G–J).
miR-205 inhibits U87 cell proliferation
and invasion while miR-195 promotes U87 cell proliferation and
invasion
To evaluate the impact of miR-205 and miR-195 on U87
cell proliferation, cell colony and cell viability assays were
employed. The results showed that miR-205 decreased U87 cell
colonies and viability, but miR-195 increased U87 cell colonies and
viability (Fig. 4A–E).
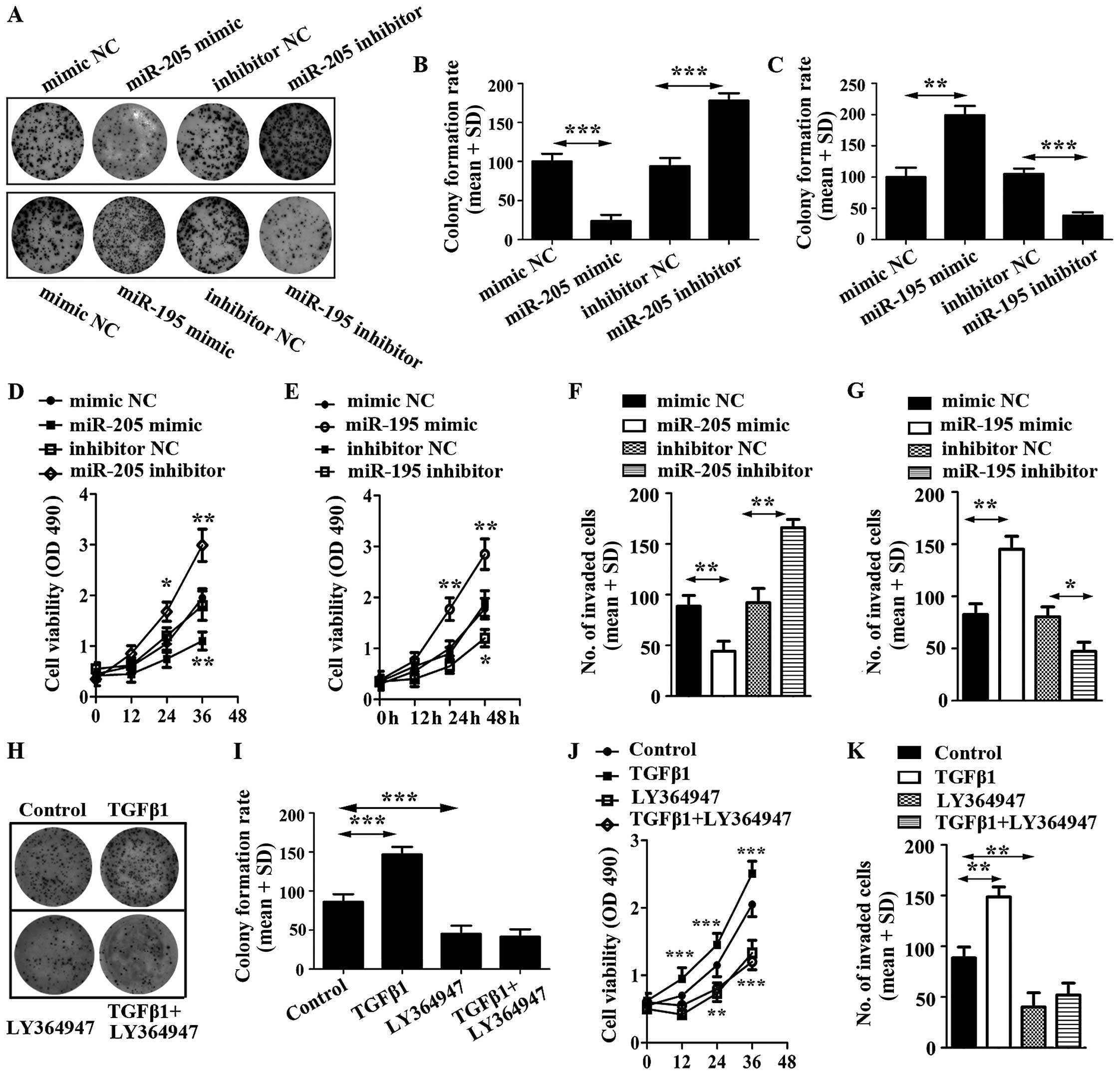 | Figure 4Effect of miR-205 mimic, miR-205
inhibitor and miR-195 mimic, miR-195 inhibitor on glioma cell
proliferation. (A) Proliferation of U87 transfected respectively
with miRNA mimic, inhibitor or control was detected and
photographed by cell colony formation assay. (B) Relative colony
formation rate was calculated in U87 cells transfected with miR-205
mimic, miR-205 inhibitor or corresponding controls. (C) Relative
colony formation rate was calculated in U87 cells transfected with
miR-195 mimic, miR-195 inhibitor or corresponding controls. (D)
Cell viability was detected in U87 cells transfected with miR-205
mimic, miR-205 inhibitor or corresponding controls. (E) Cell
viability was detected in U87 cells transfected with miR-195 mimic,
miR-195 inhibitor or corresponding controls. (F) Invasive ability
of U87 cells transfected with miR-205 mimic, miR-205 inhibitor or
corresponding controls was detected by Transwell invasion assay.
(G) Invasive ability of U87 cells transfected with miR-195 mimic,
miR-195 inhibitor or corresponding controls was detected by
Transwell invasion assay. (H) Proliferation of U87 cells treated
with TGF-β1 and its inhibitor, LY364947. (I) Relative colony
formation rate was calculated in U87 cells treated with TGF-β1 and
LY364947. (J) Cell viability was detected in U87 cells stimulated
by TGF-β1 and LY364947. (K) Cell invasion was determined in U87
cells stimulated by TGF-β1 and LY364947. |
To further explore the potential roles of miR-205
and miR-195 on glioma cells, we still overexpressed miR-205 and
miR-195 in U87 cells by transiently transfecting them with miRNAs
mimics. We then conducted cell invasion assays, which revealed that
miR-205 prohibited the invasive ability of U87 cells (Fig. 4F). In addition, miR-195 displayed a
potentiation in the invasive ability of U87 (Fig. 4G).
To corroborate the roles of miR-205 and miR-195 in
regulating cell activities, we characterized the effects of miR-205
and miR-195 blockade. We also transfected U87 cells with miR-205
and miR-195 inhibitors, and corresponding controls, respectively.
Using cell colony formation assay, cell viability detection and
cell invasion assay, it was found that the expression of
anti-miR-205 significantly enhanced proliferation and invasion of
U87; however, miR-195 inhibitor decreased proliferation and
invasion of U87 (Fig. 4)
To identify the effect of TGF-β1 on U87 cells, the
cells were stimulated with TGF-β and LY364947. The results
suggested that TGF-β1 promoted cell colony formation, increased the
viability of U87 cells and enhanced cell invasion. However,
LY364947, an inhibitor of TGF-β1, displayed a reverse function on
U87 cells and LY364947 efficiently inhibited cell proliferation
induced by TGF-β1 (Fig. 4 H–K). The
results indicated that inhibitor of TGF-β1 was able to repress the
function of miR-195.
Taken together, these observations revealed that
miR-205 functioned as a tumor inhibitor and miR-195 was possibly a
tumor promoter in U87 cells.
Discussion
Transforming growth factor-β (TGF-β) has been
suggested to regulate cellular growth, proliferation and
differentiation, and it exhibits a positive or negative function in
modulating tumor cells (30).
Classical TGF-β signaling is mediated by SMAD proteins. SMAD
proteins are divided into three groups: receptor-regulated SMADs
including SMAD1, 2, 3, 5 and 8, and common SMAD including SMAD4,
inhibitory SMAD including SMAD6 and 7 (31). TGF-β has been demonstrated to be
overexpressed in malignant glioma tissues, but undetected in normal
brain tissues.
miRNAs have recently been identified to be important
regulatory components in TGF-β signal pathway. In the present
study, we found that the mRNA level of miR-205 was decreased in
glioma patients, and miR-195 and TGF-β1 were both increased in
glioma patients. It was revealed that TGF-β1 was negatively
correlated with miR-205 mRNA level, but positively correlated with
miR-195 mRNA level. In addition, the mRNA level of miR-205 was
downregulated by TGF-β1 in a concentration-dependent manner, and
the mRNA level of miR-195 was upregulated by TGF-β1 in a
concentration-dependent manner.
miRNAs are small non-coding RNAs involved in tumor
initiation and progression. Numerous miRNAs can be regulated by
TGF-β1 and further influence the downstream of TGF-β signaling.
miR-146b is upregulated by TGF-β in a time-dependent manner in
intestinal epithelial cells (30).
miR-132 is also upregulated by TGF-β in glioma cell line U87
(32). In addition, miR-155, in
human fibroblasts, is downregulated by TGF-β. miR-182 can be
induced by TGF-β and directly target and suppress the 3′
untranslated regions (3′UTRs) of multiple genes that function as
negative regulators of NF-κB, leading to NF-κB hyperactivation and
aggressiveness of gliomas (33).
Herein, we found that miR-205 and miR-195 were mediated by TGF-β1
in glioma.
Functions of miR-205 in cancers are still vague
since it has been found to be upregulated or downregulated
targeting different genes (34,35).
In various tumors, miR-205 shows an anticancer effect, e.g.
prostate cancer, renal cancer and acute lymphoblastic leukemia
(36). Otherwise, miR-205 plays a
role as an oncogene, such as in nasopharyngeal carcinoma, and
cervical cancer (37). In the
present study, we found that elevated TGF-β1 induced miR-205
expression in U87 cells. Then, miR-205 targeting SMAD2 protein
inhibited heteromer formation of SMAD2 and SMAD4 and reduced
angiogenesis and tumor invasion. In contrast, miR-195 was promoted
by TGF-β1. miR-195 is related to tumor progression or inhibition.
Biological effects of miR-195 remain controversal. miR-195 is
reported to increase hepatocellular carcinoma and malignant
melanoma (38,39).
In the present study, it was found that miR-195
targeted 3′UTR of SMAD7 and blocked the inhibition of SMAD7. This
result is consistent with a study, that miR-195 downregulates the
SMAD7 level (40). miR-195
indirectly promoted TGF-β1 signal pathway and enhanced glioma cell
invasion and proliferation in U87 cells.
SMAD7 is not only a target gene of TGF-β1 but also
target of some inflammatory cytokines, such as IL-1, TNF-α and
IFN-γ. It is reported that SMAD7 expression is also induced by EGF,
laminar shear stress, UV irradiation and PMA (41). In general, SMAD7 gene expression is
controlled by the binding of nuclear active receptor regulated SMAD
(R-SMAD) complexes, mainly p-SMAD1-SMAD4 and p-SMAD2-SMAD4 to the
SMAD7 promoter region (42).
However, in male Sprague-Dawley rats, angiotensin II increased
phosphorylation of SMAD2 (p-SMAD2) in aortic protein, but did not
affect the level of SMAD7, which suggests that Smad7 expression can
be modulated by another mechanism besides Smad2, such as p-SMAD1
(43). In addition, in CCl4-induced
liver fibrosis rat model, TGF-β is upregulated and levels of
phospho-SMAD2/3 and its nuclear translocation are increased, but
SMAD7 is reduced in liver tissue (44). In the present study, miR-205
inhibited SMAD2, which did not influence SMAD7 expression. The
result suggested that SMAD7 was probably regulated by another
mechanism, than p-SMAD2 binding to SMAD7 promoter in glioma cell
line U87.
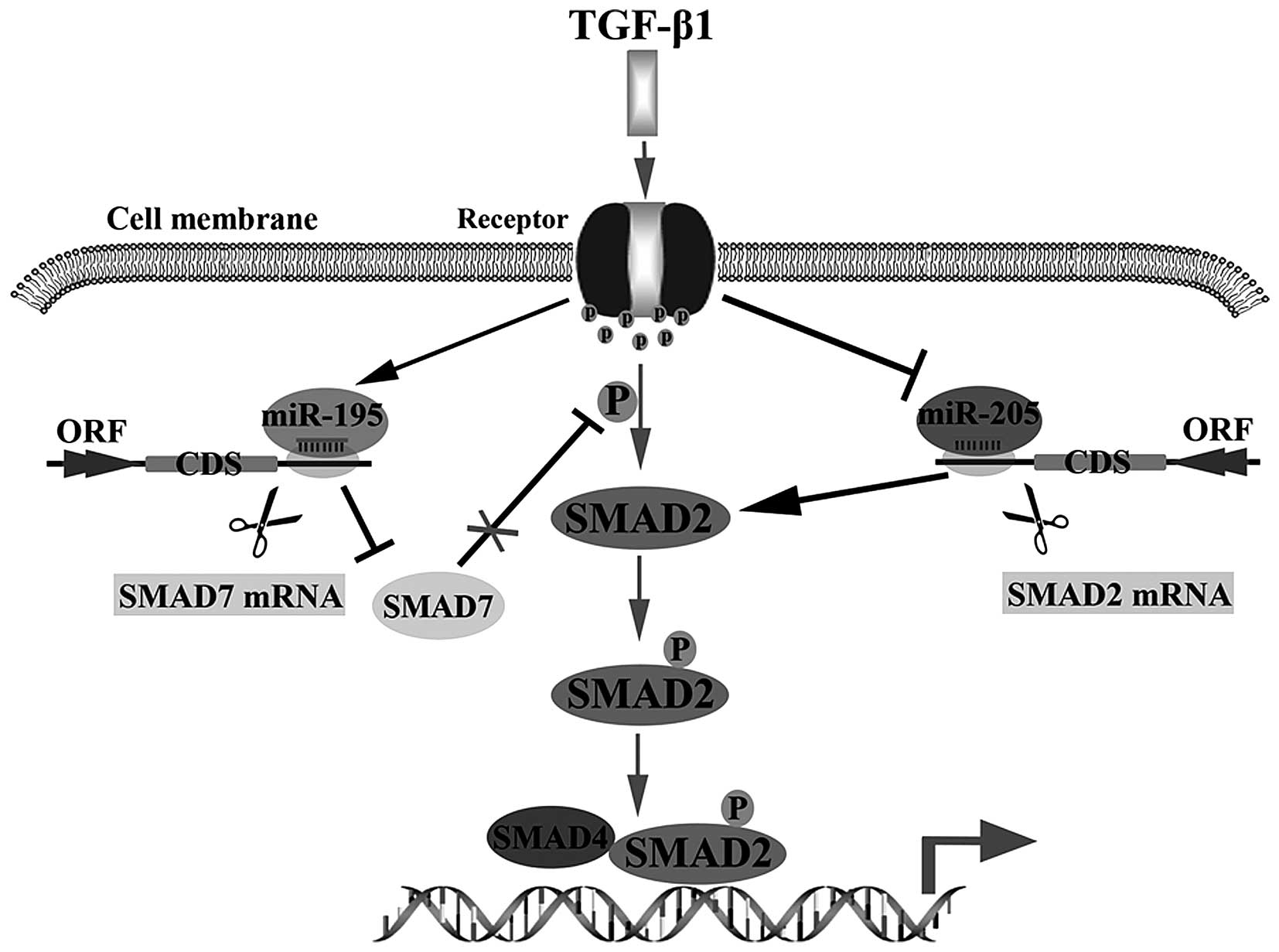
In summary, we concluded that miR-205 could be
inhibited by TGF-β1, but miR-195 could be stimulated by TGF-β1.
Then, enhancing the activation of TGF-β1 signal pathway via
inhibiting miR-205 targeting SMAD2 to reduce degradation of SMAD2
or promoting miR-195 targeting SMAD7 to increase p-SMAD2 in glioma
cells (Fig. 5). These findings
supply a potential therapeutic target for treating glioma.
Abbreviations:
|
TGF-β
|
transforming growth factor-β
|
|
UTR
|
untranslated region
|
|
miRNA
|
microRNA
|
|
GB
|
glioblastoma
|
|
FBS
|
fetal bovine serum
|
|
qRT-PCR
|
quantitative real-time PCR
|
References
|
1
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lawrence DA: Transforming growth
factor-beta: A general review. Eur Cytokine Netw. 7:363–374.
1996.PubMed/NCBI
|
|
3
|
Meulmeester E and Ten Dijke P: The dynamic
roles of TGF-β in cancer. J Pathol. 223:205–218. 2011. View Article : Google Scholar
|
|
4
|
Kang Y, Siegel PM, Shu W, Drobnjak M,
Kakonen SM, Cordón-Cardo C, Guise TA and Massagué J: A multigenic
program mediating breast cancer metastasis to bone. Cancer Cell.
3:537–549. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Flavell RA, Sanjabi S, Wrzesinski SH and
Licona-Limón P: The polarization of immune cells in the tumour
environment by TGFbeta. Nat Rev Immunol. 10:554–567. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Connolly EC, Freimuth J and Akhurst RJ:
Complexities of TGF-β targeted cancer therapy. Int J Biol Sci.
8:964–978. 2012. View Article : Google Scholar :
|
|
7
|
Alexandrow MG and Moses HL: Transforming
growth factor beta and cell cycle regulation. Cancer Res.
55:1452–1457. 1995.PubMed/NCBI
|
|
8
|
Wesolowska A, Kwiatkowska A, Slomnicki L,
Dembinski M, Master A, Sliwa M, Franciszkiewicz K, Chouaib S and
Kaminska B: Microglia-derived TGF-beta as an important regulator of
glioblastoma invasion - an inhibition of TGF-beta-dependent effects
by shRNA against human TGF-beta type II receptor. Oncogene.
27:918–930. 2008. View Article : Google Scholar
|
|
9
|
Brabletz S and Brabletz T: The ZEB/miR-200
feedback loop - a motor of cellular plasticity in development and
cancer? EMBO Rep. 11:670–677. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cottonham CL, Kaneko S and Xu L: miR-21
and miR-31 converge on TIAM1 to regulate migration and invasion of
colon carcinoma cells. J Biol Chem. 285:35293–35302. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saito A, Suzuki HI, Horie M, Ohshima M,
Morishita Y, Abiko Y and Nagase T: An integrated expression
profiling reveals target genes of TGF-β and TNF-α possibly mediated
by microRNAs in lung cancer cells. PLoS One. 8:e565872013.
View Article : Google Scholar
|
|
12
|
Katz LH, Li Y, Chen JS, Muñoz NM, Majumdar
A, Chen J and Mishra L: Targeting TGF-β signaling in cancer. Expert
Opin Ther Targets. 17:743–760. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seoane J, Le HV, Shen L, Anderson SA and
Massagué J: Integration of Smad and forkhead pathways in the
control of neuroepithelial and glioblastoma cell proliferation.
Cell. 117:211–223. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Louafi F, Martinez-Nunez RT and
Sanchez-Elsner T: MicroRNA-155 targets SMAD2 and modulates the
response of macrophages to transforming growth factor-β. J Biol
Chem. 285:41328–41336. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brower JV, Clark PA, Lyon W and Kuo JS:
MicroRNAs in cancer: Glioblastoma and glioblastoma cancer stem
cells. Neurochem Int. 77:68–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Leeper NJ, Raiesdana A, Kojima Y, Chun HJ,
Azuma J, Maegdefessel L, Kundu RK, Quertermous T, Tsao PS and Spin
JM: MicroRNA-26a is a novel regulator of vascular smooth muscle
cell function. J Cell Physiol. 226:1035–1043. 2011. View Article : Google Scholar :
|
|
17
|
Markou A, Tsaroucha EG, Kaklamanis L,
Fotinou M, Georgoulias V and Lianidou ES: Prognostic value of
mature microRNA-21 and microRNA-205 overexpression in non-small
cell lung cancer by quantitative real-time RT-PCR. Clin Chem.
54:1696–1704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li J, Li L, Li Z, Gong G, Chen P, Liu H,
Wang J, Liu Y and Wu X: The role of miR-205 in the VEGF-mediated
promotion of human ovarian cancer cell invasion. Gynecol Oncol.
137:125–133. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao P, Zhou L, Zhang J, Zheng F, Wang H,
Ma D and Tian J: Comprehensive expression profiling of microRNAs in
laryngeal squamous cell carcinoma. Head Neck. 35:720–728. 2013.
View Article : Google Scholar
|
|
20
|
Nam EJ, Lee M, Yim GW, Kim JH, Kim S, Kim
SW and Kim YT: MicroRNA profiling of a CD133+
spheroid-forming subpopulation of the OVCAR3 human ovarian cancer
cell line. BMC Med Genomics. 5:182012. View Article : Google Scholar
|
|
21
|
Greene SB, Herschkowitz JI and Rosen JM:
The ups and downs of miR-205: Identifying the roles of miR-205 in
mammary gland development and breast cancer. RNA Biol. 7:300–304.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu J, Ryan DG, Getsios S,
Oliveira-Fernandes M, Fatima A and Lavker RM: MicroRNA-184
antagonizes microRNA-205 to maintain SHIP2 levels in epithelia.
Proc Natl Acad Sci USA. 105:19300–19305. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu H, Zhu S and Mo YY: Suppression of cell
growth and invasion by miR-205 in breast cancer. Cell Res.
19:439–448. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dar AA, Majid S, de Semir D, Nosrati M,
Bezrookove V and Kashani-Sabet M: miRNA-205 suppresses melanoma
cell proliferation and induces senescence via regulation of E2F1
protein. J Biol Chem. 286:16606–16614. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Majid S, Saini S, Dar AA, Hirata H,
Shahryari V, Tanaka Y, Yamamura S, Ueno K, Zaman MS, Singh K, et
al: MicroRNA-205 inhibits Src-mediated oncogenic pathways in renal
cancer. Cancer Res. 71:2611–2621. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jia LF, Wei SB, Gong K, Gan YH and Yu GY:
Prognostic implications of micoRNA miR-195 expression in human
tongue squamous cell carcinoma. PLoS One. 8:e566342013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu J and Li Y: Trichostatin A and
Tamoxifen inhibit breast cancer cell growth by miR-204 and ERα
reducing AKT/mTOR pathway. Biochem Biophys Res Commun. 467:242–247.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bai J, Zhu X, Ma J and Wang W: miR-205
regulates A549 cells proliferation by targeting PTEN. Int J Clin
Exp Pathol. 8:1175–1183. 2015.PubMed/NCBI
|
|
29
|
Wang Y, Chen H, Fu Y, Ai A, Xue S, Lyu Q
and Kuang Y: MiR-195 inhibits proliferation and growth and induces
apoptosis of endometrial stromal cells by targeting FKN. Int J Clin
Exp Pathol. 6:2824–2834. 2013.PubMed/NCBI
|
|
30
|
Liao Y, Zhang M and Lönnerdal B: Growth
factor TGF-β induces intestinal epithelial cell (IEC-6)
differentiation: miR-146b as a regulatory component in the negative
feedback loop. Genes Nutr. 8:69–78. 2013. View Article : Google Scholar
|
|
31
|
Gratchev A: TGF-β signalling in tumour
associated macrophages. Immunobiology. S0171–2985:30096. 2016.
|
|
32
|
Wang ZH, Zhang QS, Duan YL, Zhang JL, Li
GF and Zheng DL: TGF-β induced miR-132 enhances the activation of
TGF-β signaling through inhibiting SMAD7 expression in glioma
cells. Biochem Biophys Res Commun. 463:187–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Song L, Liu L, Wu Z, Li Y, Ying Z, Lin C,
Wu J, Hu B, Cheng SY, Li M, et al: TGF-β induces miR-182 to sustain
NF-κB activation in glioma subsets. J Clin Invest. 122:3563–3578.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Iorio MV, Visone R, Di Leva G, Donati V,
Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, et
al: MicroRNA signatures in human ovarian cancer. Cancer Res.
67:8699–8707. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sempere LF, Christensen M, Silahtaroglu A,
Bak M, Heath CV, Schwartz G, Wells W, Kauppinen S and Cole CN:
Altered MicroRNA expression confined to specific epithelial cell
subpopulations in breast cancer. Cancer Res. 67:11612–11620. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dou L, Li J, Zheng D, Li Y, Gao X, Xu C,
Gao L, Wang L and Yu L: MicroRNA-205 downregulates
mixed-lineage-AF4 oncogene expression in acute lymphoblastic
leukemia. Onco Targets Ther. 6:1153–1160. 2013.PubMed/NCBI
|
|
37
|
Xie H, Zhao Y, Caramuta S, Larsson C and
Lui WO: miR-205 expression promotes cell proliferation and
migration of human cervical cancer cells. PLoS One. 7:e469902012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Amer M, Elhefnawi M, El-Ahwany E, Awad AF,
Gawad NA, Zada S and Tawab FM: Hsa-miR-195 targets PCMT1 in
hepa-tocellular carcinoma that increases tumor life span. Tumour
Biol. 35:11301–11309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bhattacharya A, Schmitz U, Wolkenhauer O,
Schönherr M, Raatz Y and Kunz M: Regulation of cell cycle
checkpoint kinase WEE1 by miR-195 in malignant melanoma. Oncogene.
32:3175–3183. 2013. View Article : Google Scholar
|
|
40
|
Chen G, Cao S, Liu F and Liu Y: miR-195
plays a role in steroid resistance of ulcerative colitis by
targeting Smad7. Biochem J. 471:357–367. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yan X and Chen YG: Smad7: Not only a
regulator, but also a cross-talk mediator of TGF-β signalling.
Biochem J. 434:1–10. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nicklas D and Saiz L: Computational
modelling of Smad-mediated negative feedback and crosstalk in the
TGF-β superfamily network. J R Soc Interface. 10:201303632013.
View Article : Google Scholar
|
|
43
|
Lin CX, Rhaleb NE, Yang XP, Liao TD,
D'Ambrosio MA and Carretero OA: Prevention of aortic fibrosis by
N-acetyl-seryl-aspartyl-lysyl-proline in angiotensin II-induced
hypertension. Am J Physiol Heart Circ Physiol. 295:H1253–H1261.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tang LX, He RH, Yang G, Tan JJ, Zhou L,
Meng XM, Huang XR and Lan HY: Asiatic acid inhibits liver fibrosis
by blocking TGF-beta/Smad signaling in vivo and in vitro. PLoS One.
7:e313502012. View Article : Google Scholar : PubMed/NCBI
|