Introduction
Hepatocellular carcinoma (HCC) is a highly
aggressive and heterogeneous disease, and is among the leading
causes of cancer-related deaths, especially in China (1). Although clinical treatments of HCC
have been developed, uncontrolled metastasis and high recurrence
lead to poor prognosis of HCC patients. A major reason for
HCC-treatment failure is the existence of a drug-resistant
subclone, either presenting at diagnosis or development during
treatment. Therefore, it is urgent to achieve new treatment options
for HCC and explore the molecular mechanisms underlying
carcinogenesis and progression of HCC.
NIMA-related expressed kinase 2 (NEK2) as one of
chromosomal instability (CIN) genes, is a member of the
serine-threonine kinase family NEK, and functional studies have
implicated that NEK2 is involved in cell division and mitotic
regulation by centrosome splitting (2,3).
Increased expression of NEK2 has been reported in certain cancers,
such as breast, cervical, prostate carcinomas, lung cancer, and
lymphoma, suggesting the involvement of NEK2 in cancer development
(4–8). Additionally, NEK2 has been proven to
play pivotal roles in cell proliferation and drug resistance of
cancer cells with poor prognosis in myeloma, in which both protein
phosphatase 1 (PP1)/Akt and Wnt pathways are involved (9). According to the current network-based
interpretation of transcript expression level, NEK2 was
significantly elevated in HCC patients compared to normal controls
(10). However, despite these
studies, the roles of NEK2 in HCC carcinogenesis and progression,
especially for drug resistance still remain unknown.
In this study, we investigated the role of NEK2 in
HCC development. We found that NEK2 is significantly increased in
both HCC tissues and cell lines, and participates in HCC
progression and drug resistance. Mechanistically, PP1/Akt and Wnt
signaling activation are significantly inhibited by NEK2 knockdown,
which implicates the role of NEK2 in promoting HCC progression.
Collectively, our data show that enhanced NEK2 expression promotes
HCC progression and drug resistance by promoting PP1/Akt and Wnt
pathway activation, which may represent a new therapeutic target
for HCC.
Materials and methods
Clinical samples
There were 64 patients who underwent resection for
HCC between 2011 and 2012 at the Department of Hepatobiliary
Surgery, the Second Xiangya Hospital of Central South University.
None of these patients received chemotherapy or radiotherapy before
the operation. The group was composed of 52 men and 12 women at the
time of operation. A summary of patient characteristics and
pathological features is presented in Table I. Tumor specimens were either cut
immediately after removal from the resected hepatic tissues, frozen
in liquid nitrogen, and then stored at −80°C or collected in 10%
formalin and then embedded in paraffin for histopathological
analysis.
 | Table IRelationship between NEK2 expression
and clinicopathological features of HCC. |
Table I
Relationship between NEK2 expression
and clinicopathological features of HCC.
| Characteristics | Total
| NEK2
|
|---|
| No. | % | Positive case | % | P-value |
|---|
| Age (years) |
| ≤50 | 33 | 51.6 | 16 | 48.5 | |
| >50 | 31 | 48.4 | 18 | 58.1 | 0.443 |
| Gender |
| Male | 52 | 81.2 | 25 | 48.1 | |
| Female | 12 | 18.8 | 82.8 | 75 | 0.092 |
| HBV |
| Positive | 53 | 82.8 | 28 | 52.8 | |
| Negative | 11 | 17.2 | 6 | 54.5 | 0.917 |
| Serum AFP
(ng/ml) |
| ≤400 | 49 | 76.6 | 24 | 49 | |
| >400 | 15 | 23.4 | 10 | 66.7 | 0.23 |
|
Differentiation |
| 1 | 7 | 10.9 | 2 | 5.9 | |
| 2 | 15 | 23.4 | 5 | 14.7 | |
| 3 | 42 | 75.7 | 27 | 79.4 | 0.045a |
| Tumor size
(cm) |
| ≤5 | 19 | 29.7 | 4 | 21 | |
| >5 | 45 | 70.3 | 30 | 66.7 | 0.001a |
| Tumor no. |
| Single | 56 | 87.5 | 30 | 51.8 | |
| Multiple | 8 | 12.5 | 29 | 62.7 | 0.57 |
| Lymph node
metastasis |
| Positive | 5 | 7.8 | 5 | 100 | |
| Negative | 59 | 92.2 | 29 | 49.2 | 0.029a |
| Hepatic
sclerosis |
| Yes | 30 | 46.9 | 15 | 50 | |
| No | 34 | 53.1 | 19 | 55.9 | 0.638 |
Antibodies, reagents and RNA
interference
Phos-Akt (Ser473), Akt (C67E7) rabbit nuclear
factor-κB (NF-κB) p65 (D14E12), phos-NF-κB p65 (Ser536), phos-GSK3
(Ser9), β-catenin (D10A8) and β-actin (8H10D10) used for
immunoprecipitation were from Cell Signaling Technology, Inc.
Antibodies to NEK2 (ab55550) for western blotting and
immunocytochemistry were from Abcam (Cambridge, MA, USA). All
antibodies were used according to the manufacturer's instructions.
5-Fluorouracil (5-FU) was purchased from Shanghai Xudong Haipu
Pharmaceutical Co., Ltd. (Shanghai, China) and was diluted directly
with cell culture medium to the desired concentration. Transient
transfection of plasmid DNA was performed using Lipofectamine Plus
reagent (Invitrogen) according to the manufacturer's instructions.
For RNA interference, siRNAs specific to NEK2, siRNA1 (5′-CAU UUG
UUG GCA CAC CUU AUU-3′), siRNA2 (5′-GCU GAG AAA CAG AUG CUU GUU-3′)
and siRNA3 (5′-UCU GUU GAA GAA CUA CAG CUU-3′) were purchased from
GenePharma Co., Ltd., and transfected into the cells using
Lipofectamine RNAi MAX (Invitrogen) according to the manufacturer's
instructions. Non-specific control siRNA (5′-AAG TAG CCG AGCT TCG
ATT GC-3′) was also used.
Cell culture
HepG2, BEL-7402, QGY-7703, SMMC7721 and Huh-7 were
from the Institute of Biochemistry and Cell Biology (Shanghai
Institutes for Biological Sciences, CAS). Cells were cultured in
RPMI-1640 (Gibco, 31800022; 1.5 g/l NaHCO3, 2.5g/l
glucose, 0.11g/l sodium pyruvate) supplemented with 10% fetal calf
serum (FCS; Invitrogen) in a humidified incubator under 5%
CO2 at 37°C.
Cell proliferation and half maximal
inhibitory concentration (IC50) values
Cell proliferation was determined using MTT assay of
cell proliferation as described before (11). Cells were cultured into a 96-well
plate at 1×104 cells/well (n=4 for each time point) in a
final volume of 100 µl. Cells were cultured for 36–48 h
after transfection with NEK2-siRNA and ctrl-siRNA (non-specific
control siRNA), respectively. Then, 20 µl MTT (5 mg/ml) was
added to each well (0.8 mg/ml final concentration) for further
incubation for 4 h. Then the medium was removed, and 100 µl
DMSO was added to dissolve the solid formazan for 15 min. The
absorbance of each well was read at 490 nm using a microplate
reader (Thermo Fisher Scientific, Inc.). For drug resistance assay,
cells were stimulated with different concentrations (0, 0.25, 0.5,
1, 20, 50 and 100 µg/ml) of 5-FU in culture medium for 48 h
after transfection with siRNA. The IC50 values were
calculated by non-linear regression analysis using SPSS 17.0
software (SPSS, Chicago, IL, USA) as describe before (12).
In vitro cell-invasion and -migration
assays
Migration and invasion assay Transwell filters
coated with collagen I or Matrigel (8-mm pore size; BD Biosciences)
were used for migration or invasion assays, respectively. Cells
(1.5×104) were seeded into the upper chamber in
RPMI-1640 with 0.5% fetal bovine serum (FBS). The bottom chamber
contained RPMI-1640 with 10% FBS. Cells were allowed to
migrate/invade at 37°C in 5% CO2 for 24 h before they
were fixed with methanol/methylene blue solution, stained with
crystal violet (Beyotime, Shanghai, China), imaged, and counted
with a microscope (Leica, UK). All experiments were performed in
triplicate. Cell counts were performed on 10 fields per filter, and
the mean was normalized to the migration/invasion cell count of
control cells.
Quantitative real-time PCR
Total RNA was extracted with TRIzol reagent
(Invitrogen) following the manufacturer's instructions. Real-time
quantitative RT-PCR analysis was performed using the LightCycler
(Roche Diagnostics) and SYBR RT-PCR kits (Takara Biotechnology Co.,
Ltd.). For mRNAs analysis, the primers were: human NEK2 forward,
5′-CCG CCC AAG TCA CAG CAG CAGA-3′ and reverse, 5′-GGG AGA AGA GCA
GCA GCA GGA AGC-3′; human β-actin forward, 5′-CAT CCT GCG TCT GGA
CCT GG-3′ and reverse, 5′-TAA TGT CAC GCA CGA TTT CC-3′; human
ABCB1 forward, 5′-AGG CTC GCC AAT GAT GC-3′ and reverse, 5′-TCC TGT
CCC AAG ATT TGC TAT-3′; human ABCG2 forward, 5′-TGA AAC CTG GTC TCA
ACGC-3′ and reverse, 5′-AGA GTG CCC ATC ACA ACA TC-3′; human ABCC1
forward, 5′-CGC CTT CGC TGA GTT CCT GC-3′ and reverse, 5′-AGT TCT
GCG GTG CTG TTG TGG-3′. The relative expression level of mRNAs was
normalized to that of internal control GAPDH by using
2−ΔΔCt cycle threshold method (13).
Cell extraction, protein electrophoresis,
and western blotting
Whole cell lysates of QGY-7703 cells were prepared
in radioimmunoprecipitation assay buffer [50 mmol Tris-HCl (pH
8.0), 150 mmol NaCl, 1% NP40, 0.5% sodium deoxycholate, and 0.1%
SDS]. Briefly, cells were washed in ice-cold 1X PBS (pH 7.4) and
lysed directly in radioimmunoprecipitation assay buffer on ice for
30 min, centrifuged at 4°C, 14,000 rpm, to remove insoluble
material and total protein concentration of the resulting
supernatant determined by bicinchoninic acid assay (Pierce
Biotechnology, Inc., Rockford, IL, USA). Lysate, 50 µg, for
each cell line was resolved by sodium dodecylsulfate-polyacrylamide
gel electrophoresis (SDS-PAGE), For immunoblot analysis, protein
samples were subjected to SDS-PAGE and transferred onto a
nitrocellulose membrane. The membrane was incubated with a primary
antibody overnight at 4°C after blocking with 5% skim milk in 0.1%
TBST (Tris-buffered saline with 0.1% Triton X-100) for 60 min. The
second day, the membrane was incubated with a horseradish
peroxidase-conjugated secondary antibody for 60 min after washing
three times with 0.1% TBST, then, the membrane was incubated with
the ECL solution after washing three times with 0.1% TBST, and
exposed to X-ray film.
Immunocytochemistry and image
processing
Immunohistochemical studies on NEK2 were performed
on formalin-fixed, paraffin-embedded tissue sections obtained from
the aforementioned patients with HCC. Tissue sections were
deparaffinized and then boiled in 0.01 mol/l sodium citrate buffer
(pH 6.0) in a 1,000 W microwave oven for 10 min to retrieve cell
antigens. The primary antibody used was rabbit polyclonal NEK2
antibody (1:200 dilution; Bioss, China). All tissue sections were
immunohistochemically stained using the avidin-biotin-peroxidase
method and were counterstained with hematoxylin. The staining was
scored by three independent investigators without knowledge of
patient outcomes. The sections were evaluated at low magnification
(×100 or ×200) to identify areas where NEK2 was evenly stained.
Statistical analysis
The data were subjected to statistical analysis
using the SPSS software package (version 17.0). The
clinicopathological parameters were tested by χ2 test
and bivariate analysis (14). All
data are presented as the mean ± SD. The statistical differences
were analyzed by Student's t-test and repeated measures of ANOVA.
The differences were considered to be statistically significant at
P<0.05.
Results
NEK2 expression is significantly elevated
in HCC
NEK2 is considered an oncogene and is overexpressed
in various tumors (8,15,16).
In order to examine the roles of NEK2 in HCC development, we
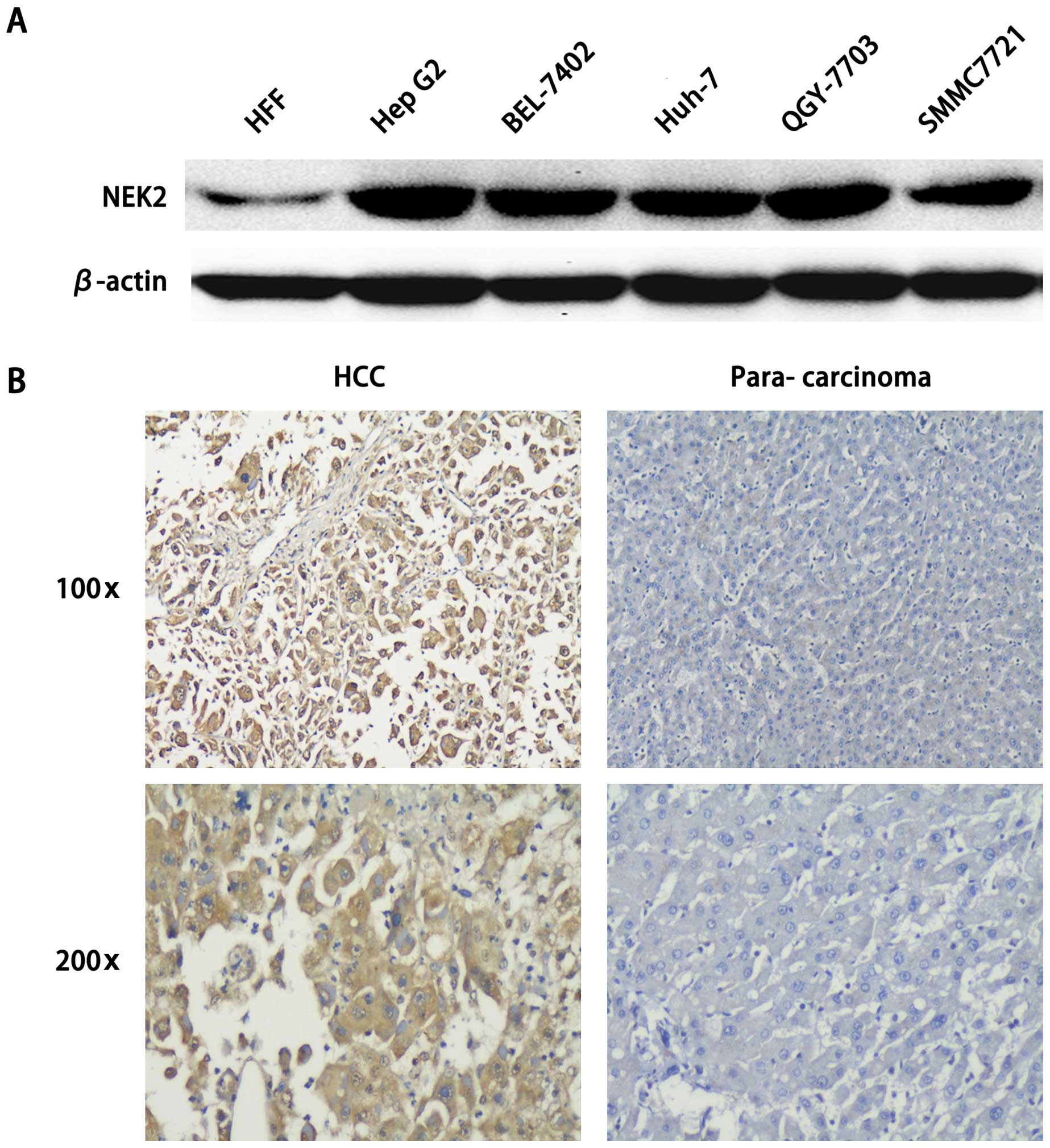
examined the expression of NEK2 in HCC tissues and cell lines. As
shown in Fig. 1A, compared with
negative control HFF (human fibroblast cell line), in HCC cell
lines HepG2, BEL-7402, QGY-7703, SMMC7721 and Huh-7, we found that
NEK2 was highly expressed. Furthermore, NEK2 expression was
significantly increased in HCC tissues as compared to that in the
matched non-tumor tissues. Hence, these data confirm that NEK2
expression is significantly increased in both HCC tissues and cell
lines, which indicates an important role in HCC carcinogenesis and
progression.
We further examined the correlation between NEK2 and
the clinicopathological characteristics of patients with HCC. As
summarized in Table I, NEK2
expression was not significantly correlated with age or
histological grades. However, NEK2 expression was significantly
correlated with tumor size, differentiation grading and lymph node
metastasis (P=0.001, 0.045 and 0.029). Together, these data suggest
that elevated NEK2 expression in HCC is correlated with the
clinical progression of HCC patients.
NEK2 promotes proliferation of HCC
cells
As NEK2 expression is increased in HCC, we next
investigated the roles of NEK2 increase in HCC development. RNA
interference has emerged as natural and highly efficient mechanism
for gene silencing (17–19). In order to test the functional role
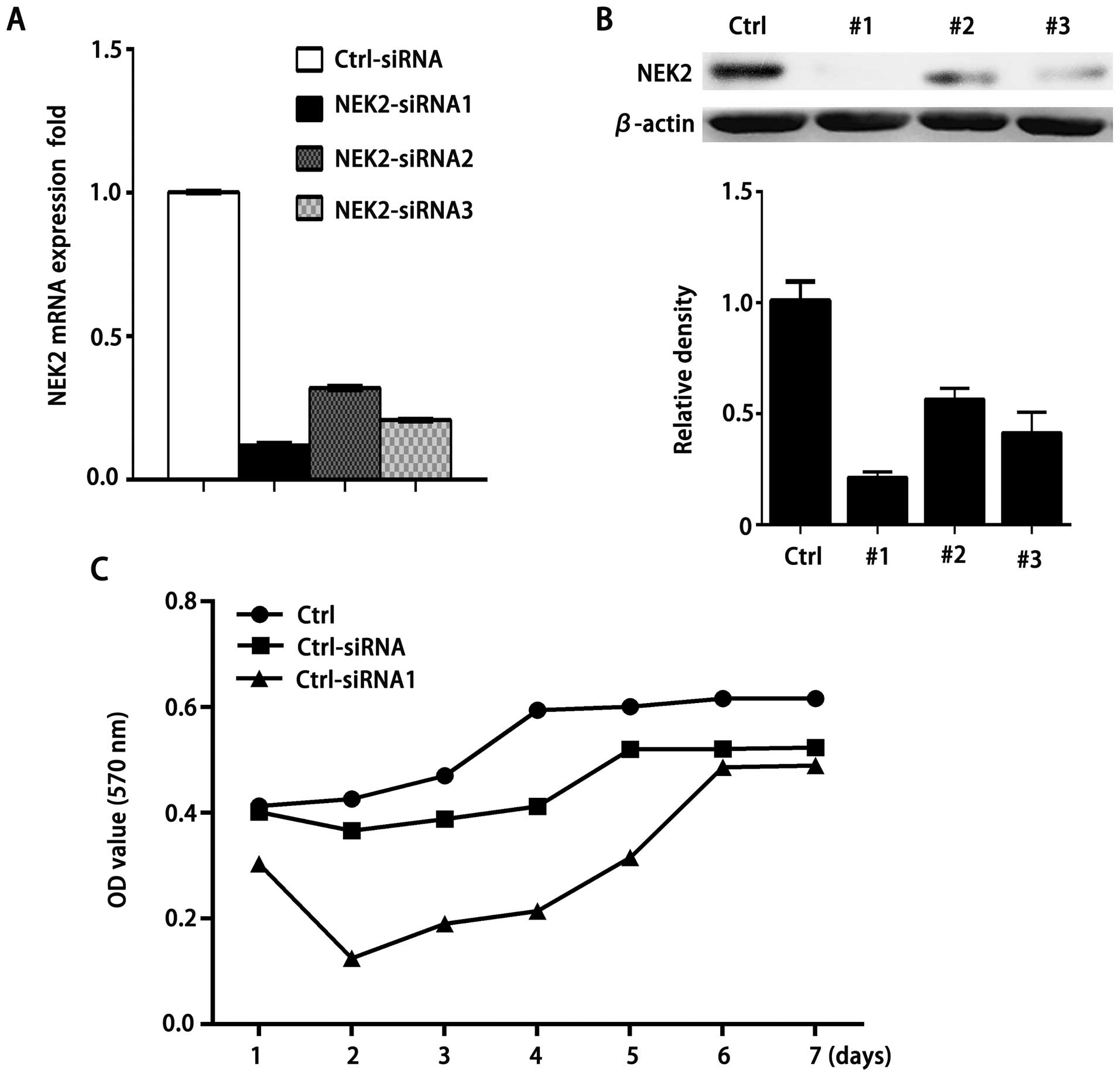
of NEK2 on cell growth, we have designed three siRNA candidates
based on NEK2 gene sequence. Real-time PCR confirmed a remarkable
downregulation of NEK2 expression in QGY-7703 cells after
transfection of these NEK2-siRNAs (Fig.
2A). Additionally, relative amounts of NEK2 protein were
decreased by siRNA1, 2 and 3 respectively, compared with those of
control siRNA-treated cells (Fig.
2B). In contrast, transfection with control siRNA did not alter
NEK2 expression significantly.
We then examined the effect of NEK2 knockdown on
cell growth of QGY-7703 cells. As shown in Fig. 2C, the growth of QGY-7703 cells was
substantially suppressed by treatment with NEK2 siRNA1 or 3
compared with control siRNA-treated cells. The results indicate
that NEK2 can promote HCC cell proliferation.
NEK2 promotes migratory and invasive
capacities of HCC cells
Because NEK2 controls microtubule organization,
overexpressed NEK2 might affect tumor invasion and migration.
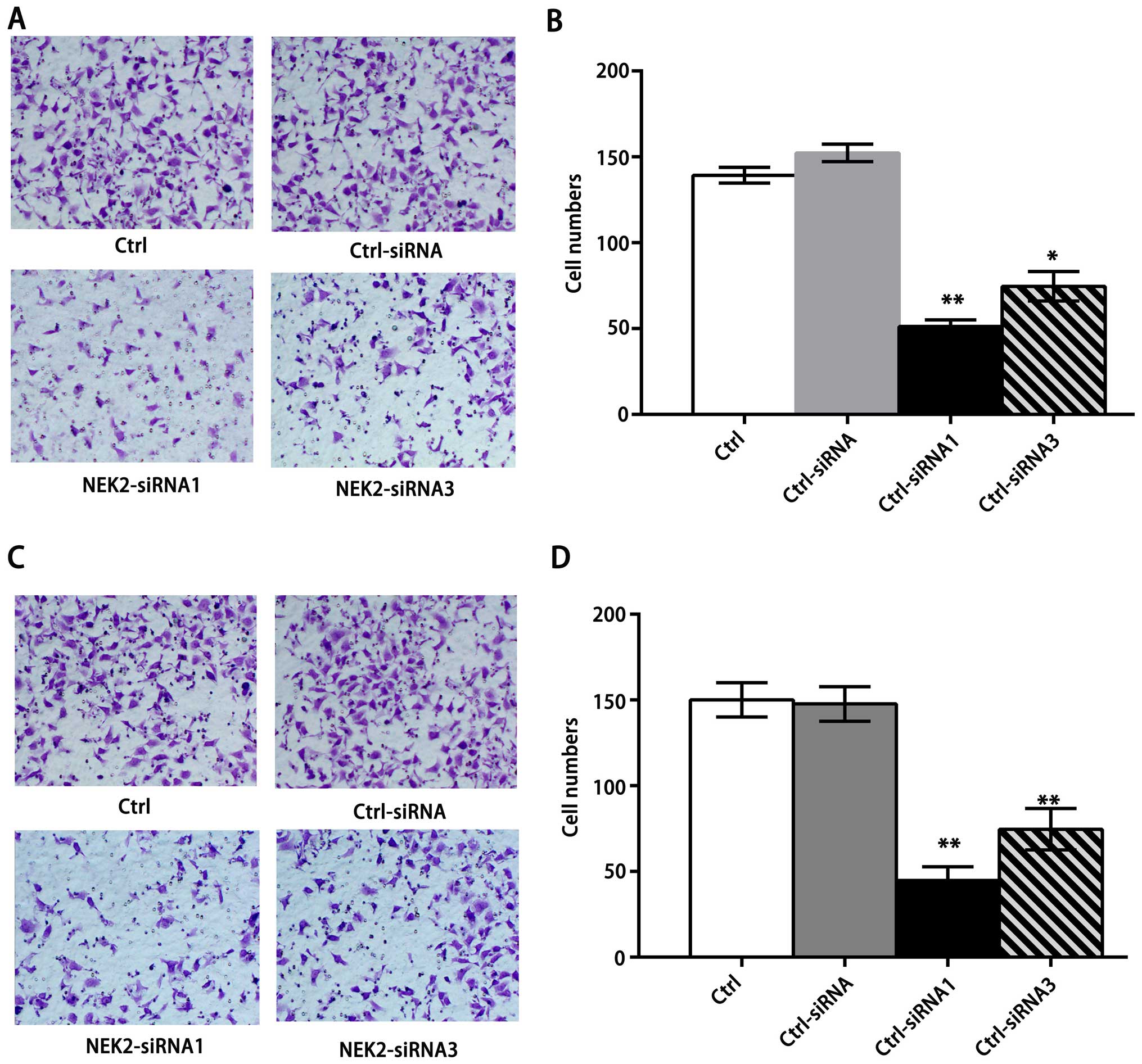
Therefore, we studied the effect of NEK2 siRNA administration on
the invasion and migration of HCC cells. Compared with their
control cells and control siRNA-treated cells, knockdown of NEK2
markedly reduced the invasion of QGY-7703 cells (Fig. 3A and B), and also showed
significantly impaired migration of QGY-7703 cells (Fig. 3C and D), suggesting that knockdown
of NEK2 may dampen the microtubule organization to inhibit their
mobility potential. Together with the role of NEK2 in the promotion
of HCC proliferation, we conclude that NEK2 promotes HCC
progression.
NEK2 knockdown frustrates drug resistance
of HCC cells
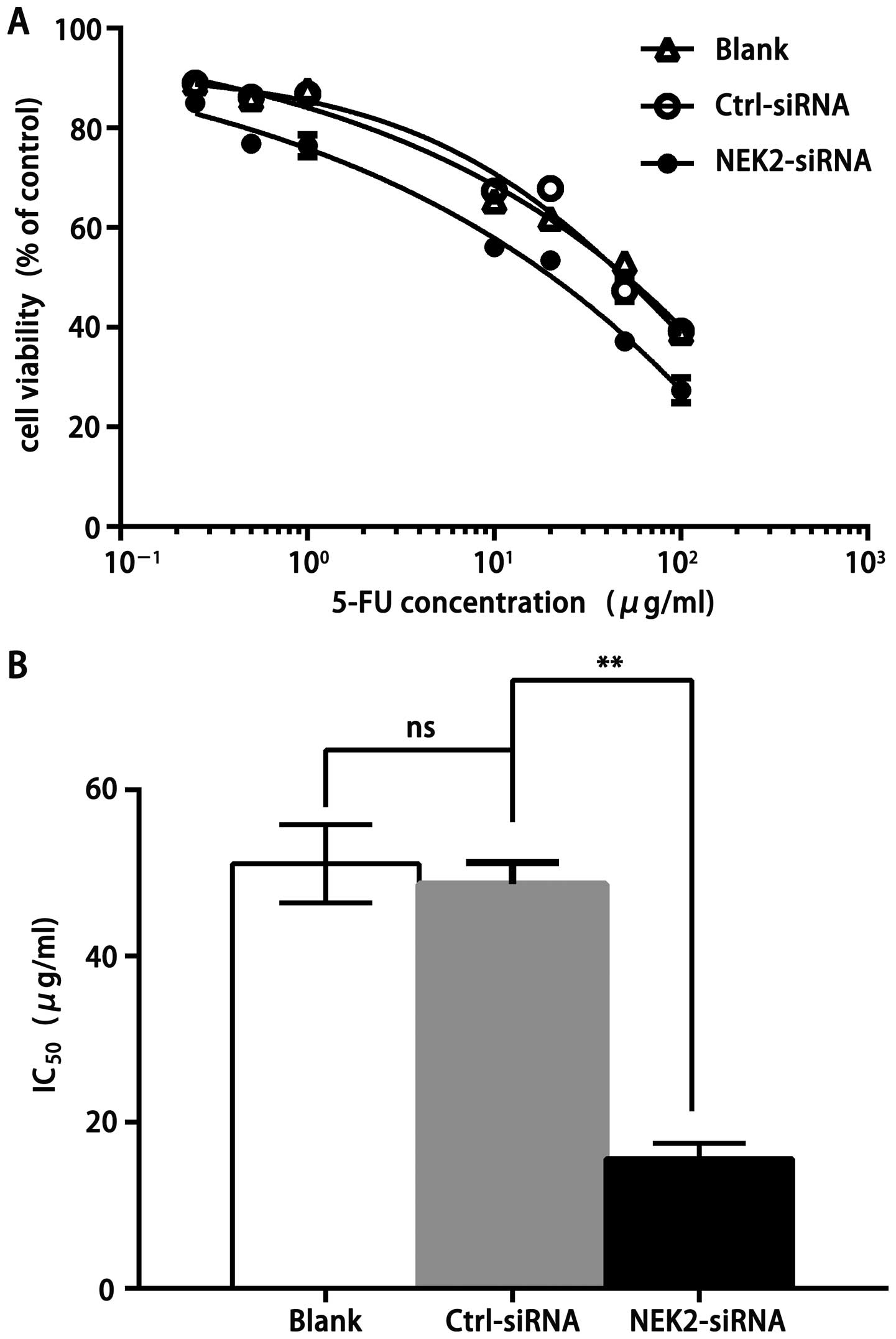
We subsequently tested if knockdown of NEK2
expression could decrease drug resistance to fluorouracil injection
(5-FU) using insensitive SMMC7721 cell lines. 5-FU inhibited cell
proliferation of the SMMC7721 cell lines in a dose-dependent manner
(Fig. 4A). Results of
IC50 from the representative SMMC7721 were 51.1±4.70,
48.69±2.57 and 15.61±1.85 µg/ml in SMMC7721 cells
non-treated, treated with ctrl-siRNA or NEK2-siRNA, respectively
(Fig. 4B). SMMC7721 cells with NEK2
knockdown showed a significant decrease of IC50,
indicating that highly elevated NEK2 could promote drug resistance
to 5-FU.
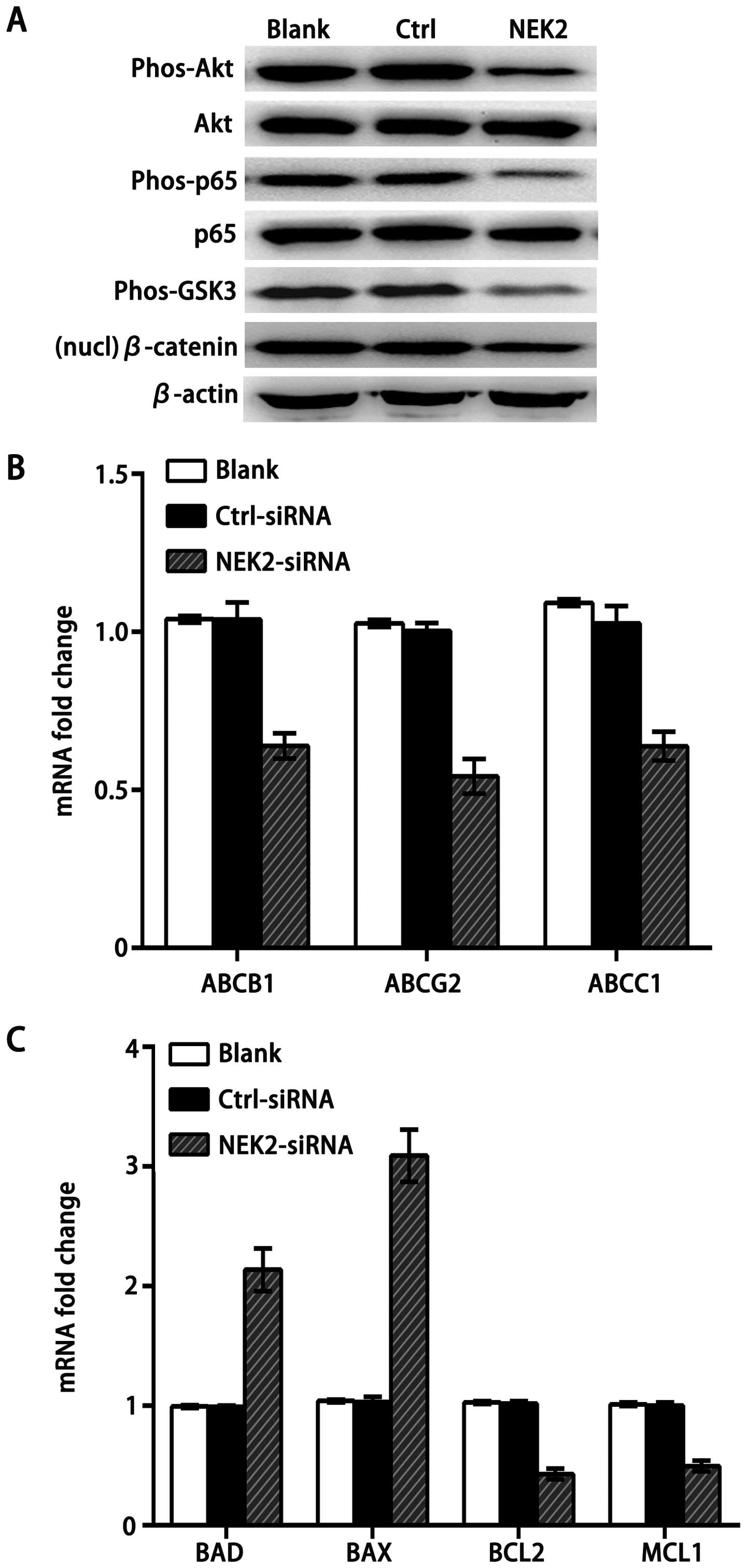
NEK2 activates both Akt and canonical Wnt
signaling
NEK2 is known to regulate the mitotic centrosome
separation through reversible phosphorylation of its substrates PP1
(20) and β-catenin (21) in yeast, which is also important for
cancer cell drug resistance and proliferation (9). Another study demonstrated that the
transcriptional level of NEK2 is increased in many aggressive types
of cancer, and at least two pathways, PP1/Akt and Wnt signaling,
are involved in NEK2-induced cancer cell progression and drug
resistance. Consistently, knockdown of NEK2 by siRNA impaired the
phosphorylation of Akt, glycogen synthase kinase-3 (GSK3), NF-κB
and decreased nuclear β-catenin (Fig.
5A), which activates drug resistance and pro-survival gene
members. Furthermore, we found that knockdown of NEK2 downregulated
mitotic checkpoint protein ABC transporter family members,
including ABCB1, the drug resistance protein ABCC1 (MRP1), and the
breast cancer resistant protein ABCG2 (BCRP) (Fig. 5B). In addition, knockdown of NEK2
decreased the expression of pro-survival gene members of the BCL2
family (BCL2 and MCL1) in SMMC7721 cell lines, while promoted the
pro-apoptotic gene members BAD and BAX, which are suppressed by Akt
(Fig. 5C). These results strongly
suggest that PP1 and β-catenin are the downstream targets of NEK2
in HCC cells, and increased NEK2 could contribute to HCC
progression by amplifying the PP1/Akt and Wnt pathway.
Discussion
HCC is the third most common cause of cancer-related
deaths worldwide (22,23). NEK2, a member of the NIMA-related
family, has several putative roles in cell differentiation,
proliferation by centrosome splitting (3,24).
Overexpression of active NEK2 leads to CIN, cell proliferation and
drug resistance, which are also commonly observed in cancers
including virtually all myelomas (9,25). Our
study showed that centrosomal kinase NEK2 expression was
significantly upregulated in HCC cell lines and tissues (Fig. 1). According to Table I, NEK2 expression is correlated with
tumor size, differentiation grading and lymph node metastasis,
which suggests that NEK2 participates in the clinical progression
of HCC patients. Our data also shown that NEK2 is mostly positive
in the cytoplasm of the HCC tissue cells and promotes HCC
progression and drug resistance by amplifying the PP1/Akt and Wnt
signaling pathway.
In this study, we found that two pathways, PP1/Akt
and Wnt signaling, are involved in NEK2-induced cancer cell
progression. It has been revealed that PP1 regulates the Akt
signaling pathway to control cell survival, invasion and migration
(26,27). Akt functions through IKK to promote
the transactivation potential and phosphorylation of NF-κB
(28), which activates
transcription of pro-survival gene members of the BCL2 family. In
the cytoplasm, GSK3 form a β-catenin destruction complex which
mediates the degradation of β-catenin, which is important for
constitutive activation of the canonical Wnt signaling pathway and
drives cell proliferation by direct induction of cell cycle
regulators (29,30). Our findings support that NEK2
promotes the expression of phosphorylated Akt, GSK3, NF-κB and
increases nuclear accumulation of β-catenin. Furthermore, we found
that knockdown of NEK2 decreased the expression of ABC
transporters, resulting in inhibited drug resistance of HCC cells.
Additionally, drug resistance is a universal problem with current
cancer therapies. CIN genes have also been associated with acquired
or intrinsic drug resistance (31).
We demonstrated that targeting NEK2 overcame drug resistance and
cell growth of SMMC7721 cells to 5-FU.
In summary, elevated expression of NEK2 in HCC
results in both impaired PP1/AKT and Wnt pathways, which are
involved in NEK2-induced cancer cell drug resistance, invasion,
migration and proliferation. Knockdown of NEK2 by siRNA inhibited
QGY-7703 cell growth and decreased drug resistance in vitro.
Thus, targeting CIN genes, such as NEK2, have the potential to
translate into very important prognostic and therapeutic clinical
tools. We are now exploring how NEK2 regulates its downstream
targets and how interaction among those pathways is regulated by
NEK2 in HCC.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (81402001). We thank Professor Lifang
Yang and Daiqiang Li for their excellent technical assistance.
References
|
1
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fry AM: The Nek2 protein kinase: A novel
regulator of centrosome structure. Oncogene. 21:6184–6194. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fletcher L, Cerniglia GJ, Nigg EA, Yend TJ
and Muschel RJ: Inhibition of centrosome separation after DNA
damage: A role for Nek2. Radiat Res. 162:128–135. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hayward DG, Clarke RB, Faragher AJ, Pillai
MR, Hagan IM and Fry AM: The centrosomal kinase Nek2 displays
elevated levels of protein expression in human breast cancer.
Cancer Res. 64:7370–7376. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsunoda N, Kokuryo T, Oda K, Senga T,
Yokoyama Y, Nagino M, Nimura Y and Hamaguchi M: Nek2 as a novel
molecular target for the treatment of breast carcinoma. Cancer Sci.
100:111–116. 2009. View Article : Google Scholar
|
|
6
|
Cappello P, Blaser H, Gorrini C, Lin DC,
Elia AJ, Wakeham A, Haider S, Boutros PC, Mason JM, Miller NA, et
al: Role of Nek2 on centrosome duplication and aneuploidy in breast
cancer cells. Oncogene. 33:2375–2384. 2014. View Article : Google Scholar
|
|
7
|
Liu X, Gao Y, Lu Y, Zhang J, Li L and Yin
F: Upregulation of NEK2 is associated with drug resistance in
ovarian cancer. Oncol Rep. 31:745–754. 2014.
|
|
8
|
Zhong X, Guan X, Dong Q, Yang S, Liu W and
Zhang L: Examining Nek2 as a better proliferation marker in
non-small cell lung cancer prognosis. Tumour Biol. 35:7155–7162.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou W, Yang Y, Xia J, Wang H, Salama ME,
Xiong W, Xu H, Shetty S, Chen T, Zeng Z, et al: NEK2 induces drug
resistance mainly through activation of efflux drug pumps and is
associated with poor prognosis in myeloma and other cancers. Cancer
Cell. 23:48–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Drozdov I, Bornschein J, Wex T, Valeyev
NV, Tsoka S and Malfertheiner P: Functional and topological
properties in hepatocellular carcinoma transcriptome. PLoS One.
7:e355102012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Min J, Li X, Huang K, Tang H, Ding X, Qi
C, Qin X and Xu Z: Phloretin induces apoptosis of non-small cell
lung carcinoma A549 cells via JNK1/2 and p38 MAPK pathways. Oncol
Rep. 34:2871–2879. 2015.PubMed/NCBI
|
|
12
|
Deng L, Ren Z, Jia Q, Wu W, Shen H and
Wang Y: Schedule- dependent antitumor effects of 5-fluorouracil
combined with sorafenib in hepatocellular carcinoma. BMC Cancer.
13:3632013. View Article : Google Scholar
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Herbst RS, Heymach JV and Lippman SM: Lung
cancer. N Engl J Med. 359:1367–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Neal CP, Fry AM, Moreman C, McGregor A,
Garcea G, Berry DP and Manson MM: Overexpression of the Nek2 kinase
in colorectal cancer correlates with beta-catenin relocalization
and shortened cancer-specific survival. J Surg Oncol. 110:828–838.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhong X, Guan X, Liu W and Zhang L:
Aberrant expression of NEK2 and its clinical significance in
non-small cell lung cancer. Oncol Lett. 8:1470–1476.
2014.PubMed/NCBI
|
|
17
|
Jinek M and Doudna JA: A three-dimensional
view of the molecular machinery of RNA interference. Nature.
457:405–412. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moazed D: Small RNAs in transcriptional
gene silencing and genome defence. Nature. 457:413–420. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Siomi H and Siomi MC: On the road to
reading the RNA-interference code. Nature. 457:396–404. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Helps NR, Luo X, Barker HM and Cohen PT:
NIMA-related kinase 2 (Nek2), a cell-cycle-regulated protein kinase
localized to centrosomes, is complexed to protein phosphatase 1.
Biochem J. 349:509–518. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bahmanyar S, Kaplan DD, Deluca JG,
Giddings TH Jr, O'Toole ET, Winey M, Salmon ED, Casey PJ, Nelson WJ
and Barth AI: beta-Catenin is a Nek2 substrate involved in
centrosome separation. Genes Dev. 22:91–105. 2008. View Article : Google Scholar :
|
|
22
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Faragher AJ and Fry AM: Nek2A kinase
stimulates centrosome disjunction and is required for formation of
bipolar mitotic spindles. Mol Biol Cell. 14:2876–2889. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hayward DG and Fry AM: Nek2 kinase in
chromosome instability and cancer. Cancer Lett. 237:155–166. 2006.
View Article : Google Scholar
|
|
26
|
Xiao L, Gong LL, Yuan D, Deng M, Zeng XM,
Chen LL, Zhang L, Yan Q, Liu JP, Hu XH, et al: Protein
phosphatase-1 regulates Akt1 signal transduction pathway to control
gene expression, cell survival and differentiation. Cell Death
Differ. 17:1448–1462. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo
P, Hu LS, Anderson MJ, Arden KC, Blenis J and Greenberg ME: Akt
promotes cell survival by phosphorylating and inhibiting a Forkhead
transcription factor. Cell. 96:857–868. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dan HC, Cooper MJ, Cogswell PC, Duncan JA,
Ting JP and Baldwin AS: Akt-dependent regulation of NF-κB is
controlled by mTOR and Raptor in association with IKK. Genes Dev.
22:1490–1500. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fodde R and Tomlinson I: Nuclear
beta-catenin expression and Wnt signalling: In defence of the
dogma. J Pathol. 221:239–241. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gehrke I, Gandhirajan RK and Kreuzer KA:
Targeting the WNT/beta-catenin/TCF/LEF1 axis in solid and
haematological cancers: Multiplicity of therapeutic options. Eur J
Cancer. 45:2759–2767. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kops GJ, Weaver BA and Cleveland DW: On
the road to cancer: Aneuploidy and the mitotic checkpoint. Nat Rev
Cancer. 5:773–785. 2005. View
Article : Google Scholar : PubMed/NCBI
|