Introduction
Bladder urothelial carcinoma (UC) ranks as one of
the most common urological malignancies worldwide. Approximately
70% of patients with bladder UC present with non-muscle invasive
bladder cancer (NMIBC). Of these, ~20% subsequently develop muscle
invasion associated with a strong propensity toward lethal
metastases (1,2). Muscle-invasive bladder cancer (MIBC)
represents a worse prognosis, with a 5-year survival of ~50% even
after curative surgery (3). In
particular, patients with pathological T1 (invading to the lamina
propria of the bladder) and/or a higher tumor grade (ʻhigh-risk'
NMIBC) are at high risk of developing MIBC.
CD44 is a major surface receptor for hyaluronate
that has been shown to be relevant to several physiological
processes including leukocyte homing and activation, intercellular
adhesion and cell-matrix adhesion, and cell migration, as well as
tumor cell invasion and metastasis (4–6). CD44
has multiple isoforms generated through alternative splicing of 10
variant exons, and has been identified as one of the major cancer
stem cell markers for various epithelial tumors (7). Recent studies have revealed that the
interaction of CD44 variants (CD44v) with the cystine transporter
subunit xCT stabilizes cancer cells and provides a defense against
reactive oxygen species (8).
In bladder UC, immunohistochemical (IHC) expression
of both standard CD44 and the CD44 variant 6 (CD44v6) isoform is
significantly reduced in relation to the pathological stage
(9). Thus, the expression of CD44
and its variant CD44v6 might have an independent prognostic value
in predicting a prolonged survival in invasive bladder cancer;
however, these findings are still controversial (10). In addition, CD44 variant 9 (CD44v9)
has been reported as a cancer stem cell marker as well as a
prognostic marker in colorectal and early gastric cancers (11,12).
Furthermore, recent studies have identified two types of molecular
classifications of MIBC, termed luminal and basal types, wherein
the latter demonstrates the worse prognoses (13).
The aim of this study was to examine whether CD44v9
expression is relevant to the new molecular classification of MIBC,
in particular to the basal type, and to explore the clinical
significance and mechanism of CD44v9 function in tumor progression.
To our knowledge this is the first study to show the
histopathological location of basal subtype marker expression and
its clinical impact in high-risk NMIBC.
Materials and methods
Patient samples
Written informed consent was obtained from all
patients to use their sample and the Institutional Review Board at
Yamaguchi University Hospital approved this study (approval no.
17). Tumor specimens were obtained from 36 patients with MIBC
treated with radical cystectomy with standard lymphadenectomy and
from 62 patients with NMIBC treated with transurethral resection
(TUR-BT) and diagnosed as high-grade pT1 (high-risk NMIBC) in
Yamaguchi University Hospital between January 2001 and December
2012. All specimens were diagnosed as UC by the pathologist.
Details of patient backgrounds are listed in Table I.
 | Table IClinicopathological characteristics of
MIBC patients treated with radical cystectomy and high-risk NMIBC
patients treated with TUR-BT. |
Table I
Clinicopathological characteristics of
MIBC patients treated with radical cystectomy and high-risk NMIBC
patients treated with TUR-BT.
A, Characteristics of
MIBC cases (n=36)
|
|---|
| Characteristics | n (%) |
|---|
| Age in years, median
(range) | 63 (42–79) |
| Gender | |
| Male | 32 (89) |
| Female | 4 (11) |
| Clinical T
category | |
| cTis | 1 (3) |
| cT1 | 3 (8) |
| cT2 | 8 (22) |
| cT3 | 16 (44) |
| cT4 | 8 (22) |
| Pathological T
category | |
| pTa | 2 (6) |
| pT1 | 8 (22) |
| pT2 | 9 (25) |
| pT3 | 10 (28) |
| pT4 | 7 (19) |
| Grade | |
| G1 | 1 (3) |
| G2 | 9 (25) |
| G3 | 23 (64) |
| Gx | 3 (8) |
| Histological
type | |
| UC | 29 (81) |
| SCC | 2 (6) |
| Other | 5 (13) |
| LVI | |
| Negative | 16 (44) |
| Positive | 16 (44) |
| Unidentified | 4 (12) |
| Neoadjuvant
therapy | |
| No | 21 (58) |
| Yes | 15 (42) |
| Adjuvant therapy | |
| No | 24 (67) |
| Yes | 12 (33) |
B, Characteristics of
high-risk NMIBC cases (n=62)
|
|---|
| Characteristics | n (%) |
|---|
| Age in years, median
(range) | 71 (51–88) |
| Gender | |
| Male | 51 (82) |
| Female | 11 (18) |
|
Primary/recurrence | |
| Primary | 55 (89) |
| Recurrence | 7 (11) |
| Histological
type | |
| UC | 55 (89) |
| UC + SCC | 3 (5) |
| UC + AC | 4 (6) |
| LVI | |
| Negative | 31 (50) |
| Positive | 5 (8) |
| Unidentified | 26 (42) |
| CIS component | |
| No | 26 (42) |
| Yes | 36 (58) |
| 2nd TUR-BT | |
| No | 38 (61) |
| Yes | 24 (39) |
Immunohistochemistry
Formalin-fixed and paraffin-embedded tissue
specimens were used for IHC staining. For each sample, 5
µm-thick sections were deparaffinized in xylene, dehydrated
in ethanol, and incubated in 0.3% hydrogen peroxide solution in
methanol for 10 min. The sections were then microwaved in 0.01 M
citrate-buffered solution (pH 6.0) for 15 min and covered in
blocking solution (IMMUNO SHOT; Cosmo Bio Co., Ltd., Tokyo, Japan)
for 30 min. After addition of a blocking solution, primary
antibodies [anti-CD44v9 (1/500 dilution, kindly provided by
Professor Saya, Keio University, Tokyo, Japan), anti-cytokeratin
5/6 (CK5/6) (1/200 dilution), or anti-cytokeratin 20 (CK20) (1/100
dilution) (both from Dako, Kyoto, Japan)] were added according to
manufacturer's instructions, followed by incubation with the
respective secondary antibody (Nichirei Bioscience, Tokyo, Japan)
for 30 min at room temperature. Double IHC studies were performed
on paraffin-embedded sections using double labeling with CD44v9 and
CK5/6 or CK20 by diaminobenzidine and fast red. To evaluate the
IHC, the H-score was used in this study. Briefly, >500 tumor
cells were counted with different three fields of views in each
case, and the H-score was subsequently calculated by multiplying
the percentage of the positive cells by the intensity (strongly
stained, 3×; moderately stained, 2×; and weakly stained, 1×),
yielding a possible range of 0–300 (14,15).
Paraffin-embedded cell pellets were obtained by freezing aliquots
of two human bladder cancer cell lines (HT1376, 5637) at −80°C
followed by fixation in 10% formalin and subsequent processing for
use in IHC staining as described (16).
Cell culture
The human bladder cancer cell lines HT1376 and 5637
and the human breast cancer cell line MDA-MB-468 were obtained from
the American Type Culture Collection (ATCC; Manassas, VA, USA). The
MDA-MB-468 cell line was selected based on previous reports of
elevated CD44v9 expression. Cells were routinely cultured in DMEM
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented
with 10% FBS (Biological Industries, Cromwell, CT, USA) and
antibiotic antimycotic solution (Sigma-Aldrich, Tokyo, Japan) in a
5% CO2 atmosphere at 37°C.
siRNA knockdown of CD44 and CD44v9
CD44, CD44v9, and control siRNAs were obtained from
Thermo Fisher Scientific, Inc. siRNA sequences were as follows:
CD44 siRNA sense, 5′-UAU UCC ACG UGG AGA AAA Att-3′ and antisense,
5′-UUU UUC UCC ACG UGG AAU Aca-3′; CD44v9 siRNA sense, 5′-CUA CUU
UAC UGG AAG GUU Att-3′ and antisense, 5′-UAA CCU UCC AGU AAA GUA
Gtt-3′. High GC duplex was used as the negative control siRNA. Each
cell line was transiently transfected with siRNA using
Lipofectamine RNAi MAX (Thermo Fisher Scientific, Inc.) according
to the manufacturer's instructions. After transfection, cells were
incubated at 37°C in a CO2 incubator for 48 h.
Quantitative evaluation of mRNA and protein expression was verified
by western blotting and RT-PCR.
RT-PCR
Total cellular RNA was extracted using TRIzol
reagent (Thermo Fisher Scientific, Inc.). A total of 1 mg RNA was
subjected to cDNA synthesis using the Reverse Transcription Kit
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. PCR reactions were carried out in the StepOnePlus
Real-Time PCR System (Thermo Fisher Scientific, Inc.) using
SYBR-Green Supermix (Takara Bio, Inc., Shiga, Japan) according to
the manufacturer's instructions. The primer sequences were as
follows: CD44 forward, 5′-CCG CTA TGT CCA GAA AGG A-3′ and
reverse, 5′-CTG TCT GTG CTG TCG GTG AT-3′; CD44 standard
isoform forward, 5′-AAA GGA GCA GCA CTT CAG GA-3′ and reverse,
5′-TGT GTC TTG GTC TCT GGT AGC-3′; CD44v9 forward, 5′-GGC
TTG GAA GAA GAT AAA GAC C-3′ and reverse, 5′-TGC TTG ATG TCA GAG
TAG AAG TTG-3′; and β-actin forward, 5′-GCA TCC TCA CCC TGA AGT
A-3′ and reverse, 5′-TGT GGT GCC AGA TTT TCT CC-3′. The differences
in gene expression levels between samples were measured using the
2−ΔΔCt method.
Flow cytometry
Aliquots of cells (5×105) were prepared
in assay tubes. After the addition of 2% FBS and 0.02% sodium azide
in PBS followed by a rinse and centrifugation, cells were incubated
in diluted primary antibody (anti-CD44v9) for 30 min at 4°C. Cells
were then washed twice with 2% FBS and 0.02% sodium azide in PBS,
and resuspended cells were incubated in phycoerythrin (PE)-labeled
secondary antibody for 30 min at 4°C in the dark. After being
rinsed twice, the conjugated cells were analyzed according to the
manufacturer's instructions (Cell Analyzer EC800; Sony, Tokyo,
Japan).
Western blotting
Protein lysates from each tested cell sample were
prepared in a radio-immunoprecipitation assay buffer with
proteinase inhibitors. Each lysate sample (30 µg) was
separated by SDS-PAGE, and electro-transferred to a PVDF membrane.
Following blocking in 5% non-fat milk or 5% BSA, these membranes
were incubated with each primary antibody overnight at 4°C. After
being washed in TBS with 0.05% Tween-20 (TBST), the membranes were
incubated with HRP-conjugated secondary antibodies for 1 h at room
temperature. After subsequent washing with TBST, membrane signals
were detected using an ECL detection system (ChemiDoc™ XRS+;
Bio-Rad Laboratories, Inc., Berkeley, CA, USA). Primary antibodies
were used at the following dilutions: anti-CD44 (1/1,000 dilution)
and anti-β-actin (1/1,000 dilution), both from Abcam (Cambridge,
UK); and anti-E-cadherin (1/1,000 dilution); anti-N-cadherin (1/500
dilution); anti-snail (1/500 dilution); and anti-slug (1/500
dilution), from Santa Cruz Biotechnology (Dallas, TX, USA). β-actin
was used for protein band normalization.
Cell invasion assay
Equal numbers of transfected and control cells
(1×105) in DMEM without FBS were added to the upper
insert invasion chambers with Matrigel (BD BioCoat Matrigel
Invasion Chamber, 12 wells, 8 µm; Becton-Dickinson, Bedford,
MA, USA) and the control chambers without Matrigel. DMEM
supplemented with 10% FBS was added to the lower compartment, and
the chambers were incubated at 37°C for 48 h. After the incubation
period, cells from the upper surface of the filter were wiped off
with a cotton swab. The lower surface of the filter was stained
with Diff-Quik (Dade Behring AG, Düdingen, Switzerland). The number
of cells having migrated to the bottom of the chamber was counted
from five randomly selected fields using a light microscope. The
mean number of cells was calculated per field. We evaluated the
invasion capacity using the ratio between the number of cells in
the invasion chamber and the number of cells in the control
chamber. Two sets of experiments were carried out, each in
triplicate.
Wound healing assay
The effect of lithium on cell migratory activity was
examined using a scratch-wound assay. Each set of transfected and
control cells was seeded in a 12-well chamber in DMEM with 10% FBS
and grown until confluency. One linear scar was drawn in the
monolayer with a 200-µl tip (width, 500 µm). Wound
closure (cell migration) was observed under a phase-contrast
microscope (×100 magnification; Olympus, Tokyo, Japan), and
photographed when the wound was made and at designated times after
wounding. Cells were counted within the initial and the remaining
wound area and the wound closure was expressed as the percentage of
migrating cells compared to the control. Two sets of experiments
were carried out, each in duplicate.
Statistical analysis
Statistical analysis was performed using JMP version
10 (SAS, Cary, NC, USA). Differences between the two groups were
analyzed using Student's t-test and the Cox proportional hazard
model was applied to determine prognostic factors for progression.
Progression-free survival and cancer specific-free survival were
calculated using the Kaplan-Meier method and compared by a log-rank
test. The Pearson's product-moment correlation coefficient was
applied to study the correlation of the H-score in IHC. P<0.05
was regarded as statistically significant. To investigate the
relation of IHC CD44v9 expression status to patient outcome, a
receiver operating characteristic (ROC) curve was applied to
determine the optimal cut-off value to discriminate cancer-specific
death. H-scores of 153 and 170 were set as the cut-offs in MIBC
[area under the curve (AUC) =0.6428] and high-risk NMIBC (AUC =
0.6354), respectively.
Results
Correlation of CD44v9 overexpression to
patient outcome
The CD44v9 expression level was evaluated by IHC in
36 MIBC and 62 high-risk NMIBC tissues. Fig. 1A shows the representative pictures
of higher and lower expression staining in MIBC and high-risk NMIBC
specimens, respectively. Table I
shows the clinicopathological characteristics of MIBC and high-risk
NMIBC patients with a median follow-up period of 39 months (range,
3–104 months) and 50 months (range, 18–125 months), respectively.
In MIBC, 25 specimens (69.4%) showed lower CD44v9 expression
(H-score <153), whereas 11 specimens (30.6%) showed higher
expression. In high-risk NMIBC, 38 specimens (61.2%) showed lower
CD44v9 expression (H-score <170), whereas 24 specimens (38.8%)
showed higher expression. Table II
shows the prognosis factors in CD44v9-positive and -negative
groups. In MIBC, the positive rate of lymphovascular invasion and
lymph node metastasis were higher in CD44v9-positive group than in
negative group (64 vs. 36%, 18 vs. 8%) (Table IIA). In univariate and multivariate
analysis, the expression of CD44v9 and recurrent tumor were
significant predictors for progression-free survival in high-risk
NMIBC (Table III; both
P<0.01). Patients with higher CD44v9 expression exhibited
significantly lower progression-free survival and cancer-specific
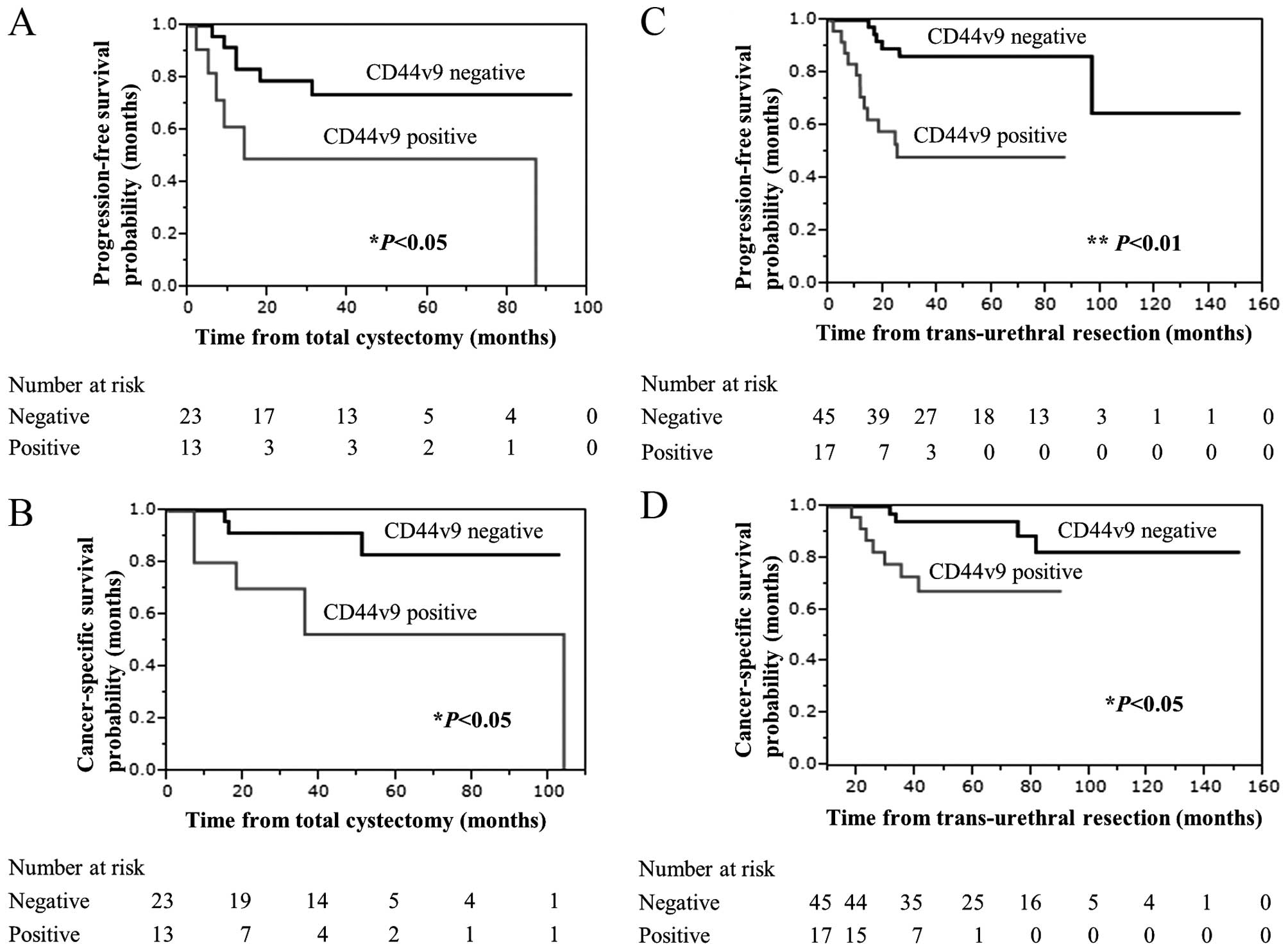
survival than those with lower CD44v9 expression in MIBC (Fig. 2A and B; both P<0.05) as well as
in high-risk NMIBC (Fig. 2C and D;
P<0.01, P<0.05).
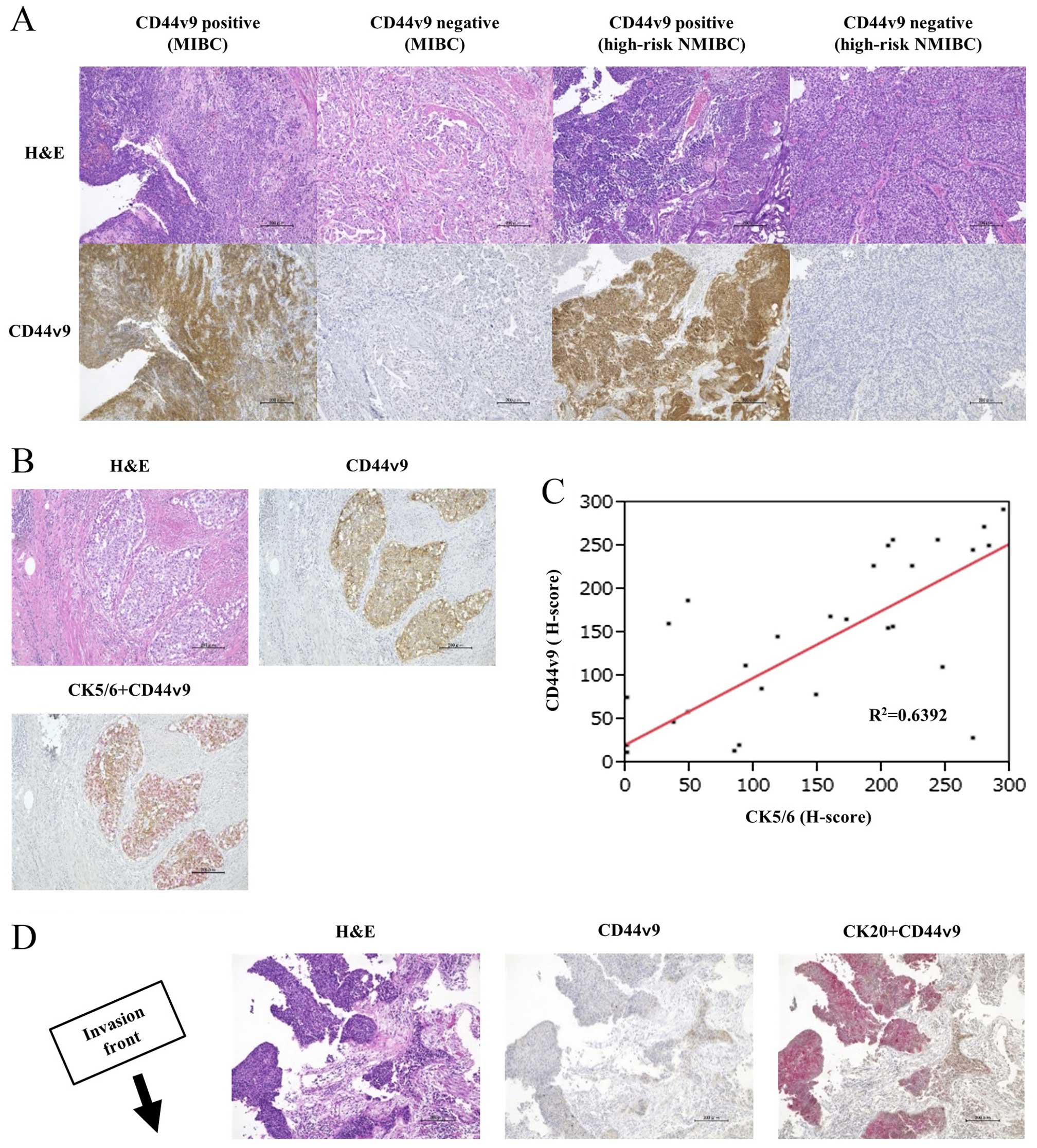 | Figure 1Histological (H&E) and IHC
staining with (A) CD44v9, (B and C) CK5/6, CK20, and double
staining of CD44v9 (brown) with CK5/6 (red) in MIBC, or (D) with
CK20 (red) in high-risk NMIBC. (A) The higher and lower expression
staining levels of CD44v9 in MIBC and high-risk NMIBC specimens.
Note that CD44v9 is predominantly located either in the cytoplasm
or in the cellular membrane. (B) A case with co-localization of
positive CD44v9 with CK5/6. Here, the tumor had spread to the
inguinal lymph nodes 7 months after radical cystectomy (pT2aN0M0),
and the patient died of bladder cancer 18 months following the
operation. (C) Significant correlation of the H-score between
CD44v9 and CK5/6 (R2=0.6392, Pearson's product-moment
correlation coefficient). (D) A case wherein the positive
expression site of CD44v9 is completely separated from that of
CK20, and is localized to the invasive front of the tumor in
high-risk NMIBC. Here, bladder recurrence occurred within 12 months
after transurethral resection (pT1/high grade) and developed to
MIBC 25 months following the operation. IHC, immunohistochemical;
CD44v9, CD44 variant 9; CK5/6, cytokeratin 5/6; CK20, cytokeratin
20; MIBC, muscle-invasive bladder cancer; NMIBC, non-muscle
invasive bladder cancer. |
 | Table IIThe relationship between the
expression of CD44v9 and clinicopathological prognosis factor for
progression in MIBC and high-risk NMIBC. |
Table II
The relationship between the
expression of CD44v9 and clinicopathological prognosis factor for
progression in MIBC and high-risk NMIBC.
A, MIBC (n=36)
|
|---|
|
Characteristics | CD44v9 negative
(n=25)
n (%) | CD44v9 positive
(n=11)
n (%) |
|---|
| Clinical T
category | | |
| <3 | 9 (36) | 3 (27) |
| ≥3 | 16 (64) | 8 (73) |
| Pathological T
category | | |
| <3 | 14 (56) | 5 (45) |
| ≥3 | 11 (44) | 6 (55) |
| Lymph node
metastasis | | |
| Negative | 23 (92) | 9 (82) |
| Positive | 2 (8) | 2 (18) |
| LVI | | |
| Negative | 13 (52) | 3 (27) |
| Positive | 9 (36) | 7 (64) |
| Unidentified | 3 (12) | 1 (9) |
B, High-risk NMIBC
(n=62)
|
|---|
|
Characteristics | CD44v9 negative
(n=38)
n (%) | CD44v9 positive
(n=24)
n (%) |
|---|
| No. of tumors | | |
| Single | 12 (32) | 4 (17) |
| Multiple | 26 (68) | 20 (83) |
| Tumor size
(cm) | | |
| <3 | 30 (79) | 19 (79) |
| ≥3 | 8 (21) | 5 (21) |
|
Primary/recurrence | | |
| Primary | 33 (87) | 21 (87) |
| Recurrence | 5 (13) | 3 (13) |
| CIS component | | |
| No | 13 (34) | 13 (54) |
| Yes | 25 (66) | 11 (46) |
 | Table IIIUnivariate and multivariate analysis
of prognosis factors associated with progression-free survival in
high-risk NMIBC. |
Table III
Univariate and multivariate analysis
of prognosis factors associated with progression-free survival in
high-risk NMIBC.
| Univariate analysis
| Multivariate
analysis
|
|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Primary or
recurrence |
| Primary vs.
recurrence | 0.25
(0.09–0.80) | 0.022 | 0.19
(0.06–0.63) | 0.009 |
| No. of tumors |
| Single vs.
multiple | 0.53
(0.12–1.61) | 0.287 | | |
| Tumor size
(cm) |
| <3 vs. ≥3 | 0.85
(0.30–3.03) | 0.787 | | |
| CIS component |
| No vs. Yes | 0.83
(0.28–2.20) | 0.725 | | |
| Expression of
CD44v9 |
| Negative vs.
positive | 0.18
(0.05–0.49) | 0.007 | 0.15
(0.05–0.43) | 0.003 |
Colocalization of CD44v9 with CK5/6
expression and specific localization of CD44v9-positive cells in
the invasive front of high-risk NMIBC samples
We compared the expression site of CK5/6, a
basal-type marker, and CK20, a luminal-type marker, to that of
CD44v9 using IHC. Fig. 1B depicts a
representative MIBC sample showing the corresponding sites of
expression of CD44v9 and CK5/6. In MIBC, the H-score of CD44v9
expression was significantly correlated with that of CK5/6
(Fig. 1C; R2= 0.6329, P<0.001).
In high-risk NMIBC, the expression sites of CD44v9 and CK5/6 were
colocalized in the invasive front of the tumor, which is defined as
the deepest invasion site in this cancer. In contrast, the
expression sites of CK20 were localized to the superficial tissue,
and distinctly separated from the expression site of CD44v9 in
high-risk NMIBC (Fig. 1D).
Effect of siRNA-induced knockdown of
CD44v9 in 5637 and HT1376 cell lines
CD44v9 expression was evaluated by IHC (Fig. 3A) and flow cytometry (Fig. 3B) and western blotting (Fig. 3C) using the 5637 and HT1376 cell
lines. The two cell lines were positive for both CK5/6 and
CD44v9/CD44 expressions, while being negative for CK20 in IHC (data
not shown). Fig. 3D shows the
inhibitory effect of CD44 and CD44v9 siRNA on protein and mRNA
expression by western blotting and RT-PCR, respectively.
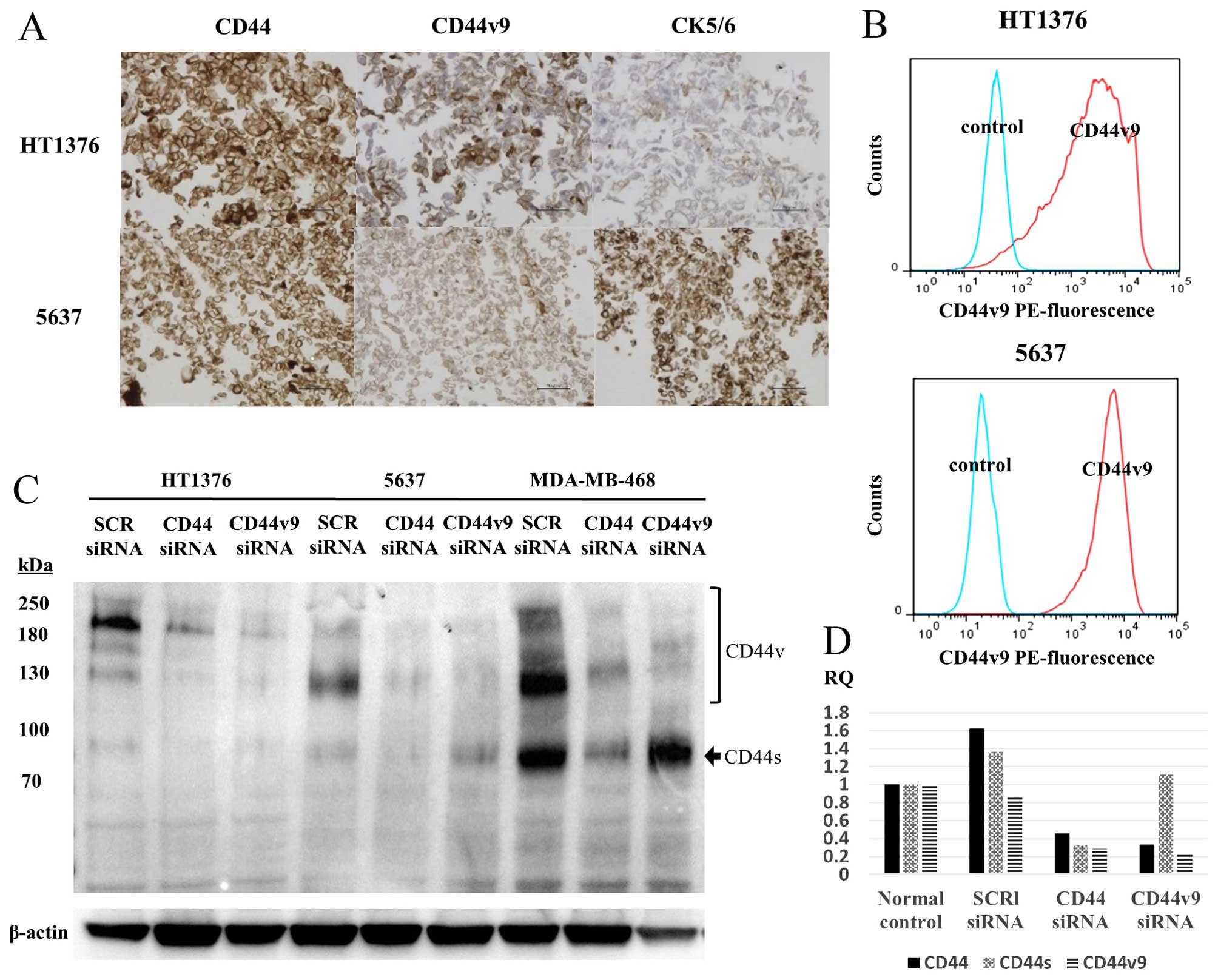 | Figure 3(A) IHC staining of CD44, CD44v9, and
CK5/6 and (B) flow cytometry analysis with an anti-CD44v9 antibody
and PE-labeled anti-rat IgG antibody in the human bladder cancer
cell lines, HT1376 and 5637. (C) Western blotting of CD44v9
expression after CD44 and CD44v9 siRNA transfection in HT1376,
5637, and MDA-MB-468 cells and (D) quantitative comparison of
CD44v9 expression between control and CD44v9 siRNA knockdown by
RT-PCR. (B) Cells expressing CD44v9 (AUC of the red line) are
predominant in both HT1376 and 5637 cell lines in flow cytometry
analysis. The MDA-MB-468 cell line, a CD44v9-positive breast cancer
cell line, was used as a CD44v9-positive control. (D) The
inhibition of CD44v9 was determined using RT-PCR in HT1376. IHC,
immunohistochemical; CD44v9, CD44 variant 9; CK5/6, cytokeratin
5/6; PE, phycoerythrin; AUC, area under the curve. |
Effect of siRNA-induced knockdown of
CD44v9 on cell migration and invasion ability in the 5637 and
HT1376 cell lines
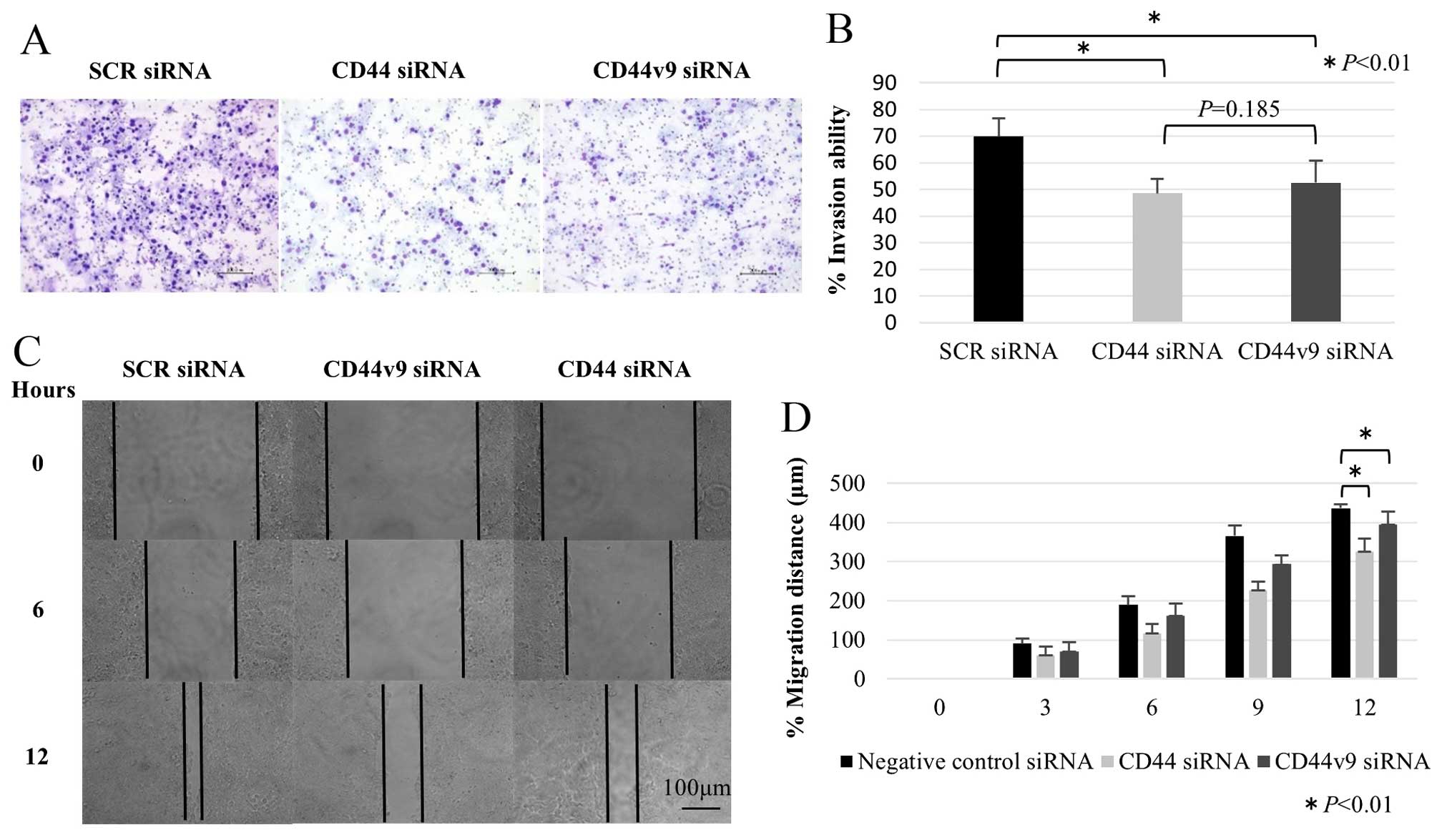
We examined the cell migration and invasion ability
associated with the suppression of CD44v9 expression. Initially, we
measured the invasion ability of HT1376 and 5637 cells after
transient transfection with scrambled or with CD44 and CD44v9 siRNA
using a Matrigel invasion assay. The invasion ability of the cells
transfected with CD44 and CD44v9 siRNA was significantly lower than
that of cells transfected with scrambled siRNA (Fig. 4A and B). Secondly, we measured the
migration ability of CD44 and CD44v9 knockdown cells using a wound
healing assay. CD44/CD44v9 knockdown cells showed significantly
decreased migration ability as compared to scrambled siRNA
transfected cells (Fig. 4C and D).
No significant difference in the proliferation ability or
resistance to cisplatin was observed between the cells transfected
with CD44/CD44v9 siRNA and those transfected with control siRNA
(data not shown).
Correlation of CD44v9 expression with
EMT
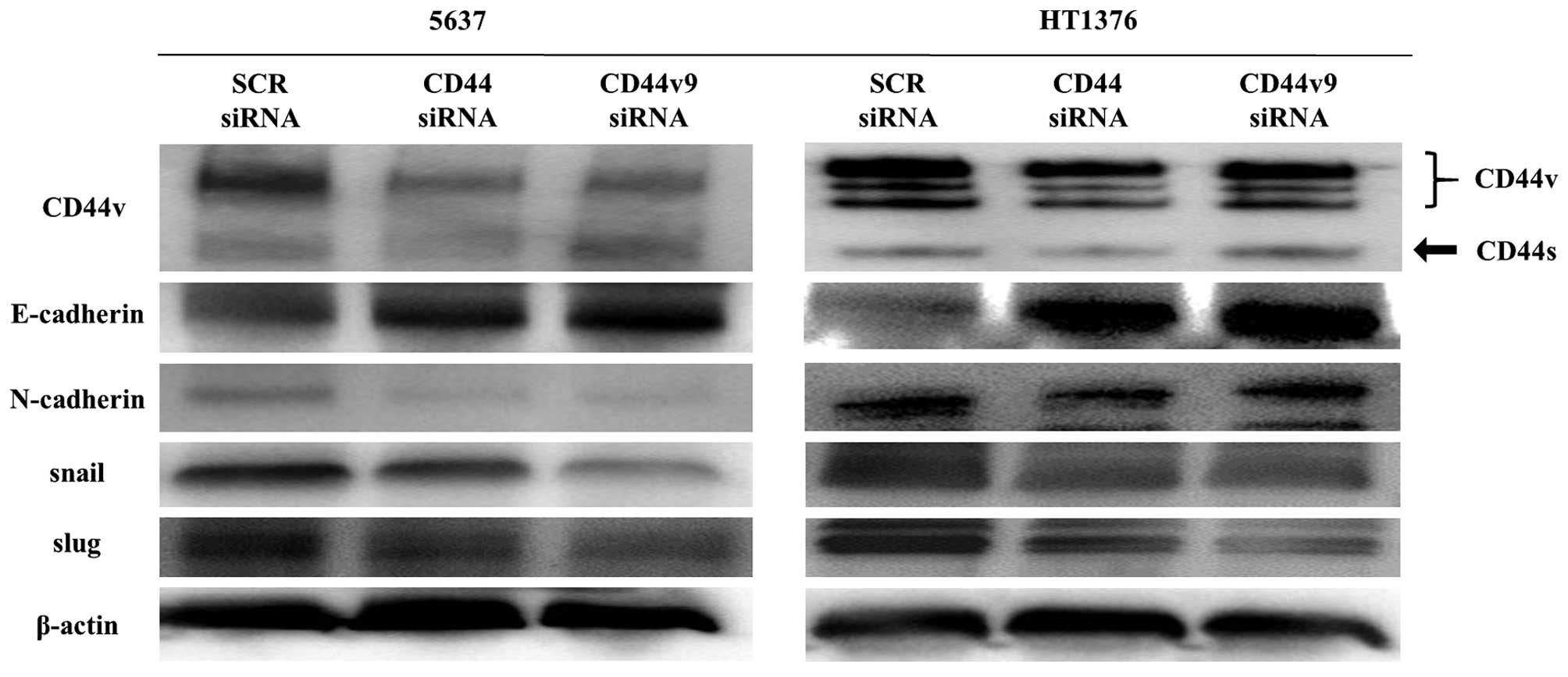
We evaluated the correlation of CD44v9 to the EMT
markers E-cadherin, N-cadherin, snail, and slug by western blotting
(Fig. 5). The cells transfected
with CD44 and CD44v9 siRNA showed of N-cadherin, snail, and slug
compared with those transfected increased expression of E-cadherin
and decreased expression with the scrambled siRNA.
Discussion
As patients with MIBC and high-risk NMIBC show poor
prognosis, the identification of the molecular markers for these
conditions is pivotal to improve the clinical management of these
UC patients. Recently, CD44v9 has been reported as a cancer stem
cell marker as well as a prognostic marker in colorectal and early
gastric cancers (11,12). In advanced head and neck cancer,
high CD44v9 expression was associated with a significantly worse
prognosis than was low expression (17). In this study, we demonstrated that
the expression of CD44v9 is closely associated with tumor
progression and cancer-associated death in MIBC as well as in
high-risk NMIBC via the possible mechanism of increasing the
properties of tumor invasion and migration and enhancing the EMT in
human bladder cancer.
Miyake et al reported that an elevated ratio
of the CD44v8-10 to CD44s ratio demonstrated a shorter disease-free
survival than did a normal ratio in patients with UC (17). In our study, the patients with
higher CD44v9 expression exhibited shorter progression-free and
cancer-specific survival in MIBC and high-risk NMIBC, respectively.
Our results are in good agreement with the previous report that
CD44v8-10 overexpression in human bladder cancer cells was
associated with the augmentation of malignant progression (18).
Recent studies have provided evidence that MIBC can
be classified as two major basal and luminal subtypes that are
similar to the intrinsic subtypes of breast cancer (13,19,20).
The basal subtype is characterized by EMT, and shows enrichment
with squamous features and better chemosensitivity than the luminal
subtype. The observed colocalization of CD44v9 with CK5/6 suggests
that CD44v9 is a potential biomarker for the basal subtype in UC.
Although CK20, a luminal subtype marker, was also expressed in most
high-risk NMIBC samples, the expression site was predominantly
localized to the surface of the tumor with a distinct separation
from the site of CD44v9 expression. Notably, the highest site of
CD44v9 expression was located at the invasion front (submucosal
layer) of the tumors with shorter progression-free survival and
cancer-specific survival within high-risk NMIBC. Our results might
be further supported by the report that tumor cells maintain an
epithelial phenotype with high CD44v expression at the invasive
front (21). The decreased invasion
as well as migration ability pursuant to CD44v9 knockdown might
explain the functional role of CD44v9 that confers an increased
malignant phenotype on the tumor at the invasion front, leading to
the development of tumor progression in high-risk NMIBC. Based on
our results and past reports, we propose that CD44v9 expression
might therefore serve as a clinically useful prognostic marker for
oncological outcome as well as a good marker for defining basal
subtype in high-risk NMIBC.
CD44v8-10, just as CD44v9, and CD44v6 have been
shown to enhance the metastatic potential of colon cancer and
melanoma cells, respectively. CD44v6 interacts with c-Met, a
receptor tyrosine kinase that binds hepatocyte growth factor, and
thereby increases the potential of melanoma cells to migrate to the
brain. CD44v9 expression has been shown to rely on the activity of
the cystine transporter subunit xCT for the control of the cell's
redox status (22). Therefore,
CD44v9-positive tumor cells have an ability to suppress the
production of reactive oxygen species, resulting in therapeutic
resistance, tumor recurrence and metastasis (8,23,24).
CD44v is predominantly expressed in epithelial cancer cells,
whereas CD44s is mainly expressed in mesenchymal cancer cells. CD44
splicing is regulated by ESRP1, redox stress-induced Wnt
activation, and TGF-β signaling. EMT-inducing transcriptional
factors (e.g., snail and slug) enhance the invasive and migratory
phenotype through the cadherin switch and a change in the
alternative splicing of CD44 (25).
The decreased expression of E-cadherin in association with
N-cadherin increase and of decreased expression EMT markers by
CD44v9 knockdown in our results are in good agreement with the
previous report, and suggest the close association of CD44v9
expression with EMT in high-risk NMIBC.
A limitation of this study is that we could not
distinguish the role of CD44v9 from that of CD44. Knockdown of CD44
had a similar effect on invasion and migration abilities as did
that of CD44v9. These results imply the possibility that the other
CD44v isoforms have similar ability. Nevertheless, the strong
association of CD44v9 expression with patient outcome, and its
specific localization to the invasion front demonstrate the
clinical benefit that the expression status of CD44v9, as
determined by IHC, might provide toward the prediction of poor
prognosis or of tumor progression in patients with high-risk
NMIBC.
In conclusion, this study demonstrated that CD44v9
is a possible prognostic marker for progression and cancer-specific
death in MIBC and high-risk NMIBC. In particular, the IHC finding
of the specific localization of CD44v9 to the invasion front using
TUR-BT specimens might aid in selecting the patients who will
likely progress to MIBC, and thereby offering such patients more
aggressive treatment options such as radical cystectomy or
cisplatin-based chemotherapy in patients in high-risk NMIBC. To
confirm these results, more cohorts with larger patient numbers
should be utilized.
Acknowledgments
We would like to thank Professor Hideyuki Saya for
providing us with CD44v9 antibody and Professor Koji Tamada for
help with technical guidance. We would like to thank Editage
(www.editage.jp) for English language editing.
References
|
1
|
Nargund VH, Tanabalan CK and Kabir MN:
Management of non-muscle-invasive (superficial) bladder cancer.
Semin Oncol. 39:559–572. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Al Hussain TO and Akhtar M: Molecular
basis of urinary bladder cancer. Adv Anat Pathol. 20:53–60. 2013.
View Article : Google Scholar
|
|
3
|
May M, Helke C, Nitzke T, Vogler H and
Hoschke B: Survival rates after radical cystectomy according to
tumor stage of bladder carcinoma at first presentation. Urol Int.
72:103–111. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Günthert U, Hofmann M, Rudy W, Reber S,
Zöller M, Haussmann I, Matzku S, Wenzel A, Ponta H and Herrlich P:
A new variant of glycoprotein CD44 confers metastatic potential to
rat carcinoma cells. Cell. 65:13–24. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nagano O and Saya H: Mechanism and
biological significance of CD44 cleavage. Cancer Sci. 95:930–935.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ponta H, Sherman L and Herrlich PA: CD44:
From adhesion molecules to signalling regulators. Nat Rev Mol Cell
Biol. 4:33–45. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kimura Y, Goi T, Nakazawa T, Hirono Y,
Katayama K, Urano T and Yamaguchi A: CD44variant exon 9 plays an
important role in colon cancer initiating cells. Oncotarget.
4:785–791. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ishimoto T, Nagano O, Yae T, Tamada M,
Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, et al:
CD44 variant regulates redox status in cancer cells by stabilizing
the xCT subunit of system xc(-) and thereby promotes tumor growth.
Cancer Cell. 19:387–400. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hong RL, Pu YS, Hsieh TS, Chu JS and Lee
WJ: Expressions of E-cadherin and exon v6-containing isoforms of
CD44 and their prognostic values in human transitional cell
carcinoma. J Urol. 153:2025–2028. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lipponen P, Aaltoma S, Kosma VM, Ala-Opas
M and Eskelinen M: Expression of CD44 standard and variant-v6
proteins in transitional cell bladder tumours and their relation to
prognosis during a long-term follow-up. J Pathol. 186:157–164.
1998. View Article : Google Scholar
|
|
11
|
Hirata K, Suzuki H, Imaeda H, Matsuzaki J,
Tsugawa H, Nagano O, Asakura K, Saya H and Hibi T: CD44 variant 9
expression in primary early gastric cancer as a predictive marker
for recurrence. Br J Cancer. 109:379–386. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamaguchi A, Urano T, Goi T, Saito M,
Takeuchi K, Hirose K, Nakagawara G, Shiku H and Furukawa K:
Expression of a CD44 variant containing exons 8 to 10 is a useful
independent factor for the prediction of prognosis in colorectal
cancer patients. J Clin Oncol. 14:1122–1127. 1996.PubMed/NCBI
|
|
13
|
Choi W, Porten S, Kim S, Willis D, Plimack
ER, Hoffman-Censits J, Roth B, Cheng T, Tran M, Lee IL, et al:
Identification of distinct basal and luminal subtypes of
muscle-invasive bladder cancer with different sensitivities to
frontline chemotherapy. Cancer Cell. 25:152–165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ishibashi H, Suzuki T, Suzuki S, Moriya T,
Kaneko C, Takizawa T, Sunamori M, Handa M, Kondo T and Sasano H:
Sex steroid hormone receptors in human thymoma. J Clin Endocrinol
Metab. 88:2309–2317. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pirker R, Pereira JR, von Pawel J,
Krzakowski M, Ramlau R, Park K, de Marinis F, Eberhardt WE,
Paz-Ares L, Störkel S, et al: EGFR expression as a predictor of
survival for first-line chemotherapy plus cetuximab in patients
with advanced non-small-cell lung cancer: Analysis of data from the
phase 3 FLEX study. Lancet Oncol. 13:33–42. 2012. View Article : Google Scholar
|
|
16
|
Subik K, Lee JF, Baxter L, Strzepek T,
Costello D, Crowley P, Xing L, Hung MC, Bonfiglio T, Hicks DG, et
al: The expression patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and
AR by immunohistochemical analysis in breast cancer cell lines.
Breast Cancer (Auckl). 4:35–41. 2010.
|
|
17
|
Miyake H, Eto H, Arakawa S, Kamidono S and
Hara I: Over expression of CD44V8-10 in urinary exfoliated cells as
an independent prognostic predictor in patients with urothelial
cancer. J Urol. 167:1282–1287. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Muramaki M, Miyake H, Kamidono S and Hara
I: Over expression of CD44V8-10 in human bladder cancer cells
decreases their interaction with hyaluronic acid and potentiates
their malignant progression. J Urol. 171:426–430. 2004. View Article : Google Scholar
|
|
19
|
Damrauer JS, Hoadley KA, Chism DD, Fan C,
Tiganelli CJ, Wobker SE, Yeh JJ, Milowsky MI, Iyer G, Parker JS, et
al: Intrinsic subtypes of high-grade bladder cancer reflect the
hallmarks of breast cancer biology. Proc Natl Acad Sci USA.
111:3110–3115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McConkey DJ, Choi W and Dinney CP: New
insights into subtypes of invasive bladder cancer: Considerations
of the clinician. Eur Urol. 66:609–610. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoshida GJ and Saya H: Inversed
relationship between CD44 variant and c-Myc due to oxidative
stress-induced canonical Wnt activation. Biochem Biophys Res
Commun. 443:622–627. 2014. View Article : Google Scholar
|
|
22
|
Yoshikawa M, Tsuchihashi K, Ishimoto T,
Yae T, Motohara T, Sugihara E, Onishi N, Masuko T, Yoshizawa K,
Kawashiri S, et al: xCT inhibition depletes CD44v-expressing tumor
cells that are resistant to EGFR-targeted therapy in head and neck
squamous cell carcinoma. Cancer Res. 73:1855–1866. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsugawa H, Suzuki H, Saya H, Hatakeyama M,
Hirayama T, Hirata K, Nagano O, Matsuzaki J and Hibi T: Reactive
oxygen species-induced autophagic degradation of Helicobacter
pylori CagA is specifically suppressed in cancer stem-like cells.
Cell Host Microbe. 12:764–777. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yae T, Tsuchihashi K, Ishimoto T, Motohara
T, Yoshikawa M, Yoshida GJ, Wada T, Masuko T, Mogushi K, Tanaka H,
et al: Alternative splicing of CD44 mRNA by ESRP1 enhances lung
colonization of metastatic cancer cell. Nat Commun. 3:8832012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoshida GJ and Saya H: The novel
anti-tumor therapy targeting the ʻfunctional' cancer stem cell
markers. Clin Exp Pharmacol. 4:1472014.
|