Introduction
Majority of patients with epithelial ovarian
carcinoma (EOC) are not diagnosed until advanced stage (III or IV)
characterized by widespread peritoneal dissemination and ascites.
Along with rapid occurrence of chemoresistance, this brings down
the 5-year survival of EOC to ~30% (1). Ovarian tumor cells primarily exfoliate
from primary site and shed into the peritoneal cavity where they
can implant onto the mesothelial layer covering the abdominal
organ, which is a crucial step in the formation of secondary foci
and EOC progression (2).
Identifying critical molecules involved in the interaction between
the exfoliated tumor cells and the peritoneal micro-environment may
not only advance our understanding of the disease, but may
ultimately enable us to develop effective therapeutic
interventions.
It is generally accepted that the initial step of
ovarian cancer metastasis is adhesion of cancer cells to the
mesothelium lining the peritoneum, mesentery and omentum (3). Impeding this attachment process could
be a way of blocking ovarian cancer dissemination. The mechanistic
explanation concerning the adherence progress remains poorly
elucidated. Integrins that mediated binding to extracellular matrix
(ECM) elements such as fibronectin (Fn), laminin and collagen, and
CD44 that facilitated binding to hyaluronan expressed on the
surface of mesothelium were identified as key orchestrators
(4,5). Among the integrin family, integrin α5
(ITGA5) and integrin β1 (ITGB1) are of particular interest in
ovarian cancer malignant behavior (6–8).
Integrin α5 primarily combines with integrin β1
subunit to form α5β1 heterodimer and recognizes its specific ligand
Fn, which is one of the most plentiful proteins in ECM of the
peritoneal cavity (9,10). Once ovarian cancer cells have
detached from the primary site, they float in the ascites as single
cells or as multicellular spheroids (11,12).
Previous studies revealed that loss of E-cadherin in ovarian cancer
cells was accompanied with the elevation of integrin α5, which
facilitates colonization of tumor cells at secondary sites
(8). Recent research showed that
the upregulation of c-Met during tumor progression, contributed to
ovarian cancer peritoneal propagation through an integrin
α5β1-dependent mechanism (13).
These accumulative findings strongly imply that integrin α5β1 could
be a potential therapeutic target, at least for a subset of EOC
patients.
A growing body of evidence suggests that miRNAs,
small non-coding RNAs ~22 nucleotides in length, are capable of
regulating gene expression through matching to target genes, either
completely or partially, at the 3′-untranslated region (3′UTR),
causing the suppression of protein translation or mRNA degradation
(14). More than 1,500 different
miRNAs have been identified related to cancer development in human,
and these miRNAs have the potential to regulate up to 30% of human
genes (15). In the present study,
we set to identify the miRNAs that regulate integrin α5β1
expression thus indirectly dominating ovarian cancer dissemination,
and to evaluate the therapeutic potential of targeting this miRNA.
Eventually, we identified hsa-mir-17 (miR-17) as a candidate.
Overexpression of miR-17 reduced integrin α5β1 expression in
ovarian cancer cells, accompanied with the inhibition of cell
adhesion and invasion. In in vivo ovarian cancer xenografts,
lentiviral transfection of miR-17 into SKOV3-Luc ovarian cancer
cells evidently reduced peritoneal dissemination. These results
suggested that miR-17 may be exploited as intervention target for
patients with ovarian cancer. Further research may be needed to
develop future clinical applications.
Materials and methods
Cells culture and transfection
The human ovarian cancer cell lines SKOV3, CaOV3,
OV-90, OVCAR3, CaOV4, ES2, TOV-21G and Tov-112D were purchased from
the American Type Culture Collection (ATCC; Manassas, VA, USA).
These cells were maintained in McCoy's 5A, RPMI-1640 medium or
Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal
bovine serum (FBS) according to the manual on ATCC website. SKOV3
cells were transduced with CMV-Fluc-IRES-GFP lentiviral plasmid
(GeneChem, Shanghai, China) and designated as SKOV3-Luc.
GFP-positive cells were enriched through FACS and further used
in vivo in adhesion assay and living imaging. Transient
transfections of the miR-17 mimics, miR-inhibitor and scrambled
negative control (RiboBio, Guangzhou, China) were conducted using
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer's instructions.
SKOV3-Luc cells were transduced with hsa-mir-17 or
mir-control lentivirus particles (GeneChem) according to the
manufacturer's protocol, the stable transfected cells were selected
by continuous exposure to puromycin (Invitrogen). The transduced
cells were designated as SKOV3-Luc-miR-17 and SKOV3-Luc-NC,
respectively.
Western blot assay
Western blotting was performed as described in our
previous study (16). The Abs of
ITGA5, ITGB1, p-ILK, matrix metalloproteinase-2 (MMP-2) and GAPDH
were obtained from Epitomics (Burlingame, CA, USA).
RNA extraction and qRT-PCR
Total RNAs were extracted from cultured cells using
the RNA simple total RNA extraction kit (Tiangen, Beijing, China)
according to the manufacturer's instructions. The expression of
ITGA5 and ITGB1 was determined by the SYBR-Green qPCR, and GAPDH
was used as the loading control. The relative gene expression
normalized to GAPDH was quantified using the comparative
CT method, and expressed as fold-change relative to the
control.
miRNA and mRNA correlation of NCI60
database
Normalized miRNA and mRNA data collections of 60
cancer cell lines were obtained from NCI60 online source
(http://discover.nci.nih.gov/cellminer/). Pearson's
correlation analysis was performed between the expressional levels
of hsa-mir-17 and the ITGA5 and ITGB1 probe set, respectively.
Dual luciferase reporter assay
The specific target sequence of miR-17 in the 3′UTR
region of human ITGA5 and ITGB1 was predicted with TargetScan
(www.TargetScan.org). ITGA5 and ITGB1
3′UTR and their variant sequence with three nucleotides mutated in
the miR-17 target site were synthesized and cloned into psi-CHECK2
vector downstream of Rellina to generate the recombinant
vectors, psi-CHECK2-ITGA5/ITGB1-3′UTR-wt and
psi-CHECK2-ITGA5/ITGB1-3′UTR-mt, respectively. SKOV3 cells were
co-transfected with the generated plasmids and miR-17 mimics or
miR-17 inhibitor. Luciferase activity was determined using the
Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA)
after 48 h of incubation. Rellina luciferase values were
normalized to firefly luciferase values.
Transwell invasion assay
The invasion assay was performed using an
8-μm pore size chamber (Corning, Corning, NY, USA) coated
with 50 μl Matrigel (BD Biosciences, Franklin Lakes, NJ,
USA) 1:6 diluted with serum-free DMEM. The lower compartment was
filled with 600 μl medium containing 20% FBS as the
chemoattractant. A total of 2×104 cells/100 μl
were seeded into the upper compartment and incubated for 24 h at
37°C. Following removal of the non-migratory cells with a cotton
swab, the remaining invaded cells at the lower surface of the
filter were fixed with cold methanol for 15 min and stained with
crystal violet for 10 min. The number of invasive cells were
calculated by counting 6 random fields at a magnification of ×100
(Leica, Solms, Germany).
In vitro and in vivo adhesion assays
For the in vitro adhesion assay, 96-well
plates were coated with 10 μg/ml Fn, 10 μg/ml type IV
collagen or 5 μg/ml laminin in phosphate-buffered saline
(PBS) overnight at 4°C. The wells were thoroughly washed with PBS,
and then blocked with 3% BSA for 1 h at 37°C. Approximately 100
μl of serum-free medium containing 104 cells in
each group were added to the coated wells and incubated for up to
1/2 h at 37°C. The wells were gently rinsed with PBS to remove
non-adherent cells. The attached cells were counted at different
fields under a light microscope. To study early adhesion of ovarian
cancer cells in mouse abdomen, in vivo adhesion assay was
performed, 106 SKOV3-GFP cells were injected into the
peritoneal cavity of female athymic nude mice. Four hours later,
mice were sacrificed, small bowel mesenterium and omentum were
excised. After the tissues were gently washed with PBS to eliminate
non-adherent cells, the adherent cells were observed and
photographed under the fluorescence.
Immunohistochemistry
Immunohistochemical staining for ITGA5, ITGB1, p-ILK
and MMP-2 expression were conducted following standard procedures.
Briefly, sections were deparaffinized and then immersed in 3%
H2O2 to inactivate endogenous peroxidase. The
slides were blocked with goat serum for 30 min and further
incubated with the antibody (ITGA5, 1:150; ITGB1, 1:100; p-ILK,
1:50; and MMP-2, 1:50) overnight at 4°C after antigen retrieval
process. The next day, the slides were exposed to horseradish
peroxidase-linked secondary antibody for 20 min. Finally the slides
were developed in DAB solution for optimal staining intensity.
Experimental metastasis model
The animal studies were approved by the Committee on
the Ethics of Animal Experiments of Tongji Medical College. Female
NOD/SCID mice (4–6 weeks old) were purchased from the Beijing HFK
Bio-Technology (Beijing, China) and maintained in a laminar flow
cabinet under specific pathogen-free conditions. SKOV3-Luc-miR-17
or SKOV3-Luc-NC cells (2×106) were injected into
peritoneal cavity (10 mice/group). The tumor growth was monitored
every 5 days until 30 days from tumor implantation. Mice were
anaesthetized with 1% pentobarbital sodium, and imaged with the
IVIS Spectrum System (Caliper; Xenogen, USA) 15 min after
intraperitoneal dosage of 100 mg/kg D-luciferin substrate. Total
flux (photons/s) and metastasis localization was analyzed using
Living Image version 4.3.1 software.
Statistical analysis
SPSS (version 16.0) software package (SPPS, Inc.,
Chicago, IL, USA) was used for all the statistical procedures. All
the experimental data are presented as means ± SD. Correlations
were analyzed using the Pearson's test. The Student's t-test
(two-tailed) was used to determine statistical differences between
two experimental groups; difference at P<0.05 was considered to
indicate statistical significance.
Results
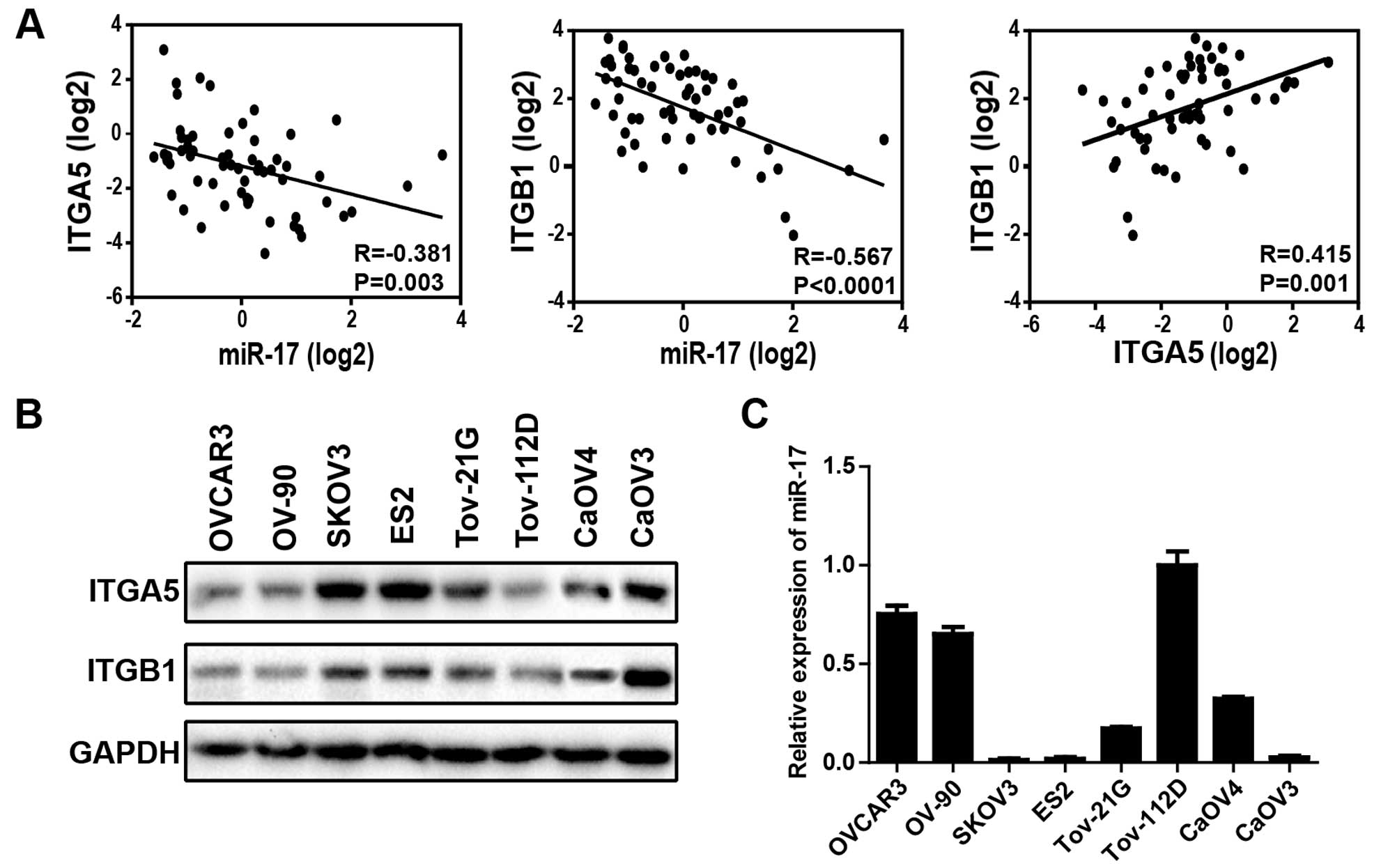
Expression level of miR-17 inversely
correlates with that of the ITGA5 and ITGB1 in ovarian cancer cell
lines
To determine whether the expression of miR-17 and
ITGA5 and ITGB1 were correlated, the US NCI60 (National Cancer
Institute) database was used, which covers 60 various human cancer
cell lines, including five ovarian cancer lines. Data were
extracted to analyze the correlation between miR-17 and ITGA5,
ITGB1 expression in different types of cancer cell lines. The
miR-17 expression level was significantly inversely correlated with
ITGA5 (R=−0.381; P=0.003) as well as ITGB1 (R=−0.567; P<0.0001)
expression, ITGA5 and ITGB1 are positively correlated (R=0.415;
P=0.001) (Fig. 1A), strongly
suggesting that miR-17 is one of the pivotal regulators of ITGA5
and ITGB1 in cancer cells. Next, we measured ITGA5 and ITGB1
expression by western blotting (Fig.
1B) and miR-17 expression by miRNA RT-PCR (Fig. 1C) in eight different ovarian cancer
cell lines. Five (SKOV3, ES2, TOV-21G, CaOV3 and CaOV3) of eight
cell lines expressed high levels of ITGA5 and ITGB1, the remaining
three (OVCAR3, OV-90 and TOV-112D) expressed relatively low level
of ITGA5 and ITGB1. Conversely, miR-17 relative expression levels
were found to be significantly lower for the five ovarian cancer
cell lines that expressed high levels of ITGA5 and ITGB1. These
observations indicated that miR-17 was a likely regulator of ITGA5
and ITGB1 in ovarian cancer cell lines.
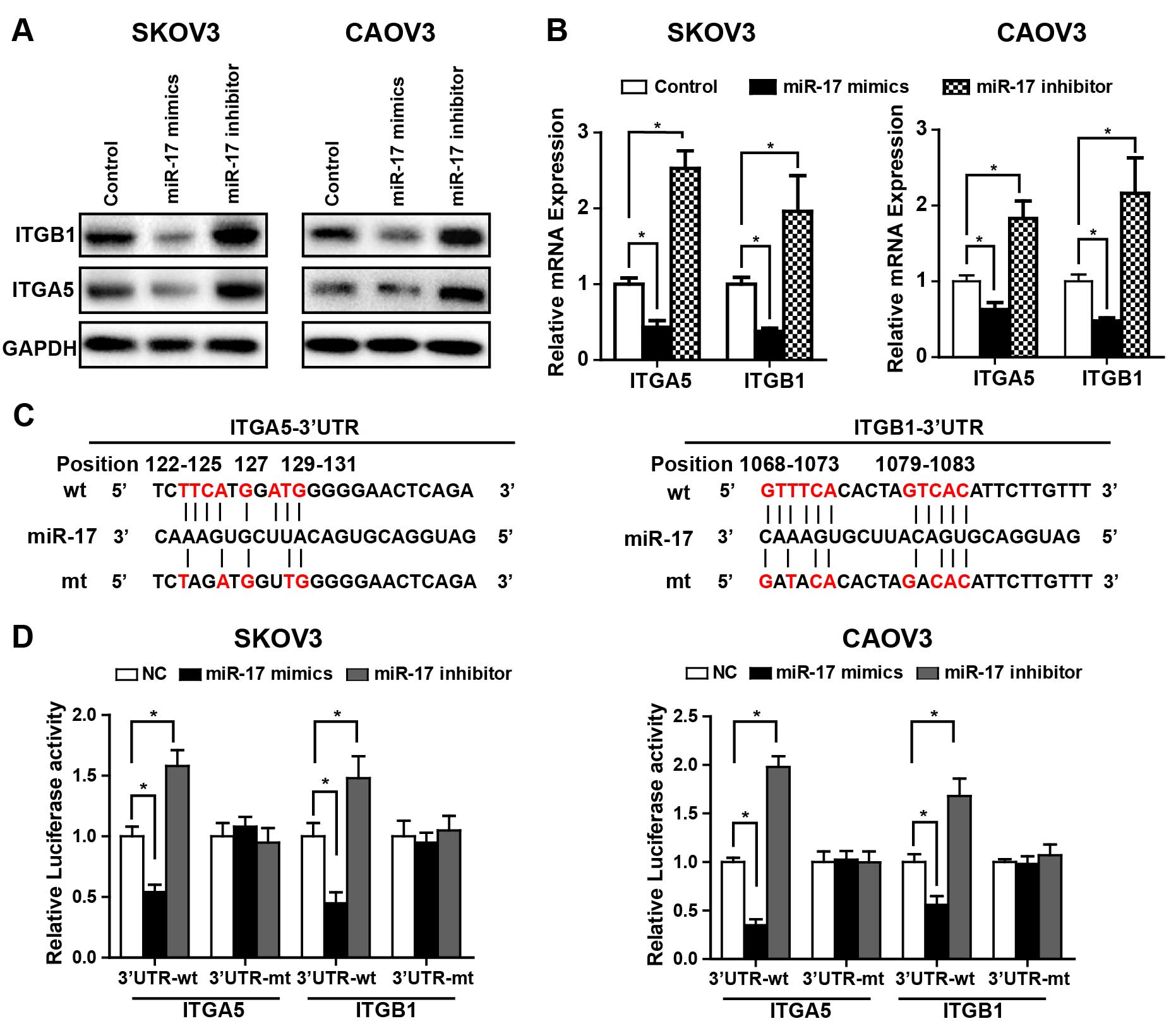
ITGA5 and ITGB1 are directly targeted by
miR-17 in ovarian cancer cell lines
Based on the above findings, we next set to clarify
whether ITGA5 and ITGB1 are direct targets of miR-17. Western blot
and qRT-PCR analyses revealed that miR-17 overexpression caused a
significant decrease in ITGA5 and ITGB1 mRNA and protein level in
SKOV-3 and CaOV3 cells while silencing of miR-17 augments ITGA5 and
ITGB1 expression in these cell lines (Fig. 2A and B). To further explore whether
miR-17 directly targets the 3′UTR of ITGA5 and ITGB1 mRNA, we
performed a dual luciferase reporter assay. The 3′UTR of both ITGA5
and ITGB1 mRNA contains conserved predicted binding site for
miR-17, two reporters, one containing wild-type ITGA5 or ITGB1
3′UTR with the miR-17 binding site and another containing mutant
3′UTR were constructed (Fig. 2C). A
significant decrease in relative luciferase activity was observed
in ovarian cancer cells (SKOV3 and CaOV3) transfected with miR-17
mimics when compared with NC miRNA. In contrast, inhibition of
miR-17 caused a notable increase in relative luciferase activity.
Nevertheless, both the miR-17 mimics and inhibitor failed to affect
the activity of mutant reporter construct, indicating a specific
direct interaction of miR-17 with the 3′UTR of ITGA5 and ITGB1
mRNA. Collectively, these data showed a directly suppression of
ITGA5 and ITGB1 by miR-17 in ovarian cancer cell lines.
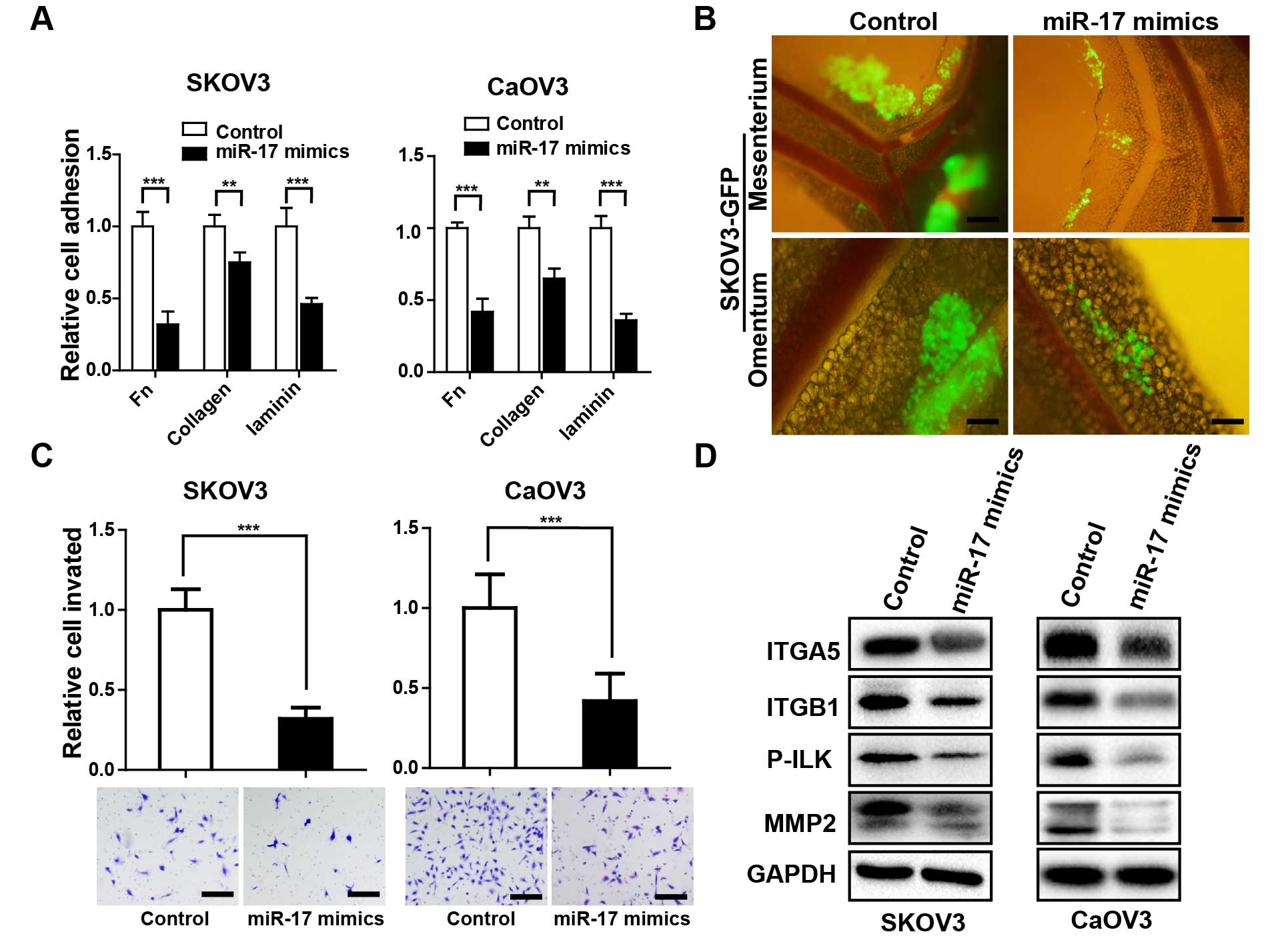
miR-17 suppresses in vitro and in vivo
adhesion, invasion capability of ovarian cancer cell lines
Due to the recognized role of ITGA5 and ITGB1 in
ovarian cancer progression (4,8), we
enforced miR-17 expression in ovarian cancer cells and evaluated
the effects on cell adhesion and invasion. miR-17 transfection
markedly diminished cell adhesion onto Fn, collagen and laminin in
SKOV3 and CaOV3 cells (Fig. 3A).
Next, we transferred the adhesion assay into the mice peritoneal
cavity, similar finding was observed that miR-17 overexpressed
SKOV3-GFP cells presented evident diminished cell aggregates
adhesion in mice mesentery and omentum region (Fig. 3B). Since invasion is the subsequent
event following adhesion in ovarian cancer metastasis (17). Then, we detected whether miR-17
overexpression also affects the invasive capacity of ovarian cancer
cells. Enforced expression of miR-17 significantly impaired the
invasion of SKOV3 and CaOV3 cells (Fig.
3C). Remodeling of cell-ECM interactions via integrins provokes
a cascade of phosphorylation of signal transduction molecules at
focal adhesions (18).
Phosphorylation of ILK is known to be particularly crucial in
integrin-mediated outside-in signal transduction pathways, which
regulate various gene expression or cell behavior, such as cell
dispersal and migration (19,20).
Enforced expression of miR-17 drastically inhibited ITGA5 and ITGB1
expression, and the phosphorylation of ILK, followed by the
downregulation of MMP-2, which is believed to play a pivotal role
in ovarian cancer invasion and metastasis (Fig. 3D) (21).
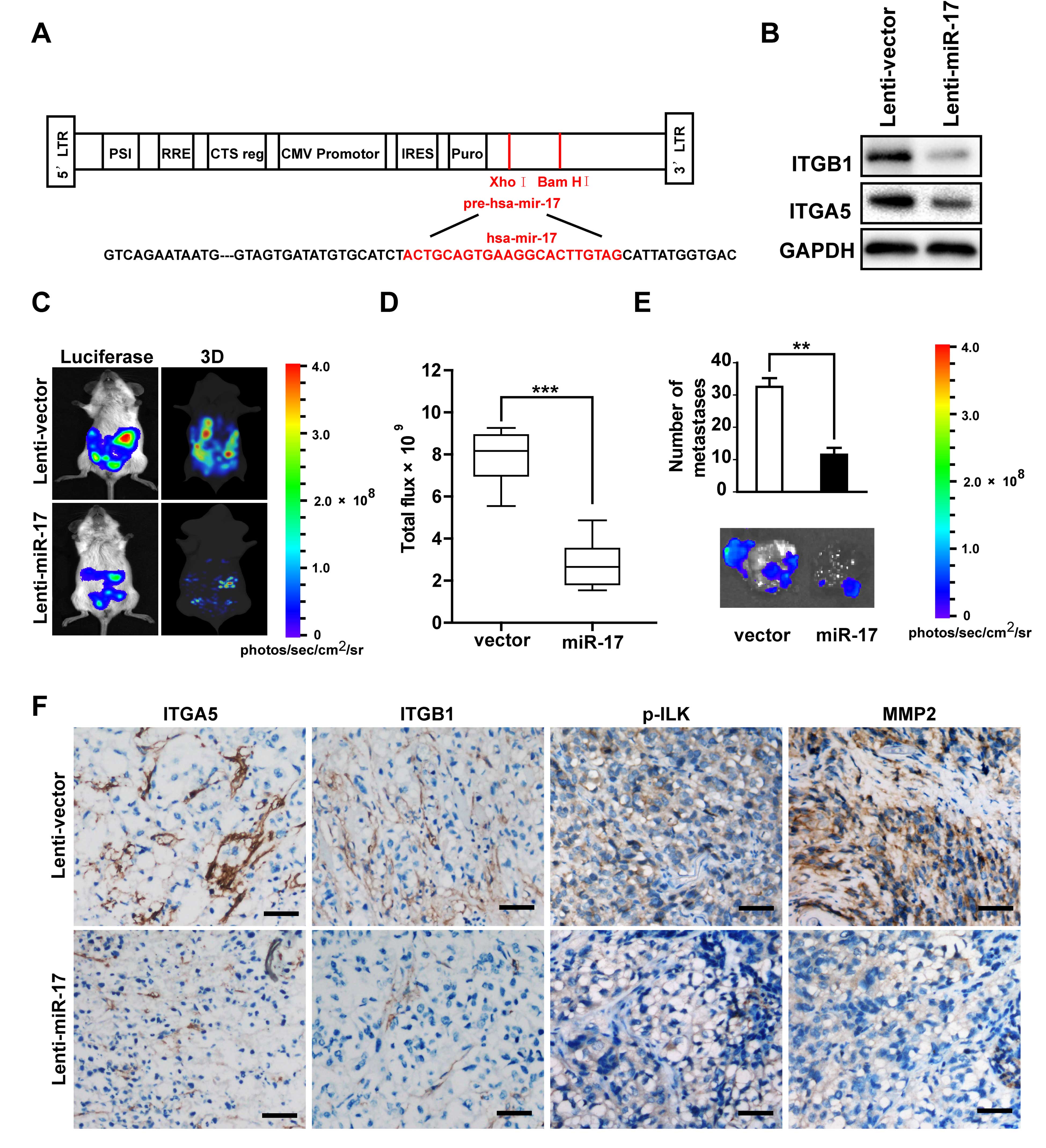
miR-17 inhibits peritoneal dissemination
in an ovarian cancer xenograft model
In light of the evident inhibitory effect of
adhesion and invasion by miR-17 in ovarian cancer cells through
repressing ITGA5 and ITGB1 expression, we further looked into the
restorative effect of enforced expression of miR-17 in ovarian
cancer i.p. implantation xenograft model. Lentiviruses with the
miR-17 precursor (Fig. 4A) or
scrambled miRNA were constructed and stably transfected into
SKOV3-Luc cells. Downregulation of ITGA5 and ITGB1 expression were
confirmed after miR-17 overexpression (Fig. 4B).
Tumor cell propagation in the peritoneal cavity of
mice were assessed 1 month after i.p. injection, in vivo
bioluminescence images showed that mice inoculated with cells that
overexpressed miR-17 presented markedly reduced metastatic lesions
in the abdomen, particularly on the peritoneal surface, small-bowel
mesentery, omentum region with 3D-reconstruction (Fig. 4C). As a result, the total tumor
burden in the SKOV3-LUC-miR-17 group was attenuated markedly
compared with the SKOV3-LUC-NC group (Fig. 4D). Consistently, the
SKOV3-LUC-miR-17 cells also developed more and larger nodules than
those generated by SKOV3-LUC-NC cells (Fig. 4E). Immunohistochemical staining
analysis also further demonstrated that miR-17 overexpression
endowed cells with reduced ITGA5 and ITGB1, as well as weaker p-ILK
and MMP-2 expression. Taken together, these in vivo results
demonstrated the inhibitory action of miR-17 on the intraperitoneal
dissemination of ovarian cancer cells.
Discussion
Several years of intensive research has elucidated
the role of integrin family as a multifunctional ingredient
involved in a myriad of tumor cell processes, including adhesion,
invasion, ECM remodeling, angiogenesis and metastasis in human
cancer (22). In ovarian cancer,
integrin α5β1, αvβ3 and α2β1 are the main integrins that contribute
to poor prognosis (4,9). However, integrin α5β1 was proven to
participate in the tumor cell adherence and clearance of
mesothelial layer on the peritoneal surfaces (23), which is a crucial step in the
establishment of secondary foci, making it a very attractive
therapeutic target in ovarian cancer. Moreover, increased integrin
α5β1 levels correlated with chemotherapy resistance and poor
patient survival (3,24), while the dysregulation mechanism of
integrin α5β1 in tumorigenesis and metastasis are poorly elucidated
to date. Thus, it would be of great interest to explore the
possible mechanism of α5β1 disorder such as miRNAs.
Recent studies have emphasized the pivotal roles of
miRNAs in cancer development and progression, they contribute to
almost all aspects of cancer biology such as proliferation,
apoptosis, angiogenesis and metastasis (14,15).
In the present study, using the US database of NCI60, we identified
that miR-17 expression was inversely correlated with both integrin
α5 and β1 levels in human cancer cells. Our experiments further
demonstrated the negative correlation between miR-17 and integrin
α5β1 in several ovarian cancer cell lines. This hypothesis was
substantially confirmed by our findings that miR-17 directly binds
to the 3′UTR region of integrin α5 and β1 in a luciferase reporter
system. miR-17 belongs to the miR-17-92 cluster, which comprises
six members: miR-17, -1 8a, -19a, -20a, -19b-1 and -92a. Previous
studies designated the miR-17-92 cluster as cancer driver for their
general overexpression in several types of cancer (25). As far as ovarian cancer, it seemed
contrasting that a miR-17-92 cluster member miR-92a had been proved
to inhibit ovarian cancer peritoneal dissemination (3). However, miR-17 had been shown to
promote the proliferation of gastric and cervical cancer cells, and
metastasis of osteosarcoma and colorectal cancer (26–29).
According to our results, miR-17 suppressed the in vitro and
in vivo adhesion of ovarian cancer cells, accompanied by a
diminished invasive capacity. These observations made us explore a
more comprehensive understanding of the role of miR-17 in ovarian
cancer peritoneal metastasis.
Evidence exists of integrin communication with ECM
molecules accelerating the adhesion and invasion of cancer cells,
and regulating the metastatic potential of tumor cells through
integrin-linked kinase (ILK)-mediated signaling (19). The data presented in the present
study clearly showed that miR-17 overexpression reduced the protein
level of integrin α5 and β1, hampering the phosphorylation of ILK,
inhibiting production of active MMP-2. Therefore, the
miR-17-mediated downregulation of α5β1 expression not only
suppressed the adhesion and invasion of tumor cells, but also
blocked ECM-α5β1-mediated signal transduction to regulate various
gene expression in tumor cells. The production of MMPs plays the
leading role in integrin-induced remodeling and degradation of ECM,
which is indispensible for tumor cell metastasis. Reducing the
amount of active MMPs in the tumor niche may be one of the reasons
contributing to inhibited α5β1-mediated invasion of tumor cells
exerted by miR-17. These results support the fact that integrin
α5β1 expression was closely associated with ovarian carcinoma
invasion involving MMP-2, which was in accordance with several
previous studies (30).
It was noteworthy that an ovarian cancer xenograft
model was chosen for further confirmation of forced expression of
miR-17 influence on peritoneal dissemination. Our present data
showed that miR-17 overexpressed SKOV3-Luc cells produced
remarkably fewer metastatic lesions, thus reduced the tumor burden
inside the abdominal cavity. Consistently, the IHC analysis
validated that ectopic expression of miR-17 caused the suppression
of integrin α5β1 as well as downstream p-ILK and active MMP-2 in
the tumor sections. In that sense, the inhibitors against integrin
α5β1 apparently indicate the possibility to be developed for
clinical use. Treatment of mice with the integrin α5β1 antibody was
reported to inhibit adhesion and peritoneal metastasis of ovarian
cancer cells (8). Monoclonal
antibody directly against integrin α5β1 (volociximab) was recently
invented and tested in preclinical trials with patients with
recurrent ovarian cancer (31).
Therefore, miR-17 may emerge as an alternative or more powerful
target for controlling ovarian cancer exacerbation.
In summary, our findings emphasized the
unanticipated regulation of integrin α5 and integrin β1 by miR-17
in ovarian cancer cells. Having provided novel evidence that miR-17
inhibited the adhesion and invasion of ovarian cancer cells by
repressing α5β1, the present study suggested that targeting miR-17
generated new possibilities in ovarian cancer treatment through the
inhibition of integrin α5β1.
References
|
1
|
Landen CN Jr, Birrer MJ and Sood AK: Early
events in the pathogenesis of epithelial ovarian cancer. J Clin
Oncol. 26:995–1005. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Engel J, Eckel R, Schubert-Fritschle G,
Kerr J, Kuhn W, Diebold J, Kimmig R, Rehbock J and Hölzel D:
Moderate progress for ovarian cancer in the last 20 years:
Prolongation of survival, but no improvement in the cure rate. Eur
J Cancer. 38:2435–2445. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ohyagi-Hara C, Sawada K, Kamiura S, Tomita
Y, Isobe A, Hashimoto K, Kinose Y, Mabuchi S, Hisamatsu T,
Takahashi T, et al: miR-92a inhibits peritoneal dissemination of
ovarian cancer cells by inhibiting integrin α5 expression. Am J
Pathol. 182:1876–1889. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lessan K, Aguiar DJ, Oegema T, Siebenson L
and Skubitz AP: CD44 and beta1 integrin mediate ovarian carcinoma
cell adhesion to peritoneal mesothelial cells. Am J Pathol.
154:1525–1537. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Strobel T and Cannistra SA:
Beta1-integrins partly mediate binding of ovarian cancer cells to
peritoneal mesothelium in vitro. Gynecol Oncol. 73:362–367. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sawada K, Ohyagi-Hara C, Kimura T and
Morishige K: Integrin inhibitors as a therapeutic agent for ovarian
cancer. J Oncol. 2012:9151402012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hodkinson PS, Elliott T, Wong WS, Rintoul
RC, Mackinnon AC, Haslett C and Sethi T: ECM overrides DNA
damage-induced cell cycle arrest and apoptosis in small-cell lung
cancer cells through beta1 integrin-dependent activation of
PI3-kinase. Cell Death Differ. 13:1776–1788. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sawada K, Mitra AK, Radjabi AR, Bhaskar V,
Kistner EO, Tretiakova M, Jagadeeswaran S, Montag A, Becker A,
Kenny HA, et al: Loss of E-cadherin promotes ovarian cancer
metastasis via alpha 5-integrin, which is a therapeutic target.
Cancer Res. 68:2329–2339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morgan MR, Byron A, Humphries MJ and Bass
MD: Giving off mixed signals–distinct functions of alpha5beta1 and
alphavbeta3 integrins in regulating cell behaviour. IUBMB Life.
61:731–738. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marelli UK, Rechenmacher F, Sobahi TR,
Mas-Moruno C and Kessler H: Tumor targeting via integrin ligands.
Front Oncol. 3:2222013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wintzell M, Hjerpe E, Åvall Lundqvist E
and Shoshan M: Protein markers of cancer-associated fibroblasts and
tumor-initiating cells reveal subpopulations in freshly isolated
ovarian cancer ascites. BMC Cancer. 12:3592012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Watanabe T, Hashimoto T, Sugino T, Soeda
S, Nishiyama H, Morimura Y, Yamada H, Goodison S and Fujimori K:
Production of IL1-beta by ovarian cancer cells induces mesothelial
cell beta1-integrin expression facilitating peritoneal
dissemination. J Ovarian Res. 5:72012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sawada K, Radjabi AR, Shinomiya N, Kistner
E, Kenny H, Becker AR, Turkyilmaz MA, Salgia R, Yamada SD, Vande
Woude GF, et al: c-Met overexpression is a prognostic factor in
ovarian cancer and an effective target for inhibition of peritoneal
dissemination and invasion. Cancer Res. 67:1670–1679. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun C, Li N, Yang Z, Zhou B, He Y, Weng D,
Fang Y, Wu P, Chen P, Yang X, et al: miR-9 regulation of BRCA1 and
ovarian cancer sensitivity to cisplatin and PARP inhibition. J Natl
Cancer Inst. 105:1750–1758. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li M, Li J, Ding X, He M and Cheng SY:
microRNA and cancer. AAPS J. 12:309–317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yi L, Zongyuan Y, Cheng G, Lingyun Z,
Guilian Y and Wei G: Quercetin enhances apoptotic effect of tumor
necrosis factor-related apoptosis-inducing ligand (TRAIL) in
ovarian cancer cells through reactive oxygen species (ROS) mediated
CCAAT enhancer-binding protein homologous protein (CHOP)-death
receptor 5 pathway. Cancer Sci. 105:520–527. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lengyel E: Ovarian cancer development and
metastasis. Am J Pathol. 177:1053–1064. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo W and Giancotti FG: Integrin
signalling during tumour progression. Nat Rev Mol Cell Biol.
5:816–826. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Persad S and Dedhar S: The role of
integrin-linked kinase (ILK) in cancer progression. Cancer
Metastasis Rev. 22:375–384. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hannigan G, Troussard AA and Dedhar S:
Integrin-linked kinase: A cancer therapeutic target unique among
its ILK. Nat Rev Cancer. 5:51–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peng JM, Chen YH, Hung SW, Chiu CF, Ho MY,
Lee YJ, Lai TC, Hsiao M, Liang CM and Liang SM: Recombinant viral
protein promotes apoptosis and suppresses invasion of ovarian
adenocarcinoma cells by targeting α5β1 integrin to down-regulate
Akt and MMP-2. Br J Pharmacol. 165:479–493. 2012. View Article : Google Scholar :
|
|
22
|
Jin H and Varner J: Integrins: Roles in
cancer development and as treatment targets. Br J Cancer.
90:561–565. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Iwanicki MP, Davidowitz RA, Ng MR, Besser
A, Muranen T, Merritt M, Danuser G, Ince TA and Brugge JS: Ovarian
cancer spheroids use myosin-generated force to clear the
mesothelium. Cancer Discov. 1:144–157. 2011. View Article : Google Scholar
|
|
24
|
Fine RN: Growth after renal
transplantation in children. J Pediatr. 110:414–416. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ebi H, Sato T, Sugito N, Hosono Y, Yatabe
Y, Matsuyama Y, Yamaguchi T, Osada H, Suzuki M and Takahashi T:
Counterbalance between RB inactivation and miR-17-92 overexpression
in reactive oxygen species and DNA damage induction in lung
cancers. Oncogene. 28:3371–3379. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu Q, Luo G, Yang Z, Zhu F, An Y, Shi Y
and Fan D: miR-17-5p promotes proliferation by targeting SOCS6 in
gastric cancer cells. FEBS Lett. 588:2055–2062. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wei Q, Li YX, Liu M, Li X and Tang H:
MiR-17-5p targets TP53INP1 and regulates cell proliferation and
apoptosis of cervical cancer cells. IUBMB Life. 64:697–704. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao Y, Luo LH, Li S and Yang C: miR-17
inhibitor suppressed osteosarcoma tumor growth and metastasis via
increasing PTEN expression. Biochem Biophys Res Commun.
444:230–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma Y, Zhang P, Wang F, Zhang H, Yang Y,
Shi C, Xia Y, Peng J, Liu W, Yang Z, et al: Elevated oncofoetal
miR-17-5p expression regulates colorectal cancer progression by
repressing its target gene P130. Nat Commun. 3:12912012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kesanakurti D, Chetty C, Dinh DH, Gujrati
M and Rao JS: Role of MMP-2 in the regulation of IL-6/Stat3
survival signaling via interaction with α5β1 integrin in glioma.
Oncogene. 32:327–340. 2013. View Article : Google Scholar
|
|
31
|
Bell-McGuinn KM, Matthews CM, Ho SN, Barve
M, Gilbert L, Penson RT, Lengyel E, Palaparthy R, Gilder K, Vassos
A, et al: A phase II, single-arm study of the anti-α5β1 integrin
antibody volociximab as monotherapy in patients with
platinum-resistant advanced epithelial ovarian or primary
peritoneal cancer. Gynecol Oncol. 121:273–279. 2011. View Article : Google Scholar : PubMed/NCBI
|