Introduction
Gastric cancer (GC) is the fourth most common cancer
and the second leading cause of cancer-related deaths worldwide
(1). Although significant progress
has been made in the diagnosis and treatment of GC patients,
one-third of these patients have already reached late-stage GC with
distant metastasis at the time of diagnosis, and prognosis remains
poor (1,2). Identification of critical factors
driving the progression of metastasis and new prognostic biomarkers
are urgently required to improve both early detection of recurrence
and prognosis of patients with advanced GC. Furthermore, in
association with recent advances in surgical techniques including
endoscopy and laparoscopy procedures, identification of GC patients
with lymph node metastasis is also needed to relieve surgical
stress and improve the quality of life of early-stage GC patients.
Therefore, identification of novel biomarkers for detecting
metastasis is a major focus of current investigations.
Metastasis from the primary site is a characteristic
event in tumor development and is established through multiple
steps, including migration of tumor cells through the stroma,
invasion of vessels into the circulation, adherence to the
microvascular endothelium, extravasation, and proliferation in the
target organ (3–6). Although accumulating evidence over the
past decades has identified hundreds of molecules that are
intimately involved in metastasis, the metastasis-associated (MTA)
tumor gene family has attracted more attention because it is one of
the most important players in the multistep invasion-metastasis
cascade (7–9). The MTA protein family mainly has three
well-known members, MTA1, 2 and 3, and dysregulation of the MTA
family is intimately involved in cancer biology via regulation of
downstream oncogene or onco-suppressor genes (10).
A previous study from our group has shown that
several MTAs are differentially expressed in advanced GC, and can
be used as biomarkers for prognosis and metastasis prediction in GC
patients (11–15). Although a growing number of studies
have demonstrated the function of the MTA family in other types of
cancer, no reports have shown any clinical significance between MTA
family expression and GC progression. In this study, we
systematically investigated the comprehensive expression profile of
the MTA family using a large cohort of specimens to clarify their
clinical significance as prognostic biomarkers and predictive
biomarkers for lymph node metastasis and recurrence in GC
patients.
Materials and methods
Patients and sample collection
A total of 145 patients (116 men and 29 women) were
included in our study according to the availability of cancer
tissue samples with complete clinical data and the quality of
isolated RNA for real-time PCR, and were consecutive patients who
underwent surgery for GC from 2000 to 2009 at Mie University
Hospital, Japan. The mean age was 67 years (range, 18–90 years). No
patient received chemotherapy or radiotherapy prior to surgery and
no perioperative mortalities were observed. The diagnosis of GC was
confirmed for all 145 patients based on clinicopathological
findings. All patients were classified according to the Japanese
Classification of Gastric Carcinoma (16) such that 21 patients had stage I
disease, 39 had stage II, 42 had stage III, and 43 were stage IV.
Ninety-nine patients received curative resection while 46 underwent
non-curative resection. Distal or total gastrectomy with D2
lymphadenectomy was performed in patients who underwent curative
resection. Patients with liver, peritoneal, or distant metastasis
underwent palliative gastrectomy with D1 lymphadenectomy. The mean
follow-up was 26 months (range, 1–79 months). During the study
period, 64 patients died from cancer-related causes.
All tissue specimens were preserved immediately
after surgical resection in RNAlater (Qiagen, Chatsworth, CA, USA)
and stored at −80°C until RNA extraction. Written informed consent
was obtained from each patient, and the study was approved by the
Institutional Review Boards of all the involved institutions.
Total RNA extraction and cDNA
synthesis
RNAlater-preserved surgical specimens were
homogenized with a Mixer Mill MM 300 homogenizer (Qiagen). Total
RNA from tissues was isolated using RNeasy Mini kits (Qiagen)
according to the manufacturer's instructions. cDNA was synthesized
from 5.0 µg of total RNA with random hexamer primers and
SuperScript III reverse transcriptase (Invitrogen Life
Technologies™, Carlsbad, CA, USA).
Real-time quantitative RT-PCR
Quantitative reverse transcriptase PCR (qRT-PCR)
analysis was performed using the StepOne Real-Time PCR system
(Applied Biosystems, Foster City, CA, USA). The MTA family (MTA1, 2
and 3) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA
expression levels were measured using Power SYBR-Green Master Mix
(life Technologies, Carlsbad, CA, USA), and primers for the MTA
family genes and GAPDH were designed using Primer3 software
(Biology Workbench version 3.2; San Diego Super Computer Center,
University of California, San Diego, CA, USA). The sequence
information of these primers is provided in Table I. We performed 40 cycles of
amplification under the following conditions: denaturation at 95°C
for 10 sec, annealing at 60°C for 10 sec, and elongation at 72°C
for 20 sec. After amplification, the products were subjected to a
temperature gradient ranging from 68 to 95°C at 0.2°C/sec under
continuous fluorescence monitoring to produce a melting curve of
the products. After proportional background adjustment, the
fit-point method was used to determine the cycle in which the
log-linear signal was distinguished from the background, and that
cycle number was used as a crossing-point value. Expression levels
of target transcripts and GAPDH were evaluated using Applied
Biosystems StepOne software v2.1.
 | Table IPrimers used for quantitative
real-time PCR analysis. |
Table I
Primers used for quantitative
real-time PCR analysis.
| Gene | Forward | Reverse |
|---|
| MTA1 |
CCTGCTGGCAGATAAAGGAG |
GCTTGTCTGTGAGTGGGTTG |
| MTA2 |
ATGGAAATGTGGAGGCAAAG |
GAAAAAGTTCCCGGTGCTTC |
| MTA3 |
AAAATGCCCACCCAGTCAG |
TTTGGACTCCCAGTGTTTCG |
| GAPDH |
GGAAGGTGAAGGTCGGAGTC |
AATGAAGGGGTCATTGATGG |
Relative mRNA expression analysis
The relative gene expression level determined by
real-time quantitative RT-PCR was calculated using the standard
curve method. Standard curves and linear equations were generated
using 5-fold serial dilutions of random-primed qPCR Human Reference
cDNA (Takara Bio, Inc., Shiga, Japan; Clontech laboratories,
Mountain View, CA, USA). Within the range analyzed, all standard
curves were linear with an acceptable correlation coefficient (R2).
The extent of target mRNA expression was calculated from the
standard curve, with quantitative normalization of the cDNA in each
sample performed using the GAPDH gene as an internal control.
Finally, target mRNA levels were expressed as ratios relative to
the GAPDH mRNA level. Real-time PCR assays were performed in
duplicate for each sample and the mean value was used to calculate
mRNA expression levels.
Immunohistochemical analysis
Immunohistochemical studies of MTA1 were performed
on surgical specimens of primary GC using avidin-biotin-peroxidase
methods (DakoCytomation, Carpinteria, CA, USA) on formalin-fixed,
paraffin-embedded (FFPE) tissues. The FFPE specimens were sliced
into sections of 2–3 µm. After deparaffinization and
dehydration, specimens were brought to a boil in 10 mM sodium
citrate buffer for antigen unmasking. Specimens were then blocked
and incubated with primary antibody overnight at 4°C. The antibody
was detected using Envision reagents (Envision kit/HRP;
DakoCytomation, Glostrup, Denmark). A primary mouse polyclonal
antibody against MTA1 (1:100; santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) was used for implementing the labeled
streptavidin-biotin method (LASB2 kit/HRP) and then stained with
3,3′-diaminobenzidine (both from DakoCytomation). All sections were
counterstained with hematoxylin, and were dehydrated and mounted.
Negative controls were also run simultaneously.
Statistical analysis
Results are expressed as the mean ± SE, and all
statistical analyses were performed using MedCalc version 12.3.0
(MedCalc software, Mariakerke, Belgium). Differences between groups
were estimated by the Mann-Whitney U test and the Kruskal-Wallis
test, as applicable. For time-to-event analyses, survival estimates
were calculated using Kaplan-Meier analysis and groups were
compared with the log-rank test. Receiver operating characteristic
(ROC) curves were established to determine the cut-off values for
analyzing prognosis by Youden's index. Overall survival (OS) was
measured from the date the patient underwent surgery until the date
of death resulting from any cause, or last known follow-up for
patients that were still alive. Disease-free survival (DFS)
analysis was measured from the date the patient underwent curative
surgery to the date of disease recurrence, death from any cause
(i.e., cancer-unrelated deaths were not censored) or until last
contact with the patient. For assessment of the performance as a
prognostic marker for OS and DFS, the power calculations are based
on the detection difference of 0.05 between favorable and
unfavorable prognosis groups. We estimated 126 and 88 patients
(distributed equally between the two groups) were needed to achieve
80% power to substantiate more than 25 and 30% differences in
prognostic and recurrent outcomes at a significance level of 0.05
using a two-sided log-rank test, respectively, and our cohort of
145 GC patients was therefore more than adequate. The Cox's
proportional hazards models were used to estimate hazard ratios
(HRS) for death. Assumption of proportionality was confirmed for
the Cox proportional hazards analyses by generating Kaplan-Meier
survival curves (e.g., high vs. low expression groups) and by
ensuring that the two curves did not intersect each other.
Multivariate logistic regression models were used to predict
factors influencing lymph node metastasis. Forced-entry regression
was used to include these variables in all multivariable equations
to analyze whether each of the predictors affected the outcome
after adjusting for known confounders. All P-values were two-sided,
and those <0.05 were considered statistically significant.
Results
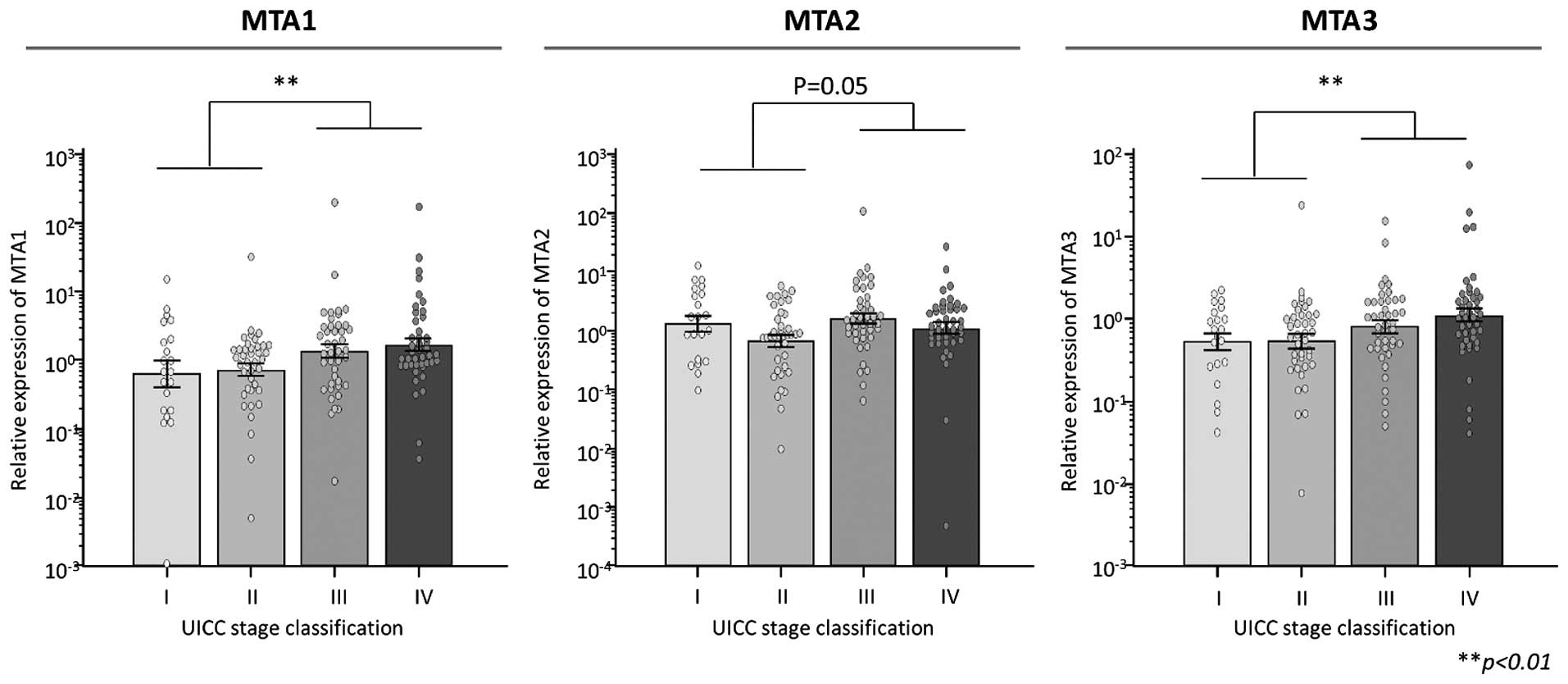
Overexpression of MTA family proteins is
associated with tumor progression in GC patients
Expression levels of MTA1, 2 and 3 in 145 GC tissues
were examined by quantitative real-time PCR. The expression levels
of both MTA1 and 3 were significantly higher in the advanced
neoplastic tissues compared with these levels in early-stage GC
tissues (MTA1, P<0.01; MTA2, P=0.05; MTA3, P<0.01; Fig. 1).
High expression of the MTA family
proteins is associated with recurrence and poor outcome in GC
patients
Next, we analyzed the expression patterns of the MTA
family members with various clinicopathological factors to
determine whether the expression status had any prognostic
significance in GC patients (Table
II). Elevated expression levels of all MTAs were significantly
correlated with progression of TNM stage classification.
Furthermore, overexpression of MTA1 and 3 was associated with
factors showing metastasis such as presence of lymph node
metastasis and distant metastasis in GC patients.
 | Table IIClinicopathological variables and MTA
family expression in GC patients. |
Table II
Clinicopathological variables and MTA
family expression in GC patients.
| Variables | n | MTA1
expression | P-value | MTA2
expression | P-value | MTA3
expression | P-value |
|---|
| Gender | | | | | | | |
| Male | 116 | 5.59±2.22 | 0.66 | 3.07±0.94 | 0.67 | 2.21±0.69 | 0.46 |
| Female | 29 | 2.09±0.53 | | 2.31±0.46 | | 1.28±0.28 | |
| Age (years) | | | | | | | |
| <70a | 71 | 2.67±0.57 | 0.94 | 2.27±0.41 | 0.23 | 1.38±0.32 | 0.7 |
| ≥70 | 74 | 7.02±3.44 | | 3.54±1.44 | | 2.65±1.04 | |
| Histological
type | | | | | | | |
| Intestinal
type | 74 | 3.86±2.28 | 0.56 | 2.08±0.28 | 0.89 | 1.98±0.98 | 0.62 |
| Diffuse type | 71 | 5.95±2.76 | | 3.8±1.52 | | 2.08±0.51 | |
| Tumor size | | | | | | | |
| ≥5.5 cmb | 72 | 7.21±3.52 | 0.79 | 3.47±1.46 | 0.6 | 2.47±1.03 | 0.33 |
| <5.5 cm | 73 | 2.6±0.62 | | 2.37±0.44 | | 1.59±0.44 | |
| Pathological T
category | | | | | | | |
| pT1/2 | 49 | 1.57±0.33 | 0.15 | 2.04±0.35 | 0.42 | 0.87±0.09 | 0.25 |
| pT3/4 | 96 | 6.58±2.67 | | 3.37±1.13 | | 2.62±0.83 | |
| Lymph node
metastasis | | | | | | | |
| N0 | 42 | 2.28±0.82 | 0.009c | 2.01±0.4 | 0.27 | 1.35±0.55 | 0.07 |
| N1 | 103 | 5.95±2.48 | | 3.29±1.06 | | 2.31±0.75 | |
| Distant
metastasis | | | | | | | |
| M0 | 102 | 3.9±1.9 | 0.08 | 3.16±1.04 | 0.78 | 1.3±0.28 | 0.021c |
| M1 | 43 | 7.24±3.98 | | 2.35±0.65 | | 3.75±1.74 | |
| TNM
classification | | | | | | | |
| Stage I/II | 60 | 1.89±0.57 | 0.007c | 1.87±0.3 | 0.05 | 1.14±0.39 | 0.007c |
| Stage III/IV | 85 | 7.0±3.0 | | 3.66±1.27 | | 2.65±0.9 | |
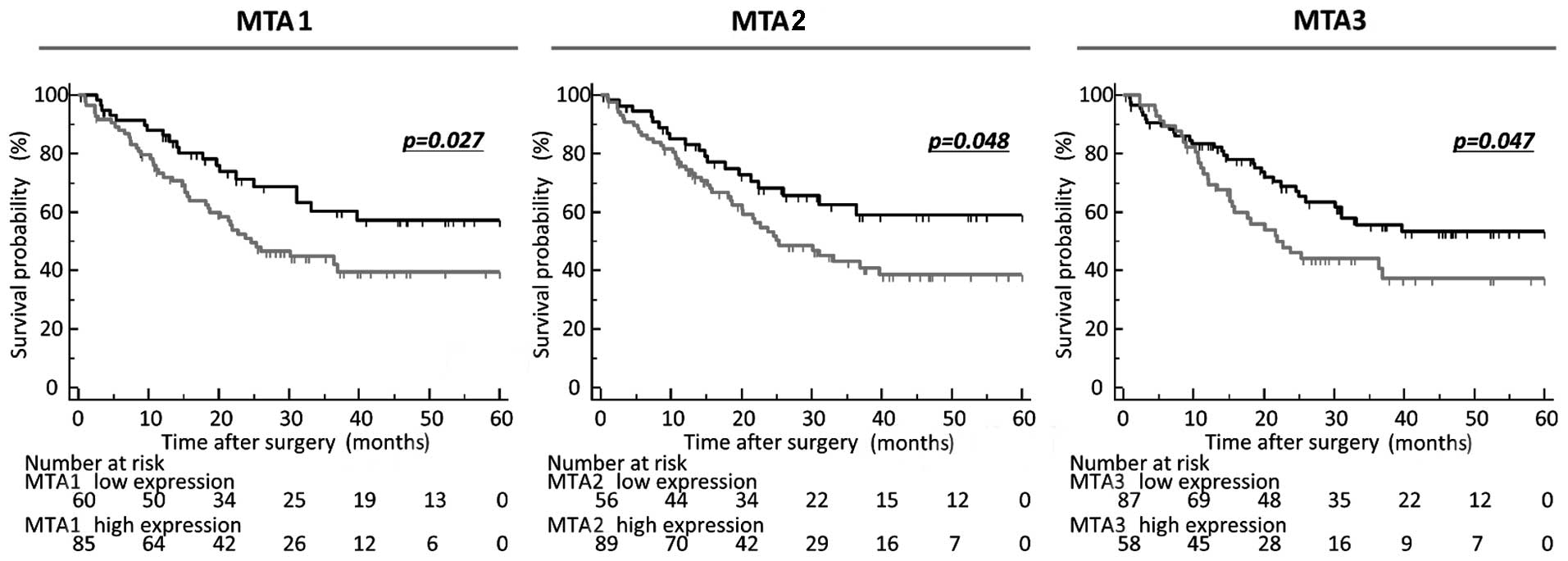
Moreover, in performing the time-to-event analysis
to evaluate the prognostic impact of these MTAs, the expression
cut-off threshold for each MTA family member was determined
according to ROC analyses with Youden's index to analyze the
prognostic impact of OS and DFS. Notably, high expression of all
the MTA family was significantly correlated with poor prognosis
compared with GCs in the low-expression group in terms of OS in
these patients (MTA1, P=0.027; MTA2, P=0.048; MTA3, P=0.047;
log-rank test; Fig. 2).
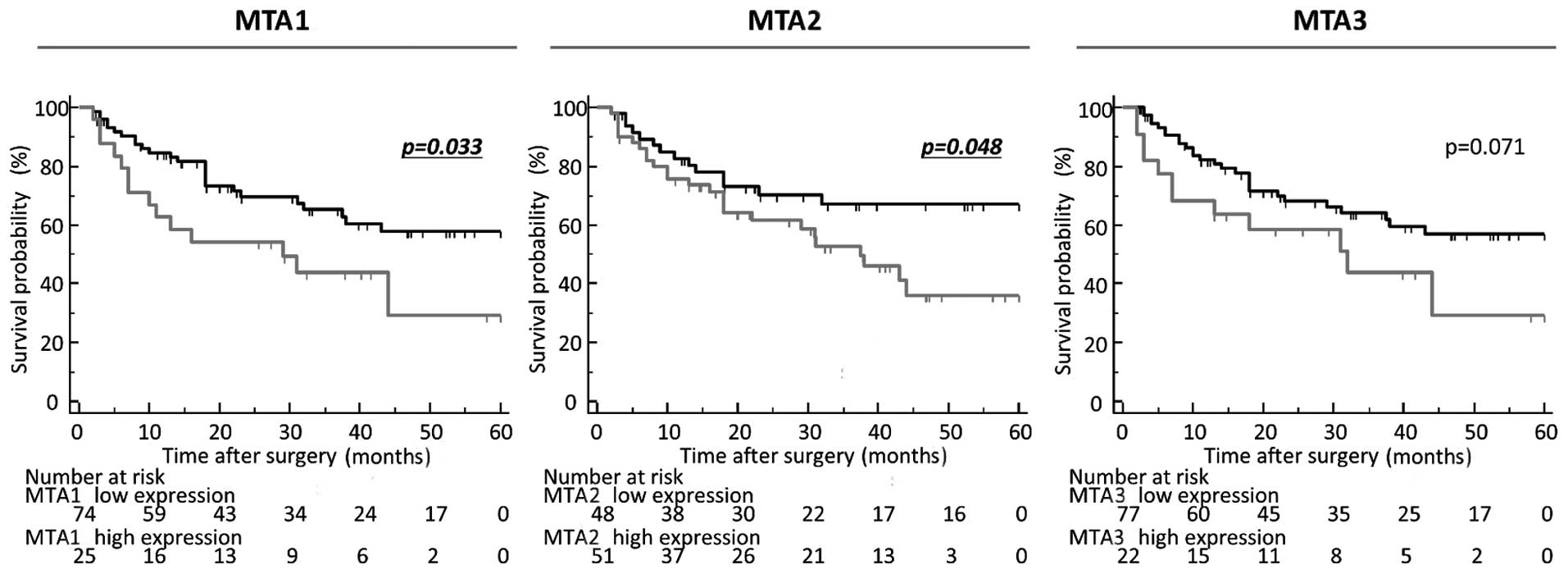
Furthermore, elevated expression of both MTA1 and 2 were
significantly correlated with poor DFS (MTA1, P=0.033; MTA2,
P=0.048; MTA3, P=0.071; log-rank test; Fig. 3). Multivariate Cox's regression
analysis showed that high MTA1 expression was an independent
prognostic factor for OS (HR, 2.01; 95% CI, 1.02–3.96; P=0.044) in
GC patients (Table III).
 | Table IIIMultivariate analysis for predictors
of OS. |
Table III
Multivariate analysis for predictors
of OS.
| Variables | Univariate
| Multivariate
|
|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Gender (male) | 0.79 | 0.44–1.43 |
0.45 | 0.63 | 0.32–1.24 | 0.18 |
| Age (≥70
years)a | 1.16 | 0.71–1.89 |
0.56 | 1.56 | 0.91–2.66 | 0.11 |
| Histological type
(intestinal type) | 0.96 | 0.59–1.57 |
0.86 | 1.19 |
0.7–1.99 | 0.52 |
| Tumor size (≥5.5
cm)b | 1.45 | 0.88–2.37 |
0.14 | 1.61 | 0.95–2.73 | 0.07 |
| T classification
(pT3/4) | 3.37 | 1.79–6.33 |
0.0002d | 2.23 | 0.89–5.61 | 0.09 |
| Lymph node
metastasis (present) | 3.57 | 1.77–7.22 |
0.0004d | 1.92 | 0.71–5.19 | 0.2 |
| Distant metastasis
(present) | 3.63 | 2.22–5.95 | <0.0001d | 2.79 | 1.52–5.1 | 0.0009d |
| TNM stage
classification (stage III/IV) | 5.03 | 2.67–9.47 | <0.0001d | 1.28 | 0.39–4.24 | 0.69 |
| High MTA1
expressionc | 1.8 | 1.06–3.05 |
0.029d | 2.01 | 1.02–3.96 | 0.044d |
| High MTA2
expressionc | 1.71 | 1.00–2.92 |
0.05 | 1.22 | 0.68–2.19 | 0.51 |
| High MTA3
expressionc | 1.64 | 1.00–2.67 |
0.049d | 0.88 | 0.47–1.62 | 0.68 |
High expression of MTA1 is a predictive
factor for the presence of lymph node metastasis in GC
patients
Of the MTA family members, only MTA1 expression
served as an independent prognostic factor for OS in GC patients.
Furthermore, overexpression of MTA1 was significantly correlated
with the presence of lymph node metastasis and recurrence in GC
patients. Based on these findings, we next undertook multivariate
logistic analysis to determine the clinical significance of MTA1
expression as a predictive biomarker of lymph node metastasis in GC
patients (Table IV). Notably,
advanced T classification and high expression of MTA1 were
independent factors for lymph node metastasis. Collectively, our
data suggest that tissue expression of MTA1 could be used as a
predictive biomarker of lymph node metastasis and identification of
high-risk GC patients.
 | Table IVMultivariate analysis for lymph node
metastasis. |
Table IV
Multivariate analysis for lymph node
metastasis.
| Variables | Univariate
| Mutivariate
|
|---|
| Odds ratio | 95% CI | P-value | Odds ratio | 95% CI | P-value |
|---|
| Gender (male) | 1.38 | 0.58–3.29 | 0.47 | 1.63 | 0.63–4.2 | 0.32 |
| Age (≥70
years)a | 1.59 | 0.77–3.28 | 0.21 | 2.23 | 0.98–5.06 | 0.056 |
| Histological type
(Intestinal type) | 1.39 | 0.67–2.85 | 0.37 | 1.55 | 0.69–3.48 | 0.28 |
| Tumor size (≥5.5
cm)b | 1.12 | 0.55–2.3 | 0.75 | 1.18 | 0.52–2.7 | 0.68 |
| T classification
(pT3/4) | 2.68 | 1.27–5.64 | 0.009d | 3.19 | 1.38–7.34 | 0.007d |
| High MTA1
expressionc | 2.48 | 1.19–5.17 | 0.014d | 2.68 | 1.04–6.9 | 0.041d |
| High MTA2
expressionc | 2.23 | 1.07–4.64 | 0.031d | 1.87 | 0.81–4.31 | 0.14 |
| High MTA3
expressionc | 1.73 | 0.81–3.71 | 0.15 | 0.83 | 0.32–2.15 | 0.7 |
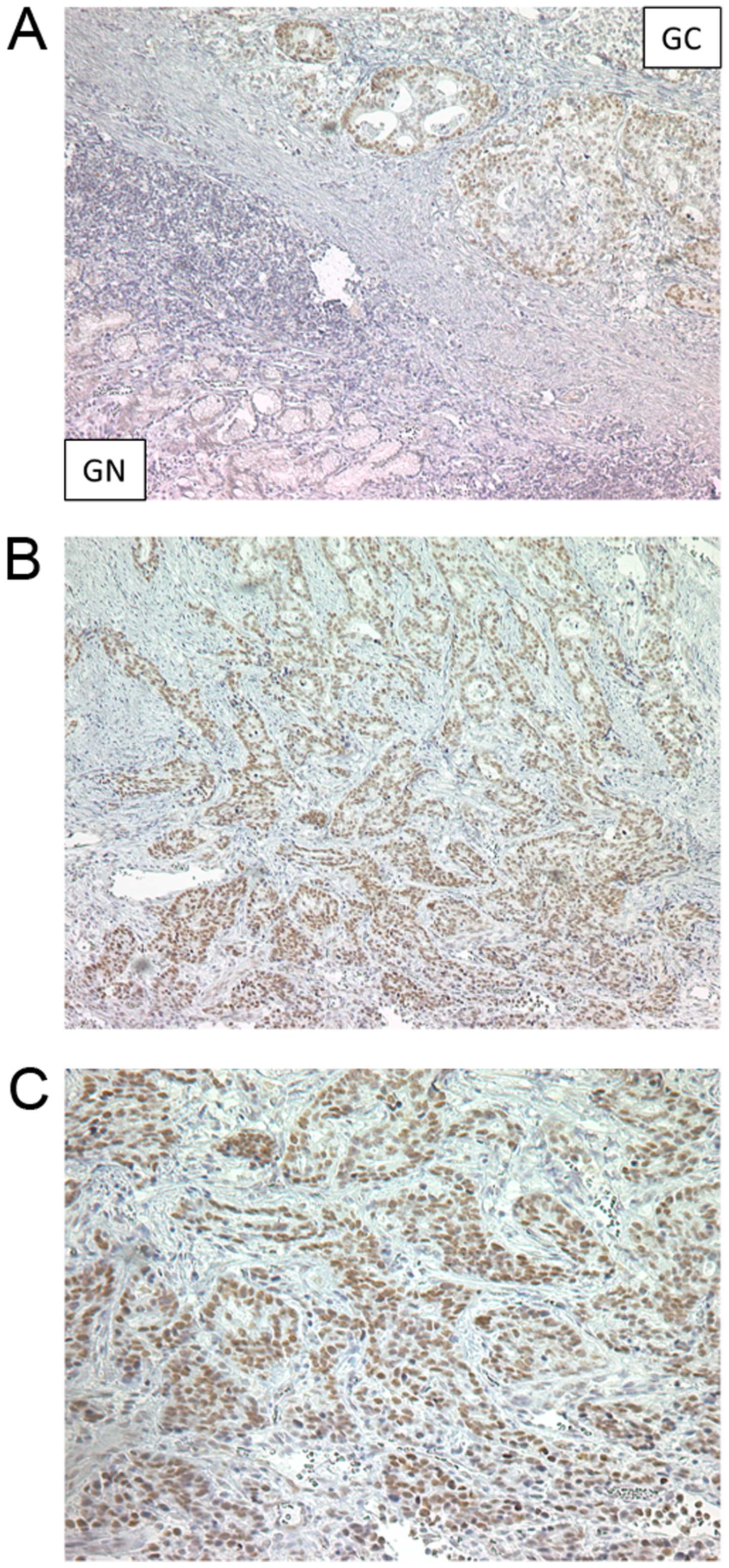
MTA1 was highly expressed in cancer cells
compared with the cancer stroma or corresponding NM
To further confirm the pathological expression
pattern of MTA1 in clinical specimens, immunohistochemical analysis
was performed on 10 primary GC tissues and corresponding adjacent
NM from this cohort. Immunohistochemical analysis revealed nuclear
staining for MTA1 in GC cells, an observation that is consistent
with previous reports in other types of cancer (17–19).
Furthermore, the MTA1 protein was predominantly expressed in the
nuclei of primary GC cells but exhibited no expression in NM and in
the cancer stroma (Fig. 4A–C).
Based on these results, MTA1 was overexpressed in the GC cells
compared with the cancer stroma or NM, and its expression was
significantly correlated with disease progression in GC
patients.
Discussion
An increasing number of studies have implicated the
involvement of the MTA family in disease progression and metastatic
processes in various types of cancer (17,20–23),
but the association between the expression pattern of MTA family
members and their clinical significance in GC, particularly their
potential as prognostic and predictive biomarkers for metastasis,
have not yet been defined. In the present study, we provide initial
evidence of the clinical impact of the dysregulation of the MTA
family in GC. Firstly, expression of almost all of the MTA family
members in primary tissues showed a significant correlation with
the presence of metastasis and advanced TNM stage classification in
GC patients. Secondly, high expression levels of all MTA family
members were significantly correlated with poor survival, and in
particular elevated expression of MTA1 in primary tissues was
significantly correlated with poor prognosis for both OS and DFS.
MTA1 expression was found to be an independent prognostic factor in
GC patients. Thirdly, we showed that overexpression of MTA1 was
deeply involved with lymph node metastasis and an independent risk
factor for lymph node metastasis in GC.
The MTA tumor gene family was originally identified
by differential cDNA screening of rat adenocarcinoma cell lines
with low and high metastatic potential (9), and mainly consists of three different
gene products, MTA1, 2 and 3 (7,8,24–26),
which are critical components of the nucleosome remodeling and
histone deacetylase (NuRD) complex that plays a negative role in
posttranslational modifications of different genes by recruiting
histone deacetylases onto their target genes (10). MTA1 was the first member of this
family to be identified, and is a ubiquitously expressed protein
that markedly increases metastasis and aggressiveness in various
types of solid cancers, including esophageal carcinoma, thymoma,
and ovarian, breast and colorectal cancer (27–32). A
recent series of studies revealed a fundamental role for MTA1 in
oncogenesis, DNA damage response, epithelial-mesenchymal
transcription, inflammation, and pathogen-driven cancers by
repressing transcription in a context-dependent manner, controlling
the steady state of proteins via affecting protein ubiquitination,
and contributing to the DNA damage response (7,10,33).
Two other members, MTA2 and 3, were subsequently identified and
found to be present in distinct NuRD complexes. MTA2 is a member of
a highly-conserved family of proteins, and is structurally
homologous to MTA1. MTA2 expression induces p53 deacetylation by
HDACs and inhibits p53-dependent transcriptional activation. MTA3
was first identified as an estrogen-inducible gene product that
forms a distinct Mi-2/NuRD complex (34), and recent evidence demonstrated that
the depletion of MTA3 expression inhibited cell proliferation and
induced cell cycle arrest at the G1/S boundary in non-small lung
cancer cell lines due to suppression of cyclin A, cyclin D1 and pRb
(23). Similar to MTA1, MTA2 and 3
dysregulation has also been demonstrated in several tumors,
including pancreatic, ovarian, and non-small cell lung cancer, and
gastroesophageal junction adenocarcinoma (23,35–39).
One of the major findings of the present study is
the prognostic impact of the MTA family expression levels in GC
patients. The expression status of all MTA family members was
significantly correlated with metastasis factors and advanced TNM
stage in GC patients. In addition, elevated expression of all of
MTA family members showed significant correlation with poor
prognosis in OS. Our results are consistent with previous studies
in various types of cancer. Recently, several studies used a series
of in vitro and in vivo experiments to demonstrate
that the MTA family members contribute to GC cell proliferation,
migration and invasion via dysregulation of downstream target
genes, including cyclin D1, interleukin-11, fibronectin, matrix
metalloproteinase (MMP)-2 and -9 (40,41).
Collectively, these studies together with our data suggest the
intimate involvement of the MTA family members in disease
progression and formation of metastasis in GC.
Another key finding of our study was the clinical
impact of MTA1 expression in GC patients. Our results clearly
demonstrated that high expression of MTA1 was a potential predictor
for recurrence and poor prognosis in GC patients. In line with
these findings, multivariate analysis revealed that high expression
of MTA1 was an independent risk factor for survival in GC patients.
Notably, high expression of MTA1 was significantly correlated with
the presence of lymph node metastasis, and logistic regression
analysis showed that elevated MTA1 expression in primary cancer
tissues was an independent risk factor for predicting lymph node
metastasis in GC patients. At present, one of the most clinically
desired biomarkers for GC patients is a predictive biomarker for
identifying patients with lymph node metastasis. Lymph node
metastasis is recognized as one of the most important risk factors
for recurrence and prognosis in GC patients, and accumulating
evidence has revealed that lymph node dissection improves survival
(42). Furthermore, thanks to
recent advances in endoscopic and surgical techniques over the past
decade, accurate detection of lymph node metastasis has become more
clinically relevant by converting to minimally invasive treatments,
such as endoscopic resection or laparoscopic-assisted gastrectomy
for patients with early GC. Therefore, identification of GC
patients with lymph node metastasis using molecular biomarkers such
as MTA1 expression will help the surgeon in decision-making
regarding lymph node dissection for these patients during surgery,
and allow the endoscopist to select subgroups of high-risk
early-stage GC patients with lymph node metastasis.
In conclusion, this study provides novel evidence
for the clinical impact of the expression of the MTA family in GC
patients. Our systemic assessment highlights the clinical
feasibility of MTA family members, in particular MTA1, as
prognostic and predictive biomarkers for lymph node metastasis and
recurrence in GC patients. We conclude that assessment of MTA1
expression in primary tumors may be used as a clinically relevant
prognostic and predictive biomarker in GC patients. Quantification
of MTA1 expression may support the accurate diagnosis of disease
staging and may help predict clinical outcomes.
Acknowledgments
The authors would like to thank Motoko Ueeda and
Yuki Orito for their excellent technical assistance. Dr Yoshinaga
Okugawa was supported in part by a grant in Aid for scientific
Research from Takeda science Foundation, Japan. The present study
was also supported in part by a grant in Aid for scientific
Research (no. 25462018) from the Ministry of Education, Culture,
Sports, Science and Technology, Japan (to M.O.).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gupta GP and Massagué J: Cancer
metastasis: building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
ten Kate M, Hofland LJ, van Grevenstein
WM, van Koetsveld PV, Jeekel J and van Eijck CH: Influence of
proinflammatory cytokines on the adhesion of human colon carcinoma
cells to lung microvascular endothelium. Int J Cancer. 112:943–950.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Basoglu M, Yildirgan MI, Taysi S, Yilmaz
I, Kiziltunc A, Balik AA, Celebi F and Atamanalp SS: Levels of
soluble inter-cellular adhesion molecule-1 and total sialic acid in
serum of patients with colorectal cancer. J Surg Oncol. 83:180–184.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fidler IJ: Critical determinants of
metastasis. Semin Cancer Biol. 12:89–96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chan DA and Giaccia AJ: Hypoxia, gene
expression, and metastasis. Cancer Metastasis Rev. 26:333–339.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Toh Y and Nicolson GL: The role of the MTA
family and their encoded proteins in human cancers: molecular
functions and clinical implications. Clin Exp Metastasis.
26:215–227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kumar R, Wang RA, Mazumdar A, Talukder AH,
Mandal M, Yang Z, Bagheri-Yarmand R, Sahin A, Hortobagyi G, Adam L,
et al: A naturally occurring MTA1 variant sequesters oestrogen
receptor-alpha in the cytoplasm. Nature. 418:654–657. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Toh Y, Pencil SD and Nicolson GL: A novel
candidate metastasis-associated gene, mta1, differentially
expressed in highly metastatic mammary adenocarcinoma cell lines.
cDNA cloning, expression, and protein analyses. J Biol Chem.
269:22958–22963. 1994.PubMed/NCBI
|
|
10
|
Li DQ, Pakala SB, Nair SS, Eswaran J and
Kumar R: Metastasis-associated protein 1/nucleosome remodeling and
histone deacetylase complex in cancer. Cancer Res. 72:387–394.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okugawa Y, Toiyama Y, Hur K, Toden S,
Saigusa S, Tanaka K, Inoue Y, Mohri Y, Kusunoki M, Boland CR, et
al: Metastasis-associated long non-coding RNA drives gastric cancer
development and promotes peritoneal metastasis. Carcinogenesis.
35:2731–2739. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okugawa Y, Tanaka K, Inoue Y, Kawamura M,
Kawamoto A, Hiro J, Saigusa S, Toiyama Y, Ohi M, Uchida K, et al:
Brain-derived neurotrophic factor/tropomyosin-related kinase B
pathway in gastric cancer. Br J Cancer. 108:121–130. 2013.
View Article : Google Scholar :
|
|
13
|
Okugawa Y, Inoue Y, Tanaka K, Kawamura M,
Saigusa S, Toiyama Y, Ohi M, Uchida K, Mohri Y and Kusunoki M: Smad
interacting protein 1 (SIP1) is associated with peritoneal
carcinomatosis in intestinal type gastric cancer. Clin Exp
Metastasis. 30:417–429. 2013. View Article : Google Scholar
|
|
14
|
Okugawa Y, Toiyama Y, Tanaka K, Matsusita
K, Fujikawa H, Saigusa S, Ohi M, Inoue Y, Mohri Y, Uchida K, et al:
Clinical significance of zinc finger E-box binding homeobox 1
(ZEB1) in human gastric cancer. J Surg Oncol. 106:280–285. 2012.
View Article : Google Scholar
|
|
15
|
Toiyama Y, Tanaka K, Kitajima T, Shimura
T, Imaoka H, Mori K, Okigami M, Yasuda H, Okugawa Y, Saigusa S, et
al: Serum angiopoietin-like protein 2 as a potential biomarker for
diagnosis, early recurrence and prognosis in gastric cancer
patients. Carcinogenesis. 36:1474–1483. 2015.PubMed/NCBI
|
|
16
|
Japanese Gastric Cancer Association:
Japanese Classification of Gastric Carcinoma (3rd English ed).
14:101–112. 2011.
|
|
17
|
Dias SJ, Zhou X, Ivanovic M, Gailey MP,
Dhar S, Zhang L, He Z, Penman AD, Vijayakumar S and Levenson AS:
Nuclear MTA1 overexpression is associated with aggressive prostate
cancer, recurrence and metastasis in African Americans. Sci Rep.
3:23312013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pakala SB, Rayala SK, Wang RA, Ohshiro K,
Mudvari P, Reddy SD, Zheng Y, Pires R, Casimiro S, Pillai MR, et
al: MTA1 promotes STAT3 transcription and pulmonary metastasis in
breast cancer. Cancer Res. 73:3761–3770. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li WF, Liu N, Cui RX, He QM, Chen M, Jiang
N, Sun Y, Zeng J, Liu LZ and Ma J: Nuclear overexpression of
metastasis-associated protein 1 correlates significantly with poor
survival in nasopharyngeal carcinoma. J Transl Med. 10:782012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang W, Yang ZL, Liu JQ, Yang LP, Yang XJ
and Fu X: Overexpression of MTA1 and loss of KAI-1 and KiSS-1
expressions are associated with invasion, metastasis, and
poor-prognosis of gallbladder adenocarcinoma. Tumori. 100:667–674.
2014.
|
|
21
|
Covington KR, Brusco L, Barone I,
Tsimelzon A, Selever J, Corona-Rodriguez A, Brown P, Kumar R,
Hilsenbeck SG and Fuqua SA: Metastasis tumor-associated protein 2
enhances metastatic behavior and is associated with poor outcomes
in estrogen receptor-negative breast cancer. Breast Cancer Res
Treat. 141:375–384. 2013. View Article : Google Scholar
|
|
22
|
Liu T, Yang M, Yang S, Ge T, Gu L and Lou
G: Metastasis-associated protein 1 is a novel marker predicting
survival and lymph nodes metastasis in cervical cancer. Hum Pathol.
44:2275–2281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li H, Sun L, Xu Y, Li Z, Luo W, Tang Z,
Qiu X and Wang E: Overexpression of MTA3 correlates with tumor
progression in non-small cell lung cancer. PLoS One. 8:e666792013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Manavathi B and Kumar R: Metastasis tumor
antigens, an emerging family of multifaceted master coregulators. J
Biol Chem. 282:1529–1533. 2007. View Article : Google Scholar
|
|
25
|
Manavathi B, Singh K and Kumar R: MTA
family of coregulators in nuclear receptor biology and pathology.
Nucl Recept Signal. 5:e0102007.
|
|
26
|
Kumar R, Wang RA and Bagheri-Yarmand R:
Emerging roles of MTA family members in human cancers. Semin Oncol.
30(Suppl 16): 30–37. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qian H, Lu N, Xue L, Liang X, Zhang X, Fu
M, Xie Y, Zhan Q, Liu Z and Lin C: Reduced MTA1 expression by RNAi
inhibits in vitro invasion and migration of esophageal squamous
cell carcinoma cell line. Clin Exp Metastasis. 22:653–662. 2005.
View Article : Google Scholar
|
|
28
|
Sasaki H, Yukiue H, Kobayashi Y, Nakashima
Y, Kaji M, Fukai I, Kiriyama M, Yamakawa Y and Fujii Y: Expression
of the MTA1 mRNA in thymoma patients. Cancer Lett. 174:159–163.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dannenmann C, Shabani N, Friese K, Jeschke
U, Mylonas I and Brüning A: The metastasis-associated gene MTA1 is
upregulated in advanced ovarian cancer, represses ERbeta, and
enhances expression of oncogenic cytokine GRO. Cancer Biol Ther.
7:1460–1467. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tong D, Heinze G, Schremmer M, Schuster E,
Czerwenka K, Leodolter S and Zeillinger R: Expression of the human
MTA1 gene in breast cell lines and in breast cancer tissues. Oncol
Res. 16:465–470. 2007. View Article : Google Scholar
|
|
31
|
Tuncay Cagatay S, Cimen I, Savas B and
Banerjee S: MTA-1 expression is associated with metastasis and
epithelial to mesenchymal transition in colorectal cancer cells.
Tumour Biol. 34:1189–1204. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li J, Ye L, Sun PH, Satherley L, Hargest
R, Zhang Z and Jiang WG: MTA1 is up-regulated in colorectal cancer
and is inversely correlated with lymphatic metastasis. Cancer
Genomics Proteomics. 12:339–345. 2015.PubMed/NCBI
|
|
33
|
Avtanski DB, Nagalingam A, Kuppusamy P,
Bonner MY, Arbiser JL, Saxena NK and Sharma D: Honokiol abrogates
leptin-induced tumor progression by inhibiting Wnt1-MTA1-β-catenin
signaling axis in a microRNA-34a dependent manner. Oncotarget.
6:16396–16410. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fujita N, Jaye DL, Kajita M, Geigerman C,
Moreno CS and Wade PA: MTA3, a Mi-2/NuRD complex subunit, regulates
an invasive growth pathway in breast cancer. Cell. 113:207–219.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ji Y, Zhang P, Lu Y and Ma D: Expression
of MTA2 gene in ovarian epithelial cancer and its clinical
implication. J Huazhong Univ Sci Technolog Med Sci. 26:359–362.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen DW, Fan YF, Li J and Jiang XX: MTA2
expression is a novel prognostic marker for pancreatic ductal
adenocarcinoma. Tumour Biol. 34:1553–1557. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu SL, Han Y, Zhang Y, Xie CY, Wang EH,
Miao Y, Li HY, Xu HT and Dai SD: Expression of
metastasis-associated protein 2 (MTA2) might predict proliferation
in non-small cell lung cancer. Target Oncol. 7:135–143. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dong H, Guo H, Xie L, Wang G, Zhong X,
Khoury T, Tan D and Zhang H: The metastasis-associated gene MTA3, a
component of the Mi-2/NuRD transcriptional repression complex,
predicts prognosis of gastroesophageal junction adenocarcinoma.
PLoS One. 8:e629862013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou C, Ji J, Cai Q, Shi M, Chen X, Yu Y,
Liu B, Zhu Z and Zhang J: MTA2 promotes gastric cancer cells
invasion and is transcriptionally regulated by Sp1. Mol Cancer.
12:1022013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yao Y, Feng S, Xiao M, Li Y, Yang L and
Gong J: MTA1 promotes proliferation and invasion in human gastric
cancer cells. Onco Targets Ther. 8:1785–1794. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou C, Ji J, Cai Q, Shi M, Chen X, Yu Y,
Zhu Z and Zhang J: MTA2 enhances colony formation and tumor growth
of gastric cancer cells through IL-11. BMC Cancer. 15:3432015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu CW, Hsiung CA, Lo SS, Hsieh MC, Chen
JH, Li AF, Lui WY and Whang-Peng J: Nodal dissection for patients
with gastric cancer: a randomised controlled trial. Lancet Oncol.
7:309–315. 2006. View Article : Google Scholar : PubMed/NCBI
|