Introduction
Medulloblastoma is the most common malignant brain
tumor in children. Patients are risk stratified according to age
(greater or less than 3 years of age), presence of disease
dissemination and extent of resection. The mainstays of therapy are
surgery, chemotherapy and irradiation. However, all of these
therapies result in long-term problems (1). Although a variety of signaling
pathways [(e.g. Sonic hedgehog (SHH) signaling, Wnt signaling
(WNT)] are known to be associated with medulloblastoma cell biology
(2–4), new therapeutic interventions for
medulloblastoma have been slow to develop. Recent genomic analyses
have identified multiple molecular subgroups with differing
outcomes (5,6), underscoring the heterogeneity of
medulloblastoma. By current international consensus there are four
main sub groups of medulloblastoma: WNT, SHH, groups 3 and 4
(7). However these molecular data
are not yet used to guide therapy (8). Therefore, there is a critical need for
new treatment agents for medulloblastoma.
We recently identified several kinases involved in
the G2/M cell cycle checkpoint that influence medulloblastoma cell
viability (9). Among these is the
monopolar spindle 1 (MPS1) kinase, which is widely conserved
amongst eukaryotes. MPS1 is involved, along with Aurora-B kinase,
in ensuring proper orientation of sister chromatids at the
kinetochore during the spindle assembly checkpoint (SAC) as well as
activation and maintenance of the checkpoint itself (10). Additionally, over-expression of
mammalian Mps1 leads to overduplication of the centrosome, and
conversely expression of the kinase-inactive allele prevents
centrosome duplication (11).
MPS1 is often dysregulated in cancers such as
thyroid papillary carcinoma, breast, gastric and lung cancer,
suggesting that increased expression of MPS1 may either promote
cancer initiation or allow survival of aneuploidy cancer cells
(10). Recently, studies have shown
that inhibition of MPS1 induces aberrant mitosis and apoptosis in a
variety of cancer cells including human breast cancer cells
(12,13). In cervical carcinoma cells
inhibition of MPS1 by a novel selective small-molecule inhibitor,
NMS-P715, led to SAC override and mitotic acceleration (14). In ovarian cancer and colon carcinoma
cells, NMS-P715 treatment also led to aneuploidy and apoptosis
(14). NMS-P715 showed significant
antiproliferative activity in colon, breast, renal and melanoma
cancer cell lines, but did not alter proliferation of normal
non-transformed cells (14).
However, the role of MPS1 in medulloblastoma has yet to be
evaluated.
In the present study, we showed that medulloblastoma
patient samples expressed higher levels of MPS1 compared to that
noted in normal cerebellum. Inhibition of MPS1 suppressed
medulloblastoma cell growth and treatment of medulloblastoma cells
with NMS-P175 suppressed medulloblastoma colony formation and
induced apoptosis.
Materials and methods
Cell lines and reagents
The Daoy and D283 medulloblastoma cell lines were
purchased from the American Type Culture Collection (ATCC)
(Rockville, MD, USA). The ONS-76 medulloblastoma cell line was
provided by Dr James T. Rutka at the University of Toronto, Canada,
and the UW228 cell line by Dr John Silber at the University of
Washington (Seattle, WA, USA). D425 and D458 cell lines were
obtained from Dr Darell D. Bigner at Duke University Medical Center
(Durham, NC, USA). Cell lines were cultured in Dulbecco' modified
Eagle's medium (DMEM) (Gibco, Carlsbad, CA, USA) supplemented with
10% fetal bovine serum (FBS) (Atlanta Biologicals, Lawrenceville,
GA, USA). Cell lines D283, D425 and D458 are all part of the group
3 genomic subgroup. Daoy, ONS-76 and UW228 cluster with the Shh
subgroup.
Primary patient samples were obtained from
Children's Hospital Colorado and were collected and used in
accordance with local and federal human research protection
guidelines and Institutional Review Board (IRB) regulations.
Informed consent was obtained for all specimens collected. Normal
brain tissue was collected from autopsy and purchased from Ambion
(Austin, TX, USA), Stratagene (Santa Clara, CA, USA) and Clontech
Laboratories, Inc. (Mountain View, CA, USA). NMS-P715 was kindly
provided by R. Colombo, Nerviano Medical Sciences (Nerviano, Italy)
or were purchased from Calbiochem, EMD Millipore Corp. (Billerica,
MA, USA). NMS-P715 was reconstituted in dimethyl sulfoxide (DMSO)
and aliquots were stored in a desiccator at −20°C. An equivalent
amount of DMSO for the highest concentration of drug was used for
each experiment as a vehicle control.
Gene expression microarray analysis
Patient tumor samples were collected at the time of
surgery and snap-frozen in liquid nitrogen. Ribonucleic acid was
extracted from all samples using an RNeasy kit (Qiagen, Valencia,
CA, USA). Samples were evaluated for gene expression using
Affymetrix U133 Plus 2.0 GeneChip microarrays as we previously
described (9,15).
Cell proliferation assay
Cell proliferation was determined by
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) assay using CellTiter 96 AQueous One Solution (Promega,
Madison, WI, USA). Seventy-two hours after transfection with shTTK1
(shMPS1), 20 µl of MTS reagent was added to the wells
already containing 100 µl of media.
For drug treatment, the cells were plated for 24 h
before adding 0.5 and 2 µm of NMS-P715. Then, 72 h after the
addition of the drug, 30 µl of MTS reagent was added to the
wells to make a final volume of 180 µl. Plates were read
using a Bio-Tek Synergy 2 plate reader (Bio-Tek, Winooski, VT, USA)
every hour for 4 h after the addition of the MTS reagent.
Experiments were carried out in triplicate and background
absorbance was subtracted from all wells before analysis.
Viability cell assay
Cell numbers after NMS-P715 treatment (2 µm)
were measured using the ViaCount assay (Millipore) on a Guava flow
cytometer as per the manufacturer's recommendations. Cells were
cultured for 72 h, and collected by centrifugation. The cells were
washed, ViaCount reagent was added, and cell counts were measured
on the Guava EasyCyte Plus flow cytometer (Millipore).
Colony formation assay
Daoy, ONS-76 and UW228 medulloblastoma cells were
seeded into 6-well plates in triplicate at a density of 500
cells/well in 3 ml medium containing 10% FBS for 24 h. The cells
were then treated with DMSO or MPS1 inhibitor, NMS-P715 (500 nm-1
µm) and further cultured for 10 days in a 37°C humidified
atmosphere containing 95% air and 5% CO2. After 10 days
of growth, the medium was aspirated, the wells were washed with
phosphate-buffered saline (PBS), and cell clones were stained for
15 min with a solution containing 0.5% crystal violet and 25%
methanol, followed by 3 rinses with tap water to remove excess dye.
The colony numbers were counted using a precise electronic counter
(Heathrow Scientific, Vernon Hills, IL, USA) and an inverted
microscope with a threshold of 50 cells necessary to constitute a
colony.
Western blotting
Protein expression levels were determined by western
blotting. Cells were lysed in 1X RIPA buffer (Thermo Scientific,
Rockford, IL, USA) containing protease inhibitor cocktail (Roche,
Indianapolis, IN, USA), 1 mM sodium vanadate and 0.1 mM sodium
molybdate. The lysates were centrifuged for 20 min and the
supernatants collected for protein concentration determination by
the Bradford reagent (Sigma, St. Louis, MO, USA). Equal amounts of
cell lysates were resolved by sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE), and western blot analysis was
performed with specific antibodies. Antibodies used for western
blot analysis were purchased from the following sources: actin
#8H10D10, MPS1#11108 (Abcam, Cambridge, MA, USA). Antibodies for
Mps1 and phosphorylated Mps1 were obtained by P.A. Eyers (16). Secondary antibodies conjugated to
horseradish-peroxidase were used in conjunction with a
chemiluminescent reagent to visualize protein bands.
Cell cycle assay
Flow cytometric analysis was performed to define the
cell cycle distribution after NMS-P715 treatment. D425 and D458
cells were seeded into 6-well plates (105 cells/well)
and 24 h later were treated with 2 µm NMS-P715. Cells were
harvested 72 h later and fixed with chilled 70% ethanol for 24 h.
Fixed cells were then washed and stained with propidium
iodide-containing cell cycle reagent (Millipore). Flow cytometric
analysis was performed on the Guava EasyCyte Plus flow
cytometer.
Cell apoptosis assay
Daoy and UW228 medulloblastoma cells were treated
with 2 µm NMS-P715 and allowed to grow in normal culture
medium for 72 h. The cell concentration was determined following
staining with Guava ViaCount reagent. Equal numbers of cells were
then stained using Guava Nexin reagent (Millipore) to detect
apoptotic cells. Samples were run on a Guava EasyCyte Plus flow
cytometer.
Results
MPS1 is highly expressed in high grade
pediatric brain tumors
Our recent study suggested that MPS1 is highly
expressed in medulloblastoma (9).
To further examine the expression of MPS1 in pediatric brain tumors
we first analyzed microarray-derived gene expression data from 90
pediatric brain tumors. MPS1 mRNA expression was significantly
elevated in high grade pediatric brain tumors including
glioblastoma (GBM), medulloblastoma (MED) and ATRT compared to
normal brain (Fig. 1A). Notably,
lower grade tumors such as pilocytic astrocytoma (PA) expressed
much lower amounts of MPS1 mRNA (Fig.
1A). There was no clear correlation between the high
MPS1-expressing samples and age, gender or outcomes.
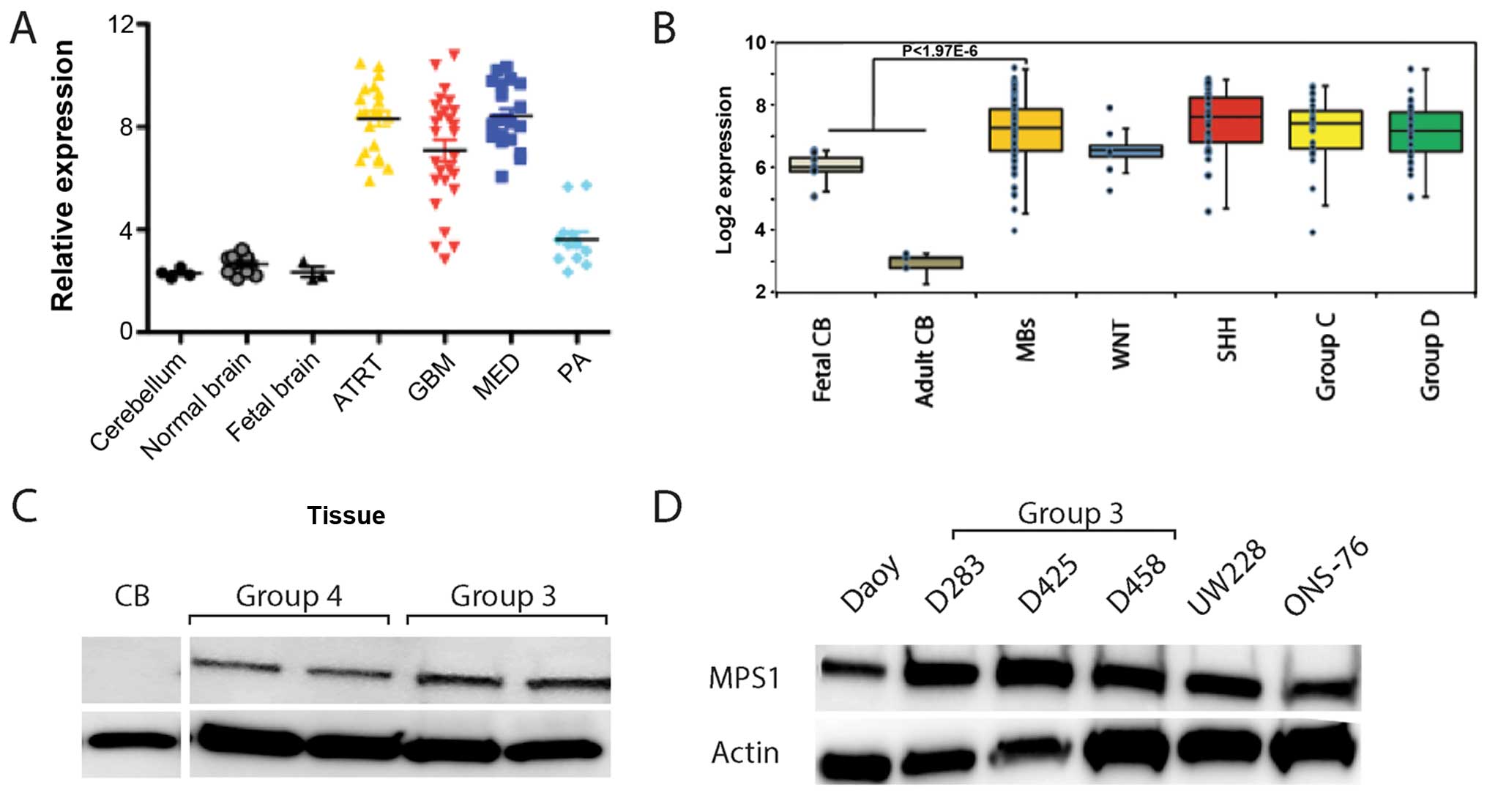 | Figure 1Overexpression of MPS1 in
pediatric brain tumors. (A) Microarray expression of MPS1
mRNA in 90 pediatric brain tumors showed significant overexpression
of MPS1 in high-grade pediatric brain tumors when compared to
normal brain. Error bars represent standard error of the mean
(SEM). (B) Microarray expression of MPS1 mRNA in 120 patients with
medulloblastoma demonstrated that MPS1 mRNA is significantly
elevated in medulloblas-toma compared to adult and fetal cerebellum
but not significantly different among the genomic subgroups (WNT,
SHH, groups 3 and 4). Error bars represent SEM. (C) MPS1 protein
expression in patient tumor samples including two group 3 and two
group 4 samples compared to normal pediatric cerebellum (CB). (D)
MPS1 protein expression in commonly used medulloblastoma cell lines
(D283, D458 and D425, group 3 genomic subgroup; Daoy, ONS-76 and
UW228, Shh genomic subgroup). ATRT, atypical teratoid/rhabdoid
tumor: GMB, glioblastoma multiforme; MED, medulloblastoma; PA,
pilocytic astrocytoma. |
Medulloblastoma consists of four major genomic
subgroups [Sonic hedgehog (SHH) signaling, Wnt signaling (WNT), and
groups 3 and 4 (7)]. To further
elucidate whether there was a correlation between the subgroups of
medulloblastoma, we examined expression of MPS1 mRNA in a cohort of
120 recently described medulloblastoma samples (5). MPS1 mRNA was significantly elevated in
medulloblastoma compared to adult and fetal cerebellum (Fig. 1B). However, MPS1 expression was not
significantly different in any of the medulloblastoma genomic
subgroups (Fig. 1B). Similarly,
MPS1 protein expression was elevated in the tumor samples from
groups 3 and 4 patients compared to normal cerebellum (Fig. 1C). All group 3 and Shh
medulloblastoma cell lines commonly used also demonstrated
significantly elevated MPS1 protein expression (Fig. 1D). These data suggest that MPS1 may
be associated with the oncogenic process in general and is not
specific to a particular molecular subgroup of medulloblastoma.
Inhibition of MPS1 suppresses
medulloblastoma cell growth
We hypothesized that overexpression of MPS1 drives
medulloblastoma cell growth and that inhibition of MPS1 would
repress medulloblastoma cell growth. To test this hypothesis we
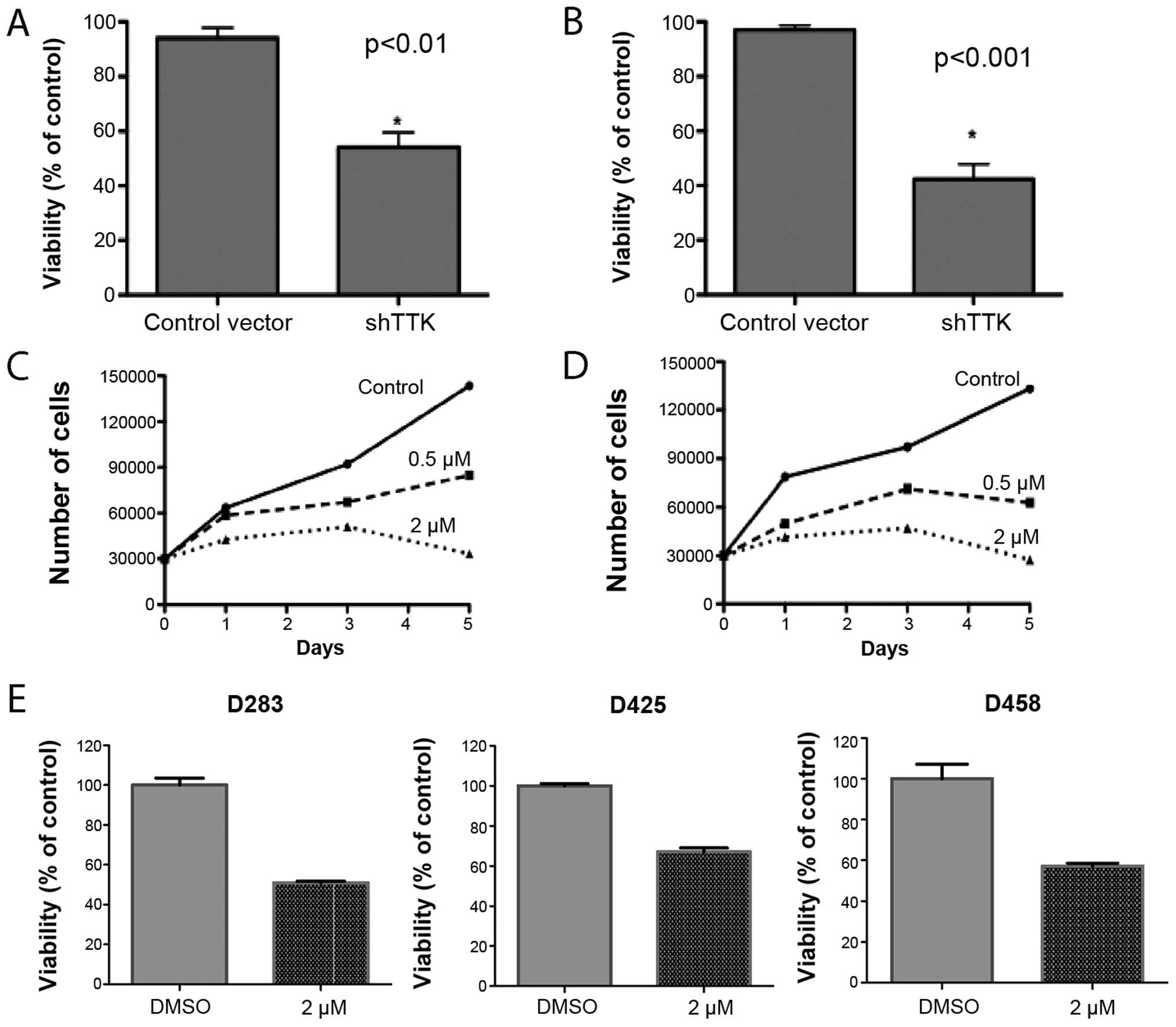
first suppressed MPS1 mRNA in 2-well characterized cell lines using
shRNA. In both Daoy and ONS-76 cell lines transfection of shTTK1
(shMPS1) significantly decreased the number of viable cells after
72 h as measured by the MTS assay (Fig.
2A and B). Knockdown of MPS1 was verified by qRT-PCR (43%
decrease in MPS1 mRNA compared to sh-control, data not shown).
To further evaluate the impact of MPS1 inhibition we
obtained a recently described MPS1 inhibitor NMS-P175 (kind gift
from Nervino Medical Sciences). NMS-P715 is an orally bioavailable,
potent and selective small-molecule inhibitor of MPS1 kinase
activity (14).
NMS-P175 potently inhibited the growth of both Daoy
and ONS-76 cells in a dose- and time-dependent manner (Fig. 2C and D). Since both Daoy and ONS-76
cell lines belong to the Shh genomic group we chose to test the
impact of chemical inhibition of MPS1 in additional cell lines. We
chose 3 cell lines with Myc translocations that are known to be
part of the group 3 medulloblastoma genomic signature. The growth
of all 3 Myc cell lines (D283, D425 and D458) was also potently
suppressed by MPS1 inhibition using NMS-P175 (Fig. 2E).
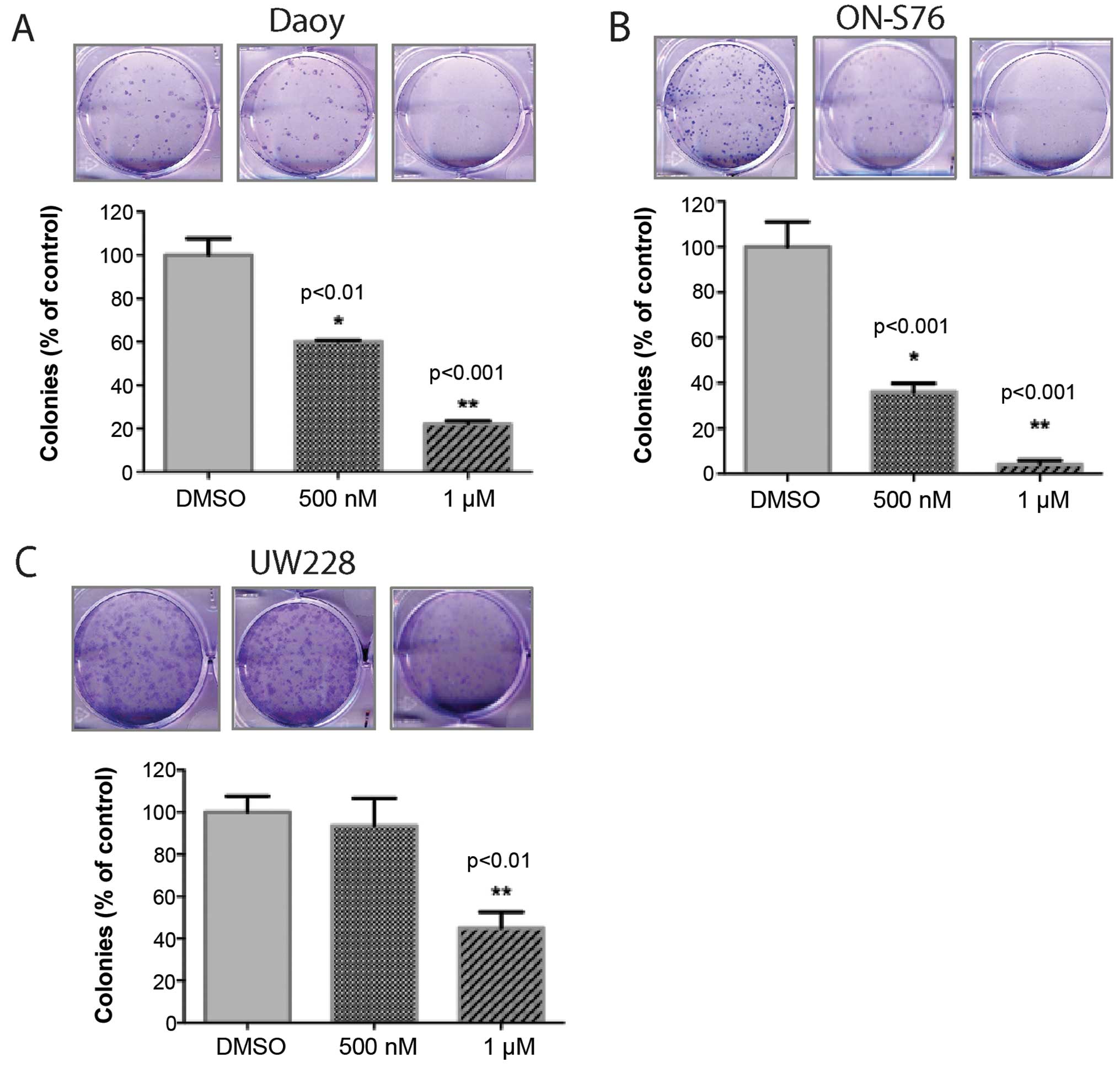
To evaluate a longer-term impact, we then performed
colony formation assays on cells treated with varying
concentrations of NMS-P715. We used lower concentrations of
NMS-P715 due to the longer-term treatment. Once again, inhibition
of MPS1 significantly decreased medulloblastoma cell growth as
measured by their ability to form colonies (Fig. 3). Notably, in the ONS-76 cells there
was a much more marked inhibition in the colony formation
capability (despite treatment with a lower dose) compared to
measuring just cell proliferation (92% inhibition vs. 46%
inhibition, respectively). These data may reflect the impact of
MPS1 on tumor cell self-renewal compared to just mitosis.
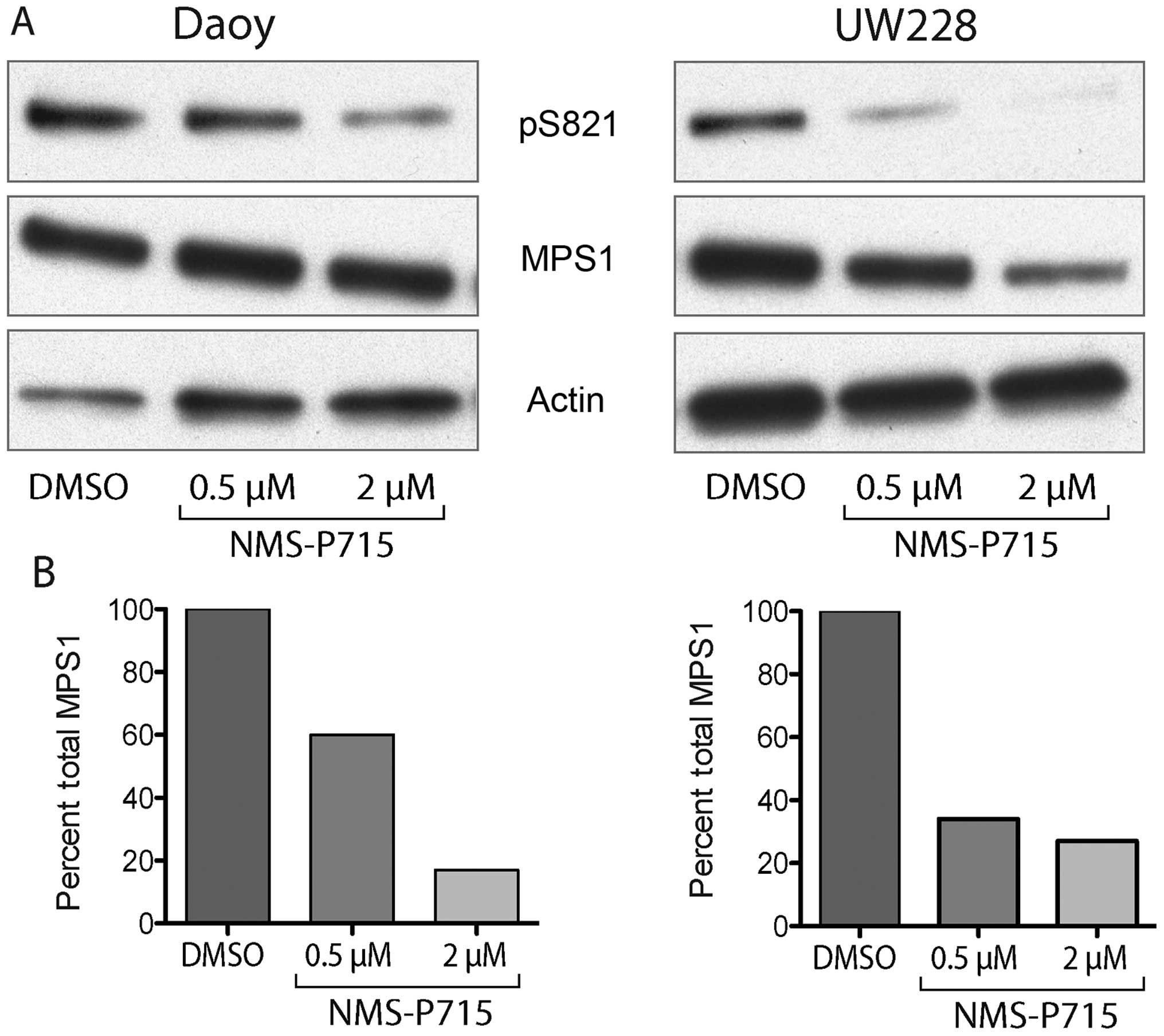
NMS-P715 suppresses kinase activity of
MPS1 and decreases MPS1 autophosphorylation
We further examined the functional activity of MPS1
inhibition in medulloblastoma cells. Recent evidence suggests that
MPS1 phosphorylates a wide range of proteins including itself
(16). To verify that inhibition of
MPS1 by NMS-P715 inhibited its kinase activity, we evaluated MPS1
autophosphorylation using protein immunoblotting. In both Daoy and
UW228 medulloblastoma cells NMS P715 strongly decreased the
phosphorylation of serine 821 a key autophosphorylation site on
MPS1 (Fig. 4A) (16). The suppression of phosphorylation
was dose-dependent for both cell lines (Fig. 4B). Total MPS1 did not change in the
Daoy cells, but there was a decrease in total MPS1 protein in the
UW228 cells suggesting that pS821 on MPS1 may be important for
protein stability.
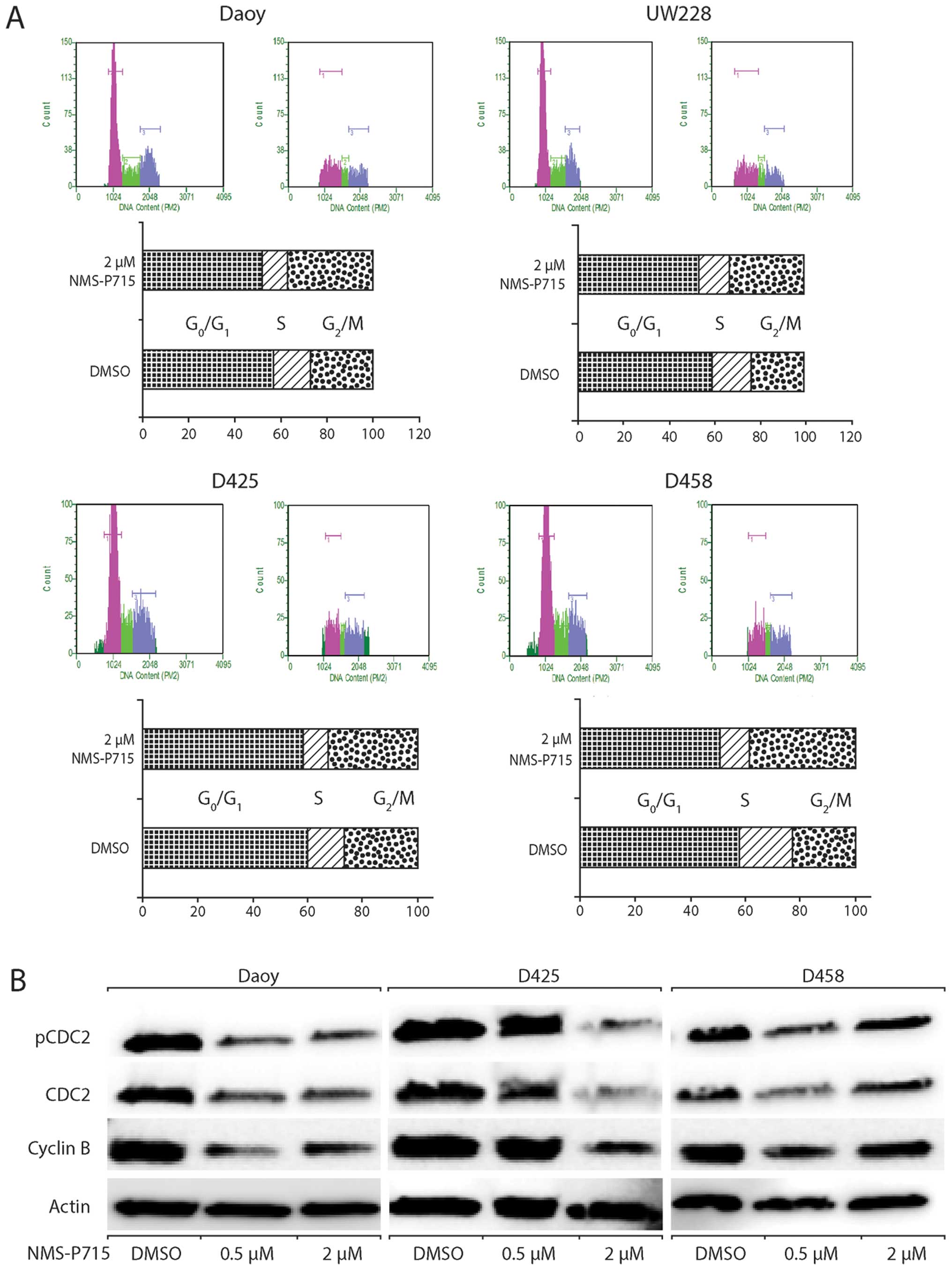
MPS1 kinase inhibitor NMS-P715 decreases
medulloblastoma cell growth by perturbing the cell cycle and
increasing apoptosis
To determine whether the decreased cell growth upon
MPS1 inhibition was due to cell cycle effects, we examined the
impact of NMS-P715 on the cell cycle distribution of
medulloblastoma cells. In all cells tested, NMS-P715 induced a G2-M
arrest as shown in Fig. 5A. These
data are consistent with previous studies showing that MPS1 is a
critical regulator of mitosis. Moreover, western blot analysis
showed that MPS1 also decreased expression of G2-M-associated
proteins CDC2 and cyclin B1 (Fig.
5B).
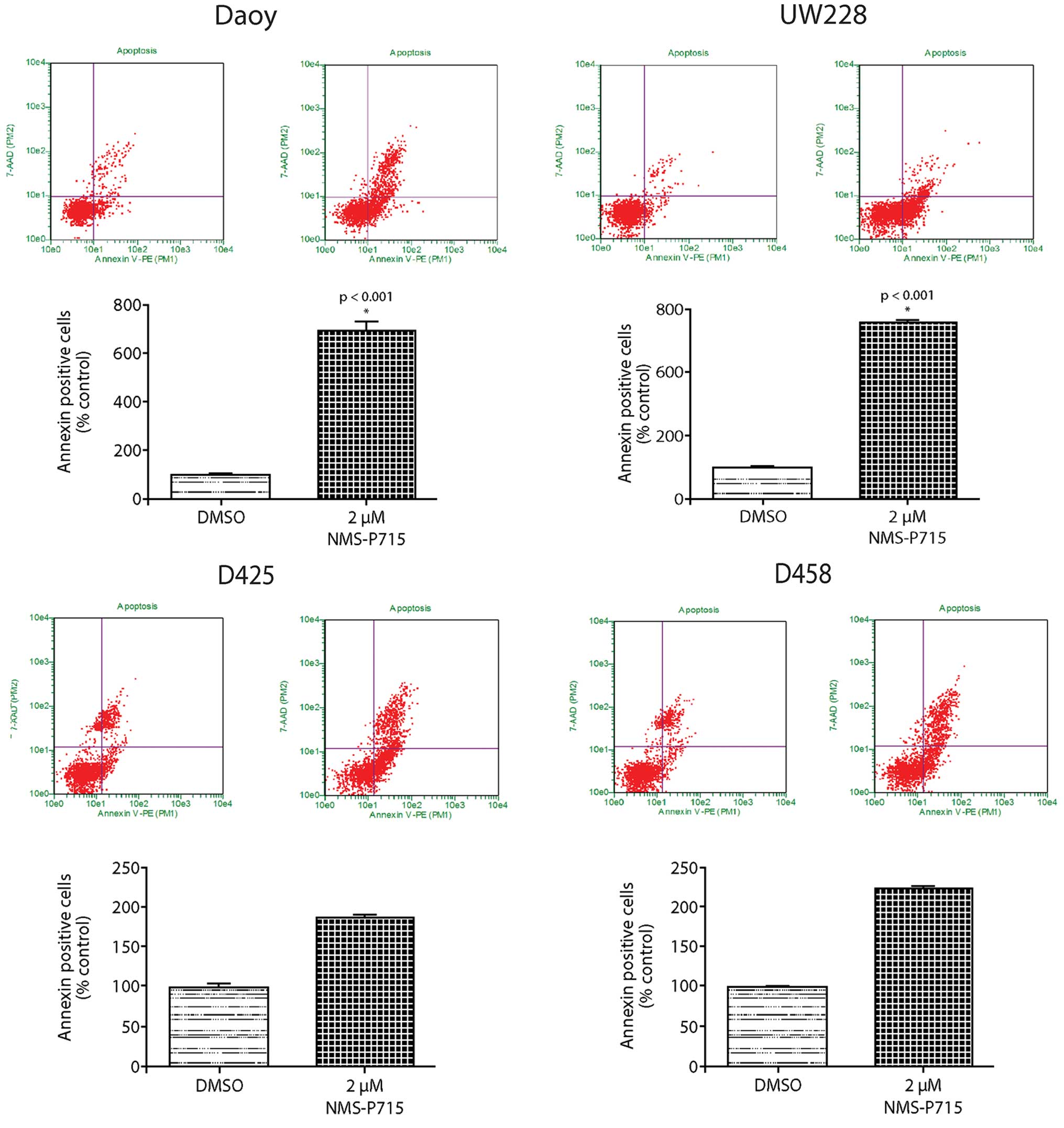
We next examined whether apoptosis was a contributor
to medulloblastoma cell growth inhibition by NMS-P715. We measured
Annexin V expression on the surface of NMS-P715-treated
medulloblastoma cells by flow cytometry. Representative plots are
shown for Daoy, UW228, D425 and D458 cells. Annexin
V-positive-7-aminoactinomycin D (7-AAD)-negative cells, indicative
of early apoptosis, were present at low levels in DMSO
control-treated cells. This population increased with increasing
doses of NMS-P715 (Fig. 6). In
addition, the Annexin V-positive-7-AAD-positive population was
significantly enhanced in the NMS-P715-treated cells, indicating
increased late apoptosis. The total percentage of apoptosis is
quantified in Fig. 6. These data
confirm that MPS1 inhibition by NMS-P715 is a strong inducer of
apoptosis in medulloblastoma.
Discussion
Therapy-associated side-effects in medulloblastoma
have led to a concentrated search for novel biologically based
therapeutic targets (17). Given
that cell cycle kinases are key regulators of tumor progression we
hypothesized that these kinases are critical for medulloblastoma
tumorigenesis (18). Analysis of
protein kinase gene expression revealed that expression of multiple
protein kinases that are components of the mitotic machinery such
as Aurora kinase A, PLK1, WEE1 and now MPS1 are significantly
deregulated in medulloblastoma (9,19,20).
Perturbing mitosis by disrupting the proper formation of mitotic
spindles required for chromosome alignment and segregation has been
shown to preferentially kill cancer cells (13).
MPS1 is a serine threonine kinase that regulates the
mitotic spindle by triggering the spindle assembly checkpoint (SAC)
(10). MPS1 plays an important role
in safeguarding proper chromosome alignment and segregation during
mitosis (14). Increased MPS1
levels have been found in numerous adult types of cancers,
including breast, pancreatic and colorectal cancer. However, the
significance of MPS1 in the pathogenesis and management of
medulloblastoma is not well understood.
In the present study, we demonstrated that MPS1 mRNA
is overexpressed in two independent medulloblastoma cohorts when
compared to normal cerebellum. Decreasing the expression of MPS1
mRNA by RNAi clearly resulted in growth suppression. Furthermore,
we showed that inhibition of MPS11 by a small-molecule inhibitor,
NMS-P715, resulted in a significant reduction in the proliferation
of medulloblastoma cells both in short-term and long-term assays.
Importantly, we showed that induction of apoptosis is a key
mechanism of NMS-P715 in medulloblastoma cells.
Notably, a recent study by Maachani et al
demonstrated that MPS1 is a promising molecular target in the
treatment of another brain tumor, glioblastoma multiforme (GBM)
(21). They showed that MPS1
inhibition can be combined with radiation to make GBM therapy more
efficacious (21). In addition,
several newer MPS1 inhibitors have been recently described
(10,22). Thus, our data along with previous
studies, strongly suggest that targeting MPS1 with small-molecule
inhibitors is both a novel strategy in the treatment of
medulloblastoma and one that warrants further study. In particular,
evaluation of resistance to MPS1 inhibitors will have to be closely
examined in medulloblastoma cells (23). Recent data suggest that cancer cells
acquire resistance by developing point mutations in the ATP binding
pocket but that the tumor cells do not develop cross resistance to
other MPS1 inhibitors (23). This
leaves open the possibility of using multiple MPS1 inhibitors in
combinatorial and or sequential fashion. The next important step
may be to test the effects of NMS-P715 in orthotopic xenograft
models of medulloblastoma. It may be important to determine whether
NMS-P715 offers a superior therapeutic index over current
treatments, and if it can be combined with other standard
treatments to improve medulloblastoma therapy.
Acknowledgments
The present study was supported by the Childhood
Brain Tumor Foundation (R.V.), Department of Pediatrics, the
University of Colorado School of Medicine, NIH-NINDS grants
K08NS059790 and RO1NS086956 (R.V.), the Childhood Brain Tumor
Foundation (R.V.), and the Morgan Adams Foundation grants (R.V. and
N.K.F.). We thank Nervino Medical Sciences for the gift of the
NMS-P715. We thank R. Colombo for helpful discussions regarding
NMS-P715. We thank P.A. Eyeres for the kind gift of the
phospho-MPS1 antibodies.
References
|
1
|
Gopalakrishnan V, Tao RH, Dobson T,
Brugmann W and Khatua S: Medulloblastoma development: Tumor biology
informs treatment decisions. CNS Oncol. 4:79–89. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fan X, Matsui W, Khaki L, Stearns D, Chun
J, Li YM and Eberhart CG: Notch pathway inhibition depletes
stem-like cells and blocks engraftment in embryonal brain tumors.
Cancer Res. 66:7445–7452. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wolter M, Reifenberger J, Sommer C,
Ruzicka T and Reifenberger G: Mutations in the human homologue of
the Drosophila segment polarity gene patched (PTCH) in sporadic
basal cell carcinomas of the skin and primitive neuroectodermal
tumors of the central nervous system. Cancer Res. 57:2581–2585.
1997.PubMed/NCBI
|
|
4
|
Wodarz A and Nusse R: Mechanisms of Wnt
signaling in development. Annu Rev Cell Dev Biol. 14:59–88. 1998.
View Article : Google Scholar
|
|
5
|
Northcott PA, Korshunov A, Witt H,
Hielscher T, Eberhart CG, Mack S, Bouffet E, Clifford SC, Hawkins
CE, French P, et al: Medulloblastoma comprises four distinct
molecular variants. J Clin Oncol. 29:1408–1414. 2011. View Article : Google Scholar
|
|
6
|
Cho YJ, Tsherniak A, Tamayo P, Santagata
S, Ligon A, Greulich H, Berhoukim R, Amani V, Goumnerova L,
Eberhart CG, et al: Integrative genomic analysis of medulloblastoma
identifies a molecular subgroup that drives poor clinical outcome.
J Clin Oncol. 29:1424–1430. 2011. View Article : Google Scholar :
|
|
7
|
Taylor MD, Northcott PA, Korshunov A,
Remke M, Cho YJ, Clifford SC, Eberhart CG, Parsons DW, Rutkowski S,
Gajjar A, et al: Molecular subgroups of medulloblastoma: The
current consensus. Acta Neuropathol. 123:465–472. 2012. View Article : Google Scholar :
|
|
8
|
Leary SE and Olson JM: The molecular
classification of medul-loblastoma: Driving the next generation
clinical trials. Curr Opin Pediatr. 24:33–39. 2012. View Article : Google Scholar :
|
|
9
|
Harris PS, Venkataraman S, Alimova I,
Birks DK, Balakrishnan I, Cristiano B, Donson AM, Dubuc AM, Taylor
MD, Foreman NK, et al: Integrated genomic analysis identifies the
mitotic checkpoint kinase WEE1 as a novel therapeutic target in
medulloblastoma. Mol Cancer. 13:722014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu X and Winey M: The MPS1 family of
protein kinases. Annu Rev Biochem. 81:561–585. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ling Y, Zhang X, Bai Y, Li P, Wei C, Song
T, Zheng Z, Guan K, Zhang Y, Zhang B, et al: Overexpression of Mps1
in colon cancer cells attenuates the spindle assembly checkpoint
and increases aneuploidy. Biochem Biophys Res Commun.
450:1690–1695. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Daniel J, Coulter J, Woo JH, Wilsbach K
and Gabrielson E: High levels of the Mps1 checkpoint protein are
protective of aneuploidy in breast cancer cells. Proc Natl Acad Sci
USA. 108:5384–5389. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Janssen A, Kops GJ and Medema RH:
Targeting the mitotic checkpoint to kill tumor cells. Horm Cancer.
2:113–116. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Colombo R, Caldarelli M, Mennecozzi M,
Giorgini ML, Sola F, Cappella P, Perrera C, Depaolini SR, Rusconi
L, Cucchi U, et al: Targeting the mitotic checkpoint for cancer
therapy with NMS-P715, an inhibitor of MPS1 kinase. Cancer Res.
70:10255–10264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Venkataraman S, Alimova I, Balakrishnan I,
Harris P, Birks DK, Griesinger A, Amani V, Cristiano B, Remke M,
Taylor MD, et al: Inhibition of BRD4 attenuates tumor cell
self-renewal and suppresses stem cell signaling in MYC driven
medulloblastoma. Oncotarget. 5:2355–2371. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tyler RK, Chu ML, Johnson H, McKenzie EA,
Gaskell SJ and Eyers PA: Phosphoregulation of human Mps1 kinase.
Biochem J. 417:173–181. 2009. View Article : Google Scholar
|
|
17
|
Samkari A, White JC and Packer RJ:
Medulloblastoma: Toward biologically based management. Semin
Pediatr Neurol. 22:6–13. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Malumbres M and Barbacid M: Cell cycle
kinases in cancer. Curr Opin Genet Dev. 17:60–65. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
El-Sheikh A, Fan R, Birks D, Donson A,
Foreman NK and Vibhakar R: Inhibition of Aurora Kinase A enhances
chemosen-sitivity of medulloblastoma cell lines. Pediatr Blood
Cancer. 55:35–41. 2010.PubMed/NCBI
|
|
20
|
Harris PS, Venkataraman S, Alimova I,
Birks DK, Donson AM, Knipstein J, Dubuc A, Taylor MD, Handler MH,
Foreman NK, et al: Polo-like kinase 1 (PLK1) inhibition suppresses
cell growth and enhances radiation sensitivity in medulloblastoma
cells. BMC Cancer. 12:802012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maachani UB, Kramp T, Hanson R, Zhao S,
Celiku O, Shankavaram U, Colombo R, Caplen NJ, Camphausen K and
Tandle A: Targeting MPS1 enhances radiosensitization of human
glioblastoma by modulating DNA repair proteins. Mol Cancer Res.
13:852–862. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wengner AM, Siemeister G, Koppitz M,
Schulze V, Kosemund D, Klar U, Stoeckigt D, Neuhaus R, Lienau P,
Bader B, et al: Novel Mps1 kinase inhibitors with potent antitumor
activity. Mol Cancer Ther. 15:583–592. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koch A, Maia A, Janssen A and Medema RH:
Molecular basis underlying resistance to Mps1/TTK inhibitors.
Oncogene. 35:2518–2528. 2016. View Article : Google Scholar :
|