Introduction
Nasal-type natural killer/T-cell lymphoma (nasal
NKTCL), is a rare subtype of non-Hodgkin's lymphoma (NHL) derived
from natural killer (NK) cells and, more rarely, T cells closely
correlate with Epstein-Barr virus (EBV) infection (1). Its frequency among all lymphoma is
~3–10% in East Asia, but <1% in Western countries (2). Even though 2/3 of nasal NKTCL patients
present with localized lesions, the prognosis of nasal NKTCL
remains dismal because of its aggressive course and resistance to
conventional chemotherapy (3,4).
Genetic variants, cellular immune deficiency, and EBV infection
have been implicated in the molecular pathogenesis of nasal NKTCL
(5). The exact etiology is still
ambiguous, and therefore the best first-line therapeutic strategies
have not been established (6).
Signal transducers and activators of transcription 3
(STAT3), a major component of the STAT family, has been established
to play important roles in the development and progression of many
human malignancies, such as glioma, pancreatic cancer, prostate
cancer, anaplastic large cell lymphoma, and T-cell large granular
lymphocyte leukemia (7–10). Phosphorylation of STAT3 triggers its
dimerization and nuclear transport, where it promotes the
transcription of genes that stimulate tumor growth. Thus
phospho-STAT3 (p-STAT3) was used as a measurement of STAT
activation. Briefly, multiple pathways unify and use STAT3 as a
central molecular hub (11–14). Inappropriately activated STAT3,
located at the converging point of various signaling networks,
ultimately activated multiple downstream genes that facilitate
tumor invasion, metastasis, and angiogenesis, but suppressed host
immune surveillance (15–18). In light of this role, inhibitors of
the STAT3 pathway are attractive therapeutic targets for cancer.
WP1066 represents a novel promising STAT3 inhibitor in antitumor
therapy (19,20). However, the involvement of STAT3
activation in the development of nasal NKTCL is still poorly
understood.
In the present study we detected the STAT3 activity
in nasal NKTCL tissues and cell line, and investigated the
inhibitory effect and the underlying mechanisms of WP1066 (small
molecule STAT3 inhibitor) in the nasal NKTCL cells.
Materials and methods
Cell line and culture
The human NKTCL SNK6 cell line was cultured in
RPMI-1640 (Gibco) supplemented with 1% penicillin/streptomycin
mixture, 2 mM L-glutamine, 1,000 U/ml interleukin (IL)-2 (Beijing
SL Pharmaceutical Co., Ltd., Beijing, China), and 10% human AB
serum provided by the Blood Center of Shandong Province (Jinan,
China) in 5% CO2 at 37°C.
Patients and tissues
The paraffin-embedded tissues from 28 cases of nasal
NKTCL (19 males and 9 females; age range 24–72 years, median 47.5
years) were collected from Shandong Provincial Hospital affiliated
to Shandong University prior to therapeutic intervention in this
study. All patients were diagnosed according to the WHO criteria
between January 2009 and June 2015. Reactive hyperplasia of lymph
node (RHLN) served as control. This study was approved by the
Medical Ethics Committee of Shandong Provincial Hospital affiliated
to Shandong University. All human samples were obtained after
informed consents had been given, according to the Declaration of
Helsinki.
Reagents
STAT3 inhibitor WP1066, purchased from Selleck
Chemicals (Boston, MA, USA), was dissolved in DMSO to make the
stock solution. Stock solution was finally diluted into cell
culture medium to make the final working concentrations.
Immunohistochemistry (IHC)
IHC of patients' paraffin-embedded tissue sections
was performed using primary rabbit antibodies for p-STAT3 (Tyr705)
(Cell Signaling Technology, Danvers, MA, USA). Briefly,
formalin-fixed, paraffin-embedded tissue sections of 4 mM thickness
were deparaffinized and hydrated. High-pressure antigen retrieval
was performed in 0.01 M sodium citrate (pH 6.0). Endogenous
peroxidase was blocked with 3% H2O2 in
methanol for 15 min at room temperature, followed by incubation
with normal serum to block non-specific staining. Primary rabbit
antibodies were diluted as p-STAT3 (Tyr705) (1:400) applied to
incubate the sections overnight in a humidified chamber at 4°C;
then the second antibody from SP reagent kit (Zhongshan
Goldenbridge Biotechnology Co., Beijing, China) was used to
incubate the sections for 1 h at room temperature. After washing,
the tissue sections were treated with biotinylated anti-rabbit
secondary antibody, followed by further incubation with
streptavidin-horseradish peroxidase complex. After staining with
3,3′-diaminobenzidine kit (DAB; Zhongshan Goldenbridge
Biotechnology Co.), the sections were counterstained with
hematoxylin and mounted. Immunohistochemical staining was assessed
in a series of randomly selected five high-power fields, which were
believed to be representative of the average in tumors at ×400
magnification, by two independent observers who were blinded to all
clinical data. Tumors displaying staining in ≥30% of the cells were
categorized as positive cases. whereas, tumors displaying staining
<30% of the cells were categorized as negative cases.
Immunofluorescence (IF)
SNK6 cells were cultured for 24 h in the presence or
absence of 5 µM WP1066. After cytospin, cells were fixed in
acetone and methanol (1:1) for 15 min at room temperature; after
three washes in PBS for 10 min each, they were blocked in PBS with
10% donkey serum for 1 h at room temperature. Then the cells were
incubated with primary rabbit anti-p-STAT3 (Tyr705) antibody
(1:100; Cell Signaling Technology) overnight at 4°C in a humidified
chamber. After three washes the following day, cells were incubated
with the secondary antibody Alexa Fluor 568-labeled donkey
anti-rabbit IgG antibody (1:500; Invitrogen, Eugene, OR, USA) for 1
h at room temperature in the dark. After washing three times with
PBS, 4′,6-diamidino-2-phenylindole (DAPI) was used to stain nuclei
followed by analysis using confocal microscopy (LSM 780; Carl
Zeiss).
Assessment of cell proliferation
The influence of WP1066 on viability of SNK6 cells
was assessed by carrying out triplicate assays with the Cell
Counting kit-8 (CCK-8; Dojindo, Kumamoto, Japan). SNK6 cells (5,000
cells/100 µl/well, respectively) were seeded into 96-well
plates at 37°C with 5% CO2 and incubated with WP1066 at
designated concentrations (0, 0.625, 1.25, 2.5, 5 and 10 µM)
for 24 h. Thereafter, the cells were incubated with 10 µl of
CCK-8 for 4 h according to the manufacturer's instructions. The
absorbance at 450 nm was measured using a SpectraMax M2 Multi-Mode
Microplate Reader (Molecular Devices, Sunnyvale, CA, USA). The
micrographs of SNK cells after WP1066 incubation were also
recorded.
Analysis of cell apoptosis
Effects of WP1066 on apoptosis of SNK6 cells were
evaluated by Annexin V-phycoerythrin (PE)/7-aminoactinomycin D
(7-AAD) assay. Flow cytometric analysis of these cells labeled with
Annexin V-PE and 7-AAD was performed according to the
manufacturer's instructions (BD Biosciences). Cells with designated
treatments were harvested, washed twice with cold PBS and
resuspended in 1X binding buffer at a concentration of
1×106 cells/ml. This was followed by transferring 100
µl of the solution to a 5 ml tube, to which 5 µl of
Annexin V-PE and 5 µl of 7-AAD were added. The tube was
gently vortexed and incubated for 15 min at room temperature in the
dark. At the end of incubation, 400 µl of 1X binding buffer
was added. The rates of cellular apoptosis were acquired
immediately on a Navios Flow Cytometer (Beckman Coulter). Viable
cells are not stained with Annexin V-PE or 7-AAD. The necrotic
cells were Annexin V-PE and 7-AAD-positive, whereas apoptotic cells
were Annexin V-PE-positive and 7-AAD-negative.
Western blot analysis
Total protein was extracted from SNK6 cells with the
treatment. Total protein was extracted using lysis buffer (Shenergy
Biocolor, Shanghai, China) and 1% PhosSTOP (Roche, Mannheim,
Germany). The protein concentration was determined by the BCA assay
(Shenergy Biocolor). Cell lysate was then electrophoresed on 10%
SDS-polyacrylamide gels and transferred onto polyvinylidene
difluoride (PVDF) membranes. The membranes were blocked with 5%
skim-milk in Tris-saline buffer with 0.1% Tween-20, and then
incubated with primary antibodies at 4°C overnight. After washing
with TBST, secondary antibody conjugated with the horseradish
peroxidase (Zhongshan Goldenbridge Biotechnology Co.) was added to
the membranes. Proteins were detected using the chemiluminescence
detection kit (Millipore, USA). Primary antibodies against p-STAT3
(Tyr705) (1:2,000), STAT3 (1:1,000), c-Myc (1:1,000), cyclin D1
(1:1,000), and Bcl-2 (1:1,000) were purchased from Cell Signaling
Technology. The expression level of β-actin was used as the loading
control for the western blot analysis. Western blot analysis
results were analyzed using the LAS 4000 Image software and Multi
Gauge version 3.0 software (Fujifilm Life Science, Japan).
Real-time quantitative polymerase chain
reaction (RT-qPCR)
The expression of genes was detected by RT-qPCR
analysis. Total RNA was extracted by TRIzol reagent (Takara,
Dalian, China) from cells following incubation of WP1066 at
different concentrations for 24 h. Then the reverse transcription
reaction was conducted by means of Takara reverse transcription
reagents (Takara). Amplification reactions were performed using a
SYBR Premix Ex Taq II kit (Takara) on a LightCycler 480 real-time
PCR system (Roche), using β-actin as an internal reference. The
2−ΔΔCt method was used for data analysis with
LightCycler 480 Gene Scanning version 1.5 software (Roche). The
primers of related genes are provided in Table II.
 | Table IIPCR primer sequences. |
Table II
PCR primer sequences.
| Gene | Primer
sequence |
|---|
| c-Myc |
5′-GGCTCCTGGCAAAAGGTCA-3′ |
|
5′-AGTTGTGCTGATGTGTGGAGA-3′ |
| Cyclin D1 |
5′-CAAATGGAGCTGCTCCTGGTG-3′ |
|
5′-CTTCGATCTGCTCCTGGCAGG-3′ |
| Bcl-2 |
5′-ATGTGTGTGGAGAGCGTCAA-3′ |
|
5′-ACAGTTCCACAAAGGCATCC-3 |
| β-actin |
5′-TGACGTGGACATCCGCAAAG-3′ |
|
5′-CTGGAAGGTGGACAGCGAGG-3′ |
Statistical analysis
Results are expressed as mean ± standard error of
mean (SEM). One-way analysis of variance (ANOVA) or t-tests were
used to test for differences between groups. Fisher's exact
probability test was used to analyze the correlation between the
immunohistochemical expression of p-STAT3 and the
clinicopathological parameters of the 28 nasal NKTCL patients.
P<0.05 was accepted as evidence of significance.
Results
Protein expression of p-STAT3 in nasal
NKTCL tissues and cell line, and the correlation between p-STAT3
and clinicopathological parameters of nasal NKTCL patients
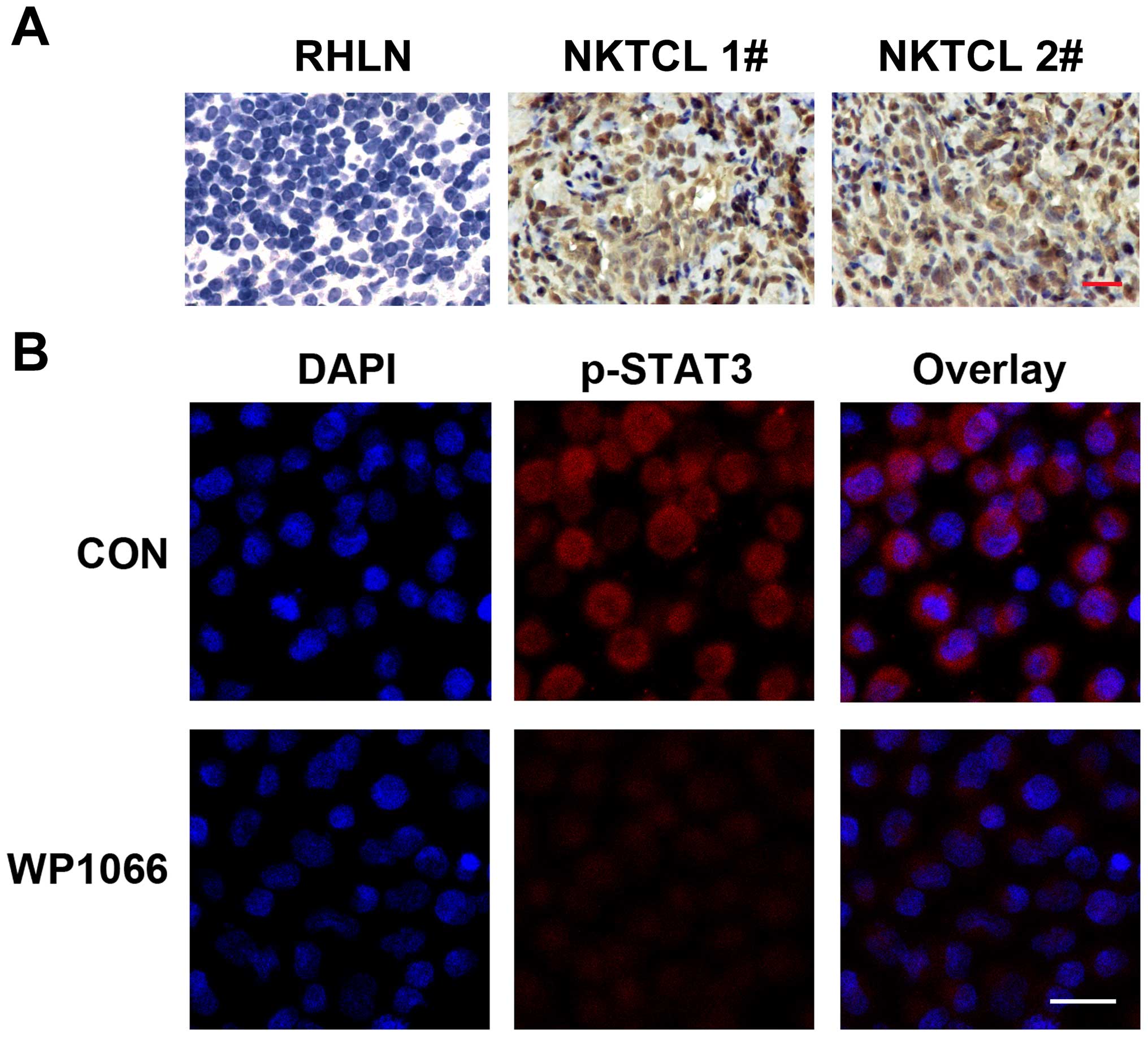
We examined the expression of p-STAT3 in tissues
from 28 cases of nasal NKTCL by IHC. The positive expression of
p-STAT3 was detected in 21/28 (75.0%) cases, when tumor tissues
displaying staining in ≥30% of the cells were categorized as
positive cases. The expression of p-STAT3 was detected in both
tumor and stromal cells, indicating the involvement of p-STAT3 in
the tumor microenvironment (Fig.
1A). However, p-STAT3 staining was too weak to be detected in
the control RHLN tissues. Moreover, Fisher's exact probability test
showed that the immunohistochemical expression of p-STAT3 was
positively correlated with Ki-67 levels (proliferative index) of
these tumor tissues. However, no correlation was identified between
p-STAT3 expression and the other clinical histopathological
parameters (Table I). The
expression of p-STAT3 in NKTCL cell line SNK6 was also demonstrated
by IF using confocal microscopy (Fig.
1B). The blue color labeled the nucleus staining with DAPI, and
red color represented the p-STAT3 expression in SNK6 cells.
 | Table ICorrelation between p-STAT3 protein
expression in nasal NKTCL tissues and the clinicopathological
parameters of the 28 nasal NKTCL patients. |
Table I
Correlation between p-STAT3 protein
expression in nasal NKTCL tissues and the clinicopathological
parameters of the 28 nasal NKTCL patients.
| Total no. | p-STAT3
|
|---|
| Positive | Negative | P-value |
|---|
| Gender | | | | |
| Male | 19 | 15 | 4 | 0.398 |
| Female | 9 | 6 | 3 | |
| Age (years) | | | | |
| ≥45 | 15 | 12 | 3 | 0.412 |
| <45 | 13 | 9 | 4 | |
| EBER | | | | |
| Positive | 20 | 16 | 4 | 0.306 |
| Negative | 8 | 5 | 3 | |
| Ki-67 (%) | | | | |
| ≥60 | 20 | 18 | 2 | 0.009a |
| <60 | 8 | 3 | 5 | |
WP1066 inhibits proliferation and induces
apoptosis of SNK6 cells
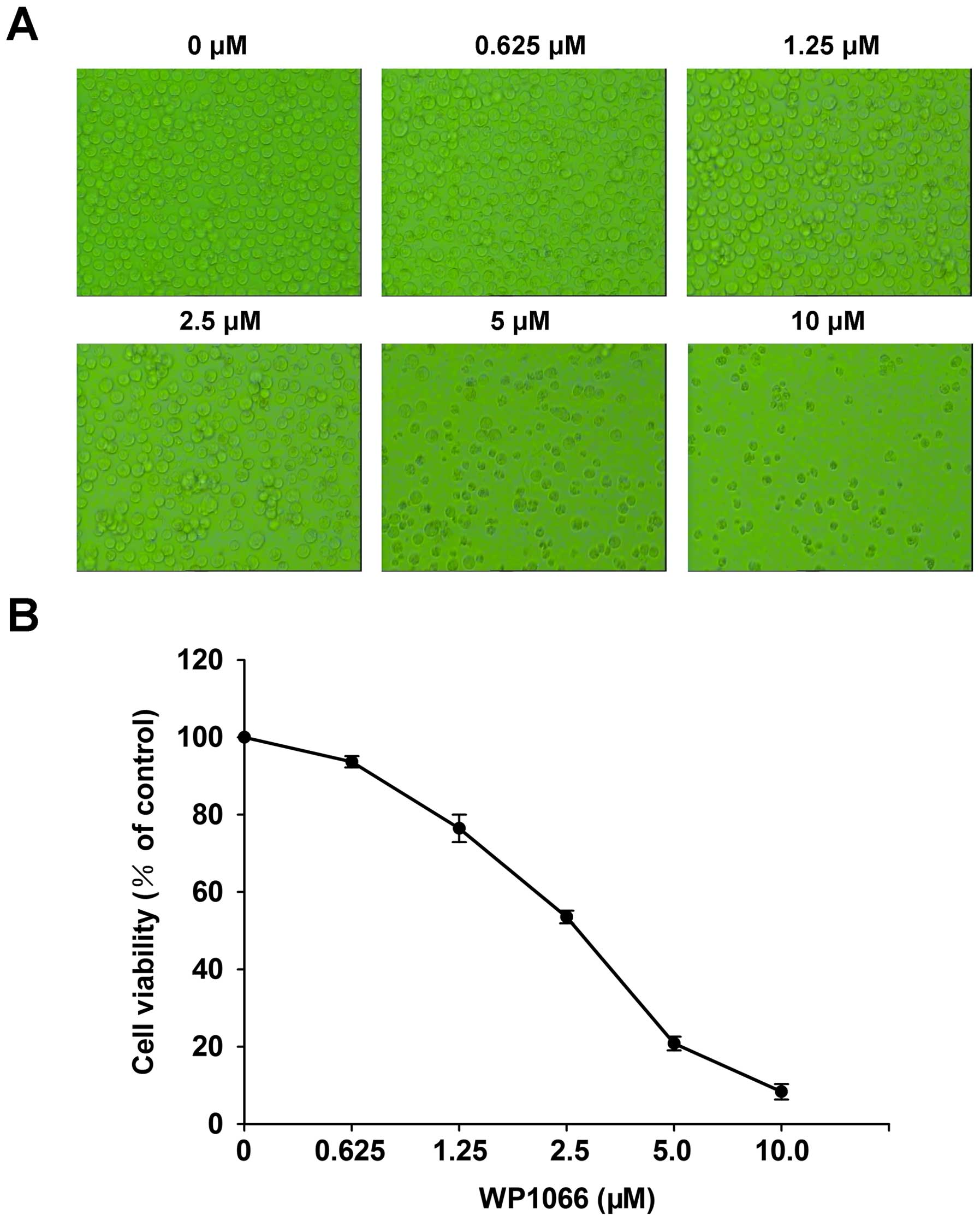
Cytotoxicity of WP1066 on SNK6 cells was evaluated
by morphology and the CCK-8 assay after a 24-h WP1066 treatment at
different concentrations (0, 0.625, 1.25, 2.5, 5 and 10 µM).
Morphology observations found that the number of SNK6 cells with
WP1066 incubation decreased significantly compared to the control
group (Fig. 2A). Simultaneously,
CCK-8 analysis confirmed that the viability of the SNK6 cells
decreased in a dose-dependent manner after a 24-h WP1066 treatment
(Fig. 2B). The half maximal
inhibitory concentration (IC50) value of WP1066 in SNK6
cells at 24 h was calculated as 2.62±0.28 µM. Effect of
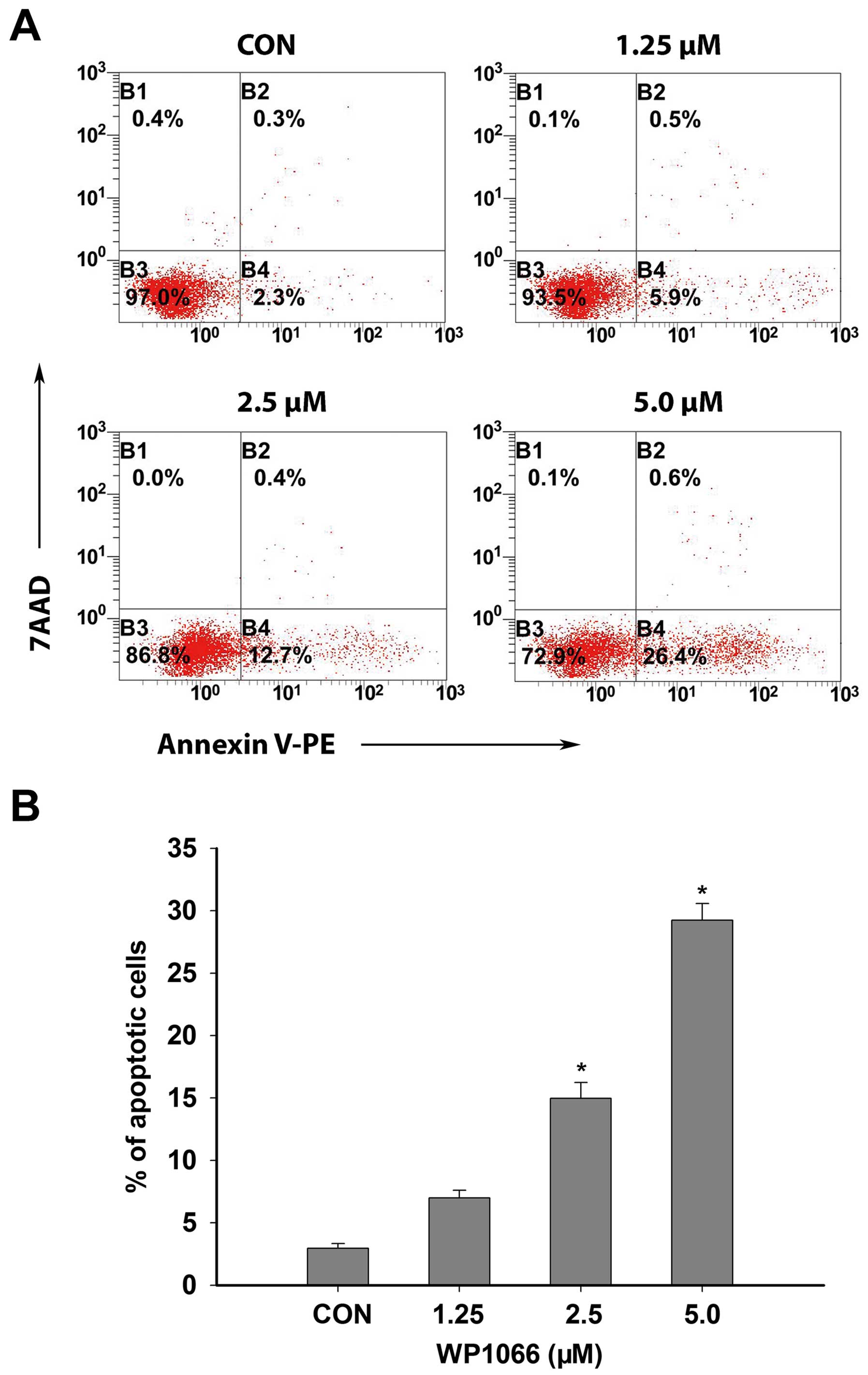
WP1066 on apoptosis of SNK6 cells was assessed after a 24-h WP1066
treatment at different concentrations. Flow cytometric analysis
demonstrated that the percentage of apoptotic cells increased
significantly at 24 h following medium- to high-concentration
WP1066 treatment compared with the solvent treatment groups
(control groups) (P<0.05, n=3) (Fig.
3A and B).
Influence of WP1066 on protein expression
of p-STAT3 and downstream molecules in SNK6 cells
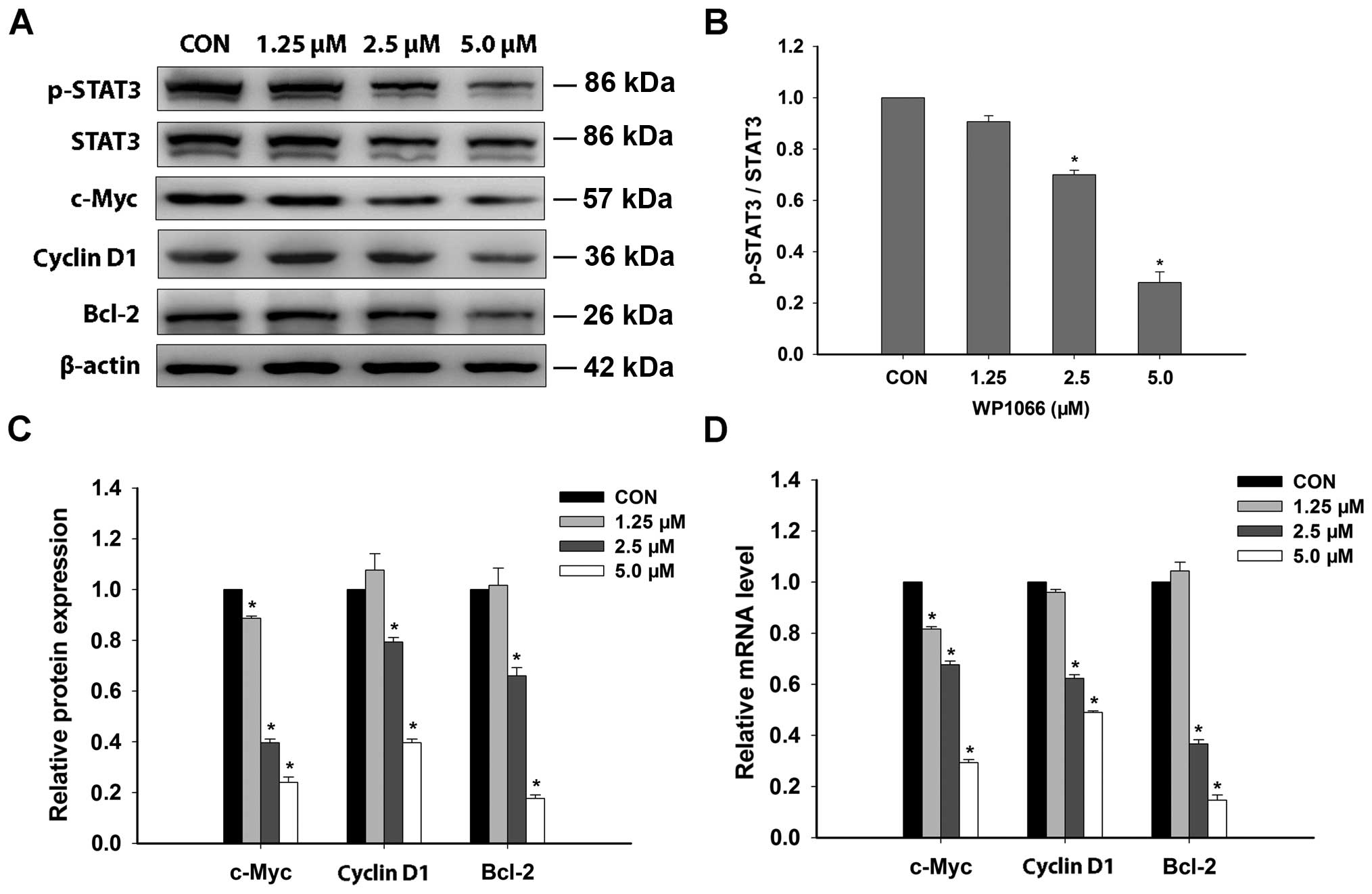
Western blot analysis exhibited the decrease of
protein expression for p-STAT3 in SNK6 cells after a 24-h treatment
of WP1066 in a dose-dependent manner (Fig. 4A and B). Concomitantly, there was a
significant reduction in the protein expression of c-Myc, cyclin
D1, and Bcl-2 after WP1066 treatment from moderate to high
concentration in SNK6 cells (Fig.
4C). The inhibitory effect of WP1066 on phosphorylation of
STAT3 in SNK6 cells was also confirmed by IF analysis. WP1066
treatment brought about a significant downregulation of p-STAT3 in
SNK6 cells (Fig. 1B). The
inhibition of these pro-survival factors downstream of STAT3
pathway may be the potential mechanisms contributing to the
antitumor effect of WP1066 in nasal NKTCL cells.
Effect of WP1066 on mRNA levels of
pro-survival genes related to STAT3 pathway in SNK6 cells
To investigate the regulation of WP1066 on the
oncogenic genes downstream of STAT3 pathway, the mRNA levels of
c-Myc, cyclin D1, Bcl-2 genes were determined by RT-qPCR after
WP1066 treatment with different concentrations for 24 h. The mRNA
levels of these three genes were significantly downregulated after
WP1066 treatment from moderate to high concentration in SNK6 cells
(Fig. 4D).
Discussion
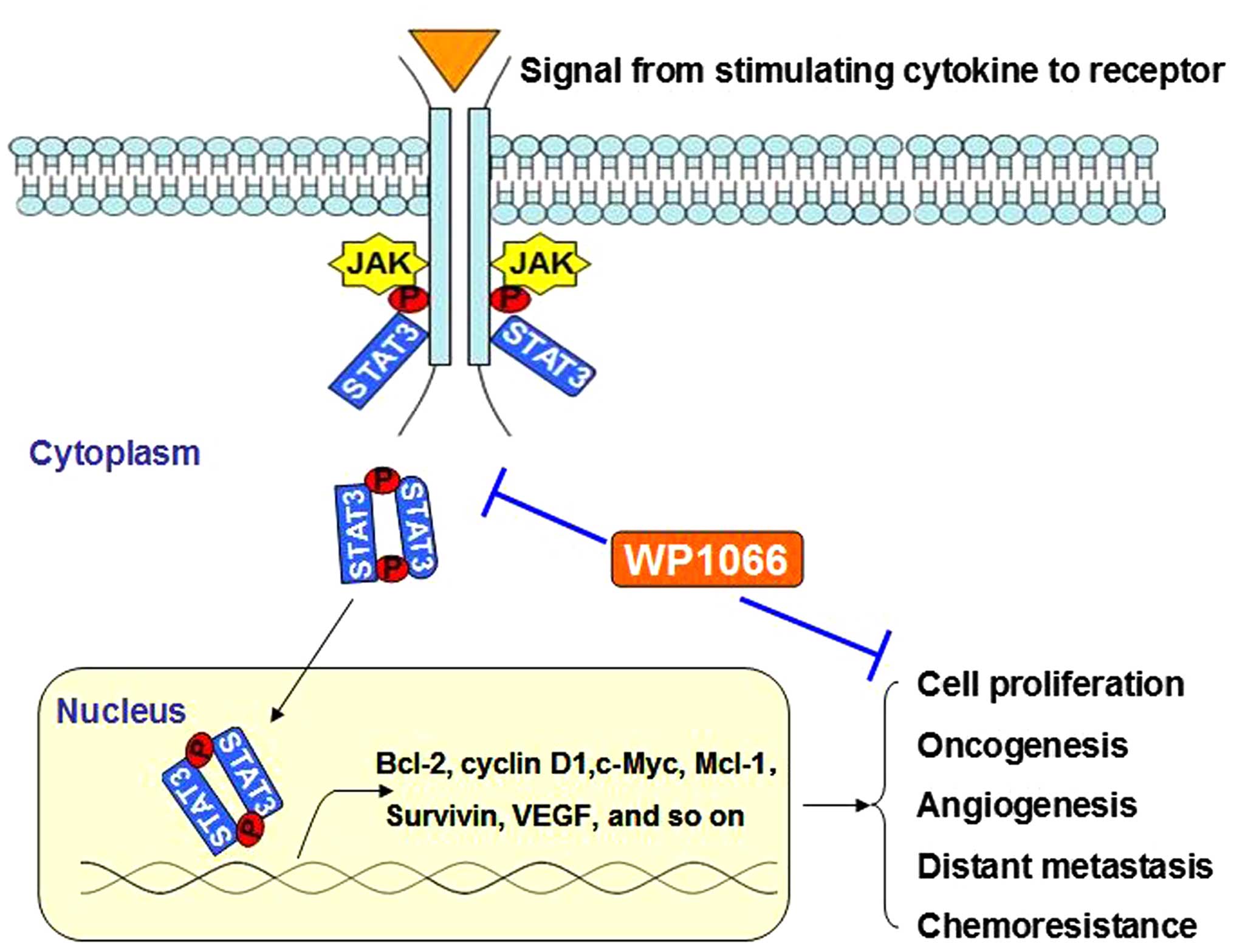
STAT3 protein is known as an important effector of
multiple growth factor receptors and cytokine signals to
participate in tumorigenesis by preventing apoptosis, enhancing
proliferation, angiogenesis, invasiveness, chemoresistance, and
immune evasion. Constitutive activation of the STAT3 pathway has
been noted in a wide range of cancers and typically occurs in
response to stimulation by tumor-promoting factors, including
epidermal growth factor, IL-6, Tyr kinase, and many others
(21,22). Inhibition of aberrantly activated
STAT3 signaling pathway may present a novel antitumor strategy
(Fig. 5). In our present study,
constitutively protein expression of p-STAT3 was detected in both
nasal NKTCL tissues and cell line (SNK6), suggesting the activation
of STAT3 signal in this neoplasm. Moreover, immunohistochemical
expression of p-STAT3 in nasal NKTCL tissues was found to be
positively correlated with the Ki-67 levels in these cases with
Fisher's exact test analysis. This was in accordance with previous
studies of glioma and colorectal cancers in which STAT3 activation
was generally correlated with poor prognosis (23,24).
Based on the published data, STAT3 has gained
notoriety as a hub to relay multiple oncogenic signals modulating
transcription of target genes. STAT3 was shown to promote growth
and protect tumor cells against apoptosis by regulating genes
encoding multiple oncogenic proteins, such as Bcl-2, Mcl-1, VEGF,
c-Myc, and cyclin D1 (25–28). The evidence suggested that STAT3 was
a promising candidate for antitumor target. WP1066 was a novel
small molecule STAT3 inhibitor synthesized by modifying the
structure of AG490 (a tyrphostins to block JAK2 activity) (29,30).
AG490 inhibited STAT3 only at high concentrations (IC50,
50–100 µM) and did not show significant antitumor effect in
animal models (31,32). In contrast, WP1066 has exhibited
significant antitumor activity against human cancer cells in
vitro and in a xenograft mouse model, including gastric cancer,
acute myelogenous leukaemia and melanoma (33–35).
Up to date, the exact working mechanism of WP1066 is
largely unknown. This study evaluated the effect and mechanism of
WP1066 in nasal NKTCL cells, and we demonstrated that WP1066
inhibited STAT3 activation and induced apoptosis in SNK6 nasal
NKTCL cell line. Our findings indicated that the downregulation of
c-Myc, cyclin D1, and Bcl-2 may partly contribute to the potential
mechanisms for the antitumor effect of WP1066 in nasal NKTCL cells.
The proliferation of SNK6 cells was significantly inhibited by
WP1066 with IC50 concentration as 2.62±0.28 µM at
24 h, which was similar to previous data described in renal cancer
and erythroleukemia cell lines. Our results suggested that using
WP1066 to inhibit the STAT3 signaling pathway could be a novel
therapeutic strategy against nasal NKTCL.
To the best of our knowledge, our study is the first
to report the antitumor effect and working mechanism of WP1066
through downregulation of p-STAT3 and downstream pro-survival
molecules in nasal NKTCL. These findings showed that inhibitors of
the STAT3 signaling pathway have enormous potential in the
treatment of nasal NKTCL. Although crosstalk between STAT3 and
other oncogenic signaling pathways deserve more exploration, this
novel study contributes to further investigation on the useful
biomarkers and potential therapeutic targets in nasal NKTCL. WP1066
is still undergoing preclinical and clinical trials which will
provide novel insights into their antitumor activity,
pharmacokinetic, and toxicity (36). Moreover, the small molecule STAT3
inhibitor may be a promising combination for conventional
chemotherapies to overcome or delay tolerance of nasal NKTCL and
improve the prognosis of this disease.
Acknowledgments
This study was partly supported by the National
Natural Science Foundation (nos. 81473486 and 81270598), the
National Public Health Grand Research Foundation (no. 201202017),
the Natural Science Foundation of Shandong Province (nos.
ZR2012HZ003 and 2009ZRB14176), the Technology Development Projects
of Shandong Province (nos. 2014GSF118021, 2010GSF10250 and
2008GG2NS02018), the Program of Shandong Medical Leading Talent,
and the Taishan Scholar Foundation of Shandong Province.
References
|
1
|
Lima M: Aggressive mature natural killer
cell neoplasms: From epidemiology to diagnosis. Orphanet J Rare
Dis. 8:952013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roman E and Smith AG: Epidemiology of
lymphomas. Histopathology. 58:4–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lim ST, Hee SW, Quek R, Lim LC, Yap SP,
Loong EL, Sng I, Tan LH, Ang MK, Ngeow J, et al: Comparative
analysis of extra-nodal NK/T-cell lymphoma and peripheral T-cell
lymphoma: Significant differences in clinical characteristics and
prognosis. Eur J Haematol. 80:55–60. 2008.
|
|
4
|
Lee J, Park YH, Kim WS, Lee SS, Ryoo BY,
Yang SH, Park KW, Kang JH, Park JO, Lee SH, et al: Extranodal nasal
type NK/T-cell lymphoma: Elucidating clinical prognostic factors
for risk-based stratification of therapy. Eur J Cancer.
41:1402–1408. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suzuki R: NK/T-cell lymphomas:
Pathobiology, prognosis and treatment paradigm. Curr Oncol Rep.
14:395–402. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tse E and Kwong YL: Practical management
of natural killer/T-cell lymphoma. Curr Opin Oncol. 24:480–486.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li H, Huang C, Huang K, Wu W, Jiang T, Cao
J, Feng Z and Qiu Z: STAT3 knockdown reduces pancreatic cancer cell
invasiveness and matrix metalloproteinase-7 expression in nude
mice. PLoS One. 6:e259412011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Don-Doncow N, Escobar Z, Johansson M,
Kjellström S, Garcia V, Munoz E, Sterner O, Bjartell A and Hellsten
R: Galiellalactone is a direct inhibitor of the transcription
factor STAT3 in prostate cancer cells. J Biol Chem.
289:15969–15978. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spaccarotella E, Pellegrino E, Ferracin M,
Ferreri C, Cuccuru G, Liu C, Iqbal J, Cantarella D, Taulli R,
Provero P, et al: STAT3-mediated activation of microRNA cluster
17~92 promotes proliferation and survival of ALK-positive
anaplastic large cell lymphoma. Haematologica. 99:116–124. 2014.
View Article : Google Scholar :
|
|
10
|
Koskela HL, Eldfors S, Ellonen P, van
Adrichem AJ, Kuusanmäki H, Andersson EI, Lagström S, Clemente MJ,
Olson T, Jalkanen SE, et al: Somatic STAT3 mutations in large
granular lymphocytic leukemia. N Engl J Med. 366:1905–1913. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sansone P and Bromberg J: Targeting the
interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol.
30:1005–1014. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin HO, Lee YH, Park JA, Kim JH, Hong SE,
Kim HA, Kim EK, Noh WC, Kim BH, Ye SK, et al: Blockage of Stat3
enhances the sensitivity of NSCLC cells to PI3K/mTOR inhibition.
Biochem Biophys Res Commun. 444:502–508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang C, Guo F, Xu G, Ma J and Shao F:
STAT3 cooperates with Twist to mediate epithelial-mesenchymal
transition in human hepatocellular carcinoma cells. Oncol Rep.
33:1872–1882. 2015.PubMed/NCBI
|
|
14
|
Yan CM, Zhao YL, Cai HY, Miao GY and Ma W:
Blockage of PTPRJ promotes cell growth and resistance to 5-FU
through activation of JAK1/STAT3 in the cervical carcinoma cell
line C33A. Oncol Rep. 33:1737–1744. 2015.PubMed/NCBI
|
|
15
|
Munoz J, Dhillon N, Janku F, Watowich SS
and Hong DS: STAT3 inhibitors: Finding a home in lymphoma and
leukemia. Oncologist. 19:536–544. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oh MK, Park HJ, Kim NH, Park SJ, Park IY
and Kim IS: Hypoxia-inducible factor-1alpha enhances haptoglobin
gene expression by improving binding of STAT3 to the promoter. J
Biol Chem. 286:8857–8865. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen L, Liu D, Zhang Y, Zhang H and Cheng
H: The autophagy molecule Beclin 1 maintains persistent activity of
NF-κB and Stat3 in HTLV-1-transformed T lymphocytes. Biochem
Biophys Res Commun. 465:739–745. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu ZQ, Han YC, Fang JM, Hu F, Zhang X and
Xu Q: WITHDRAWN: Hypoxia-induced STAT3 contributes to
chemoresistance and epithelial-mesenchymal transition in prostate
cancer cells. Biochem Biophys Res Commun. S0006-291X(15)00244-2.
2015.
|
|
19
|
Horiguchi A and Asano T, Kuroda K, Sato A,
Asakuma J, Ito K, Hayakawa M, Sumitomo M and Asano T: STAT3
inhibitor WP1066 as a novel therapeutic agent for renal cell
carcinoma. Br J Cancer. 102:1592–1599. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu K, Chen N, Zhou XX, Ge XL, Feng LL, Li
PP, Li XY, Geng LY and Wang X: The STAT3 inhibitor WP1066
synergizes with vorinostat to induce apoptosis of mantle cell
lymphoma cells. Biochem Biophys Res Commun. 464:292–298. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eiring AM, Page BD, Kraft IL, Mason CC,
Vellore NA, Resetca D, Zabriskie MS, Zhang TY, Khorashad JS, Engar
AJ, et al: Combined STAT3 and BCR-ABL1 inhibition induces synthetic
lethality in therapy-resistant chronic myeloid leukemia. Leukemia.
29:586–597. 2015. View Article : Google Scholar :
|
|
22
|
Mills LD, Zhang Y, Marler RJ,
Herreros-Villanueva M, Zhang L, Almada LL, Couch F, Wetmore C,
Pasca di Magliano M and Fernandez-Zapico ME: Loss of the
transcription factor GLI1 identifies a signaling network in the
tumor microenvironment mediating KRAS oncogene-induced
transformation. J Biol Chem. 288:11786–11794. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morikawa T, Baba Y, Yamauchi M, Kuchiba A,
Nosho K, Shima K, Tanaka N, Huttenhower C, Frank DA, Fuchs CS, et
al: STAT3 expression, molecular features, inflammation patterns,
and prognosis in a database of 724 colorectal cancers. Clin Cancer
Res. 17:1452–1462. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abou-Ghazal M, Yang DS, Qiao W,
Reina-Ortiz C, Wei J, Kong LY, Fuller GN, Hiraoka N, Priebe W,
Sawaya R, et al: The incidence, correlation with tumor-infiltrating
inflammation, and prognosis of phosphorylated STAT3 expression in
human gliomas. Clin Cancer Res. 14:8228–8235. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
de Groot J, Liang J, Kong LY, Wei J, Piao
Y, Fuller G, Qiao W and Heimberger AB: Modulating antiangiogenic
resistance by inhibiting the signal transducer and activator of
transcription 3 pathway in glioblastoma. Oncotarget. 3:1036–1048.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kanterman J, Sade-Feldman M and Baniyash
M: New insights into chronic inflammation-induced
immunosuppression. Semin Cancer Biol. 22:307–318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang T, Yuan J, Zhang J, Tian R, Ji W,
Zhou Y, Yang Y, Song W, Zhang F and Niu R: Anxa2 binds to STAT3 and
promotes epithelial to mesenchymal transition in breast cancer
cells. Oncotarget. 6:30975–30992. 2015.PubMed/NCBI
|
|
28
|
Yang C, He L, He P, Liu Y, Wang W, He Y,
Du Y and Gao F: Increased drug resistance in breast cancer by
tumor-associated macrophages through IL-10/STAT3/bcl-2 signaling
pathway. Med Oncol. 32:3522015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Iwamaru A, Szymanski S, Iwado E, Aoki H,
Yokoyama T, Fokt I, Hess K, Conrad C, Madden T, Sawaya R, et al: A
novel inhibitor of the STAT3 pathway induces apoptosis in malignant
glioma cells both in vitro and in vivo. Oncogene. 26:2435–2444.
2007. View Article : Google Scholar
|
|
30
|
Hussain SF, Kong LY, Jordan J, Conrad C,
Madden T, Fokt I, Priebe W and Heimberger AB: A novel small
molecule inhibitor of signal transducers and activators of
transcription 3 reverses immune tolerance in malignant glioma
patients. Cancer Res. 67:9630–9636. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Meydan N, Grunberger T, Dadi H, Shahar M,
Arpaia E, Lapidot Z, Leeder JS, Freedman M, Cohen A, Gazit A, et
al: Inhibition of acute lymphoblastic leukaemia by a Jak-2
inhibitor. Nature. 379:645–648. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Horiguchi A, Oya M, Marumo K and Murai M:
STAT3, but not ERKs, mediates the IL-6-induced proliferation of
renal cancer cells, ACHN and 769P. Kidney Int. 61:926–938. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Judd LM, Menheniott TR, Ling H, Jackson
CB, Howlett M, Kalantzis A, Priebe W and Giraud AS: Inhibition of
the JAK2/STAT3 pathway reduces gastric cancer growth in vitro and
in vivo. PLoS One. 9:e959932014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ferrajoli A, Faderl S, Van Q, Koch P,
Harris D, Liu Z, Hazan-Halevy I, Wang Y, Kantarjian HM, Priebe W,
et al: WP1066 disrupts Janus kinase-2 and induces caspase-dependent
apoptosis in acute myelogenous leukemia cells. Cancer Res.
67:11291–11299. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hatiboglu MA, Kong LY, Wei J, Wang Y,
McEnery KA, Fuller GN, Qiao W, Davies MA, Priebe W and Heimberger
AB: The tumor microenvironment expression of p-STAT3 influences the
efficacy of cyclophosphamide with WP1066 in murine melanoma models.
Int J Cancer. 131:8–17. 2012. View Article : Google Scholar :
|
|
36
|
Assi HH, Paran C, VanderVeen N, Savakus J,
Doherty R, Petruzzella E, Hoeschele JD, Appelman H, Raptis L,
Mikkelsen T, et al: Preclinical characterization of signal
transducer and activator of transcription 3 small molecule
inhibitors for primary and metastatic brain cancer therapy. J
Pharmacol Exp Ther. 349:458–469. 2014. View Article : Google Scholar : PubMed/NCBI
|