Introduction
T-cell dependent tumor immune responses have been
demonstrated in clinical studies and the CD28 family of receptors
and the B7 family of ligands are thought to be most attractive as
T-cell regulatory targets for cancer immunotherapy or for the
control of immunological disorders (1–4). B7
homolog 4 (B7-H4) negatively regulates T-cell proliferation and
cytokine production (5,6). B7-H4 mRNA is expressed widely in
multiple tissues to a low degree (6,7), but
its receptor on activated lymphocytes has yet to be identified
(8,9).
B7-H4 is overexpressed in many human cancers
(10–13), and B7-H4 levels in patient sera are
upregulated in ovarian cancer and renal cell carcinoma (RCC)
(14,15). However, low levels of B7-H4 can also
be detected in healthy human sera. Endothelial cells of the RCC
tumor vasculature express B7-H4, whereas normal renal vessels and
tissues exhibit little to no expression. Notably, patients with RCC
expressing both B7-H1 and B7-H4 have a poor prognosis compared to
patients presenting with single expression alone or patients with
no expression at all (12). By
contrast, high expression of B7-H4 in breast cancer is correlated
with an improved recurrence-free survival (16).
Ovarian cancer patients frequently express the B7-H4
ligand in the cytoplasm and on membranes but not on the cell
surface (17,18), and the expression level is inversely
correlated with patient survival (19). However, B7-H4-expressing
infiltrating macrophages, but not tumor cells, suppress
tumor-associated antigen (TAA)-specific T-cell immunity (18). Tumor cell-derived B7-H4 could
function in immunosuppression even though the physiological
implications are not apparent.
IL-6 and IL-10 induce B7-H4 expression on
tumor-associated macrophages (TAMs) and on other immune cells
involved in Treg development (18,19),
but IL-6 and IL-10 receptors rarely exist on normal tissue cells
(20) and non-hematopoietic
malignanct cell lines (21), which
indicates that B7-H4 expression in tumor cells is regulated in a
different manner than in immune cells. By contrast, IFN-γ induces
B7-H4 expression on mouse embryonic fibroblasts (16), and its receptor is widely expressed
on both normal and tumor cells. This indicates that IFN-γ is
prossibly a B7-H4 inducer in tumors. Consequently, ovarian cancer
rapidly loses B7-H4 expression after a few days in vitro,
which suggests that the microenvironment can influence B7-H4
expression (22).
B7-H4 is a candidate immunoregulatory target and may
also be a direct therapeutic target against tumors. B7-H4 knockdown
by morpholino oligos improved TAA-specific T-cell immunity
(18) and knockout protected mice
from experimental lung metastasis (23). However, antibody-dependent cellular
cytotoxicity (ADCC) or tumor growth suppression was not reported
except during B7-H4 forced expression (11). Notably, Lee et al reported
that B7-H4 is not detected on immune cells of either humans or mice
by flow cytometry before or even after stimulation, and this
observation is inconsistent with previous studies (24).
In clinical studies targeting immune checkpoint
molecules of the B7/CD28 families, a B7-H1 (PD-L1)-targeting
inhibitory antibody has shown potent antitumor effects in advanced
melanoma (3), although
B7-H4-targeting clinical trials have not yet been performed.
In this study, we performed comprehensive whole
exome sequencing and gene expression profiling based on 1,058
cancer patient-derived tumor tissues and consequently found high
B7-H4 expression in non-small cell lung cancer and breast cancer
patients. Furthermore, we characterized unstable expression of the
B7-H4 molecule in cancer cells using antibodies generated in house.
Finally, we discuss how to use anti-B7-H4 antibodies for cancer
immunotherapy.
Materials and methods
Patient materials
This clinical research project using comprehensive
whole exome sequencing and gene expression profiling of various
tumor tissues, called the High-tech Omics-based Patient Evaluation
(HOPE) for Cancer Therapy, has been conducted in accordance with
the ʻEthical Guidelines for Human Genome and Genetic Analysis
Researchʼ, revised in 2013. Informed consents were obtained from
all patients participating in the HOPE project, which was approved
by the Institutional Review Board of the Shizuoka Cancer Center
(SCC), Japan. Tumor tissues, along with surrounding normal tissues,
were dissected from surgical specimens by trained pathologists.
Animal experiments
BALB/cA mice and nude mice (BALB/cA-nu/nu) were
obtained from Nippon Clea (Tokyo, Japan). All animals were cared
for and used humanely according to the Guidelines for the Welfare
and Use of Animals in Cancer Research (25). All procedures were approved by the
Animal Care and Use Committee of the Shizuoka Cancer Center (SCC)
Research Institute.
Gene expression analysis of the B7 family
of genes
Total RNA was extracted from ~10 mg of tissue
samples using the miRNeasy Mini Kit (Qiagen) according to the
manufacturer's instructions. Following assessment using an Agilent
2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA),
total RNA with an RNA integrity number (RIN) of six or higher was
used for DNA microarray analysis. Gene expression analysis was
performed using SurePrint G3 Human GE 8×60K v2.0 arrays (Agilent
Technologies) according to the manufacturer's instructions. Signal
data analysis was carried out using GeneSpring version 13.1.1
software (Agilent Technologies). The ratio of tumor tissue vs.
surrounding non-cancerous tissue was calculated from the normalized
values.
Generation of anti-B7-H4 monoclonal
antibodies
The human B7-H4 isoform 1 extracellular domain was
constructed with a 6X histidine tag in a pcDNA3.3 expression
vector, and the B7-H4 extracellular soluble free form was harvested
from Expi293F™ cell (Life Technologies Corporation) culture
supernatant, affinity-purified, and used for experiments. B7-H1 and
B7-DC were produced in the same way for use as negative controls.
After the immunization of BALB/cA mice, an antibody secreting
hybridoma was generated by a common method using the mouse myeloma
cell line P3X63Ag8.653 (American Type Culture Collection; ATCC,
Manassas, VA, USA).
ELISA
Specificity was validated by sandwich ELISA and
fluorescent staining of B7-H4-transfected HEK293 cells. Briefly,
purified newly-generated antibodies were immobilized on a 96-well
microplate Nunc Immobilizer Amino Surface (Thermo Fisher
Scientific, Inc.) prior to the addition of 3% bovine serum albumin
for overnight blocking and 10 µg/ml B7-H4 (soluble form) or
controls. After being washed, 2 µg/ml biotinylated antibody
(Ab) clone #25 was added and detected by HRP-conjugated
streptavidin (Thermo Fisher Scientific, Inc.).
Flow cytometry
HEK293 cells that transformed with the full B7-H4
sequence or cancer cell lines were incubated with 8 µg/ml Ab
clones and later incubated with 4 µg/ml PE-labeled
polyclonal anti-mouse Ig Ab (BD Biosciences) on ice. Fluorescence
intensity was determined by the flow cytometer FACSCanto (BD
Biosciences).
Affinity kinetics determination of
anti-B7-H4 antibodies
Surface plasmon resonance (SPR) analysis was
performed on a Biacore ×100 (GE Healthcare) in order to determine
the kinetics of the anti-B7-H4 Ab and free form of B7-H4. All
reagents and sensor chips were purchased from GE Healthcare.
Immobilization of anti-B7-H4 IgG antibodies on the CM5 sensor chip
was performed at pH 5.0, and the Ab binding was targeted to 1000
response units (RU). To regenerate the sensor chip, 10 mM
glycine-HCl pH 1.7 was used. The binding kinetics were monitored by
injecting multiple concentrations of B7-H4 in HBS buffer (10 mM
HEPES pH 7.4, containing 0.15 M NaCl, 3 mM EDTA, and 0.05%
Tween-20) at a flow rate of 30 µl/min at 25°C. The
dissociation phase was also monitored by HBS buffer flow. The
binding kinetic parameters were calculated by Biacore ×100
evaluation software.
Western blot analysis
Breast cancer cell line lysates harvested with
Laemmli sample buffer, and over-confluent culture supernatants were
used for western blot analysis. Cell lysates and supernatants were
electrophoresed through a SDS-PAGE gradient gel and transferred to
a PVDF membrane. After overnight blocking with 5% skim milk and
washing with 0.05% Tween-20-PBS (T-PBS), the membrane was incubated
for 1 h at room temperature (RT) with rabbit anti-human B7-H4 Ab
(clone, EP1165). After being washed with T-PBS, the membrane was
incubated with a secondary Ab, HRP-conjugated donkey anti-rabbit
IgG polyclonal Ab (GE Healthcare) diluted 1:10,000. ECL Plus (GE
Healthcare) was used for detection according to the manufacturer's
instructions.
Immunohistochemistry
Female nude mice (BALB/cA-nu/nu, 5–6 weeks old) were
transplanted subcutaneously with human breast cancer MDA-MB-468 or
lung cancer NCI-H2170 cells. Formalin-fixed paraffin-embedded
(FFPE) cancer tissue blocks were made. To evaluate B7-H4
expression, sections from the cancers were immunostained with
anti-B7-H4 Ab (clone #25) or mouse IgG1 isotype control (BD
Pharmingen) and later stained with hematoxylin.
Tumor growth inhibition assay
MDA-MB-468 cells (1×107) were inoculated
into the mammary fat pad of BALB/cA-nu/nu mice. Antibody clone #25,
at doses of 2 or 10 mg/kg, was administered by intraperitoneal
injection twice a week from day 9 to day 27 after tumor
inoculation. To evaluate the antitumor activity against inoculated
tumors, tumor volume was calculated based on the National Cancer
Institute formula as follows: Tumor volume (mm3) =
length (mm) × [width (mm)]2 × 1/2.
Indirect ADCC experiment
Briefly, effector cells were isolated from healthy
volunteer peripheral blood by Ficoll-Paque PLUS (GE Healthcare Life
Sciences, Buckinghamshire, UK) and then enriched by depletion of
CD14+ or CD19+ cells using MicroBeads and
autoMACS (Miltenyi Biotec K.K., Tokyo, Japan). The effector cells
were incubated on ice for 10 min with 0.2 mg/ml (high
concentration) anti-CD3 Ab (OKT3) diluted with staining buffer
(0.5% BSA, 0.1% azide, 1 mM glucose-PBS), followed by washing.
Cells were then incubated with 0.5 mg/ml polyclonal rabbit
anti-mouse immunoglobulins (DakoCytomation, Glostrup, Denmark) and
after being washed, they were incubated with 0.5 mg/ml of each
anti-B7-H4 antibody. The effector cells were washed and resuspended
to a concentration of ~107 cells/ml in 10% FBS-RPMI-1640
and co-cultured with non-radioactive reagent-labeled MDA-MB-468
target cells in a 96-well round-bottom plate at 37°C for 3 h.
Target cell lysis was performed with DELFIA® EuTDA
Cytotoxicity Assay Reagents and measured using a Wallac 1420 ARVO
SX multilabel counter (PerkinElmer, Waltham, MA, USA). The
percentage of specific lysis was determined by the following
formula: Percentage of specific lysis = (experimental release −
spontaneous release)/(maximal release − spontaneous release) ×
100.
Statistical analysis
Statistically differences were analyzed using the
Student's paired two-tailed t-test. Values of P<0.01 were
considered to indicate statistically significant differences.
Results
B7-H4 expression in cancer tissues and
cell lines
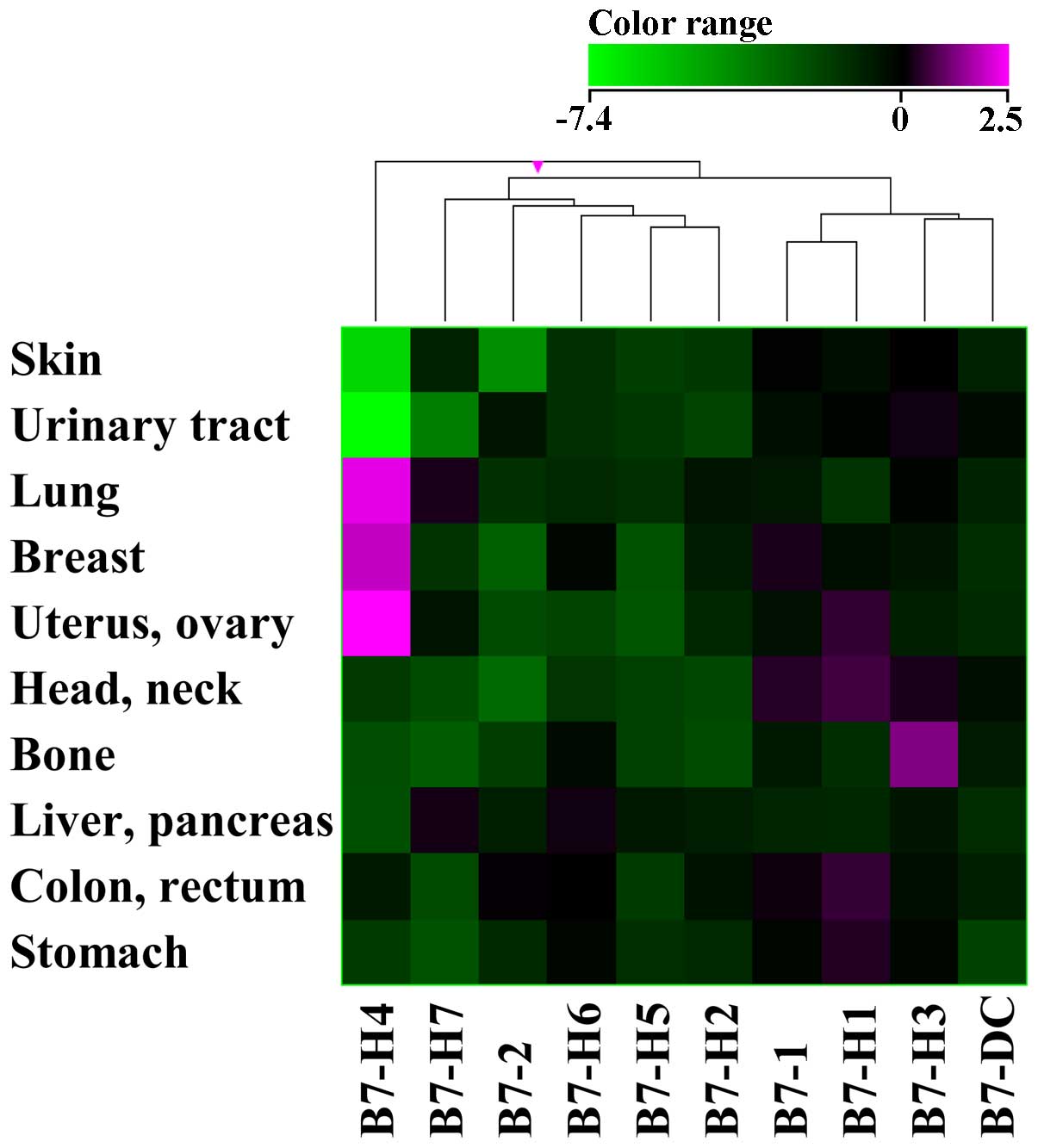
We found that B7-H4 expression was upregulated in
some types of cancer when compared to expression in non-cancerous
normal tissues (Fig. 1). B7-H4
expression was upregulated in breast, lung, and gynecological
cancers and downregulated in skin and urinary tract cancers.
Expression of other genes from the B7 family was not apparently
altered. B7-H4 expression was detected in breast and ovarian cancer
cell lines by RT-PCR in accordance with the previous study (data
not shown), but the soluble free form of B7-H4 in the culture
medium was not, as determined by ELISA. The splice variants of
B7-H4 that were detected corresponded mainly to isoforms 1, 2, and
4 and partially to isoform 3 which has a different sequence.
Production and characterization of
anti-B7-H4 monoclonal antibodies
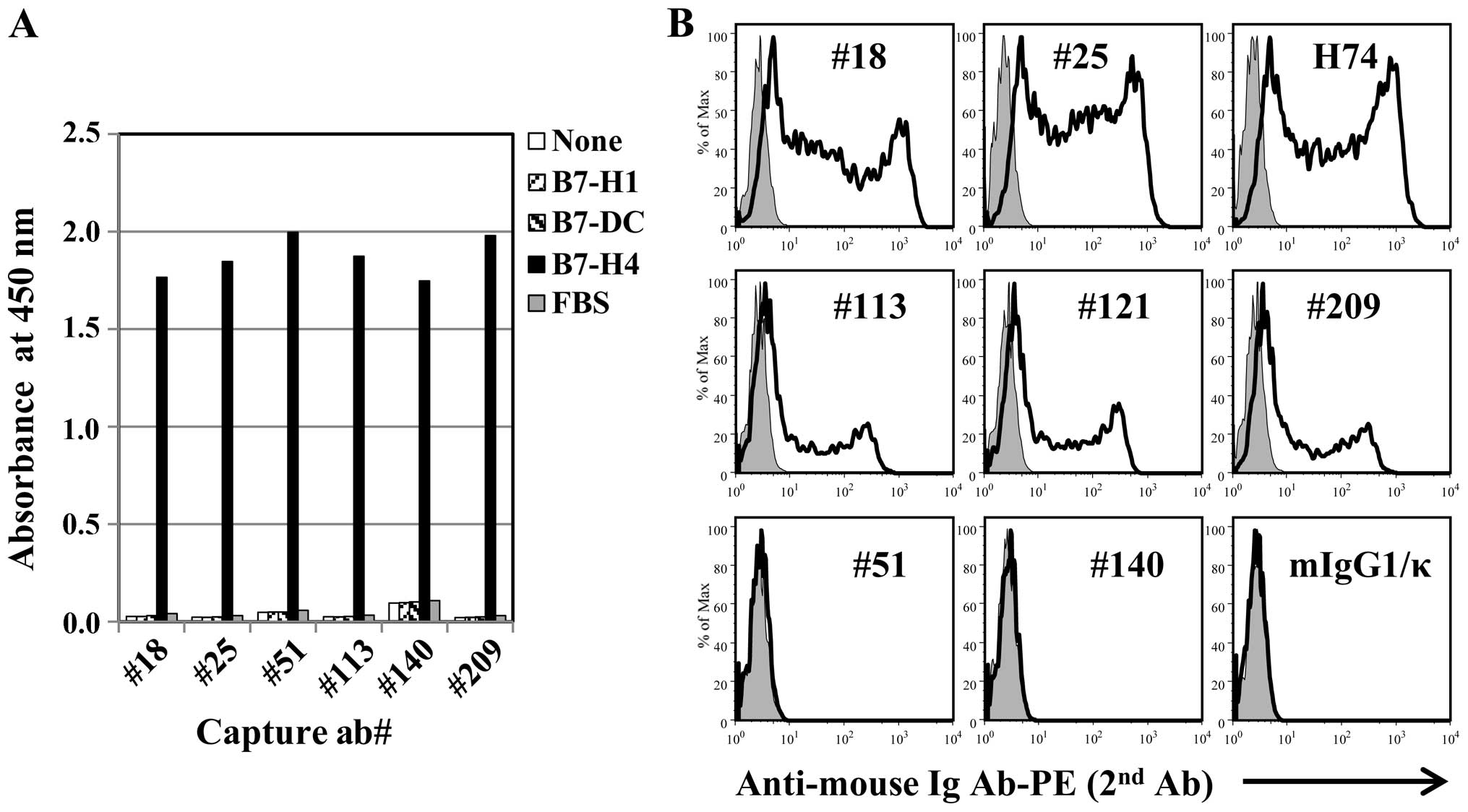
We generated 19 hybridoma clones of anti-human B7-H4
isoform 1 for detection of the natural form, and purified eight
antibody clones which could be cultured in vitro under
serum-free conditions. The antibody clones bound B7-H4 but not B7
homologs PD-L1 or PD-L2 (B7-DC) (Fig.
2A), and the staining of HEK293 cells transfected with the full
B7-H4 isoform 1 sequence showed two different types of staining,
strong (#18, #25) and weak (#113, #121, #209), by flow cytometry.
All mAb clones were confirmed to have different DNA sequences
(Fig. 2B).
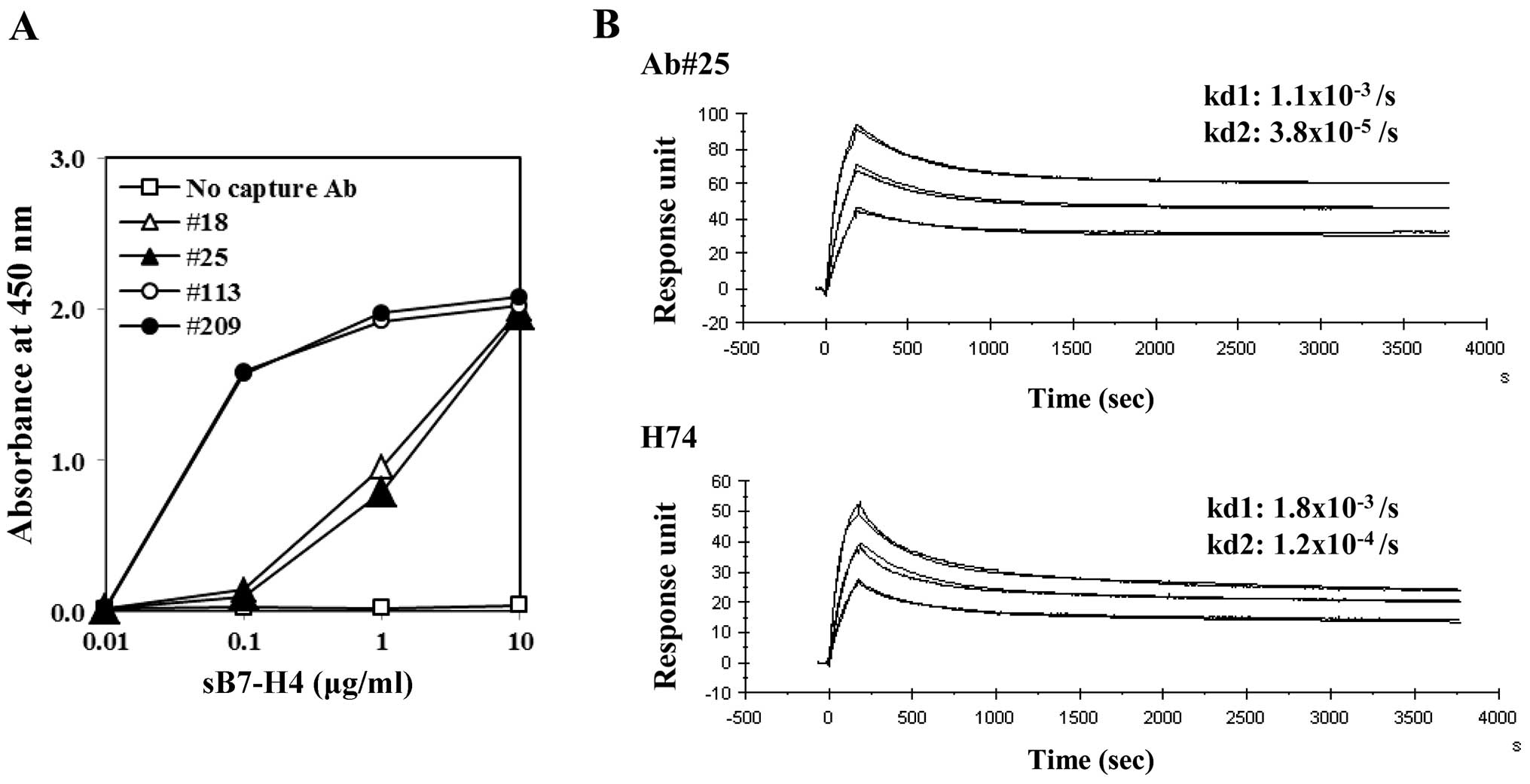
B7-H4 forms homodimers
Sandwich ELISAs using antibody clone #25 as both the
capture and detector antibody detected the free form of B7-H4;
ELISA using clone #18 as the capture Ab showed a similar curve.
These two capture Abs had lower sensitivities than the other
clones, which contrasted to staining of HEK293 transfectants
(Figs. 3A and 2B). B7-H4 is a monovalent molecule, and
thus these observations were indicative of B7-H4 homodimerization
and bivalent antigenicity. In determining the affinity kinetics of
the antibodies to the free form of B7-H4, both clone #25 and the
commercially available clone-H74 showed two-state reaction curves,
including rapid dissociation and stable dissociation curves, which
can be explained by dimer dissociation and monomer dissociation,
respectively (Fig. 3B). Each rapid
dissociation rate was equivalent.
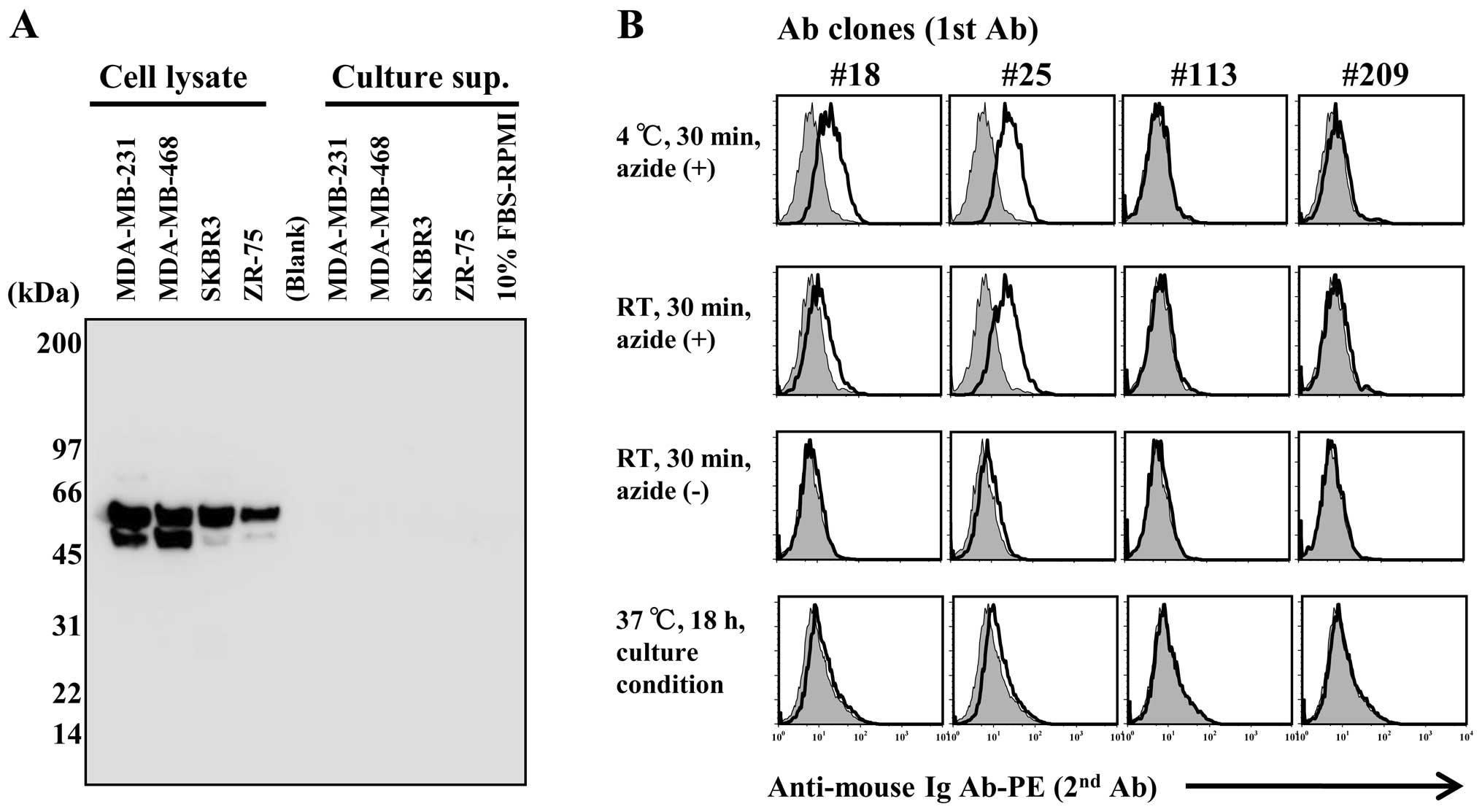
B7-H4 ligand instability on the tumor
cell surface
B7-H4 expression in the breast cancer cell lines was
validated by western blot analysis. Predicted molecular weight of
B7-H4 is 29 kDa but it aligns with a size of 50~70 kDa owing to
glycosylation and isoform variations (14,26).
B7-H4 was detected in cell lysates but not in culture supernatants
(Fig. 4A). The soluble-free forms
have reportedly been detected in patient sera, but it is difficult
to detect in vitro in culture supernatant.
We compared B7-H4 staining in the MDA-MB 468 cell
line under suppressive (azide-containing at 4°C) or normal
(azide-free at RT) conditions by flow cytometry (Fig. 4B). B7-H4 staining was positive under
suppressive conditions using clone #18 and clone #25 antibodies;
however, there was no detection under normal conditions. In
addition, B7-H4 staining was also not apparent in samples that
underwent an overnight incubation at 37°C and 5%
CO2.
In vivo experiments
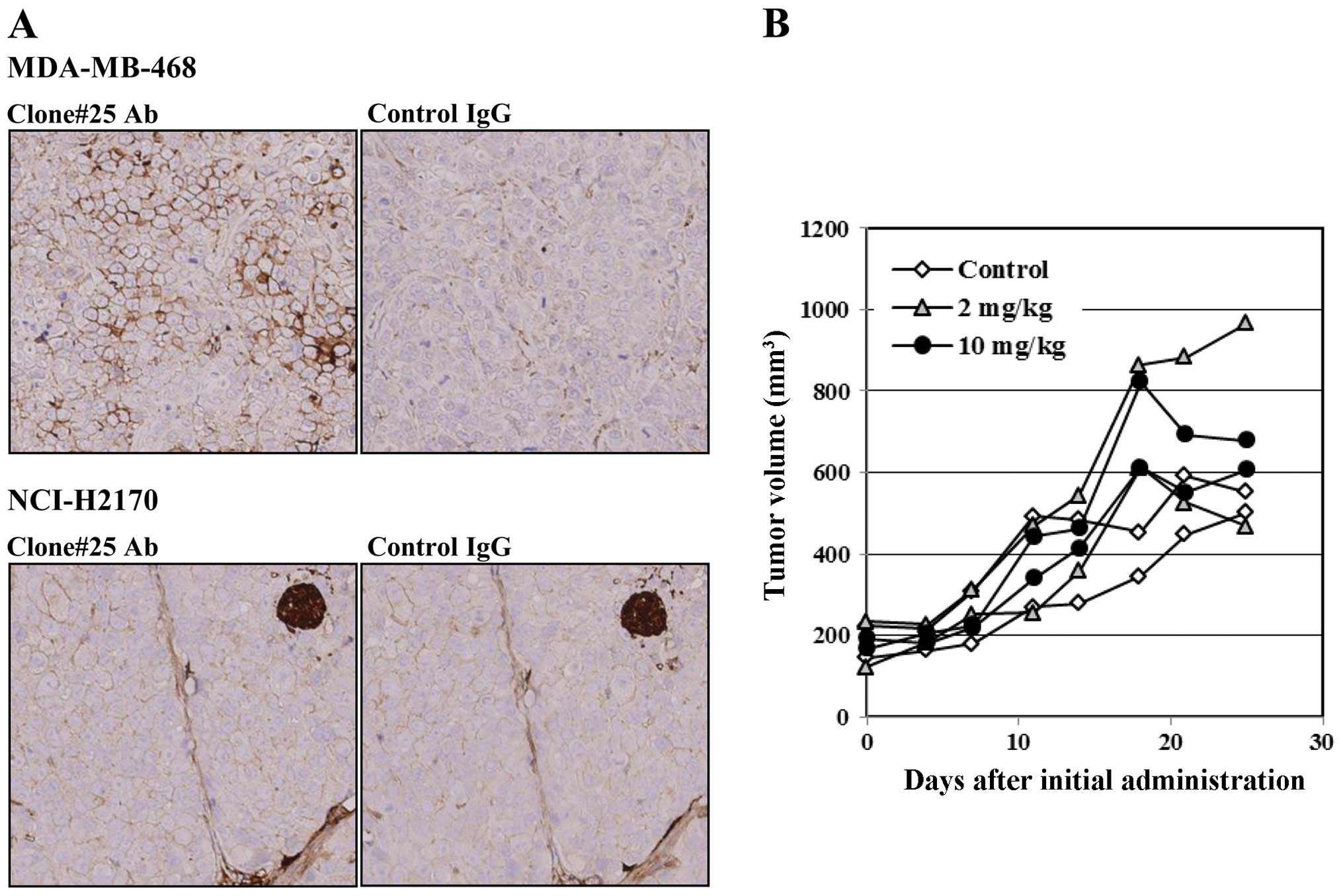
In the MDA-MB-468 transplanted nude mouse model, the
strong B7-H4 expression on the tumor cell membrane was observed by
immunohistochemistry (IHC), and the intensity of the staining was
heterogeneous in the tumor tissue (Fig.
5A). By contrast, the B7-H4-negative NCI-H2170 cell line
(control) did not show any B7-H4 expression in tumor tissues. We
administered antibody clone #25 (mouse IgG1) to the MDA-MB-468
transplanted mouse model, but no tumor suppressive effect was
observed (Fig. 5B).
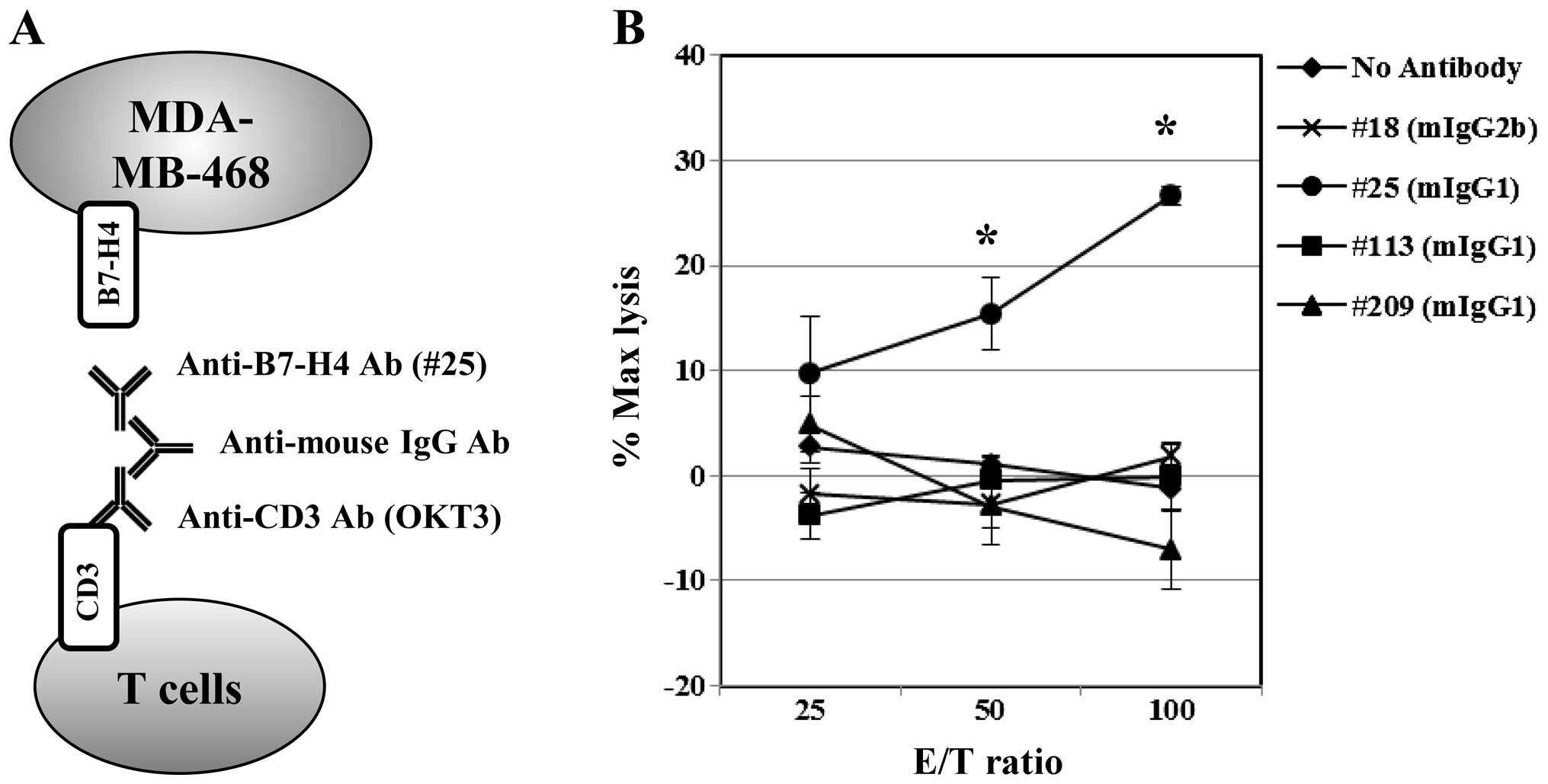
Indirect ADCC elicited tumor cell
death
Based on the observations that i) there was loss of
B7-H4 expression on the tumor cell surfaces under usual conditions
and ii) there was lack of antitumor activity due to non-functional
ADCC in vivo, we employed an indirect ADCC-redirecting
T-cell cytotoxicity assay to study B7-H4 using polyclonal
anti-mouse IgG-mediated linking of anti-CD3 and anti-B7-H4
antibodies. Freshly enriched T cells indirectly targeting B7-H4
exhibited clone #25-dependent cellular cytotoxicity against the
MDA-MB468 cells (Fig. 6). By
contrast, clones #18 (mIgG2b), #113 (mIgG1) and #209 (mIgG1) did
not induce targeted cell death.
Discussion
A comprehensive whole exome sequencing and gene
expression analysis of surgically resected tumor tissues derived
from 2,000 patients registered in the HOPE project was performed by
the Shizuoka Cancer Center (SCC), Japan over the last 2 years.
Focusing on the B7 and CD28 gene families, we found that B7-H4 was
altered in several types of cancer (Fig. 1). Unlike other B7 family genes,
B7-H4 is also expressed in many normal tissues at the mRNA level.
B7-H4 expression in cancer cell lines is low level except for
ovarian and breast cancers, while mammary gland epithelium normally
expresses B7-H4 (14,20). In vitro-cultured cell lines
reflect a similar B7-H4 expression profile as in normal tissues,
but expression is not comparable to that of lung cancer tissues
in vivo because of B7-H4 modulation.
Our monoclonal antibody clones #113 and #209 bound
to B7-H4-transfected HEK293 cells but not to the breast cancer cell
line MDA-MB-468 expressing B7-H4 (Figs.
2A and 4B). No B7-H4 gene
mutations in the MDA-MB-468 cell line were found, so we
hypothesized that the natural expression possibly harbors some type
of antibody-binding site as a result of homodimer or heterodimer
formation on the cell surface. In addition, we observed that B7-H4
intensity varied as a result of staining temperature and cellular
activity (Fig. 4B). Considering
that B7-H4 was not identified in culture supernatants (Fig. 4A), tumor cell surface-expressed
B7-H4 is likely internalized. The B7-H4 molecule consists of merely
two amino acids in the cytoplasm and does not have any signaling
function. B7-H4 may be involved in immune regulation as a ligand or
decoy ligand for receptors, or it may form a heterodimer with other
known B7 family ligands (27).
B7-H4 nuclear localization was reported (28), but the expressed isoforms and
constructs appear to mainly function on the cell membrane to
regulate intercellular signaling.
The studies concerning B7-H4 expression on tumor
tissues are not consistent, which may be explained partially by
incompatible antibody use (29).
However, differences in expression on immune cells suggest immune
receptor mobility such as the TCR, CD19 and CD28 families;
cross-linking of these antibodies could easily result in receptor
internalization on activated lymphocytes (30–33).
The disappearance of B7-H4 on immune cells may be due to the
cross-linking of antibodies (24).
Mobility of the B7-H4 ligand on tumor cells was not accounted for
(Fig. 4B) and puzzled expression in
ovarian cancer (17,18) may explain this phenomenon. This may
therefore explain why ADCC could not be performed on tumor cells
naturally expressing B7-H4 (Fig.
5B).
The B7-H4 antibody was able to induce cytotoxicity
of tumor cells expressing hB7-H4 or mB7-H4 by retroviral vectors
through ADCC or complement-dependent cytotoxicity (11), but these observations have not been
demonstrated in tumors spontaneously expressing B7-H4. We examined
whether the clone #25 Ab could act for MDA-MB-468 tumors
transplanted in nude mice, but tumor growth was not suppressed,
despite B7-H4 expression in tumors in vivo (Fig. 5A). This is possibly the result of
antigen disappearance on the cell surface (Fig. 4B). Cancer therapy targeting immune
regulatory molecules are difficult to test in a mouse model, and
the benefit of targeting human B7-H4 in human tumors has not yet
been shown. In addition, GPI-anchored glycine in mouse B7-H4 is not
conserved in humans, and thus B7-H4 may not behave similarly in
mice and humans (6). Recently,
single-chain Fv fragments (scFv) against hB7-H4 were reported but
did not exhibit tumor growth suppression in vivo. Small
molecule antibodies such as scFv leak through the kidneys (34-36),
and decreased affinity of scFv (37,38)
may be incompatible with this inhibitory effect.
Dynamic and unstable cell surface antigens are
probably not a target of conventional ADCC activity, but by
redirecting T-cell cellular cytotoxicity using specific antibodies
recognizing cell surface CD19 antigens instead of a peptide/MHC
complex and T-cell clonal specificity (39) have been successful. Clinical studies
of chimeric antigen receptor (CAR) T cells transfected with the CD3
gene linked to antigen-specific scFv have been successful and
extended further to other tumor targets. Bispecific scFv linked to
CD19 and CD3 was also succeeded in clinical study (40).
Here, we used indirect ADCC assays targeting B7-H4
(Fig. 6) and demonstrated a clone
#25 Ab-dependent cellular cytotoxicity in breast cancer cells.
Since clone #25 (mouse IgG1) does not bind to human Fc receptors,
this tumor lysis appears to result from effector T cells but not
natural killer cells. Ab clone #18 (IgG2b) and others that bound to
B7-H4 on HEK293 cells did not induce breast cancer cell death in
this study. These observations are the first to report anti-B7H4
Ab-mediated ADCC activity against tumors spontaneously expressing
B7-H4. Therefore, anti-B7H4 Ab-mediated cell death may be
considered an alternative therapy for PD-L1-negative and
B7H4-positive cancers.
B7-H4 upregulation in lung cancer tissues (Fig. 1) could be induced by cytokines
secreted from tumor-infiltrating lymphocytes or by the accumulation
of B7-H4-expressing suppressive macrophages derived from abundant
intrinsic alveolar-associated macrophages (41,42).
In cancer, tumor-infiltrating macrophages and regulatory T cells
collectively suppress tumor-associated antigen-specific T cells via
B7-H4 and consequently protect tumor cells from immune attacks
(18,22). Therefore, approaches to targeting
immune suppressor cells expressing B7-H4 are needed for effective
cancer therapy, in addition to targeting tumor cells. Elimination
of B7-H4-expressing tumor cells and immune-suppressive macrophages
by redirecting cytotoxic T cells could be a novel approach for
next-generation therapies against cancer.
Abbreviations:
|
ADCC
|
antibody-dependent cellular
cytotoxicity
|
|
B7-H4
|
B7 family homolog 4
|
|
Ab
|
antibody
|
Acknowledgments
This study was supported by a grant to Yasuto
Akiyama by JSPS KAKENHI (grant no. 25430166), Japan.
References
|
1
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hamid O, Robert C, Daud A, Hodi FS, Hwu
WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al:
Safety and tumor responses with lambrolizumab (anti-PD-1) in
melanoma. N Engl J Med. 369:134–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rizvi NA, Hellmann MD, Snyder A, Kvistborg
P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al: Cancer
immunology. Mutational landscape determines sensitivity to PD-1
blockade in non-small cell lung cancer. Science. 348:124–128. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sica GL, Choi IH, Zhu G, Tamada K, Wang
SD, Tamura H, Chapoval AI, Flies DB, Bajorath J and Chen L: B7-H4,
a molecule of the B7 family, negatively regulates T cell immunity.
Immunity. 18:849–861. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Prasad DV, Richards S, Mai XM and Dong C:
B7S1, a novel B7 family member that negatively regulates T cell
activation. Immunity. 18:863–873. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zang X, Loke P, Kim J, Murphy K, Waitz R
and Allison JP: B7x: a widely expressed B7 family member that
inhibits T cell activation. Proc Natl Acad Sci USA.
100:10388–10392. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen L: Co-inhibitory molecules of the
B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol.
4:336–347. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Choi IH, Zhu G, Sica GL, Strome SE,
Cheville JC, Lau JS, Zhu Y, Flies DB, Tamada K and Chen L: Genomic
organization and expression analysis of B7-H4, an immune inhibitory
molecule of the B7 family. J Immunol. 171:4650–4654. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jeon H, Vigdorovich V, Garrett-Thomson SC,
Janakiram M, Ramagopal UA, Abadi YM, Lee JS, Scandiuzzi L,
Ohaegbulam KC, Chinai JM, et al: Structure and cancer immunotherapy
of the B7 family member B7x. Cell Reports. 9:1089–1098. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Krambeck AE, Thompson RH, Dong H, Lohse
CM, Park ES, Kuntz SM, Leibovich BC, Blute ML, Cheville JC and Kwon
ED: B7-H4 expression in renal cell carcinoma and tumor vasculature:
Associations with cancer progression and survival. Proc Natl Acad
Sci USA. 103:10391–10396. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miyatake T, Tringler B, Liu W, Liu SH,
Papkoff J, Enomoto T, Torkko KC, Dehn DL, Swisher A and Shroyer KR:
B7-H4 (DD-O110) is overexpressed in high risk uterine endometrioid
adenocarcinomas and inversely correlated with tumor T-cell
infiltration. Gynecol Oncol. 106:119–127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Simon I, Zhuo S, Corral L, Diamandis EP,
Sarno MJ, Wolfert RL and Kim NW: B7-h4 is a novel membrane-bound
protein and a candidate serum and tissue biomarker for ovarian
cancer. Cancer Res. 66:1570–1575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thompson RH, Zang X, Lohse CM, Leibovich
BC, Slovin SF, Reuter VE, Cheville JC, Blute ML, Russo P, Kwon ED,
et al: Serum-soluble B7x is elevated in renal cell carcinoma
patients and is associated with advanced stage. Cancer Res.
68:6054–6058. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rahbar R, Lin A, Ghazarian M, Yau HL,
Paramathas S, Lang PA, Schildknecht A, Elford AR, Garcia-Batres C,
Martin B, et al: B7-H4 expression by nonhematopoietic cells in the
tumor microenvironment promotes antitumor immunity. Cancer Immunol
Res. 3:184–195. 2015. View Article : Google Scholar
|
|
17
|
Zang X, Sullivan PS, Soslow RA, Waitz R,
Reuter VE, Wilton A, Thaler HT, Arul M, Slovin SF, Wei J, et al:
Tumor associated endothelial expression of B7-H3 predicts survival
in ovarian carcinomas. Mod Pathol. 23:1104–1112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kryczek I, Zou L, Rodriguez P, Zhu G, Wei
S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, et al: B7-H4
expression identifies a novel suppressive macrophage population in
human ovarian carcinoma. J Exp Med. 203:871–881. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kryczek I, Wei S, Zhu G, Myers L, Mottram
P, Cheng P, Chen L, Coukos G and Zou W: Relationship between B7-H4,
regulatory T cells, and patient outcome in human ovarian carcinoma.
Cancer Res. 67:8900–8905. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
National Cancer Institute: Genotype-Tissue
Expression (GTEx). http://www.gtexportal.org/home.
2015
|
|
21
|
Broad institute: The Cancer Cell Line
Encyclopedia (CCLE). https://www.broadinstitute.org/ccle/home.
2015
|
|
22
|
Dangaj D, Lanitis E, Zhao A, Joshi S,
Cheng Y, Sandaltzopoulos R, Ra HJ, Danet-Desnoyers G, Powell DJ Jr
and Scholler N: Novel recombinant human b7-h4 antibodies overcome
tumoral immune escape to potentiate T-cell antitumor responses.
Cancer Res. 73:4820–4829. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abadi YM, Jeon H, Ohaegbulam KC,
Scandiuzzi L, Ghosh K, Hofmeyer KA, Lee JS, Ray A, Gravekamp C and
Zang X: Host b7x promotes pulmonary metastasis of breast cancer. J
Immunol. 190:3806–3814. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee JS, Scandiuzzi L, Ray A, Wei J,
Hofmeyer KA, Abadi YM, Loke P, Lin J, Yuan J, Serreze DV, et al:
B7x in the periphery abrogates pancreas-specific damage mediated by
self-reactive CD8 T cells. J Immunol. 189:4165–4174. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Workman P, Aboagye EO, Balkwill F, Balmain
A, Bruder G, Chaplin DJ, Double JA, Everitt J, Farningham DAH,
Glennie MJ, et al: Guidelines for the welfare and use of animals in
cancer research. Br J Cancer. 102:1555–1577. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tringler B, Zhuo S, Pilkington G, Torkko
KC, Singh M, Lucia MS, Heinz DE, Papkoff J and Shroyer KR: B7-h4 is
highly expressed in ductal and lobular breast cancer. Clin Cancer
Res. 11:1842–1848. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Butte MJ, Keir ME, Phamduy TB, Sharpe AH
and Freeman GJ: Programmed death-1 ligand 1 interacts specifically
with the B7-1 costimulatory molecule to inhibit T cell responses.
Immunity. 27:111–122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang L, Wu H, Lu D, Li G, Sun C, Song H,
Li J, Zhai T, Huang L, Hou C, et al: The costimulatory molecule
B7-H4 promote tumor progression and cell proliferation through
translocating into nucleus. Oncogene. 32:5347–5358. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Smith JB, Stashwick C and Powell DJ Jr:
B7-H4 as a potential target for immunotherapy for gynecologic
cancers: A closer look. Gynecol Oncol. 134:181–189. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pulczynski S, Boesen AM and Jensen OM:
Antibody-induced modulation and intracellular transport of CD10 and
CD19 antigens in human B-cell lines: An immunofluorescence and
immunoelectron microscopy study. Blood. 81:1549–1557.
1993.PubMed/NCBI
|
|
31
|
Gerber HP, Kung-Sutherland M, Stone I,
Morris-Tilden C, Miyamoto J, McCormick R, Alley SC, Okeley N, Hayes
B, Hernandez-Ilizaliturri FJ, et al: Potent antitumor activity of
the anti-CD19 auristatin antibody drug conjugate hBU12-vcMMAE
against rituximab-sensitive and -resistant lymphomas. Blood.
113:4352–4361. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schaffar L, Dallanegra A, Breittmayer JP,
Carrel S and Fehlmann M: Monoclonal antibody internalization and
degradation during modulation of the CD3/T-cell receptor complex.
Cell Immunol. 116:52–59. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Boyer C, Auphan N, Gabert J, Blanc D,
Malissen B and Schmitt-Verhulst AM: Comparison of phosphorylation
and internalization of the antigen receptor/CD3 complex, CD8, and
class I MHC-encoded proteins on T cells. Role of intracytoplasmic
domains analyzed with hybrid CD8/class I molecules. J Immunol.
143:1905–1914. 1989.PubMed/NCBI
|
|
34
|
He J, Wang Y, Feng J, Zhu X, Lan X, Iyer
AK, Zhang N, Seo Y, VanBrocklin HF and Liu B: Targeting prostate
cancer cells in vivo using a rapidly internalizing novel human
single-chain antibody fragment. J Nucl Med. 51:427–432. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Willuda J, Kubetzko S, Waibel R, Schubiger
PA, Zangemeister-Wittke U and Plückthun A: Tumor targeting of
mono-, di-, and tetravalent anti-p185HER-2
miniantibodies multimerized by self-associating peptides. J Biol
Chem. 276:14385–14392. 2001.PubMed/NCBI
|
|
36
|
Deyev SM, Waibel R, Lebedenko EN,
Schubiger AP and Plückthun A: Design of multivalent complexes using
the barnase·barstar module. Nat Biotechnol. 21:1486–1492. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pleckaityte M, Mistiniene E, Lasickiene R,
Zvirblis G and Zvirbliene A: Generation of recombinant single-chain
antibodies neutralizing the cytolytic activity of vaginolysin, the
main virulence factor of Gardnerella vaginalis. BMC Biotechnol.
11:1002011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Willuda J, Honegger A, Waibel R, Schubiger
PA, Stahel R, Zangemeister-Wittke U and Plückthun A: High thermal
stability is essential for tumor targeting of antibody fragments:
Engineering of a humanized anti-epithelial glycoprotein-2
(epithelial cell adhesion molecule) single-chain Fv fragment.
Cancer Res. 59:5758–5767. 1999.PubMed/NCBI
|
|
39
|
Porter DL, Levine BL, Kalos M, Bagg A and
June CH: Chimeric antigen receptor-modified T cells in chronic
lymphoid leukemia. N Engl J Med. 365:725–733. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bargou R, Leo E, Zugmaier G, Klinger M,
Goebeler M, Knop S, Noppeney R, Viardot A, Hess G, Schuler M, et
al: Tumor regression in cancer patients by very low doses of a T
cell-engaging antibody. Science. 321:974–977. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Richters A, Sherwin RP and Richters V: The
lymphocyte and human lung cancers. Cancer Res. 31:214–222.
1971.PubMed/NCBI
|
|
42
|
Cohen AB and Cline MJ: The human alveolar
macrophage: Isolation, cultivation in vitro, and studies of
morphologic and functional characteristics. J Clin Invest.
50:1390–1398. 1971. View Article : Google Scholar : PubMed/NCBI
|