Introduction
Breast cancer is the most prevalent malignant cancer
in women in the world. It is highly heterogenic disease, both in
terms of its molecular characteristics, as well as clinical course
and prognosis (1). Despite of an
improvement in early diagnosis and detection of early stages of
breast cancer, the growing trend for morbidity and mortality due to
this disease is still observed (1).
Periostin (POSTN) is multi-functional, homodimeric
glycoprotein with molecular mass of about 93.3 kDa (2,3).
POSTN, originally named as osteoblast-specific factor 2, (OSF-2)
was first identified in 1993 as a putative cell adhesion protein
for preosteoblasts in a mouse osteoblastic MC3T3-E1 cell line
(2), and then classified as a new
extracellular matrix (ECM) protein (4). The protein structure of POSTN is
composed of an N-terminal secretory signal peptide followed by an
EMI domain rich in cysteine, 4 internal repeated and conserved
fascilin (FAS)-1 domains and a C-terminal variable hydrophilic
domain (3,5,6). POSTN
plays an important role in collagen fibrillogenesis (7), cell adhesion and wound healing process
(8). It also takes part in ECM
remodelling by interacting with other proteins, i.e. fibronectin,
tenascin-C and type V collagen (4).
It is believed that POSTN has also a key influence on the
carcinogenesis process. POSTN interacts with multiple cell-surface
receptors, most notably integrins (αvβ3,
αvβ5, α6β4), and signals mainly via the
PI3-K/Akt and other pathways to promote cancer cell survival,
epithelial-mesenchymal transition (EMT), invasion, and metastasis
(3,4,6,9–11).
There is also a number of studies indicating a
significant role of tumour stroma in carcinoma progression
(12). Tumour stroma, which is an
essential part of the tumour, is formed by a stromal matrix in
which cancer cells and the peritumoural stromal cells are embedded.
One of the main cellular components of the tumour stroma, in
addition to inflammatory cells and endothelial cells, are
cancer-associated fibroblasts (CAFs), which may be an important
source of POSTN. CAFs play several key functions, among others they
promote tumour growth by secretion of growth factors (e.g. EGF,
IGF-1, TGF-α). Some of the factors secreted by CAFs, i.e.
hepatocyte growth factor (HGF/SF), significantly influence the
migration of cancer cells (13).
CAFs regulate the angiogenesis process and organise the stroma by
producing ECM components (mainly collagen type I, III and V,
fibronectin, laminin); simultaneously, they secrete
metalloproteinases (MMPs), i.e. MMP-1 and MMP-3 are involved in
proteolytic modifications and remodelling of ECM, which are crucial
in the process of cell migration, angiogenesis and metastasis
(12,14).
For several years studies have indicated increased
expression of POSTN in various type of human cancer, among others
in breast cancer (15–18), non-small cell lung carcinoma (NSCLC)
(19–21), gastric cancer (22–25),
colorectal cancer (26–28), ovary (29–31),
prostate (32–34) and brain cancers (35–37).
However, up till now, there are no data regarding expression of
POSTN in stromal cells, the CAFs, either in pre-invasive (DCIS) or
invasive (IDC) breast cancers. Taking into account the
above-mentioned facts related to the potential role of POSTN in the
promotion of cancer cell invasiveness and metastasis processes, it
seems important to conduct studies to evaluate POSTN expression in
CAFs in IDC and in DCIS and to correlate the results with
clinicopathological parameters.
Materials and methods
Patients and clinical samples
The study was performed on archival material of 21
cases of FC (control group), 70 paraffin-embedded samples of IDC
and 44 paraffin-embedded samples of DCIS diagnosed from 2000 to
2007. Paraffin blocks containing IDC were obtained from Lower
Silesian Oncology Centre in Wroclaw, and samples of DCIS and FC
were obtained from the Department of Tumour Pathology of the Maria
Sklodowska-Curie Institute of Oncology in Krakow. The present study
was approved by the Bioethics Commission at the Wroclaw Medical
University. The patient clinicopathological data are listed in
Table I. Histopathological
evaluation of the hematoxylin and eosin (H&E) stained slides
was used to determine the type and the malignancy grade of the
tumours (G) according to WHO criteria (38). Most patients were treated by
mastectomy or quadrantectomy, and a subsequent axillary lymph node
resection. In 53 (75.7%) cases, adjuvant chemotherapy was applied.
Only 1 (1.4%) patient received neoadjuvant chemotherapy prior to
primary tumour resection. The mean patient age at diagnosis was
59±12.2 years. Molecular investigations were performed on frozen
IDC fragments, sampled from 41 patients diagnosed from 2004 to
2006, including 11 cases that have been subjected to laser capture
microdissection (LCM). All the samples were collected before
treatment initiation.
 | Table IClinicopathological characteristics
of the 70 invasive ductal breast carcinoma (IDC) patients. |
Table I
Clinicopathological characteristics
of the 70 invasive ductal breast carcinoma (IDC) patients.
| Parameters | No. | (%) |
|---|
| Age | | |
| ≤55 | 16 | 22.9 |
| >55 | 54 | 77.1 |
| Tumour size | | |
| pT1 | 29 | 41.4 |
| pT2 | 33 | 47.2 |
| pT3 | 5 |
7.1 |
| pT4 | 3 |
4.3 |
| Grade | | |
| G1 | 4 | 5.7 |
| G2 | 30 | 42.9 |
| G3 | 36 | 51.4 |
| Menopausal
status | | |
| Pre | 19 | 27.2 |
| Post | 51 | 72.8 |
| Lymph nodes | | |
| N0 | 39 | 55.7 |
| N1, N2, N3 | 30 | 42.9 |
| NA | 1 | 1.42 |
| ER | | |
| Positive | 51 | 72.9 |
| Negative | 19 | 27.1 |
| PR | | |
| Positive | 45 | 64.3 |
| Negative | 25 | 35.7 |
| HER-2 | | |
| Positive | 51 | 72.9 |
| Negative | 19 | 27.1 |
Immunohistochemistry
Immunohistochemical (IHC) reactions were performed
using Dako Autostainer Link 48 (DakoCytomation, Glostrup, Denmark)
equipment. Rabbit polyclonal antibody directed against Periostin
(Novus Biologicals, Littleton, CO, USA) and murine monoclonal
antibodies directed against D2-40 (ready-to-use, RTU, Dako),
vimentin (Vim, RTU, Dako), α-smooth muscle actin (α-SMA, RTU,
Dako), oestrogen receptor (ER) (clone 1D5; 1:100, Dako),
progesterone receptor (PR) (clone 635; 1:100, Dako) were
utilized.
The sections were first boiled in Target Retrieval
Solution buffer using a Pre-Treatment link platform (in order to
deparaffinise, rehydrate, and unmask the antigens) and subsequently
cooled in a rinsing buffer (TBS/0.1% Tween). The activity of
endogenous peroxidase was blocked by 5 min incubation with EnVision
FLEX peroxidase-blocking reagent. EnVision FLEX System was used to
visualize the antigens. After rinsing the slides in TBS/0.1% Tween
buffer, the primary antibodies were applied for 20 min at room
temperature. Next, the slides were incubated for 20 min with
secondary antibodies, EnVision FLEX/HRP. To visualize the reaction,
sections were incubated for 10 min with EnVision FLEX working
solution, where 3,3′-diaminobenzidine (DAB) was used as a
chromogen. All slides were counterstained with EnVision FLEX
hematoxylin. Subsequently, the sections were dehydrated in alcohol
and xylene, and then mounted in SUB-X mounting medium. HER-2
receptors were localised with the use of Hercept Test (Dako).
Evaluation of IHC reactions
All IHC sections were evaluated using a BX41 light
microscope (Olympus, Tokyo, Japan) by two independent pathologists
(P.D. and J.G.) who were blinded to the patient clinical data.
POSTN expression in CAFs was assessed using the immunoreactive
score (IRS) of Remmele and Stegner (39).
This scale evaluates the percentage of cells with
positive reaction (A) and the intensity of the reaction (B). The
final score represents the sum of the two values, ranging within
the scope of 0–12 (AxB) (Table
II). For the assessment of ER and PR expression a four grade
scoring system based on tumour cell positivity in the whole tumour
section was used: 0 (0% cells stained), 1 (1–10% cells stained), 2
(11–50% cells stained), 3 (51–100% cells stained). The evaluation
of expression of HER2 receptors was performed with the use of a
scale, in which both the intensity of colour of membrane reaction,
as well as the percent of stained cancer cells are taken into
account (40).
 | Table IISemi-quantitative IRS scale of
Remmele and Stegner (39). |
Table II
Semi-quantitative IRS scale of
Remmele and Stegner (39).
| Points | A
Percentage of positive cells | Points | B
Intensity of colour reaction |
|---|
| 0 | No positive
cells | 0 | No staining |
| 1 | Up to 10% positive
cells | 1 | Low intensity of
staining |
| 2 | 11–50% positive
cells | 2 | Moderate intensity
of staining |
| 3 | 51–80% positive
cells | 3 | Intense
staining |
| 4 | >80% positive
cells | | |
Laser capture microdissection (LCM)
Fresh frozen IDC specimens were obtained during
resections in Lower Silesian Oncology Centre in Wroclaw from 11
patients. The obtained tissues were snap-frozen in liquid nitrogen
and stored at −80°C until stromal and cancer cells LCM was
performed. To this end, tissue sections (8 µm) were cut on a
Leica CM1950 cryostat (Leica Microsytems, Wetzlar, Germany) and
placed on a PET membrane slide (MMI, Glattbrugg, Switzerland). The
slides were then fixed in 100% isopropyl alcohol and stained using
the H&E staining kit Plus for LCM (MMI). LCM was performed
using the MMI CellCut Plus system (MMI). Dissected samples were
collected on the adhesive lids of 500 µl tubes (MMI).
Real-time PCR
Total RNA from 41 IDC cases was isolated with the
use of RNeasy Mini kit (Qiagen, Hilden, Germany), according to
manufacturer's instructions. Reverse transcription reactions were
performed with the use of High-Capacity cDNA Reverse Transcription
kits (Applied Biosystem, Foster City, CA, USA). In turn, total RNA
from 11 microdissected stromal and cancer cells were isolated with
the use of RNeasy Micro kit (Qiagen), according to manufacturer's
instructions. QuantiTect Reverse Transcription kit (Qiagen) was
used for cDNA synthesis. Expression of POSTN mRNA was determined by
quantitative real-time PCR with using a 7500 Real-time PCR System
and iTag Universal Probes Supermix (Bio-Rad, Hercules, CA, USA)
according to the manufacturer's protocol. As reference gene β-actin
(ACTB) was used. The primers and TaqMan probes used were:
Hs00170815_m1 for POSTN, Hs99999903_m1 for β-actin (Applied
Biosystem). All reactions were performed in triplicates under
following conditions: initial denaturation at 94°C for 30 sec
followed by 45 cycles of denaturation at 94°C for 15 sec, and
annealing and elongation at 60°C for 60 sec. The relative mRNA
expression levels of POSTN were calculated using the ΔΔCt
method.
Statistical analysis
Prism 5.0 (Graphpad Software, La Jolla, CA, USA)
statistical software was used to analyse the results. The
non-parametric Mann-Whitney U test (for unpaired observations) and
the Wilcoxon signed-rank test (for paired observations) were used
to compare groups of data. The associations between clinical and
pathological parameters and the expression of the studied IHC
markers were analysed using χ2-test. Survival times were
determined by the Kaplan-Meier method, and the significance of the
differences was determined by a log-rank test. For each variable,
the hazard ratio (HR) and the 95% confidence interval (95% CI) were
estimated. In all the analyses, the results were considered
statistically significant at p<0.05.
Results
With the use of IHC, POSTN expression was found in
70 (100%) cases. Expression of POSTN was noted mainly in tumour
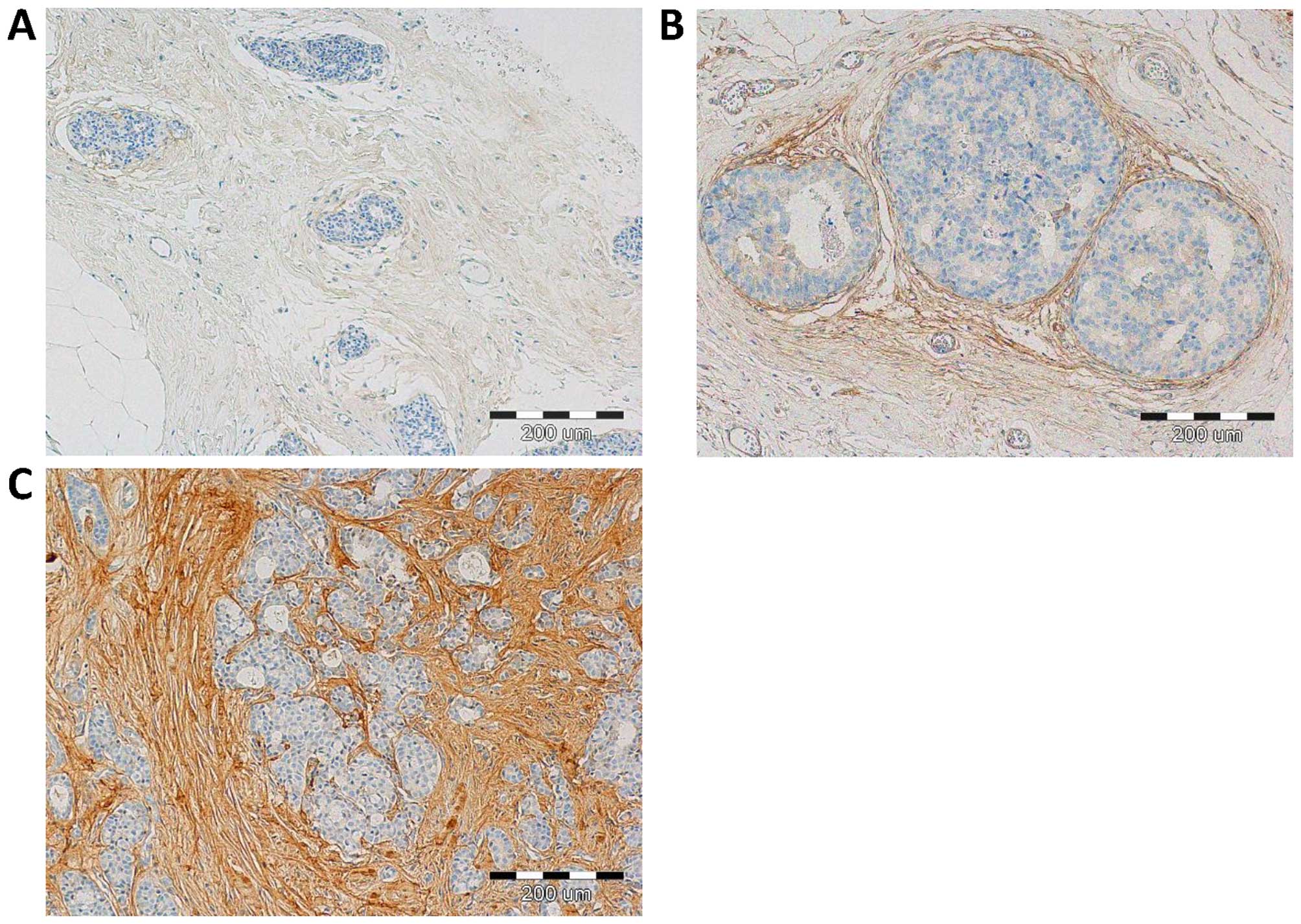
stromal cells, i.e. CAFs, as evidenced by the positive IHC reaction
for α-SMA, vimentin and podoplanin (D2-40), - a characteristic
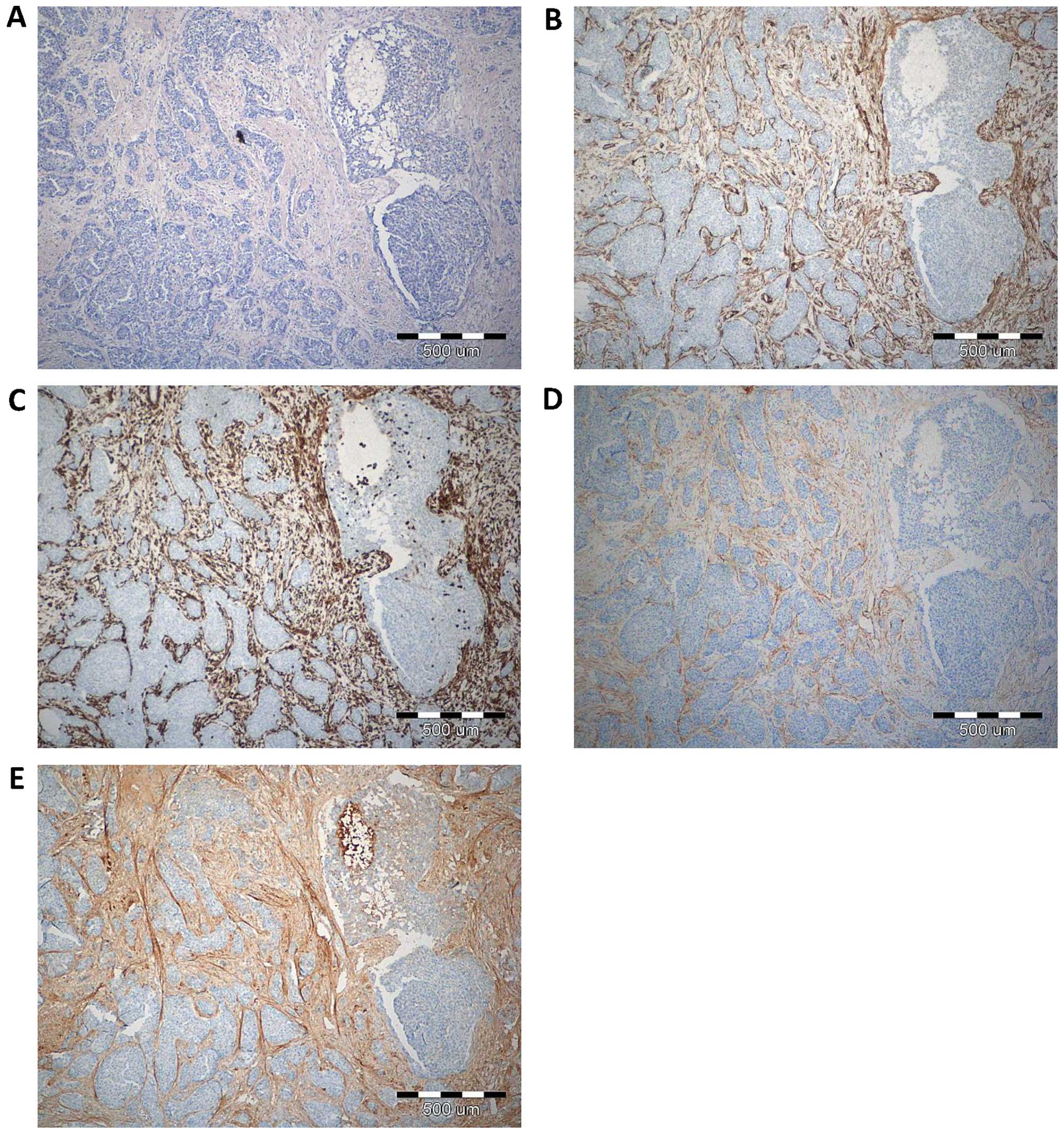
marker of CAFs, (Fig. 1). IHC
reaction with POSTN expression in CAFs in IDC, DCIS and FC is shown
in Fig. 2. Our results showed, that
70% of analysed tumour cases demonstrate high level of POSTN
expression in CAFs, evaluated for 8–12 IRS points. The mean value
of POSTN expression in CAFs was 8.41±2.21. Statistically
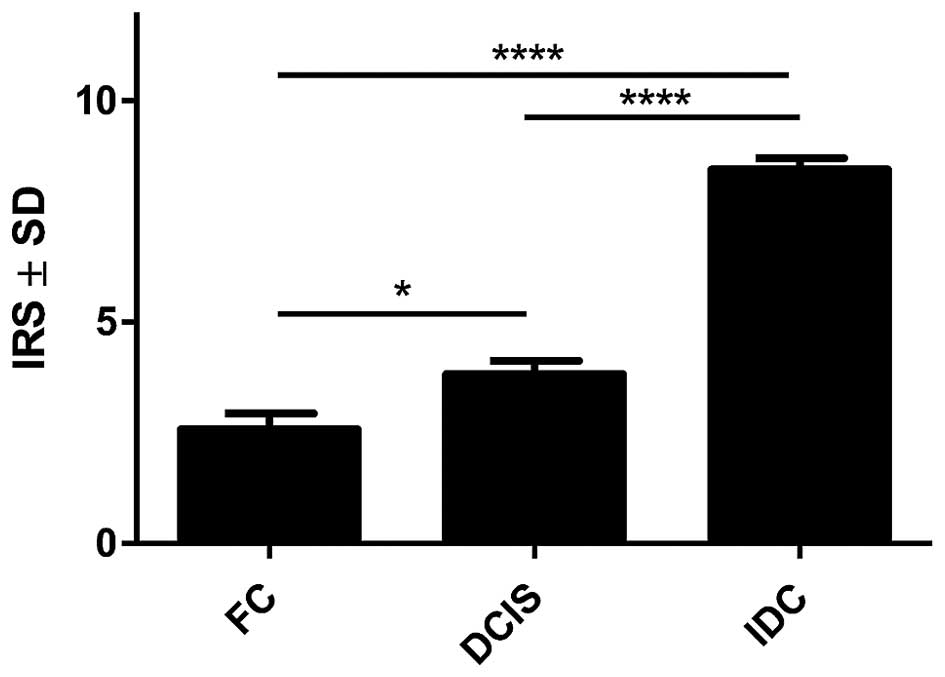
significant higher expression level of POSTN in CAFs in IDC as
compared to the cases of FC (p<0.0001 Mann-Whitney U test;
Fig. 3) was observed. Additionally,
statistically elevated POSTN expression in CAFs in IDC relative to
the analysed cases of DCIS (p<0.0001) and significantly
increased expression of POSTN in CAFs in DCIS in comparison to FC
(p=0.0158, Mann-Whitney U test; Fig.
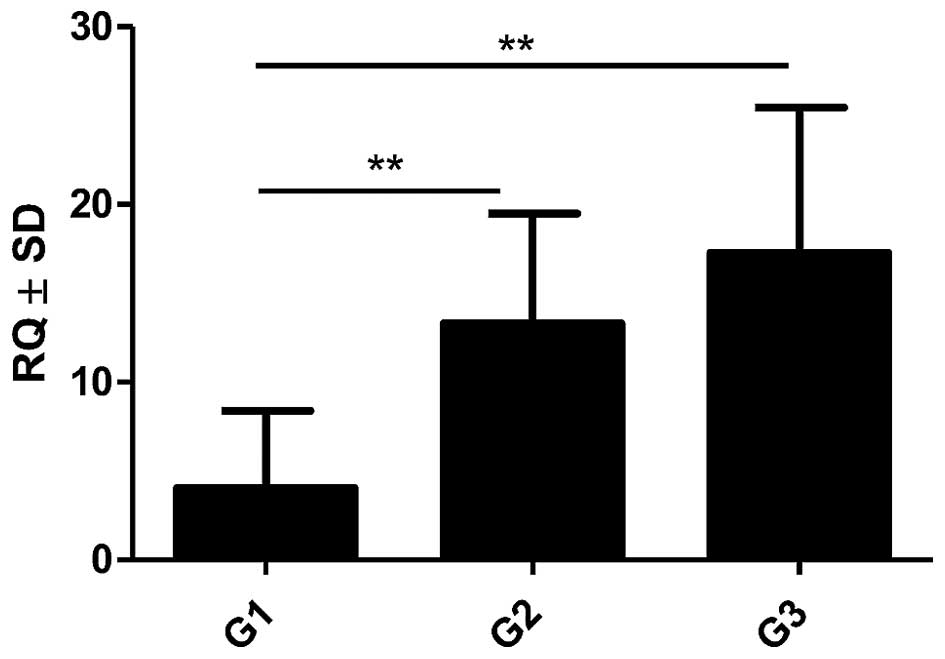
3) was also shown. Moreover, an increasing level of POSTN
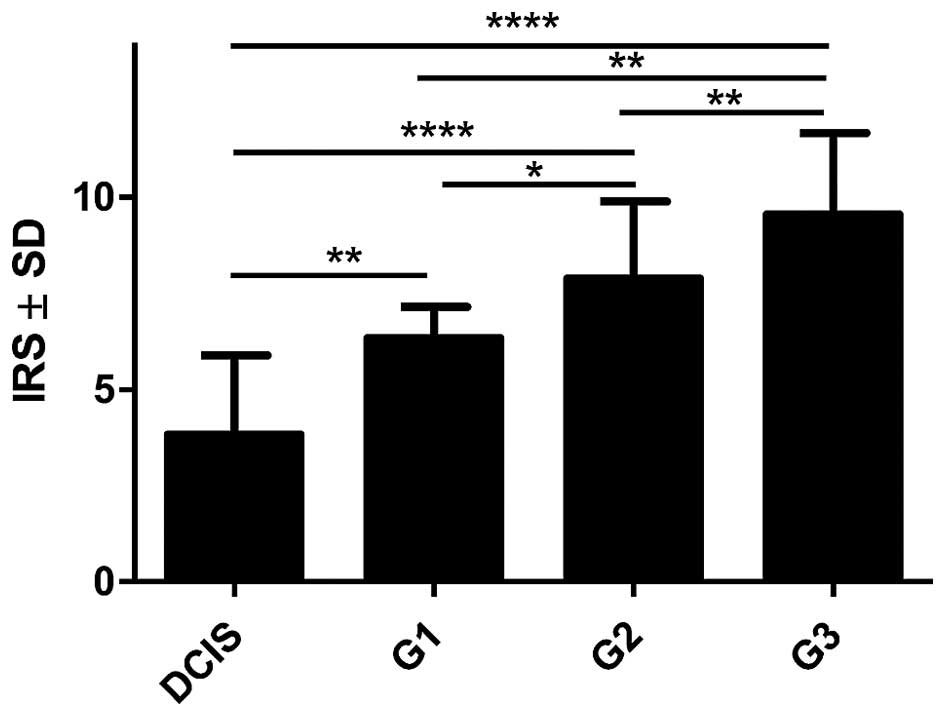
expression in CAFs with the growing malignancy grade of the tumours
(G) was shown in the analysed group of IDC cases. Significant
differences were noted using the Mann-Whitney U test between G1
tumours and those of G2 and G3 (p=0.0454 and p=0.0011,
respectively). In addition, significant differences were observed
between G2 and G3 tumours (p= 0.0036, Mann-Whitney U test, Fig. 4). Furthermore, significantly
increased expression level of POSTN in CAFs in IDC in different
grades of tumour malignancy (G) was found in comparison to the
expression of POSTN in DCIS. Significant differences were noted
between G1, G2 and G3 tumours relative to DCIS (p=0.0023,
p<0.0001, p<0.0001, respectively, Mann-Whitney U test;
Fig. 4).
The correlations between the presence of POSTN
expressing CAFs in IDC and the clinicopathological parameters of
the patients are summarized in Table
III. With the χ2-test, significant correlations were
found between high level of POSTN expression in CAFs in IDC (>8
IRS points) and tumour malignancy grade (G) (p=0.0070, Fisher's
exact test). However, no significant correlation was found between
POSTN expression in CAFs in IDC and the expression of ER, PR and
HER2 receptor, the size of primary tumour (pT), metastasis to lymph
nodes (pN), menopausal status or the age of patients.
 | Table IIICorrelations between periostin
(POSTN) expression by CAFs and selected clinical pathological
characteristics in 70 patients with invasive ductal breast
carcinoma (IDC). |
Table III
Correlations between periostin
(POSTN) expression by CAFs and selected clinical pathological
characteristics in 70 patients with invasive ductal breast
carcinoma (IDC).
|
Characteristics | No. (%) | POSTN expression by
CAFs, No. (%)
| P-value
(χ2) |
|---|
| IRS≤8 | IRS>8 |
|---|
| Age | | | | |
| ≤55 | 16 (22.9) | 9 (12.9) | 7 (10.0) | 0.9345 |
| >55 | 54 (77.1) | 31 (44.2) | 23 (44.2) | |
| Tumour size | | | | |
| pT1 | 29 (41.4) | 20 (28.6) | 14 (20.0) | 0.5348 |
| pT2 | 33 (47.2) | 19 (27.1) | 13 (18.6) | |
| pT3 | 5 (7.1) | 1 (1.42) | 1 (1.42) | |
| pT4 | 3 (4.3) | 0 (0.0) | 2 (2.86) | |
| Grade | | | | |
| G1 | 4 (5.7) | 6 (8.6) | 0 (0.0) | 0.0070 |
| G2 | 30 (42.9) | 23 (32.9) | 12 (17.1) | |
| G3 | 36 (51.4) | 11 (15.7) | 18 (25.7) | |
| Menopausal
status | | | | |
| Pre | 19 (27.2) | 7 (17.5) | 33 (82.5) | 0.4214 |
| Post | 51 (72.8) | 28 (40.0) | 23 (32.9) | |
| Lymph nodes | | | | |
| N0 | 39 (55.7) | 24 (34.3) | 15 (21.4) | 0.4028 |
| N1, N2, N3 | 30 (42.9) | 16 (22.9) | 14 (20.0) | |
| N/A | 1 (1.42) | 0 (0.0) | 1 (1.42) | |
| ER | | | | |
| Positive | 51 (72.9) | 31 (44.3) | 22 (31.4) | 0.6875 |
| Negative | 19 (27.1) | 9 (12.9) | 8 (11.4) | |
| PR | | | | |
| Positive | 45 (64.3) | 27 (38.6) | 18 (25.7) | 0.5169 |
| Negative | 25 (35.7) | 13 (18.6) | 12 (17.1) | |
| HER-2 | | | | |
| Positive | 51 (72.9) | 25 (35.7) | 19 (27.2) | 0.5752 |
| Negative | 19 (27.1) | 15 (21.4) | 11 (15.7) | |
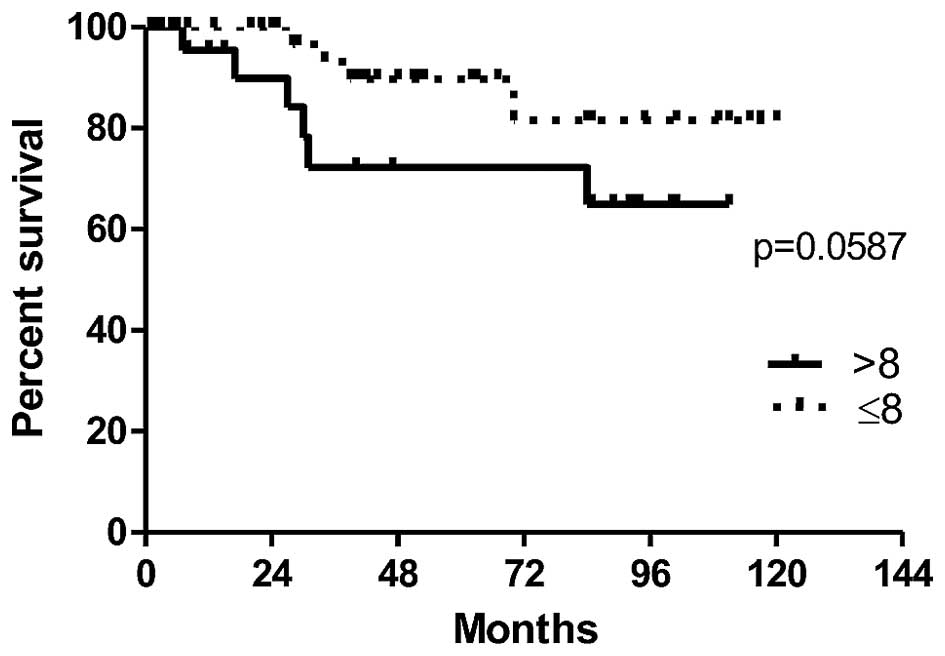
In order to evaluate the prognostic value of POSTN
expression by CAFs in IDC cases, the survival of patients depending
on the expression level of POSTN was analysed. The association
between expression of POSTN in CAFs and overall patients survival
time (OS) was found on the border of statistical significance
(p=0.0587). The results demonstrate a clear tendency towards a
shorter survival in a group of patients with high POSTN expression
in CAFs in IDC (>8 IRS points), (Fig. 5). Moreover, univariate survival
analysis in a studied group of patients showed that the presence of
lymph node metastases (pN) (p=0.046) and the menopausal status
(p=0.021) were associated with poor patient survival (Table IV).
 | Table IVUnivariate Cox proportional hazard
analysis in 70 patients with invasive ductal breast carcinoma
(IDC). |
Table IV
Univariate Cox proportional hazard
analysis in 70 patients with invasive ductal breast carcinoma
(IDC).
|
Characteristics | Overall survival
| P-value |
|---|
| HR | 95% CI |
|---|
| Age (≤55 vs.
>55) | 1.5578 | 0.4376–5.5448 | 0.49378 |
| Tumour size (T1 vs.
T2–T4) | 3.1271 | 0.8055–12.139 | 0.09946 |
| Histological Grade
(G1.G2 vs. G3) | 0.8573 | 0.1066–6.8947 | 0.88494 |
| Menopausal status
(Pre vs. Post) | 0.4927 | 0.2695–0.9009 | 0.02152 |
| Lymph node
involvement (N− vs. N+) | 4.8197 | 1.0219–22.730 | 0.04687 |
| ER (positive vs.
negative) | 0.7881 | 0.2034–3.0539 | 0.73047 |
| PR (positive vs.
negative) | 0.6280 | 0.1813–2.1745 | 0.46291 |
| HER2 (positive vs.
negative) | 0.9668 | 0.8541–1.0943 | 0.59344 |
Expression of POSTN in 41 cases was evaluated also
at the mRNA level by using real-time PCR technique. Additionally,
in the 11 of the above-mentioned IDC cases, POSTN mRNA expression
was analysed in the microdissected stromal and cancer cells.
Expression of POSTN gene was noted in 100% (41) of analysed IDC cases. The obtained
data showed increasing levels of POSTN mRNA expression which
correlated to increased G of the tumours. A statistically higher
mRNA expression of POSTN in G2 and G3 cases relative to G1 cases
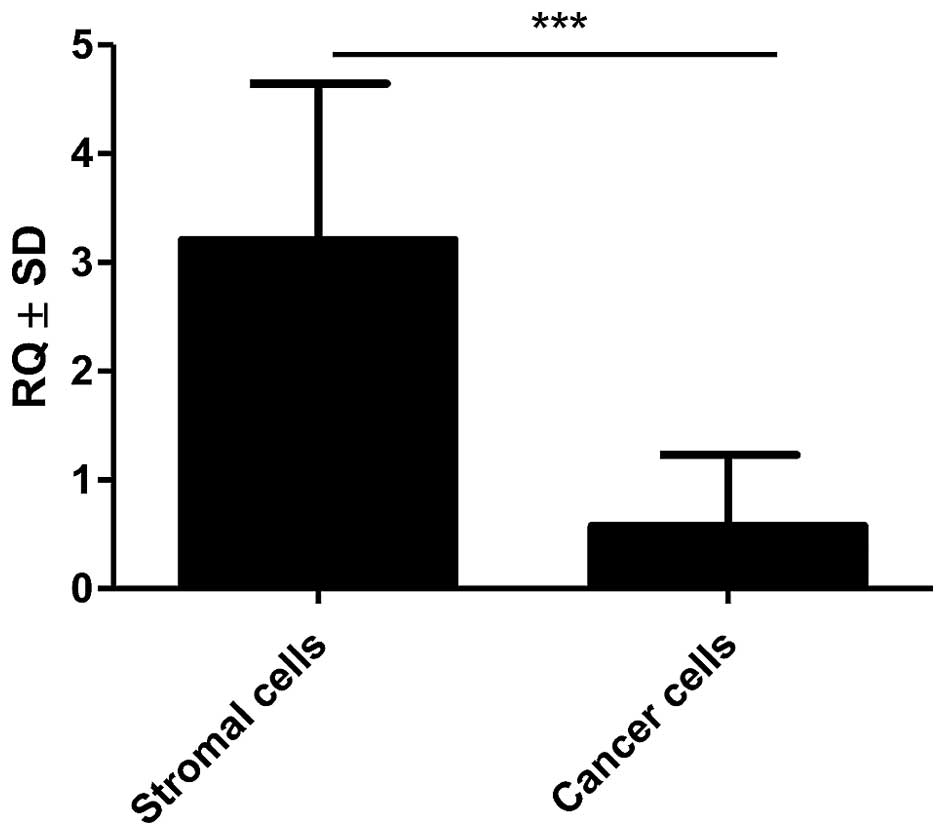
(p=0.0037 and p=0.0023, respectively, Mann-Whitney U test; Fig. 6) was found. Additionally,
significantly higher expression of mRNA POSTN in stromal cells
(CAFs) relative to cancer cells (p<0.001, Mann-Whitney U test;
Fig. 7) was shown in the material
from LCM.
Discussion
One of the main mechanisms responsible for cancer
invasiveness and metastasis is the EMT process, characterised by
the cells losing their epithelial phenotype (i.e. loss of
intercellular adhesion, basal-apical polarity and depletion of
E-cadherin expression) and acquiring mesenchymal features enabling
their migration, invasion or metastasis, such as an increased
expression of fibronectin, vimentin and N-cadherin (41,42).
As shown in many studies, EMT may be initiated by signalling
pathways activated by receptors with tyrosine or serine-threonine
kinase activity (42). It is
believed that POSTN is one of the factors influencing regulation of
intracellular pathway related to 3 phosphatidylinositol kinase
(PI3K) and serine-threonine AKT/PKB protein kinase (6,27,43).
POSTN is a glycoprotein that belongs to
matricellular proteins (8). This
protein plays an important role in numerous biological processes
related to carcinogenesis, i.e. proliferation (26), migration (44), angiogenesis (45,46),
invasion, and metastasis (45,47).
It is believed that POSTN may be a marker for progression in
various cancer types. However, the accurate mechanisms responsible
for the influence of POSTN on cancer progression and metastasis
still remain a subject of further studies.
So far, most research on the role of POSTN
expression in breast cancer confirmed POSTN expression localized
mainly in cancer cells and, in few cases, in tumour stroma
(18). In our work, POSTN
expression by stromal cells - CAFs, was shown for the first time,
both in pre-invasive (DCIS) and invasive (IDC) tumours. The study
confirming POSTN expression in CAFs is positive by IHC reaction
performed on the serial sections for α-SMA, vimentin and D2-40,
i.e. the CAFs, characteristic markers, was shown in our earlier
studies (48).
In the present study, statistically increased
expression of POSTN in CAFs in IDC in comparison to cases of FC was
found with the use IHC method. It was also shown that the
expression of POSTN was significantly higher in CAFs in IDC
relative to cases of DCIS. POSTN expression by CAFs seems quite
interesting taking into account that Lv et al (23) in their work on gastric cancer showed
that the role of POSTN in tumorigenesis is strongly dependent on
the type of cells it is derived from. Above-mentioned authors found
that epithelial cell-derived POSTN can function as a tumour
suppressor in gastric cancer through stabilizing p53 and E-cadherin
proteins via the Rb/E2F1/p14ARF/Mdm2 signalling pathway (23).
On the other hand, Kikuchi et al (22) confirmed increased stromal POSTN
expression with the increase of the stage of gastric cancer of both
intestinal and diffuse type. Similarly to the results obtained by
us it was noted that CAFs are the primary source of POSTN, which
facilitates tumour cell invasion by inducing EMT and by
establishing a neoplastic niche in gastric cancers (22). The research done by the same team in
colorectal cancer with the use of IHC method and double
immunofluorescent staining confirmed that POSTN is expressed by
CAFs, which, is in line with our results (49). However, in contrast to the results
of our study, these authors did not analyse the correlation between
POSTN expression in CAFs and the tumour malignancy grade (G)
(49). Similarly, underwood et
al (50) showed a significant
role of tumour stroma in oesophageal adenocarcinoma (EAC)
progression. It was found that CAFs promote tumour cell invasion
in vitro and growth in vivo by signalling to EAC
cells via secretion of the ECM protein, POSTN (50).
Comparably to the results obtained by us, it was
shown also in the studies conducted by Choi et al (31) that ovarian cancer CAFs are
responsible for the deposition of POSTN in the stroma. It was also
shown that POSTN expression in CAFs may have prognostic relevance
in ovarian cancer (31). Similarly,
Ryner et al (51) showed
significant role of interactions between ovarian cancer and its
microenvironment. The authors of the study emphasised that
identification of reactive stromal components, including POSTN, may
be helpful in the development of novel diagnostic and therapeutic
strategies for overcoming chemoresistance in ovarian cancer
(51). Li et al (52) also obtained comparable results in
their studies and showed expression of POSTN mainly in the stroma
of nasopharyngeal cancer. Additionally, with the use of LCM method
and mass spectroscopy, the authors identified different protein
expression profiles between the stroma of nasopharyngeal cancer and
normal nasopharyngeal mucosa (52).
Therefore, it is suggested that the stromal components, including
POSTN, may play a crucial role in tumour metastasis and,
potentially, they may become the target of pharmacotherapy
(52).
The latest proteomic research by Reddy et al
(53) also confirmed that the
proteome of tumour-adjacent IDC stroma differs from that of
tumour-distal stroma, which may have an important meaning in the
understanding of the role of stromal cells, especially CAFs, in IDC
progression process. It is believed that the studies on tumour
stroma may set new directions for searching for target points for
new therapies. In line with our results and with the use of IHC
method, Zhang et al (16)
also confirmed an increased expression of POSTN in breast cancer,
which was observed mainly in the stromal compartment and, in few
cases, in the cytoplasm of cancer cells. These authors showed also
significantly increased expression of POSTN in breast cancers
relative to corresponding normal tissues, which is consistent with
the results obtained by us (16).
Zhang et al (16) observed
also positive association between expression of POSTN and the
clinical stage of breast cancer, thus indicating an important role
of POSTN in progression of this cancer.
Additionally, Puglisi et al (15) also described significantly increased
expression of POSTN in breast cancer in comparison to the control
group that included benign lesions of this organ. However, opposite
to the results obtained by us, POSTN expression was localized
mainly in the cytoplasm of cancer cells and, in 12% of cases, a
nuclear reactivity was observed (15). Slightly different results of IHC
were also obtained by Xu et al (17), where expression of POSTN was
indicated mainly in the cytoplasm of breast cancer cells.
Noteworthy are also studies by Ishiba et al (54). They showed significantly increased
expression of POSTN in tumour phyllodes (mostly benign breast
cancer), relative to fibroadenoma. POSTN expression was present
mainly in tumour stroma, however, contrary to our results, no POSTN
expression in CAFs was shown (54).
Additionally, with the use of immunoprecipitation technique and
mass spectrometry, the authors showed that in tumour phyllodes and
in the in vitro studies POSTN forms a complex with decorin,
small leucine-rich proteoglycan (54), which can delay tumour growth by
blocking transforming growth factor β (TGF-β) (55) and by interaction with E-cadherin
(55,56). It was also proved that knockdown of
POSTN results in translocation of decorin from the cytoplasm to the
extracellular space, leading to the inhibition of cancer cell
migration and invasion. Therefore it is suggested that
POSTN-decorin complex may become a potential target for anticancer
therapy (54).
In the presented work, in order to confirm obtained
results that determine the level of POSTN protein in IDC, the
studies on the expression of POSTN gene (mRNA) with the use of
quantitative method (real-time PCR) were also performed. In IDC,
significant increase of expression of mRNA encoding for POSTN in
parallel to growing malignancy grade (G) was found. High level of
expression of mRNA POSTN reflected increased level of the protein
in CAFs in IDC, as shown by us. Additionally, with the use of LCM
method, we confirmed statistically significant higher expression of
mRNA POSTN in stromal cells the CAFs in comparison to cancer cells.
Significantly higher POSTN mRNA expression in breast cancer
relative to normal breast tissue was also shown in the studies by
Zhang et al (16) and Shao
et al (46). The authors of
these studies confirmed obtained results also with the use of IHC
method and showed overexpression of POSTN in breast cancer in
comparison to the corresponding control tissues, which is
consequently in line with the results of our studies.
Moreover, prognostic impact of POSTN expression in
stromal cells - CAFs was analysed regarding patient OS. The results
showed the association of shorter overall survival time of patients
with high expression of POSTN in CAFs, which was on the border of
statistical significance. Therefore, it seems that high POSTN
expression in CAFs may be associated with poorer prognosis, which
was also confirmed by Nuzzo et al (18). Similarly, Hong et al
(21) confirmed that in NSCLC, the
3-year overall survival rate for the patients with low levels of
POSTN expression in tumour stroma was much higher than for those
with high levels of POSTN expression. Such correlation was also
observed by Ben et al (57)
which showed that in pancreatic cancer, high expression of POSTN in
tumour stroma and in cancer cells is associated with shorter
patient survival time. The relationship between high POSTN
expression in tumour stroma and shorter patient survival time was
reported also in prostate cancer (32,33).
Therefore it is suggested that in both breast cancer and in other
types of cancers with epithelial origin, POSTN expression in tumour
stroma, and particularly in CAFs, may be an unfavourable prognostic
marker.
The results of our studies are the first that
tackled the subject of POSTN expression in CAFs in IDC and DCIS.
POSTN expression by CAFs was confirmed on protein and mRNA level
with the use of LCM method. Additionally, we showed significantly
increased expression of POSTN in CAFs IDC in comparison with FC
cases. Significantly elevated expression of POSTN in CAFs in in IDC
relative to DCIS was noted, which may indicate the role of POSTN
expression by CAFs in the process of cancerous transformation.
Moreover, POSTN expression correlated with increasing malignancy
grade (G) of the analysed tumours, both on protein and on mRNA
level.
In conclusion, POSTN may be a factor that plays an
important role in the mechanism of cancer transformation and
progression, which raises hope for the possible future usage of
this glycoprotein as a target for therapy.
Acknowledgments
The study was subsidized from research funds from
2014 to 2016, as a research project no. Pbmn 141. This study was
partially financially supported by the 'Wrovasc-Integrated
Cardiovascular Centre' project, co-financed by the European
Regional Development Fund, within the Innovative Economy
Operational Program, 2007–2013.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takeshita S, Kikuno R, Tezuka K and Amann
E: Osteoblast-specific factor 2: Cloning of a putative bone
adhesion protein with homology with the insect protein fasciclin I.
Biochem J. 294:271–278. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nuzzo PV, Buzzatti G, Ricci F, Rubagotti
A, Argellati F, Zinoli L and Boccardo F: Periostin: A novel
prognostic and therapeutic target for genitourinary cancer? Clin
Genitourin Cancer. 12:301–311. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ruan K, Bao S and Ouyang G: The
multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci.
66:2219–2230. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Horiuchi K, Amizuka N, Takeshita S,
Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF and Kudo A:
Identification and characterization of a novel protein, periostin,
with restricted expression to periosteum and periodontal ligament
and increased expression by transforming growth factor beta. J Bone
Miner Res. 14:1239–1249. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morra L and Moch H: Periostin expression
and epithelial-mesenchymal transition in cancer: A review and an
update. Virchows Arch. 459:465–475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Norris RA, Damon B, Mironov V, Kasyanov V,
Ramamurthi A, Moreno-Rodriguez R, Trusk T, Potts JD, Goodwin RL,
Davis J, et al: Periostin regulates collagen fibrillogenesis and
the biomechanical properties of connective tissues. J Cell Biochem.
101:695–711. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hamilton DW: Functional role of periostin
in development and wound repair: Implications for connective tissue
disease. J Cell Commun Signal. 2:9–17. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kudo Y, Siriwardena BS, Hatano H, Ogawa I
and Takata T: Periostin: Novel diagnostic and therapeutic target
for cancer. Histol Histopathol. 22:1167–1174. 2007.PubMed/NCBI
|
|
10
|
Michaylira CZ, Wong GS, Miller CG,
Gutierrez CM, Nakagawa H, Hammond R, Klein-Szanto AJ, Lee JS, Kim
SB, Herlyn M, et al: Periostin, a cell adhesion molecule,
facilitates invasion in the tumor microenvironment and annotates a
novel tumor-invasive signature in esophageal cancer. Cancer Res.
70:5281–5292. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ratajczak-Wielgomas K and Dziegiel P: The
role of periostin in neoplastic processes. Folia Histochem
Cytobiol. 53:120–132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Franco OE, Shaw AK, Strand DW and Hayward
SW: Cancer associated fibroblasts in cancer pathogenesis. Semin
Cell Dev Biol. 21:33–39. 2010. View Article : Google Scholar :
|
|
13
|
Pennacchietti S, Michieli P, Galluzzo M,
Mazzone M, Giordano S and Comoglio PM: Hypoxia promotes invasive
growth by transcriptional activation of the met protooncogene.
Cancer Cell. 3:347–361. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim HM, Jung WH and Koo JS: Expression of
cancer-associated fibroblast related proteins in metastatic breast
cancer: An immunohistochemical analysis. J Transl Med. 13:2222015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Puglisi F, Puppin C, Pegolo E, Andreetta
C, Pascoletti G, D'Aurizio F, Pandolfi M, Fasola G, Piga A, Damante
G, et al: Expression of periostin in human breast cancer. J Clin
Pathol. 61:494–498. 2008. View Article : Google Scholar
|
|
16
|
Zhang Y, Zhang G, Li J, Tao Q and Tang W:
The expression analysis of periostin in human breast cancer. J Surg
Res. 160:102–106. 2010. View Article : Google Scholar
|
|
17
|
Xu D, Xu H, Ren Y, Liu C, Wang X, Zhang H
and Lu P: Cancer stem cell-related gene periostin: A novel
prognostic marker for breast cancer. PLoS One. 7:e466702012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nuzzo PV, Rubagotti A, Zinoli L, Salvi S,
Boccardo S and Boccardo F: The prognostic value of stromal and
epithelial periostin expression in human breast cancer: Correlation
with clinical pathological features and mortality outcome. BMC
Cancer. 16:952016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sasaki H, Dai M, Auclair D, Fukai I,
Kiriyama M, Yamakawa Y, Fujii Y and Chen LB: Serum level of the
periostin, a homologue of an insect cell adhesion molecule, as a
prognostic marker in nonsmall cell lung carcinomas. Cancer.
92:843–848. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Morra L, Rechsteiner M, Casagrande S, von
Teichman A, Schraml P, Moch H and Soltermann A: Characterization of
periostin isoform pattern in non-small cell lung cancer. Lung
Cancer. 76:183–190. 2012. View Article : Google Scholar
|
|
21
|
Hong LZ, Wei XW, Chen JF and Shi Y:
Overexpression of periostin predicts poor prognosis in non-small
cell lung cancer. Oncol Lett. 6:1595–1603. 2013.PubMed/NCBI
|
|
22
|
Kikuchi Y, Kunita A, Iwata C, Komura D,
Nishiyama T, Shimazu K, Takeshita K, Shibahara J, Kii I, Morishita
Y, et al: The niche component periostin is produced by
cancer-associated fibroblasts, supporting growth of gastric cancer
through ERK activation. Am J Pathol. 184:859–870. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lv H, Liu R, Fu J, Yang Q, Shi J, Chen P,
Ji M, Shi B and Hou P: Epithelial cell-derived periostin functions
as a tumor suppressor in gastric cancer through stabilizing p53 and
E-cadherin proteins via the Rb/E2F1/p14ARF/Mdm2 signaling pathway.
Cell Cycle. 13:2962–2974. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li B, Wang L and Chi B: Upregulation of
periostin prevents P53-mediated apoptosis in SGC-7901 gastric
cancer cells. Mol Biol Rep. 40:1677–1683. 2013. View Article : Google Scholar
|
|
25
|
Liu Y and Liu BA: Enhanced proliferation,
invasion, and epithelial-mesenchymal transition of
nicotine-promoted gastric cancer by periostin. World J
Gastroenterol. 17:2674–2680. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tai IT, Dai M and Chen LB: Periostin
induction in tumor cell line explants and inhibition of in vitro
cell growth by anti-periostin antibodies. Carcinogenesis.
26:908–915. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu
M, Shao R, Anderson RM, Rich JN and Wang XF: Periostin potently
promotes metastatic growth of colon cancer by augmenting cell
survival via the Akt/PKB pathway. Cancer Cell. 5:329–339. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiao ZM, Wang XY and Wang AM: Periostin
induces chemoresistance in colon cancer cells through activation of
the PI3K/Akt/survivin pathway. Biotechnol Appl Biochem. 62:401–406.
2015. View
Article : Google Scholar
|
|
29
|
Zhu M, Fejzo MS, Anderson L, Dering J,
Ginther C, Ramos L, Gasson JC, Karlan BY and Slamon DJ: Periostin
promotes ovarian cancer angiogenesis and metastasis. Gynecol Oncol.
119:337–344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu M, Saxton RE, Ramos L, Chang DD,
Karlan BY, Gasson JC and Slamon DJ: Neutralizing monoclonal
antibody to periostin inhibits ovarian tumor growth and metastasis.
Mol Cancer Ther. 10:1500–1508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Choi KU, Yun JS, Lee IH, Heo SC, Shin SH,
Jeon ES, Choi YJ, Suh DS, Yoon MS and Kim JH: Lysophosphatidic
acid-induced expression of periostin in stromal cells: Prognostic
relevance of periostin expression in epithelial ovarian cancer. Int
J Cancer. 128:332–342. 2011. View Article : Google Scholar
|
|
32
|
Tian Y, Choi CH, Li QK, Rahmatpanah FB,
Chen X, Kim SR, Veltri R, Chia D, Zhang Z, Mercola D, et al:
Correction: Overexpression of periostin in stroma positively
associated with aggressive prostate cancer. PLoS One.
10:e01303332015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nuzzo PV, Rubagotti A, Zinoli L, Ricci F,
Salvi S, Boccardo S and Boccardo F: Prognostic value of stromal and
epithelial periostin expression in human prostate cancer:
Correlation with clinical pathological features and the risk of
biochemical relapse or death. BMC Cancer. 12:6252012. View Article : Google Scholar
|
|
34
|
Chen J, Xi J, Tian Y, Bova GS and Zhang H:
Identification, prioritization, and evaluation of glycoproteins for
aggressive prostate cancer using quantitative glycoproteomics and
antibody-based assays on tissue specimens. Proteomics.
13:2268–2277. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou W, Ke SQ, Huang Z, Flavahan W, Fang
X, Paul J, Wu L, Sloan AE, McLendon RE, Li X, et al: Periostin
secreted by glioblastoma stem cells recruits M2 tumour-associated
macrophages and promotes malignant growth. Nat Cell Biol.
17:170–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mikheev AM, Mikheeva SA, Trister AD,
Tokita MJ, Emerson SN, Parada CA, Born DE, Carnemolla B, Frankel S,
Kim DH, et al: Periostin is a novel therapeutic target that
predicts and regulates glioma malignancy. Neuro Oncol. 17:372–382.
2015.
|
|
37
|
Tian B, Zhang Y and Zhang J: Periostin is
a new potential prognostic biomarker for glioma. Tumour Biol.
35:5877–5883. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tavassoli FA, Ortiz-Hidalgo C,
Baquera-Heredia J and Grassi P: Images in pathology: The hearts of
a breast pathologist, a hematopathologist, and of a
cytotechnologist. Int J Surg Pathol. 10:2952002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.In German. PubMed/NCBI
|
|
40
|
Mueller-Holzner E, Fink V, Frede T and
Marth C: Immunohistochemical determination of HER2 expression in
breast cancer from core biopsy specimens: A reliable predictor of
HER2 status of the whole tumor. Breast Cancer Res Treat. 69:13–19.
2001. View Article : Google Scholar
|
|
41
|
Hugo H, Ackland ML, Blick T, Lawrence MG,
Clements JA, Williams ED and Thompson EW: Epithelial-mesenchymal
and mesenchymal-epithelial transitions in carcinoma progression. J
Cell Physiol. 213:374–383. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Guarino M: Epithelial-mesenchymal
transition and tumour invasion. Int J Biochem Cell Biol.
39:2153–2160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Baril P, Gangeswaran R, Mahon PC, Caulee
K, Kocher HM, Harada T, Zhu M, Kalthoff H, Crnogorac-Jurcevic T and
Lemoine NR: Periostin promotes invasiveness and resistance of
pancreatic cancer cells to hypoxia-induced cell death: Role of the
beta4 integrin and the PI3k pathway. Oncogene. 26:2082–2094. 2007.
View Article : Google Scholar
|
|
44
|
Gillan L, Matei D, Fishman DA, Gerbin CS,
Karlan BY and Chang DD: Periostin secreted by epithelial ovarian
carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5)
integrins and promotes cell motility. Cancer Res. 62:5358–5364.
2002.PubMed/NCBI
|
|
45
|
Siriwardena BS, Kudo Y, Ogawa I, Kitagawa
M, Kitajima S, Hatano H, Tilakaratne WM, Miyauchi M and Takata T:
Periostin is frequently overexpressed and enhances invasion and
angiogenesis in oral cancer. Br J Cancer. 95:1396–1403. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shao R, Bao S, Bai X, Blanchette C,
Anderson RM, Dang T, Gishizky ML, Marks JR and Wang XF: Acquired
expression of periostin by human breast cancers promotes tumor
angiogenesis through up-regulation of vascular endothelial growth
factor receptor 2 expression. Mol Cell Biol. 24:3992–4003. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kudo Y, Ogawa I, Kitajima S, Kitagawa M,
Kawai H, Gaffney PM, Miyauchi M and Takata T: Periostin promotes
invasion and anchorage-independent growth in the metastatic process
of head and neck cancer. Cancer Res. 66:6928–6935. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pula B, Jethon A, Piotrowska A,
Gomulkiewicz A, Owczarek T, Calik J, Wojnar A, Witkiewicz W, Rys J,
Ugorski M, et al: Podoplanin expression by cancer-associated
fibroblasts predicts poor outcome in invasive ductal breast
carcinoma. Histopathology. 59:1249–1260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kikuchi Y, Kashima TG, Nishiyama T,
Shimazu K, Morishita Y, Shimazaki M, Kii I, Horie H, Nagai H, Kudo
A, et al: Periostin is expressed in pericryptal fibroblasts and
cancer-associated fibroblasts in the colon. J Histochem Cytochem.
56:753–764. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Underwood TJ, Hayden AL, Derouet M, Garcia
E, Noble F, White MJ, Thirdborough S, Mead A, Clemons N, Mellone M,
et al: Cancer-associated fibroblasts predict poor outcome and
promote periostin-dependent invasion in oesophageal adenocarcinoma.
J Pathol. 235:466–477. 2015. View Article : Google Scholar :
|
|
51
|
Ryner L, Guan Y, Firestein R, Xiao Y, Choi
Y, Rabe C, Lu S, Fuentes E, Huw LY, Lackner MR, et al: Upregulation
of periostin and reactive stroma is associated with primary
chemoresistance and predicts clinical outcomes in epithelial
ovarian cancer. Clin Cancer Res. 21:2941–2951. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li M, Li C, Li D, Xie Y, Shi J, Li G, Guan
Y, Li M, Zhang P, Peng F, et al: Periostin, a stroma-associated
protein, correlates with tumor invasiveness and progression in
nasopharyngeal carcinoma. Clin Exp Metastasis. 29:865–877. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Reddy LA, Mikesh L, Moskulak C, Harvey J,
Sherman N, Zigrino P, Mauch C and Fox JW: Host response to human
breast Invasive Ductal Carcinoma (IDC) as observed by changes in
the stromal proteome. J Proteome Res. 13:4739–4751. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ishiba T, Nagahara M, Nakagawa T, Sato T,
Ishikawa T, Uetake H, Sugihara K, Miki Y and Nakanishi A: Periostin
suppression induces decorin secretion leading to reduced breast
cancer cell motility and invasion. Sci Rep. 4:70692014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yamaguchi Y, Mann DM and Ruoslahti E:
Negative regulation of transforming growth factor-beta by the
proteoglycan decorin. Nature. 346:281–284. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bi X, Pohl NM, Qian Z, Yang GR, Gou Y,
Guzman G, Kajdacsy-Balla A, Iozzo RV and Yang W: Decorin-mediated
inhibition of colorectal cancer growth and migration is associated
with E-cadherin in vitro and in mice. Carcinogenesis. 33:326–330.
2012. View Article : Google Scholar :
|
|
57
|
Ben QW, Jin XL, Liu J, Cai X, Yuan F and
Yuan YZ: Periostin, a matrix specific protein, is associated with
proliferation and invasion of pancreatic cancer. Oncol Rep.
25:709–716. 2011.PubMed/NCBI
|