Introduction
Lung cancer has one of the highest morbidity and
mortality rates worldwide. The majority of lung cancers are
diagnosed at late stages, with locally advanced disease and distant
metastases, and its long-term survival remains desperately poor.
Radiotherapy is one of the most important therapies in these
patients while recurrence and metastasis, signs of malignancy and
the main cause of death in cancer patients (1), and the risk of tumor radio-resistance
limit the effect of radiotherapy. Many studies have also found that
radiotherapy plays a role in stimulating tumor cell growth and
promoting invasiveness of tumor cells in addition to a number of
common adverse reactions. Thus, looking for the effective
combination therapy to enhance the antitumor effect is still
urgent.
Low-molecular-weight heparins (LMWHs), obtained by
the methods of enzymatic hydrolysis or chemical degradation from
unfractionated heparin (UFH), relative molecular weight 3–9 kDa,
was approved by the FDA in 1998 for anticoagulant therapy and has
been administrated as effective anticoagulants in the prevention
and treatment of thrombosis safely for many years (2), along with having potential anticancer
effects and improving survival in cancer patients (3,4).
Furthermore, recent studies have showed that LMWHs can directly
induce the inhibition of the invasion and metastasis of the cancer
cells, change the cell cycle, increase the sensitivity of
chemotherapy and reduce the extent of radiation-induced liver
injury (5–8). Whether it has synergistic antitumor
effects to radiotherapy has rarely been reported. On the basis of
present studies, we used two doses of nadroparin, a kind of LMWH,
combined with X-ray irradiation to treat A549 cells in order to
assess the relevant interactions between nadroparin and
radiotherapy in this study.
Materials and methods
Main instruments and reagents
A549 cell line used in this study was kindly
supplied by the Shanghai Institute of Life Science, Chinese Academy
of Sciences (Shanghai, China) and cultured in RPMI-1640 medium
(Biowest, Nuaillé, France) supplemented with 10% fetal bovine serum
(FBS). Cultures were maintained at 37°C in a humidified atmosphere
containing 5% CO2. Nadroparin (Fraxiparina®;
GlaxoSmithKline, London, UK) was obtained as standard drug
formulations. The Cell Counting Kit-8 (CCK-8; Dojindo Laboratories,
Kumamoto, Japan) was used to detect cell proliferation and
activity. Levels of transforming growth factor-β1 (TGF-β1) in the
culture supernatants was measured using a standard Quantikine
enzyme-linked immunosorbent assay (ELISA) kit from R&D Systems,
Inc. (Minneapolis, MN, USA). Annexin V-fluorescein isothiocyanate
(FITC) detection kit was purchased from Becton-Dickinson (San Jose,
CA, USA) to detect cell apoptosis. Matrigel and Transwell chambers
were purchased from Becton-Dickinson as well. Rabbit monoclonal
antibodies against survivin, goat anti-rabbit horseradish
peroxidase labelled secondary antibody, anti-GAPDH mouse monoclonal
antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). The linear accelerator was Precise 5839
(Elekta, Stockholm, Sweden).
Experimental method and grouping
This study contained six groups with different kinds
of treatments: control group; irradiation group (X-ray, treated
with 10 Gy X-ray irradiation); LMWH50 group
(L50, treated with 50 IU/ml of nadroparin);
LMWH100 group (L100, treated with 100 IU/ml
of nadroparin); LMWH50+X-ray irradiation group and
LMWH100+X-ray irradiation group (X+L50,
X+L100, treated with two doses of nadroparin and 10 Gy
X-ray irradiation). Cells were seeded and incubated for 24 h, then
changed to 10% FBS-1640 with or without nadroparin overnight.
Twenty-four hours later, X-ray irradiation was administered
according to the experimental design. A variety of detection was
performed in the next 24 and 48 h after radiation.
Cell viability assay
Cells (5×103 cells/well) were incubated
in the 96-well plates and treatments were conducted as mentioned
above. The CCK-8 solution (10 µl/well) was added at the
indicated time, then incubated for further 1 h at 37°C. The
absorbance of cells in each well was measured at 450 nm. The cell
viability was expressed as percentage of the absorbance present in
the treated group compared to the control group:
(ODtreated/ODcontrol) × 100%.
Assessment of cell apoptosis
In brief, 2×05 cells/well were applied to
six-well plates and treated exactly as described above. The cells
were harvested and centrifuged at 24 and 48 h after radiation and
then washed twice with PBS. The cells were resuspended in 500
µl of binding buffer containing 5 µl FITC conjugated
Annexin V and 5 µl propidium iodide (PI), and incubated in
the dark at room temperature for 15 min. Cells were then analysed
in a FACSCalibur flow cytometer (Becton-Dickinson) to differentiate
apoptotic cells (Annexin V-positive and PI-negative, lower right
quadrant) from necrotic cells (Annexin V/PI-positive, upper right
quadrant). Fifteen thousand events were recorded for each treatment
group.
Measurement of TGF-β1 release
The cell culture supernatants were collected at 24
and 48 h after radiation. The culture supernatants were separated
by centrifugation and stored at −80°C. The concentration of TGF-β1
was measured simultaneously using an ELISA kit (R&D Systems,
Inc.).
Determination of migration and
invasion
About 2×105 cells/well were seeded to
six-well plates and treated exactly as described above. The cells
were harvested and centrifuged at 24 h after radiation and
maintained in serum-free RPMI-1640 for 12 h. Cell invasion and
migration were determined with or without Matrigel-coated Transwell
chambers. Cells (1×104) were removed for migration and
2×104 cells for invasion of these treated cells were
placed into the upper compartment with 100 µl serum-free
RPMI-1640, and the lower compartment was filled with RPMI-1640
containing 15% FBS. The chamber was then cultivated in 5%
CO2 at 37°C for 24 h to detect the cell migration and
invasion. The Matrigel and cells in the upper chamber were removed,
and the attached cells in the lower section were stained with 0.1%
crystal violet. These cells were counted in five high-power
microscope fields of vision and photo graphed.
Protein extraction and western
blotting
Cells (2×105) were seeded in six-well
plates and treated as described above. At the end of the treatment
period (24 and 48 h after radiation), cells were washed three times
in ice-cold PBS and lysed for at least 30 min on ice in the cold
lysis buffer with 1 mM phenylmethanesulfonyl fluoride (PMSF).
Protein concentrations were measured using the Coomassie Blue Fast
staining solution (Beyotime, Shanghai, China). Proteins (50
µg) in each group was separated on 10–12% SDS-PAGE and
transferred to a PVDF membrane (Millipore Corp., Billerica, MA,
USA) at 80 V for 90 min. Membranes were blocked by TBS/T containing
5% skim milk for 3 h, and incubated with the CD147, matrix
metalloproteinase-2 (MMP-2) and survivin antibody (1:500–1:1,000
dilution) overnight at 4°C, while the GAPDH and β-actin antibody
(1:10,000 dilution) was used as an endogenous reference for
quantification. Then the membranes were incubated with the
secondary antibodies (1:4,000 dilution) at room temperature for 1 h
after three washes in TBS/T. After several washes with TBS/T, the
blots were detected using Immobilon™ Western Chemiluminescent HRP
substrate (Millipore Corp.) and quantified using Tanon-4500 Gel
Imaging System with GIS ID Analysis Software v4.1.5 (Tanon Science
& Technology Co., Ltd., Shanghai, China).
Statistical analysis
Statistical analysis was performed using the SPSS
software version 20.0. Data were expressed as means ± standard
error (SE). Student's t-test was used to test the differences
between groups. Differences resulting in p<0.05 were considered
to be statistically significant. All data are reported from three
independent experiments.
Results
Effects on cell viability
This study examined the cell viability of A549 cells
treated with two doses of nadroparin and X-ray radiation at
different time-points (24 and 48 h) using the CCK-8 assay. As shown
in Fig. 1, the cell viability was
inhibited to different extent compared to the control group after
treatments. With the prolongation of treat ment time, no
significant change was observed in the inhibition of cell viability
treated with low dose of nadroparin alone (L50 group),
while the cell viability was inhibited in a dose- and
time-dependent manner after the other treatments (irradiation
alone, high dose of nadroparin alone (L100 group) and
different dose of nadroparin combined with X-ray irradiation).
Furthermore, the cell viability was significantly inhibited in the
high dose of nadroparin combined with X-ray irradiation group
compared to the other groups (p<0.05).
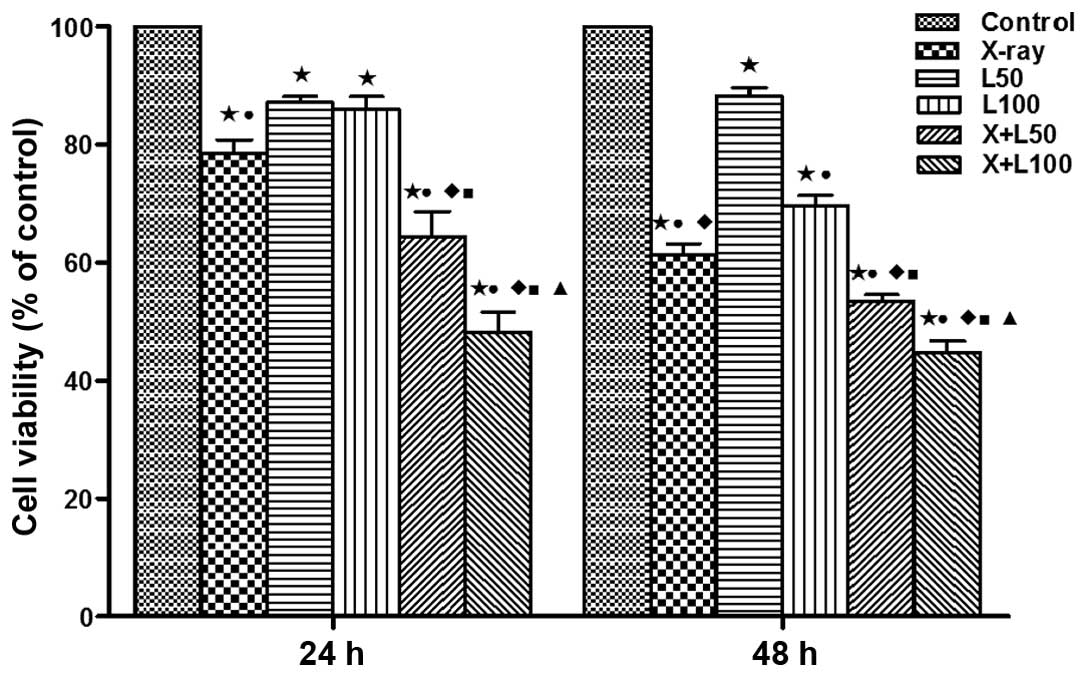 | Figure 1Effect of nadroparin and X-ray
irradiation on A549 cell viability. Cell Counting Kit-8 was used to
detect the cell viability of A549 cells after different treatments
at different time-points, respectively. Significant differences
were observed in the treated groups compared to the control group
(*p<0.05, t=9.492, 12.889, 6.753, 8.153 and 14.704 at 24 h,
t=20.413, 7.990, 17.770, 44.692 and 29.615 at 48 h). A difference
from X-ray irradiation group (■p<0.05, t=2.883 and
7.256 at 24 h, t=3.639 and 6.214 at 48 h); a difference from
L50 (●p<0.05, t=3.483, 5.083 and 10.643 at
24 h, t=11.152, 19.150 and 18.200 at 48 h, p<0.05); a difference
from L100 (♦p<0.05, t=4486 and 9.473 at 24
h, t=3.264, 8.095 and 9.826 at 48 h); a difference from
X+L50 (▲p<0.05, t=2.886 at 24 h, t=4050 at
48 h). |
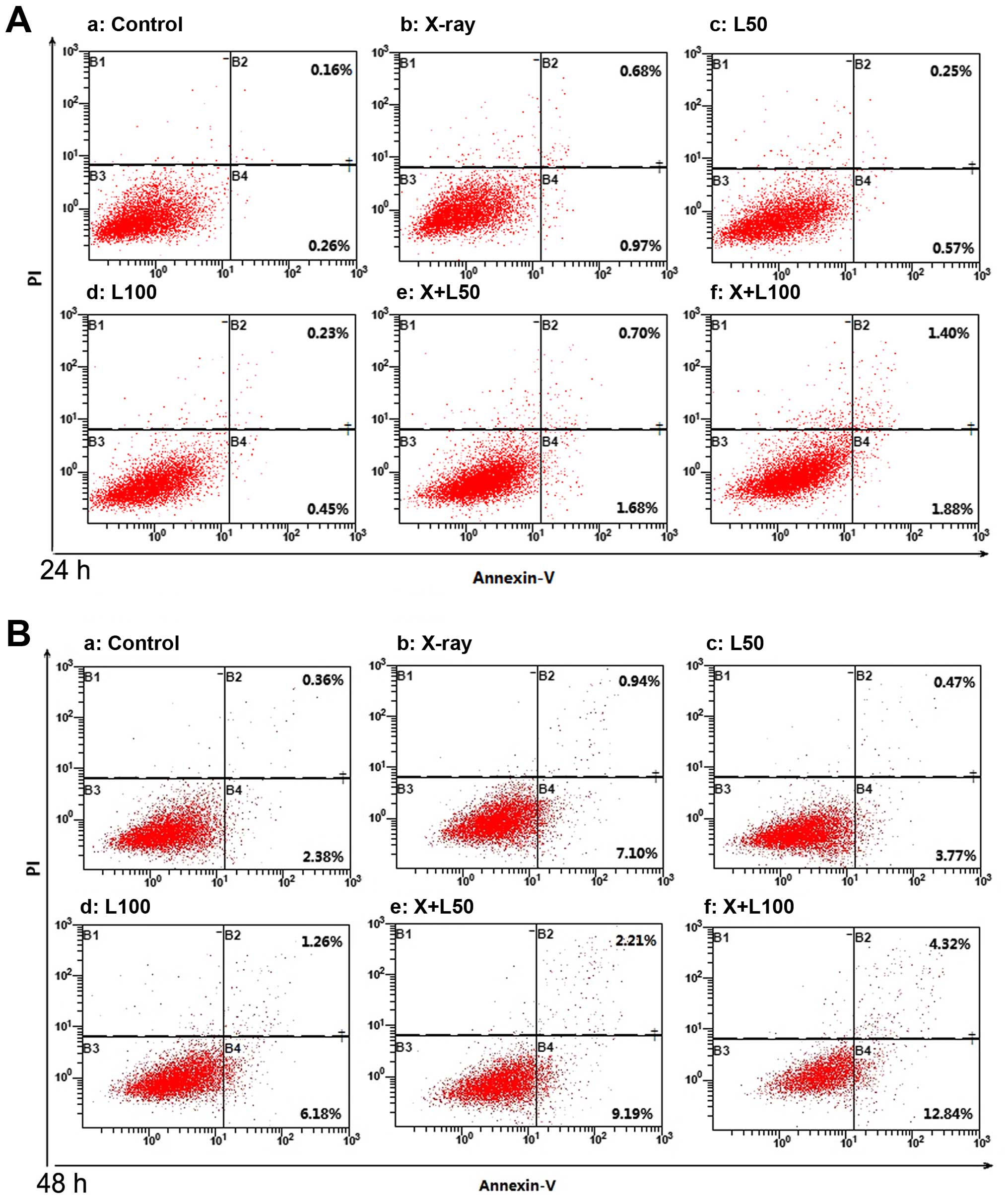
Cell apoptosis
LWMHs induced apoptosis of tumor cells in
vitro and in vivo (9,10). Our
study examined whether nadroparin had synergetic contribution to
tumor cell apoptosis when combined with X-ray irradiation. As shown
in Figs. 2 and 3, each treatment remarkably induced A549
cell apoptosis dose- and time-dependently compared to the control
group (p<0.05). The promotion of apoptosis induced by X-ray
irradiation alone was more significant than by different dose of
nadroparin alone (p<0.05). Furthermore, the apoptosis rate
reached 17.41±0.63% in X+L100 group 48 h after
treatment, and was the highest among the experimental groups
(p<0.05), which revealed that the higher the dose of nadroparin
combined with X-ray irradiation, the more effective the result
was.
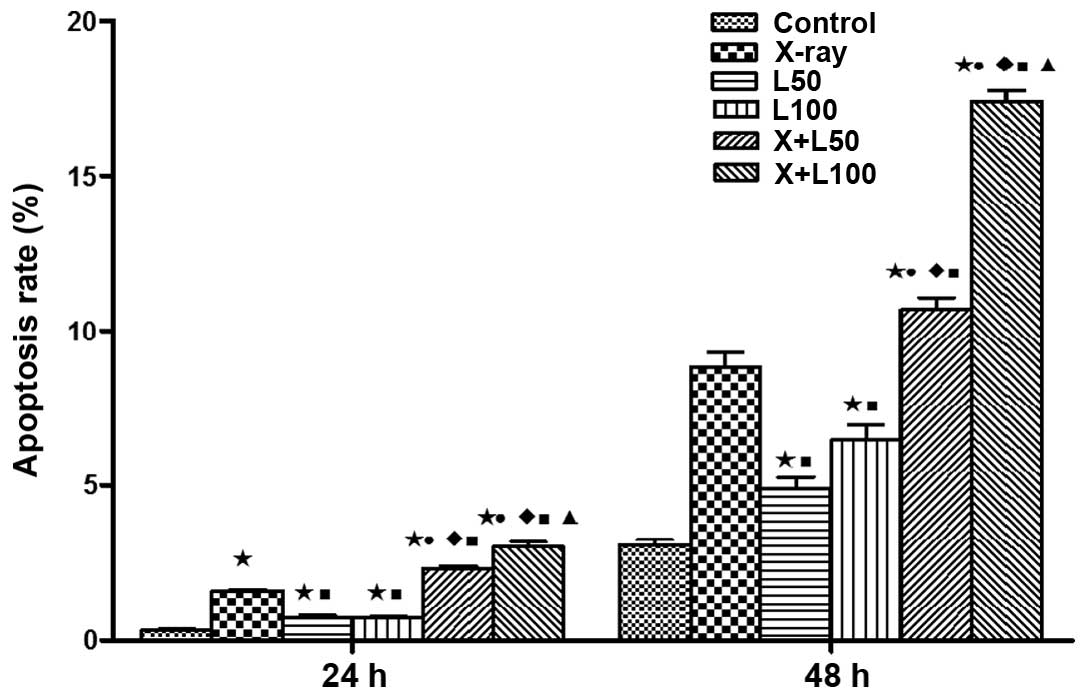 | Figure 3Apoptotic fraction of A549 cells in
different experimental groups. Significant differences of A549 cell
apoptosis in the treated groups were observed compared to the
control group (*p<0.05, t=17.307, 3.635, 4.964, 18.636 and
15.353 at 24 h, t=11.251, 4.517, 6.443, 18.380 and 35.032 at 48 h).
The promotion of apoptosis induced by X-ray irradiation alone was
more significant than by different dose of nadroparin alone but
less effect was observed when combined (■p<0.05,
t=9683, 15.043, 7.899 and 8.523 at 24 h, t=6.606, 3.432, 3.096 and
14.371 at 48 h). A difference from L50
(●p<0.05, t=13.338 and 12.525 at 24 h, t=11.279 and
24.579 at 48 h, p<0.05); a difference from L100
(♦p<0.05, t=16.415 and 13.501 at 24 h, t=6.842 and
17.866 at 48 h); a difference from X+L50
(▲p<0.05, t=3.846 at 24 h, t=13.020 at 48 h). |
Release of TGF-β1
The concentrations of TGF-β1 in the cell
supernatants at the time-points (24 and 48 h) after irradiation
were compared among each group in Fig.
4. We found that the TGF-β1 levels were increased with the
prolonging of time in the control group, X-ray irradiation group
and the L50 group, and the increase of the control group
was the most obvious. On the contrary, with the prolonging of time,
the TGF-β1 levels were significantly decreased in the
L100 group and two combined treatment groups. The
concentrations of TGF-β1 in X+L100 group were 71.88±5.87
and 37.35±2.92 pg/ml, respectively, at 24 and 48 h after treatment,
which was the lowest among the experimental groups (p<0.05).
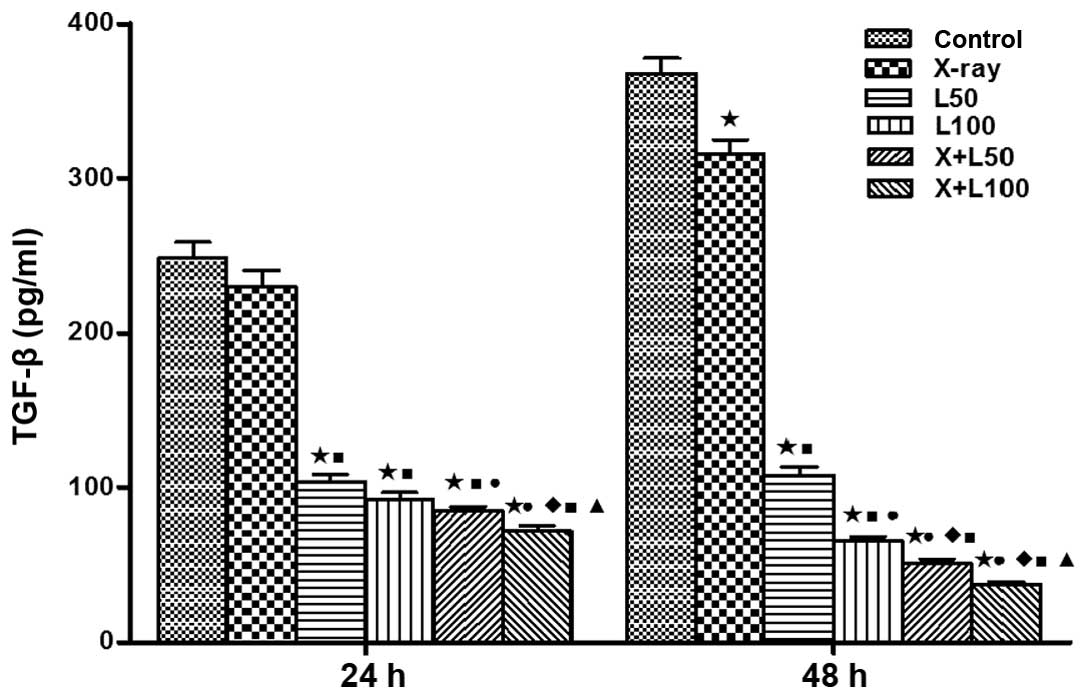 | Figure 4Concentration of transforming growth
factor-β1 (TGF-β1) in cell culture supernatants. Enzyme-linked
immunosorbent assay kit was used to measure TGF-β1 levels in cell
culture supernatants. The levels were of statistically significant
differences among the groups. A difference from the control group
(*p<0.05, t=13.294, 14.419, 16.238 and 17.173 at 24 h, t=3.817,
22.470, 28.463, 29.598 and 31.885 at 48 h); a difference from X-ray
irradiation group (■p<0.05, t=11.173, 12.253, 13.802
and 14.759 at 24 h, t=20.145, 27.037, 28.312 and 31.065 at 48 h); a
difference from L50 (●p<0.05, t=3.384 and
5.356 at 24 h, t=6.987,9.147 and 12.513 at 48 h); a difference from
L100 (p<0.05, t=3.392 at 24 h, t=3.370 and 8.318 at
48 h); a difference from X+L50 (p<0.05, t=3.014 at 24
h, t=3.703 at 48 h). |
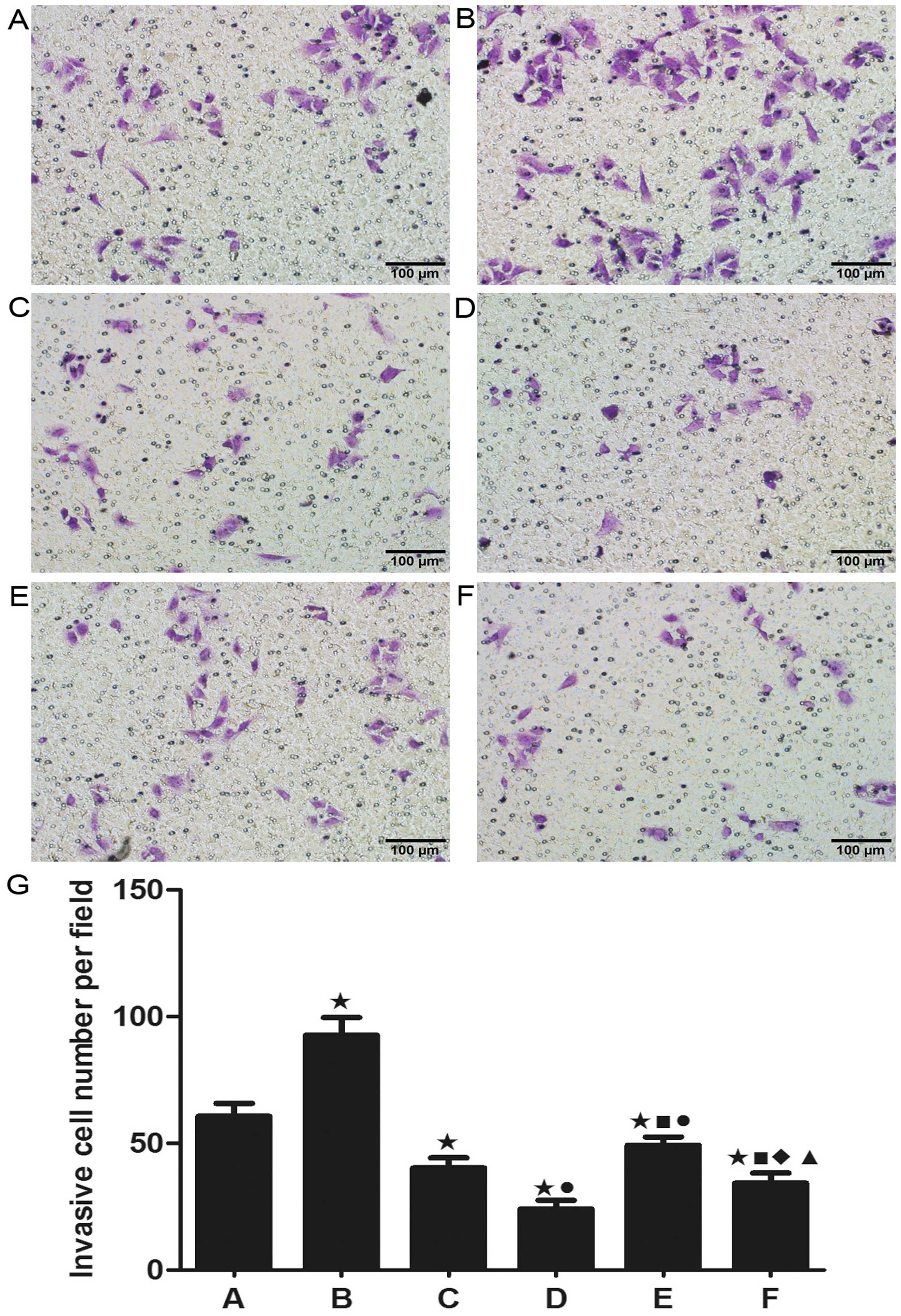
Cell migration and invasion
Cell migration and invasion are critical for the
spreading of cancer and the formation of metastasis in vivo.
As shown in Fig. 5, X-ray
irradiation alone resulted in a significant increase in A549 cell
migration while less migration was observed in the nadroparin alone
groups in a dose-dependent manner. Furthermore, nadroparin
inhibited the increase in A549 cell migration induced by X-ray
irradiation in the combined treatment groups. Additionally, as
shown in Fig. 6, similar results
were seen in A549 cell invasion. Our result showed that X-ray
irradiation promoted the ability of invasion and migration in A549
cells while nadroparin inhibited this side effect
dose-dependently.
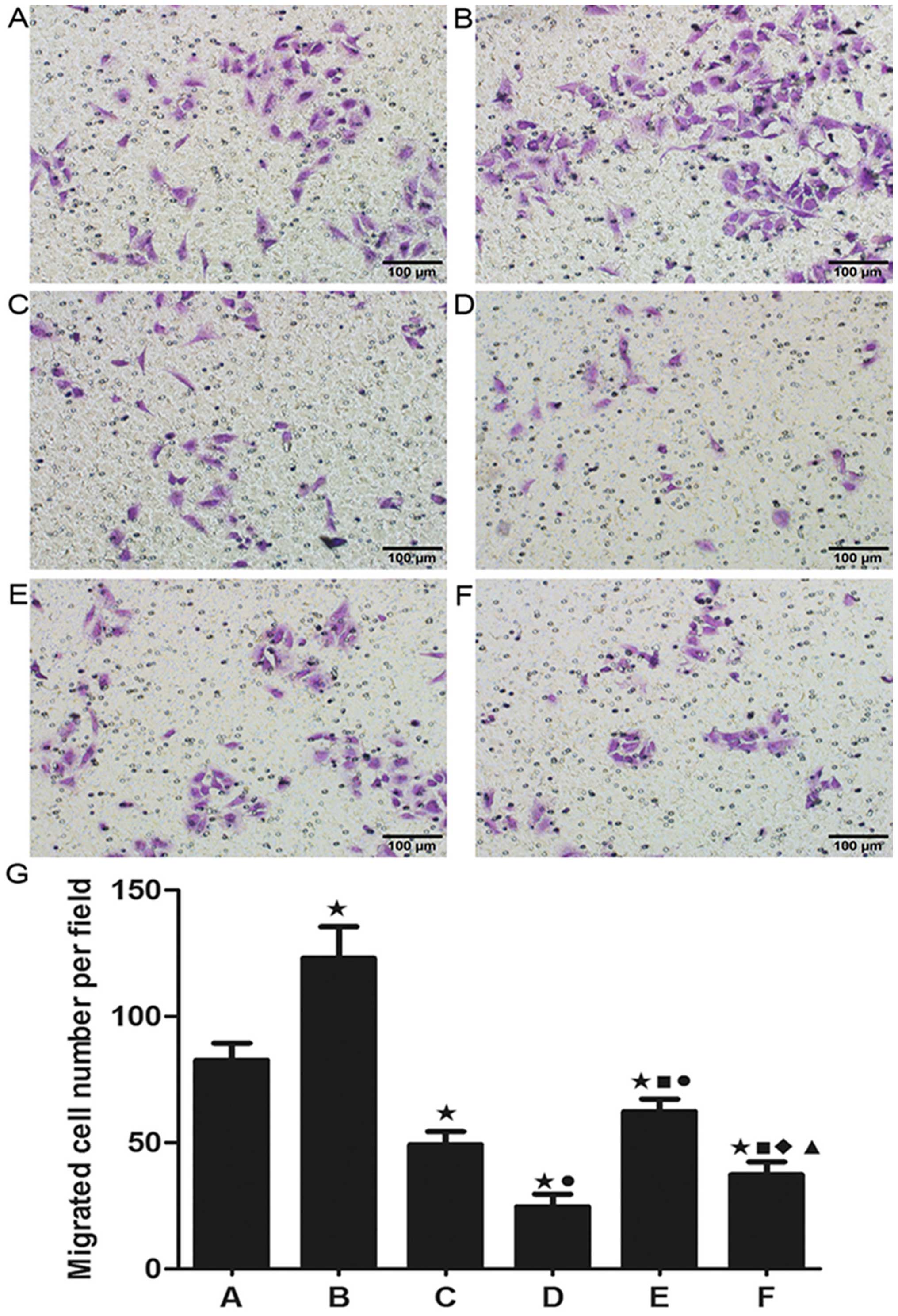 | Figure 5Detection of cell migration. (A)
Control group, (B) X-ray irradiation group, (C) L50
group, (D) L100 group, (E) X+L50 group, (F)
X+L100 group. Original amplification, ×200; scale bar,
100 µm. (G) The migrated cell number per field in each
experimental group. Twenty-four hours after treatment, the ability
of cell migration was increased in the X-ray irradiation group
while nadroparin alone or the combined treatments decreased the
cell migration compared to the control group (*p<0.05, t=4.899,
6.773, 11.867, 4.819 and 9.211). Nadroparin inhibited the promotion
of A549 cell migration induced by X-ray irradiation in a
dose-dependent manner. A difference from X-ray irradiation group
(■p<0.05, t=7803 and 10.959); a difference from
L50 (●p<0.05, t=5.944 and 3.163); a
difference from L100 (♦p<0.05,t=3.052); a
difference from X+L50 (▲p<0.05,
t=6.083). |
Expression levels of CD147, MMP-2 and
survivin
We examined the protein expression levels of
survivin, CD147 and MMP-2 using western blotting in our study to
investigate the related molecular mechanism of the antitumor effect
associated with nadroparin combined with X-ray radiation on A549
cells. As shown in Fig. 7, the
expression levels of CD147 in A549 cells were inhibited by
nadroparin in a dose-and time-dependent manner while no significant
change of CD147 expression was observed in the X-ray irradiation
group. Furthermore, the expression levels of CD147 were the lowest
in the combined treatment groups and L100 group at the
time of 48 h after treatment (p<0.05). As shown in Fig. 8, at the time of 24 h after
treatment, the expression levels of MMP-2 were upregulated in the
X-ray irradiation group while down-regulated in the groups treated
with nadroparin alone (p<0.05). Interestingly, we observed that
the upregulated effects induced by radiation were inhibited by
nadroparin in the combined treatment groups (p<0.05). Similar
tendency of survivin expressions was observed at the time of 24 h
after treatment, which is shown in Fig.
9. On the other hand, at the time of 48 h after treatment, the
expression levels of MMP-2 were inhibited in the treated groups
compared to the control group and the expression levels of survivin
were downregulated in the treated groups compared to the control
group while the down regulation effect induced by X-ray irradiation
alone or the combined treatment was more significant than
nadroparin alone (p<0.05).
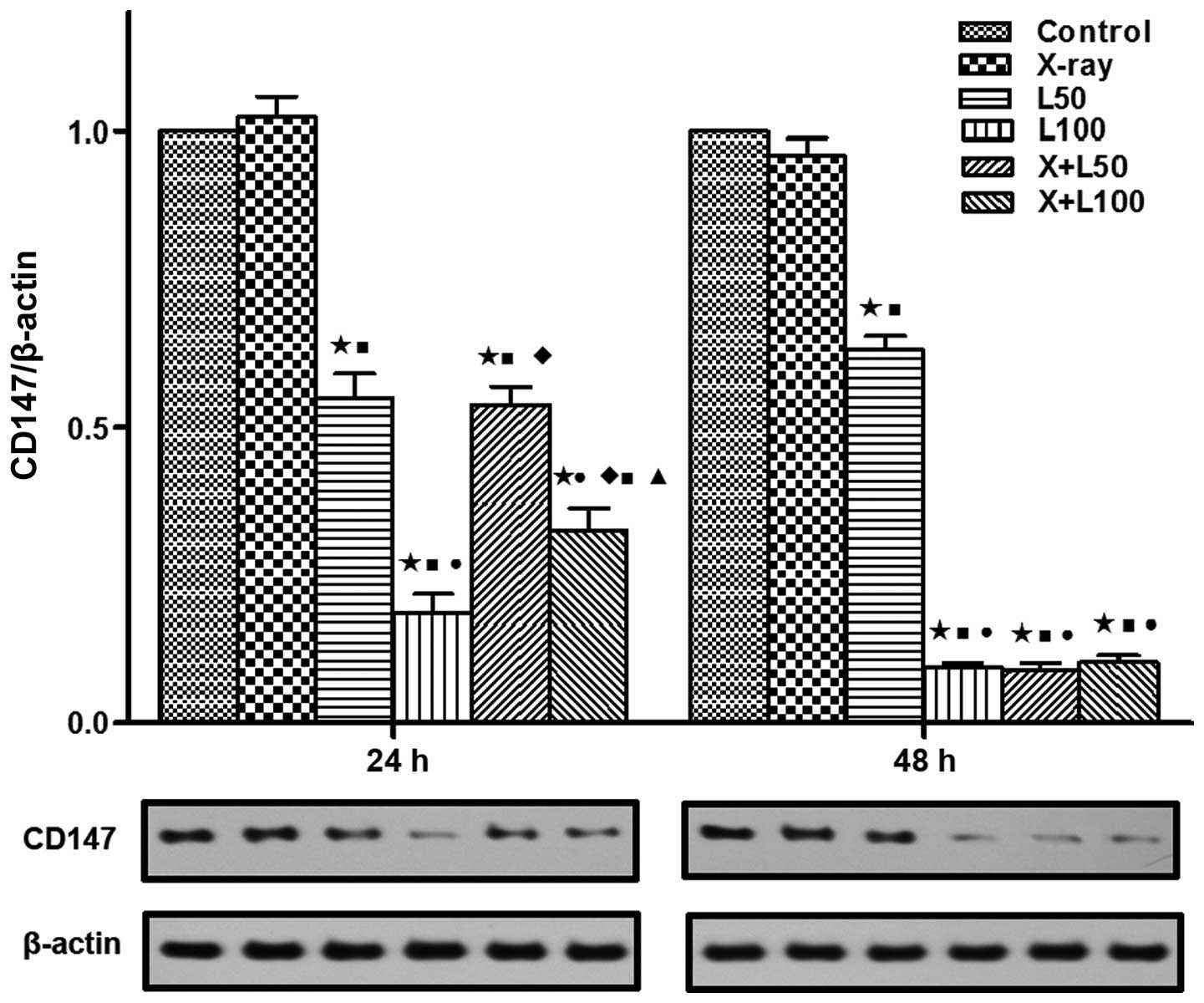 | Figure 7Expression of CD147. Western blotting
demonstrated that CD147 protein expression was significantly
different among each group at 24 and 48 h after treatment in the
lower part of the picture while the upper part shows the relative
expression in each treatment group compared to the control group. A
difference from the control group (*p<0.05, t=11.303, 24.867,
15.121 and 17.772 at 24 h, t=15.858, 114.959, 80.418 and 81.934 at
48 h); a difference from X-ray irradiation group
(■p<0.05, t=8.918, 17.447, 10.419 and 13.493 at 24 h,
t=98.968, 39.360, 28.454 and 28.151 at 48 h); a difference from
L50 (●p<0.05, t=7088 and 4.083 at 24 h,
t=21.811, 20.886 and 20.507 at 48 h); a difference from
L100 (♦p<0.05, t=7902 and 2.811 at 24 h);
a difference from X+L50 (▲p<0.05, t=4374
at 24 h). |
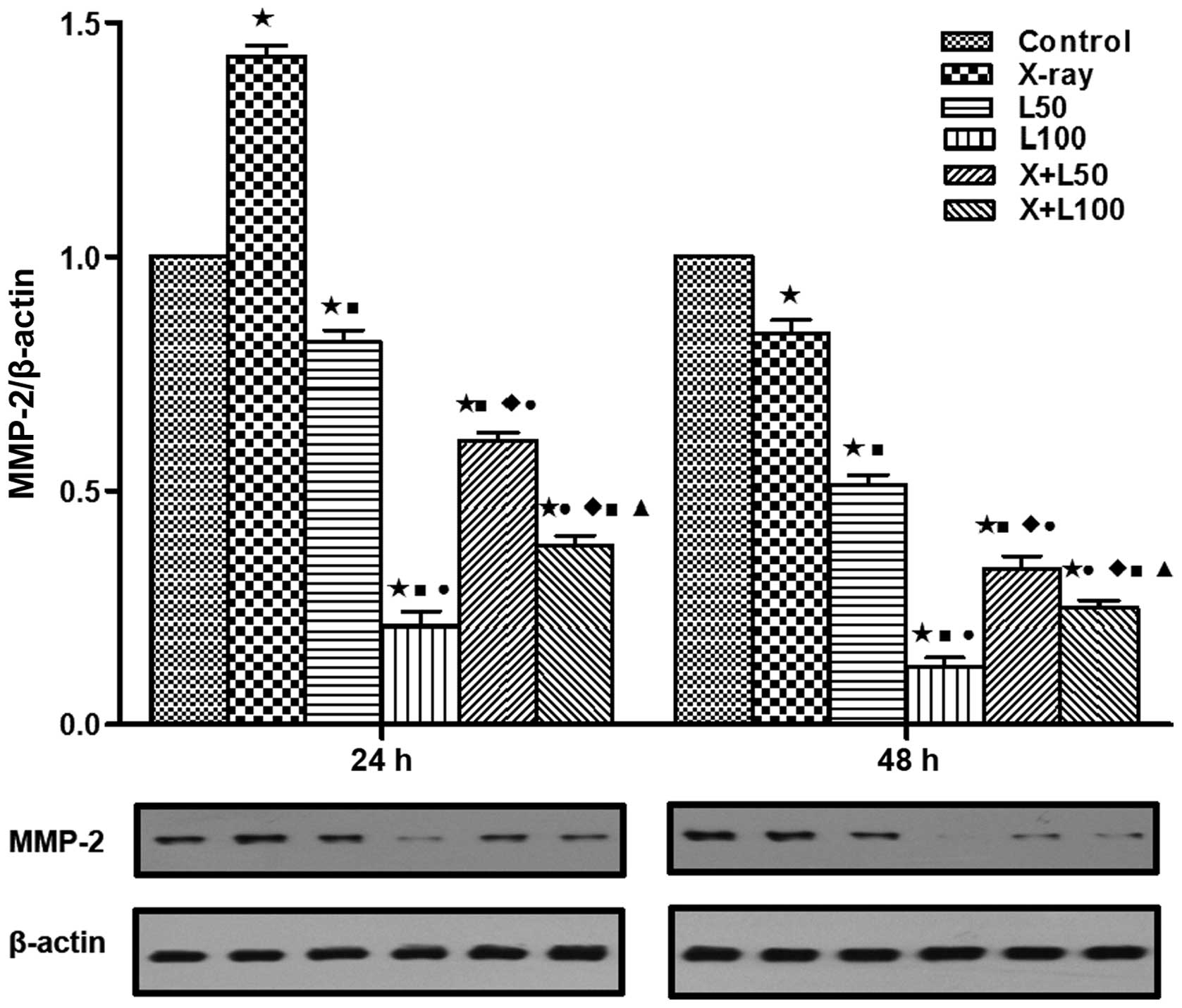 | Figure 8Expression of matrix
metalloproteinase-2 (MMP-2). MMP-2 protein expression was
significantly different among each group at 24 and 48 h after
treatment by western blotting assay in the lower part of the
picture. The upper part shows the relative expression in each
treatment group compared to the control group. A difference from
the control group (*p<0.05, t=17.347, 7.186, 26.075, 21.443 and
30.536 at 24 h, t=5.932, 20.911, 47.308, 25.276 and 45.072 at 48
h); a difference from X-ray irradiation group
(■p<0.05, t=17.277, 31.163, 26.693 and 32.715 at 24
h, t=9.128, 21.711, 13.307 and 18.514 at 48 h); a difference from
L50 (●p<0.05, t=15.407, 6.763 and 13.405
at 24 h, t=12.979, 5.006 and 9.173 at 48 h); a difference from
L100 (♦p<0.05, t=11.207 and 4.796 at 24 h,
t=6.553 and 4.956 at 48 h); a difference from X+L50
(▲p<0.05, t=8.160 at 24 h, t=2.801 at 48 h). |
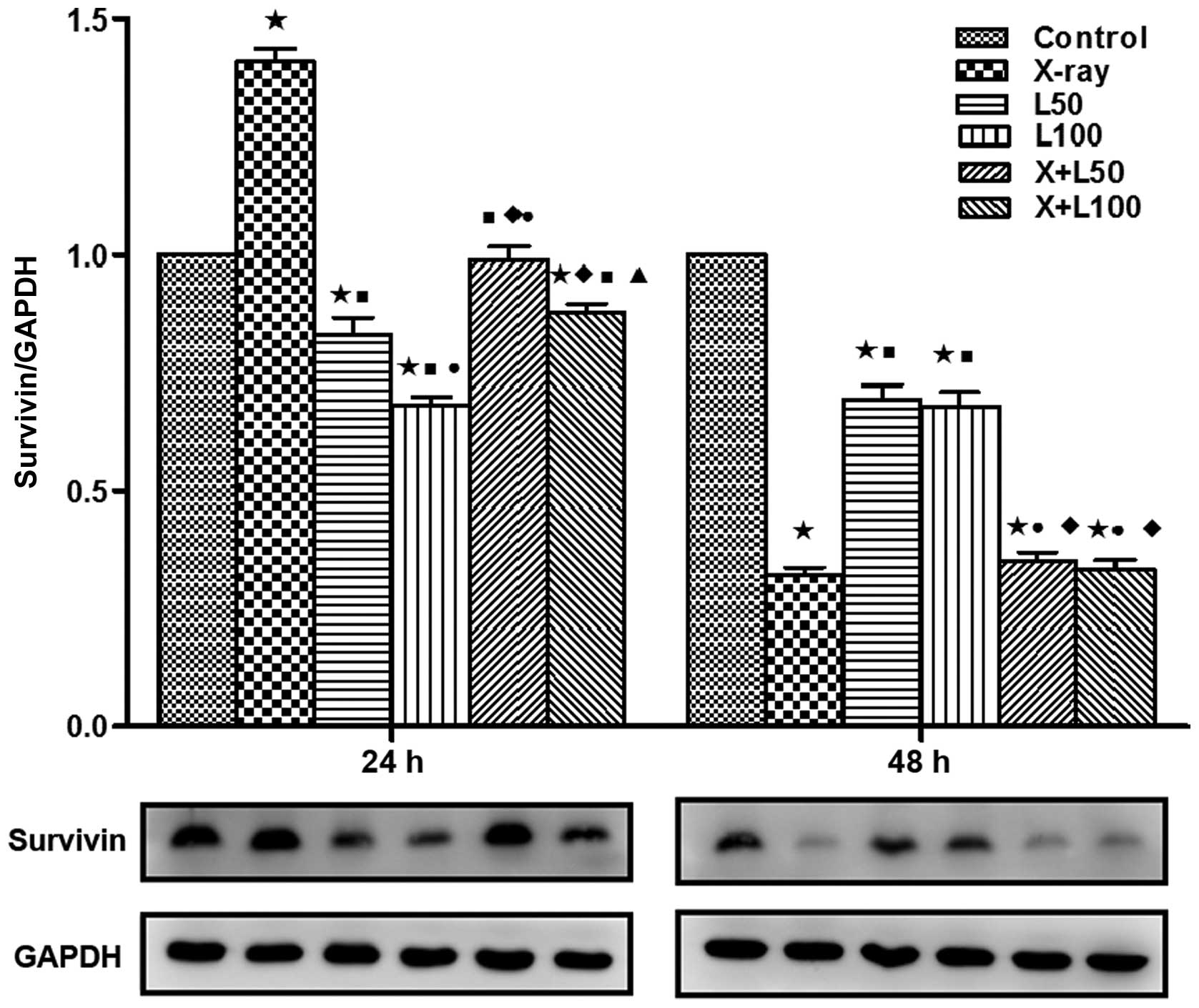 | Figure 9Expression of survivin. Similar to
CD147 and matrix metalloproteinase-2 (MMP-2), survivin protein
expression was also significantly different among each group at 24
and 48 h after treatment by western blotting. A difference from the
control group (*p<0.05, t=14.589, 4.740, 17.331 and 6.550 at 24
h, t=39.077, 10.495, 10.076, 35.048 and 31.658 at 48 h); a
difference from X-ray irradiation group (■p<0.05,
t=12.797, 21.690, 10.421 and 15.791 at 24 h, t=1 1.130 and 9.924 at
48 h); a difference from L50 (●p<0.05,
t=3.789 and 3.448 at 24 h, t=10.006 and 10.131 at 48 h); a
difference from L100 (♦p<0.05, t=8.997 and
7.581 at 24 h, t=8.903 and 9.084 at 48 h); a difference from
X+L50 (▲p<0.05, t=3.231 at 24 h). |
Discussion
Radiotherapy plays an important role in the
treatment of advanced lung cancer. However, the therapeutic
efficacy is compromised due to the resistance to X-rays, tumor
recurrence and metastasis. In addition, radiation has been shown to
stimulate the tumor cell growth and promote invasiveness of
different types of tumor cells in vitro by the upregulation
of secreted proteases, such as MMPs and plasminogen activators
(11–14). Other researchers have made similar
findings in vivo. For instance, Camphausen et al
(15) have reported that radiation
therapy to a primary tumor accelerates metastatic growth in the
Lewis lung cancer mouse model. So it is particularly important to
find the effective combination therapy in order to get the best
therapeutic efficacy in tumor treatment. LMWH has been reported
effective for the treatment of metastasis to some extent, the
mechanisms are proposed as anti-coagulation, inhibition of
heparanase, selectins, adhesion, angiogenesis mediated by the tumor
cells, and the effects on cell cycle and apoptosis (6,16,17).
Furthermore, clinical studies have suggested that LMWHs improve
life expectancy of lung cancer patients (18–20).
So, based on the above, we undertook this experiment to find out
the therapeutic effect of nadroparin combined with radiation, and
if so, whether there was a dose and time-response relationship for
nadroparin also could be analyzed.
Results of our study showed that nadroparin and
X-ray irradiation have synergistic antitumor effect in
vitro. Nadroparin or X-ray irradiation alone could slightly
inhibit the cell viability and enhance the cell apoptosis of A549
cells. The therapeutic effect of X-ray irradiation alone was more
significant than that of nadroparin alone. Furthermore, the
antitumor effect was greatly improved in a dose- and time-dependent
manner when these two treatments were combined.
TGF-β, a kind of multifunctional polypeptide, is
closely related to the development of tumors. Three subunits
defined as TGF-β1, T GF - β2 and TGF-β3 have been cloned in mammals
and the study of TGF-β1 is the most profound and active. Many
studies have shown that TGF-β can enhance growth in a progressive
tumor and the possible mechanisms for these growth enhancing
effects were quite complex, including the enhanced angiogenesis
(21,22), increased peritumoral stroma
formation and induced immunosuppression. In our study, with the
prolonging of time, TGF-β1 levels in the cell supernatants were
increased in the control group while X-ray irradiation or low dose
of nadroparin alone could slightly inhibit the increase of TGF-β1
level. Moreover, TGF-β1 levels were decreased when A549 cells were
treated with high dose of nadroparin and the extent of this
decrease was enhanced when combined with X-ray irradiation. This
result suggested that the antitumor effect of nadroparin combined
with X-ray irradiation may partially relate to the inhibition of
TGF-β1 secreted by A549 cells.
CD147, a member of the immunoglobulin superfamily,
is overexpressed in a number of epithelial cell-derived carcinomas
and is associated with tumor development and metastasis by
activating the fibroblasts producing MMPs (23,24).
Wu et al (25) found that
CD147 induced resistance to ionizing radiation in hepatocellular
carcinoma cells in vitro and in vivo. In our study,
we found that nadroparin alone could inhibit the expression of
CD147 and MMP-2 in a dose- and time-dependent manner. The
expression levels of CD147 and MMP-2 were significantly lower in
the combined treatment groups than the control group or X-ray
irradiation group at the time of 24 and 48 h after treatment.
Survivin, the smallest member of the inhibitor of apoptosis protein
(IAP) family, encoded by a single gene located on the human 17q25
chromosome with a molecular mass of 16.5 kDa, was first isolated by
Ambrosini et al (26) at
Yale University. The biological functions of survivin are also
quite complex, including inhibiting tumor cell apoptosis (27,28),
promoting cell proliferation, participating in the regulation of
cell cycle (29–31) and promoting blood vessel formation
(32), which plays an important
role in the development of cancer. Recently, increasing number of
reports have shown that survivin is an independent prognostic
factor for tumor therapy (33,34).
It has also showed that survivin overexpression has been correlated
with elevated resistance to radiotherapy and chemotherapy in recent
studies (35, 36). Investigations have reported that
ionizing radiation at doses of 1 to 8 Gy is known to significantly
elevate survivin levels in malignant cells (37–39).
Therefore, blocking tumor cell survivin function or inhibiting its
expression may inhibit cell apoptosis and proliferation and enhance
its sensitivity to radiotherapy and chemotherapy. In our study, we
found that nadroparin alone could inhibit the expression of
survivin, which was dose-dependent, but not time-dependent. The
expression of survivin was significantly upregulated by X-ray
irradiation at the time of 24 h after treatment and this effect was
inhibited by nadroparin in a dose-dependent manner. Based on the
results above, we can conclude that the expression of CD147, MMP-2
and survivin was effectively and enduringly inhibited by nadroparin
combined with X-ray irradiation in the whole process of the
treatment. This result suggested that the antitumor effect of
nadroparin combined with X-ray irradiation may partially relate to
the inhibited expressions of CD147, MMP-2 and survivin in A549
cells.
Taken together, addition of nadroparin to
combination radiotherapy resulted in a powerful synergistic
antitumor effect in lung adenocarcinoma A549 cells. The mechanism
may be associated with the induction of cell apoptosis, reduction
of TGF-β1 level, inhibition of cell invasion and migration and
downregulated expression of CD147, MMP-2 and survivin. Importantly,
these results revealed that nadroparin could inhibit the promotion
of cell migration and invasion induced by radiotherapy in a
dose-dependent manner. In brief, nadroparin combined with
radiotherapy exerted a synergistic antitumor function, which may
provide a novel strategy for cancer treatment. However, further
investigations are required due to the complexity of structure and
function of different LMWHs.
Acknowledgments
This study was supported by the Personnel Training
Plan of Jinshan Hospital (2015–7).
References
|
1
|
Padera TP, Kadambi A, di Tomaso E,
Carreira CM, Brown EB, Boucher Y, Choi NC, Mathisen D, Wain J, Mark
EJ, et al: Lymphatic metastasis in the absence of functional
intratumor lymphatics. Science. 296:1883–1886. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mitrovska S and Jovanova S: Low-molecular
weight heparin enoxaparin in the treatment of acute coronary
syndromes without ST segment elevation. Bratisl Lek Listy.
110:45–48. 2009.PubMed/NCBI
|
|
3
|
Mousa SA and Petersen LJ: Anti-cancer
properties of low-molecular-weight heparin: Preclinical evidence.
Thromb Haemost. 102:258–267. 2009.PubMed/NCBI
|
|
4
|
Icli F, Akbulut H, Utkan G, Yalcin B,
Dincol D, Isikdogan A, Demirkazik A, Onur H, Cay F and Büyükcelik
A: Low molecular weight heparin (LMWH) increases the efficacy of
cisplatinum plus gemcitabine combination in advanced pancreatic
cancer. J Surg Oncol. 95:507–512. 2007. View Article : Google Scholar
|
|
5
|
Yu CJ, Ye SJ, Feng ZH, Ou WJ, Zhou XK, Li
LD, Mao YQ, Zhu W and Wei YQ: Effect of Fraxiparine, a type of low
molecular weight heparin, on the invasion and metastasis of lung
adenocarcinoma A549 cells. Oncol Lett. 1:755–760. 2010.PubMed/NCBI
|
|
6
|
Carmazzi Y, Iorio M, Armani C, Cianchetti
S, Raggi F, Neri T, Cordazzo C, Petrini S, Vanacore R, Bogazzi F,
et al: The mechanisms of nadroparin-mediated inhibition of
proliferation of two human lung cancer cell lines. Cell Prolif.
45:545–556. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Niu Q, Wang W, Li Y, Ruden DM, Wang F, Li
Y, Wang F, Song J and Zheng K: Low molecular weight heparin ablates
lung cancer cisplatin-resistance by inducing proteasome-mediated
ABCG2 protein degradation. PLoS One. 7:e410352012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Seidensticker M, Seidensticker R, Damm R,
Mohnike K, Pech M, Sangro B, Hass P, Wust P, Kropf S, Gademann G,
et al: Prospective randomized trial of enoxaparin, pentoxifylline
and ursodeoxycholic acid for prevention of radiation-induced liver
toxicity. PLoS One. 9:e1127312014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bae SM, Kim JH, Chung SW, Byun Y, Kim SY,
Lee BH, Kim IS and Park RW: An apoptosis-homing peptide-conjugated
low molecular weight heparin-taurocholate conjugate with antitumor
properties. Biomaterials. 34:2077–2086. 2013. View Article : Google Scholar
|
|
10
|
Erduran E, Tekelioğlu Y, Gedik Y and
Yildiran A: Apoptotic effects of heparin on lymphoblasts,
neutrophils, and mononuclear cells: Results of a preliminary in
vitro study. Am J Hematol. 61:90–93. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jadhav U and Mohanam S: Response of
neuroblastoma cells to ionizing radiation: Modulation of in vitro
invasiveness and angiogenesis of human microvascular endothelial
cells. Int J Oncol. 29:1525–1531. 2006.PubMed/NCBI
|
|
12
|
Kaliski A, Maggiorella L, Cengel KA, Mathe
D, Rouffiac V, Opolon P, Lassau N, Bourhis J and Deutsch E:
Angiogenesis and tumor growth inhibition by a matrix
metalloproteinase inhibitor targeting radiation-induced invasion.
Mol Cancer Ther. 4:1717–1728. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park CM, Park MJ, Kwak HJ, Lee HC, Kim MS,
Lee SH, Park IC, Rhee CH and Hong SI: Ionizing radiation enhances
matrix metalloproteinase-2 secretion and invasion of glioma cells
through Src/epidermal growth factor receptor-mediated p38/Akt and
phosphatidylinositol 3-kinase/Akt signaling pathways. Cancer Res.
66:8511–8519. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhai GG, Malhotra R, Delaney M, Latham D,
Nestler U, Zhang M, Mukherjee N, Song Q, Robe P and Chakravarti A:
Radiation enhances the invasive potential of primary glioblastoma
cells via activation of the Rho signaling pathway. J Neurooncol.
76:227–237. 2006. View Article : Google Scholar
|
|
15
|
Camphausen K, Moses MA, Beecken WD, Khan
MK, Folkman J and O'Reilly MS: Radiation therapy to a primary tumor
accelerates metastatic growth in mice. Cancer Res. 61:2207–2211.
2001.PubMed/NCBI
|
|
16
|
Norrby K: Low-molecular-weight heparins
and angiogenesis. APMIS. 114:79–102. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Debergh I, Van Damme N, Pattyn P, Peeters
M and Ceelen WP: The low-molecular-weight heparin, nadroparin,
inhibits tumour angiogenesis in a rodent dorsal skinfold chamber
model. Br J Cancer. 102:837–843. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kakkar AK, Levine MN, Kadziola Z, Lemoine
NR, Low V, Patel HK, Rustin G, Thomas M, Quigley M and Williamson
RC: Low molecular weight heparin, therapy with dalteparin, and
survival in advanced cancer: The fragmin advanced malignancy
outcome study (FAMOUS). J Clin Oncol. 22:1944–1948. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Altinbas M, Coskun HS, Er O, Ozkan M, Eser
B, Unal A, Cetin M and Soyuer S: A randomized clinical trial of
combination chemotherapy with and without low-molecular-weight
heparin in small cell lung cancer. J Thromb Haemost. 2:1266–1271.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Klerk CP, Smorenburg SM, Otten HM, Lensing
AW, Prins MH, Piovella F, Prandoni P, Bos MM, Richel DJ, van
Tienhoven G, et al: The effect of low molecular weight heparin on
survival in patients with advanced malignancy. J Clin Oncol.
23:2130–2135. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tuxhorn JA, McAlhany SJ, Yang F, Dang TD
and Rowley DR: Inhibition of transforming growth factor-beta
activity decreases angiogenesis in a human prostate cancer-reactive
stroma xenograft model. Cancer Res. 62:6021–6025. 2002.PubMed/NCBI
|
|
22
|
Li C, Guo B, Bernabeu C and Kumar S:
Angiogenesis in breast cancer: The role of transforming growth
factor beta and CD105. Microsc Res Tech. 52:437–449. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kanekura T and Chen X: CD147/basigin
promotes progression of malignant melanoma and other cancers. J
Dermatol Sci. 57:149–154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weidle UH, Scheuer W, Eggle D, Klostermann
S and Stockinger H: Cancer-related issues of CD147. Cancer Genomics
Proteomics. 7:157–169. 2010.PubMed/NCBI
|
|
25
|
Wu J, Li Y, Dang YZ, Gao HX, Jiang JL and
Chen ZN: HAb18G/CD147 promotes radioresistance in hepatocellular
carcinoma cells: A potential role for integrin β1 signaling. Mol
Cancer Ther. 14:553–563. 2015. View Article : Google Scholar
|
|
26
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tamm I, Wang Y, Sausville E, Scudiero DA,
Vigna N, Oltersdorf T and Reed JC: IAP-family protein survivin
inhibits caspase activity and apoptosis induced by Fas (CD95), Bax,
caspases, and anticancer drugs. Cancer Res. 58:5315–5320.
1998.PubMed/NCBI
|
|
28
|
Suzuki A, Ito T, Kawano H, Hayashida M,
Hayasaki Y, Tsutomi Y, Akahane K, Nakano T, Miura M and Shiraki K:
Survivin initiates procaspase 3/p21 complex formation as a result
of interaction with Cdk4 to resist Fas-mediated cell death.
Oncogene. 19:1346–1353. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li F, Ambrosini G, Chu EY, Plescia J,
Tognin S, Marchisio PC and Altieri DC: Control of apoptosis and
mitotic spindle checkpoint by survivin. Nature. 396:580–584. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Skoufias DA, Mollinari C, Lacroix FB and
Margolis RL: Human survivin is a kinetochore-associated passenger
protein. J Cell Biol. 151:1575–1582. 2000. View Article : Google Scholar
|
|
31
|
Suzuki A, Hayashida M, Ito T, Kawano H,
Nakano T, Miura M, Akahane K and Shiraki K: Survivin initiates cell
cycle entry by the competitive interaction with Cdk4/p16(INK4a) and
Cdk2/cyclin E complex activation. Oncogene. 19:3225–3234. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nassar A, Lawson D, Cotsonis G and Cohen
C: Survivin and caspase-3 expression in breast cancer: Correlation
with prognostic parameters, proliferation, angiogenesis, and
outcome. Appl Immunohistochem Mol Morphol. 16:113–120. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Monzó M, Rosell R, Felip E, Astudillo J,
Sánchez JJ, Maestre J, Martín C, Font A, Barnadas A and Abad A: A
novel anti-apoptosis gene: Re-expression of survivin messenger RNA
as a prognosis marker in non-small-cell lung cancers. J Clin Oncol.
17:2100–2104. 1999.PubMed/NCBI
|
|
34
|
Kren L, Brazdil J, Hermanova M, Goncharuk
VN, Kallakury BV, Kaur P and Ross JS: Prognostic significance of
anti-apoptosis proteins survivin and bcl-2 in non-small cell lung
carcinomas: A clinicopathologic study of 102 cases. Appl
Immunohistochem Mol Morphol. 12:44–49. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yoshida H, Sumi T, Hyun Y, Nakagawa E,
Hattori K, Yasui T, Morimura M, Honda K, Nakatani T and Ishiko O:
Expression of survivin and matrix metalloproteinases in
adenocarcinoma and squamous cell carcinoma of the uterine cervix.
Oncol Rep. 10:45–49. 2003.
|
|
36
|
Ikeguchi M, Liu J and Kaibara N:
Expression of survivin mRNA and protein in gastric cancer cell line
(MKN-45) during cisplatin treatment. Apoptosis. 7:23–29. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jin XD, Gong L, Guo CL, Hao JF, Wei W, Dai
ZY and Li Q: Survivin expressions in human hepatoma HepG2 cells
exposed to ionizing radiation of different LET. Radiat Environ
Biophys. 47:399–404. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rödel F, Reichert S, Sprenger T, Gaipl US,
Mirsch J, Liersch T, Fulda S and Rödel C: The role of survivin for
radiation oncology: Moving beyond apoptosis inhibition. Curr Med
Chem. 18:191–199. 2011. View Article : Google Scholar
|
|
39
|
Rödel C, Haas J, Groth A, Grabenbauer GG,
Sauer R and Rödel F: Spontaneous and radiation-induced apoptosis in
colorectal carcinoma cells with different intrinsic
radiosensitivities: Survivin as a radioresistance factor. Int J
Radiat Oncol Biol Phys. 55:1341–1347. 2003. View Article : Google Scholar : PubMed/NCBI
|