Introduction
Glioblastoma is the most common malignant tumor in
the brain and central nervous system (CNS) with only a 5% five-year
survival rate, and glioblastoma accounts for the majority of
gliomas in the USA (1). In Korea,
glioblastoma is the most common neuroepithelial tumor accounting
for 15.1% of all primary brain and CNS tumors and 34.4% of all
gliomas (2). Glioblastoma is one of
the most difficult cancers to treat, because of the highly invasive
nature and the recurrence at new sites of migration (3). In light of the overall poor prognosis
of glioblastoma, understanding glioblastoma invasion is crucial for
the development of efficient glioblastoma treatment.
Epithelial-mesenchymal transition (EMT) is a
necessary event in the invasion and metastasis of various types of
human cancers including glioblastoma. EMT is a drastic alteration
of epithelial cells involving a shedding of their characteristic
morphology and gene expression patterns and the assumption of
mesenchymal characteristics including motility which occurs during
embryogenesis, wound healing, and cancer progression (4–7). Many
cellular changes such as loss of cell junctions and polarity, and
reorganization of the cytoskeleton are associated with EMT,
resulting in a cellular transformation in terms of morphology,
invasiveness and motility (6).
Rho GTPases belong to the Ras superfamily. RhoA,
Rac1 and Cdc42 are critical effectors of actin cytoskeleton
reorganization, cellular contraction and migration during the EMT,
although the precise molecular mechanisms remain to be elucidated
(8,9). According to previous studies, RhoA,
Rac1 and Cdc42 are highly expressed or activated in glioblastoma
and promote the invasive behavior of glioma cells (10–16). A
most common characteristic of glioblastoma is the overexpression of
receptors such as MET and/or EGFR that activate the downstream
signaling pathways, PI3K, MAPK and the Rho family of GTPases
(17–19). Activated signaling pathways
including Rho-GTPases facilitate cancer cell migration and invasion
thus inducing metastasis (20,21).
Although astrocyte elevated gene-1 (AEG-1) was first
discovered as an HIV-induced gene in primary human fetal
astrocytes, studies in the past decade have indicated that AEG-1
plays crucial roles in tumor progression such as transformation,
survival, invasion, metastasis, angiogenesis and drug resistance in
various types of human cancers (22–28).
Furthermore, recent reports have revealed that AEG-1 participates
in the regulation of the EMT process under diverse cellular
conditions (29–35). However, its role in the regulation
of EMT in glioblastoma has not been uncovered yet. In the present
study, we identified AEG-1 as a mesenchymal transition inducer to
mediate invasion and migration of human glioblastoma cells through
increasing actin stress fiber formation by activating Rho GTPase
signals.
Materials and methods
Cell cultures
Human glioma cell lines U87MG and U373MG (Korean
Cell Line Bank (KCLB), Seoul, Korea), and an immortalized primary
human fetal astrocyte cell line IM-PHFA were previously described
(36,37). The Lenti-X 293T cell line for
lentivirus packaging was purchased from Clontech (Mountain View,
CA, USA). All cells were cultured in Dulbecco's modified Eagle's
medium (DMEM) supplemented with 10% heat-inactivated fetal bovine
serum (FBS) and 100 U/ml of antibiotic-antimycotic (both from
Lonza, Walkersville, MD, USA) at 37°C in a humidified incubator
with 5% CO2, and the viability of the cultured cells was
monitored by LUNA-FL Automated Cell Counter (Logos Biosystems,
Gyeonggi-do, Korea).
Reagents
4′,6-Diamidino-2-phenylindole (DAPI) and
rhodamine-phalloidin were purchased from Invitrogen Life
Technologies (Carlsbad, CA, USA). G418 and puromycin were purchased
from Biomax (Seoul, Korea). Polybrene was purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). Y-27632 was purchased from
Sigma-Aldrich. The antibodies used were purchased as following:
TCF8/ZEB1, Slug, E-cadherin, N-cadherin, ZO-1, RhoA, Cdc42,
Rac1/2/3, phospho-Rac1/Cdc42, and active Rho detection kit from
Cell Signaling Technology (Danvers, MA, USA), and β-actin from
Sigma-Aldrich (St. Louis, MO, USA). Horseradish
peroxidase-conjugated anti-mouse IgG and anti-rabbit IgG were
purchased from Jackson ImmunoResearch Laboratories, Inc. (West
Grove, PA, USA).
Lentivirus production and infection
Lentiviral vectors expressing AEG-1 shRNA were
purchased from Sigma-Aldrich (SHCLNG-NM_178812). Control viral
vector pLKO.1 GFP shRNA plasmid (Addgene; plasmid #30323) was a
gift from Dr David Sabatini (Massachusetts Institute of Technology,
Cambridge, MA, USA), and the envelope vector pMD2.G (plasmid
#12259) and packaging plasmid psPAX2 (plasmid #12260; both from
Addgene) were kindly provided by Dr Didier Trono (Ecole
Polytechnique Federale de Lausanne, Lausanne, Switzerland). The
lentiviral vector expressing AEG-1 was purchased from OriGene
(RC207238L1). Each lentivirus expressing AEG-1 (lenti-AEG-1), AEG-1
shRNA (lenti-AEG-1sh) or GFP shRNA (lenti-GFPsh) was produced by
co-transfection with each expression plasmid, pMD2.G and psPAX2
into Lenti-X 293T cells. Transfections were carried out using
Lipofector-2000 (AptaBio, Gyeonggi-do, Korea) according to the
manufacturer's instructions. Media were harvested at 48, 72 and 96
h post-transfection and the media were centrifuged at 1,500 rpm for
5 min and filtered using a 0.45 µm syringe filter for
removing inadvertently collected cells. Every infection was carried
out in the presence of 8 µg/ml of Polybrene. After
transduction, cells were selected with 2 µg/ml
puromycin.
Western blot analysis and active Rho
pull-down assays
Whole cell lysates were prepared, and western blot
analysis was performed as previously described (38). Active Rho detection was performed
with an active Rho detection kit (Cell Signaling Technology; #8820)
according to the manufacturer's protocol. Seven hundred micrograms
of total proteins were used to pull down active Rho with GST-RBD of
Rhotekin. Primary antibodies for TCF8/ZEB1 (1:1,000), Slug
(1:1,000), ZO-1 (1:1,000), N-cadherin (1:1,000), E-cadherin
(1:1,000), RhoA (1:1,000), Rac1/2/3 (1:1,000), Cdc42 (1:1,000),
phospho-Rac1/Cdc42 (1:1,000), active-Rho (1:1,000) and β-actin
(1:10,000) were used for immunoblotting followed by horseradish
peroxidase-conjugated anti-mouse IgG or anti-rabbit IgG (1:5,000)
for 1 h, and visualized using the enhanced chemiluminescence
detection system.
Phalloidin staining
Cells were plated in 8-well chamber slides and
incubated for 24 h in complete medium. Then the cells were fixed
with 4% paraformaldehyde in PBS for 30 min and the slides were
incubated with 0.1% Triton X-100 in PBS for 5 min for
permeabilization and blocked with 1% BSA in PBS for 20 min. Then
the cells were incubated with rhodamine-phalloidin for 20 min and
DAPI for 1 min at room temperature. Images were taken with a
FluoView FV1000 confocal microscope (Olympus) and iRiS™ Digital
Cell Imaging System (Logos Biosystems).
Invasion assays
In vitro invasion assays were performed using
48-well Boyden chambers (Nuero Probe, Inc., Gaithersburg, MD, USA).
A polycarbonate membrane with 8-µm pore size (Nuero Probe)
was coated with Matrigel and dried overnight. The Boyden chambers
were filled with medium containing 10% FBS in the lower
compartment, and the coated membrane was mounted in the chamber.
Fifty microliters of U87MG cells in serum-free media were placed in
the upper chamber of the apparatus and allowed to settle onto the
Matrigel-coated membrane. Boyden chambers were incubated at 37°C in
a CO2 incubator for 16 h. After incubation, the
membranes were removed, fixed, and stained with Diff-Quick staining
kit. Non-motile cells were removed with cotton swabs from the upper
surface of the membrane. Motile cells on the bottom face of the
membrane were photographed and the stained cells were counted in
three randomly chosen fields using a microscope.
Wound healing assays
U373MG cells were seeded into 6-well plates and
incubated for 24 h until reaching 80–90% confluency. A
200-µl pipette tip was used to make scratches in each well.
Several regions were marked and photographed at 0 and 24 h after
the scratches were made. Phase-contrast microscopy images were
taken using Zeiss Axio. Cell motility was calculated as the area
covered by the cells between the edges at the time of measurement.
Migration rate = (mean area occupied by cells/mean original area) ×
100. Each test group was assayed in triplicate at least.
Statistical analysis
Data are presented as the mean ± standard error of
the mean (SEM) and were analyzed for statistical significance using
the unpaired Student's t-test. P<0.05 was considered
statistically significant.
Results
AEG-1 induces mesenchymal markers in
human glioblastoma cells
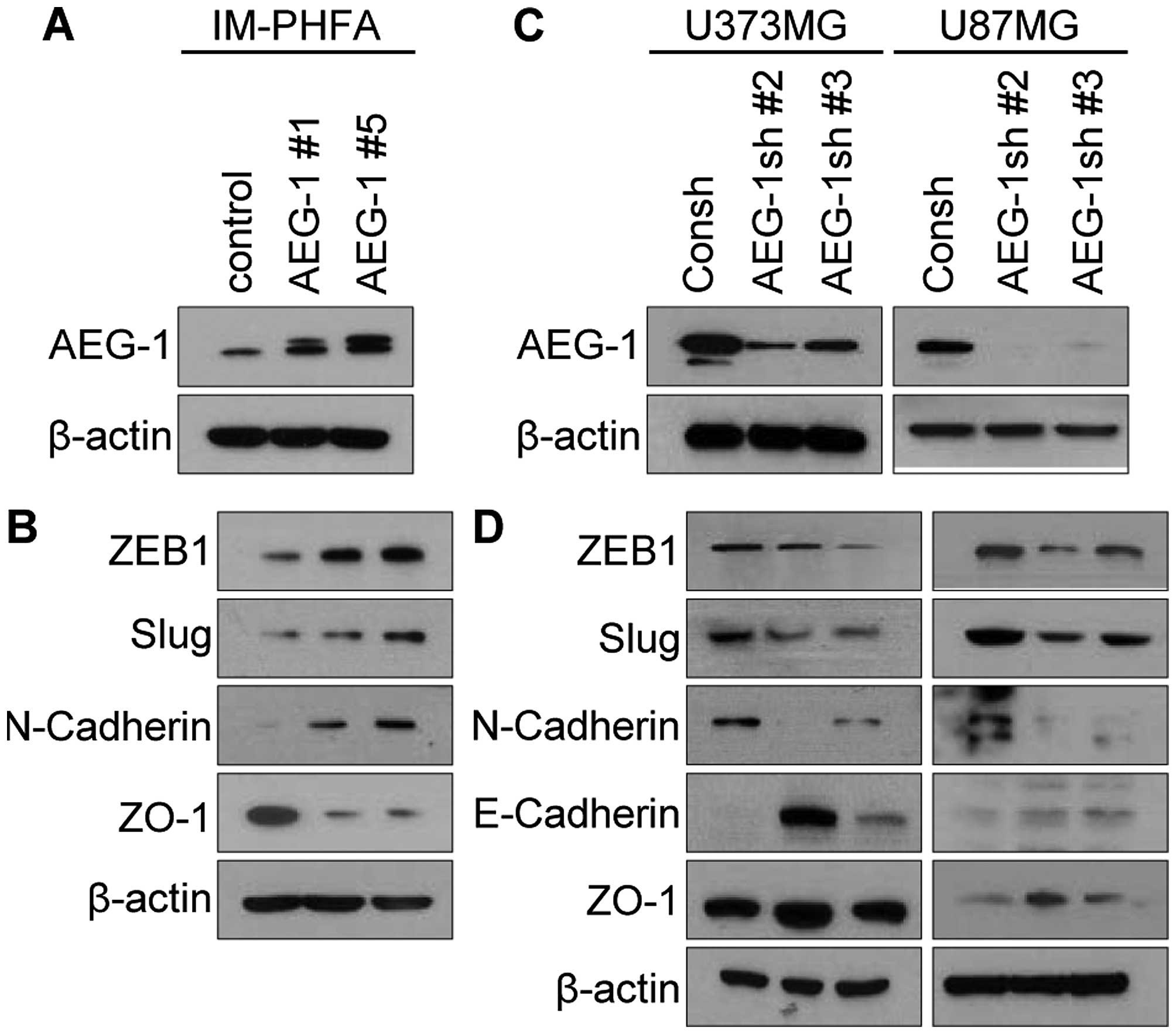
To examine whether AEG-1 promotes mesenchymal
transition of glioblastoma cells, we modulated AEG-1 expression in
normal astrocytes (IM-PHFA), and glioblastoma cells (U373MG and
U87MG) (Fig. 1A and C).
Overexpression of AEG-1 in IM-PHFA cells intensively induced
expression levels of the mesenchymal markers ZEB1, Slug and
N-cadherin, but reduced expression of the epithelial marker ZO-1
(Fig. 1B). Consistently knockdown
of AEG-1 in human glioblastoma cells decreased expression of
mesenchymal markers ZEB1, Slug and N-cadherin, but increased
expression of epithelial markers E-cadherin and ZO-1 (Fig. 1D). These results indicate that AEG-1
may promote mesenchymal transition of human glioblastoma cells.
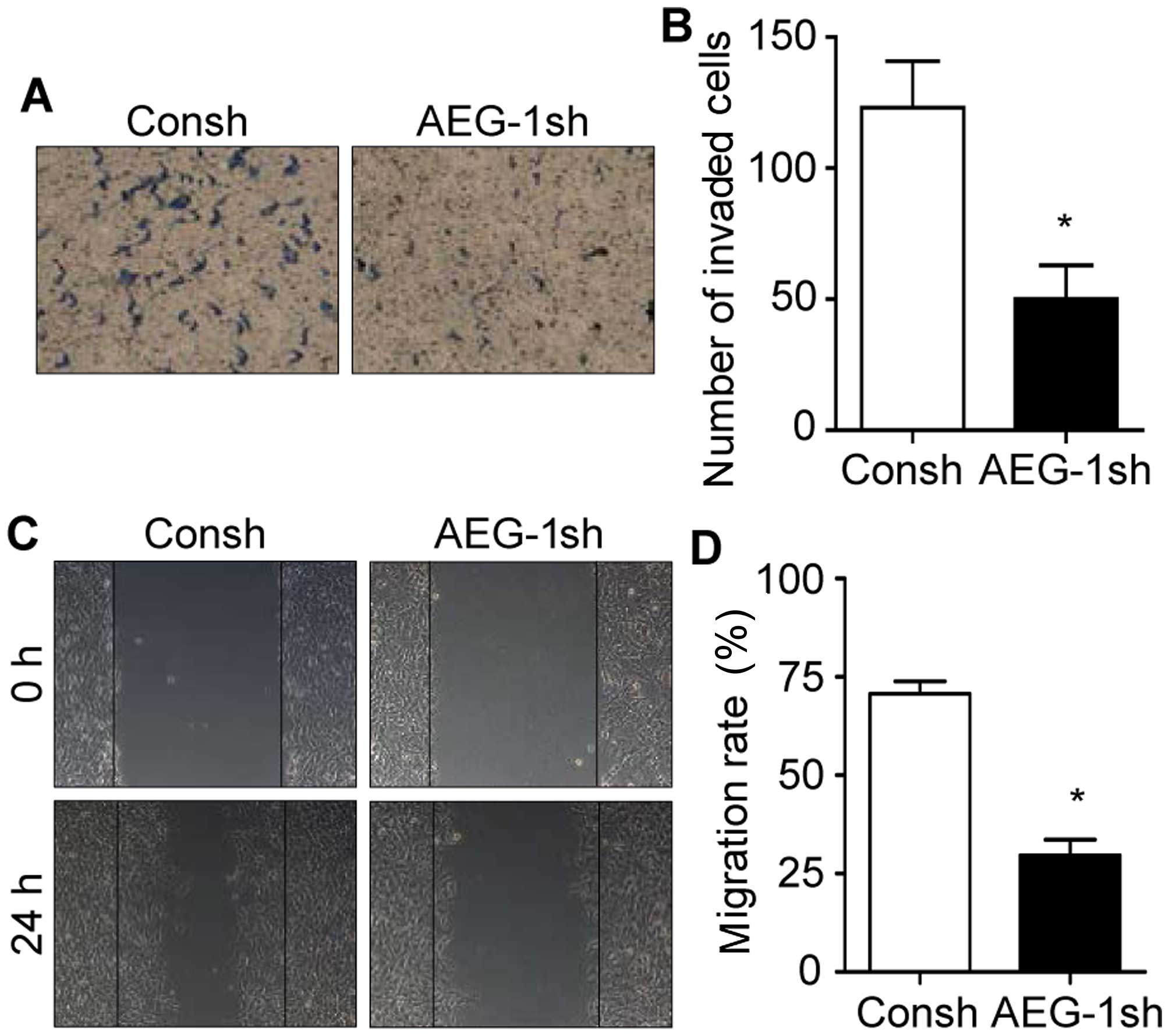
Knockdown of AEG-1 suppresses invasion
and migration of human glioblastoma cells
Since mesenchymal transition of glioblastoma is
involved in the invasion and migration of cancer cells, we then
aimed to ascertain whether AEG-1 affects invasive ability and
motility of human glioblastoma cells. As shown in Fig. 2A and B, knockdown of AEG-1 in
glioblastoma cells decreased more than 58% of invaded cells
compared with the control. In addition, wound healing assays showed
that knockdown of AEG-1 significantly inhibited the migration of
glioblastoma cells (Fig. 2C and D).
Collectively, these results suggest that AEG-1 may play an
important role in the invasion and migration of glioblastoma cells
through the induction of mesenchymal transition.
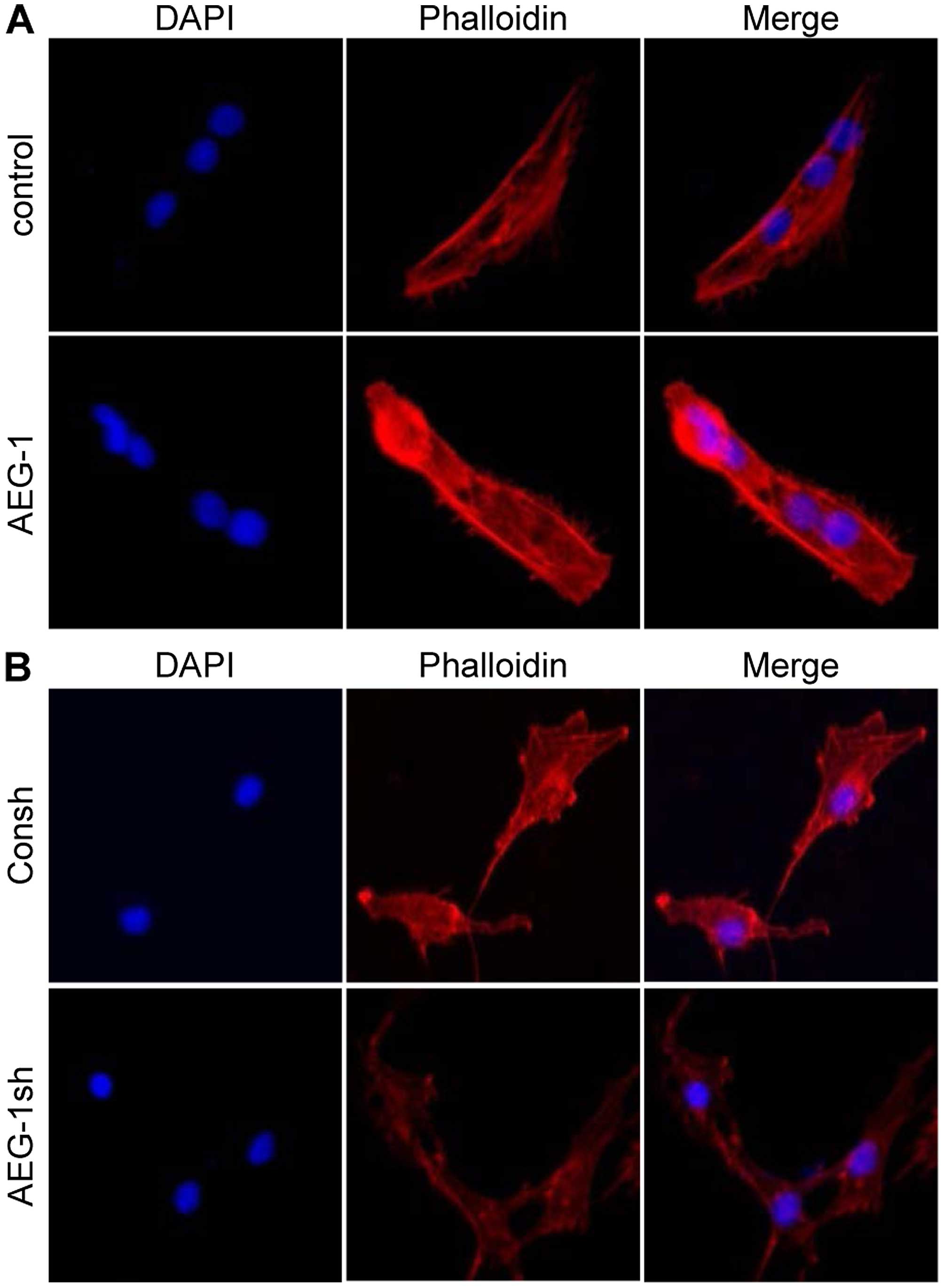
AEG-1 induces formation of stress fibers
in human glioblastoma cells
Actin stress fibers that induce cytoskeleton
remodeling are associated with cellular contractility providing
force for increased cell movement during mesenchymal transition
(39). Thus, we examined whether
AEG-1 modulates stress fiber formation. Overexpression of AEG-1 in
IM-PHFA cells significantly increased actin stress fibers (Fig. 3A). In accordance, knockdown of AEG1
in glioblastoma cells dramatically decreased stress fibers
(Fig. 3B). These results indicate
that AEG-1 increases actin stress fiber formation to induce changes
in actin cytoskeleton structures and the motility of human
glioblastoma cells.
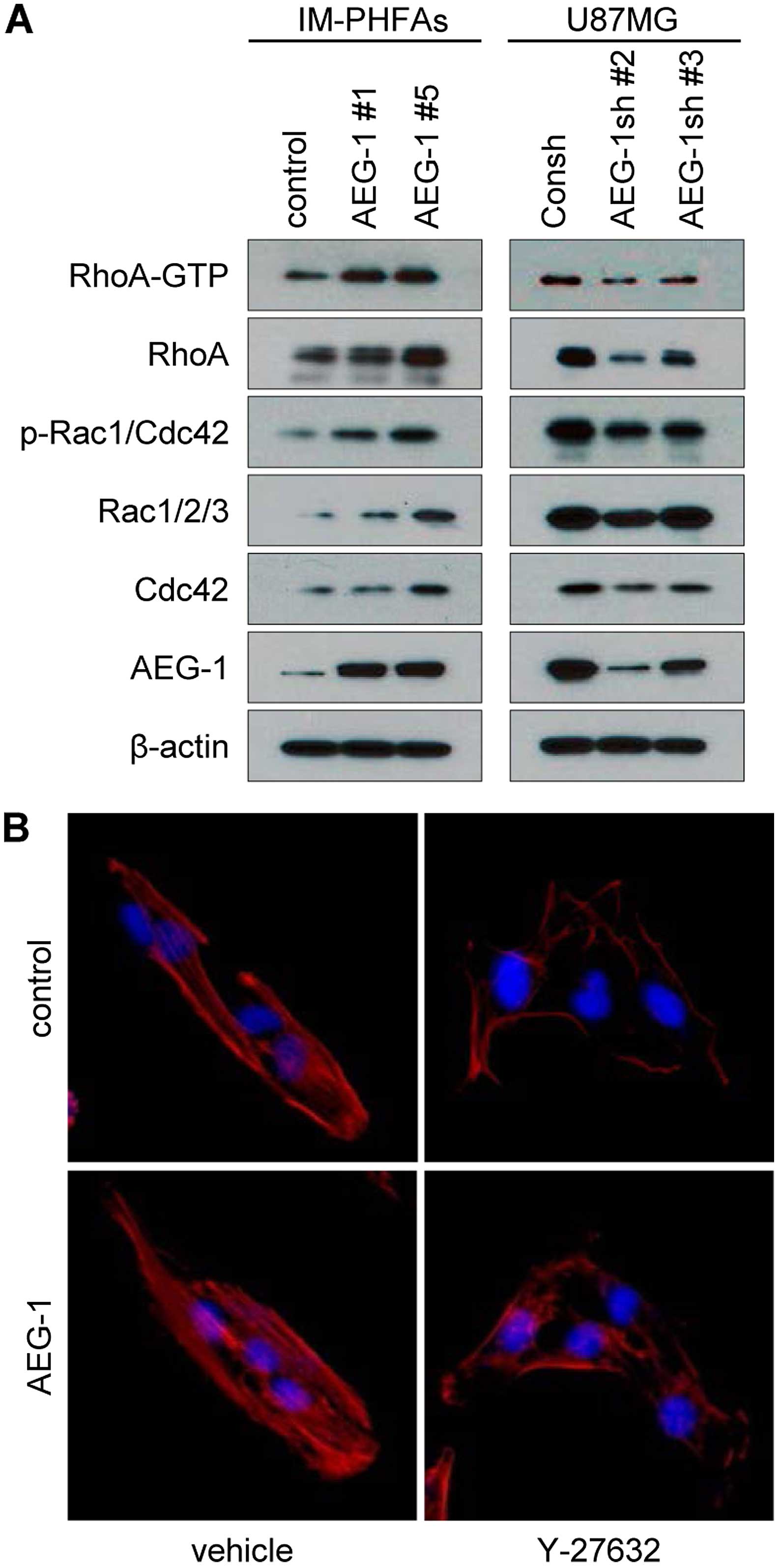
AEG-1 activates Rho family GTPases in
human glioblastoma cells
During mesenchymal transition, cells require dynamic
actin rearrangement and cytoskeleton remodeling via regulation of
Rho family GTPases (39).
Overexpression of AEG-1 in IM-PHFA cells intensively increased the
active form of RhoA (RhoA-GTP) and phosphorylation of Rac1 and
Cdc42, and also increased the protein levels of the Rho family
GTPases (Fig. 4A). Knockdown of
AEG-1 in glioblastoma cells significantly reduced expression of the
Rho family proteins as well as their activation (Fig. 4A). Rho-associated,
coiled-coil-containing protein kinase 1 (ROCK1) is a major
downstream protein of RhoA, which promotes F-actin stabilization
and induction of Rac signaling (39). To confirm whether AEG-1-induced RhoA
activation is critical for mesenchymal transition, we used Y-27632
a specific inhibitor of ROCK1. As shown in Fig. 4B, treatment with Y-27632 attenuated
AEG-1-induced stress fibers. Collectively these results revealed
that AEG-1 activates Rho signals to induce stress fiber formation
and mesenchymal transition.
Discussion
Glioblastoma is commonly classified as a malignant
form of glioma with highly invasive behavior and a poor survival
rate and arises from astrocytes. The invasive ability of
glioblastoma into the brain parenchyma is the major obstacle to
treatment, because tumor cells can evade surgical resection and
radiation therapy (3). Although
diffuse invasion of malignant glioma is one of its most adverse
characteristics and thus has been vigorously investigated,
relatively few mechanisms involved in the induction of mesenchymal
transition and invasion of glioma cells have been uncovered.
Since AEG-1 was initially identified as an
HIV-1-inducible gene, evidence that AEG-1 is related with cancer
progression and thus functions as an oncogene has accumulated
(40–42). AEG-1 is elevated in various types of
human cancers including carcinomas of the liver, gallbladder,
kidney, breast, lung, prostate, ovary, esophagus, stomach and
colon, as well as glioma, neuroblastoma, melanoma, and osteosarcoma
(40,43). Increased AGE-1 in various types of
human cancers participates in diverse signaling pathways such as
the PI3K/AKT, NF-κB, Wnt/β-catenin and MAPK pathways leading to
proliferation, survival, drug resistance, EMT, invasion and
metastasis of cancer (44).
Particularly, AEG-1 plays crucial roles during EMT in
hepatocellular carcinoma, non-small cell lung cancer and cervical
cancer by activating diverse signals such as Wnt/β-catenin and
TGF-β (29–35). In the present study we revealed that
AEG-1 also participated in the induction of mesenchymal transition
and then invasion and motility of glioblastoma.
Dissociation of cell-cell junctions, loss of
polarity, reorganization of the cytoskeletal structure, acquisition
of a front-rear polarity, and increase in motility are key events
during EMT (45,46). Alteration of the cytoskeletal
architecture results in stress fiber formation that is one of the
common EMT markers (47). Induction
of actin dynamics and rearrangement require activation of the Rho
family of proteins (48). RhoA,
Rac1 and Cdc42 play crucial roles in the formation of actin stress
fiber, lamellipodia and filopodia, respectively (49–51).
As shown in Figs. 3 and 4, AEG-1 promotes actin stress fiber
formation by activating RhoA in glioblastoma cells. In addition,
AEG-1 induces activation of RhoA, Rac1 and Cdc42 as well as their
expression. These results indicate that AEG-1 regulates the actin
cytoskeleton structure by modulating the Rho family of proteins in
human glioblastoma cells.
Collectively, these results suggest that AEG-1
promotes mesenchymal transition in human glioblastoma cells through
increasing formation of actin stress fibers by activation and also
by the induction of the Rho family of proteins. This may be an
important mechanism by which AEG-1 induces diffuse invasion of
glioblastoma into the brain parenchyma. Therefore, further studies
to investigate the role of AEG-1 in the formation of lamellipodia
and filopodia in glioblastoma are warranted to reveal the more
detailed mechanisms of glioblastoma invasion and motility and then
to pave the way for developing novel therapeutic interventions and
ameliorating the suffering of glioblastoma patients from this most
aggressive and fatal disease.
Acknowledgments
This study was supported by research grants from the
National Research Foundation of Korea (grant nos. 2007-0054931,
NRF-2013R1A1A2007263 and NRF-2013R1A2A2A01069099) and from the
National R&D Program for Cancer Control of the Ministry of
Health and Welfare, Republic of Korea (grant no. 1320120).
References
|
1
|
Ostrom QT, Gittleman H, Liao P, Rouse C,
Chen Y, Dowling J, Wolinsky Y, Kruchko C and Barnholtz-Sloan J:
CBTRUS statistical report: Primary brain and central nervous system
tumors diagnosed in the United States in 2007–2011. Neuro-oncol.
16(Suppl 4): iv1–iv63. 2014. View Article : Google Scholar
|
|
2
|
Jung KW, Ha J, Lee SH, Won YJ and Yoo H:
An updated nationwide epidemiology of primary brain tumors in
Republic of Korea. Brain Tumor Res Treat. 1:16–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cuddapah VA, Robel S, Watkins S and
Sontheimer H: A neuro-centric perspective on glioma invasion. Nat
Rev Neurosci. 15:455–465. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chapman HA: Epithelial-mesenchymal
interactions in pulmonary fibrosis. Annu Rev Physiol. 73:413–435.
2011. View Article : Google Scholar
|
|
5
|
Huang RY, Guilford P and Thiery JP: Early
events in cell adhesion and polarity during epithelial-mesenchymal
transition. J Cell Sci. 125:4417–4422. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haudenschild DR, D'Lima DD and Lotz MK:
Dynamic compression of chondrocytes induces a Rho kinase-dependent
reorganization of the actin cytoskeleton. Biorheology. 45:219–228.
2008.PubMed/NCBI
|
|
9
|
Nobes CD and Hall A: Rho, Rac, and Cdc42
GTPases regulate the assembly of multimolecular focal complexes
associated with actin stress fibers, lamellipodia, and filopodia.
Cell. 81:53–62. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bigarella CL, Borges L, Costa FF and Saad
ST: ARHGAP21 modulates FAK activity and impairs glioblastoma cell
migration. Biochim Biophys Acta. 1793:806–816. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fortin SP, Ennis MJ, Schumacher CA,
Zylstra-Diegel CR, Williams BO, Ross JT, Winkles JA, Loftus JC,
Symons MH and Tran NL: Cdc42 and the guanine nucleotide exchange
factors Ect2 and trio mediate Fn14-induced migration and invasion
of glioblastoma cells. Mol Cancer Res. 10:958–968. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Johnston AL, Lun X, Rahn JJ, Liacini A,
Wang L, Hamilton MG, Parney IF, Hempstead BL, Robbins SM, Forsyth
PA, et al: The p75 neurotrophin receptor is a central regulator of
glioma invasion. PLoS Biol. 5:e2122007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Malchinkhuu E, Sato K, Maehama T, Mogi C,
Tomura H, Ishiuchi S, Yoshimoto Y, Kurose H and Okajima F:
S1P2 receptors mediate inhibition of glioma cell
migration through Rho signaling pathways independent of PTEN.
Biochem Biophys Res Commun. 366:963–968. 2008. View Article : Google Scholar
|
|
14
|
Salhia B, Tran NL, Chan A, Wolf A, Nakada
M, Rutka F, Ennis M, McDonough WS, Berens ME, Symons M, et al: The
guanine nucleotide exchange factors trio, Ect2, and Vav3 mediate
the invasive behavior of glioblastoma. Am J Pathol. 73:1828–1838.
2008. View Article : Google Scholar
|
|
15
|
Tran NL, McDonough WS, Savitch BA, Fortin
SP, Winkles JA, Symons M, Nakada M, Cunliffe HE, Hostetter G,
Hoelzinger DB, et al: Increased fibroblast growth factor-inducible
14 expression levels promote glioma cell invasion via Rac1 and
nuclear factor-kappaB and correlate with poor patient outcome.
Cancer Res. 66:9535–9542. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan B, Chour HH, Peh BK, Lim C and
Salto-Tellez M: RhoA protein expression correlates positively with
degree of malignancy in astrocytomas. Neurosci Lett. 407:124–126.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fortin Ensign SP, Mathews IT, Symons MH,
Berens ME and Tran NL: Implications of Rho GTPase signaling in
glioma cell invasion and tumor progression. Front Oncol. 3:2412013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kwiatkowska A and Symons M: Signaling
determinants of glioma cell invasion. Adv Exp Med Biol.
986:121–141. 2013. View Article : Google Scholar
|
|
19
|
Nakada M, Nakada S, Demuth T, Tran NL,
Hoelzinger DB and Berens ME: Molecular targets of glioma invasion.
Cell Mol Life Sci. 64:458–478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang L and Goldman RD: Intermediate
filaments mediate cytoskeletal crosstalk. Nat Rev Mol Cell Biol.
5:601–613. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Helfand BT, Chang L and Goldman RD:
Intermediate filaments are dynamic and motile elements of cellular
architecture. J Cell Sci. 117:133–141. 2004. View Article : Google Scholar
|
|
22
|
Emdad L, Lee SG, Su ZZ, Jeon HY, Boukerche
H, Sarkar D and Fisher PB: Astrocyte elevated gene-1 (AEG-1)
functions as an oncogene and regulates angiogenesis. Proc Natl Acad
Sci USA. 106:21300–21305. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gnosa S, Shen YM, Wang CJ, Zhang H,
Stratmann J, Arbman G and Sun XF: Expression of AEG-1 mRNA and
protein in colorectal cancer patients and colon cancer cell lines.
J Transl Med. 10:1092012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gnosa S, Zhang H, Brodin VP, Carstensen J,
Adell G and Sun XF: AEG-1 expression is an independent prognostic
factor in rectal cancer patients with preoperative radiotherapy: A
study in a Swedish clinical trial. Br J Cancer. 111:166–173. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang T, Zhu A, Zhu Y and Piao D: Clinical
implications of AEG-1 in liver metastasis of colorectal cancer. Med
Oncol. 29:2858–2863. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ke ZF, He S, Li S, Luo D, Feng C and Zhou
W: Expression characteristics of astrocyte elevated gene-1 (AEG-1)
in tongue carcinoma and its correlation with poor prognosis. Cancer
Epidemiol. 37:179–185. 2013. View Article : Google Scholar
|
|
27
|
Ke ZF, Mao X, Zeng C, He S, Li S and Wang
LT: AEG-1 expression characteristics in human non-small cell lung
cancer and its relationship with apoptosis. Med Oncol. 30:3832013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li C, Liu J, Lu R, Yu G, Wang X, Zhao Y,
Song H, Lin P, Sun X, Yu X, et al: AEG-1 overexpression: A novel
indicator for peritoneal dissemination and lymph node metastasis in
epithelial ovarian cancers. Int J Gynecol Cancer. 21:602–608. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He W, He S, Wang Z, Shen H, Fang W, Zhang
Y, Qian W, Lin M, Yuan J, Wang J, et al: Astrocyte elevated
gene-1(AEG-1) induces epithelial-mesenchymal transition in lung
cancer through activating Wnt/β-catenin signaling. BMC Cancer.
15:1072015. View Article : Google Scholar
|
|
30
|
Li WN, Wei JL, Wu M, Wu W, Huang Y, Xie MW
and Han H: AEG-1 participates in high glucose-induced activation of
Rho kinase and epithelial-mesenchymal transition in proximal
tubular epithelial cells. Asian Pac J Trop Med. 8:1076–1078. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu K, Guo L, Guo Y, Zhou B, Li T, Yang H,
Yin R and Xi T: AEG-1 3′-untranslated region functions as a ceRNA
in inducing epithelial-mesenchymal transition of human non-small
cell lung cancer by regulating miR-30a activity. Eur J Cell Biol.
94:22–31. 2015. View Article : Google Scholar
|
|
32
|
Song E, Yu W and Xiong X, Kuang X, Ai Y
and Xiong X: Astrocyte elevated gene-1 promotes progression of
cervical squamous cell carcinoma by inducing epithelial-mesenchymal
transition via Wnt signaling. Int J Gynecol Cancer. 25:345–355.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wei J, Li Z, Chen W, Ma C, Zhan F, Wu W
and Peng Y: AEG-1 participates in TGF-beta1-induced EMT through p38
MAPK activation. Cell Biol Int. 37:1016–1021. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang J, Zhu D, Lv Q, Yi Y, Li F and Zhang
W: The key role of astrocyte elevated gene-1 in CCR6-induced EMT in
cervical cancer. Tumour Biol. 36:9763–9767. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zheng J, Li C, Wu X, Yang Y, Hao M, Sheng
S, Sun Y, Zhang H, Long J and Hu C: Astrocyte elevated gene-1 is a
novel biomarker of epithelial-mesenchymal transition and
progression of hepatocellular carcinoma in two China regions.
Tumour Biol. 35:2265–2269. 2014. View Article : Google Scholar
|
|
36
|
Lee SG, Su ZZ, Emdad L, Sarkar D, Franke
TF and Fisher PB: Astrocyte elevated gene-1 activates cell survival
pathways through PI3K-Akt signaling. Oncogene. 27:1114–1121. 2008.
View Article : Google Scholar
|
|
37
|
Park D, Ha IJ, Park SY, Choi M, Lim SL,
Kim SH, Lee JH, Ahn KS, Yun M and Lee SG: Morusin induces TRAIL
sensitization by regulating EGFR and DR5 in human glioblastoma
cells. J Nat Prod. 79:317–323. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee SG, Kim K, Kegelman TP, Dash R, Das
SK, Choi JK, Emdad L, Howlett EL, Jeon HY, Su ZZ, et al: Oncogene
AEG-1 promotes glioma-induced neurodegeneration by increasing
glutamate excitotoxicity. Cancer Res. 71:6514–6523. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee SG, Kang DC, DeSalle R, Sarkar D and
Fisher PB: AEG-1/MTDH/LYRIC, the beginning: Initial cloning,
structure, expression profile, and regulation of expression. Adv
Cancer Res. 120:1–38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sarkar D and Fisher PB: Advances in Cancer
Research. AEG-1/MTDH/LYRIC implicated in multiple human cancers.
Preface. Adv Cancer Res. 120:xi–xiv. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Su ZZ, Kang DC, Chen Y, Pekarskaya O, Chao
W, Volsky DJ and Fisher PB: Identification and cloning of human
astrocyte genes displaying elevated expression after infection with
HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid
subtraction hybridization, RaSH. Oncogene. 21:3592–3602. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huang Y and Li LP: Progress of cancer
research on astrocyte elevated gene-1/Metadherin (Review). Oncol
Lett. 8:493–501. 2014.PubMed/NCBI
|
|
44
|
Emdad L, Das SK, Dasgupta S, Hu B, Sarkar
D and Fisher PB: AEG-1/MTDH/LYRIC: Signaling pathways, downstream
genes, interacting proteins, and regulation of tumor angiogenesis.
Adv Cancer Res. 120:75–111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hay ED: An overview of
epithelial-mesenchymal transformation. Acta Anat (Basel). 154:8–20.
1995. View Article : Google Scholar
|
|
46
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Haynes J, Srivastava J, Madson N, Wittmann
T and Barber DL: Dynamic actin remodeling during
epithelial-mesenchymal transition depends on increased moesin
expression. Mol Biol Cell. 22:4750–4764. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jaffe AB and Hall A: Rho GTPases:
Biochemistry and biology. Annu Rev Cell Dev Biol. 21:247–269. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Etienne-Manneville S and Hall A: Rho
GTPases in cell biology. Nature. 420:629–635. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ridley AJ: Rho GTPases and actin dynamics
in membrane protrusions and vesicle trafficking. Trends Cell Biol.
16:522–529. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Valencia A, Chardin P, Wittinghofer A and
Sander C: The ras protein family: Evolutionary tree and role of
conserved amino acids. Biochemistry. 30:4637–4648. 1991. View Article : Google Scholar : PubMed/NCBI
|