Introduction
Gastric cancer is one of prevailing cancers
worldwide accounting for nearly 8–10% of cancer mortality (1). According to published reports, there
are approximately one million gastric cancer cases with 73,800
deaths in 2008 (2). In recent
years, many advances have taken place in diagnosis and treatment
(3). However, the mortality of
gastric cancer remains 70% especially with late stage patients
(4). Pharmacotherapy remains the
effective strategy for treatment of gastric cancer (5). Screening effective agents from herb
plants have been proved efficient (6). Many constituent have been demonstrated
with antitumor effects both in vitro and in vivo, for
instance: celastrol (7), bigelovin
(8), and parthenolide (9). Other natural compounds also
demonstrated many functions leading to cell proliferation, cell
differentiation, apoptosis and cell cycle changes (10,11).
Sophora flavescens have been widely planted
in East Asian countries as herbal plants and cosmetics (12). The root of Sophora flavescens
was frequently used for treatment of mental health, inflammation,
asthmas, and microbial activities (13–15).
Trifolirhizin is a natural pterocarcan flavonoid extracted from
Sophora flavescens (16).
Published reports demonstrated its chemical structure (Fig. 1A) (17). Trifolirhizin has many
pharmacological activities, such as hepatoprotective (18), anti-inflammatory (19), anti-proliferation (20), and skin-whitening (21). Trifolirhizin is also a biologically
active constituent of Zeng-Sheng-Ping, a commercial Chinese
traditional medicine for cancer prevention (22). Accumulating reports manifested that
trifolirhizin had anti-proliferative activities in melanoma B16
cells, human A2780 ovarian and H23 lung cancer cells in
vitro (19,21). Trifolirhizin also induced apoptosis
in human leukemia HL-60 cells in vitro (23). Despite reports on the trifolirhizin
cytotoxicity effect on various cells, the intrinsic mechanism of
cytotoxicity and apoptosis triggered by trifolirhizin remains
unknown. Herein, it is demonstrated that trifolirhizin induce
apoptosis and cell cycle arrest while we explored also the
mechanism of activation.
Materials and methods
Reagents and trifolirhizin
preparation
Trifolirhizin standard preparation (purity >98%)
were purchased from Chinese National Institute for the Control of
Pharmaceutical and Biological Products (Beijing, China). All other
chemicals used were provided from Sigma-Aldrich (St. Louis, MQ,
USA). Trifolirhizin was dissolved in DMSO. Cells were treated with
increasing concentrations of trifolirhizin for designated hours.
Control groups were exposed to the same volumes of DMSO under the
same conditions.
Cell culture and cell viability
assay
MKN45, L02, HEK293 cells were purchased from the
Cell Bank of Chinese Academy of Sciences (Shanghai, China). All
cells were maintained in RPMI-1640 medium (Gibco, Carlsbad, CA,
USA) with 10% fetal calf serum (Gibco) containing antibiotics (100
IU/ml penicillin and 100 IU/ml streptomycin) at 37°C in an
atmosphere containing 5% CO2. The 3 types of cell lines
were treated with various concentrations of trifolirhizin for
suitable times. Cell viability of control and experiment cells were
investigated using the 3-(4, 5-dimethylthiazol-2-yl)-2,
5-diphenyl-2H-tetrazolium bromide (MTT) assay. The absorbance at
570 nm was assessed using a microplate reader (Perkin-Elmer Inc.,
Waltham, MA, USA).
Apoptosis detection of Hoechst 33342
staining and TUNEL staining
MKN45 cells (105) were cultured on
cover-slide and treated as described above. For Hoechst 33342
staining, cells were added with Hoechst 33342 (5 µg/ml) at
4°C for 10 min in the dark (Beyotime, Haimeng, China). For terminal
deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling
(TUNEL) staining (Roche, Basel, Switzerland), TUNEL kit was used to
analyze cell DNA fragmentation in apoptotic cells according to the
manufacturer's instructions. Trifolirhizin-treated cells after
fixing was added by moderate TUNEL and DAPI (Beyotime). Finally,
apoptotic cells were observed using an inverted fluorescence
microscope (D5100, Nicon, Tokyo, Japan).
Flow cytometry assay for ratio of
apoptosis
MKN45 cell (2–5×105) apoptosis
quantification was analyzed according to the Annexin V&PI kit
manufacturer's instructions (Selleck Chemicals, Houston, TX, USA).
A minimum of 105 trifolirhizin-treated cells were
counted for each detection and immediately assessed by FACSCalibur
flow cytometry (BD Biosciences, San Jose, CA, USA). Final sample
data were reported as percentage of apoptotic cells (sum of
early-phase apoptosis at right down quadrant and late-apoptosis at
right upper quadrant).
Assay of mitochondrial membrane potential
(MMP)
The JC-10 assay kit (AAT Bioquest, CA, USA) was used
to assess the change tendency in mitochondrial membrane potential
(MMP). The kit was applied according to the manufacturer's
instructions. MKN45 cells were incubated in 96-well plate and
exposed to trifolirhizin under desired concentrations. JC-10
working solutions (50 µl) were added into each well. After
incubation and re-suspending in buffer B, the treated and control
MKN45 cell suspensions were measured with an excitation filter of
490 nm while emission filter of 525 nm (green) and another
excitation filter of 490 nm while emission filter of 590 nm (red)
by a microplate reader (Perkin-Elmer).
Cell cycle assay
PI was employed for cycle analysis (Beyotime). After
trifolirhizin treatment at 48 h, MKN45 carcinoma cells were
harvested from 6-cm culture plates and subsequently fixed with 70%
pre-cooled ethanol. After washing and centrifufation, cell pellets
were re-suspended in staining solutions containing RNase A (10
µg/ml) with PI (5 µg/ml) under darkness. Cells
treated with trifolirhizin and control were incubated for 30 min
and then tested with a FACSCalibur cytometer system (BD
Biosciences). Cell cycles were measured using ModFit LT3.2
software.
Western blot analysis
Western blotting was used to investigate the levels
of apoptotic and related signaling pathway proteins. Concisely,
cells treated with trifolirhizin and control were lysed with lysis
buffer (Beyotime) and centrifuged. Cell proteins were fractionated
by 8–12% SDS-PAGE. The proteins were electro-transferred onto the
nitrocellulose membranes, using the BCA kit to adjust protein
levels (Beyotime). The proteins levels were assessed using primary
antibody, including Bcl-2, Bax, PARP, c-Myc, caspase-3, caspase-9,
p-JNK, p-ERK, p-P38, JNK, P38, ERK, P53, β-actin (CST, Beverly, MA,
USA), followed by incubation with anti-mouse/rat HRP-labeled
secondary antibodies. The β-actin antibody was performed as
control. The signals were invested by ECL chemiluminescence (BD
Biosciences). The tendency of increasing or decreasing of proteins
levels were analyzed comparing to control.
Animals
The animal study was licensed by the Institutional
Animal Care and Use Committee of Fudan University and performed
strictly accordance with the Declaration of Helsinki and the Guide
for the Care and the Use of Laboratory Animals. The 6-week-old
BALB/C nude mice were obtained from Shanghai Slac Laboratory Animal
Corporation and maintained in a sterile humidified environment with
average temperature at 22°C. The mice were divided into 4 groups: 1
control group and 3 treatment groups (N=6) with increasing
trifolirhizin. MKN45 cells were harvested, diluted to desired
concentrations (5×106) and injected intraperitoneally
into the right flank of each mouse. Then, trifolirhizin was
injected into the BALB/C nude mice when tumor volume achieved a
mean group size of 100 mm3. The saline was injected into
control group. All mouse groups were injected every 3 days. BALB-nu
mice were sacrificed at 24 h when tumor volume achieved average
group size of 1,000 mm3. MKN45 tumor xenografts were
evaluated and fixed for further experiments. Tumor volumes were
evaluated as (W2 × L)/2 per 3 days using micrometer
calipers, and tumor weights were calculated.
Immunohistochemistry
The xenografted MKN45 tumor tissue samples were
removed and cut to desired small pieces. samples (5 µm) were
fixed and stained by cleaved-caspase-3 and Ki67 antibodies,
respectively, for immunohistochemistry. We observed the visual
images using an inverted fluorescence microscope (D5100, Nicon).
The immunohistochemistry positive cells were counted and analyzed
using ImageJ Pro 6.0 (National Institutes of Health).
Statistical analysis
We performed triplicate experiments using
independent samples. Results were expressed as mean values ±
standard deviation (mean ± SD). Statistical significance of our
present results was determined using the one-way ANOVA. All
statistical analyses were performed with commercial SPSS software
(SPSS19.0, Chicago, IL, USA).
Results
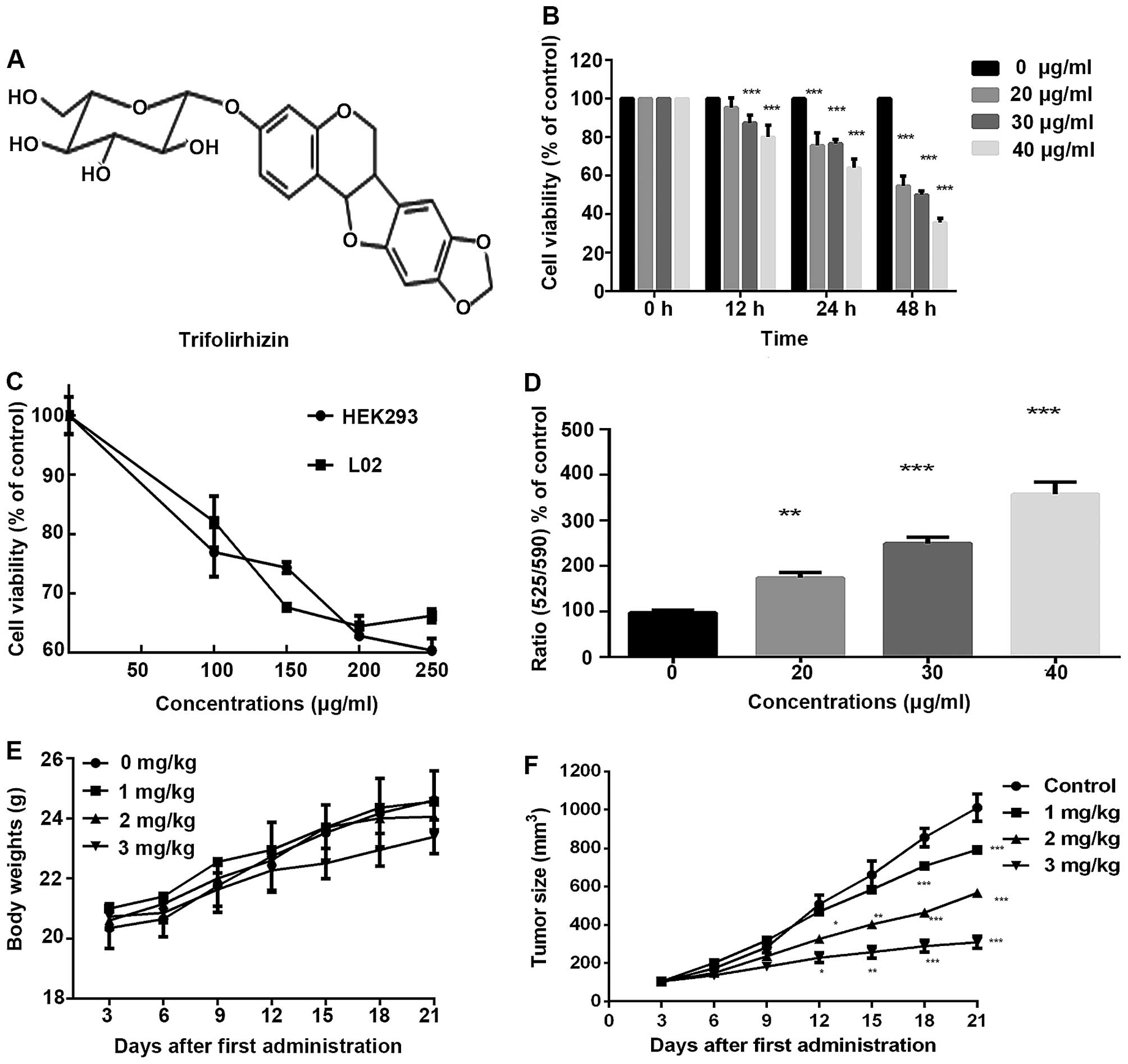
Trifolirhizin exerts anti-proliferation
effects in human gastric cancer cells
Published reports have manifested that trifolirhizin
is cytotoxic on cancer cells. MTT assay was used to assess the
numbers of viable MKN45 cells. Exposure under trifolirhizin results
in a concentration- and time-dependent suppression of MKN45 cell
viability (Fig. 1B). The
trifolirhizin exposure time was 48 h in the present examination.
The IC50 value of trifolirhizin on MKN45 cells was
33.27±2.06 µg/ml. We also conducted MTT assay to evaluate
the efficacy of trifolirhizin on a human normal liver cell line and
human normal kidney cell line in vitro. To our satisfaction,
>60% tested cells were viable when treated with increasing
trifolirhizin dose ≤250 µg/ml. The apparently higher
IC50 value of trifolirhizin on two normal human cell
lines exhibited more safety and further implication possibility
(Fig. 1C).
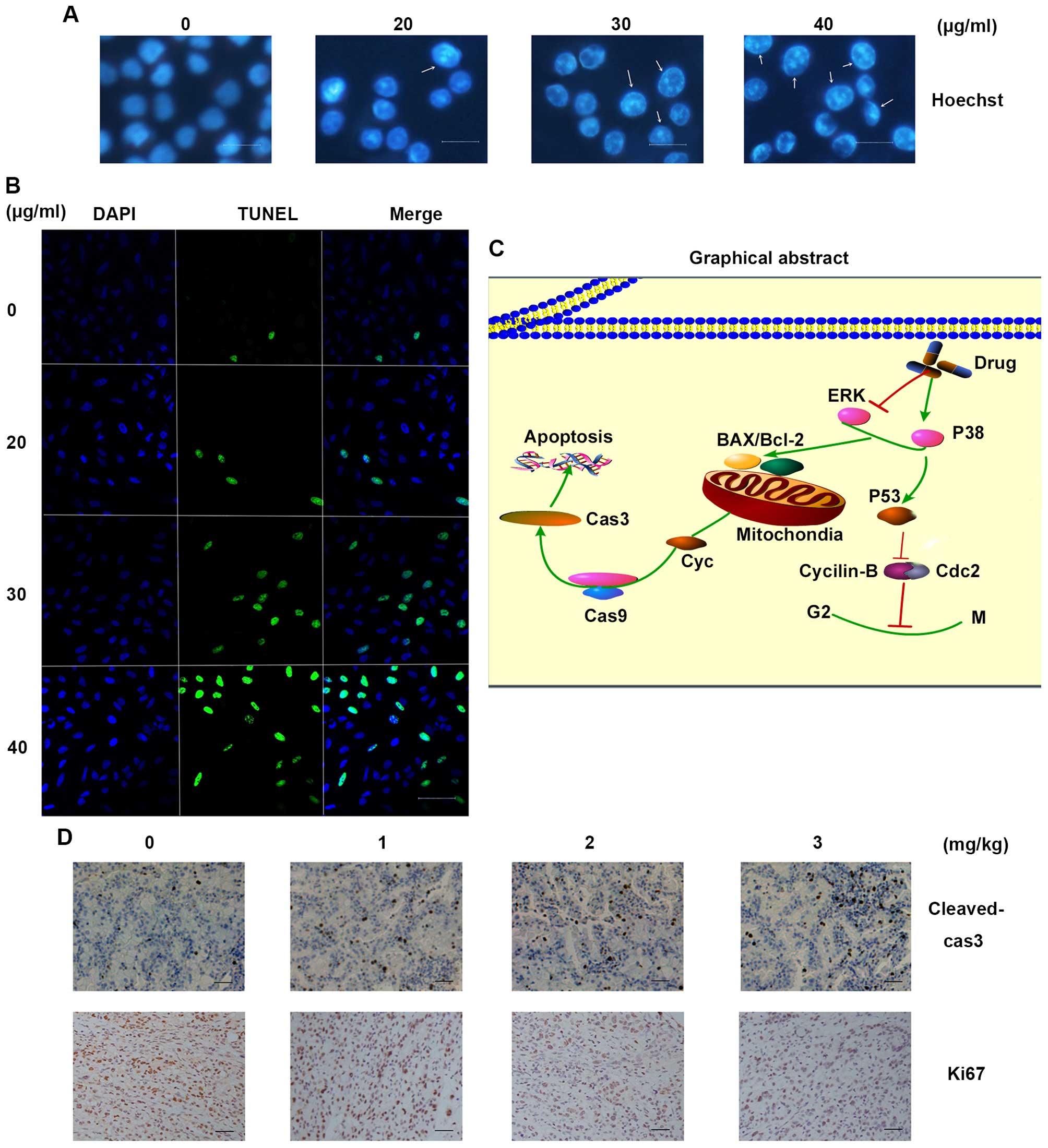
Trifolirhizin induces apoptosis in human
stomach cancer cells in vitro
MKN45 cells were treated with desired concentrations
of trifolirhizin as above at 48 h. Hoechst staining exhibited
visual images with cell shrinkage, nuclear fragmentation and
chromatin compaction of apoptosis (Fig.
4A). Images indicated that trifolirhizin lead to increasing
population of apoptotic cells. Consistently, TUNEL staining showed
a significant difference between trifolirhizin treated cells vs.
the vehicle control, the ratio of green staining (apoptotic) vs
blue staining (normal) arise with increasing trifolirhizin (20–40
µg/ml, shown in Fig. 4B).
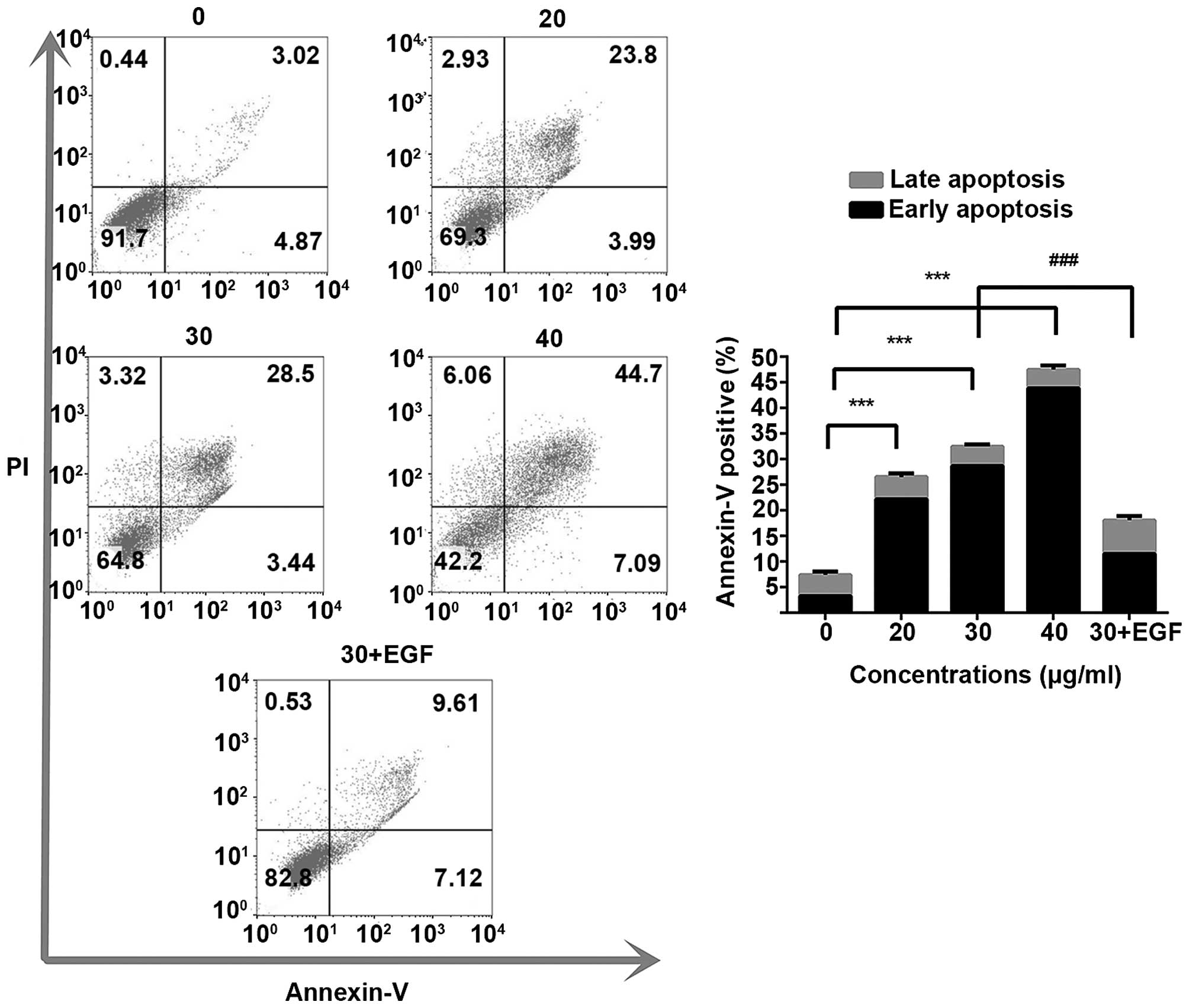
Annexin V/PI assay was performed to evaluate the apoptotic
population quantitation after trifolirhizin exposure. In this
study, trifolirhizin significantly triggered apoptosis. The
apoptotic population in trifolirhizin (20–40 µg/ml) induced
MKN45 cells increased (Fig. 2). The
JC-10 assay was used to indicate the mitochondrial outer membrane
permeabilization after trifolirhizin treatment in early phase.
Fig. 1D demonstrated that red
fluorescence (normal cells) was decreased while green fluorescence
(apoptotic cells) increased with trifolirhizin treatment (20–40
µg/ml) at 24 h, so the upgrade ratio of green/red
fluorescence reflected that the MMP (∆Ψm) in MKN45 after
trifolirhizin treatment (20–40 µg/ml) was decreased.
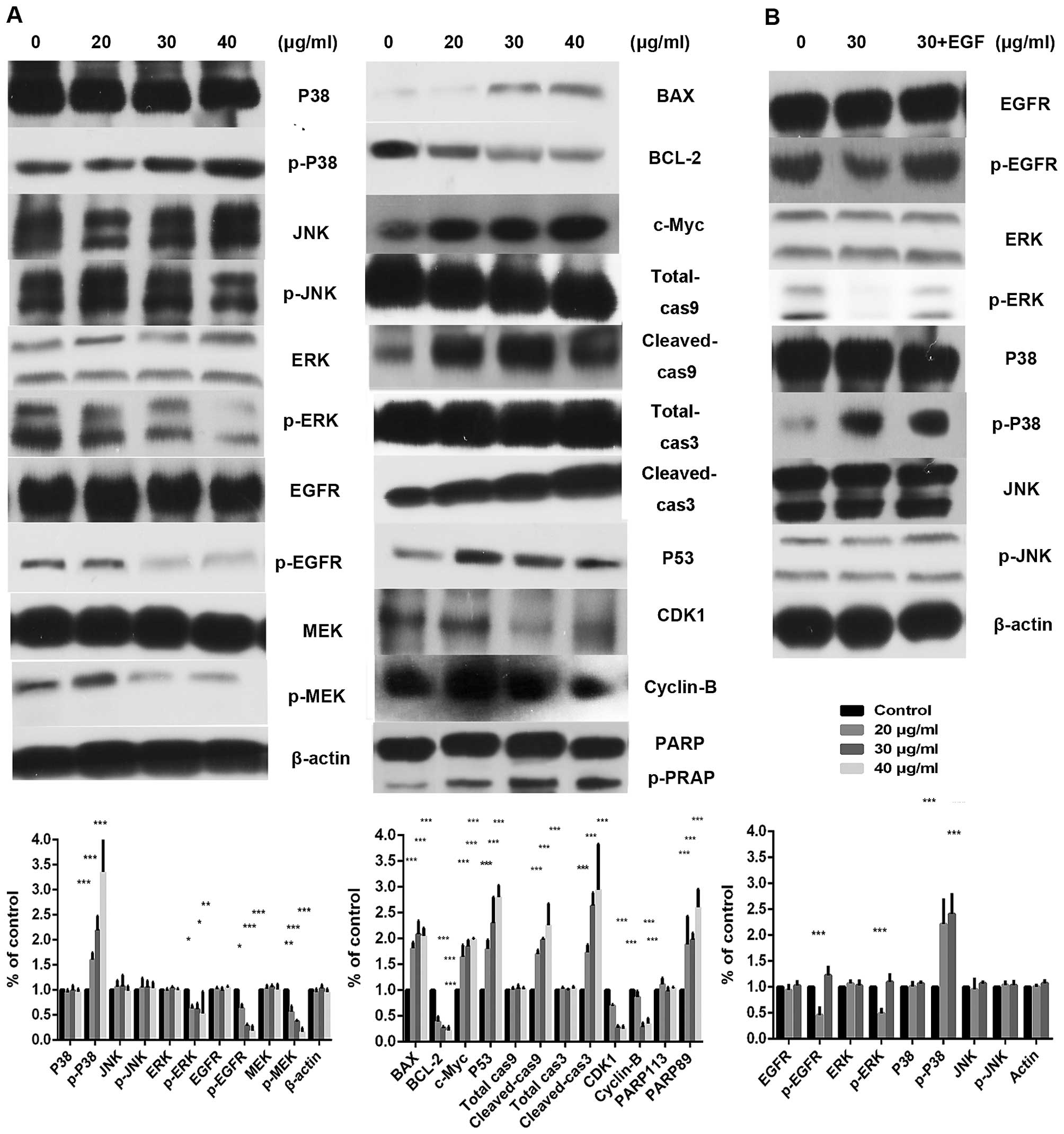
The expression levels of the epidermal growth factor
receptor (EGFR) signaling pathways and its downstream mitogen
activated protein kinase (MAPK) signaling pathway were shared
involving multiple anticancer agent molecular activities. The EGFR
levels, containing RAS/RAF/MEK/ERK are downregulated with the
increasing trifolirhizin, Fig. 4A
indicates that trifolirhizin (20–40 µg/ml) triggered the
increase of p38 and elicited no impact on p-JNK. Bcl-2 and Bax
family members are the critical regulators in mitochondrial
apoptosis pathways. Trifolirhizin leads to disorder of expression
ratio of Bcl-2 and Bax. The induction of apoptosis by trifolirhizin
was subsequently mediated through activities of c-Myc, caspase-9,
and caspase-3.
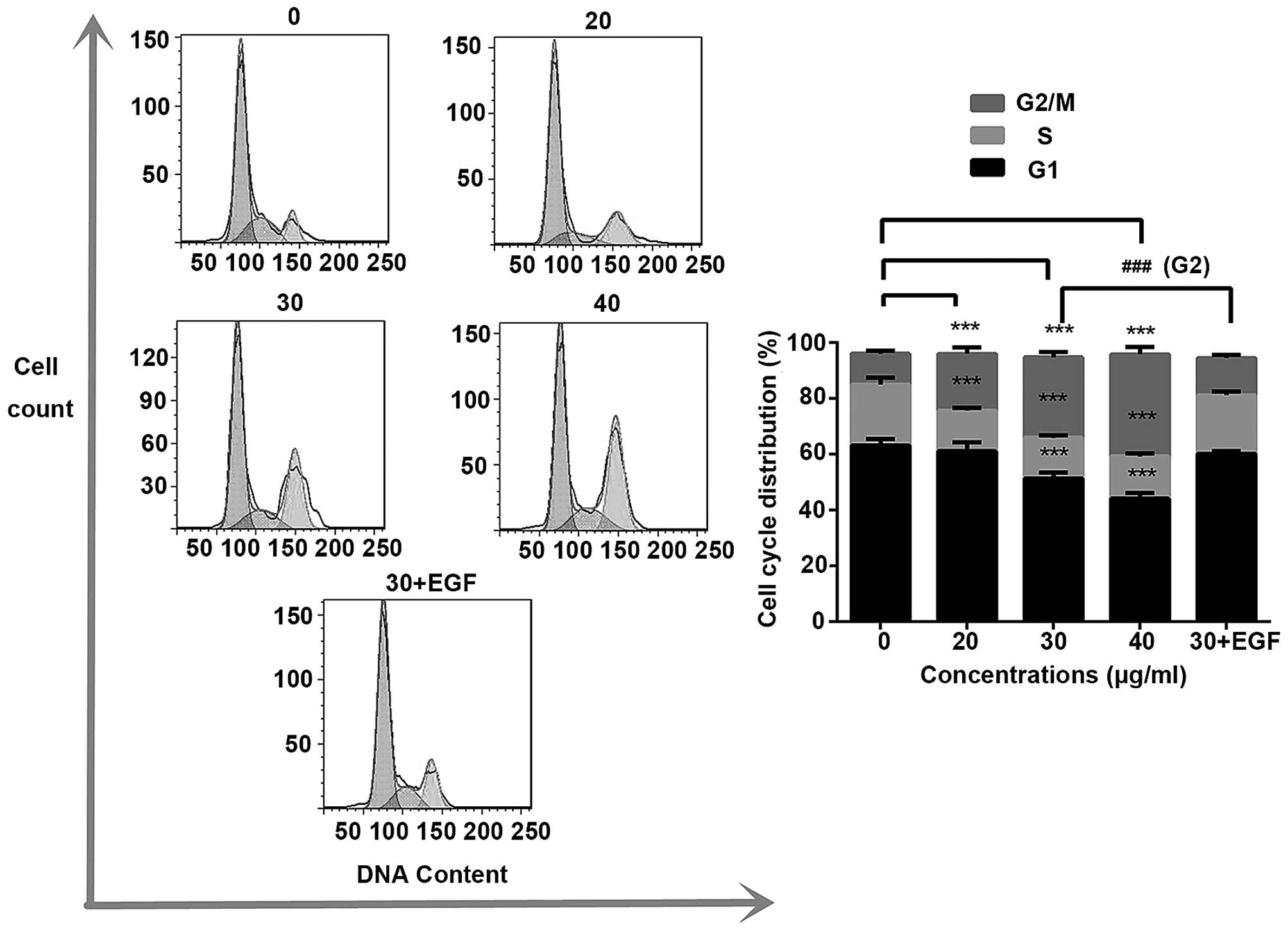
Trifolirhizin causes cell cycle arrest in
human gastric cancer cells in vitro
PI was employed to determine the cell cycle
distribution under trifolirhizin exposure. Fig. 3 shows that trifolirhizin exposure
(20–40 µg/ml) resulted in increasing accumulation in
G2/M phase in MKN45 cells. Furthermore, trifolirhizin
exhibited impact on the cell cycle through regulation of cell cycle
related proteins. There are an appreciable downregulation of cyclin
B as well as Cdc2 in trifolirhizin treatment (20–40 µg/ml).
The P53 protein, which is located upstream of cyclin complex and
downstream of EGFR signaling pathways, is a key point between
signaling pathway and cellular response in cell cycle arrest.
Trifolirhizin treatment (20–40 µg/ml) elicited P53
activation (Fig. 5A).
Anti-proliferation effect of
trifolirhizin on MKN45 xenograft tumors
Efficacy of trifolirhizin was also confirmed because
there were few changes in the mouse body weight in the experiment
(Fig. 1E). These results certified
the relative safety of trifolirhizin in vivo. After 21
administration days, xenograft tumors treated with trifolirhizin
(1–3 mg/kg) were smaller than control treated with saline (Table I and Fig. 1F). The significantly decreasing
levels of Ki67-positive cells were found in the study, which hinted
the anti-proliferation effect of trifolirhizin (1–3 mg/kg) in
vivo. Cleaved-caspase-3 positive cells were increased which
reflected the trifolirhizin apoptotic effect (Fig. 4D). These results also manifested the
antitumor effects of trifolirhizin.
 | Table IThe inhibitory effect of
trifolirhizin on MKN45 implantation tumor growth in BALB/C-nu
mice. |
Table I
The inhibitory effect of
trifolirhizin on MKN45 implantation tumor growth in BALB/C-nu
mice.
| Groups (mg/kg) | No. of animals
survived | Body weights (g)
| Tumor weight
(g) | Inhibition rate
(%) |
|---|
| Start | End |
|---|
| Control | 6 | 20.35±0.68 | 24.6±0.98 | 1.42±0.42 | |
| 1 | 6 | 20.88±0.36 | 24.58±0.74 | 0.90±0.23a | 36.37 |
| 2 | 6 | 20.71±0.38 | 24.28±1.30 | 0.61±0.24b | 57.29 |
| 3 | 6 | 20.73±0.42 | 23.38±0.56 | 0.34±0.22c | 76.35 |
Discussion
A previous published study indicated that
trifolirhizin had cytotoxicity in several cancer cells (19). Despite the cytotoxicity on human
cells by previous reports, the markedly higher IC50 in
our finding manifested the tissue specific effect of trifolirhizin
on HEK293 (normal kidney cell) and L02 (normal liver cell).
Therefore, trifolirhizin could be a potent safe anticancer
candidate although the administration or the structure of
trifolirhizin needs further efforts in future. In 2004, it was
indicated that trifolirhizin induces apoptosis in HL-60 cells
(23). However, the underlying
apoptotic mechanism remains unclear. Thus, it was chosen as one
candidate agent in our large scale natural anticancer compound
screening for further efforts. The MKN45 cell is a typical gastric
cancer cell commonly conducted as an anti-tumor model (24,25).
Our assay indicated that trifolirhizin inhibited the proliferation
of MKN45 gastric carcinoma cells in a time- and dose-dependent
manner. Apoptosis hypothesis was further confirmed by flow
cytometry data and visual contact under a microscope.
Many studies have indicated that EGFR-MAPK pathway
has the ability to affect the Bcl-2 family (26). For instance, JNK can inactivate
Bcl-2 while eliciting mitochondrial translocation of Bax (27). The Bcl-2 protein family is a crucial
regulator of apoptosis (28). Bcl-2
protein family contain two main types of members: pro-apoptotic
members Bax, anti-apoptotic members Bcl-2. The ratio of Bax/Bcl-2
leads to impaired apoptosis (29).
In this study, imbalance in expression level of Bcl-2/Bax was
clearly detected in the trifolirhizin treated MKN45 cells. MMP
decreased significantly in trifolirhizin treated MKN45 cells. Loss
of MMP contributes to the caspase pathway cascade (30). Caspases, a family of cysteine
proteases, are important proteins in regulating the apoptotic
response (31). Caspase-9
represents the intrinsic cascade pathway, while caspase-3 is the
key executioner in apoptosis (32).
Based on our western blot assay results, trifolirhizin induced the
activation of caspase-9, caspase-3, and cleavage of PARP. Then
apoptosis triggered cells to death. On the other hand, most
cytotoxic agents usually inhibit cell proliferation via arresting
the cycle in G1/S or G2/M phase (33,34).
This arrest process is mediated with cyclin-dependent protein
(Cdcs) with specific cyclin proteins. Trifolirhizin treatment leads
to mediating accumulation in blocking of Cdc2 and inhibition of
cyclin B resulting in a G2/M phase arrest.
We used the EGF (100 ng/ml) to re-activate EGFR
signal pathway for the mechanism validation (35). The apoptosis ratio in trifolirhizin
(30 µg/ml) combined with EGF using flow cytometry assay was
decreased compared with trifolirhizin (30 µg/ml, Fig. 2). The cell cycle distributions were
also transformed (30 µg/ml, Fig.
3). The EGFR protein levels were upregulated in the combination
group (30 µg/ml, Fig. 5B).
The examination manifested that EGFR signaling pathway may be
paramount in the effect of trifolirhizin on MKN45 cells.
In conclusion, trifolirhizin induced apoptosis in
MKN45 cancer cells in vitro and in vivo mediated via
EGFR-MAPK pathways. Trifolirhizin also arrest G2/M cycle
through impact on Cdc2/cyclin B complex (Fig. 4C). The results of this study implied
that trifolirhizin may a feasible agent for anti-stomach tumor
treatment, further scientific and therapeutic development should be
established.
Acknowledgments
This study was supported by State Administration of
Traditional Chinese Medicine 'Twelfth Five Year Plan' Key Specialty
(Chinese Medicine Geriatrics). The Shanghai Key Laboratory of
Clinical Geriatric Medicine provided important technical
support.
References
|
1
|
Tang X, Hu G, Xu C, Ouyang K, Fang W,
Huang W, Zhang J, Li F, Wang K, Qin X, et al: HZ08 reverse the
aneuploidy-induced cisplatin-resistance in gastric cancer by
modulating the p53 pathway. Eur J Pharmacol. 720:84–97. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saika K and Sobue T: Cancer statistics in
the world. Gan To Kagaku Ryoho. 40:2475–2480. 2013.In Japanese.
PubMed/NCBI
|
|
3
|
Nomoto-Kojima N, Aoki S, Uchihashi K,
Matsunobu A, Koike E, Ootani A, Yonemitsu N, Fujimoto K and Toda S:
Interaction between adipose tissue stromal cells and gastric cancer
cells in vitro. Cell Tissue Res. 344:287–298. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fock KM: Review article: The epidemiology
and prevention of gastric cancer. Aliment Pharmacol Ther.
40:250–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hacker U and Lordick F: Current standards
in the treatment of gastric cancer. Deutsche medizinische
Wochenschrift. 140:1202–1205. 2015.In German.
|
|
6
|
Safarzadeh E, Sandoghchian Shotorbani S
and Baradaran B: Herbal medicine as inducers of apoptosis in cancer
treatment. Adv Pharm Bull. 4(Suppl 1): 421–427. 2014.PubMed/NCBI
|
|
7
|
Li HY, Zhang J, Sun LL, Li BH, Gao HL, Xie
T, Zhang N and Ye ZM: Celastrol induces apoptosis and autophagy via
the ROS/JNK signaling pathway in human osteosarcoma cells: An in
vitro and in vivo study. Cell Death Dis. 6:e16042015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang HH, Kuang S, Wang Y, Sun XX, Gu Y,
Hu LH and Yu Q: Bigelovin inhibits STAT3 signaling by inactivating
JAK2 and induces apoptosis in human cancer cells. Acta Pharmacol
Sin. 36:507–516. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xin Y, Yin F, Qi S, Shen L, Xu Y, Luo L,
Lan L and Yin Z: Parthenolide reverses doxorubicin resistance in
human lung carcinoma A549 cells by attenuating NF-κB activation and
HSP70 up-regulation. Toxicol Lett. 221:73–82. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin LL, Hsia CR, Hsu CL, Huang HC and Juan
HF: Integrating transcriptomics and proteomics to show that
tanshinone IIA suppresses cell growth by blocking glucose
metabolism in gastric cancer cells. BMC Genomics. 16:412015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ye B, Li J, Li Z, Yang J, Niu T and Wang
S: Anti-tumor activity and relative mechanism of ethanolic extract
of Marsdenia tenacissima (Asclepiadaceae) against human hematologic
neoplasm in vitro and in vivo. J Ethnopharmacol. 153:258–267. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kocevski D, Du M, Kan J, Jing C, Lačanin I
and Pavlović H: Antifungal effect of Allium tuberosum, Cinnamomum
cassia, and Pogostemon cablin essential oils and their components
against population of Aspergillus species. J Food Sci.
78:M731–M737. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim DW, Chi YS, Son KH, Chang HW, Kim JS,
Kang SS and Kim HP: Effects of sophoraflavanone G, a prenylated
flavonoid from Sophora flavescens, on cyclooxygenase-2 and in vivo
inflammatory response. Arch Pharm Res. 25:329–335. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hwang JS, Lee SA, Hong SS, Lee KS, Lee MK,
Hwang BY and Ro JS: Monoamine oxidase inhibitory components from
the roots of Sophora flavescens. Arch Pharm Res. 28:190–194. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jung HJ, Kang SS, Hyun SK and Choi JS: In
vitro free radical and ONOO- scavengers from Sophora flavescens.
Arch Pharm Res. 28:534–540. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fujise Y, Toda T and Itô S: Isolation of
trifolirhizin from Ononis spinosa L. Chem Pharm Bull (Tokyo).
13:93–95. 1965. View Article : Google Scholar
|
|
17
|
Zhang C, Ma Y, Gao HM, Liu XQ, Chen LM,
Zhang QW, Wang ZM and Li AP: Non-alkaloid components from Sophora
flavescens. Zhongguo Zhong Yao Za Zhi. 38:3520–3524. 2013.In
Chinese.
|
|
18
|
Abdel-Kader MS: Preliminary
pharmacological study of the pterocarpans macckian and
trifolirhizin isolated from the roots of Ononis vaginalis. Pak J
Pharm Sci. 23:182–187. 2010.PubMed/NCBI
|
|
19
|
Zhou H, Lutterodt H, Cheng Z and Yu LL:
Anti-inflammatory and antiproliferative activities of
trifolirhizin, a flavonoid from Sophora flavescens roots. J Agric
Food Chem. 57:4580–4585. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang N, Liang B, Srivastava K, Zeng J,
Zhan J, Brown L, Sampson H, Goldfarb J, Emala C and Li XM: The
Sophora flavescens flavonoid compound trifolirhizin inhibits
acetylcholine induced airway smooth muscle contraction.
Phytochemistry. 95:259–267. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hyun SK, Lee WH, Jeong DM, Kim Y and Choi
JS: Inhibitory effects of kurarinol, kuraridinol, and trifolirhizin
from Sophora flavescens on tyrosinase and melanin synthesis. Biol
Pharm Bull. 31:154–158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yin T, Yang G, Ma Y, Xu B, Hu M, You M and
Gao S: Developing an activity and absorption-based quality control
platform for Chinese traditional medicine: Application to
Zeng-Sheng-Ping (Antitumor B). J Ethnopharmacol. 172:195–201. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aratanechemuge Y, Hibasami H, Katsuzaki H,
Imai K and Komiya T: Induction of apoptosis by maackiain and
trifolirhizin (maackiain glycoside) isolated from sanzukon (Sophora
Subprostrate Chen et T. Chen) in human promyelotic leukemia HL-60
cells. Oncol Rep. 12:1183–1188. 2004.PubMed/NCBI
|
|
24
|
Lindén SK, Driessen KM and McGuckin MA:
Improved in vitro model systems for gastrointestinal infection by
choice of cell line, pH, microaerobic conditions, and optimization
of culture conditions. Helicobacter. 12:341–353. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Giard DJ, Aaronson SA, Todaro GJ, Arnstein
P, Kersey JH, Dosik H and Parks WP: In vitro cultivation of human
tumors: Establishment of cell lines derived from a series of solid
tumors. J Natl Cancer Inst. 51:1417–1423. 1973.PubMed/NCBI
|
|
26
|
Thomas S, Quinn BA, Das SK, Dash R, Emdad
L, Dasgupta S, Wang XY, Dent P, Reed JC, Pellecchia M, et al:
Targeting the Bcl-2 family for cancer therapy. Expert Opin Ther
Targets. 17:61–75. 2013. View Article : Google Scholar
|
|
27
|
Yu H, Zhang T, Cai L, Qu Y, Hu S, Dong G,
Guan R, Xu X and Xing L: Chamaejasmine induces apoptosis in human
lung adenocarcinoma A549 cells through a Ros-mediated mitochondrial
pathway. Molecules. 16:8165–8180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Llambi F and Green DR: Apoptosis and
oncogenesis: Give and take in the BCL-2 family. Curr Opin Genet
Dev. 21:12–20. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bah N, Maillet L, Ryan J, Dubreil S,
Gautier F, Letai A, Juin P and Barillé-Nion S: Bcl-xL controls a
switch between cell death modes during mitotic arrest. Cell Death
Dis. 5:e12912014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
David R: Apoptosis: A lipid trigger of
MOMP. Nat Rev Mol Cell Biol. 13:208–209. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cohen GM: Caspases: The executioners of
apoptosis. Biochem J. 326:1–16. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brentnall M, Rodriguez-Menocal L, De
Guevara RL, Cepero E and Boise LH: Caspase-9, caspase-3 and
caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell
Biol. 14:322013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Carduner L, Picot CR, Leroy-Dudal J, Blay
L, Kellouche S and Carreiras F: Cell cycle arrest or survival
signaling through αv integrins, activation of PKC and ERK1/2 lead
to anoikis resistance of ovarian cancer spheroids. Exp Cell Res.
320:329–342. 2014. View Article : Google Scholar
|
|
34
|
Romanov V, Whyard TC, Waltzer WC, Grollman
AP and Rosenquist T: Aristolochic acid-induced apoptosis and G2
cell cycle arrest depends on ROS generation and MAP kinases
activation. Arch Toxicol. 89:47–56. 2015. View Article : Google Scholar
|
|
35
|
Li L, Gao Y, Zhang L, Zeng J, He D and Sun
Y: Silibinin inhibits cell growth and induces apoptosis by caspase
activation, down-regulating survivin and blocking EGFR-ERK
activation in renal cell carcinoma. Cancer Lett. 272:61–69. 2008.
View Article : Google Scholar : PubMed/NCBI
|