Introduction
Hepatocellular carcinoma (HCC) is among the leading
diseases in the world today, being the fifth most common malignant
tumor, representing 85% of primary liver tumors and accounting for
nearly two thirds of death among cancers. The prognosis is
generally poor due to rapid tumor growth and the absence of
symptoms at the beginning of the disease (1,2).
The chronic inflammatory state appears to be
necessary for the initiation and development of liver cancer and
the HCC is an example of inflammation-related cancer (3,4). The
presence of underlying diseases, such as hepatitis B and C, is a
major cause of tumor (5). HCC is
among the top ten cancers that affect the world population. Its
incidence has increased in recent years, mainly due to infection by
the hepatitis C virus (6).
Among the various types of cancer therapies,
surgical resection, radiotherapy and chemotherapy are employed, the
latter being the most widely used method. Therapies may be used
singly or in combination (3). Even
with different options, treatment against cancer is difficult to be
handled, mainly due to the low specificity of some drugs and the
narrow therapeutic window, which are closely related to toxicity.
Thus, the search and development of new therapies is extremely
necessary (7). A recent innovation
in anticancer therapy is the inhibition of mTOR (mammalian target
of rapamycin), which has been shown to suppress the growth of liver
tumors and metastasis (8,9). These results are encouraging, but the
HCC often shows higher resistance to rapamycin when used alone.
Therefore, new studies have investigated the association of
rapamycin with other antitumor drugs to reduce or avoid treatment
resistance (10,11).
Rapamycin, a macrocyclic lactone produced by
Streptomyces hygroscopicus, molecular formula C51 H79 NO13
and molecular weight 914.2, is a highly effective chemotherapy, its
mechanism of action is the inhibition of mTORC1. Since its approval
by the US Food and drug Administration in 1999, rapamycin has been
administered to patients who received kidney transplant due to its
immunosuppressive activity. In the 80s, scientists also found that
the drug inhibits the growth of tumors and, since 2007, two of its
derivatives, temsirolimus (Wyeth) and everolimus (Novartis) have
been approved against various cancers, including liver cancer,
administered alone or in combination with other drugs (12,13).
The mechanism of action of rapamycin occurs
intracellularly where these inhibitors form a complex with the
protein bound to FK506 12 (FKBP-12) that is recognized by mTOR. The
formation of the complex results in the inhibition of the activity
of mTOR, and the s6K expression of the protein will result in the
inhibition of cell cycle progression, survival and angiogenesis
(14,15).
Clinical and experimental evidence shows strong
correlation between the dosage and the toxicity. Rapamycin induces
side-effects such as nausea, vomiting, anemia, hyperlipidemia,
respiratory, cardiovascular, and nephrotoxicity, which are
dose-dependent and limit the administration of higher dosages,
thus, compromising therapeutic efficacy. Nephrotoxicity is one of
the major limitations and, for this reason, the monitoring of the
renal function is required during treatment (16,17).
This makes inadequate doses the most significant obstacle in the
exact definition of the clinical role of rapamycin, and probably in
expanding its activity. Therefore, when high doses are
administered, rapamycin is essential to identify the effective drug
carrier that can prevent or counteract the side-effects of
rapamycin (16). One of them is
fructose-1,6-bisphosphate (FBP), a sugar which has mechanisms that
promote renal protection (18).
Previous studies reported the antioxidant and
anti-inflammatory therapeutic properties plus a nephroprotecting
effect in animal models of FBP. Knowing that the process of
carcinogenesis involves inflammatory mediators such as cytokines,
chemokines, and reactive oxygen species, we believe that the FBP
can be helpful in maintaining the therapeutic effect of rapamycin
and inhibition of adverse effects. Therefore, the aim of the
present study was to evaluate the effect of rapamycin alone and in
combination with fructose-1,6-bisphosphate on cell death and
proliferation, inflammation and oxidative stress parameters in
liver carcinoma cells (HepG2). The objective of this study was to
evaluate the activity of rapamycin in combination with the FBP in
HepG2 cell proliferation and mechanisms involved, looking to
determine whether rapamycin ineffective doses can decrease cell
proliferation and toxicity, when associated with FBP.
Materials and methods
Cell culture
Human hepatocarcinoma cell line (HepG2) was obtained
from the American Type Culture Collection (ATCC; Manassas, VA,
USA). The medium used for the culture of cells was Dulbecco's
modified Eagle's medium (DMEM) supplemented with fetal bovine serum
(FBS 10%) under a humidified atmosphere containing 5%
CO2. HepG2 cells, after being cultured and presenting
~70% confluence, were detached from culture bottles and transferred
to 96-well culture plates at a uniform cell density.
Treatment with rapamycin and
fructose-1,6-bisphosphate
The plates were incubated at 37°C in a humidified
incubator with 5% CO2 for 72 h, with the objective to
establish a dose-response curve using rapamycin (Wyeth
Pharmaceuticals Co., Collegeville, PA, USA), tested in different
concentrations of 10, 20, 30, 40 and 50 nM (19,20)
and fructose 1,6 bisphosphate (Sigma-Aldrich, St. Louis, MO, USA)
at doses of 5 and 10 mM, and in DMEM medium in order to establish
dose correlation of cell growth and proliferation. The choice of
72-h treatment time was based on other studies by our laboratory,
that have shown that the best growth evaluation time is 72 h, and
that at this time the cell growth is ~100%, in other words, the
control group doubles the number of cells, which is taken as the
ideal internationally to measure proliferation. The FBP
concentrations were in agreement with experiments performed in our
laboratory on HepG2 cells as was rapamycin on impact articles.
Evaluation of cellular proliferation
The assessment of the viability and cellular
growth/proliferation was performed by cell counting in a Neubauer
chamber. The experiments were performed in triplicate and repeated
three times. After this evaluation the rapamycin doses of 10 and 10
mM FBP were selected.
Measurement of lactate dehydrogenase
(LDH)
The test LDH (lactate dehydrogenase) is a marker of
membrane integrity. The enzyme lactate dehydrogenase is present
throughout the cell cytoplasm, and when the membrane is damaged it
is released to the external environment. The LDH allows the
analyzes of the number of total inviable (dead) cells (21).
Cytotoxicity was assessed by the presence of the
enzyme LDH measured in both supernatants and cell lysate of
treatments in HepG2 cells, using the UV kinetic method
(Lactate-Pyruvate) by Labtest Diagnostic Kit SA. For the control of
cell lysis, a 5% Tween was used.
Quantification of cytokines
To measure cytokine production we used the BD
Cytometric Bead Array (CBA), Human Inflammatory Cytokine CBA kit.
According to kit manual, the BD CBA system uses the sensitivity of
amplified fluorescence detection by flow cytometry to measure
soluble analytes in a particle-based immunoassay. Each bead in a BD
CBA kit provides a capture surface for a specific protein and is
analogous to an individually coated well in an ELISA plate. The BD
CBA capture bead mixture is in suspension to allow for the
detection of multiple analytes in a small volume sample (22). The treated HepG2 cells were
incubated for 72 h, supernatants were collected and stored at −20°C
for later analysis.
Evaluation of apoptosis, senescence and
autophagy
HepG2 cells were treated in 24-well plates for a
preview of apoptosis, senescence and autophagy. Apoptosis and
senescence were evaluated by DAPI (4′,6-diamidino-2-phenylindole),
a fluorescent staining that binds strongly to regions rich in
adenine and thymine in DNA sequences (23). The evaluation of autophagy was by
acridine orange (AO), a vital acidotropic fluorescent dye (24). The results were visualized by
fluorescent microscope and the apoptotic and senescent nuclei were
quantified using Image-Pro Plus software.
Evaluation of oxidative stress
Oxidative stress of liver carcinoma cells was
measured by the method of thiobarbituric acid (TBARS) by
fluorimetry.
Statistical analysis
The results are presented using descriptive
statistics (average and standard deviation). For the comparison of
average between group analysis of variance (ANOVA) and post hoc
Tukey's test for multiple comparisons were used. In the presence of
asymmetry the corresponding non-parametric was used. The level of
significance was set at P<0.05 with a 95% confidence interval
and the data were analyzed by SPSS software (Statistical Package
for Social Sciences) for Windows, version 15.0. (SPSS, Inc.,
Cincinnati, OH, USA).
Results
The evaluation of cell proliferation of rapamycin
(R) at concentrations of 10, 20, 30, 40 and 50 nM, FBP 5 and 10 mM
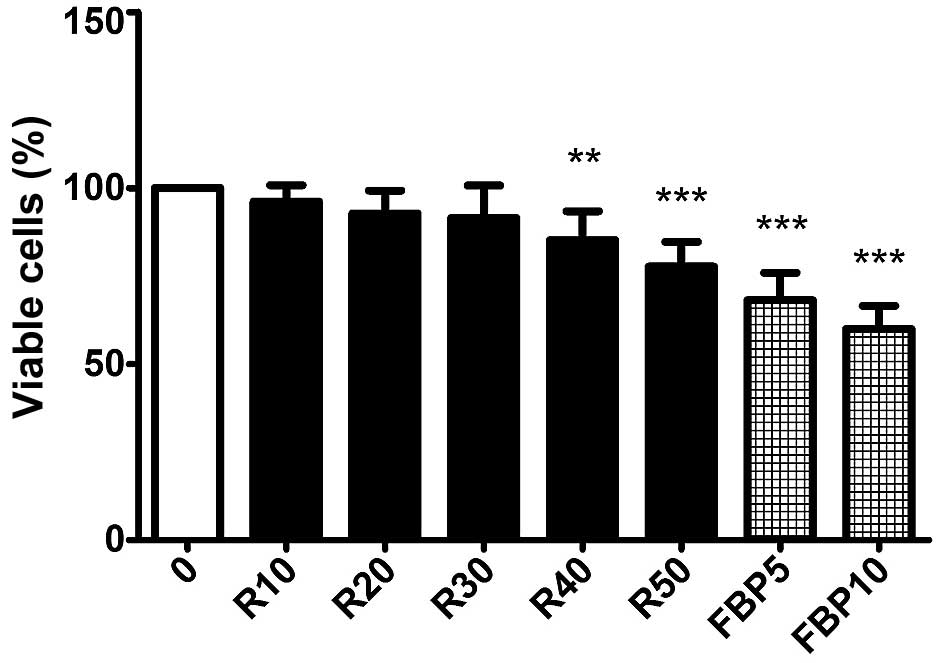
and the association of the two substances was performed. Fig. 1, presents that rapamycin (R) causes
a significant decrease in concentrations of 40 and 50 nM and the
FBP causes a reduction of cell growth at concentrations of 5 and 10
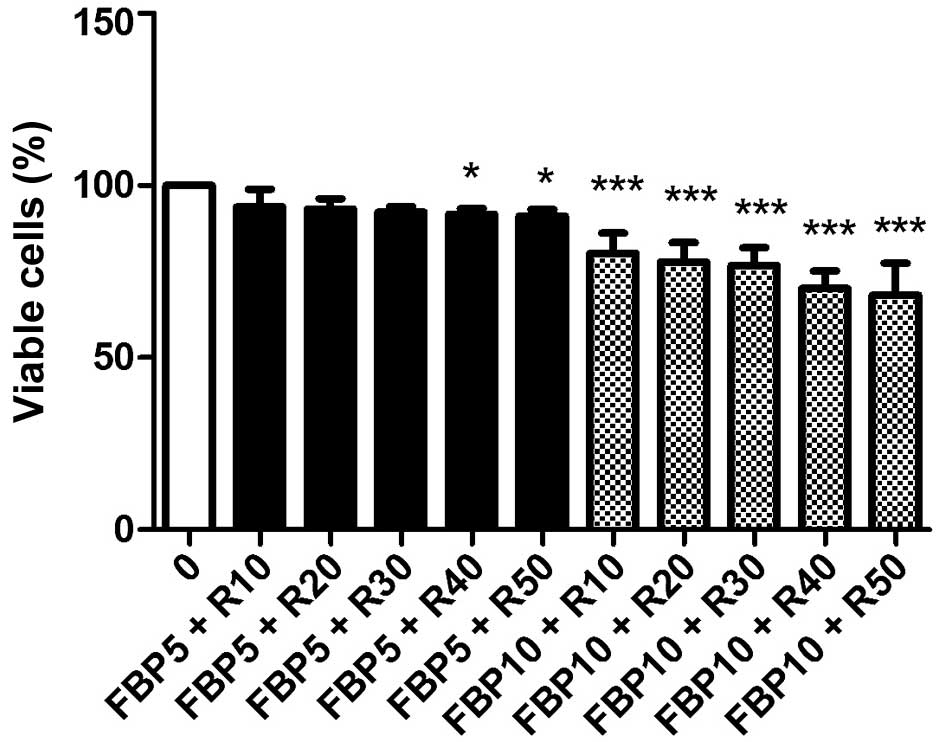
mM. In Fig. 2, it is evident that
the association with FBP 10 mM makes the subtherapeutic doses of
10, 20 and 30 nM of rapamycin (R) effective. Already in combination
with 5 nM of FBP, rapamycin (R) did not decrease cell proliferation
in any of the concentrations. For this reason, the doses of 10 mM
FBP and 10 nM rapamycin (R) were selected for the following
experiments.
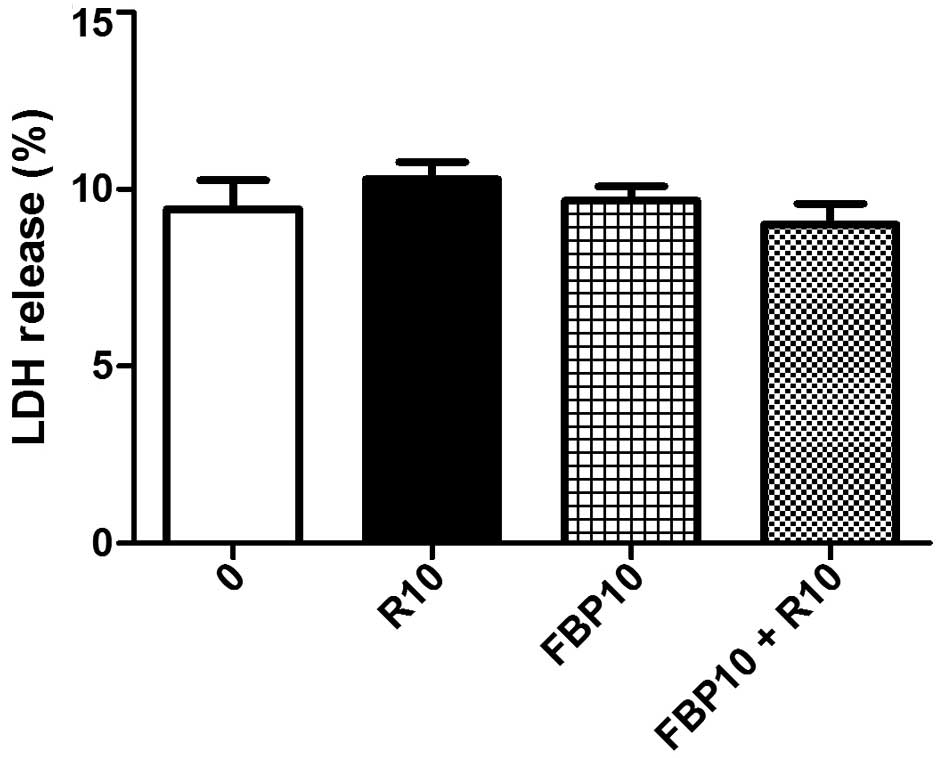
The integrity of the membrane of HepG2 cells treated
with rapamycin (R) and FBP, isolated and in combination, was
evaluated through the measurement of LDH in the cell culture
supernatant. There was no significant decrease in the percentage of
LDH released in any of the groups analyzed, demonstrating that
there is no significant increase in cell death associated with
necrosis in the treated groups in comparison to the control group
(Fig. 3).
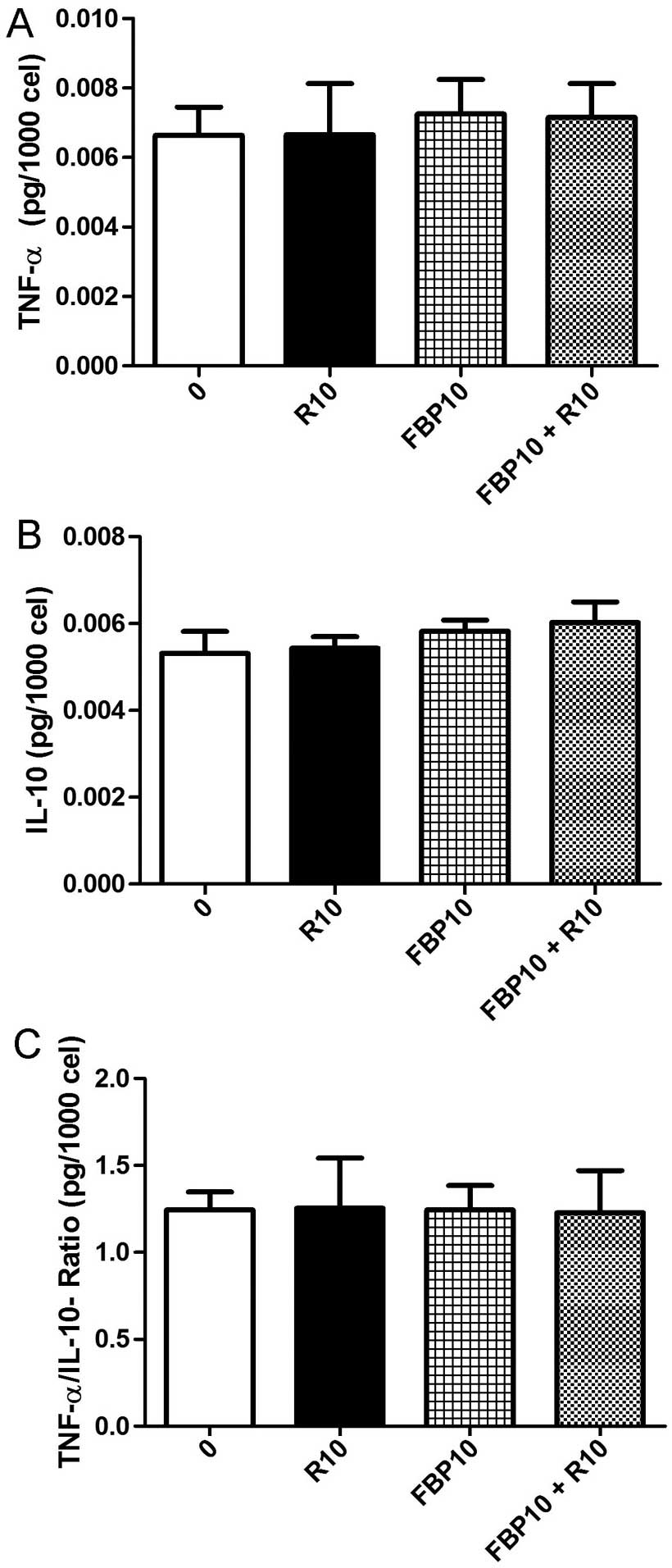
Inflammation and cancer are associated, for this
reason we evaluated the pro-inflammatory and anti-inflammatory
cytokines. We selected tumor necrosis factor α, TNF-α (Fig. 4A) and interleukin 10, IL-10
(Fig. 4b). The ratio between the
cytokines was also calculated (Fig.
4C). Changes of cytokines in relation to the control were not
observed and the ratio gave the balance between pro- and
anti-inflammatory cytokines.
To check whether the decrease of cellular
proliferation was by apoptosis or senescence, DAPI staining was
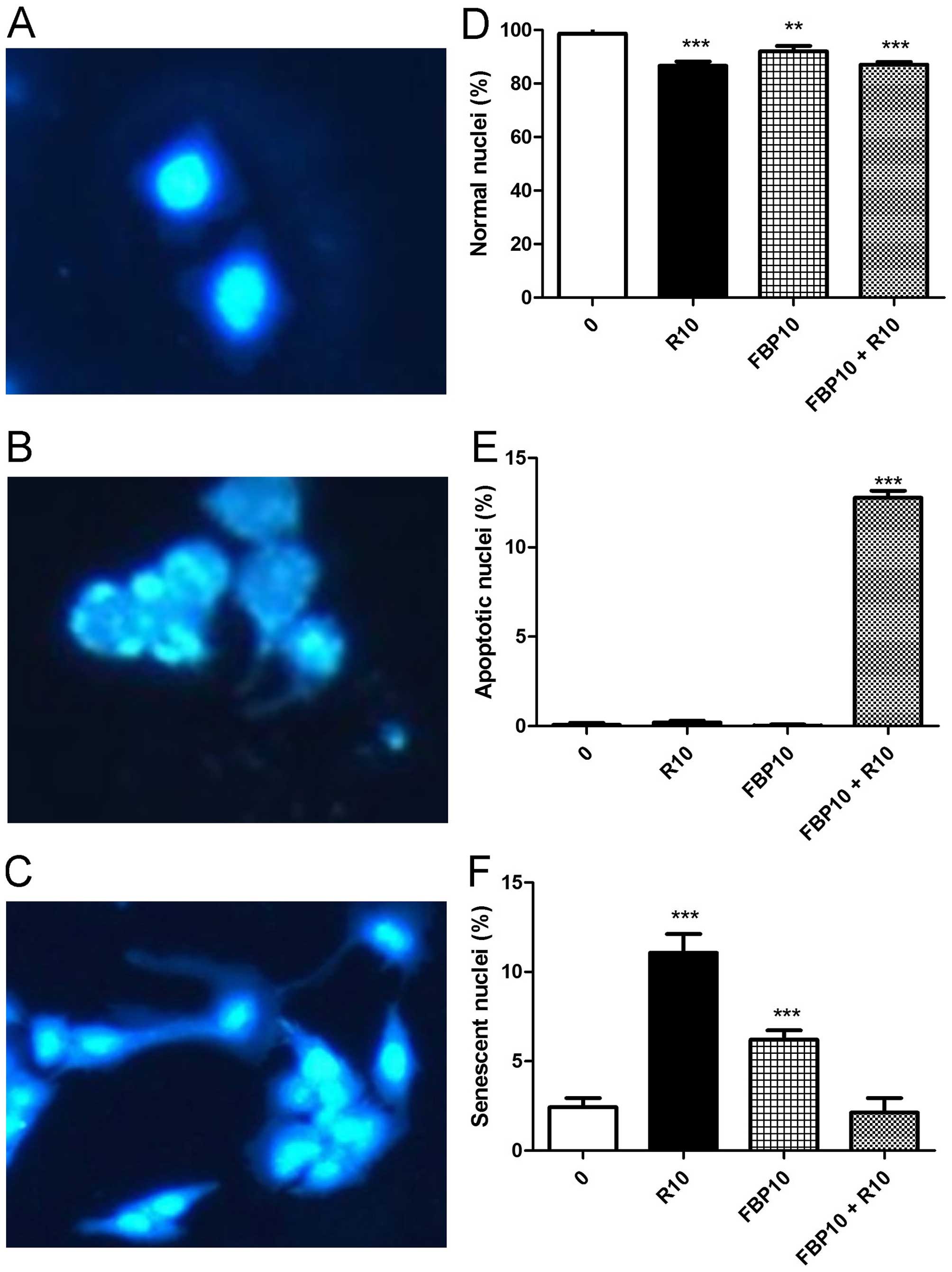
used. The Fig. 5A presents a
picture of normal nuclei, Fig. 5B
apoptotic nuclei and Fig. 5C
senescent nuclei. In the analysis of normal nuclei, R and FBP,
isolated and in combination, had a significant decrease (Fig. 5d). Associated with that result, we
had a significant increase of apoptotic nuclei percentage in the
combination (Fig. 5E) and a
significantly increased senescent nuclei in the R and FBP isolated,
when compared to control group.
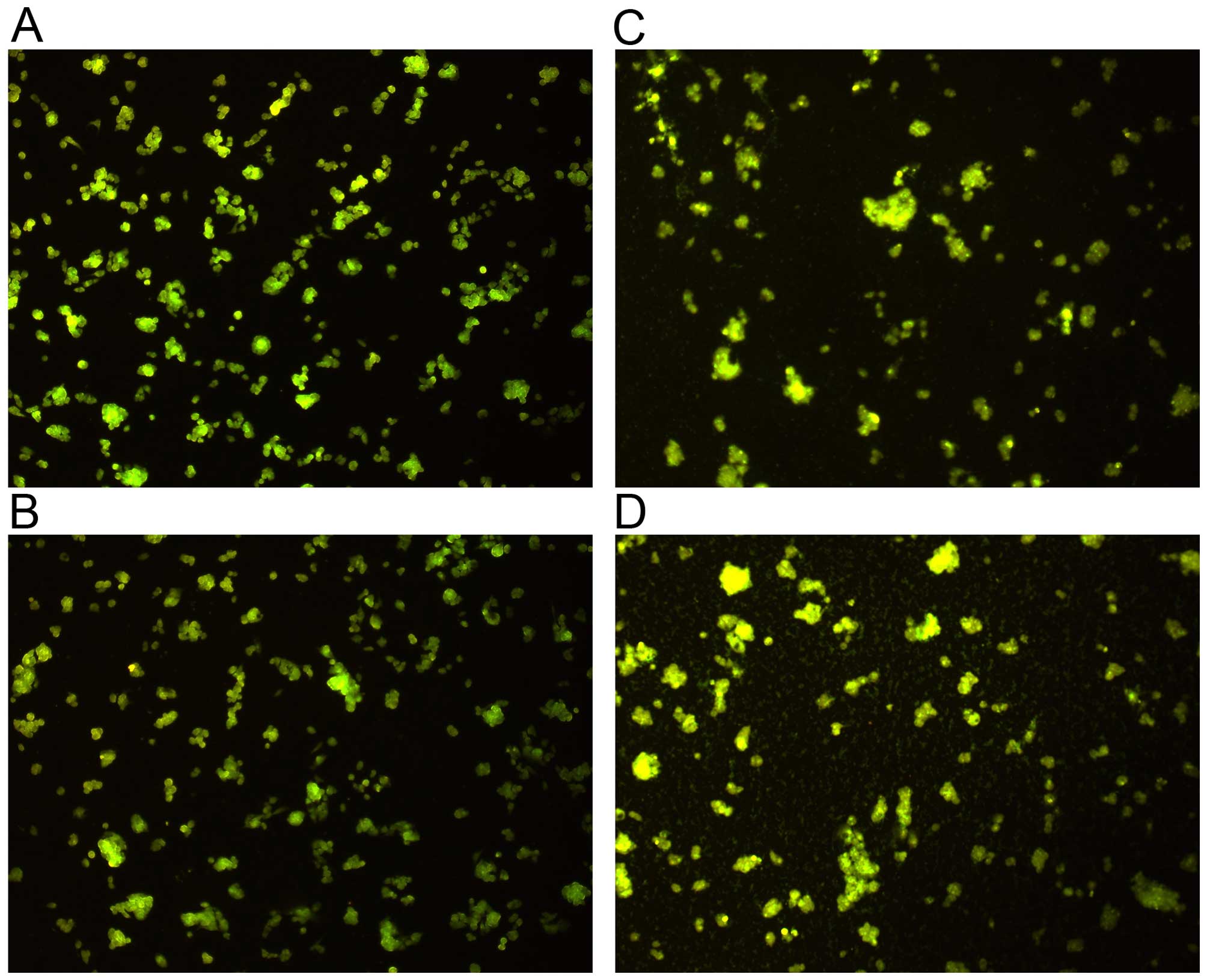
Autophagy, another cellular death mechanism that
involves cell degradation of unnecessary or dysfunctional
components (25), was analyzed in
HepG2 cells through the use of acridine orange dye. No significant
differences were fund between the study groups (Fig. 6).
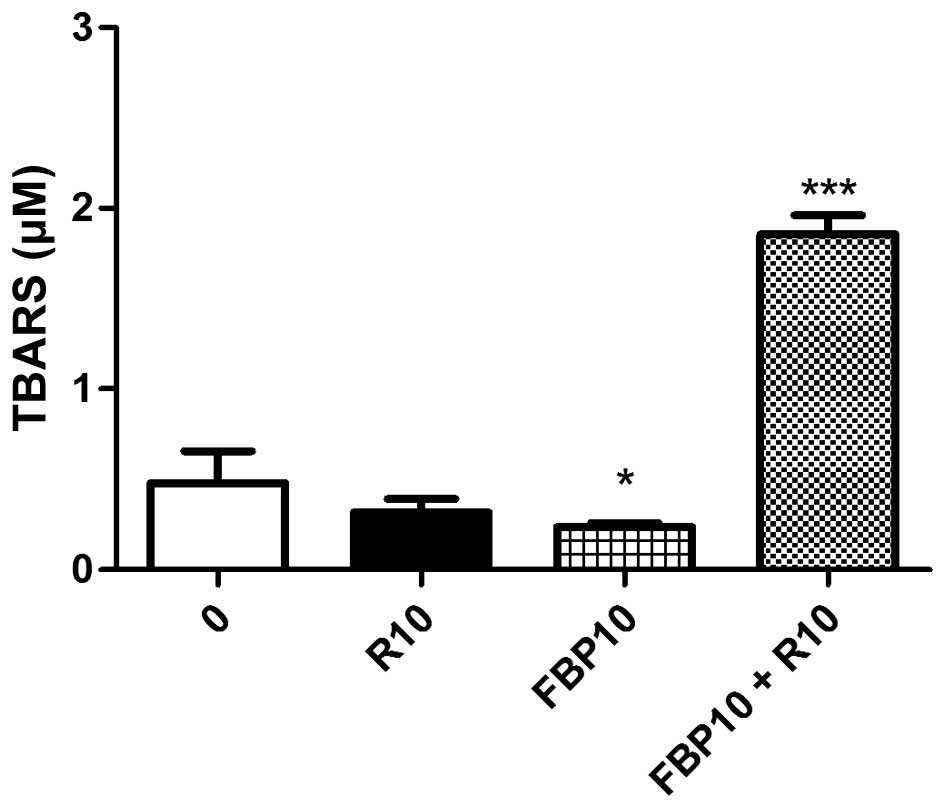
The oxidative stress can provoke cellular damage.
For this reason, we analyzed thiobarbituric acid reactive
substances (TBARS) (26). Our
results showed an antioxidant effect of FBP, but in association
with R, it provokes a significant increase in the release of free
radicals in relation to the other groups (Fig. 7).
Discussion
HCC is closely linked with chronic inflammation.
This association involves the time factor: the longer the
inflammation persists, the greater the risk of developing cancer
(27).
Rapamycin, a known immunosuppressant used in kidney
transplant patients, also acts by inhibiting the complex of the
mammalian target of rapamycin, mTOR. Also known as FKBP12-rapamycin
associated protein (FRAP), mTOR is a serine/threonine protein
kinase that promotes cell proliferation and differentiation
(28). Therefore, rapamycin is
currently being used to treat certain types of cancer (29). However, its use is limited due to
its toxicity and resistance to prolonged use, which causes adverse
events, mainly nephrotoxicity (30). For this reason, the association with
other substances in order to avoid adverse events becomes
essential. The FBP, a sugar belonging to glycolytic cellular route
that has some therapeutic effects in inflammatory diseases, such as
rheumatoid arthritis and septicemia (31), was chosen for the combination with
rapamycin.
Our first results showed that rapamycin and FBP
singly decreases the proliferation of HepG2 cells. However, when
used in combination, rapamycin was effective at lower doses,
suggesting a decrease in the therapeutic dosage. In essence,
rapamycin has a high degree of toxicity and when associated with
FBP showed an antiproliferative effect, which indicated that the
association could result in greater effectiveness of rapamycin at
concentrations that do not significantly decrease proliferation.
Despite the combination decrease the efficiency of FBP, increased
the efficiency of 10 nM rapamycin, which was our target. This
effect may have been caused by necrosis (cytotoxicity), apoptosis,
autophagy or senescence. Therefore, with the intention of proving
that this association has no cytotoxicity, we measured LDH that did
not demonstrate correlation between the ceasing of cell
proliferation and death due to necrosis, showing that the
combination of the substances does not cause cellular toxicity.
Inflammatory mediators, such as cytokines, free
radicals, prostaglandins, and growth factors can induce changes in
cellular homeostasis, leading to the development and progression of
cancer. Several inflammatory mechanisms are involved in cancer. One
of the most important is the genomic instability caused by
inflammation. The activation of leukocytes, especially macrophages
and granulocytes, leads to the synthesis of reactive oxygen species
(ROS) and nitrogen (RNS) that can cause damage to DNA, proteins and
lipids, which may cause mutations in the cells. The free radical
damage can be caused by a proinflammatory enzyme, cyclooxygenase-2
(COX-2), which leads to production of high levels of peroxides
within the cells. Therefore, therapies that reduce inflammation
prior to immunization can increase the efficacy of immunotherapy
(32,33).
In the last two decades, evidence emerged that the
molecular level of most chronic diseases, including cancer, are
caused by a dysregulated inflammatory response. The tumor necrosis
factor α (TNF-α) exerts regulatory role, by stimulating the
biosynthesis of growth factors. It is directly cytotoxic to
endothelial cells and can induce the biosynthesis of collagenases,
proteases, reactive oxygen intermediates and arachidonic acid
metabolites. On the other hand, anti-inflammatory interleukins like
interleukin-10 (IL-10) have multifaceted properties; such
properties include the inhibition of prototypic inflammatory
transcription factor nuclear factor kappa B, leading to suppressed
cytokine production, reduction of tissue factor expression, and
inhibition of apoptosis of macrophages and monocytes after
infection (34).
To check if the decrease of cellular proliferation
is occurring by the inflammatory route, we analyzed
pro-inflammatory cytokines TNF-α and anti-inflammatory IL-10,
besides the ratio between them. None of the parameters show changes
in relation to the control, and the ratio demonstrated the
inflammatory balance between cytokines.
Apoptosis, a programmed cell death, is decreased in
cancer cells. Many therapies try to increase apoptosis of these in
order to reduce their proliferation.
Another way of cell death that is also reduced in
cancer is senescence. Senescence is the aging of the cell that
occurs when they stop dividing to replace other cells that, for
some reason, failed to metabolize. On the other hand, cancer cells
have an enzyme called telomerase that regenerates telomeres of the
cell, allowing it to multiply indefinitely (35).
Through the use of fluorescent staining DAPI and the
quantification of the images generated by fluorescent microscope,
we noted a significant increase of the apoptosis in the association
of rapamycin and FBP doses and this test result can be correlated
with the percentage of the antiproliferative effect found in the
viability assay. Also, there was a significant increase in
senescence of nuclei in the isolated rapamycin and FBP compared to
the control group. By comparing the results of cell proliferation
of the isolated drugs it is safe to conclude that, in spite of
being a potential anticancer factor, senescence did not seem to
influence the decrease of cell proliferation.
Autophagy, a mechanism that may protect against
cancer by isolating damaged organelles, allowing cell
differentiation, increasing and promoting cell death of cancerous
cells (25), may be related to
senescence or apoptosis, because while autophagy decreases,
apoptosis and/or senescence increases (36).
When the tumor is established and autophagy is
functional it can help the tumor to survive and grow, in the
beginning, autophagy can phagocytize the mutated cells and
suppresses the tumor. In contrast, apoptosis and senescence
processes are irreversible. As no difference was observed between
the treatment and control in acridine orange staining analyzes, and
we had the result of apoptosis in combination, we did not continue
with more specific tests.
The cytoperoxidation is a cell membrane damage
caused by free radicals. This toxic effect can be assessed by the
formation of thiobarbituric acid reactive substances (TBARS),
especially malondialdehyde (MDA) (26). Our results showed that, while FBP
singly decreases the cytoperoxidation, the combination of the two
drugs cause a significant injury provoked by free radicals,
suggesting that this phenomenon might be the cause of cell death by
apoptosis.
Apoptosis and senescence, the main routes that limit
the growth of tumors, occurs in response to DNA damage or stress.
The decision between life and death can be determined by the extent
of damage or the duration of stress (37). Based on this, it is observed that
due to the increased oxidative stress (TBARS) in cells treated with
the combination, they are induced to apoptosis, while cells treated
with isolated R or F has no increased oxidative stress and become
senescent.
The present study promotes for the first time the
combination of these two drugs and addresses the importance of
trying the combination of substances such as FBP with other drugs
commonly used to treat cancer, such as rapamycin, for a more
effective result and the promotion of life quality for patients in
treatment.
In conclusion, our results show that the two drugs
individually cause decreased cell proliferation by senescence,
however, when combined, increase cell death by apoptosis. These
results are very important, since the decrease in apoptosis is one
of the main factors that leads the cell to proliferate
uncontrollably.
Based on this we can conclude that the concomitant
use of rapamycin and FBP could be a promising treatment for
patients with hepatocellular carcinoma, because the combination of
rapamycin with FBP significantly reduces cell proliferation and,
most importantly, brings to reality the possibility of achieving
the goal of making an effective subtherapeutic dose, minimizing the
serious known reactions to drugs used in cancer therapy today by
the increase of free radicals and apoptosis when the association is
used.
References
|
1
|
Organization WHO: The top 10 causes of
death. Journal. 2014.
|
|
2
|
Gonzalez SA: Novel biomarkers for
hepatocellular carcinoma surveillance: Has the future arrived?
Hepatobiliary Surg Nutr. 3:410–414. 2014.
|
|
3
|
Baird A, Lee J, Podvin S, Kurabi A, Dang
X, Coimbra R, Costantini T, Bansal V and Eliceiri BP: Esophageal
cancer-related gene 4 at the interface of injury, inflammation,
infection, and malignancy. Gastrointest Cancer. 2014:131–142. 2014.
View Article : Google Scholar
|
|
4
|
Capece D, Fischietti M, Verzella D,
Gaggiano A, Cicciarelli G, Tessitore A, Zazzeroni F and Alesse E:
The inflammatory micro-environment in hepatocellular carcinoma: A
pivotal role for tumor-associated macrophages. Biomed Res Int.
2013:1872042013. View Article : Google Scholar
|
|
5
|
Bharadwaj S and Gohel TD: Perspectives of
physicians regarding screening patients at risk of hepatocellular
carcinoma. Gastroenterol Rep (Oxf) gou089. 2015.
|
|
6
|
Salhab M and Canelo R: An overview of
evidence-based management of hepatocellular carcinoma: A
meta-analysis. J Cancer Res Ther. 7:463–475. 2011. View Article : Google Scholar
|
|
7
|
Almeida JRCd: Farmacêuticos em Oncologia:
uma nova realidade. Atheneu; Aracaju: pp. 3582004, In
Portuguese.
|
|
8
|
Wang Z, Zhou J, Fan J, Qiu SJ, Yu Y, Huang
XW and Tang ZY: Effect of rapamycin alone and in combination with
sorafenib in an orthotopic model of human hepatocellular carcinoma.
Clin Cancer Res. 14:5124–5130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Z, Zhou J, Fan J, Tan CJ, Qiu SJ, Yu
Y, Huang XW and Tang ZY: Sirolimus inhibits the growth and
metastatic progression of hepatocellular carcinoma. J Cancer Res
Clin Oncol. 135:715–722. 2009. View Article : Google Scholar
|
|
10
|
Wang C, Gao D, Guo K, Kang X, Jiang K, Sun
C, Li Y, Sun L, Shu H, Jin G, et al: Novel synergistic antitumor
effects of rapamycin with bortezomib on hepatocellular carcinoma
cells and orthotopic tumor model. BMC Cancer. 12:1662012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Y, Speeg KV, Washburn WK and Halff G:
Sirolimus plus sorafenib in treating HCC recurrence after liver
transplantation: A case report. World J Gastroenterol.
16:5518–5522. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang J: 203985 Everolimus Clinpharm BPCA.
2012, http://www.fda.gov/downloads/drugs/developmentapprovalprocess/developmentresources/ucm320466.pdf.
Accessed February 11, 2016.
|
|
13
|
Zirkelbach JF: 22088 Temsirolimus
Clinpharm BPCA. 2011, http://www.fda.gov/downloads/drugs/developmentapprovalprocess/developmentresources/ucm307049.pdf.
Accessed February 11, 2016.
|
|
14
|
Porta C, Paglino C and Mosca A: Targeting
PI3K/Akt/mTOR Signaling in Cancer. Front Oncol. 4:642014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sahin F, Kannangai R, Adegbola O, Wang J,
Su G and Torbenson M: mTOR and P70 S6 kinase expression in primary
liver neoplasms. Clin Cancer Res. 10:8421–8425. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cuconati A, Mills C, Goddard C, Zhang X,
Yu W, Guo H, Xu X and Block TM: Suppression of AKT anti-apoptotic
signaling by a novel drug candidate results in growth arrest and
apoptosis of hepatocellular carcinoma cells. PLoS One.
8:e545952013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cendales L, Bray R, Gebel H, Brewster L,
Elbein R, Farthing D, Song M, Parker D, Stillman A, Pearson T, et
al: Tacrolimus to belatacept conversion following hand
transplantation: A case report. Am J Transplant. 15:2250–2255.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seok SM, Park TY, Park HS, Baik EJ and Lee
SH: Fructose-1,6-bisphosphate suppresses lipopolysaccharide-induced
expression of ICAM-1 through modulation of toll-like receptor-4
signaling in brain endothelial cells. Int Immunopharmacol.
26:203–211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang JF, Liu JJ, Lu MQ, Cai CJ, Yang Y,
Li H, Xu C and Chen GH: Rapamycin inhibits cell growth by induction
of apoptosis on hepatocellular carcinoma cells in vitro. Transpl
Immunol. 17:162–168. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dai ZJ, Gao J, Ma XB, Kang HF, Wang BF, Lu
WF, Lin S, Wang XJ and Wu WY: Antitumor effects of rapamycin in
pancreatic cancer cells by inducing apoptosis and autophagy. Int J
Mol Sci. 14:273–285. 2012. View Article : Google Scholar
|
|
21
|
Philipp AB, Nagel D, Stieber P, Lamerz R,
Thalhammer I, Herbst A and Kolligs FT: Circulating cell-free
methylated DNA and lactate dehydrogenase release in colorectal
cancer. BMC Cancer. 14:2452014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Human Inflammatory Cytokines Kit
Instruction Manual. Journal. 2008, http://www.bdbiosciences.com/documents/CBA_Human_Inf_Cytokine_manual.pdf.
Accessed February 11, 2016.
|
|
23
|
Kim TM, Shin SK, Kim TW, Youm SY, Kim DJ
and Ahn B: Elm tree bark extract inhibits HepG2 hepatic cancer cell
growth via pro-apoptotic activity. J Vet Sci. 13:7–13. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chiela ECF: Protocol for measuring
autophagy. Journal. http://www.ufrgs.br/labsinal/autofagia.htm.
Accessed February 11, 2016.
|
|
25
|
Nepal S and Park PH: Regulatory role of
autophagy in globular adiponectin-induced apoptosis in cancer
cells. Biomol Ther (Seoul). 22:384–389. 2014. View Article : Google Scholar
|
|
26
|
Carlos SP, Dias AS, Forgiarini Júnior LA,
Patricio PD, Graciano T, Nesi RT, Valença S, Chiappa AM, Cipriano G
JR, Souza CT, et al: Oxidative damage induced by cigarette smoke
exposure in mice: impact on lung tissue and diaphragm muscle. J
Bras Pneumol. 40:411–420. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rutkowski MR and Conejo-Garcia JR: Size
does not matter: Commensal microorganisms forge tumor-promoting
inflammation and anti-tumor immunity. Oncoscience. 2:239–246. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Asnaghi L, Bruno P, Priulla M and Nicolin
A: mTOR: A protein kinase switching between life and death.
Pharmacol Res. 50:545–549. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McGranahan N, Favero F, De Bruin EC,
Birkbak NJ, Szallasi Z and Swanton C: Clonal status of actionable
driver events and the timing of mutational processes in cancer
evolution. Sci Transl Med. 7:283ra542015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fervenza FC, Fitzpatrick PM, Mertz J,
Erickson SB, Liggett S, Popham S, Wochos DN, Synhavsky A, Hippler
S, Larson TS, et al Mayo Nephrology Collaborative Committee: Acute
rapamycin nephrotoxicity in native kidneys of patients with chronic
glomerulopathies. Nephrol Dial Transplant. 19:1288–1292. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Azambuja AA, Lunardelli A, Nunes FB,
Gaspareto PB, Donadio MV, Poli de Figueiredo CE and de Oliveira JR:
Effect of fructose-1,6-bisphosphate on the nephrotoxicity induced
by cisplatin in rats. Inflammation. 34:67–71. 2011. View Article : Google Scholar
|
|
32
|
Seelaender M, Neto JC, Pimentel GD,
Goldszmid RS and Lira FS: Inflammation in the disease: Mechanism
and therapies 2014. Mediators Inflamm. 2015:1698522015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Raza H, John A and Benedict S:
Acetylsalicylic acid-induced oxidative stress, cell cycle arrest,
apoptosis and mitochondrial dysfunction in human hepatoma HepG2
cells. Eur J Pharmacol. 668:15–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Goswami B, Rajappa M, Mallika V, Shukla DK
and Kumar S: TNF-alpha/IL-10 ratio and C-reactive protein as
markers of the inflammatory response in CAD-prone North Indian
patients with acute myocardial infarction. Clin Chim Acta.
408:14–18. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Raouf S, Weston C and Yucel N:
Reproducibility Project: Cancer Biology: Registered report:
Senescence surveillance of pre-malignant hepatocytes limits liver
cancer development. Elife. Jan 26–2015. View Article : Google Scholar
|
|
36
|
Vessoni AT, Filippi-Chiela EC, Menck CF
and Lenz G: Autophagy and genomic integrity. Cell Death differ.
20:1444–1454. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Suzuki K and Matsubara H: Recent advances
in p53 research and cancer treatment. J BioMed Biotechnol.
2011:9783122011. View Article : Google Scholar : PubMed/NCBI
|