Introduction
Breast cancers are one of the major diseases that
endanger human life and health. Breast cancer ranks first among the
malignant tumours diagnosed in women and is a major disease that
threatens women's health. The incidence of breast cancer has shown
a continuing upward trend year after year (1–3). Early
diagnosis and effective treatment are critical for most breast
cancer patients to achieve prolonged survival and improved
prognosis. Therefore, clarification of the molecular mechanisms
underlying the development and progression of breast cancer and the
identification of more effective diagnostic markers and therapeutic
targets are required to improve the overall efficiency of breast
cancer treatments.
δ-opioid receptor (DOR) belongs to the G
protein-coupled receptor (GPCR) family (4,5). The
endogenous ligands of DOR are opioid peptides (6,7). DOR
is widely distributed in the body, including the heart (8), the gastrointestinal (GI) tract
(9–11), the immune system (12) and the reproductive system (13–15).
Studies have revealed that DOR is involved in the development and
progression of certain malignant tumours. Debruyne et al
found that DOR agonists affect the invasion of HCT-8/E11 colon
cancer cells in a concentration-dependent manner (16). Kuniyasu et al found that the
functional status of DOR plays an important regulatory role in the
metastasis of colorectal cancer to the liver (17). Schreiber et al found that DOR
is highly expressed in small cell lung cancer, and the sequence of
DOR mRNA expressed in lung cancer shares homology with the DOR
sequence in the brain (18). These
findings indicate that DOR exerts certain biological activities on
various types of tumours. The µ-opioid receptor gene
polymorphism observed in breast cancer is a highly significant risk
factor for the development and progression of breast cancer
(19). The present study found that
the activation of DOR promoted the proliferation of human breast
cancer cells.
Protein kinase C (PKC) is widely distributed
throughout the human body. PKC regulates the proliferation and
differentiation of a variety of cells (20–22);
furthermore, activated PKC promotes the proliferation of a variety
of tumour cells (23), including
breast cancer cells (24,25). Moreover, DOR-specific agonists
activate PKC, which induces the activation of extracellular
signal-regulated kinases (ERKs) (26). ERKs belong to the mitogen-activated
protein kinase (MAPK) family and include two subtypes, the ERK1 and
2. ERKs participate in various biological processes, including cell
growth, cell proliferation and apoptosis (27). Studies have found that DOR promotes
cell proliferation through ERK pathways. This phenomenon has been
confirmed in PC12 cells, SH-SY5Y cells and cardiomyocytes (28–30).
Therefore, we hypothesized that DOR regulates the progression of
breast cancer via the PKC/ERK signalling pathway.
The goal of the present study was to verify the
effect of DOR on the development and progression of breast cancer
through the examination of DOR expression in breast cancer tissues
and cells. Our results showed that DOR was widely expressed in
breast cancer tissues and cells, exogenous activation of DOR
promoted the proliferation of breast cancer cells in a
concentration-dependent manner and the PKC/ERK signalling pathway
played an important role in these processes.
Materials and methods
Collection of breast cancer tissue
samples
This study was approved by the Ethics Committee of
the Affiliated Hospital of Guilin Medical College. Tissue samples
and clinical data were collected after the patients or their
families signed the informed consent documents. All 62 breast
cancer and paracancerous tissue samples (tissues located >2 cm
away from the tumour resection margins) examined in the present
study were derived from the surgical resection specimens preserved
during the 2007–2009 period at the Department of Breast Surgery,
Affiliated Hospital of Guilin Medical College. The tissue samples
were subjected to pathological examination and associated with
complete clinical information. All surgical resection specimens
were separately packed and stored in liquid nitrogen within 30 min
of resection. None of the patients received palliative surgery,
radiotherapy, chemotherapy or other related treatments prior to the
surgical resection.
The patients were comparable in terms of age,
menopausal status, family history of cancer, ethnicity,
pathological type and grade, tumour size, axillary lymph node
metastasis and clinical stage. The differences in these parameters
were not statistically significant. The histological grade of the
breast tumours were assessed according to the World Health
Organization (WHO) standards and were classified into grades I–III.
Tumour stage was determined based on the seventh edition of the
tumour-node-metastasis (TNM) classification of malignant tumours
published by the American Joint Committee on Cancer (AJCC) and the
Union for International Cancer Control (UICC). The 62 patients were
followed up for five consecutive years and were required to undergo
a computed tomography (CT) scan and ultrasound once every 6
months.
Cell culture
MCF-10F human breast epithelial cells and MCF-7,
MDA-MB-231 and SKBR-3 human breast cancer cells were purchased from
the Cell Bank of Shanghai Institute of Cell Biology, Chinese
Academy of Sciences. The human breast epithelial cells and breast
cancer cells were cultured in Dulbecco's modified Eagle's medium
(DMEM) and Roswell Park Memorial Institute (RPMI)-1640 medium,
respectively. All media were supplemented with 10% fetal bovine
serum (FBS), 100 U/ml of penicillin and 100 U/ml of streptomycin.
The cells were cultured at 37°C in a humidified 5% CO2
incubator.
Cell transfection
Breast cancer cells were maintained at a logarithmic
growth phase until 75% confluency. The cells were then transduced
with 5 µl of virus (titre, 1.0×109 TU/ml). In
addition, polybrene was added to each well of cells. After 12 h of
viral infection, the virus-containing medium was replaced with
fresh conventional culture medium, and the cultured cells remained
at 37°C in a 5% CO2 incubator. At 4 days after viral
infection, fluorescence within the cells was observed and
photographed. In addition, the mRNA or total protein was extracted
based on the goals of each experiment.
Reverse transcription polymerase chain
reaction (RT-PCR)
The total RNA was extracted from breast cancer
tissues and cells using the TRIzol method. After quantification,
total RNA was reverse transcribed into total complementary DNA
(cDNA) using the Takara RT kit in accordance with the
manufacturer's instructions. The PCR was performed using the cDNA
as a template and the appropriate proportion of pre-synthesized PCR
primers. The sequences of the DOR primers were
5′-ACCAAGATCTGCGTGTTCCT-3′ for the upstream primer and
5′-CGATGACGAAGATGTGGATG-3′ for the downstream primer. The sequences
of the β-actin primers were 5′-CTGGGACGACATGGAGAAAA-3′ for the
upstream primer and 5′-AAGGAAGGCTGGAAGAGTGC-3′ for the downstream
primer. The volume of the PCR reaction was 50 µl. The PCR
reaction conditions consisted of 31 cycles of 94°C for 2 min,
denaturation at 94°C for 30 sec, annealing at 55°C for 30 sec and
elongation at 72°C for 30 sec. The obtained PCR products were
subjected to agarose gel electrophoresis (1.5% gel). The gel was
scanned and analysed using a gel imaging system.
Western blot analysis
The total protein was extracted from tissues and
cells and quantified using the bicinchoninic acid (BCA) assay. The
protein samples were subjected to sodium dodecyl
sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and then
transferred to nitrocellulose membranes. The membrane was blocked
at 4°C overnight in a Tris-buffered saline/Tween-20 (TBST) blocking
solution containing 5% non-fat dry milk. After blocking, the
membrane was incubated with the primary antibody at 37°C for 1 h.
The membrane was washed three times for 10 min each wash with TBST
and then incubated with the secondary antibody at 37°C for 1 h. The
membrane was washed three more times for 10 min each wash with
TBST. Subsequently, the proteins were visualized using enhanced
chemiluminescence (ECL) reagents. The images were scanned and
analysed.
Cell viability assay
Logarithmically growing cells were selected. Each
group of cells was treated for 48 h. Subsequently, the cells were
incubated in the presence of 20 µl of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
(5 mg/ml) for 4 h at 37°C in a 5% CO2 incubator. The
spent solution was discarded and 150 µl of dimethyl
sulphoxide was added to each well of cells. The cells were then
agitated at low speed for 10 min on a shaker at room temperature.
The optical density (OD490) value was determined using a
microplate reader.
Cell cycle analysis
The cells were collected and fixed with pre-cooled
70% ethanol at 4°C overnight. The cell density was adjusted to
1×106 cells/ml. Subsequently, 50 mg/l of RNase, 100 mg/l
of propidium iodide (PI) and 1 ml/l of Triton X-100 were added to
the cells. The cells were incubated in the dark for 15 min, and the
labelled cells were examined by flow cytometry.
Examination of apoptosis
The cells were digested with trypsin and then
resuspended at a density of 1×106 cells/ml. After the
addition of 5 µl of Annexin V-fluorescein isothiocyanate
(FITC) and PI, the cells were incubated at 37°C for 15 min in the
dark. Subsequently, the cells were analysed by flow cytometry.
Generation of tumour-bearing nude
mice
The animal experiments were conducted after
receiving approval from the Medical Ethics Committee of Guilin
Medical College. All the mice used in the present study were
purchased from the Experimental Animal Centre of Guilin Medical
College. The mice were male, aged 6–8 weeks, and weighed 20±0.5 g.
The mice were randomly divided into two groups, and each group
contained five mice. Xenograft tumours were established using the
conventional method of subcutaneous inoculation. Four weeks later,
the mice were euthanized by cervical dislocation. The xenograft
tumours were collected for subsequent experiments.
Statistical analysis
All experimental data were subjected to statistical
analysis using SPSS 16.0 statistical software. The quantitative
data were expressed as the means ± standard deviation. Comparisons
between two groups were conducted using t-tests. The relationship
between DOR and clinical pathology data was assessed using the
Chi-square test. The differences in survival rate were assessed
using the Kaplan-Meier survival analysis. In addition, survival
rates were compared between the groups using the log-rank test.
P<0.05 indicated a statistically significant difference between
two groups.
Results
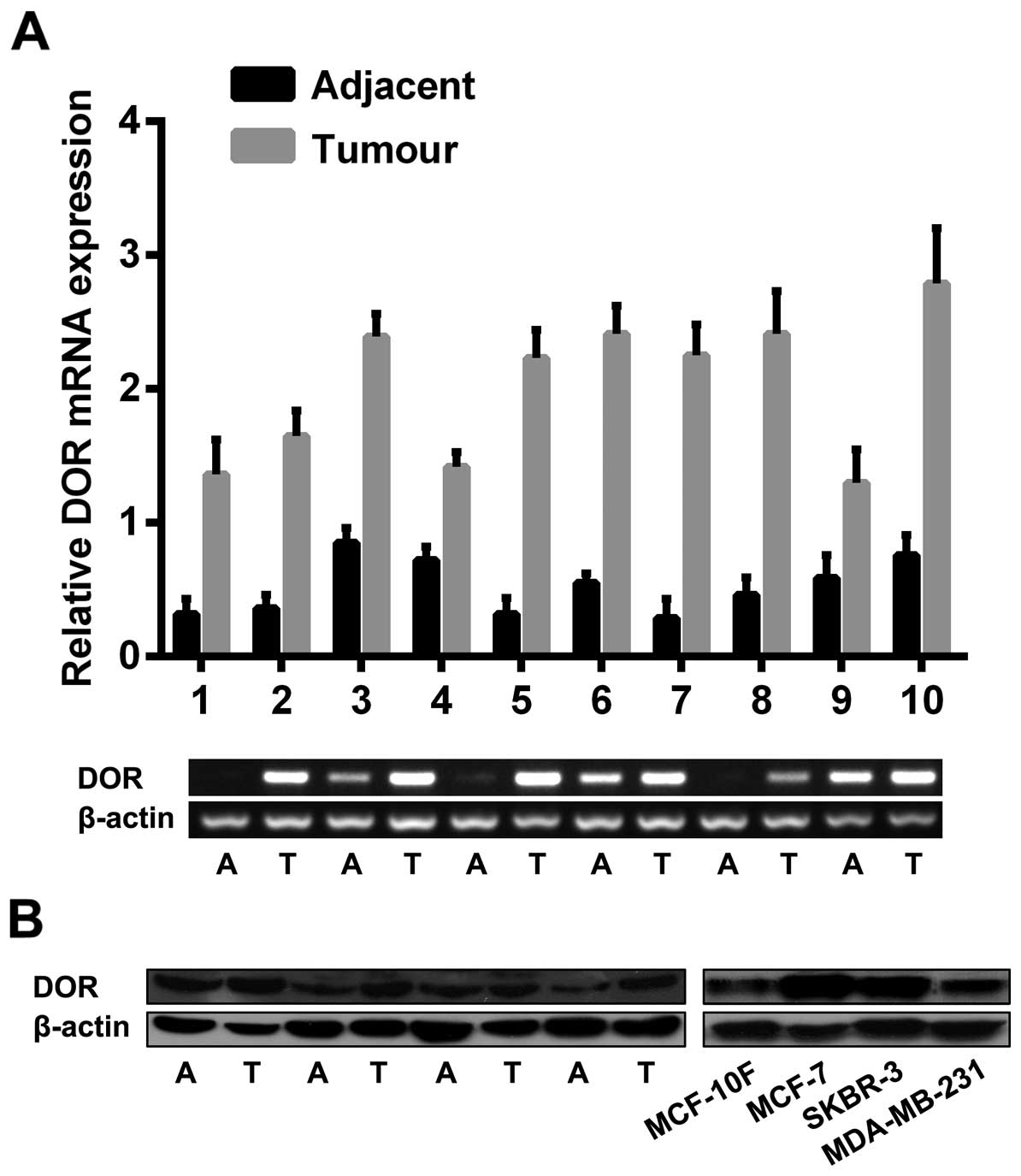
DOR is highly expressed in human breast
cancer tissues and cells
RT-PCR and western blot analysis were completed to
analyse DOR expression in breast cancer tissues and cells. The
examination of the 62 breast cancer and paracancerous tissues
revealed that DOR mRNA was highly expressed in cancer tissues. In
contrast, the corresponding paracancerous tissues either expressed
a low level of DOR mRNA or showed no DOR mRNA expression
(p<0.05). Among the examined cell lines, MCF-7 and SKBR-3 cells
highly expressed DOR, and MDA-MB-231 and MCF-10F cells showed low
or no DOR expression (p<0.05) (Fig.
1A). To further verify the RT-PCR results, a western blot
analysis was performed. The results showed that the expression
level of DOR protein was significantly higher in breast cancer
tissues than in the corresponding paracancerous tissues
(p<0.05). In addition, the expression level of DOR protein was
higher in various types of breast cancer cells than in normal
breast epithelial cells (p<0.05) (Fig. 1B).
The relationship between DOR expression
and the clinical characteristics of breast cancer
The relationship between DOR expression in breast
cancer and clinical pathology data was examined. Among the 62
breast cancer patients, 54 patients (83.1%) showed high DOR
expression in cancer tissues, whereas only 19 patients (30.6%)
showed DOR expression in paracancerous tissues. This difference was
statistically significant (p<0.05). DOR expression was
positively correlated with tumour metastasis (p<0.05) and
clinical stage (p<0.05, Table
I). In contrast, no correlation existed between DOR expression
and other clinical parameters, including age, tumour size, tumour
location and the presence/absence of tumour recurrence.
 | Table IClinical characteristics. |
Table I
Clinical characteristics.
| No. | χ2 | P-value |
|---|
| Age (years) | | 0.087 | 0.768 |
| ≤45 | 24 | | |
| >45 | 30 | | |
| Tumour size
(cm) | | 0.245 | 0.620 |
| ≤2 | 32 | | |
| >2 | 22 | | |
| Tumour
location | | 0.006 | 0.940 |
| Left breast | 21 | | |
| Right breast | 33 | | |
| Axillary lymph
node | | 23.852 | 0.000 |
| 0 | 6 | | |
| 1–3 | 35 | | |
| >4 | 13 | | |
| Clinical stage | | 4.059 | 0.044 |
| I–II | 35 | | |
| III | 19 | | |
| Relapse | | 0.221 | 0.638 |
| Yes | 18 | | |
| No | 36 | | |
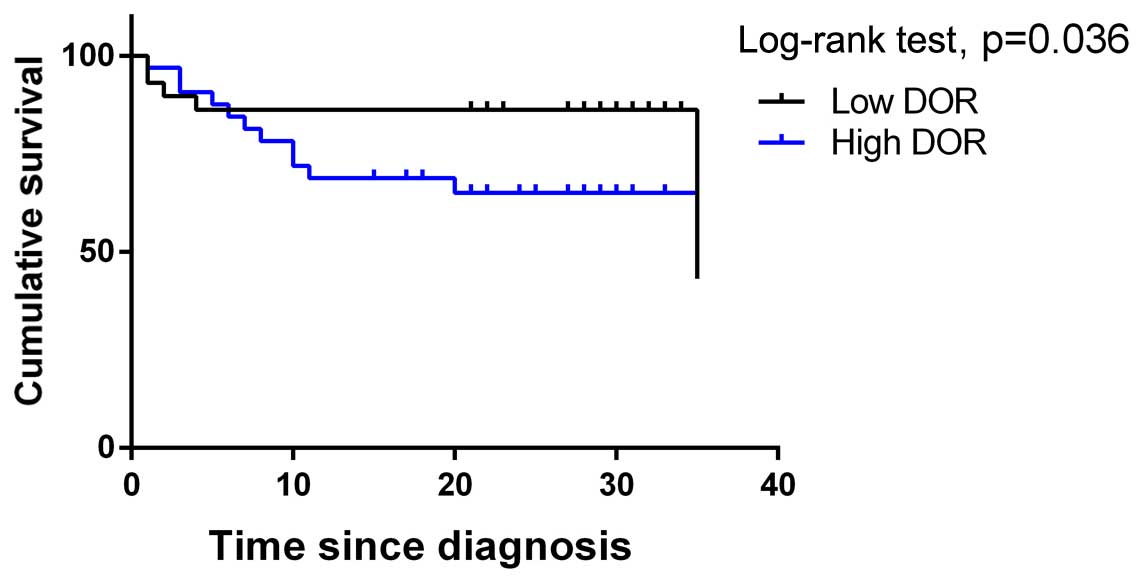
The relationship between DOR expression
and survival
All of the patients were subjected to regular
follow-ups. The Kaplan-Meier survival curves were analysed, and the
results showed that the overall survival rate was significantly
lower in patients from the DOR-positive group when compared with
the DOR-negative group (Fig. 2).
The survival rates were compared between the groups using a
log-rank test, and the results revealed that high DOR expression
was related to a poor prognosis in breast cancer patients
(p<0.05). The results indicate that high DOR expression exerted
an adverse effect on the prognosis of patients with breast
cancer.
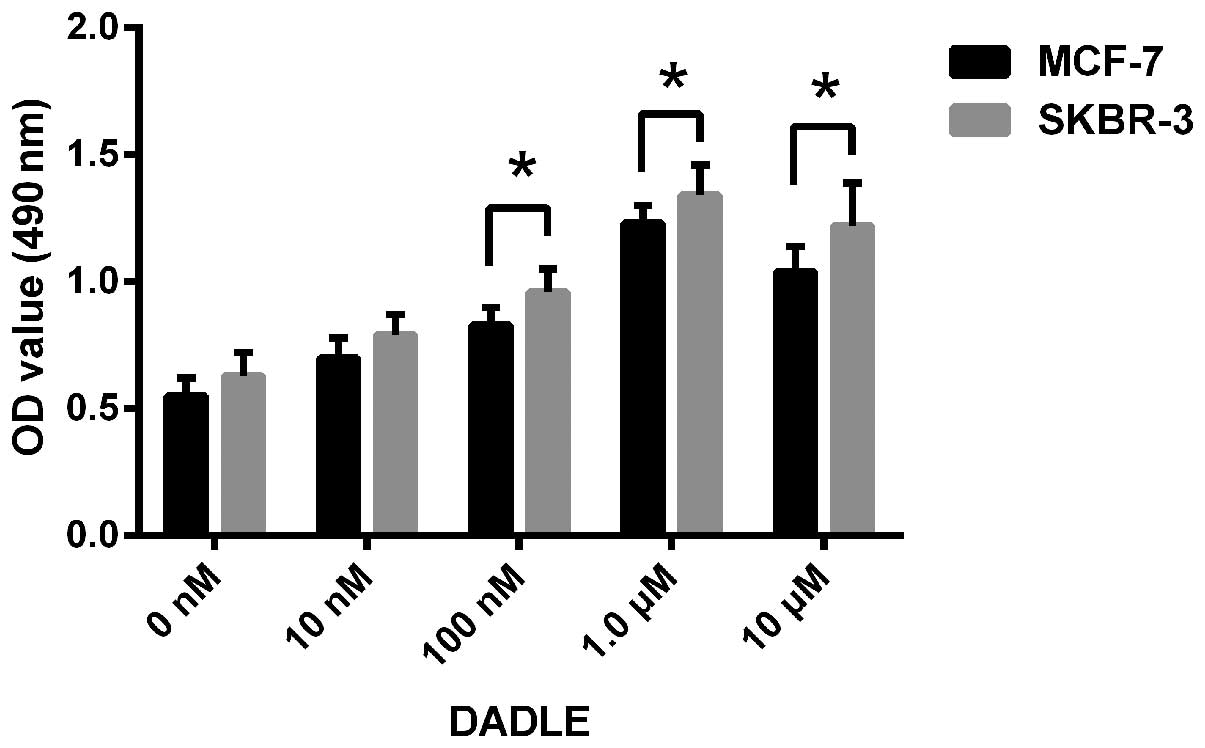
Activation of DOR promotes the
proliferation of human breast cancer cells
Based on the expression status of DOR in breast
cancer cells, we selected two cell lines, MCF-7 and SKBR-3, to
investigate the effect of DOR on the proliferation of breast cancer
cells. [D-Ala2, D-Leu5]enkephalin (DADLE) is
a specific DOR agonist. The proliferation rates of the breast
cancer cells after DADLE treatment were determined using an MTT
assay. The OD490 value of the breast cancer cells showed
a gradual dose-dependent increase at DADLE concentrations between
10 nM and 1.0 µM. In contrast, the OD490 value of
the control group did not significantly change (p<0.05).
However, when the concentration of DADLE exceeded 1.0 µM,
the OD490 value of the breast cancer cells did not show
a further increase (Fig. 3). The
results demonstrate that activation of DOR promoted the
proliferation of human breast cancer cells. In addition, this DOR
effect was concentration-dependent.
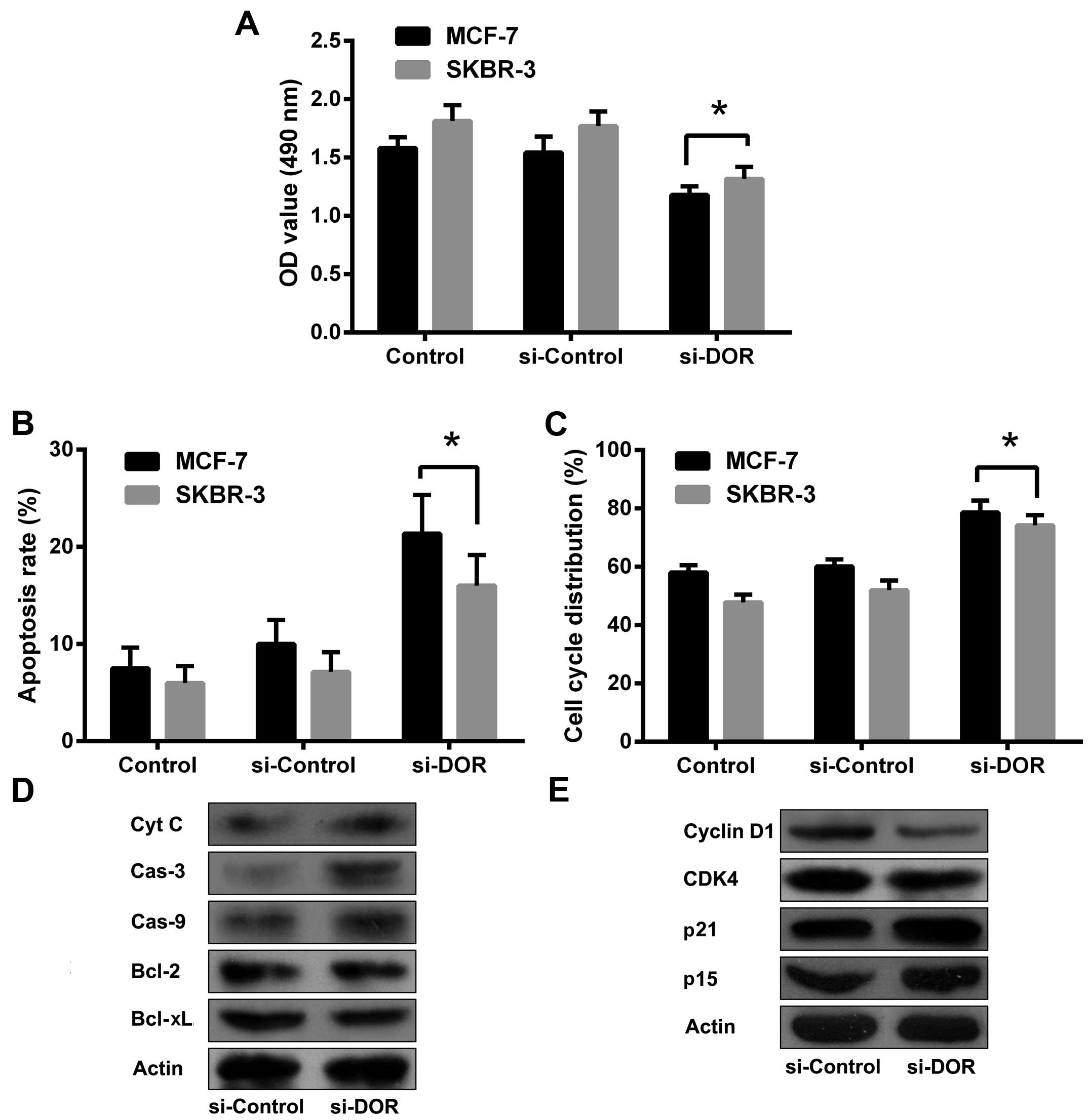
DOR inhibits breast cancer progression in
vitro
The RNA interference (RNAi) technique was employed
to silence DOR expression in the breast cancer cell line MCF-7. A
48-h siDOR transfection led to the successful silencing of DOR
expression. The OD490 value was significantly lower in
the siDOR-transfected group when compared with the control group
and the group transfected with a negative control oligonucleotide
(p<0.05) (Fig. 4A). The results
indicate that DOR is capable of inhibiting breast cancer cell
proliferation. To further clarify the mechanism used to affect the
proliferation of human breast cancer cells, flow cytometry was
performed to examine apoptosis and the cell cycle. The apoptotic
rate was significantly increased in breast cancer cells after
RNAi-mediated silencing of DOR when compared with the control group
and the negative control oligonucleotide-transfected group
(p<0.05). The expression levels of caspase-3, caspase-9 and
cytochrome C protein were also elevated in breast cancer cells
after DOR silencing when compared with the control group and the
negative control oligonucleotide-transfected group. The expression
levels of B-cell lymphoma 2 (Bcl-2) and B-cell lymphoma-extra large
(Bcl-xL) were reduced in breast cancer cells after DOR silencing
compared to those in the control group and the negative control
oligonucleotide-transfected group. The majority of the tumour cells
were arrested in the G1 phase after silencing of DOR (p<0.05).
In addition, the levels of cyclin-dependent kinase 4 (CDK4) and
cyclin D1 were decreased and the levels of p15 and p21 were
increased in these cells (Fig.
4B–E). These results indicate that the inhibitory effect of DOR
on breast cancer progression is closely related to the promotion of
apoptosis and the blockage of cell cycle progression.
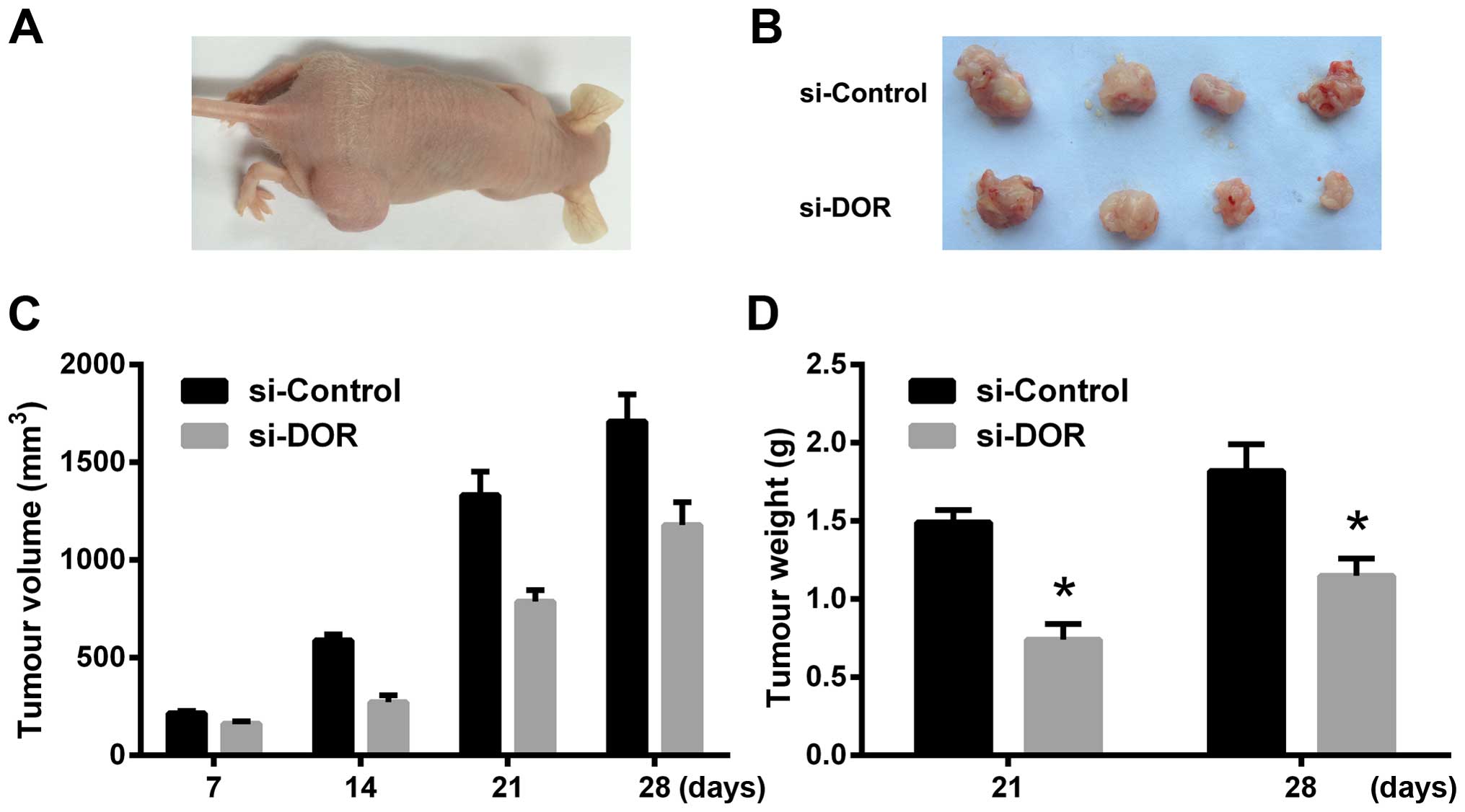
The effect of DOR on xenograft tumours in
nude mice
Four weeks after the subcutaneous inoculation of
tumour cells, the rate of tumour formation reached 100% in the
si-Control group. The rate of tumour formation was 80% in the
si-DOR group. The xenograft tumour grew more rapidly in the
si-Control group than in the si-DOR group. In addition, the volume
and weight of the xenograft tumours were significantly reduced in
the si-DOR group compared to those in the si-Control group at all
time points examined (p<0.05) (Fig.
5A–D). The results indicate that DOR inhibits tumourigenesis in
nude mice.
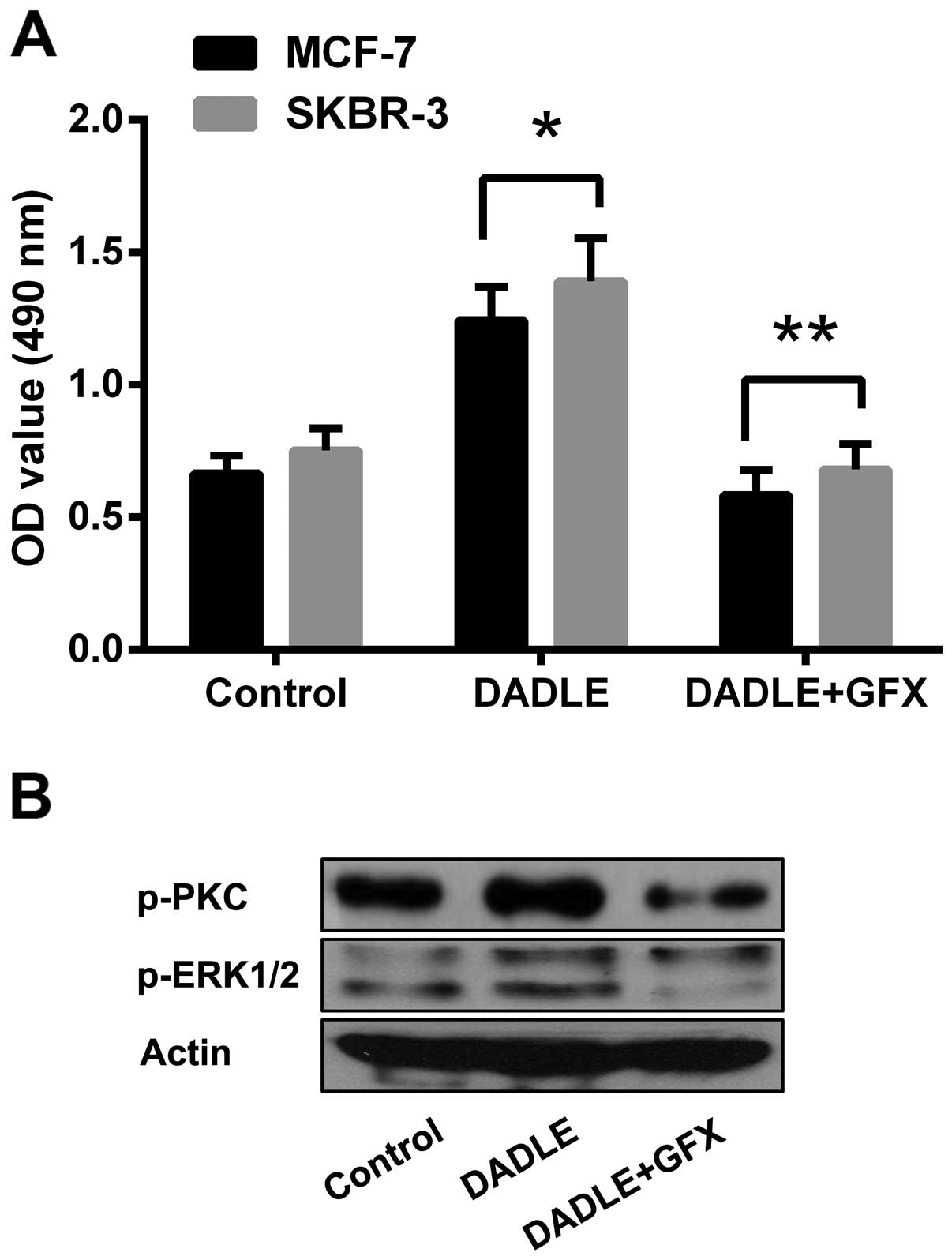
Role of the PKC/ERK pathway in
DOR-mediated effects on breast cancer progression
The phosphorylation levels of PKC and ERK proteins
were examined to verify the specific role of the PKC/ERK signalling
pathway in the DOR-mediated effects on human breast cancer
progression. The results showed that activation of DOR led to
significantly increased phosphorylation of the PKC protein in the
cytoplasm (p<0.05). In addition, the intracellular
phosphorylation level of the ERK proteins increased accordingly
(p<0.05). The results demonstrate that DOR induced the
phosphorylation of PKC and ERK proteins. However, treatment of the
tumour cells with PKC-specific antagonist GF109203X (10 µM)
significantly inhibited the proliferation of tumour cells,
regardless of DOR activation. Moreover, blocking the PKC pathway
resulted in a decline in ERK phosphorylation levels (Fig. 6). The results demonstrate that the
PKC pathway plays a critical role in the DOR-mediated effects on
the progression of human breast cancer.
Discussion
Breast cancer is a common malignancy in women.
Breast cancer originates in the breast ductal epithelium and mainly
occurs in women between 40–60 years of age. Breast cancer ranks
first among malignant tumours in women and is a major disease that
threatens women's health. The incidence of breast cancer continues
to rise year after year (31,32).
An earlier detection of breast cancer correlates with an improved
therapeutic efficacy. However, the pathogenesis of breast cancer is
not entirely clear. Therefore, understanding the mechanisms
underlying the development and progression of breast cancer is
vital for breast cancer research and treatment.
Opioid receptors are the receptors for endogenous
opioids and opioid analgesics (33). Opioid receptors belong to the
superfamily of seven-transmembrane GPCRs, including µ, κ and
δ receptors. The binding of opioid receptors to the natural ligands
present in the organisms, such as endorphins, dynorphin, enkephalin
and nociceptin, activates inhibitory G proteins in the cytoplasmic
face of the plasma membrane and triggers downstream molecular
mechanisms through various signal transduction pathways (34). DOR is widely distributed in the
heart (8), GI tract (9–11),
immune system (12), reproductive
system (13–15) and other tissues (35). Among all of the opioid receptor
family members, DOR is believed to be the most closely related to
cell survival and cell proliferation (35,36)
and is involved in the development and progression of certain
malignant tumours. Studies that assessed the role of DOR in breast
cancer are rare. In the present study, we found that DOR was highly
expressed in human breast cancer tissues and cells. Subsequently,
DOR expression was analysed in combination with the clinical
pathological data of breast cancer patients. Positive DOR
expression was closely related to lymph node metastasis, distant
metastasis and the clinical stage of tumours. The results indicate
that DOR is closely associated with the development, progression
and clinical treatment of breast cancer.
Tumourigenesis is a multistep, prolonged interactive
process that involves a variety of factors. Tumourigenesis is
closely related to abnormal cell proliferation and apoptosis. A
recent study showed that among the members of the opioid receptor
superfamily, DOR is closely related to cell survival and
proliferation (36). Zhao et
al found that DOR activation promotes the proliferation of
ventricular myocytes in neonatal rats (28). Avella et al found that
endogenous opioid peptides promote liver cancer cell proliferation
through DOR activation in the membrane of liver cells (37). The present study found that changes
in DOR activity had a major impact on breast cancer cell
proliferation and apoptosis. Activation of DOR promoted the
proliferation of human breast cancer cells in vitro. The
results of the present study are consistent with the aforementioned
published research findings. However, a study conducted by Kuniyasu
et al showed that methionine enkephalin inhibits the
proliferation and invasion of colorectal cancer cells (17). This finding is contradictory to the
results of the present study. The contradiction is likely due to
the differences in tumour characteristics.
Delayed cell cycle progression is another important
factor that affects the proliferation of tumour cells (38). The present study found that
silencing DOR expression induced G1-phase arrest in breast cancer
cells, thereby inhibiting tumour cell proliferation. Targeted
silencing of DOR also induced apoptosis in breast cancer cells. In
addition, the silencing of DOR expression effectively inhibited the
progression of xenograft tumours in tumour-bearing nude mice. These
results demonstrate that DOR is closely related to breast cancer
progression.
It is well-known that PKC is widely distributed in
living organisms. PKC regulates the proliferation and
differentiation of various types of cells. Studies have shown that
activated PKC inhibits apoptosis in various cell types (39–41).
Oskoueian et al (24) and Li
et al (25) found that PKC
participates in breast cancer cell proliferation and
differentiation. Recently, the consensus PKC phosphorylation
sequence was discovered in DOR. The treatment of NG108-15 cells
with DOR agonists activates PKC in a dose-dependent manner, whereas
DOR-mediated PKC-activation is blocked by DOR antagonists (42). Therefore, DOR-mediated intracellular
signalling pathways are closely related to the PKC pathway. The
present study found that DOR activation in breast cancer cells led
to the phosphorylation of the PKC protein. In addition, the
phosphorylation level of ERK proteins was also increased. ERK
belongs to the MAPK family and is related to cell proliferation,
transformation and differentiation. The ERK pathway plays an
important role in tumour development, progression and metastasis;
thus, members of the pathway have become the molecular targets for
novel cancer drug development (43). In recent years, studies have shown
that DOR activation induces cell proliferation through the ERK
signal transduction pathway (28–30).
Zhu et al found that a DOR-specific agonist activated PKC
and subsequently induced ERK activation (26). The results of the present study are
consistent with the aforementioned previously reported results.
To prove that the PKC/ERK pathway is related to the
effect of DOR on breast cancer progression, we artificially
suppressed the PKC pathway. Inhibition of the PKC pathway resulted
in the suppression of breast cancer cell proliferation, regardless
of DOR activation. In addition, the phosphorylation level of ERK
was decreased. These results indicate that the PKC and ERK pathways
are involved in the apoptosis of MCF-7 cells induced by DOR
downregulation; furthermore, DOR activation may promote the
development and progression of breast cancer through the PKC/ERK
pathway. A recent study found that DOR is related to tumour
chemosensitivity (44). DOR is also
involved in cell cycle progression. Further investigation is
required to determine whether DOR affects chemotherapy resistance
in breast cancer.
In summary, the present study demonstrated that DOR
is highly expressed in breast cancer tissues and cells and is
closely related to the progression of human breast cancer.
Moreover, the present study suggests that the effects of DOR occur
via the activation of the PKC/ERK signal transduction pathways.
Future clarification of the mechanisms of DOR function is vital in
order to understand the role of DOR in breast cancer progression
and the development of treatment strategies focused on this
potential therapeutic target.
Acknowledgments
This study was funded by the Health Department of
the Guangxi Zhuang Autonomous Region (project nο. Z2013488).
References
|
1
|
McPherson K, Steel CM and Dixon JM: ABC of
breast diseases. Breast cancer-epidemiology, risk factors, and
genetics. BMJ. 321:624–628. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Benson JR and Jatoi I: The global breast
cancer burden. Future Oncol. 8:697–702. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anderson WF, Katki HA and Rosenberg PS:
Incidence of breast cancer in the United States: Current and future
trends. J Natl Cancer Inst. 103:1397–1402. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kieffer BL, Befort K, Gaveriaux-Ruff C and
Hirth CG: The delta-opioid receptor: Isolation of a cDNA by
expression cloning and pharmacological characterization. Proc Natl
Acad Sci USA. 89:12048–12052. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Evans CJ, Keith DE Jr, Morrison H,
Magendzo K and Edwards RH: Cloning of a delta opioid receptor by
functional expression. Science. 258:1952–1955. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bzdega T, Chin H, Kim H, Jung HH, Kozak CA
and Klee WA: Regional expression and chromosomal localization of
the delta opiate receptor gene. Proc Natl Acad Sci USA.
90:9305–9309. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Simonin F, Befort K, Gavériaux-Ruff C,
Matthes H, Nappey V, Lannes B, Micheletti G and Kieffer B: The
human delta-opioid receptor: Genomic organization, cDNA cloning,
functional expression, and distribution in human brain. Mol
Pharmacol. 46:1015–1021. 1994.PubMed/NCBI
|
|
8
|
Howells RD, Kilpatrick DL, Bailey LC, Noe
M and Udenfriend S: Proenkephalin mRNA in rat heart. Proc Natl Acad
Sci USA. 83:1960–1963. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fickel J, Bagnol D, Watson SJ and Akil H:
Opioid receptor expression in the rat gastrointestinal tract: A
quantitative study with comparison to the brain. Brain Res Mol
Brain Res. 46:1–8. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Neidle A, Manigault I and Wajda IJ:
Distribution of opiate-like substances in rat tissues. Neurochem
Res. 4:399–410. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wittert G, Hope P and Pyle D: Tissue
distribution of opioid receptor gene expression in the rat. Biochem
Biophys Res Commun. 218:877–881. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Radulović J and Janković BD: Opposing
activities of brain opioid receptors in the regulation of humoral
and cell-mediated immune responses in the rat. Brain Res.
661:189–195. 1994. View Article : Google Scholar
|
|
13
|
Kilpatrick DL, Howells RD, Noe M, Bailey
LC and Udenfriend S: Expression of preproenkephalin-like mRNA and
its peptide products in mammalian testis and ovary. Proc Natl Acad
Sci USA. 82:7467–7469. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen CL, Chang CC, Krieger DT and Bardin
CW: Expression and regulation of proopiomelanocortin-like gene in
the ovary and placenta: Comparison with the testis. Endocrinology.
118:2382–2389. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Civelli O, Douglass J, Goldstein A and
Herbert E: Sequence and expression of the rat prodynorphin gene.
Proc Natl Acad Sci USA. 82:4291–4295. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Debruyne D, Leroy A, DE Wever O, Vakaet L,
Mareel M and Bracke M: Direct effects of delta opioid receptor
agonists on invasion-associated activities of HCT-8/E11 colon
cancer cells. Anticancer Res. 30:9–17. 2010.PubMed/NCBI
|
|
17
|
Kuniyasu H, Luo Y, Fujii K, Sasahira T,
Moriwaka Y, Tatsumoto N, Sasaki T, Yamashita Y and Ohmori H: CD10
enhances metastasis of colorectal cancer by abrogating the
anti-tumoural effect of methionine-enkephalin in the liver. Gut.
59:348–356. 2010. View Article : Google Scholar
|
|
18
|
Schreiber G, Campa MJ, Prabhakar S,
O'Briant K, Bepler G and Patz EF Jr: Molecular characterization of
the human delta opioid receptor in lung cancer. Anticancer Res.
18:1787–1792. 1998.PubMed/NCBI
|
|
19
|
Cieślińska A, Sienkiewicz-Szłapka E,
Kostyra E, Fiedorowicz E, Snarska J, Wroński K, Tenderenda M,
Jarmołowska B and Matysiewicz M: µ-Opioid receptor gene (OPRM1)
polymorphism in patients with breast cancer. Tumour Biol.
36:4655–4660. 2015. View Article : Google Scholar
|
|
20
|
Molè D, Gentilin E, Gagliano T, Tagliati
F, Bondanelli M, Pelizzo MR, Rossi M, Filieri C, Pansini G, degli
Uberti EC, et al: Protein kinase C: A putative new target for the
control of human medullary thyroid carcinoma cell proliferation in
vitro. Endocrinology. 153:2088–2098. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wickley PJ, Ding X, Murray PA and Damron
DS: Propofol-induced activation of protein kinase C isoforms in
adult rat ventricular myocytes. Anesthesiology. 104:970–977. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saberi B, Shinohara M, Ybanez MD, Hanawa
N, Gaarde WA, Kaplowitz N and Han D: Regulation of H(2)O(2)-induced
necrosis by PKC and AMP-activated kinase signaling in primary
cultured hepatocytes. Am J Physiol Cell Physiol. 295:C50–C63. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ali AS, Ali S, El-Rayes BF, Philip PA and
Sarkar FH: Exploitation of protein kinase C: A useful target for
cancer therapy. Cancer Treat Rev. 35:1–8. 2009. View Article : Google Scholar
|
|
24
|
Oskoueian E, Abdullah N and Ahmad S:
Phorbol esters from Jatropha meal triggered apoptosis, activated
PKC-δ, caspase-3 proteins and down-regulated the proto-oncogenes in
MCF-7 and HeLa cancer cell lines. Molecules. 17:10816–10830. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Z, Wang N, Fang J, Huang J, Tian F, Li
C and Xie F: Role of PKC-ERK signaling in tamoxifen-induced
apoptosis and tamoxifen resistance in human breast cancer cells.
Oncol Rep. 27:1879–1886. 2012.PubMed/NCBI
|
|
26
|
Zhu M, Li M, Yang F, Ou X, Ren Q, Gao H,
Zhu C and Guo J: Mitochondrial ERK plays a key role in δ-opioid
receptor neuroprotection against acute mitochondrial dysfunction.
Neurochem Int. 59:739–748. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sancho P, Galeano E, Estañ MC, Gañán-Gómez
I, Boyano-Adánez MC and García-Pérez AI: Raf/MEK/ERK signaling
inhibition enhances the ability of dequalinium to induce apoptosis
in the human leukemic cell line K562. Exp Biol Med (Maywood).
237:933–942. 2012. View Article : Google Scholar
|
|
28
|
Zhao M, Wang HX, Yang J, Su YH, Su RJ and
Wong TM: delta-Opioid receptor stimulation enhances the growth of
neonatal rat ventricular myocytes via the extracellular
signal-regulated kinase pathway. Clin Exp Pharmacol Physiol.
35:97–102. 2008. View Article : Google Scholar
|
|
29
|
Hayashi T, Tsao LI and Su TP:
Antiapoptotic and cytotoxic properties of delta opioid peptide
[D-Ala(2),D-Leu(5)] enkephalin in PC12 cells. Synapse. 43:86–94.
2002. View Article : Google Scholar
|
|
30
|
Bilecki W, Zapart G, Ligeza A,
Wawrzczak-Bargiela A, Urbański MJ and Przewłocki R: Regulation of
the extracellular signal-regulated kinases following acute and
chronic opioid treatment. Cell Mol Life Sci. 62:2369–2375. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
American Cancer Society: Cancer facts and
figures. 2006, http://www.cancer.org/downloads/STT/CAFF2006PWSecured.pdf
Accessed February 7, 2007.
|
|
32
|
Kuhl CK, Schrading S, Leutner CC,
Morakkabati-Spitz N, Wardelmann E, Fimmers R, Kuhn W and Schild HH:
Mammography, breast ultrasound, and magnetic resonance imaging for
surveillance of women at high familial risk for breast cancer. J
Clin Oncol. 23:8469–8476. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brownstein MJ: A brief history of opiates,
opioid peptides, and opioid receptors. Proc Natl Acad Sci USA.
90:5391–5393. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Al-Hasani R and Bruchas MR: Molecular
mechanisms of opioid receptor-dependent signaling and behavior.
Anesthesiology. 115:1363–1381. 2011.PubMed/NCBI
|
|
35
|
Su TP: Delta opioid peptide[D-
Ala(2),D-Leu(5)]enkephalin promotes cell survival. J Biomed Sci.
7:195–199. 2000.PubMed/NCBI
|
|
36
|
Kim H, Lee SW, Park JS, Min JH and Kim HK:
Genomic analysis of [d-Ala2, d-Leu5]
enkephalin preconditioning in cortical neuron and glial cell injury
after oxygen deprivation. Brain Res. 1447:91–105. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Avella DM, Kimchi ET, Donahue RN, Tagaram
HR, McLaughlin PJ, Zagon IS and Staveley-O'Carroll KF: The opioid
growth factor-opioid growth factor receptor axis regulates cell
proliferation of human hepatocellular cancer. Am J Physiol Regul
Integr Comp Physiol. 298:R459–R466. 2010. View Article : Google Scholar :
|
|
38
|
Stewart ZA, Westfall MD and Pietenpol JA:
Cell-cycle dysregulation and anticancer therapy. Trends Pharmacol
Sci. 24:139–145. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Allen TR, Krueger KD, Hunter WJ III and
Agrawal DK: Evidence that insulin-like growth factor-1 requires
protein kinase C-epsilon, PI3-kinase and mitogen-activated protein
kinase pathways to protect human vascular smooth muscle cells from
apoptosis. Immunol Cell Biol. 83:651–667. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Agudo-López A, Miguel BG, Fernández I and
Martínez AM: Role of protein kinase C and mitochondrial
permeability transition pore in the neuroprotective effect of
ceramide in ischemia-induced cell death. FEBS Lett. 585:99–103.
2011. View Article : Google Scholar
|
|
41
|
Peng Y, Hu Y, Feng N, Wang L and Wang X:
L-3-n-butyl-phthalide alleviates hydrogen peroxide-induced
apoptosis by PKC pathway in human neuroblastoma SK-N-SH cells.
Naunyn Schmiedebergs Arch Pharmacol. 383:91–99. 2011. View Article : Google Scholar
|
|
42
|
Heiss A, Ammer H and Eisinger DA:
delta-Opioid receptor-stimulated Akt signaling in neuroblastoma ×
glioma (NG108-15) hybrid cells involves receptor tyrosine
kinase-mediated PI3K activation. Exp Cell Res. 315:2115–2125. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huang D, Ding Y, Luo WM, Bender S, Qian
CN, Kort E, Zhang ZF, VandenBeldt K, Duesbery NS, Resau JH, et al:
Inhibition of MAPK kinase signaling pathways suppressed renal cell
carcinoma growth and angiogenesis in vivo. Cancer Res. 68:81–88.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tang B, Du J, Gao ZM, Liang R, Sun DG, Jin
XL and Wang LM: DADLE suppresses the proliferation of human liver
cancer HepG2 cells by activation of PKC pathway and elevates the
sensitivity to cis-diammine dichloridoplatium. Zhonghua Zhong Liu
Za Zhi. 34:425–429. 2012.In Chinese. PubMed/NCBI
|