Introduction
Chronic infection by hepatitis B virus (HBV) is
strongly associated with the initiation and progression of
hepatocellular carcinoma (HCC) (1,2), and
HBV X protein (HBx) is a major risk factor in the molecular
pathogenesis of HBV-related HCC (3,4). HBx
is encoded by the HBV genome, and it is required for mammalian
hepadnavirus infectivity and replication (5). In human hepatocytes, HBx has multiple
molecular functions by interacting with different transcription
factors and modulating numerous cellular signaling pathways
(6–8).
microRNAs (miRNAs) are a class of endogenous small
RNAs 19 to 23 nucleotides (nt) in length, which have been studied
as regulators of gene expression in biological processes, including
cell development, differentiation, apoptosis, and proliferation
(9). Recent studies have shown that
HBx protein can induce the differential expression of miRNAs, such
as miR-520 (10), miR-145, miR-222,
miR-21 (11) and miR-146 (12). Oncogenic miR-21 was reported as an
important miRNA induced by HBx, and promotes cell proliferation
(13) and transformation (14), but the related mechanism is not yet
fully elucidated.
Interleukin-12 (IL-12) was discovered as a 'natural
killer-stimulating factor' and a 'cytotoxic lymphocyte maturation
factor' (15,16). IL-12 is produced by monocytes,
macrophages, B cells and dendritic cells. IL-12 is known as a T
cell-stimulating factor, which can stimulate the production of
interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) from T
cells and natural killer (NK) cells, and favors the differentiation
of naive CD4+ T cells into mature Th1 cells (17). Studies have reported that IL-12 as a
cytokine has antitumor therapeutic activities, and it has been
shown to inhibit tumorigenesis and induce regression of established
tumors. IL-12 promotes the effective destruction of cancer cells by
inducing proliferation of NK and T cells, and IL-12 enhances the
generation and activity of cytotoxic T lymphocytes (CTLs) (18,19).
In addition, several other mechanisms of IL-12 strongly contribute
to antitumor activities (20,21),
and the antitumor activity of IL-12 can be improved by its
combination with various therapeutics (22,23).
In this study, we first report that IL-12 was
regulated by HBx-induced miR-21 in human hepatocytes, especially in
HCC cells. The study aimed to reveal the role of HBx-induced miR-21
and IL-12 in HCC biology.
Materials and methods
Cell culture and transfection
Human HCC cell lines HepG2 and HepG2 2.2.15, normal
liver L02 cells and human embryonic kidney 293 (HEK293) cells were
maintained in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS) containing
penicillin-streptomycin antibiotics (all from Thermo Fisher
Scientific, Waltham, MA, USA) at 37°C in a humidified incubator
with 5% CO2.
miR-21 mimics and the inhibitor were transfected
into cells for upregulation or downregulation, respectively, of
miR-21 expression, and an miR-21 sequence-scrambled RNA was used as
the miRNA negative control (NC_miR). Pre-designed siRNAs were used
to inhibit the expression of HBx. cDNA of HBx cloned into the
pEGFP-N3 vector (Clontech Laboratories, Mountain View, CA, USA) was
used as an HBx transgene (pHBx) in hepatic cells, and an empty
vector (vector) was used as a negative control. The cells were
transfected in vitro with the miRNAs, siRNAs and plasmids
using Lipofectamine® 2000 transfection reagent (Thermo
Fisher Scientific) according to the manufacturer's instructions.
miR-21 mimics, inhibitor, siRNAs and negative control were obtained
from Biomics Biotechnologies Co., Ltd. (Nantong, China) and the
sequences are shown in Table I.
 | Table ISequences of the RT-qPCR primers. |
Table I
Sequences of the RT-qPCR primers.
| Genes | Sequences
(5′–3′) |
|---|
| HBx | F:
GTCTGTGCCTTCTCATCTG |
| R:
GGTCGGTCGTTGACATTG |
| IL-12 | F:
CTCCTCCTTGTGGCTACC |
| R:
TGAAGGCATGGGAACATTC |
| β-actin | F:
AATCGTGCGTGACATTAAG |
| R:
GAAGGAAGGCTGGAAGAG |
Dual-luciferase reporter (DLR) assay
The cDNA of the 3′UTR region of IL-12 mRNA was
constructed into the pGL3-vector (Promega Corp., Madison, WI, USA)
as a dual-luciferase miRNA target expression vector (pGL3-IL-12
3′UTR wild-type) to evaluate miR-21 activity. The IL-12 3′UTR
mutant vector was also constructed as the negative control
(pGL3-IL-12 3′UTR mutant). HEK293 cells were seeded in a 24-well
plate. After 24 h, the cells were co-transfected with pGL3-IL-12
3′UTR wild-type or mutant vector and miR-21 mimics or NC_miR;
pRL-TK (Promega Corp.) was co-transfected as internal control.
Luciferase activities were measured 48-h post-transfection using
the DLR assay system (Promega Corp.) according to the
manufacturer's instructions.
Real-time quantitative PCR (RT-qPCR)
Total RNA of the hepatocytes for mRNA detection was
extracted using TRIzol® reagent (Thermo Fisher
Scientific). Small RNA enriched with miRNAs was isolated using
mirPremier® microRNA isolation kit (Sigma-Aldrich, St.
Louis, MO, USA) according to the manufacturer's instructions.
Stem-loop RT-qPCR was performed for miR-21
expression detection as described previously (24), U6 small RNAs were used as an
internal control (25). RT-qPCR was
carried out using the SuperScript® III
Platinum® SYBR® Green One-Step RT-qPCR kit
(Thermo Fisher Scientific) according to the manufacturer's
instructions. The relative expression was evaluated by the
2−ΔΔCt method (26). The
primer sequences are shown in Table
II.
 | Table IISequences of the HBx-targeted
siRNAs. |
Table II
Sequences of the HBx-targeted
siRNAs.
| siRNAs | Sequences
(5′–3′) |
|---|
| HBx_si1 | Sense: |
GGACUCUCUGCAAUGUCAAdTdT |
| Antisense: |
UUGACAUUGCAGAGAGUCCdTdT |
| HBx_si2 | Sense: |
GGGAGGAGAUUAGAUUAAAdTdT |
| Antisense: |
UUUAAUCUAAUCUCCUCCCdTdT |
| HBx_si3 | Sense: |
GCGGGACGUCCUUUGUUUAdTdT |
| Antisense: |
UAAACAAAGGACGUCCCGCdTdT |
| HBx_si4 | Sense: |
GAAUGUUGCCCAAGGUCUUdTdT |
| Antisense: |
AAGACCUUGGGCAACAUUCdTdT |
| NC_siR | Sense: |
UUCUCCGAACGUGUCACGUdTdT |
| Antisense: |
ACGUGACACGUUCGGAGAAdTdT |
Western blot analysis
Cells were plated in 6-well plates and treated as
described above. Post 48-h treatment, the cells were harvested and
lysed in ice-cold cell RIPA lysis and extraction buffer (Thermo
Fisher Scientific). After centrifugation for collecting, the
proteins were separated by polyacrylamide gel electrophoresis and
electro-transferred to polyvinylidene fluoride (PVDF) membranes
(Millipore Corp., Billerica, MA, USA), and then incubated with the
anti-IL-12 (1:1,000 dilution), anti-HBx antigen (1:1,000 dilution)
or mouse anti-human β-actin antibody (1:5,000 dilution) (all from
Abcam, Cambridge, MA, USA) as internal control. After washing with
TBST, the membrane was incubated with horseradish peroxidase
(HRP)-conjugated secondary antibody for 1.5 h at room temperature,
and then washed in TBST. Finally, the specific proteins were
detected with ECL substrate (Thermo Fisher Scientific).
Cell proliferation assay
Cell proliferation was measured using the
Vybrant® MTT Cell Proliferation assay kit (Thermo Fisher
Scientific). Hepatocytes were seeded on a 96-well plate at a
concentration of 5×103 cells/well before transfection
and grown to about 70% confluency for 24 h. After treatments for 0,
24, 48, 72 and 96 h, the medium was removed and replaced with 100
μl of fresh culture medium, and then 10 μl of the 12
mmol/l MTT stock solution was added to each well. A negative
control of 10 μl of the MTT stock solution added to 100
μl of medium alone was included. After incubation at 37°C
for 4 h, 100 μl of the SDS-HCl (0.01 mol/l) solution was
added to each well and mixed thoroughly. The microplate was
incubated at 37°C for 4 h in a humidified chamber. Each sample was
mixed again and the absorbance was read at 570 nm using a
microplate reader (BioTek, Winooski, VT, USA).
Cell apoptosis assay
Cell apoptosis following the different treatments
was determined by flow cytometric (FCM) analysis with Annexin
V-FITC/PI double staining. Briefly, post 48-h treatments as
described above, 1×105 cells/well were harvested and
washed in phosphate-buffered saline (PBS) in a 6-well plate, then
re-suspended in Annexin-binding buffer, followed by incubation with
Annexin V-FITC conjugate and PI for 15 min at room temperature. The
stained cells were detected by FCM, and the results were analyzed
by BD CellQuest software (BD Biosciences, Franklin Lakes, NJ,
USA).
Statistical analysis
All the experiments were performed independently
three times. The data are shown as mean values ± standard deviation
(SD). Statistical analyses were performed using SPSS 19.0 software,
and the results were analyzed using one way ANOVA followed by post
hoc test to assess statistical significance. All P-values are based
on a two-sided statistical analysis and P<0.05 was considered to
indicate statistical significance.
Results
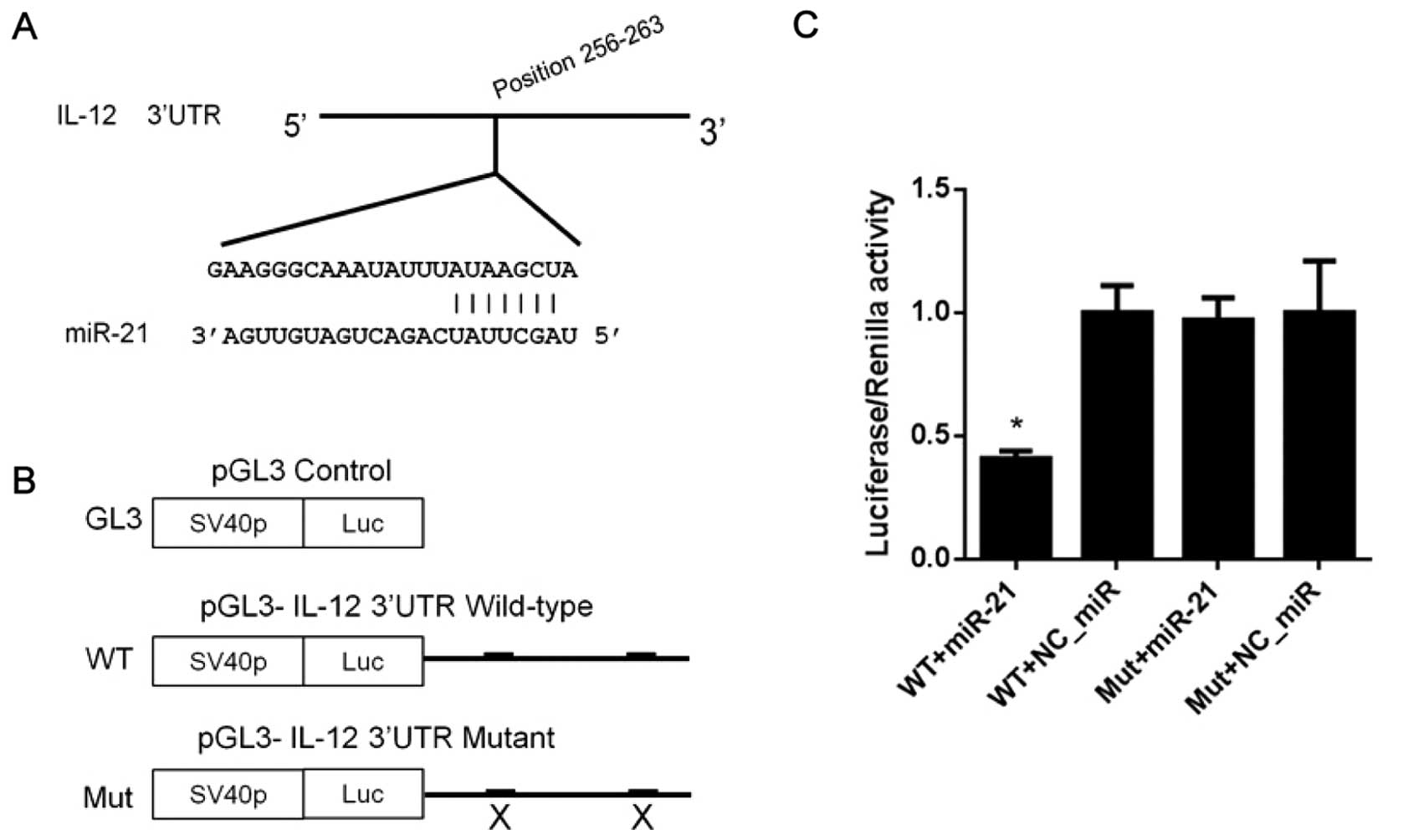
3′UTR of IL-12 mRNA is a direct target of
miR-21
miRNA target predication software was used to
identify the potential miRNAs which regulate IL-12 in TargetScan
database (http://www.targetscan.org/). A
putative miR-21 binding site was predicted in the 3′UTR region of
IL-12 mRNA (Fig. 1A). To
investigate the regulatory effects of miR-21 on IL-12, pGL3-IL-12
3′UTR wild-type and mutant vector were constructed (Fig. 1B). The results of the DLR assay
showed that co-transfection of HEK293 cells with the miR-21 mimics
(miR-21) and pGL3-IL-12 3′UTR wild-type or mutant vector, led to an
obvious reduction in luciferase activity compared to the NC_miR
(Fig. 1C). In contrast, the
luciferase activity of the pGL3-IL-12 3′UTR mutant vector was not
affected by upregulation of miR-21 (Fig. 1C), which further validated the
direct binding between the miR-21 seed sequence and 3′UTR of IL-12
mRNA, and indicates that IL-12 is a novel direct target of
miR-21.
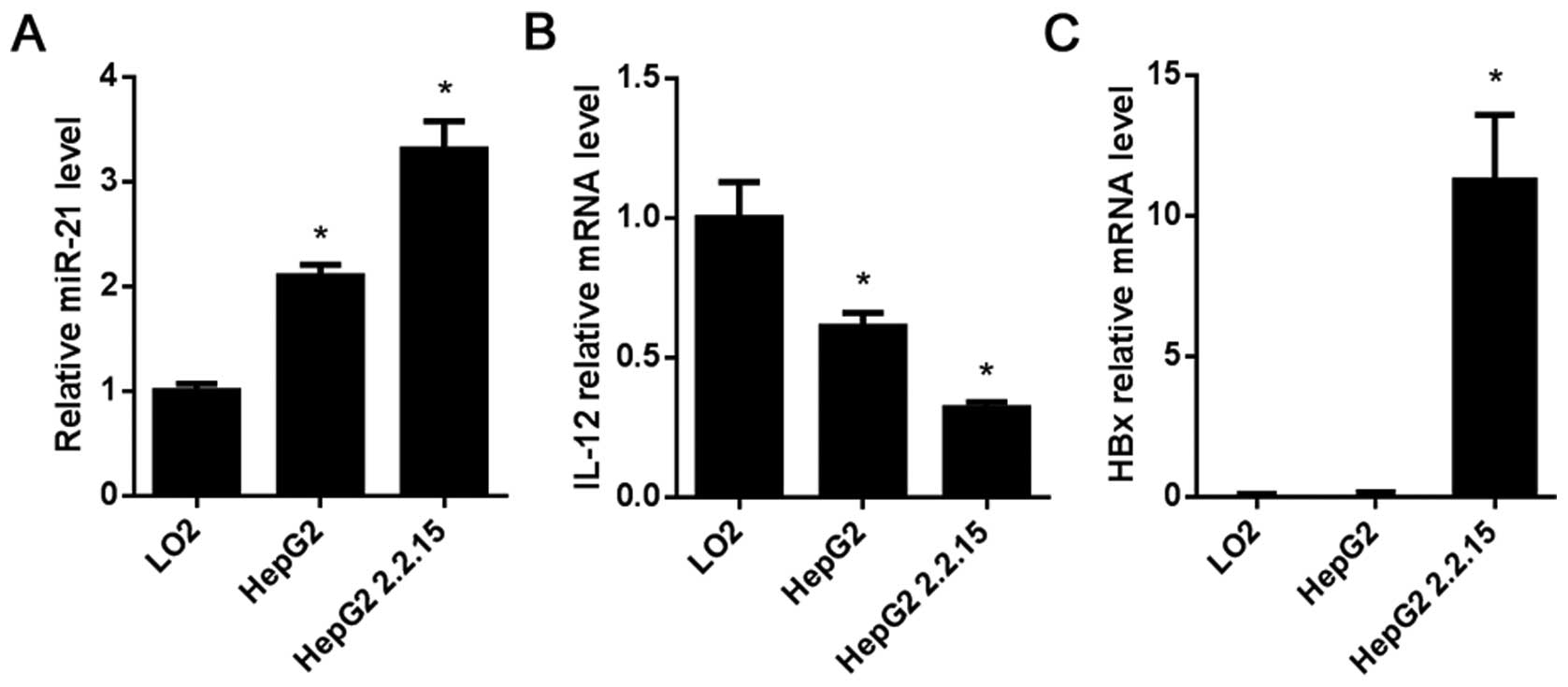
Expression of miR-21, IL-12 and HBx in
the HCC cells
miR-21 expression and IL-12 and HBx mRNA levels in
the HCC cell lines HepG2 and HepG2 2.2.15 were determined by
RT-qPCR. Compared with the L02 cells, the results showed that
miR-21 was highly expressed in the HepG2 and HepG2 2.2.15 cells
(P<0.05; Fig. 2A), while the
mRNA level of IL-12 in the HepG2 and HepG2 2.2.15 cells was lower
than that in the L02 cells (P<0.05; Fig. 2B), HBx was highly expressed in the
HepG2 2.2.15 cells (P<0.05; Fig.
2C).
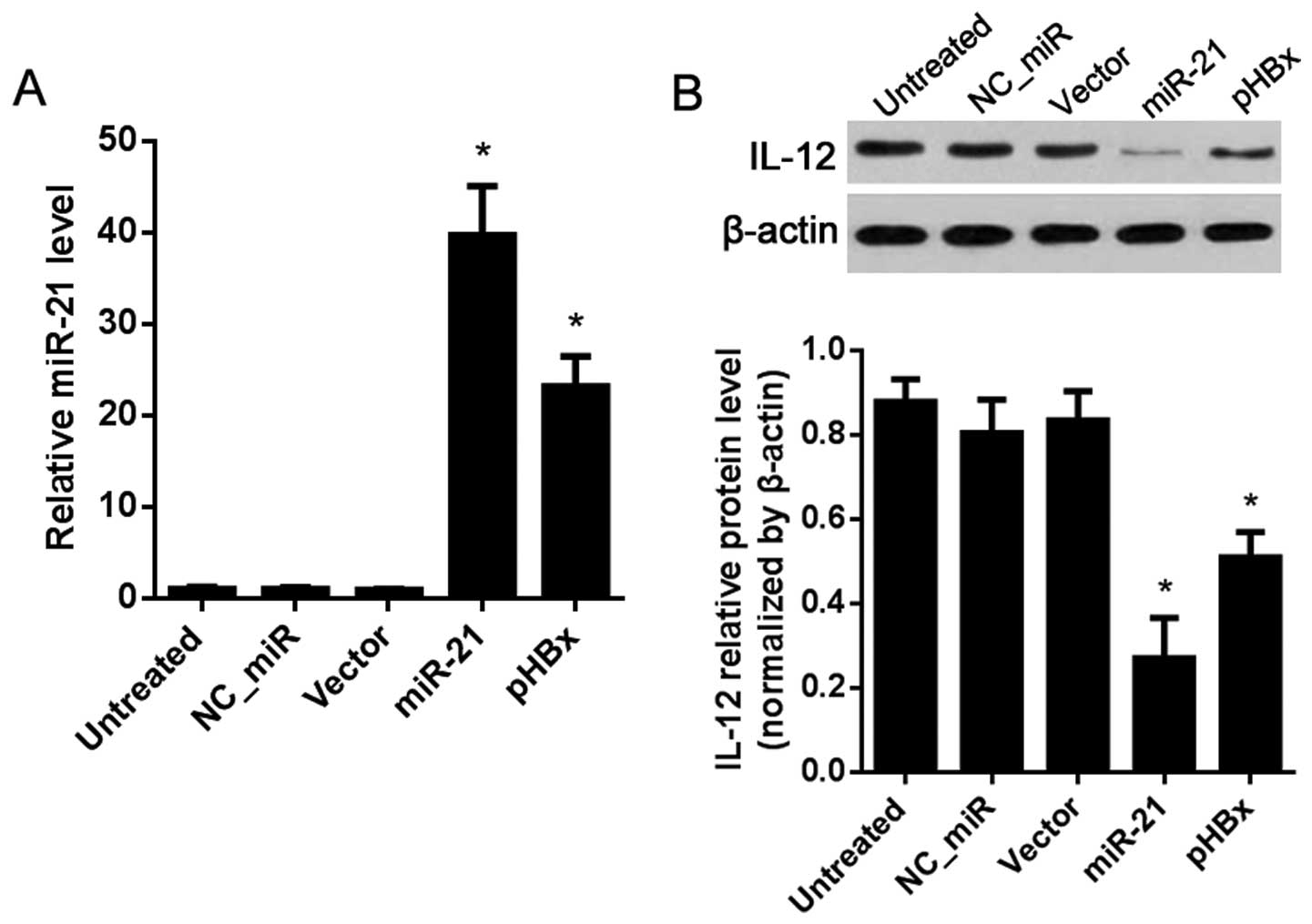
IL-12 is regulated by miR-21 in the L02
cells
The above results (Fig.
2) showed that miR-21 was weakly expressed in the L02 cells,
and there was no HBx expression. As shown in Fig. 3, the expression level of miR-21 was
significantly upregulated post-miR-21 mimic or HBx overexpression
vector (pHBx) transfection compared with that in the NC_miR or
vector-treated group (P<0.05; Fig.
3A). The protein level of IL-12 was obviously decreased in the
miR-21 and pHBx-treated group compared with the levels in the
NC_miR or vector-treated group (P<0.05; Fig. 3B).
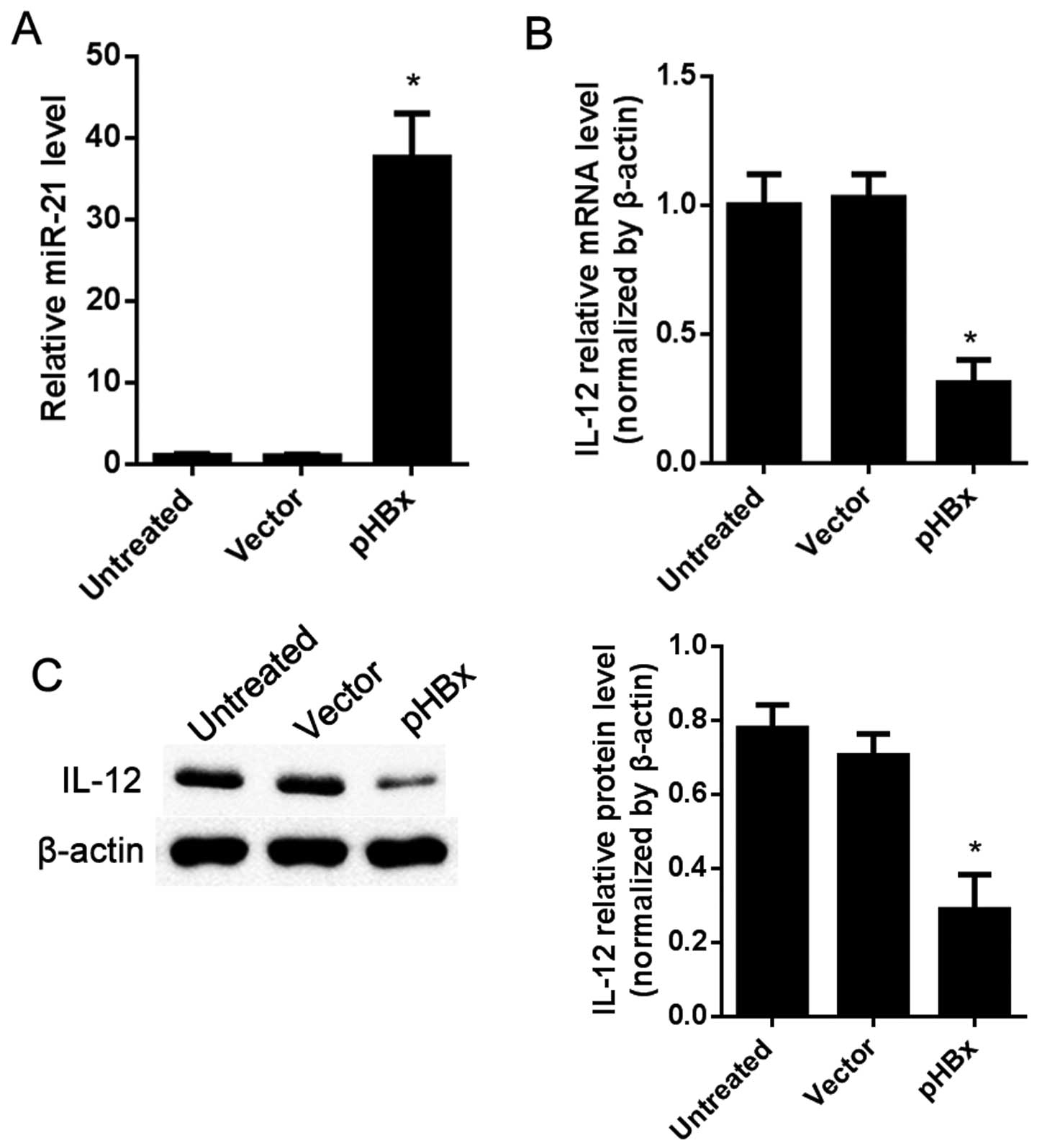
IL-12 is regulated by HBx in the HepG2
cells
HepG2, an HCC cell line with no HBx was used to
observe the expression level of IL-12 as affected by HBx. The
results showed that, compared with the vector-treated cells, miR-21
was significantly upregulated in the pHBx-treated group (P<0.05;
Fig. 4A). The mRNA and protein
levels of IL-12 were both decreased in the pHBx-treated cells as
detected by RT-qPCR and western blot analysis separately (Fig. 4B and C).
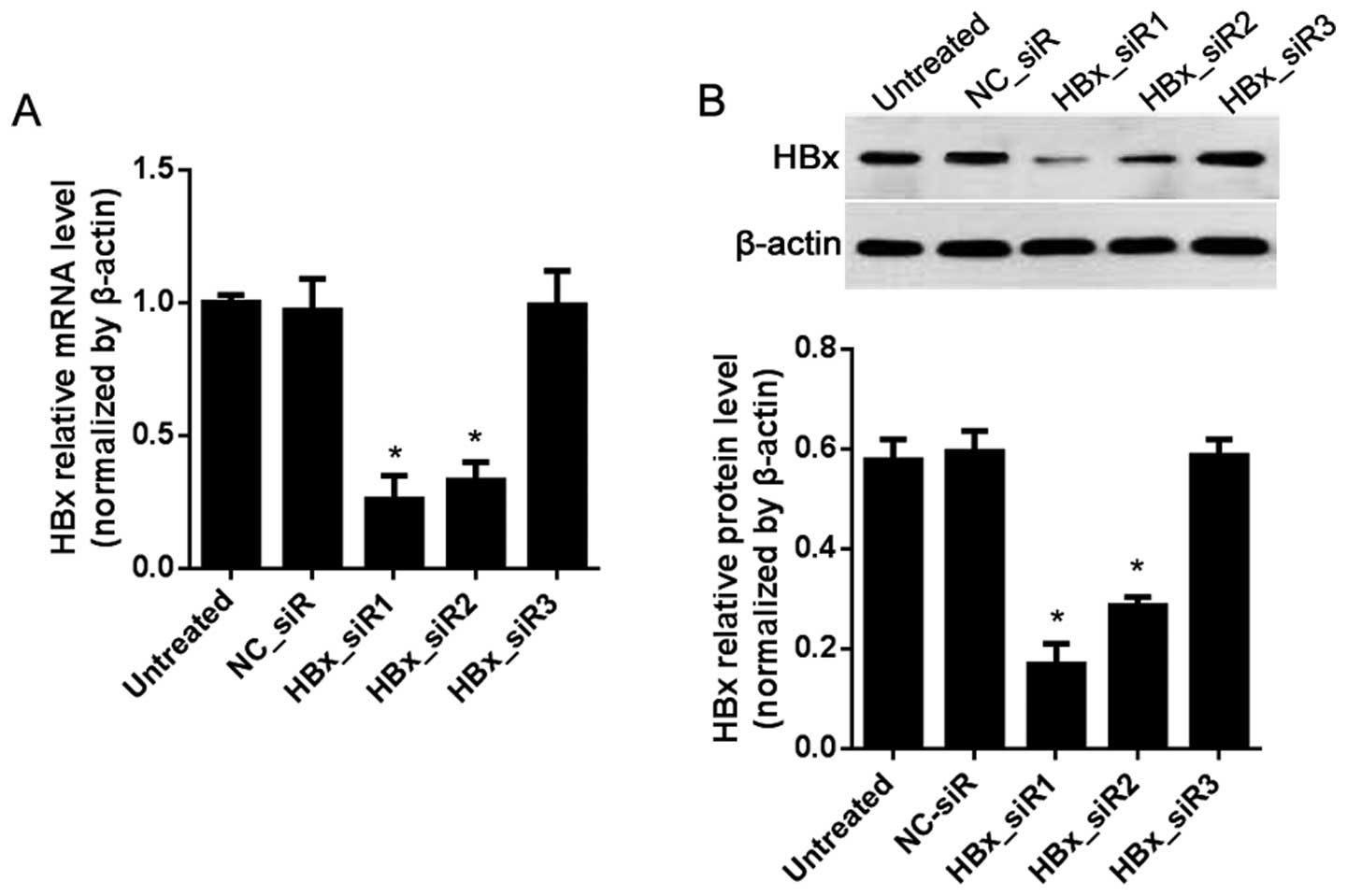
HBx is inhibited by siRNAs in the HepG2
2.2.15 cells
To clarify the correlation of HBx and/or IL-12 with
miR-21, HBx-targeted siRNAs were designed for suppression of HBx in
the HepG2 2.2.15 cells via RNAi method. In comparison with the
NC_siR-treated cells, the mRNA and protein levels of HBx were both
inhibited by HBx_siR1 and HBx_siR2 (P<0.05; Fig. 5), and HBx_siR1 was the most
effective siRNA.
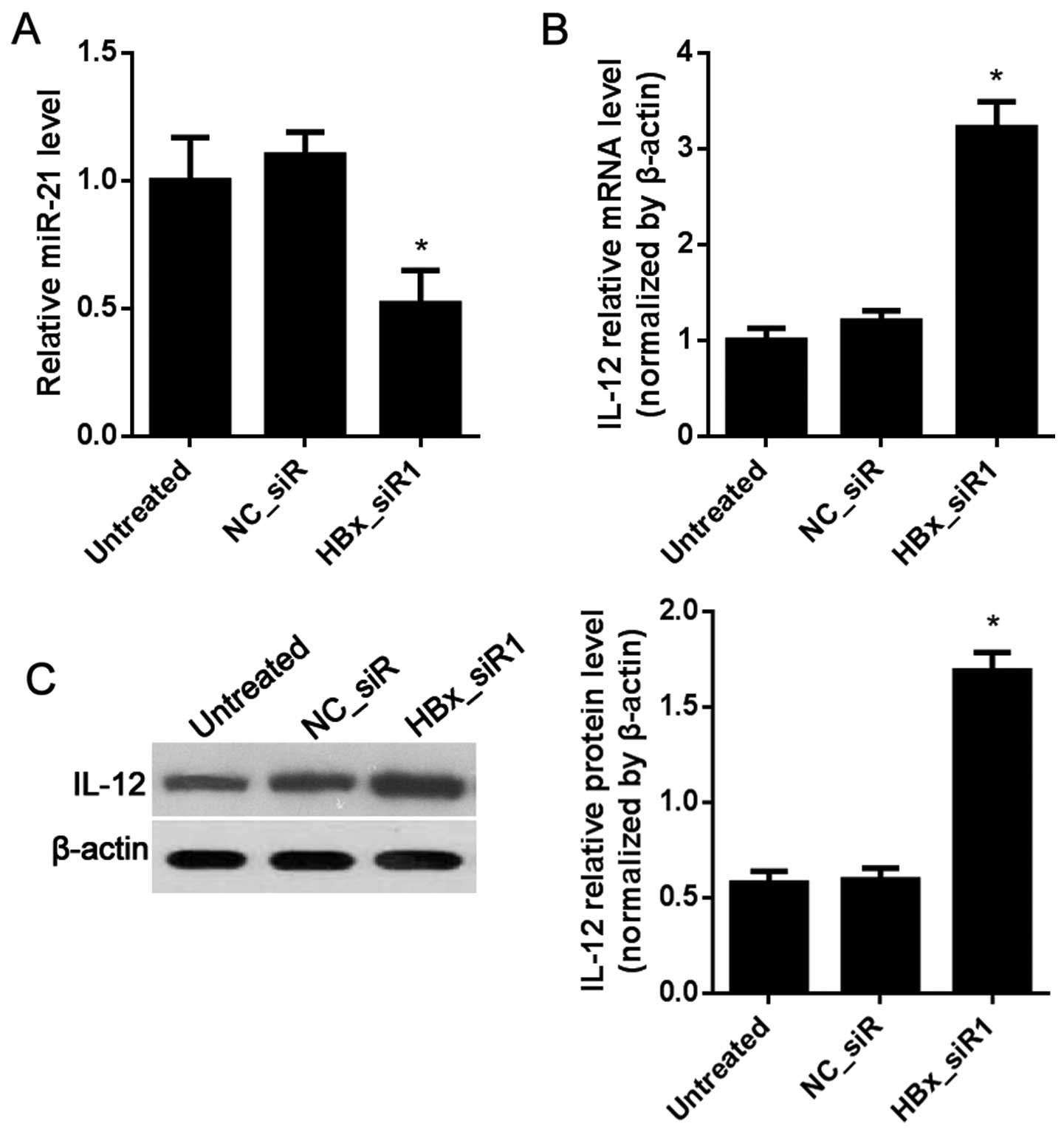
IL-12 is regulated by miR-21 in the HepG2
2.2.15 cells
IL-12 was weakly expressed in the HepG2 2.2.15 cells
as shown in Fig. 2B. The effects of
the regulation of IL-12 by miR-12 when HBx or miR-21 is
downregulated or upregulated were observed. Compared with the
NC_siR-treated cells, miR-21 was significantly decreased in the
HBx_siR1-treated cells (Fig. 6A).
The mRNA and protein levels of IL-12 were both increased in the
HBx_siR1-treated cells (Fig. 6B and
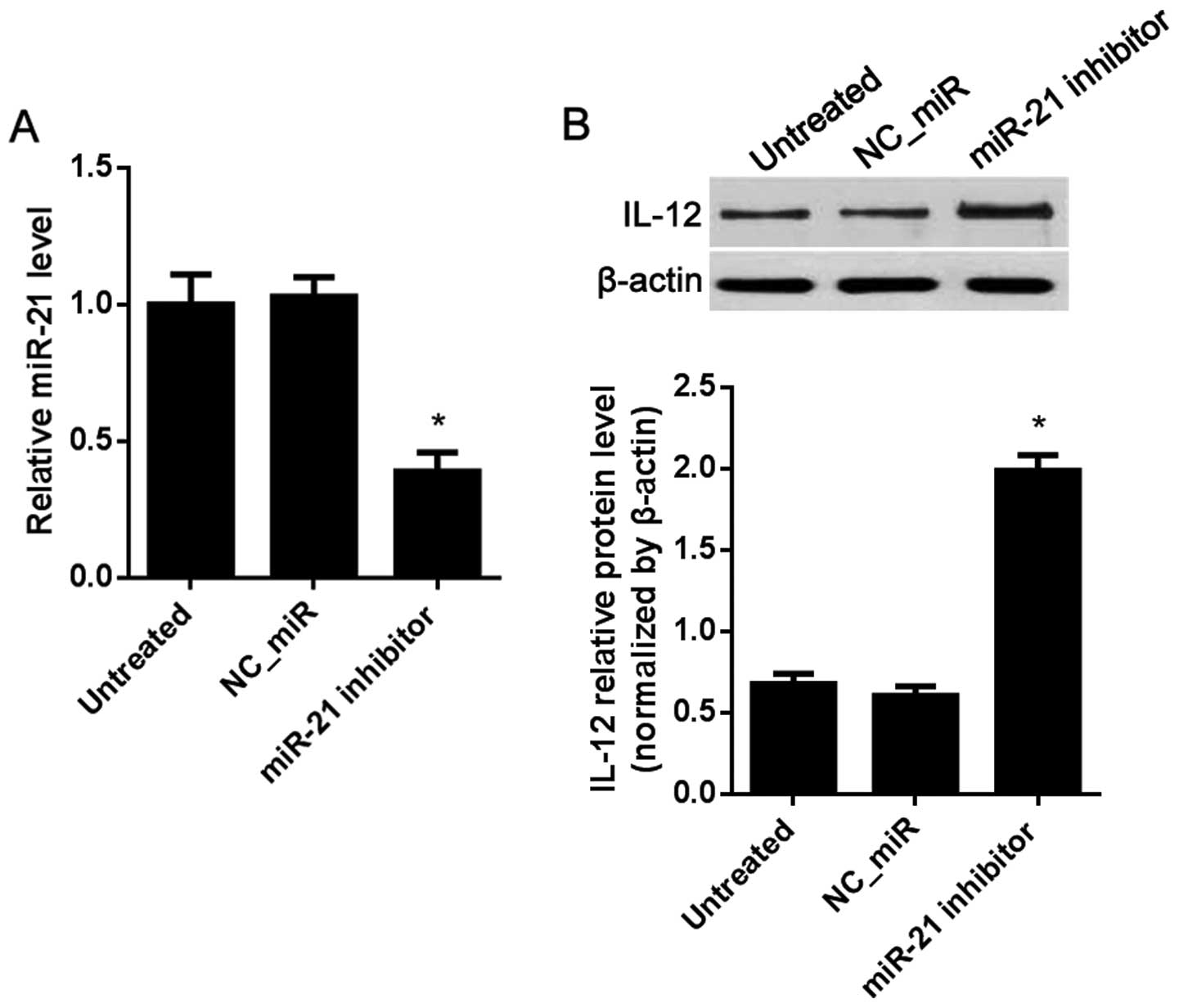
C). To inhibit miR-21, the miR-21 inhibitor was used. The
results showed that, compared with the NC_miR-treated cells, miR-21
was significantly decreased in the miR-21 inhibitor-treated cells
(Fig. 7A), while the protein level
of IL-12 was increased (Fig.
7B).
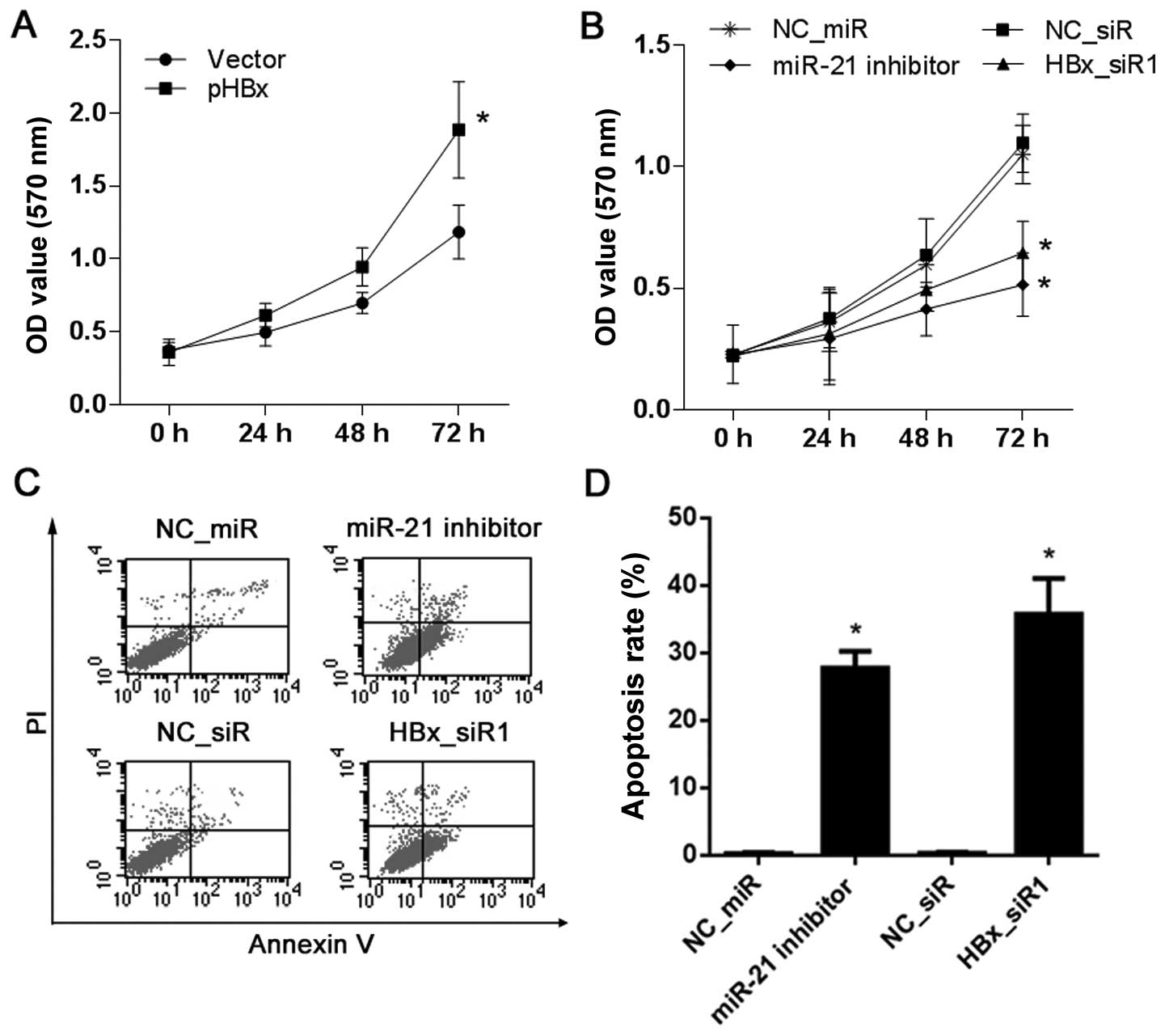
Proliferation and apoptosis of the HCC
cells are affected by HBx or miR-21
HepG2 cells with no HBx expression and HepG2 2.2.15
cells with high HBx expression were used to observe cell
proliferation affected by HBx or miR-21 by MTT assay. Compared with
the vector-treated cells, the results showed that the proliferation
ability of the HepG2 cells was increased after treatment with pHBx
(Fig. 8A). Compared with the NC_miR
or NC_siR-treated cells, the proliferation ability of the HepG2
2.2.15 cells was decreased after treatment with the miR-21
inhibitor or HBx_siR1 (Fig.
8B).
In addition, the role of HBx or miR-21 on the
apoptosis of HepG2 2.2.15 cells was examined by FCM analysis with
Annexin V-FITC/PI double staining. Compared with the NC_miR or
NC_siR-treated cells, treatment with the miR-21 inhibitor or
HBx_siR1 resulted in a significant increase in apoptosis
(P<0.05; Fig. 8C and D).
Discussion
IL-12 used as an anticancer therapeutic in several
clinical trials has demonstrated beneficial results by gene
therapy, and currently antitumor studies based on IL-12 are ongoing
with the key focus of reducing toxicities and side effects
(27–29). Undoubtedly, cancer treatment using
direct administration of IL-12 protein or IL-12 expression vector
is preferred, but the production of IL-12 protein or IL-12 gene
delivery is problematic to define. In the present study, we aimed
to identify an optional route for the cell endogenous
IL-12-inducing pathway in HCC, especially in HBx-related HCC.
HBx plays an important role in the development of
HBV-related HCC (30). HBx has been
shown to induce various signaling pathways and cellular proteins
that could link HCC with HBV infection (31–33).
Studies have shown that HBx protein induces the expression of
oncogenic miR-21, but the molecular mechanism of the role of miR-21
in HBx-induced proliferation and apoptosis in HCC cells is still
unknown. In this study, we investigated the effect of HBx on the
expression of miR-21 and its role in inducing the proliferation and
apoptosis induced by targeting IL-12 in HCC cells.
The results of our study showed that IL-12 is a
direct target of miR-21 by binding to 3′UTR of IL-12 and DLR assay
validation (Fig. 1). Furthermore,
HepG2 cells with no HBx expression, and HepG2 2.2.15 cells with
high expression of HBx (Fig. 2C)
were used as an HCC in vitro cell model, and normal
hepatocyte L02 cells were used as a control. The result of RT-qPCR
showed that miR-21 was highly expressed in both HepG2 and HepG2
2.2.15 cells compared with the L02 cells (Fig. 2A), while IL-12 was weakly expressed
in the two HCC cell lines (P<0.05; Fig. 2B). In the L02 cells with low miR-21
and no HBx expression, the expression of IL-12 was downregulated
significantly after miR-21 was increased or HBx was overexpressed
(Fig. 3). In the HepG2 cells with
no HBx, overexpression of HBx resulted in significant miR-21
upregulation (Fig. 4A).
Upregulation of HBx resulted in a decrease in the mRNA and protein
levels of IL-12 (Fig. 4B and C). In
the HepG2 2.2.15 cells with high expression of HBx and high miR-21
level, HBx inhibited by siRNAs resulted in a significant decrease
in miR-21 (Fig. 6A). The mRNA and
protein levels of IL-12 were both increased (Fig. 6B and C). miR-21 inhibited by the
miR-21 inhibitor resulted in the increase in the protein level of
IL-12 (Fig. 7). These results
showed that IL-12 is regulated by HBx-induced miR-21.
Previous studies have shown that HBx inhibits
apoptosis and enhances cellular proliferation in hepatoma cells
(34,35), while the mechanism is unknown. Thus,
further validation by MTT assay and FCM analysis showed that the
proliferation ability of the HepG2 cells was increased when HBx was
overexpressed (Fig. 8A). The
proliferation ability of the HepG2 2.2.15 cells was decreased when
miR-21 was inhibited by the miR-21 inhibitor or HBx_siR1 (Fig. 8B). Treatment with miR-21 inhibitor
or HBx_siR1 resulted in a significant increase in HepG2 2.2.15 cell
apoptosis (Fig. 8C and D).
Our study confirmed that IL-12 is a direct target of
miR-21 and miR-21 can be upregulated by HBx protein in hepatic
cells. A high level of HBx also resulted in the inhibition of
IL-12. A high level of miR-21 resulted in a significant decrease in
IL-12 expression and an increase in proliferation. Inhibition of
miR-21 resulted in a significant increase in IL-12 expression and
an increase in apoptosis. The results suggest that the suppression
of apoptosis in HCC cells was at least partially carried out
through HBx-induced miR-21 by targeting IL-12.
Acknowledgments
The present study was supported by the Youth
Foundation of Nantong Health and Family Planning Commission
(WQ2015018 and WQ2014005).
References
|
1
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tan A, Yeh SH, Liu CJ, Cheung C and Chen
PJ: Viral hepatocarcinogenesis: From infection to cancer. Liver
Int. 28:175–188. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lupberger J and Hildt E: Hepatitis B
virus-induced oncogenesis. World J Gastroenterol. 13:74–81. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marra M, Sordelli IM, Lombardi A, Lamberti
M, Tarantino L, Giudice A, Stiuso P, Abbruzzese A, Sperlongano R,
Accardo M, et al: Molecular targets and oxidative stress biomarkers
in hepatocellular carcinoma: An overview. J Transl Med. 9:1712011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McClain SL, Clippinger AJ, Lizzano R and
Bouchard MJ: Hepatitis B virus replication is associated with an
HBx-dependent mitochondrion-regulated increase in cytosolic calcium
levels. J Virol. 81:12061–12065. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pan J, Lian Z, Wallett S and Feitelson MA:
The hepatitis B x antigen effector, URG7, blocks tumour necrosis
factor alpha-mediated apoptosis by activation of phosphoinositol
3-kinase and beta-catenin. J Gen Virol. 88:3275–3285. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang B and Bouchard MJ: The hepatitis B
virus X protein elevates cytosolic calcium signals by modulating
mitochondrial calcium uptake. J Virol. 86:313–327. 2012. View Article : Google Scholar :
|
|
8
|
Yamashita T, Budhu A, Forgues M and Wang
XW: Activation of hepatic stem cell marker EpCAM by
Wnt-beta-catenin signaling in hepatocellular carcinoma. Cancer Res.
67:10831–10839. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Soifer HS, Rossi JJ and Saetrom P:
MicroRNAs in disease and potential therapeutic applications. Mol
Ther. 15:2070–2079. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang W, Lu Z, Kong G, Gao Y, Wang T, Wang
Q, Cai N, Wang H, Liu F, Ye L, et al: Hepatitis B virus X protein
accelerates hepatocarcinogenesis with partner survivin through
modulating miR-520b and HBXIP. Mol Cancer. 13:1282014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bandopadhyay M, Banerjee A, Sarkar N,
Panigrahi R, Datta S, Pal A, Singh SP, Biswas A, Chakrabarti S and
Chakravarty R: Tumor suppressor microRNA miR-145 and onco microRNAs
miR-21 and miR-222 expressions are differentially modulated by
hepatitis B virus X protein in malignant hepatocytes. BMC Cancer.
14:7212014. View Article : Google Scholar
|
|
12
|
Li JF, Dai XP, Zhang W, Sun SH, Zeng Y,
Zhao GY, Kou ZH, Guo Y, Yu H, Du LY, et al: Upregulation of
microRNA-146a by hepatitis B virus X protein contributes to
hepatitis development by downregulating complement factor H. MBio.
6:e02459-e142015. View Article : Google Scholar
|
|
13
|
Damania P, Sen B, Dar SB, Kumar S, Kumari
A, Gupta E, Sarin SK and Venugopal SK: Hepatitis B virus induces
cell proliferation via HBx-induced microRNA-21 in hepatocellular
carcinoma by targeting programmed cell death protein4 (PDCD4) and
phosphatase and tensin homologue (PTEN). PLoS One. 9:e917452014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li CH, Xu F, Chow S, Feng L, Yin D, Ng TB
and Chen Y: Hepatitis B virus X protein promotes hepatocellular
carcinoma transformation through interleukin-6 activation of
microRNA-21 expression. Eur J Cancer. 50:2560–2569. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kobayashi M, Fitz L, Ryan M, Hewick RM,
Clark SC, Chan S, Loudon R, Sherman F, Perussia B and Trinchieri G:
Identification and purification of natural killer cell stimulatory
factor (NKSF), a cytokine with multiple biologic effects on human
lymphocytes. J Exp Med. 170:827–845. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stern AS, Podlaski FJ, Hulmes JD, Pan YC,
Quinn PM, Wolitzky AG, Familletti PC, Stremlo DL, Truitt T and
Chizzonite R: Purification to homogeneity and partial
characterization of cytotoxic lymphocyte maturation factor from
human B-lymphoblastoid cells. Proc Natl Acad Sci USA. 87:6808–6812.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li L, Jiang Y, Lao S, Yang B, Yu S, Zhang
Y and Wu C: Mycobacterium tuberculosis-specific
IL-21+IFN-γ+CD4+ T cells are
regulated by IL-12. PLoS One. 11:e01473562016. View Article : Google Scholar
|
|
18
|
Otani T, Nakamura S, Toki M, Motoda R,
Kurimoto M and Orita K: Identification of IFN-gamma-producing cells
in IL-12/IL-18-treated mice. Cell Immunol. 198:111–119. 1999.
View Article : Google Scholar
|
|
19
|
Zeh HJ III, Hurd S, Storkus WJ and Lotze
MT: Interleukin-12 promotes the proliferation and cytolytic
maturation of immune effectors: Implications for the immunotherapy
of cancer. J Immunother Emphasis Tumor Immunol. 14:155–161. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yuzhalin AE and Kutikhin AG:
Interleukin-12: Clinical usage and molecular markers of cancer
susceptibility. Growth Factors. 30:176–191. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lasek W, Zagożdżon R and Jakobisiak M:
Interleukin 12: Still a promising candidate for tumor
immunotherapy? Cancer Immunol Immunother. 63:419–435. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alatrash G, Hutson TE, Molto L, Richmond
A, Nemec C, Mekhail T, Elson P, Tannenbaum C, Olencki T, Finke J,
et al: Clinical and immunologic effects of subcutaneously
administered interleukin-12 and interferon alfa-2b: Phase I trial
of patients with metastatic renal cell carcinoma or malignant
melanoma. J Clin Oncol. 22:2891–2900. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gollob JA, Veenstra KG, Parker RA, Mier
JW, McDermott DF, Clancy D, Tutin L, Koon H and Atkins MB: Phase I
trial of concurrent twice-weekly recombinant human interleukin-12
plus low-dose IL-2 in patients with melanoma or renal cell
carcinoma. J Clin Oncol. 21:2564–2573. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee
DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, et al:
Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic
Acids Res. 33:e1792005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li J, Fu H, Xu C, Tie Y, Xing R, Zhu J,
Qin Y, Sun Z and Zheng X: miR-183 inhibits TGF-beta1-induced
apoptosis by downregulation of PDCD4 expression in human
hepatocellular carcinoma cells. BMC Cancer. 10:354–363. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
Motzer RJ and Bukowski RM: Targeted
therapy for metastatic renal cell carcinoma. J Clin Oncol.
24:5601–5608. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cody JJ, Scaturro P, Cantor AB, Yancey
Gillespie G, Parker JN and Markert JM: Preclinical evaluation of
oncolytic δγ(1)34.5 herpes simplex virus expressing interleukin-12
for therapy of breast cancer brain metastases. Int J Breast Cancer.
2012:6286972012. View Article : Google Scholar
|
|
29
|
Parker JN, Gillespie GY, Love CE, Randall
S, Whitley RJ and Markert JM: Engineered herpes simplex virus
expressing IL-12 in the treatment of experimental murine brain
tumors. Proc Natl Acad Sci USA. 97:2208–2213. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Benhenda S, Cougot D, Buendia MA and
Neuveut C: Hepatitis B virus X protein molecular functions and its
role in virus life cycle and pathogenesis. Adv Cancer Res.
103:75–109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kong GY, Zhang JP, Zhang S, Shan CL, Ye LH
and Zhang XD: Hepatitis B virus X protein promotes hepatoma cell
proliferation via upregulation of MEKK2. Acta Pharmacol Sin.
32:1173–1180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xia LM, Huang WJ, Wu JG, Yang YB, Zhang Q,
Zhou ZZ, Zhu HF, Lei P, Shen GX and Tian DA: HBx protein induces
expression of MIG and increases migration of leukocytes through
activation of NF-kappaB. Virology. 385:335–342. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang WC, Chen WS, Chen YJ, Wang LY, Hsu
SC, Chen CC and Hung MC: Hepatitis B virus X protein induces IKKα
nuclear translocation via Akt-dependent phosphorylation to promote
the motility of hepatocarcinoma cells. J Cell Physiol.
227:1446–1454. 2012. View Article : Google Scholar
|
|
34
|
Gaur AB, Holbeck SL, Colburn NH and Israel
MA: Downregulation of Pdcd4 by mir-21 facilitates glioblastoma
proliferation in vivo. Neuro-oncol. 13:580–590. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou X, Ren Y, Moore L, Mei M, You Y, Xu
P, Wang B, Wang G, Jia Z, Pu P, et al: Downregulation of miR-21
inhibits EGFR pathway and suppresses the growth of human
glioblastoma cells independent of PTEN status. Lab Invest.
90:144–155. 2010. View Article : Google Scholar : PubMed/NCBI
|