Introduction
Hepatocellular carcinoma (HCC) is a common malignant
cancer worldwide and is diagnosed in an increasing number of
individuals (1). According to the
data since 2000, HCC is the fifth most common malignant cancer
among males and the eighth most common cancer among females
(2). As a probable radical cure,
surgical resection remains the optimal method for patients in China
and many other countries (3).
Through regular application of ultrasound screening for patients
suffering liver cirrhosis or hepatitis, many patients are
diagnosted who are suitable for hepatectomy. With the development
of surgical techniques and perioperative management, hepatectomy
has become a secure method for HCC with rare complications
(4). In the past 30 years, the
survival rate of HCC patients after resective surgery has been
significantly improved. However, in China and Southeast Asia, as
HCC is secondary to viral hepatitis and liver cirrhosis,
postoperative recurrence is the main cause of treatment failure
(5).
HCC is a disease with a high incidence in China.
Hepatic fibrosis refers to a disease characterized by
over-deposition of extracellular matrix (ECM) and hepatic function
damage caused by various types of continuous hepatic trauma repair
reacting to chronic diseases (6).
TGF-β is the most important cytokine in fibrosis, as it plays an
essential role in promoting hepatic stellate cells (HSCs) to
secrete collagens (6). TGF-β is
generally secreted in an inactive form. However, when under a
stress state, the potential TGF-β1 compound will be activated by a
tissue damage-specific mechanism to exert its biological effects.
As a messenger protein molecule after the specific receptor of the
TGF-β superfamily, Smad exerts intermediary functions by
introducing cell nucleus from cytomembrane through mediating TGF-β
signals. The basic procedure of the TGF-β/Smad signaling pathway
expression is as follows. After stimulating factors which act on
HSCs, the auto-phosphorylated TβRII combines with the TGFβ ligand
under the auxiliary function of TβRIII to activate transmembrane
TβR. Subsequently, after phosphorylation of transmembrane
receptors, the Smad proteins combine with synergistic Smad of Smad4
to form compounds, which will be transmitted into the cell nucleus.
Finally, the activated compounds combine with cofactors of two DNAs
to determine the transcriptional activity of target genes. After
entering the nucleus, Smad complexes combine with the specific
sequence of the promoters of target genes to form stable compounds
to regulate target genes indirectly. Meanwhile, Smad complexes
combine directly with DNA in the cell nucleus to activate the
transcription of target genes. After the activation of HSCs, an
extensive amoount of ECM will be deposited in hepatic cells.
Furthermore, the activated HSCs can also secrete TGF-β to form
cascade reactions, which promote the incidence of fibrosis
(7).
Smurf2 can selectively act on R-Smad proteins. With
the PPXY sequence, Smad2 and Smad3 can combine with Smurf 2 through
WW structural domain. Without the proline-tyrosine PPXY sequence,
Smad4 cannot be directly combined with Smad2, but can be regulated
only through indirect pathways (8).
Studies have shown that the Smad4 level cannot be changed when
cells are separately transfected by Smad7 genes while they can only
be downregulated when cells are independently transfected with
Smads2. However, if cells are transfected by Smad7 and Smurf2
simultaneously, the expression of Smad4 will significantly decrease
(9). In addition, it is observed
that Smad7 can activate enzymatic activities through facilitating
Smurf2 to gather E2, which plays multiple functions in regulating
TGF-β pathways (8).
Playing an essential role in HCC, miRNAs directly
participate in proliferation, apoptosis, differentiation, invasion
and metastasis of HCC cells (10).
Studies also verify that the expression profiles of miRNAs are
closely associated with the pathological features of HCC such as
pathological patterns, stage and grades of malignancy (11), indicating that miRNAs can be
utilized for not only diagnosis and individualized treatment but
also as a technique to assess the prognosis of HCC (12). Therefore, identification of the
miRNAs which play a vital role in HCC development and its function
is expected to provide a new approach for HCC diagnosis and
treatment. Notably, miR-15b is located on sites of genomes related
with blood pressure, diabetes and prostatic cancer, suggesting that
miR-15b may participate in regulating the processes of these
diseases (13). miR-15b and miR-16
have various types of target genes, including proteins related to
cell proliferation, cell cycle and apoptosis, such as cyclin Dl,
cyclin D3, cyclin El and CDK6 which are associated with cell cycle
regulation. These proteins play crucial roles in cell cycle
progression from G1 stage to other stages. In the present study, we
demonstrated that the high expression of miR-15b is a predictor of
the poor prognosis of HCC after curative hepatectomy.
Materials and methods
Patients and clinical specimens
All HCCs in the patients in this study were
confirmed by histological diagnosis. Consent from all patients and
approval from the Ethics Committee of the Chinese PLA General
Hospital (Beijing, China) were obtained. Thirteen patients with HCC
diagnosed from March 2014 and May 2014 at the Chinese PLA General
Hospital were enrolled. Their tumor, para-tumor (≤2.0 cm distance
from the tumor edge) and normal (>2.0 cm distance from the tumor
edge) tissue samples were acquired after patient consent. A
consecutive 156 untreated patients with HCC who received curative
hepatectomy were enrolled from May 2008 to March 2009 at the
Chinese PLA General Hospital. Curative hepatectomy conformed to the
following criteria: cancer tissue sample ≤3, no vascular and bile
duct invasion; microscopically complete removal of cancers; and no
lymph node or distant metastasis. The main clinical and
pathological variables of all patients are described in detail in
Table I.
 | Table ICorrelations between miR-15b
expression and clinicopathological characteristics. |
Table I
Correlations between miR-15b
expression and clinicopathological characteristics.
| High
miR-15b
(n=65) | Low miR-15b
(n=91) | P-value |
|---|
| Gender | | | |
| Male | 57 | 78 | 0.713 |
| Female | 8 | 13 | |
| Age (years) | 46.2±11.1 | 45.9±12.6 | 0.581 |
| ≤55 | 55 | 74 | |
| >55 | 10 | 17 | |
| AFP level
(ng/ml) | | | 0.974 |
| ≤400 | 33 | 48 | |
| >400 | 32 | 43 | |
| Tumor size (cm) | 5.84±2.9 | 6.41±3.1 | 0.069 |
| ≤5 | 34 | 47 | |
| >5 | 31 | 44 | |
| Tumor
encapsulation | | | 0.809 |
| Complete | 33 | 31 | |
| Incomplete | 32 | 60 | |
| TNM stage | | | 0.029 |
| I | 29 | 42 | |
| II–III | 36 | 49 | |
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNAs were extracted from different tissue
samples using TRIzol reagent (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer's protocol. Five micrograms of total
RNA was transcripted with DNase I (Promega) for 15 min at room
temperature and then polyA using polymerase (NEB, Ipswich, MA, USA)
was added at 37°C for 1 h. Total RNA (1.0 g) was transcripted with
River-Tra Ace (Toyobo, Tokyo, Japan), oligo(dT)20, RNase
inhibitor, 5-RT buffer, and dNTP mixture. RT-PCR was performed at
42°C for 20 min and then at 95°C for 5 min using 1.0 g of RNA per
reaction. The ABI 7300 HT sequence detection system (Applied
Biosystems, Foster City, CA, USA) was used to execute RT-PCR to
detect the expression of miR-15b in all tissue and cell samples.
The primers of miR-15b are listed in Table II. The quantitative miR-15b, TGF-β
and Smad2 expression was determined using the 2−ΔΔCt
method.
 | Table IIPrimers for miR-15b, TGF-β and
Smad2. |
Table II
Primers for miR-15b, TGF-β and
Smad2.
| Gene | Primers |
|---|
| miR-15b | F:
5′-GCGAGCACAGAATTAATACGACTCAC-3′ |
| miR-15b | R:
5′-GCGAGCACAGAATTAATACGACTCACTATAGG-3′ |
| U6 | F:
5′-GCTTGCTTCGGCAGCACATATAC-3′ |
| U6 | R:
5′-TGCATGTCATCCTTGCTCAGGG-3′ |
Immunohistochemistry
The tissue samples from the tumor and para-tumor
samples were fixed with formalin, embedded in paraffin, cut into
4-μm thick sections, de-waxed in xylene and rehydrated with
graded concentrations of ethanol. The sections were blocked with
0.3% hydrogen peroxide, and then incubated with antigens in a
microwave oven in 10 mM citrate buffer for 30 min. Next, the
sections were washed three times using PBS and then incubated with
anti-TGF-β (1:500) and Smad2 (1:500) (both from Abcam, Cambridge,
MA, USA) overnight at 4°C. The sections were incubated with
horseradish peroxidase-conjugated secondary antibody and developed
with diaminobenzidine tetrahydrochloride (DAB; Beyotime Institute
of Biotechnology, Jiangsu, China) and counterstained with
hematoxylin.
Follow-up
In the first 2 years after surgery, follow-up of the
patients included physical examination and routine laboratory
testing every 3 months. Following 3 to 5 years after surgery,
follow-up of the patients included a physical examination and
routine laboratory testing every 6 months.
Cell culture and cell transfection
Human hepatocyte cell line MIHA and human HCC cell
lines HepG2, PLC and 97H were cultured in Dulbecco's modified
Eagle's medium (DMEM; Hyclone, Logan, UT, USA) supplemented with
10% fetal bovine serum (FBS; Gibco, USA) at 37°C in a 5%
CO2 incubator. The oligonucleotides of anti-miR-15b
(inhibitor) were synthesized by GenePharma (Shanghai, China) bases
with the following sequences: sense, 5′-UUCUCCGAACGUGUCACGUTT-3′
and antisense, 5′-ACGUGACACGUUCGGAGAATT-3′. HepG2 cells
(2×105) were placed into each well of 6-well plates for
24 h. Anti-miR-15b (inhibitor) was transfected with Lipofectamine
2000 (Invitrogen) according to the manufacturer's instructions and
incubated for 8 h before a change in medium.
MTT assay
The transfected HepG2 cells were seeded into 96-well
plates at 3×103 cells/well. Fifty microliters of 3-(4,
5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
dilution (5 mg/ml; KeyGen, Jiangsu, China) was added into each well
for an additional 4 h. The supernatant was removed and 200
μl of DMSO (Invitrogen) was added to each well to dissolve
the precipitate. The cell growth of the HepG2 cells was measured at
a wavelength of 570 nm.
Apoptosis assay
The transfected HepG2 cells were seeded into 6-well
plates at 1×106 cells/well. Ten microliters of Annexin V
FITC and 5 μl PI (both from KeyGen) were used to
double-stain the HepG2 cells in the dark for 30 min at room
temperature. Then, apoptosis was detected and quantified by flow
cytometry (Becton-Dickinson, USA).
ELISA analysis of caspase-3 activity
The transfected HepG2 cells were seeded into 6-well
plates at 1×106 cells/well. Total proteins were
extracted and the protein concentration was determined by the BSA
method (KeyGen). Fifty micrograms of total proteins were incubated
with DEVD-pNA at 37°C for 30 min. Caspase-3 activity was measured
at a wavelength of 405 nm.
Western blot analysis
The transfected HepG2 cells were seeded into 6-well
plates at 1×106 cells/well. Total proteins were
extracted and the protein concentration was determined by the BSA
method (KeyGen). Fifty micrograms of total proteins were subjected
to SDS-PAGE on 10–12% acrylamide gel and transferred to NC
membranes (Millipore Corporation, USA). The membrane was blocked in
5% nonfat milk in TBST (10 mM Tris-HCl buffer, pH 8.0, 150 mM NaCl,
0.1% Tween-20) and incubated with diluted antibodies against TGF-β
(1:2,000), TβRI (1:3,000), Smad2 (1:4,000), cyclin D1 (1:4,000),
Bax (1:4,000) and β-actin (1:5,000) (all from Santa Cruz
Biotechnology, USA) overnight at 4°C followed by incubation with
HRP-conjugated secondary antibody (1:3,000; Santa Cruz
Biotechnology).
Statistical analysis
Statistical analyses were performed using SPSS 17.0
statistical software (SPSS Company, Chicago, IL, USA). The
correlation between the expression of miR-15b and
clinicopathological characteristics of the HCC patients were
analyzed with the Chi-square test. Results are expressed as mean ±
SD. Differences with P<0.05 were considered statistically
significant.
Results
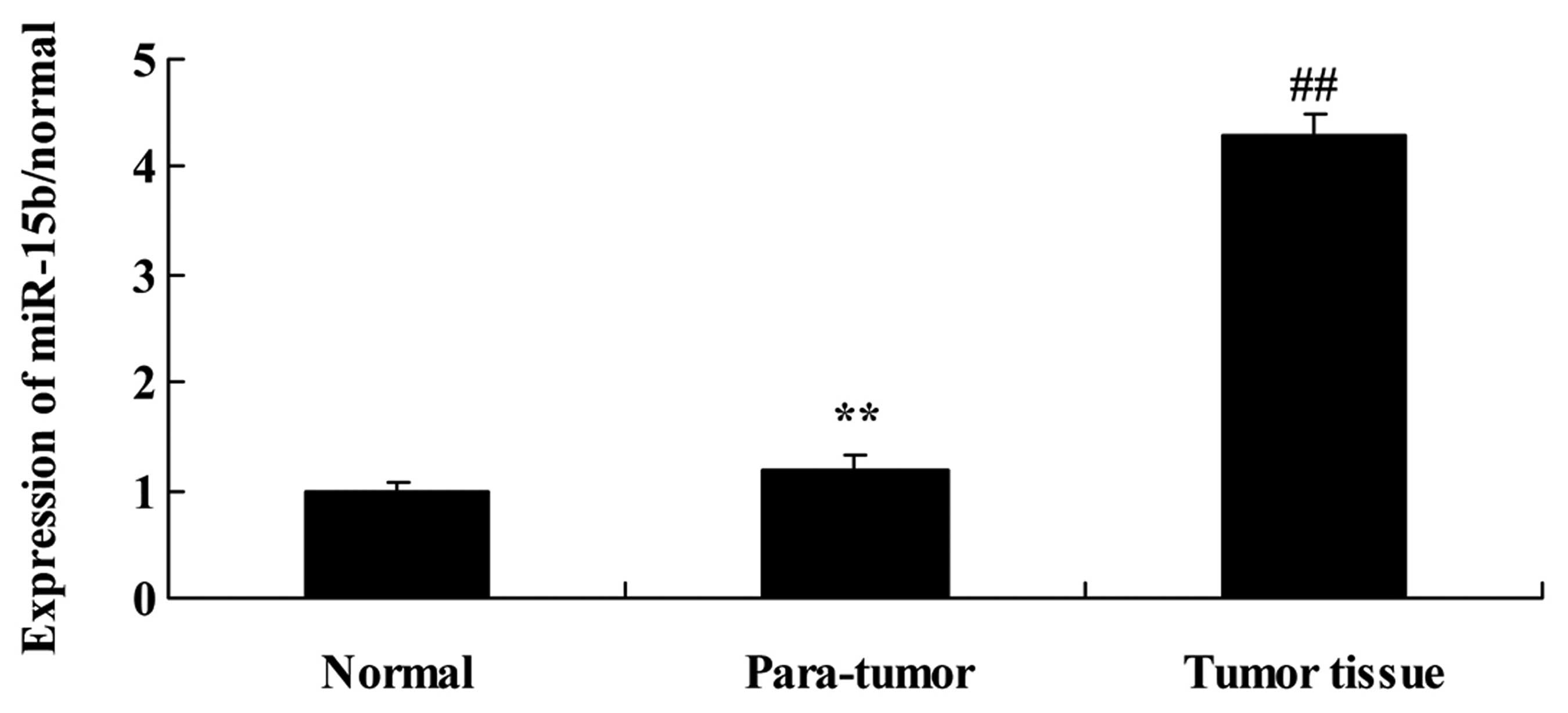
RT-PCR analysis of miR-15b expression in
normal, para-tumor and tumor tissues
To explore miR-15b expression in normal, para-tumor
and tumor tissues, the expression of miR-15b was examined by RT-PCR
analysis. As shown in Fig. 1, the
expression of miR-15b in the para-tumor tissue samples was higher
than that in the normal tissue samples. The results of RT-PCR
analysis also revealed that the expression of miR-15b in the tumor
tissue samples was higher than that in the para-tumor tissue
samples (Fig. 1).
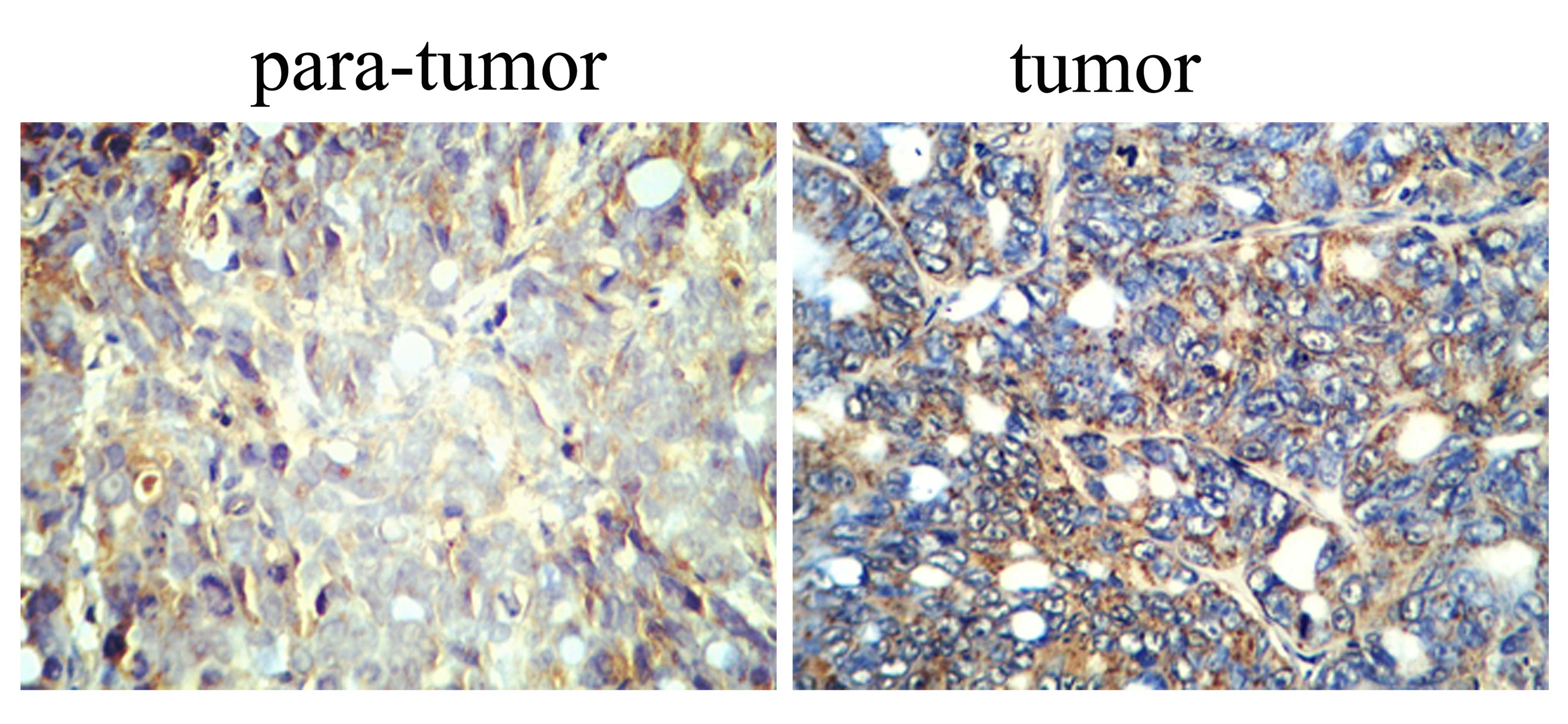
Immunohistochemical analysis of TGF-β in
the tumor and para-tumor tissues
To determine the biological functions of TGF-β in
the tumor and para-tumor tissues, we used immunohistochemical
analysis of TGF-β protein in the HCC patient tumor and para-tumor
tissues. The results of the immunohistochemistry showed that TGF-β
protein in the para-tumor tissue samples was extremely lower than
the level detected in the tissue samples (Fig. 2).
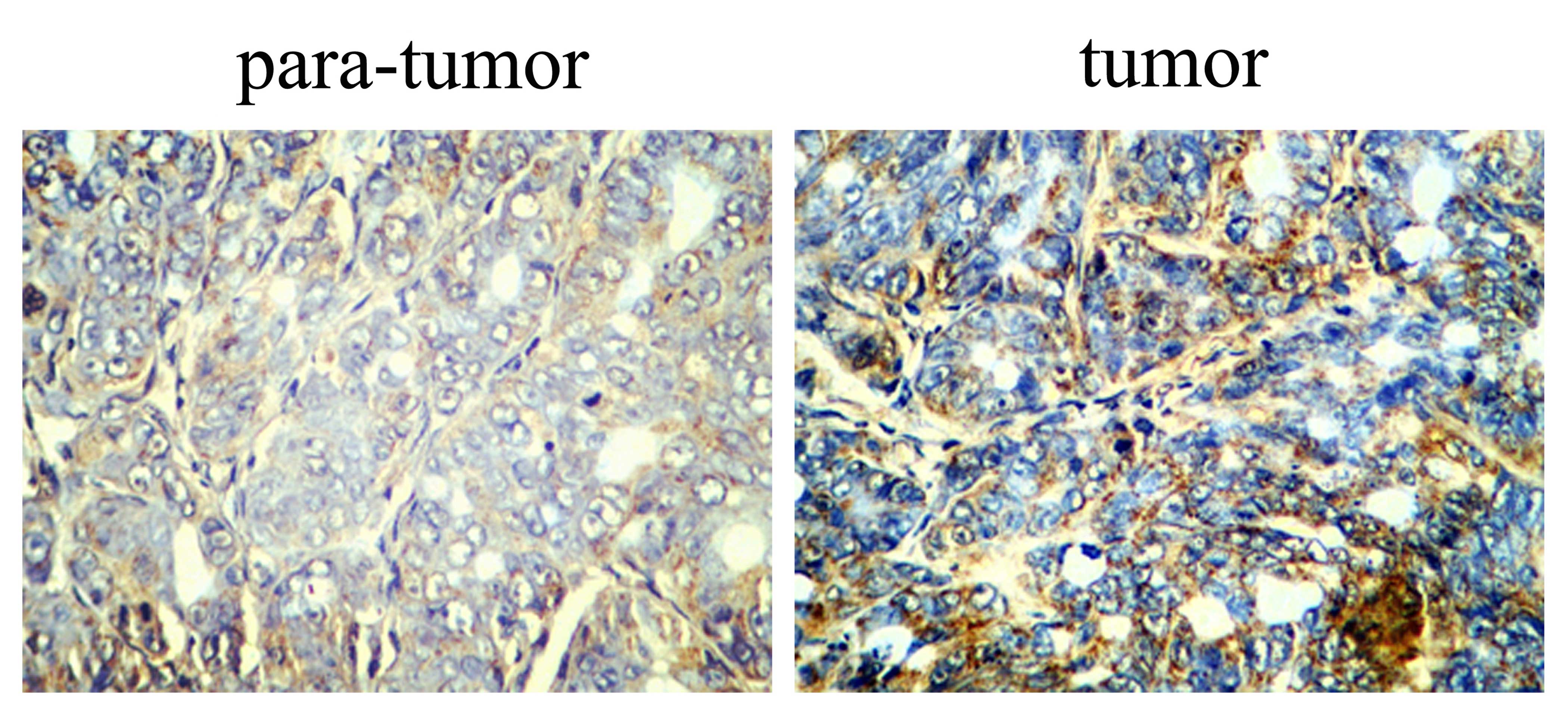
Immunohistochemical analysis of Smad2 in
the tumor and para-tumor tissues
The impact of Smad2 was identified in the tumor and
para-tumor tissues. Immunohistochemical analysis was employed to
determine the protein expression of Smad2 in the HCC tumor and
para-tumor tissues. Fig. 3 shows
that Smad2 protein expression was downregulated in the para-tumor
tissue samples, compared with the level detected in the tumor
tissue samples.
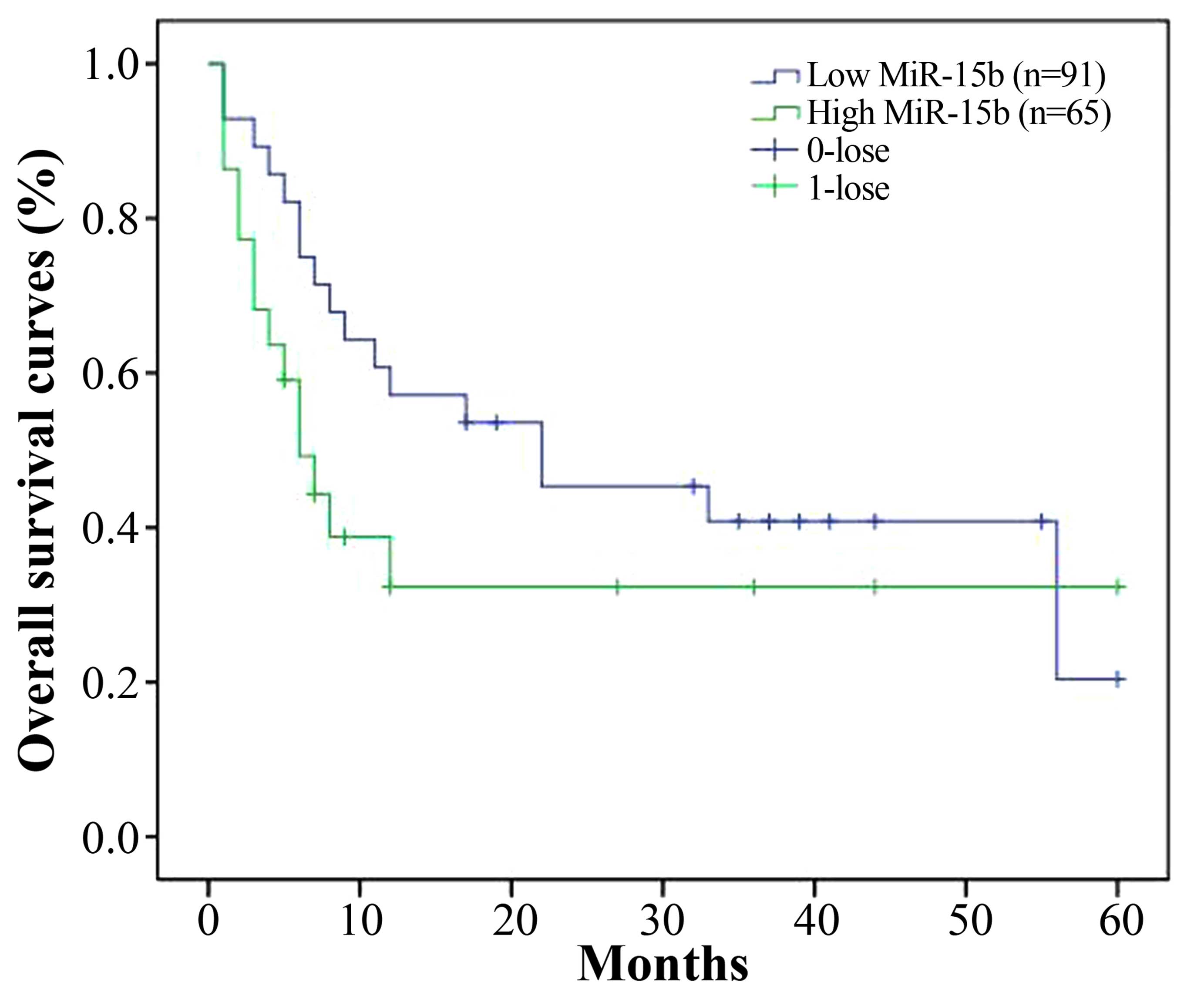
Overall survival curves of HCC patients
after curative hepatectomy assessed according to miR-15b
expression
To investigate the association between miR-15b and
overall survival (OS) of the HCC patients after curative
hepatectomy, we analyzed the OS curves of HCC patients after
curative hepatectomy as assessed by Kaplan-Meier analysis. As shown
in Fig. 4, OS of the HCC patients
with low miR-15b was increased, compared with the OS of the
patients with high miR-15b.
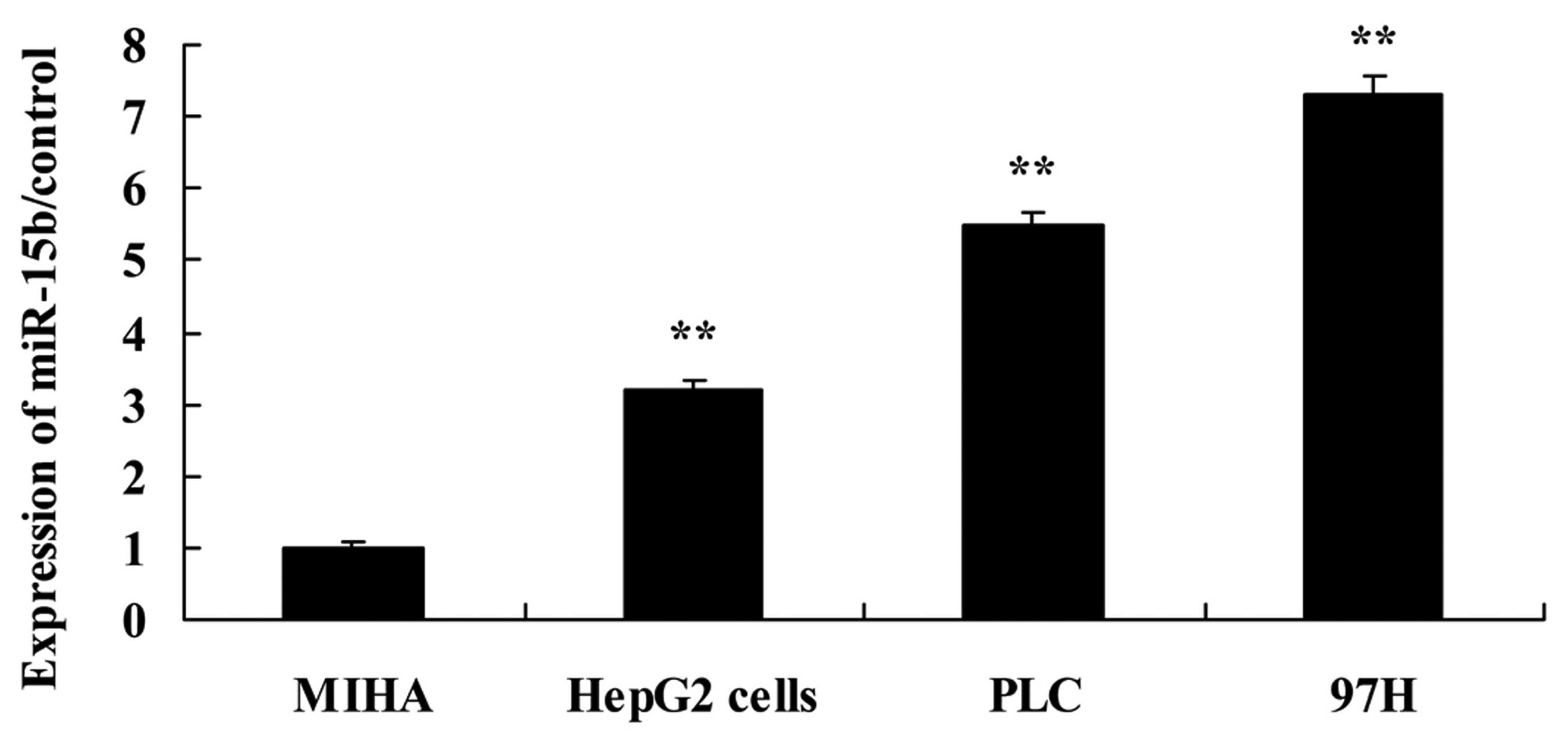
miR-15b expression in the HCC cells
To investigate the expression of miR-15b in human
hepatocyte cell lines MIHA and human hepatocellular carcinoma cell
lines HepG2, PLC and 97H cells, miR-15b expression was analyzed
using RT-PCR analysis. As shown in Fig.
5, RT-PCR analysis revealed that expression of miR-15b in the
HepG2, PLC and 97H cells was significantly activated, compared with
the level in the MIHA cells.
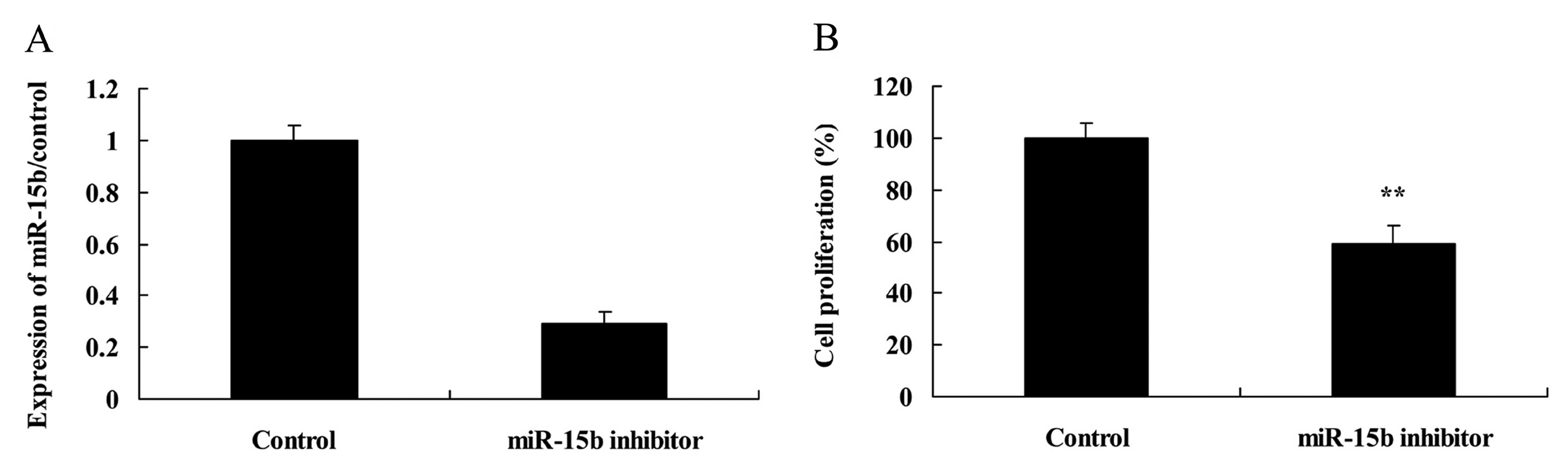
Effect of the downregulation of miR-15b
expression on the growth of HepG2 cells
To further examine the effect of miR-15b on the cell
growth of HepG2 cells, cell proliferation of HepG2 cells was
measured using the MTT assay. Importantly, anti-miR-15b (inhibitor)
effectively diminished miR-15b expression (Fig. 6A) and suppressed cell proliferation
of HepG2 cells, compared with the control group (Fig. 6B).
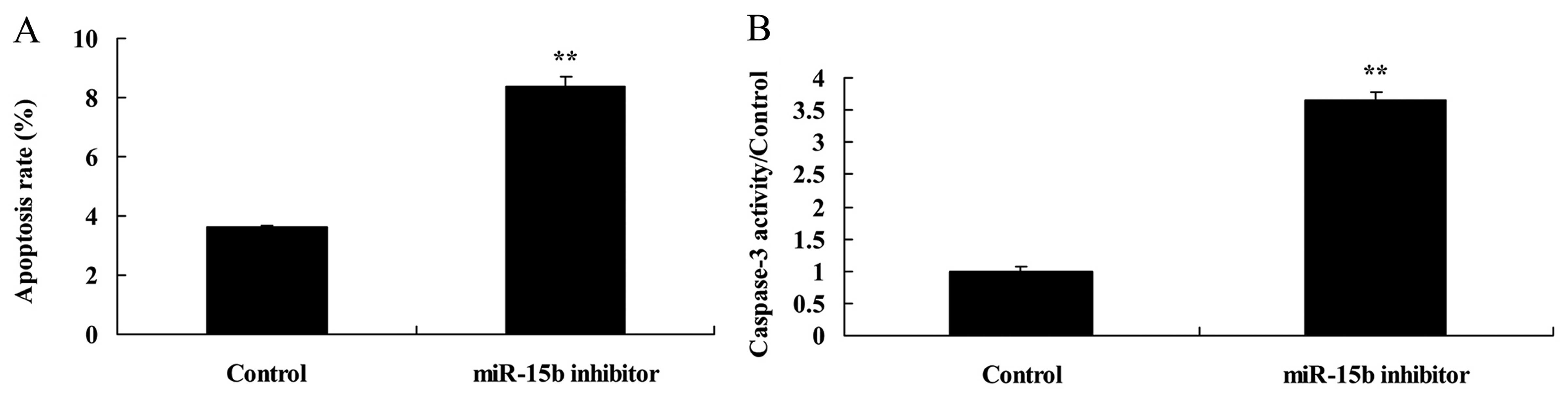
Effect of the downregulation of miR-15b
expression on apoptosis and caspase-3 in the HepG2 cells
Next, we analyzed the effect of the downregulation
of miR-15b expression on apoptosis and caspase-3 in the HepG2
cells. As expected, HepG2 cells transfected with the miR-15b
inhibitor displayed an enhanced apoptosis rate (Fig. 7A) and caspase-3 activity (Fig. 7B), compared with the control
group.
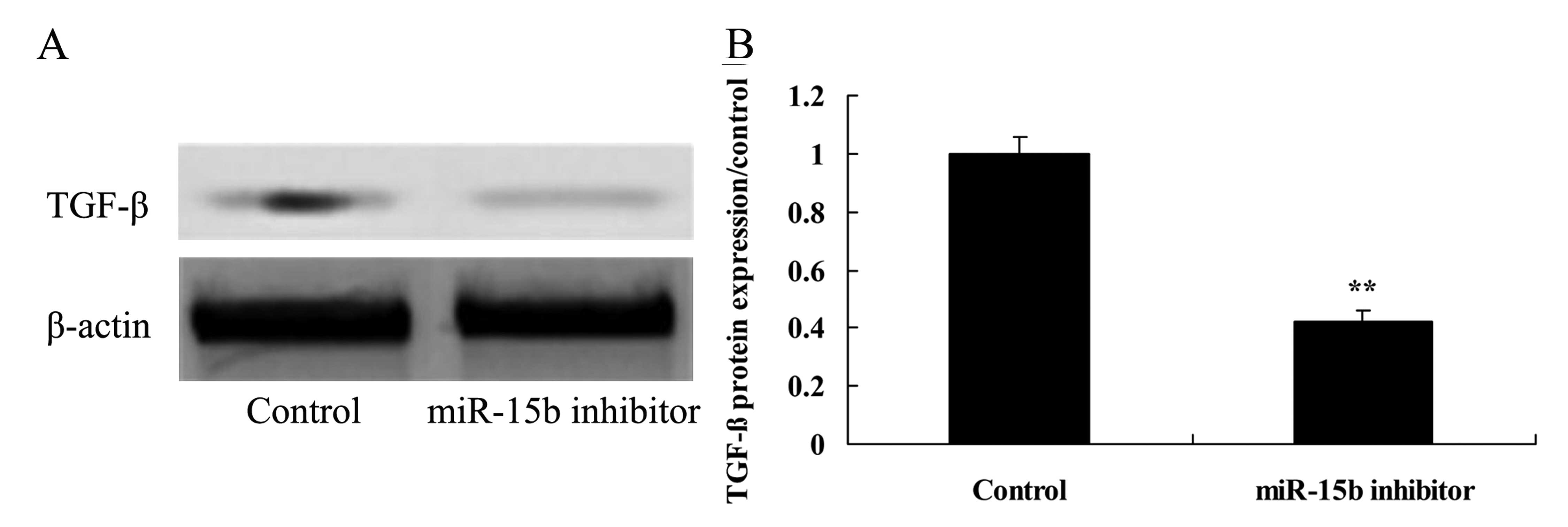
Effect of the downregulation of miR-15b
expression on TGF-β in the HepG2 cells
We then assessed the relationship of miR-15b and
TGF-β signaling in the HepG2 cells. Downregulation of miR-15b
expression reduced TGF-β signaling in the HepG2 cells compared with
the control group (Fig. 8).
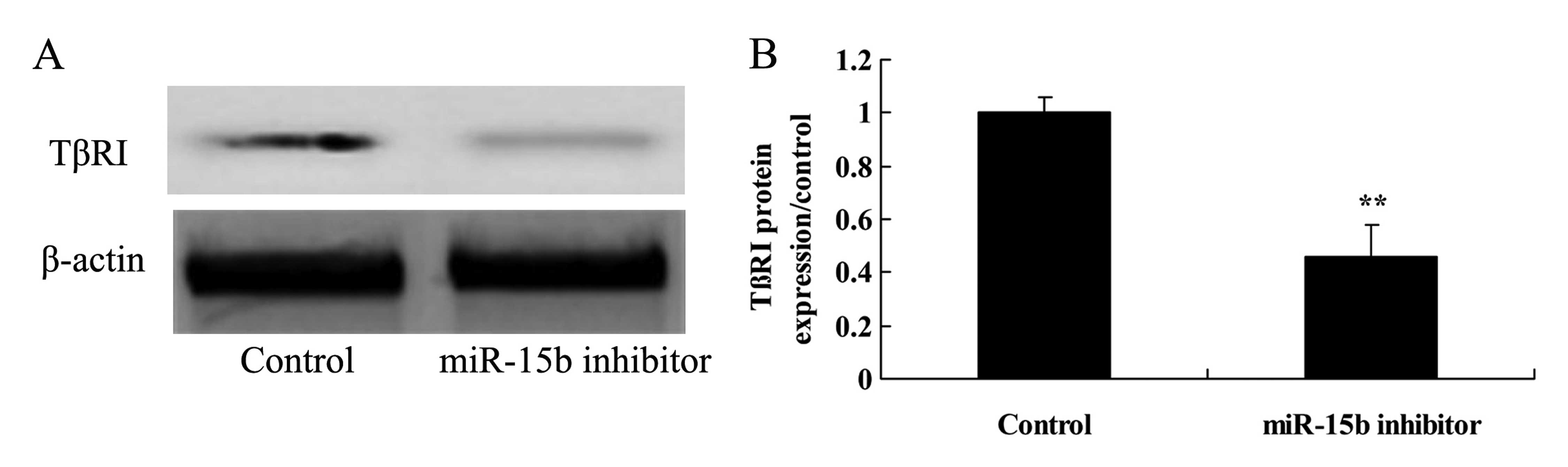
Effect of the downregulation of miR-15b
expression on TβRI in the HepG2 cells
To reveal the effect of the downregulation of
miR-15B on TβRI in the HepG2 cells, TβRI protein expression was
assessed using western blot analysis. The protein expression of
TβRI was effectively inhibited by the downregulation of miR-15b,
compared with the control group (Fig.
9).
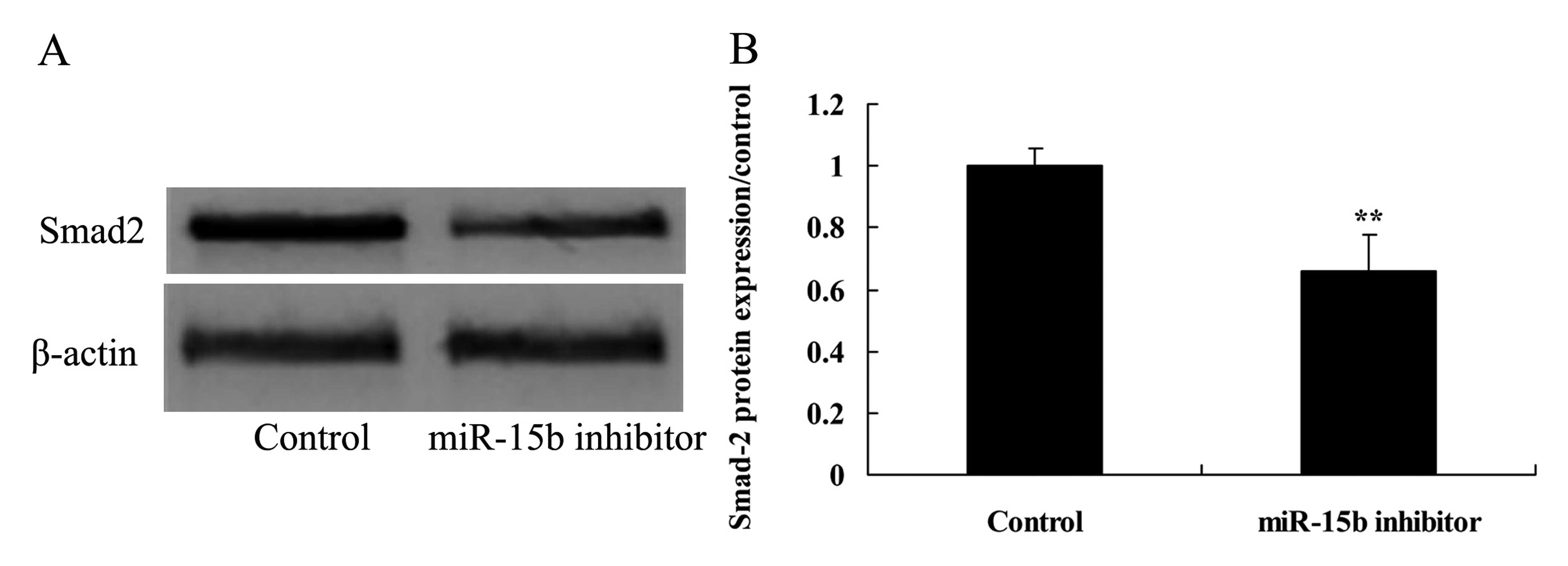
Effect of the downregulation of miR-15b
expression on Smad2 in the HepG2 cells
To further understand the functional effect of Smad2
in the HepG2 cells transfected by anti-miR-15b, Smad2 protein
expression was determined using western blot analysis. The results
of the western blot analysis demonstrated that downregulation of
miR-15b observably suppressed the protein expression of Smad2 in
the HepG2 cells, compared with the control group (Fig. 10).
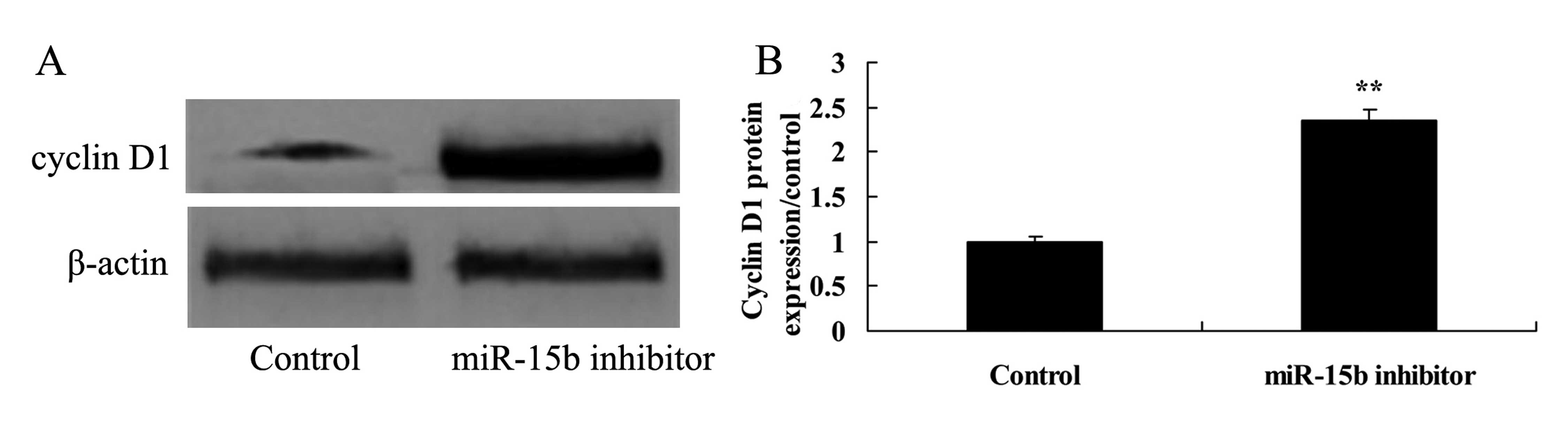
Effect of the downregulation of miR-15b
expression on the level of cyclin D1 in the HepG2 cells
Next, we assessed the effect of the downregulation
of miR-15b expression on cyclin D1 in the HepG2 cells. Cyclin D1
protein expression was detected using western blot analysis. As
shown in Fig. 11, cyclin D1
protein expression was markedly enhanced by the downregulation of
the expression of miR-15b, compared with the control group.
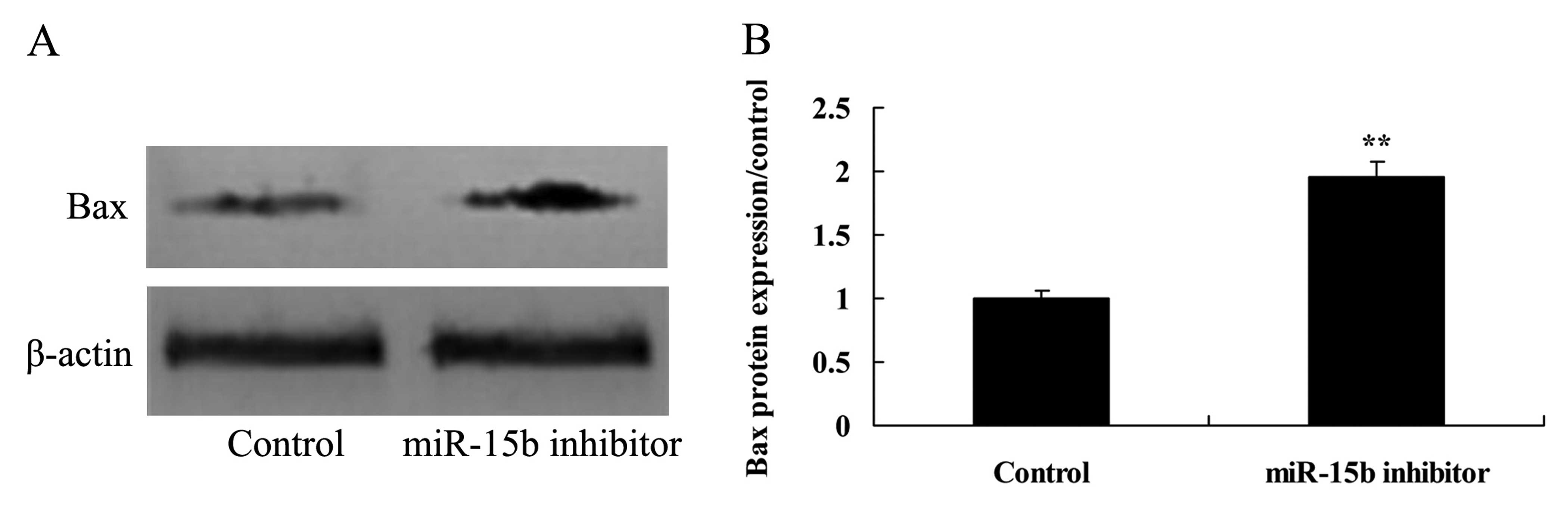
Effect of the downregulation of miR-15b
expression on the level of Bax in the HepG2 cells
To this end, we co-transfected HepG2 cells with
anti-miR-15b, and the protein expression of Bax was analyzed using
western blot analysis. Bax protein expression was significantly
activated by the downregulation of the expression of miR-15b,
compared with the control group (Fig.
12).
Discussion
Identification of the risk factors of post-operative
recurrence for HCC are an urgent quest. Research suggests that
these high risk factors involve many aspects, such as surgical
techniques, HCC background, tumor characteristics and perioperative
treatment (1). These factors are
reciprocal and synergistic. It is important for strict monitoring
to detect postoperative recurrence (2). However, it is obviously unsuitable
from the perspective of health economics to provide all patients
with frequent comprehensive examinations. Consequently, it is
essential for hepatic surgeons to properly control the factors
associated with postoperative recurrence and identify high-risk
populations to carry out suitable reexaminations and conduct
subsequent adjuvant therapy (3).
Here our results suggest that miR-15b promoted the proliferation of
HCC cells, and downregulation of the expression of miR-15b
suppressed cell growth and induced the apoptosis of HepG2
cells.
In the liver, TGF-β can be secreted by various types
of cells, including parenchymal and mesenchymal cells, such as
endothelial cells, hepatic cells and HSCs (4). During developmental processes of
hepatic fibrosis, TGF-β can activate fibrotic cytokines and promote
HSCs to differentiate into myofibroblasts (5). Continuous stimulation of TGF-β can
result in the accumulation of ECM and the synthesis of tissue
inhibitor of metalloproteinases facilitating the production of
hepatic fibrosis (6). In the
present study, we showed that downregulation of the expression of
miR-15b suppressed the growth of HCC HepG2 cells through the TGF-β
signal. Furthermore, recent reports have identified that the
microRNA-15 family inhibits the TGFβ pathway in the heart (7).
Smad protein molecules inhibit hepatic fibrosis by
inhibiting the formation, phosphorylation and nuclear translocation
of Smad protein molecules (8).
After injection of adenovirus vector with Smad7 cDNA to rats, rat
collagen contents were found to be reduced while the activation of
HSCs was inhibited (8).
Furthermore, in an in vitro experiment it was found that
Smad7 influenced hepatic fibrosis through inhibition of
phosphorylation of Smad2/3 and nuclear translocation of activated
Smad (9). Hepatic fibrosis can be
inhibited by reducing the contents of Smad3 and Smad4 or decreasing
their activities. Importantly, our data suggest that downregulation
of the expression of miR-15b observably suppressed Smad2 signaling
in the HepG2 cells.
Smad7 could freeze activated Smad1 and Smad2 to
degrade them. Therefore, ectopic expression of Smad2 decreases
expression of Smad1 and Smad2 while expression of Smad3 cannot be
decreased (10). Smads2 is
increased in the livers of rats with fibrosis while it was
decreased in cirrhotic rats and humans (11). Overexpressed Smad2 reduces the
production of HSC collagen. In humans, overexpression of Smad2
decreased levels of TβRI and Smad7 (12). Meanwhile, levels of ECM and laminin
were decreased. Stimulated by TGF-β, Smad2 recruits Smad7 to form
compounds in the cell nucleus (9).
Under stimulation of TGF-β, compounds are transferred into the
cytoplasm. Subsequently, these compounds interact with TβRI. Our
observations revealed that downregulation of the expression of
miR-15b observably inhibited TGF-β protein expression in the HepG2
cells through TβRI signal. Zhang et al demonstrated that
miR-15b promotes epithelial-mesenchymal transition of pancreatic
cancer through TGF-β/TβRI signaling (13)
The Bcl-2 family are apoptosis factors and Bcl-2 is
the strongest anti-apoptotic factor which is closely associated
with the incidence and tolerance of tumors (14). Bcl-2 and Bax are antagonists and
function in the form of a dipolymer. If expression levels are
balanced, the cell lifespan is normal (15). When the quantity of Bcl-2 is
relatively high, the heterodimer Bcl-2/Bax is formed. When the
quantity of Bax is relatively high, the homodimer Bax/Bax is
formed, which promotes apoptosis by inhibiting the anti-apoptotic
functions of Bcl-2 (16,17). Therefore, it is generally thought
that Bcl-2 and Bax play a fundamental role in cell apoptosis.
Caspases directly participate in the early initiation of apoptosis
and the transmission of apoptotic signaling while at the advanced
stage they produce apoptosis characteristics such as shrinkage,
nuclear division and DNA fracture (18). Caspases are located at the central
position of the apoptosis process. Caspase-3 is the key
administrator of apoptosis (19).
It is clear from our data, that downregulation of the expression of
miR-15b significantly activated Bax protein expression and
caspase-3 activity in the HepG2 cells. Liu et al found that
knockdown of NDRG2 sensitized cervical cancer HeLa cells through
miR-15b and miR-16 expression mediated by Bcl-2 and Bax (17).
Taken together, our results demonstrate that miR-15b
plays a role in the poor prognosis of HCC patients after curative
hepatectomy. This outcome suggests that miR-15b is a potential
therapeutic target for HCC. Our results showed that miR-15b
mediates cell growth and induces apoptosis of liver cancer,
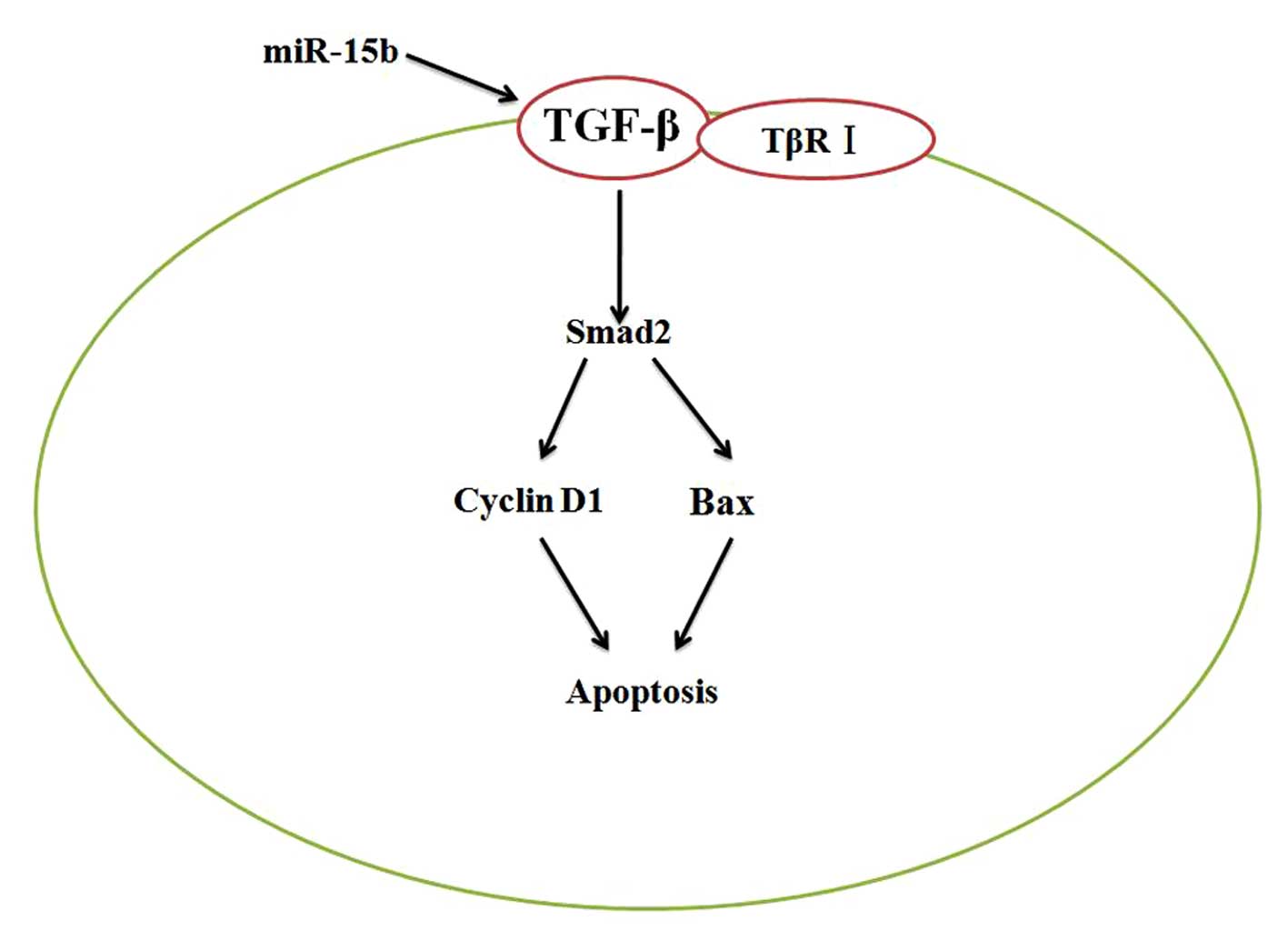
possibly by directly targeting the TGF-β/TβRI-Smad2-cyclin D1/Bax
signaling pathway (Fig. 13).
Collectively, these data suggest that miR-15b and the
TGF-β/TβRI-Smad2-cyclin D1/Bax signaling pathway may be potential
therapeutic targets for HCC after curative hepatectomy and deserve
further study.
References
|
1
|
El-Kady NM, Esmat G, Mahmoud EH, Darweesh
SK, Mahmoud SH and Elagawy WA: Hypertonic saline-enhanced
radiofrequency versus chemoembolization sequential radiofrequency
in the treatment of large hepatocellular carcinoma. Eur J
Gastroenterol Hepatol. 25:628–633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fernandez-Banet J, Lee NP, Chan KT, Gao H,
Liu X, Sung WK, Tan W, Fan ST, Poon RT, Li S, et al: Decoding
complex patterns of genomic rearrangement in hepatocellular
carcinoma. Genomics. 103:189–203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sarwar S, Khan AA and Tarique S: Validity
of alpha fetoprotein for diagnosis of hepatocellular carcinoma in
cirrhosis. J Coll Physicians Surg Pak. 24:18–22. 2014.PubMed/NCBI
|
|
4
|
Jiang F, Wang X, Liu Q, Shen J, Li Z, Li Y
and Zhang J: Inhibition of TGF-β/SMAD3/NF-κB signaling by
microRNA-491 is involved in arsenic trioxide-induced
anti-angiogenesis in hepatocellular carcinoma cells. Toxicol Lett.
231:55–61. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lou XL, Deng J, Deng H, Ting Y, Zhou L,
Liu YH, Hu JP, Huang XF and Qi XQ: Aspirin inhibit platelet-induced
epithelial-to-mesenchymal transition of circulating tumor cells
(Review). Biomed Rep. 2:331–334. 2014.PubMed/NCBI
|
|
6
|
Yang J, Zheng J, Wu L, Shi M, Zhang H,
Wang X, Xia N, Wang D, Liu X, Yao L, et al: NDRG2 ameliorates
hepatic fibrosis by inhibiting the TGF-β1/Smad pathway and altering
the MMP2/TIMP2 ratio in rats. PLoS One. 6:e277102011. View Article : Google Scholar
|
|
7
|
Tijsen AJ, van der Made I, van den
Hoogenhof MM, Wijnen WJ, van Deel ED, de Groot NE, Alekseev S,
Fluiter K, Schroen B, Goumans MJ, et al: The microRNA-15 family
inhibits the TGFβ-pathway in the heart. Cardiovasc Res. 104:61–71.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gal A, Sjöblom T, Fedorova L, Imreh S,
Beug H and Moustakas A: Sustained TGF beta exposure suppresses Smad
and non-Smad signalling in mammary epithelial cells, leading to EMT
and inhibition of growth arrest and apoptosis. Oncogene.
27:1218–1230. 2008. View Article : Google Scholar
|
|
9
|
Chegini N, Luo X, Ding L and Ripley D: The
expression of Smads and transforming growth factor beta receptors
in leiomyoma and myometrium and the effect of gonadotropin
releasing hormone analogue therapy. Mol Cell Endocrinol. 209:9–16.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jeong MH, Kim SJ, Kang H, Park KW, Park
WJ, Yang SY and Yang DK: Cucurbitacin I attenuates cardiomyocyte
hypertrophy via inhibition of connective tissue growth factor
(CCN2) and TGF-β/Smads signalings. PLoS One. 10:e01362362015.
View Article : Google Scholar
|
|
11
|
Choi K, Lee K, Ryu SW, Im M, Kook KH and
Choi C: Pirfenidone inhibits transforming growth factor-β1-induced
fibrogenesis by blocking nuclear translocation of Smads in human
retinal pigment epithelial cell line ARPE-19. Mol Vis.
18:1010–1020. 2012.
|
|
12
|
Zheng F, Lu W, Wu F, Li H, Hu X and Zhang
F: Recombinant decorin ameliorates the pulmonary structure
alterations by downregulating transforming growth
factor-beta1/SMADS signaling in the diabetic rats. Endocr Res.
35:35–49. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang WL, Zhang JH, Wu XZ, Yan T and Lv W:
miR-15b promotes epithelial-mesenchymal transition by inhibiting
SMURF2 in pancreatic cancer. Int J Oncol. 47:1043–1053.
2015.PubMed/NCBI
|
|
14
|
Li CL, Chang L, Guo L, Zhao D, Liu HB,
Wang QS, Zhang P, Du WZ, Liu X, Zhang HT, et al: β-elemene induces
caspase-dependent apoptosis in human glioma cells in vitro through
the upregulation of Bax and Fas/FasL and downregulation of Bcl-2.
Asian Pac J Cancer Prev. 15:10407–10412. 2014. View Article : Google Scholar
|
|
15
|
Reed JC: Proapoptotic multidomain
Bcl-2/Bax-family proteins: Mechanisms, physiological roles, and
therapeutic opportunities. Cell Death Differ. 13:1378–1386. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cory S and Adams JM: Killing cancer cells
by flipping the Bcl-2/Bax switch. Cancer Cell. 8:5–6. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu J, Yang L, Zhang J, Zhang J, Chen Y,
Li K, Li Y, Li Y, Yao L and Guo G: Knock-down of NDRG2 sensitizes
cervical cancer Hela cells to cisplatin through suppressing Bcl-2
expression. BMC Cancer. 12:3702012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ryoo HD and Bergmann A: The role of
apoptosis-induced proliferation for regeneration and cancer. Cold
Spring Harb Perspect Biol. 4:a0087972012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen H, Yang X, Feng Z, Tang R, Ren F, Wei
K and Chen G: Prognostic value of caspase-3 expression in cancers
of digestive tract: A meta-analysis and systematic review. Int J
Clin Exp Med. 8:10225–10234. 2015.PubMed/NCBI
|