Introduction
Intrahepatic cholangiocarcinoma (ICC), the second
most common primary liver cancer after hepatocellular carcinoma
(HCC), has been categorized as mass-forming type, periductular
infiltrating type and intraductal growth type according to category
of the gross type (1). With a
worldwide increase in the incidence rate in recent years (1–3),
however, ICC remains less understood and always has worse prognosis
compared with HCC (3–5), and practice guideline for treatment
has not been universally accepted and thoroughly studied for ICC in
advanced stage (6). It has been
generally believed that surgical resection is indicated for all
potentially resectable ICC for the benefit of better survival
(3,6–10) and
for the lack of other effective treatment options, but the
prognosis remains dismal because the disease is usually advanced at
the time of diagnosis or after surgery for quite a number of
patients (6,8,11). Up
to 60% of ICC patients presented with disease in so advanced stage
that made them not suitable for curative resection (3,6–8), as
recurrence occurs frequently even after extended surgical resection
(6,8,10).
Although recent studies suggest that curative/palliative resection
can have beneficial impact on overall survival (OS) for some
certain patients with advanced ICC (6,8,10,12–14),
the range or extent of ICC tumor in advanced stage justifying
aggressive surgery, however, has not reached a consensus for
surgeons in different countries.
Current studies of surgical treatments for ICC
usually enrolled patients with tumors in all stages according to
the 7th edition American Joint Committee on Cancer (AJCC) staging
system (8,15–19).
Owing to the relative paucity of this disease, most of the existing
studies, however, usually involved small numbers of cases, spanned
over a study period of decades, or were multicenter results with
very small numbers per center, and in some studies patient
inclusion was highly selective (concentrating on tumors in
AJCC-stage I–III), leading to significant difference in OS among
different studies and to controversies over surgical management of
ICC especially in AJCC-stage IV. So, there is an increasing need
for more comprehensive and more close data from large series to
determine exact role of surgical treatment for ICC in AJCC-stage
IV. In this retrospective study, we investigated surgical outcome,
survival and the prognostic factors in a large series of
consecutive patients with ICC in AJCC-stage IV at the time of
diagnosis or after surgery (all of tumors were locally advanced but
potentially resectable preoperatively) between 2007 and 2011 at a
single institution, with the aim to evaluate safety and efficacy of
surgical treatment, and the prognostic accuracy of AJCC staging
system for patients with ICC in stage IV, and among which, to
identify who would benefit from surgery.
Patients and methods
Patient recruiting and data
collection
All consecutive patients with ICC who were admitted
to the Eastern Hepatobiliary Surgery Hospital, a high-volume center
in China, for initial surgical treatment from January 2007 to
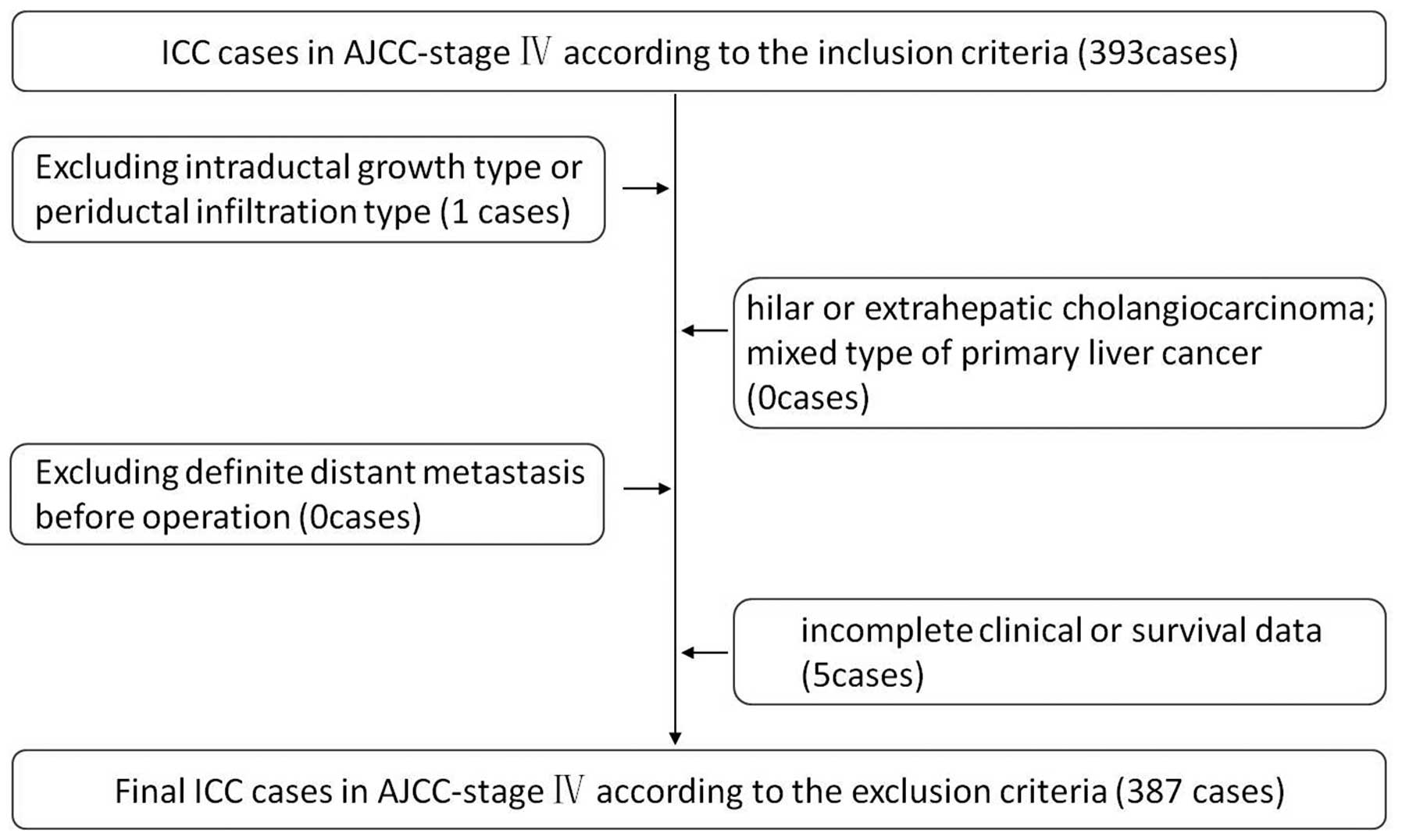
December 2011, were retrospectively reviewed. Inclusion criteria
included the following: no history of previous anticancer therapy;
no history of other malignancies; no severe comorbidity that can
affect survival or act as contra-indications for surgery; all the
tumors were locally advanced but potentially resectable
preoperatively, and were proven to be in stage IV according to AJCC
staging system at the time of diagnosis or after surgery; all of
the cases were histopathologically proven. Exclusion criteria were
as follows: hilar or extrahepatic cholangiocarcinoma; mixed type of
primary liver cancer; intraductal growth type or periductal
infiltration type of ICC (because the case numbers of the other two
pathological type were very small and bio-characteristics of the
other two types were different from mass-forming type); definite
distant metastasis before operation (localized/diffuse occult
distant metastasis within abdomen were sometimes found during
surgical exploration, and some of such patients obtained
resection); and incomplete clinical or survival data (Fig. 1). Demographic data for all patients,
including age, gender, symptoms, underlying liver diseases, image
findings, and laboratory test were collected. This study was
approved by the local Ethics Committee.
Preoperative workup and surgery
Routine preoperative workup consisted of, but was
not limited to, ultrasound scanning, three-phase computed
tomography (CT) or magnetic resonance imaging (MRI) of the liver,
endoscopic examination and laboratory tests. Patients underwent
surgical exploration if preoperative image indicated a potentially
resectable ICC and there were no general contra-indications for
surgery. However, radiographic studies had a limited ability to
determine respectability. Most of the liver resections were carried
out under vascular control, and both anatomical and non-anatomic
hepatectomy were performed depending on the size and location of
tumor. The surgical radicality and margins were evaluated and
examined for the presence of residual tumor which was described by
the following classification: R0, no residual tumor and resection
margin was >0 mm; R1, microscopic residual tumor or resection
margin was nil; R2, macroscopic residual tumor (4,20,21) or
macroscopic unresectable LN metastasis. All of the patients
enrolled in this study underwent resection of lesions with curative
(R0) or relatively curative (R1) intention, even when occult
distant metastasis within abdomen such as localized
peritoneal/diaphragm seeding, and localized omentum metastasis were
found. Palliative resection (R2) intention was never suggested to
apply before operation, neither was exploratory laparotomy with
biopsy, except when the findings of intraoperative exploration were
beyond preoperative evaluation of ICC and R0/R1 resection could not
be obtained, such as disseminated intrahepatic tumor spread,
disseminated peritoneal/diaphragm seeding, diffuse/unresectable
fixed LN metastasis with vascular invasion were found beyond
preoperative evaluation of ICC, depending on intraoperative
assessment of value and safety of surgery. In patients with
suspected LN metastasis (preoperative and during operation), liver
resection together with lymph node dissection (LND) was performed
when possible (regional and extended LND but seldom into the
paraaortic regions); liver resection with only lymph node biopsy
(LNB) was performed, when diffuse/unresectable fixed LN metastasis
with vascular invasion was encountered and the intrahepatic tumor
was localized at the same time; LNB was also performed when the
primary tumors were unresectable to help to confirm metastasis; in
other patients with intrahepatic tumor curatively resected and with
no evidence of macroscopic LN enlargement, preventive
skeletonization of the hepatoduodenal ligament was performed, with
the aim to confirm the stage.
Pathological evaluation
Diagnosis ICC was based on macroscopic examination,
H&E staining, and immunohistochemical study of the resected or
biopsy specimens. Pathological characteristics, including tumor
size and number, capsule formation, LN metastasis, vascular
invasion, perineural invasion and tumor cell differentiation, were
collected for all the patients. Each tumor was staged according to
AJCC staging system for ICC (19,22),
and classified by the category of the gross type of ICC by the
Liver Cancer Study Group of Japan (23). Based on the factors of LN metastasis
and distant metastasis, we divided patients with tumors in stage
AJCC-stage IV B into three subgroups according to our new staging
system: patients with tumors (AnyTN2-3M0), patients with tumors
(AnyTN0-1M1), and patients with tumors (AnyTN2-3M1). Differences of
our new staging system and AJCC 7th staging system are listed in
Table I.
 | Table IDifference between American Joint
Committee on Cancer 7th Edition staging system and our staging
system for intrahepatic cholangiocarcinoma. |
Table I
Difference between American Joint
Committee on Cancer 7th Edition staging system and our staging
system for intrahepatic cholangiocarcinoma.
| AJCC 7th
edition | Present study |
|---|
| TNM
classification | TNM
classification |
| T1 | Solitary tumor
without vascular invasiona | T1 | Solitary tumor
without vascular invasiona |
| T2a | Solitary tumor with
vascular invasiona | T2a | Solitary tumor with
vascular invasiona |
| T2b | Multiple tumors,
with or without vascular invasiona | T2b | Multiple tumors,
with or without vascular invasiona |
| T3 | Tumor perforating
the visceral peritoneum or involving local extrahepatic structures
by direct invasion | T3 | Tumor perforating
the visceral peritoneum or involving local extrahepatic structures
by direct invasion |
| T4 | Tumor with
periductal invasionb | T4 | Tumor with
periductal invasionb |
| N0 | No regional lymph
node metastasis | N0 | No regional lymph
node metastasis |
| N1 | Regional lymph node
metastasis | N1 | Regional lymph node
metastasis |
| | N2–3 | Nodal involvement
of the celiac, periaortic, or caval lymph nodes |
| M0 | No distant
metastasis | M0 | No distant
metastasis |
| M1 | Distant metastasis;
nodal involvement of the celiac, periaortic, or caval lymph nodes
(N2-3) is considered to be M1 | M1 | Distant
metastasis |
| Stage
groupings | | Stage
groupings | |
| Stage I | T1 N0 M0 | Stage I | T1 N0 M0 |
| Stage II | T2 N0 M0 | Stage II | T2 N0 M0 |
| Stage III | T3 N0 M0 | Stage III | T3 N0 M0 |
| Stage IV A | T4 N0 M0, Any T N1
M0 | Stage IV A | T4 N0 M0, Any T N1
M0, AnyTN2-3M0 |
| Stage IV B | AnyTN2-3M0,
AnyTN0M1, AnyTN2-3M1 | Stage IV B | Any T Any N M1 |
Follow-up
All patients were followed up by ultrasound scan and
tests for liver function and the levels of α-fetal protein (AFP),
carbohydrate antigen 19-9 (CA19-9) and carcinoembryonic antigen
(CEA) at an interval of 1 month for the first year, 1–2 months for
the second year, and every 3 months the year after, if there was no
recurrence. When tumor recurrences were suspected, CT or MRI scan
would be performed to confirm the diagnosis. Treatments of
recurrent disease included surgery, transarterial chemoembolization
(TACE), radiotherapy, and supportive therapy. Patients were
followed for survival until death or the study deadline date of 30
September, 2014.
Statistical analysis
Continuous variables were presented as the mean ±
SD. Independence tests were performed using unpaired t-test for
continuous variables and Chi-square or Wilcoxon test for
categorical variables. OS rates were calculated with the
Kaplan-Meier method. The possible prognostic factors were analyzed
by the univariate analysis and evaluated using the Kaplan-Meier
method and compared by the log-rank test. The multivariate analysis
was performed using the Cox proportional hazards model to identify
the independent prognostic factors. Statistical analysis was
performed with SPSS for Windows (version 19; SPSS, Inc., Chicago,
IL, USA). Statistical significance was defined as P<0.05.
Results
Demographic and clinicopathological
features
Between January 2007 and December 2011, a total of
387 surgically treated patients with mass-forming (MF) type of ICC
in AJCC-stage IV were included in this study, among which, 298
patients had ICC in AJCC-stage IV A, while 89 patients were in
AJCC-stage IV B. Among patients with ICC in AJCC-stage IV B, 23
patients had ICC in AnyTN2-3M0 stage, 12 patients had ICC in
AnyTN0-1M1 stage, and 54 patients had ICC in AnyTN2-3M1 stage
according to our new staging system. There were 256 (66.1%) males
and 131 (33.9%) females, with a mean age of 54.04±11.17 years
(range, 18–82 years). At diagnosis, most patients (76.7%) had
symptoms such as epigastric pain, hepatomegaly and jaundice. In
some patients, ICC was accompanied by other concurrent liver
diseases, including hepatitis B virus HBV infection (33.1%),
cirrhosis 198 patients(14.0%). Elevated serum levels of CA19-9
and/or CEA were detected in 288 (74.4%) cases. At pathological
examination, 189 patients (48.8%) had solitary tumor whereas
(51.2%) had multiple tumors, and the mean tumor size was 7.83±3.60
cm (range, 0.4–21.0 cm), with a median tumor size of 7.0 cm. ICC
with vascular invasion was found in 47 patients (12.1%), with
perineural invasion in 41 patients (10.6%), and with capsule
formation in 2 patients (0.5%). Difference of clinicopathological
characteristics in the patients with tumors in stage IV A and IV B
are listed in Table II, and with
more details in Table III.
 | Table IIComparisons of clinicopathological
characteristics in the patients with tumors in stage IV A and IV
B. |
Table II
Comparisons of clinicopathological
characteristics in the patients with tumors in stage IV A and IV
B.
|
Characteristics | IV A (N=298) | IV B (N=89) | P-value |
|---|
| Age (years) | 53.73±11.43 | 55.06±10.23 | 0.327 |
| Gender | | | 0.774 |
| Male | 196 (65.8) | 60 (67.4) | |
| Female | 102 (34.2) | 29 (32.6) | |
| HBsAg (+) | 104 (34.9) | 24 (27.0) | 0.163 |
| Cirrhosis | 42 (14.1) | 12 (13.5) | 0.884 |
| CA19-9 (+) and/or
CEA (+) | 216 (72.5) | 72 (80.9) | 0.110 |
| Tumor size
(cm)a | 7.46±3.46 | 9.05±3.81 | <0.001 |
| Tumor number | | | 0.006 |
| Single | 157 (52.7) | 32 (36.0) | |
| Multiple | 141 (47.3) | 57 (64.0) | |
| Capsule
formation | 1 (0.3) | 1 (1.1) | 0.363 |
| Lymphatic
metastasis | 298 (100) | 77 (86.5) | <0.001 |
| Vascular
invasion | 39 (13.1) | 8 (9.0) | 0.299 |
| Perineural
invasion | 33 (11.1) | 8 (9.0) | 0.575 |
| Surgical margin
status | | | <0.001 |
| R0 | 22 (7.4) | 0 (0) | |
| R1 | 121 (40.6) | 12 (13.5) | |
| R2 | 141 (47.3) | 47 (52.8) | |
| Exploration | 14 (4.7) | 30 (33.7) | |
 | Table IIIComparisons of clinicopathological
characteristics in the patients with tumors in different stages (IV
A, AnyTN2-3M0, AnyTN0-1M1, and AnyTN2-3M1). |
Table III
Comparisons of clinicopathological
characteristics in the patients with tumors in different stages (IV
A, AnyTN2-3M0, AnyTN0-1M1, and AnyTN2-3M1).
|
Characteristics | IVA
(N=298) |
AnyTN2-3M0
(N=23) |
AnyTN0-1M1
(N=12) |
AnyTN2-3M1
(N=54) | P-value |
|---|
| Age (years) | 53.73±11.43 | 56.52±9.95 | 57.50±6.38 | 53.89±10.98 | 0.472 |
| Gender | | | | | 0.896 |
| Male | 196 (65.8) | 16 (69.6) | 9 (75.0) | 35 (64.8) | |
| Female | 102 (34.2) | 7 (30.4) | 3 (25.0) | 19 (35.2) | |
| HBsAg (+) | 104 (34.9) | 7 (30.4) | 1 (8.3) | 16 (29.6) | 0.248 |
| Cirrhosis | 42 (14.1) | 6 (26.1) | 0 (0) | 6 (11.1) | 0.162 |
| CA19-9 (+) and/or
CEA (+) | 216 (72.5) | 17 (73.9) | 8 (66.7) | 47 (87.0) | 0.14 |
| Tumor size
(cm) | 7.46±3.46 | 7.60±2.96 | 10.69±3.24 | 9.31±4.08 | <0.001 |
| Tumor number | | | | | <0.001 |
| Single | 157 (52.7) | 17 (73.9) | 2 (16.7) | 13 (24.1) | |
| Multiple | 141 (47.3) | 6 (26.1) | 10 (83.3) | 41 (75.9) | |
| Capsule
formation | 1 (0.3) | 0 (0) | 0 (0) | 1 (1.9) | 0.523 |
| Lymphatic
metastasis | 298 (100) | 23 (100) | 0 (0) | 54 (100) | <0.001 |
| Vascular
invasion | 39 (13.1) | 6 (26.1) | 0 (0) | 2 (3.7) | 0.021 |
| Perineural
invasion | 33 (11.1) | 4 (17.4) | 1 (8.3) | 3 (5.6) | 0.439 |
| Surgical margin
status | | | | | <0.001 |
| R0 | 22 (7.4) | 0 (0) | 0 (0) | 0 (0) | |
| R1 | 121 (40.6) | 6 (26.1) | 1 (8.3) | 5 (9.3) | |
| R2 | 141 (47.3) | 17 (73.9) | 2 (16.7) | 28 (51.9) | |
| Exploration | 14 (4.7) | 0 (0) | 9 (75.0) | 21 (38.9) | |
Surgical results
Of the 387 ICC patients, 343 received liver
resection, with an overall resectability rate of 88.7%. R0, R1 and
R2 resections were obtained in 22 (5.7%), 133 (34.3%) and 188
(48.6%) patients, respectively, and the remaining 44 (11.4%)
patients had only laparotomy and biopsy (tumor and/or LN) because
of extensive intrahepatic metastases or peritoneal seeding. LN
metastasis occurred in 375 (96.9%) patients, among which, 154
patients obtained complete LND, including LN around the
hepatoduodenal ligament, the left gastric artery, the common
hepatic artery, the celiac trunk, and even the paraaortic regions,
in addition to liver resection. In other 186 patients with LN
metastasis, liver resection with only LNB was performed, which were
the mainstay of R2 resection in this study, with residual tumor in
the liver as the second most common cause. The remaining 35
patients with LN metastasis were among those who had only
laparotomy exploration and biopsy. In 12 patients without LN
metastasis, 3 patients obtained tumor resection with preventive
skeletonization of the hepatoduodenal ligament, the other 9
patients had only laparotomy exploration and biopsy of tumor.
Operative death, which was defined as death within 30 days of
surgery or death that occurred during same admission period
(24), occurred in three cases with
an operative mortality of 0.8%. Of the three operative deaths, two
cases occurred in IV A group, while the other one occurred in
AnyTN2-3M1 group, and all of these cases occurred in R2 resection
group. According to the Clavien-Dindo classification (24), postoperative complications developed
in 14 (3.6%) cases, with 10/298 (3.4%) in IV A group, 2/23 (8.7%)
in AnyTN2-3M0 group, 1/12 (8.3%) cases in AnyTN0-1M1 group, and
1/54 (1.9%) in AnyTN2-3M1 group. Of the cases with postoperative
complications, 5/133 cases (3.8%) occurred in R1 resection group,
9/188 cases (4.8%) occurred in R2 resection group, and there were
no cases in R0 resection group and laparotomy group. Except 3
patients who died of hepatic failure or multiple organ failure, all
the patients successfully recovered from the complications.
Adjuvant therapy
Adjuvant chemotherapy was not recommended for
patients with R0 resection, while 42.9% (57) of patients with R1
resection received transarterial chemoembolization (TACE) 4 weeks
after surgery and 31.5% (73) of the patients with unresectable
disease or with R2 resection received adjuvant chemo/radiotherapy.
The most common chemotherapy regimen was 5-fluorouracil (FU)
combined with cisplatin and gemcitabine, and three dimensional
conformal radiotherapy was the standard radiation therapy that was
used mainly for residual positive LN.
Survival
Tumor stage and and its influence on
survival
The median follow-up period was 47 months (range,
1–93 months). The overall 1-, 3- and 5-year survival rates of the
whole cohort were 31.3, 6.7 and 1.6%, respectively, with a median
survival time (MST) of 8.1 months (range, 1–89 months). The 1-, 3-
and 5-year OS rates of patients with stage IV A tumors were 35.9,
7.7 and 1.9%, respectively (MST, 9.0 months); with corresponding
rates of 15.7, 3.4 and 0% for patients with stage IV B tumors,
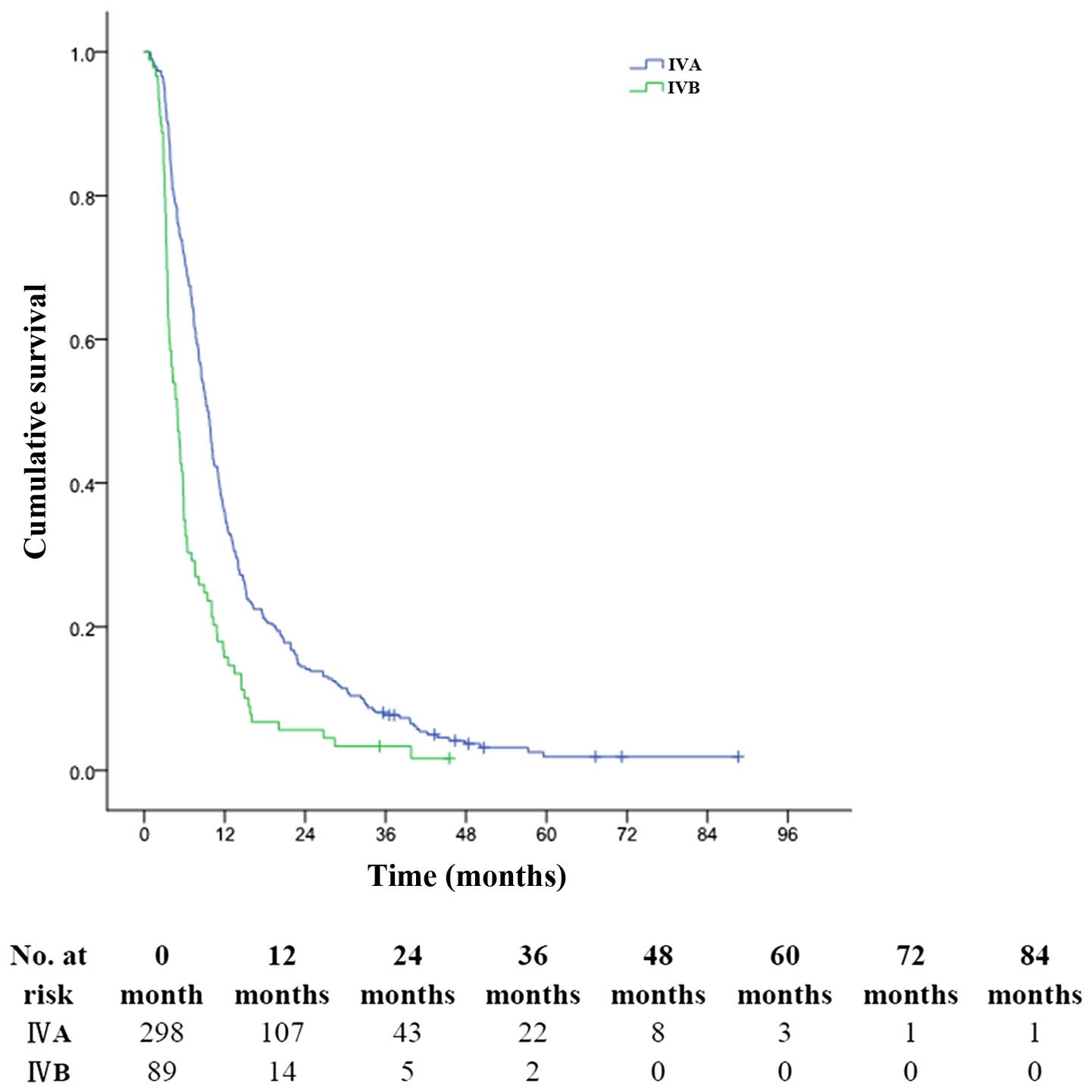
respectively (MST, 5.0 months) (P<0.001, Fig. 2). The 1-, 3- and 5-year OS rates for
patients with stage AnyTN2-3M0 tumors were 30.4, 8.7 and 0.0%,
respectively (MST, 9.0 months); much better than 8.3, 0.0 and 0.0%
for patients with stage AnyTN0M1 tumors (MST, 3.0 months)
(P<0.001, Fig. 3); and better
than 11.1, 1.9 and 0.0% for patients with stage AnyTN2-3M1 tumors
(MST, 4.0 months) (P<0.001, Fig.
3).
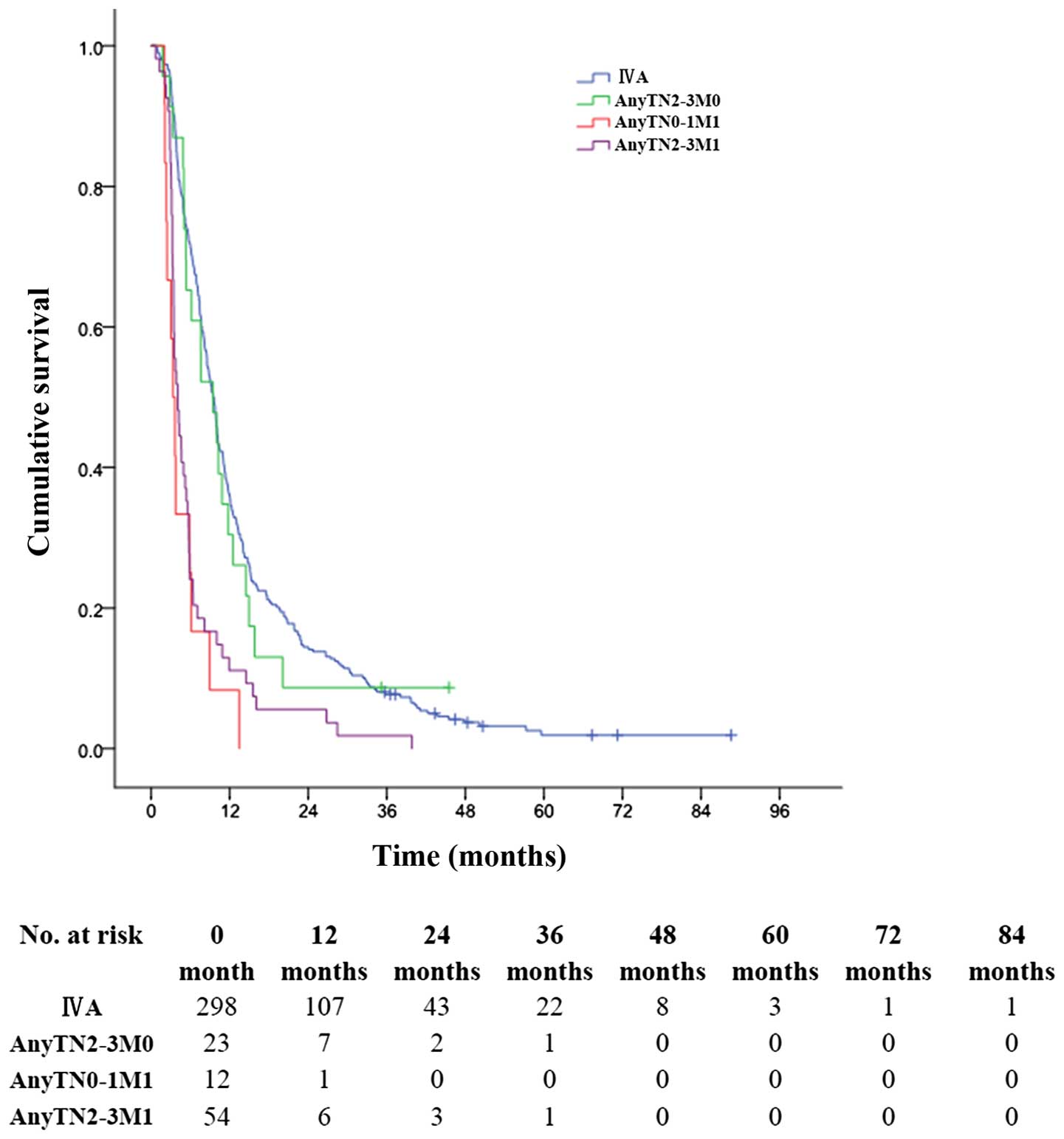 | Figure 3OS for patients with ICC in different
subgroups after surgery. The 1-, 3- and 5-year OS rates for
patients with stage IV A tumors were 35.9, 7.7 and 1.9%,
respectively, better than corresponding rates of 30.4, 8.7 and 0%,
respectively, for patients with tumors in stage AnyTN2-3M0; much
better than 8.3, 0 and 0%, respectively, for patients with tumors
in stage AnyTN0-1M1; and than 11.1, 1.9 and 0%, respectively, for
patients with tumors in stage AnyTN2-3M1 (P<0.001). |
Status of residual tumor after surgery
and its influence on survival
In patients with ICC in AJCC-stage IV, those who
obtained R0 resection, the 1-, 3- and 5-year OS rates were 68.2,
13.6 and 0%, respectively, with an MST of 14.0 months; while for
those who obtained R1 resection, the 1-, 3- and 5-year OS rates
were 49.6, 11.9 and 3.3%, respectively, with an MST of 12.0 months
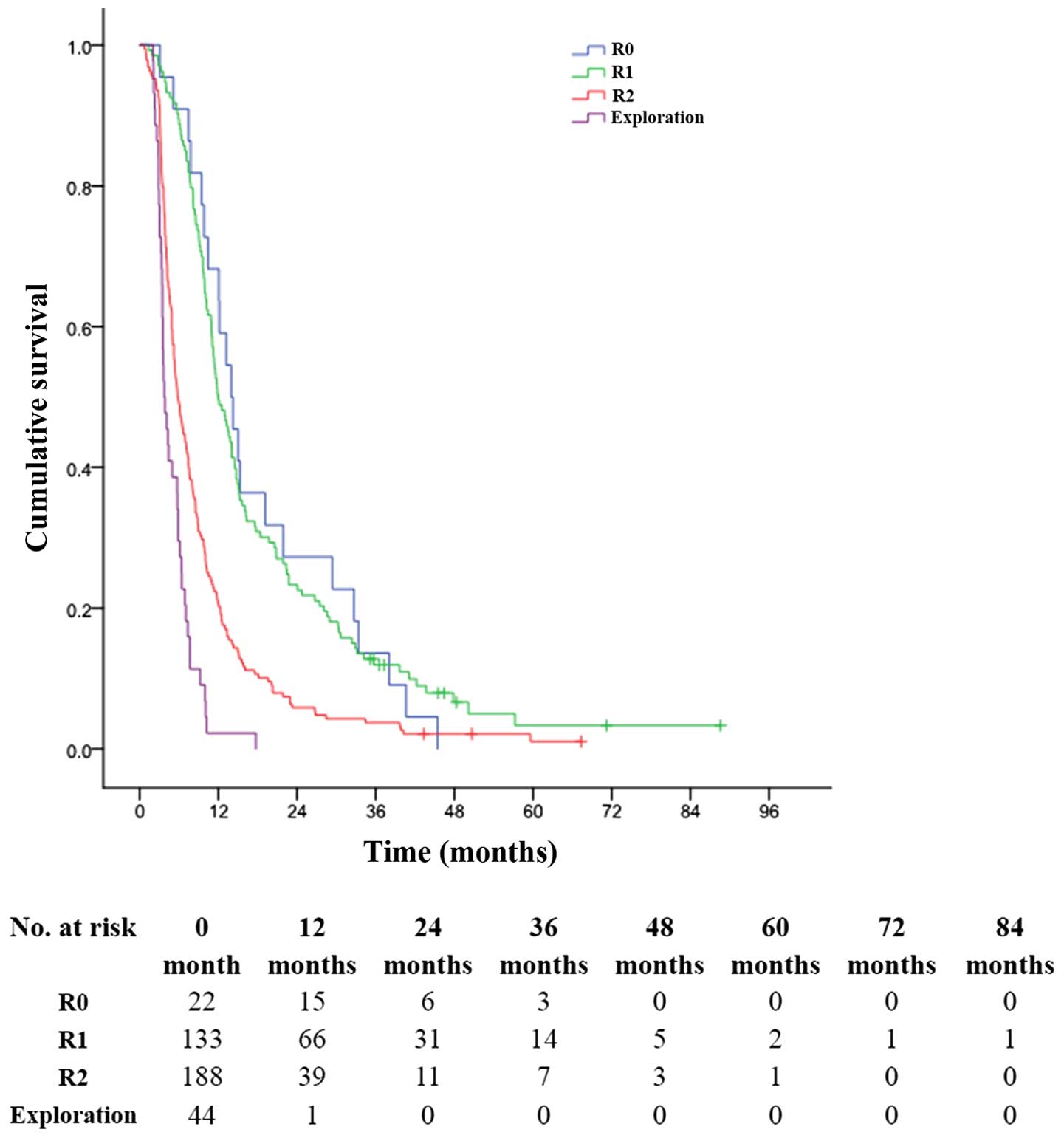
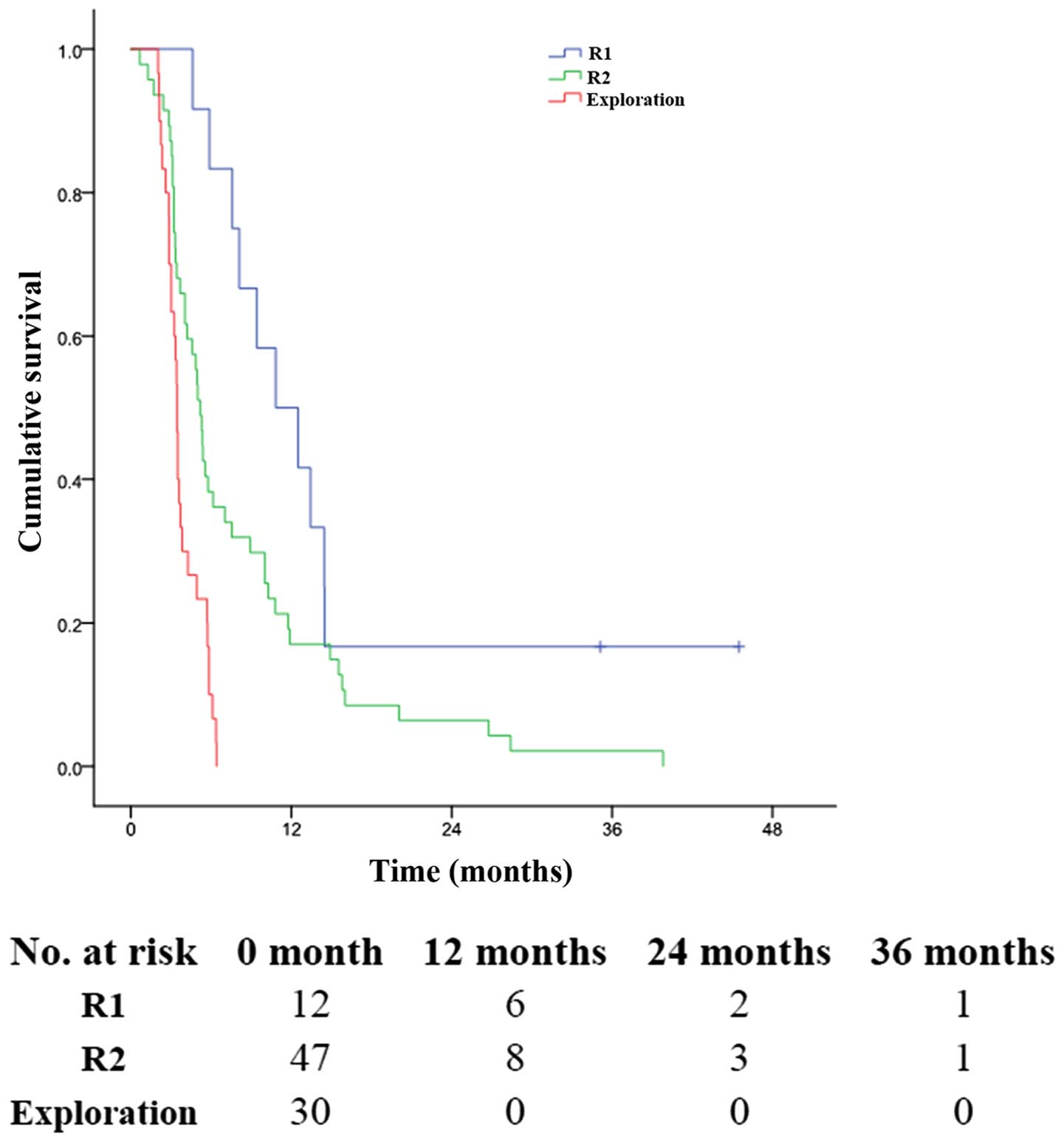
(P<0.001, Fig. 4). The 1-, 3-
and 5-year OS rates were 20.7, 3.7 and 1.1%, respectively, in
patients with R2 resection (MST, 6.0 months), much better than 2.3,
0.0 and 0.0% in patients with only exploratory laparotomy and
biopsy (MST, 4.0 months) (P<0.001, Fig. 4).
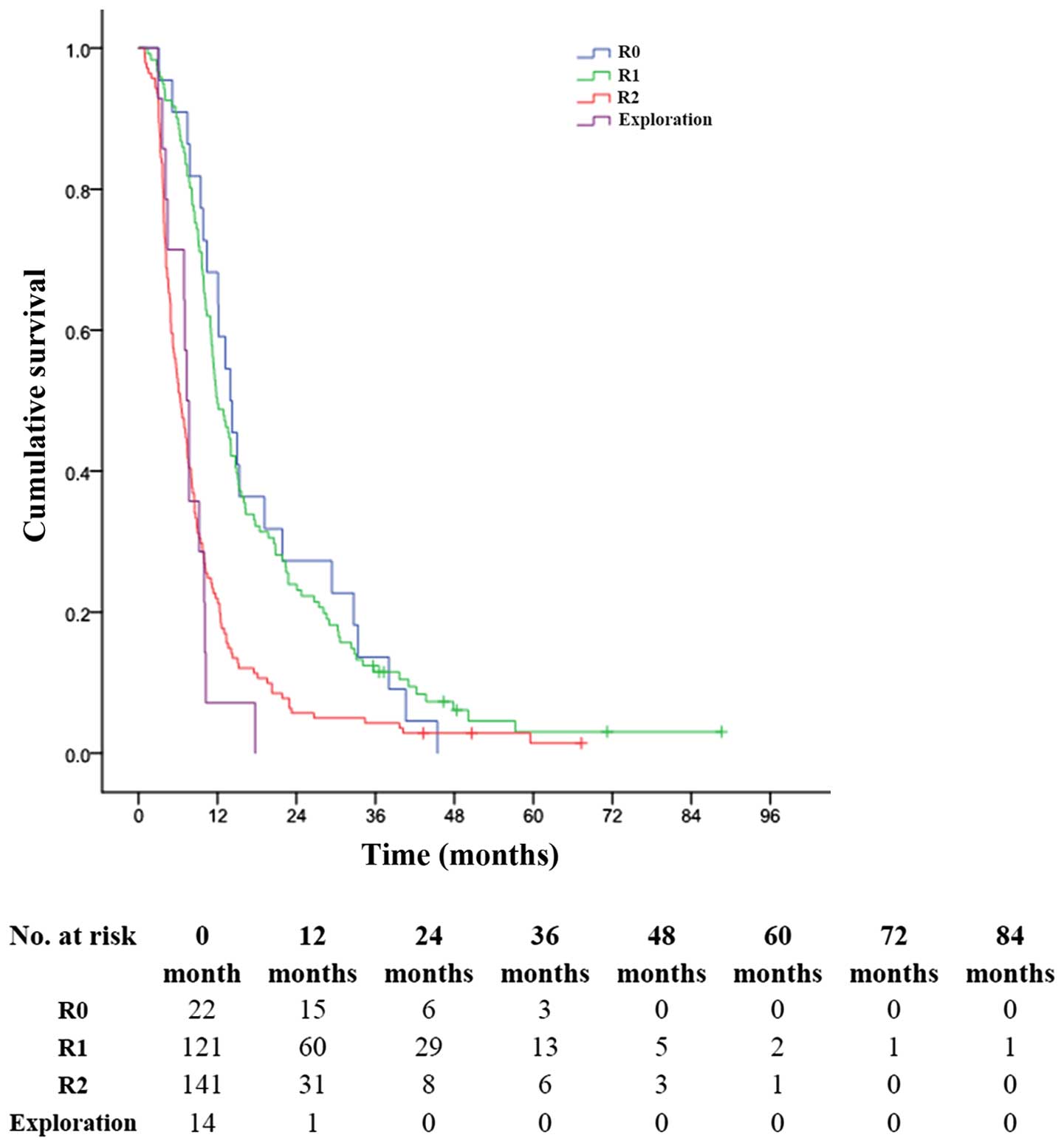
In patients with ICC in AJCC-stage IV A, those who
obtained R0 resection, the 1-, 3- and 5-year OS rates were 68.2,
13.6 and 0%, respectively, with an MST of 14.0 months; while for
those who obtained R1 resection, the 1-, 3- and 5-year OS rates
were 49.6, 11.5 and 3.1%, respectively, with an MST of 12.0 months
(P<0.001, Fig. 5). The 1-, 3-
and 5-year OS rates were 22.0, 4.3 and 1.4%, respectively, in
patients with R2 resection (MST, 6.0 months), much better than 7.1,
0.0 and 0.0% in patients with only exploratory laparotomy and
biopsy (MST, 7.0 months) (P<0.001, Fig. 5).
In patients with ICC in AJCC-stage IV B, nobody
obtained R0 resection in this group, and the 1-, 3- and 5-year OS
rates were 50.0, 16.7 and 0.0%, respectively, for those who
obtained R1 resection (MST, 11.0 months). The 1-, 3- and 5-year OS
rates were 17.0, 2.1 and 0.0%, respectively, in patients with R2
resection (MST, 5.0 months), much better than 0.0, 0.0 and 0.0% in
patients with only exploratory laparotomy and biopsy (MST, 3.0
months) (P<0.001, Fig. 6).
Status of the lymph node and its
influence on survival
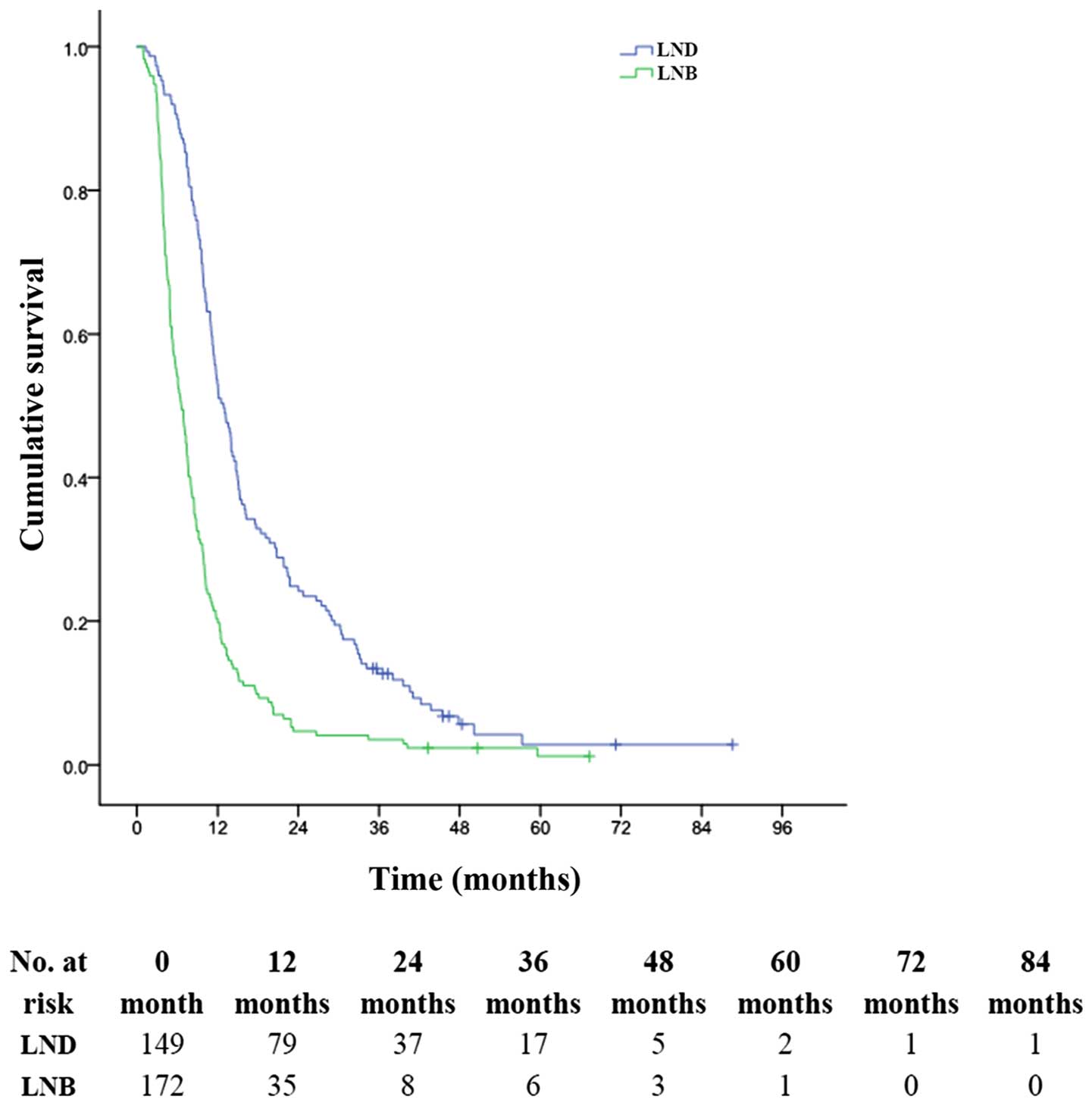
In this study, the patients with LN metastasis had
1-, 3- and 5-year OS rates of 32.0, 6.9 and 1.7%, respectively
(MST, 8.0 months) significantly longer than those without LN who
had 1-, 3- and 5-year OS rates of 8.3, 0 and 0%, respectively (MST,
3.0 months). Among the patients with LN metastasis and without
distant metastasis (stage IV A + AnyTN2-3M0), the 1-, 3- and 5-year
OS rates were 53.0, 12.7 and 2.8%, respectively (MST, 13.0 months)
for patients undergoing LND, and 20.3, 3.5 and 1.2%, respectively
(MST, 7.0 months) for patients who underwent LNB (P<0.001,
Fig. 7).
Discussion
To the best of our knowledge, this is the largest
study involving surgically treated patients with ICC in AJCC-stage
IV, so the results of our study would reflect the current profile
of surgical treatment of such patients. ICC has been proved to be a
highly malignant neoplasm, and extraordinary attention has been
paid on the factors that influence the prognosis of ICC following
surgical resection in our previous study (25) and other reports (4,12,15–18,21,26–31),
but these studies usually related to potential resectable ICC in
all stages, and never focused only on ICC in AJCC-stage IV. We
would not operate on patients with M1 disease definitely diagnosed
before operation, however, occult distant metastasis within abdomen
was sometimes found during surgical exploration, and some of such
patients obtained resection, thus, it turned out that patients with
M1 disease were enrolled in this study. Although patients with ICC
in stage IV would rarely undergo a resection in the Western world,
our study demonstrated that surgery is still valuable for at least
part of these patients, because natural history without any
treatment for ICCs in advanced stage was even worse with an MST of
only 3.0 months (203 cases) (32),
and similiar patients with supportive therapy alone had a MST of
4.9 months (12 cases) (33). An MST
of 12.9 months was reported for advanced ICC by palliative
chemoradiotherapy (33), which
seemed superior than our result (MST, 8.1 months), however, the
number of reported patients was small (28 cases) and the evidence
was weak. This study demonstrated that the prognosis of ICC in
AJCC-stage IV was unfavorable even after surgical management, with
an MST of 8.1 months, because compared with the former studies
(4,6,8,11,12,15–18,25,27),
ICC in this series was more frequently associated with lymph node
involvement, multiple tumors, peritoneal dissemination, and low R0
resection rate. However, such patients could definitely benefit
from surgery when R0 or R1 resection was available.
Generally speaking, resection was attempted for ICC
whenever possible (3,6–11),
leading to varied surgical margin statuses, such as R0, R1, R2
resection, and exploratory laparotomy. Our study, like some
reported studies (6,12,21,34),
showed that the resection margin status was an independent variable
that influenced the prognosis of ICC following surgery. In this
series (Fig. 3), surgery was again
proven valuable for that R0 resection (MST, 14.0 months) and R1
resection (MST, 12.0 months) provided better survival than R2
resection (MST, 6.0 months) and non-resective biopsy (MST, 4.0
months). However, R0 resection is not feasible in a considerable
proportion especially for ICC cases with tumor in stage IV, and in
this study, only ~5.7% patients obtained R0 resection, much lower
than that of our previous study (25) and the literatures (ranging from 19.8
to 80.0%) (3–5,12,15–17,21,
26–31,35,36),
owing to different inclusion criteria. However, in present series,
the overall resectability rate was 88.7%, which was, to our
knowledge, much higher than the reported series including patients
with tumors even in earlier stage (ranging from 18 to 77%)
(7,8,10,15,17,23,30).
All these data might account for low R0 resection rate in this
study, which was suggested to be responsible for poor prognosis of
ICC in stage IV following surgery, and R0 resection is definitely
recommended and valuable when possible for such patients.
In the present study, R1 resection occurred in 34.4%
of the whole cohort, owing to disadvantageous factors such as large
tumors (median size, 7.0 cm), multiple tumors (51.2%), centrally
located tumors, and incomplete tumor capsule with ill-defined
borders. Although in this study, R1 resection had significant
unfavorable influence on patient survival when compared to R0
resection (MST: 14 months vs 12 months), and although there has
been controversy about the effect of R1 resection on patient
survival in the literature (4,6,12,16,20,21,28,34),
patients obtained R1 resection survived significantly longer than
those with R2 resection or laparotomy (Figs. 4Figure 5–6). Therefore, R1 resection is recommended
when R0 resection cannot be obtained, not only for its relative
curativity but also for ubiquitous unfavorable pathological
features of ICC in stage IV.
Previously it was suggested that all types of
cancer-directed surgery including palliative surgery may offer a
survival advantage over no surgery (32,37).
Controversy exists over the justification of R2 resection. Some
authors reported that R2 resection did not provide any survival
benefit but only bore the risks of major hepatic surgery (6,34,38),
whereas others believed that some patients could benefit from R2
resection (12,13,36).
In current series, more than half of the patients with LN
metastasis obtained only liver resection with residue positive
nodal, which was the most common cause of R2. Although patients
obtained R2 resection survived a little longer than those
undergoing only exploratory laparotomy (MST, 6.0/4.0 months)
(Figs. 4Figure 5–6), it showed no significant advantage over
those with chemoradiotherapy, or supportive therapy alone, or
without any treatment (32,33). Thus, during surgical exploration, R2
resection is not recommended.
Up till now, routine lymphadenectomy did not receive
unified agreement during surgery especially for ICC in stage IV,
not only considering the stage of tumor and the extent of LN
metastasis, but also as the debate existed both in the literatures
(9,11,29,39–42)
and within the surgeons of our hospital for whether such patients
could necessarily benefit from standardized LN dissection for
prolonged survival. In current series, complete LND was achieved in
154 patients with relatively localized tumors and better range of
LN metastasis, while 221 patients underwent LNB because of either
diffuse/unresectable fixed LN metastasis or unresectable tumor.
Although observations in hilar cholangiocarcinoma (43) and ICC (8) revealed that paraaortic lymphadenectomy
may result in improved long-term survival in macroscopically
negative but microscopically positive paraaortic LN, extended LN
dissection was obtained in celiac trunk, retropancreatic and even
into paraaortic regions only in a small number of patients (R1
resection in IV B) give consideration to the unsatisfactory
surgical radicality of primary liver tumor itself. In this study,
results showed that patients with liver resection with LN
dissection survived significantly longer than those with LN biopsy
for patients without distant metastasis (stage IV A + AnyTN2-3M0),
suggesting that LN dissection should be performed when suitable for
the benefit of better survival.
Different from former studies (9,11,25,29,39–42),
LN metastasis, one of the key elements constituting AJCC staging
system (19,22), was proved to be a favorable
prognostic factor for ICC patients only in this series. In fact,
there were 375 (96.9%) patients with LN metastasis in this cohort,
with 298 (100%) patients in IV A group and 77 (86.5%) patients in
IV B group, leading to more advanced stage and worse prognosis for
tumors of patients without LN metastasis. On the other hand, in IV
B group of this series, patients with extended LN metastasis (N2-3)
may not have significantly poorer survival than those with regional
(N1) LN metastasis or without LN metastasis (Fig. 2), was inconsistent with current AJCC
staging system (19,22), where extended LN metastasis (N2-3)
was emphasized as an extremely poor prognostic factor in such a
strong way that it was classified as distant metastasis (M1). In
this study, we found that, patients in group IV A and/or subgroup
AnyTN2-3M0 had better survival than in subgroup AnyTN0-1M1 and/or
AnyTN2-3M1, while survival was better in patients in subgroup
AnyTN2-3M1 than subgroup AnyTN0-1M1. Survival was similar between
patients in group IV A and subgroup AnyTN2-3M0. These results all
suggested that M1 was a stronger poor prognostic factor for ICC
than extended LN metastasis (N2-3), which was different from
opinions of current AJCC staging system (19,22).
Moreover, the above data of our study indicated that, using N2-3
instead of M1 to classify extended LN metastasis with involvement
of the celiac, periaortic, or caval lymph nodes was more accurate
not only in staging ICC but also in estimating survival of patients
with ICC
A prognostic and staging system of ICC should be
developed based on the outcomes of all the cases for providing
guideline on the appropriate of surgery as treatment option. An
evaluation of the prognostic accuracy of the 7th edition AJCC
staging system is missing for ICC in AJCC-stage IV as yet.
Specifically, the N and M classification of current AJCC staging
system for ICC seemed to be prognostically inaccurate. We use N2-3
instead of M1 for extended LN metastasis classification to stage
advanced ICC, thus ICC in stage IV B (AJCC 7th) were divided into
three subgroups: AnyTN2-3M0, AnyTN0-1M1, and AnyTN2-3M1. The 1- and
3-year OS for patients in group AnyTN2-3M0 were different but close
to group IV A; while the 1- and 5-year OS for patients in group
AnyTN0M1 were different but close to group AnyTN2-3M1. Thus,
AnyTN2-3M0 should be included in stage IV A, while AnyTN0-1M1 and
AnyTN2-3M1 should be included in stage IV B (Table I). This classification was more
precise for prediction of outcome in patients with advanced
ICC.
However, the current study has several limitations.
It is a retrospective single institution study over a 5-year
period, during which significant changes in surgical technic,
adjuvant therapies and standpoints of surgeons occurred. Our study
represents more recent data compared with the former studies in the
literature. In addition, there is likely a selection bias; patients
with unreconstructable vasculature, significant preoperative
comorbidity, and extremely advanced tumor biology (as demonstrated
by rapid tumor progression or metastases) were likely not offered
resection and thus not included in this study.
In conclusion, liver resections, including R0 and R1
resections, could provide survival benefit to the patients with ICC
in stage IV. Patients with ICC in stage IV A and AnyTN2-3M0 are
candidates for surgery especially when R0 or R1 resections could be
obtained, not only for safety, but also for possibility of
long-term survival in these patients. R2 resection should be
avoided for patients with ICC in both stage IV A and IV B, for
whom, surgery should be attempted if there are no sign of M1 before
operation, and should be ceased when any evidence of M1 was found
during surgical exploration. The current AJCC staging system on N
and M classification for ICC does not accurately stratify patients
with regard to prognosis. Staging of advanced ICC by N2-3 instead
of M1 for extended LN metastasis classification is superior in
comparison with current AJCC staging system.
Abbreviations:
|
AJCC
|
American Joint Committee on Cancer
|
|
HBV
|
hepatitis B virus
|
|
HBsAg
|
hepatitis B surface antigen
|
|
ICC
|
intrahepatic cholangiocarcinoma
|
|
HCC
|
hepatocellular carcinoma
|
|
LN
|
lymph node
|
|
CA19-9
|
carbohydrate antigen 19-9
|
|
CEA
|
carcinoembryonic antigen
|
|
AFP
|
α-fetoprotein
|
|
OS
|
overall survival
|
|
MST
|
median survival time
|
References
|
1
|
Sempoux C, Jibara G, Ward SC, Fan C, Qin
L, Roayaie S, Fiel MI, Schwartz M and Thung SN: Intrahepatic
cholangiocarcinoma: New insights in pathology. Semin Liver Dis.
31:49–60. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shaib YH, Davila JA, McGlynn K and
El-Serag HB: Rising incidence of intrahepatic cholangiocarcinoma in
the United States: A true increase? J Hepatol. 40:472–477. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Endo I, Gonen M, Yopp AC, Dalal KM, Zhou
Q, Klimstra D, D'Angelica M, DeMatteo RP, Fong Y, Schwartz L, et
al: Intrahepatic cholangiocarcinoma: Rising frequency, improved
survival, and determinants of outcome after resection. Ann Surg.
248:84–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tamandl D, Herberger B, Gruenberger B,
Puhalla H, Klinger M and Gruenberger T: Influence of hepatic
resection margin on recurrence and survival in intrahepatic
cholangiocarcinoma. Ann Surg Oncol. 15:2787–2794. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou XD, Tang ZY, Fan J, Zhou J, Wu ZQ,
Qin LX, Ma ZC, Sun HC, Qiu SJ, Yu Y, et al: Intrahepatic
cholangiocarcinoma: Report of 272 patients compared with 5,829
patients with hepatocellular carcinoma. J Cancer Res Clin Oncol.
135:1073–1080. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lang H, Sotiropoulos GC, Frühauf NR,
Dömland M, Paul A, Kind EM, Malagó M and Broelsch CE: Extended
hepatectomy for intrahepatic cholangiocellular carcinoma (ICC):
When is it worthwhile? Single center experience with 27 resections
in 50 patients over a 5-year period. Ann Surg. 241:134–143.
2005.
|
|
7
|
Tan JC, Coburn NG, Baxter NN, Kiss A and
Law CH: Surgical management of intrahepatic cholangiocarcinoma - a
population-based study. Ann Surg Oncol. 15:600–608. 2008.
View Article : Google Scholar
|
|
8
|
Jonas S, Thelen A, Benckert C, Biskup W,
Neumann U, Rudolph B, Lopez-Hänninen E and Neuhaus P: Extended
liver resection for intrahepatic cholangiocarcinoma: A comparison
of the prognostic accuracy of the fifth and sixth editions of the
TNM classification. Ann Surg. 249:303–309. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ohtsuka M, Ito H, Kimura F, Shimizu H,
Togawa A, Yoshidome H and Miyazaki M: Results of surgical treatment
for intrahepatic cholangiocarcinoma and clinicopathological factors
influencing survival. Br J Surg. 89:1525–1531. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakagohri T, Asano T, Kinoshita H,
Kenmochi T, Urashima T, Miura F and Ochiai T: Aggressive surgical
resection for hilar-invasive and peripheral intrahepatic
cholangiocarcinoma. World J Surg. 27:289–293. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shimada M, Yamashita Y, Aishima S, Shirabe
K, Takenaka K and Sugimachi K: Value of lymph node dissection
during resection of intrahepatic cholangiocarcinoma. Br J Surg.
88:1463–1466. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Konstadoulakis MM, Roayaie S, Gomatos IP,
Labow D, Fiel MI, Miller CM and Schwartz ME: Fifteen-year,
single-center experience with the surgical management of
intrahepatic cholangiocarcinoma: Operative results and long-term
outcome. Surgery. 143:366–374. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lang H, Sotiropoulos GC, Sgourakis G,
Schmitz KJ, Paul A, Hilgard P, Zöpf T, Trarbach T, Malagó M, Baba
HA, et al: Operations for intrahepatic cholangiocarcinoma:
Single-institution experience of 158 patients. J Am Coll Surg.
208:218–228. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Urahashi T, Yamamoto M, Ohtsubo T,
Katsuragawa H, Katagiri S and Takasaki K:
Hepatopancreatoduodenectomy could be allowed for patients with
advanced intrahepatic cholangiocarcinoma. Hepatogastroenterology.
54:346–349. 2007.PubMed/NCBI
|
|
15
|
Wu ZF, Zhang HB, Yang N, Zhao WC, Fu Y and
Yang GS: Postoperative adjuvant transcatheter arterial
chemoembolisation improves survival of intrahepatic
cholangiocarcinoma patients with poor prognostic factors: Results
of a large monocentric series. Eur J Surg Oncol. 38:602–610. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cho SY, Park SJ, Kim SH, Han SS, Kim YK,
Lee KW, Lee SA, Hong EK, Lee WJ and Woo SM: Survival analysis of
intrahepatic cholangiocarcinoma after resection. Ann Surg Oncol.
17:1823–1830. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shen WF, Zhong W, Xu F, Kan T, Geng L, Xie
F, Sui CJ and Yang JM: Clinicopathological and prognostic analysis
of 429 patients with intrahepatic cholangiocarcinoma. World J
Gastroenterol. 15:5976–5982. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Uenishi T, Yamazaki O, Yamamoto T,
Hirohashi K, Tanaka H, Tanaka S, Hai S and Kubo S: Serosal invasion
in TNM staging of mass-forming intrahepatic cholangiocarcinoma. J
Hepatobiliary Pancreat Surg. 12:479–483. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Edge S, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th edition.
Springer; New York, NY: 2010
|
|
20
|
Farges O, Fuks D, Boleslawski E, Le Treut
YP, Castaing D, Laurent A, Ducerf C, Rivoire M, Bachellier P,
Chiche L, et al: Influence of surgical margins on outcome in
patients with intrahepatic cholangiocarcinoma: A multicenter study
by the AFC-IHCC-2009 study group. Ann Surg. 254:824–829; discussion
830. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shimada K, Sano T, Sakamoto Y, Esaki M,
Kosuge T and Ojima H: Clinical impact of the surgical margin status
in hepatectomy for solitary mass-forming type intrahepatic
cholangiocarcinoma without lymph node metastases. J Surg Oncol.
96:160–165. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liver Cancer Study Group of Japan: The
general rules for the clinical and pathological study of primary
liver cancer. Jpn J Surg. 19:98–129. 1989. View Article : Google Scholar
|
|
24
|
Clavien PA, Barkun J, de Oliveira ML,
Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J,
Slankamenac K, Bassi C, et al: The Clavien-Dindo classification of
surgical complications: Five-year experience. Ann Surg.
250:187–196. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luo X, Yuan L, Wang Y, Ge R, Sun Y and Wei
G: Survival outcomes and prognostic factors of surgical therapy for
all potentially resectable intrahepatic cholangiocarcinoma: A large
single-center cohort study. J Gastrointest Surg. 18:562–572. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Y, Li J, Xia Y, Gong R, Wang K, Yan
Z, Wan X, Liu G, Wu D, Shi L, et al: Prognostic nomogram for
intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin
Oncol. 31:1188–1195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tamandl D, Kaczirek K, Gruenberger B,
Koelblinger C, Maresch J, Jakesz R and Gruenberger T: Lymph node
ratio after curative surgery for intrahepatic cholangiocarcinoma.
Br J Surg. 96:919–925. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Paik KY, Jung JC, Heo JS, Choi SH, Choi DW
and Kim YI: What prognostic factors are important for resected
intrahepatic cholangiocarcinoma? J Gastroenterol Hepatol.
23:766–770. 2008. View Article : Google Scholar
|
|
29
|
Uchiyama K, Yamamoto M, Yamaue H, Ariizumi
S, Aoki T, Kokudo N, Ebata T, Nagino M, Ohtsuka M, Miyazaki M, et
al: Impact of nodal involvement on surgical outcomes of
intrahepatic cholangiocarcinoma: A multicenter analysis by the
Study Group for Hepatic Surgery of the Japanese Society of
Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci.
18:443–452. 2011. View Article : Google Scholar
|
|
30
|
Shirabe K, Mano Y, Taketomi A, Soejima Y,
Uchiyama H, Aishima S, Kayashima H, Ninomiya M and Maehara Y:
Clinicopathological prognostic factors after hepatectomy for
patients with mass-forming type intrahepatic cholangiocarcinoma:
Relevance of the lymphatic invasion index. Ann Surg Oncol.
17:1816–1822. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
de Jong MC, Nathan H, Sotiropoulos GC,
Paul A, Alexandrescu S, Marques H, Pulitano C, Barroso E, Clary BM,
Aldrighetti L, et al: Intrahepatic cholangiocarcinoma: An
international multi-institutional analysis of prognostic factors
and lymph node assessment. J Clin Oncol. 29:3140–3145. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Park J, Kim MH, Kim KP, Park H, Moon SH,
Song TJ, Eum J, Lee SS, Seo DW and Lee SK: Natural History and
Prognostic Factors of Advanced Cholangiocarcinoma without Surgery,
Chemotherapy, or Radiotherapy: A Large-Scale Observational Study.
Gut Liver. 3:298–305. 2009. View Article : Google Scholar
|
|
33
|
Dhanasekaran R, Hemming AW, Zendejas I,
George T, Nelson DR, Soldevila-Pico C, Firpi RJ, Morelli G, Clark V
and Cabrera R: Treatment outcomes and prognostic factors of
intrahepatic cholangiocarcinoma. Oncol Rep. 29:1259–1267.
2013.PubMed/NCBI
|
|
34
|
Puhalla H, Schuell B, Pokorny H, Kornek
GV, Scheithauer W and Gruenberger T: Treatment and outcome of
intrahepatic cholangiocellular carcinoma. Am J Surg. 189:173–177.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou HB, Wang H, Li YQ, Li SX, Wang H,
Zhou DX, Tu QQ, Wang Q, Zou SS, Wu MC, et al: Hepatitis B virus
infection: A favorable prognostic factor for intrahepatic
cholangiocarcinoma after resection. World J Gastroenterol.
17:1292–1303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang BG, Sun LL, Yu WL, Tang ZH, Zong M
and Zhang YJ: Retrospective analysis of histopathologic prognostic
factors after hepatectomy for intrahepatic cholangiocarcinoma.
Cancer J. 15:257–261. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Karpeh MS Jr: Palliative treatment and the
role of surgical resection in gastric cancer. Dig Surg. 30:174–180.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Suh KS, Chang SH, Lee HJ, Roh HR, Kim SH
and Lee KU: Clinical outcomes and apomucin expression of
intrahepatic cholangiocarcinoma according to gross morphology. J Am
Coll Surg. 195:782–789. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
DeOliveira ML, Cunningham SC, Cameron JL,
Kamangar F, Winter JM, Lillemoe KD, Choti MA, Yeo CJ and Schulick
RD: Cholangiocarcinoma: Thirty-one-year experience with 564
patients at a single institution. Ann Surg. 245:755–762. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Inoue K, Makuuchi M, Takayama T, Torzilli
G, Yamamoto J, Shimada K, Kosuge T, Yamasaki S, Konishi M,
Kinoshita T, et al: Long-term survival and prognostic factors in
the surgical treatment of mass-forming type cholangiocarcinoma.
Surgery. 127:498–505. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Patel T: Increasing incidence and
mortality of primary intrahepatic cholangiocarcinoma in the United
States. Hepatology. 33:1353–1357. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Grobmyer SR, Wang L, Gonen M, Fong Y,
Klimstra D, D'Angelica M, DeMatteo RP, Schwartz L, Blumgart LH and
Jarnagin WR: Perihepatic lymph node assessment in patients
undergoing partial hepatectomy for malignancy. Ann Surg.
244:260–264. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kitagawa Y, Nagino M, Kamiya J, Uesaka K,
Sano T, Yamamoto H, Hayakawa N and Nimura Y: Lymph node metastasis
from hilar cholangiocarcinoma: Audit of 110 patients who underwent
regional and paraaortic node dissection. Ann Surg. 233:385–392.
2001. View Article : Google Scholar : PubMed/NCBI
|