Introduction
As a highly aggressive malignant disease, pancreatic
ductal adenocarcinomax (PDAC) is the fourth leading cause of
cancer-related death and has a median survival of 6 months
(1). Despite extensive clinical
efforts, the mortality of patients with PDAC has not significantly
altered, and the 5-year survival rate remains unacceptably low.
During the diagnosis of pancreatic cancer, early metastasis is
often found, eliminating the option of curative surgery (2–4).
However, in clinical practice, few markers other than
tumor-node-metastasis (TNM) stage can be used as independent
prognostic factors of tumour progression. Moreover, the molecules
involved in the progression of metastasis may be markers for the
early detection of recurrence or metastasis as well as prognostic
indicators for surgical intervention. Therefore, it is necessary to
further explore new indicators for the prediction and evaluation of
tumour progression and patient prognosis.
As an evolutionarily conserved signalling pathway,
the Notch signalling pathway has been shown involved in cell-fate
determination, tissue patterning and morphogenesis, and cell
differentiation, proliferation and death (5,6). Since
the Notch signalling pathway participates in progression processes
such as proliferation and apoptosis, it may be associated with
tumourigenesis (7). Previous
studies indicated that many signalling pathways, including the
Notch signalling pathway, may play an important role in PDAC
(8). The Notch signalling pathway
plays a critical role in the control of cell proliferation,
differentiation, apoptosis, invasion and metastasis in PDAC
(9). Studies frequently show that
the expression of Notch receptors and their ligands increase in
PDAC (10). Inhibition of Notch1 is
the main cause of decreased proliferation, migration, and invasion
and increased apoptosis in PDAC cells (11,12).
In an in vivo experiment, downregulation of the Notch
signalling pathway led to inhibition of the canceration and
development of PDAC cells (13).
Contradictorily, the Notch signalling pathway may play an oncogenic
or onco-suppressive role in tumourigenesis, and its function is
also context-dependent in PDAC (14). Surprisingly, Notch1 may have an
onco-suppressive role in K-ras-induced PDAC (15). All of these findings indicate that
further research is needed to explore the role of the Notch
signalling pathway in PDAC since the relationship between the
expression of Notch3 and survival in PDAC patients is unclear.
In the present study, we used immunohistochemistry
to investigate the protein expression of Notch3. This is the first
study to explore the potential relationship between the protein
expression of Notch3 and the prognosis of patients with PDAC.
Furthermore, we also explored the role of Notch3 in the migration
and invasion of PDAC.
Materials and methods
Patients and tissue specimens
We collected PDAC and adjacent non-cancerous tissues
(at least 1.5 cm from the tumour) from 101 patients who underwent
surgery for primary PDAC at the Department of Hepatobiliary Surgery
at Xijing Hospital (Xi'an, China) between 2002 and 2010. These
patients had not received preoperative treatments such as
chemotherapy, ethanol injection or transarterial chemoembolization.
A total of 59 male and 42 female patients participated in the
present study. The median age of the patients was 55.3 years
(range, 39–82 years). Our research was approved by the Ethics
Committee of the Fourth Military Medical University and conformed
to the ethical guidelines of the 2004 Declaration of Helsinki.
Written informed consent was obtained from each patient or from
his/her legal guardian. To ensure the validity of the experiment,
histopathologic examinations were performed to confirm that the
tumour samples contained an adequate number of cancer cells and
that no cancer cells had contaminated the non-cancerous tissues.
All specimens were fixed in 10% formalin and embedded in paraffin,
and 4-μm serial sections were examined using
immunohistochemistry. We assessed clinical parameters such as
gender, age, tumour grade, metastasis and American Joint Committee
on Cancer (AJCC) TNM stage. Of the 38 cases diagnosed with
metastasis, 26 had venous invasion, 11 had bile duct tumour
thrombi, and 17 had lymph node metastasis. A 1-year follow-up study
was conducted to perform survival calculations for these
patients.
Cell culture and reagents
The human pancreatic non-tumour cell line (HPDE6c7)
and human PDAC cell lines (ASPC-1, BXPC-3, CFPAC-1 and PANC-1)
cultivated in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% foetal calf serum (Sigma Chemical Co., St.
Louis, MO, USA). The PDAC cells were seeded into 6-well cell
culture plates at a density of 1×105 cells/well. Primary
antibodies against Notch3, CD44v6, E-cadherin, matrix
metalloproteinase-2 (MMP-2), MMP-9, vascular endothelial growth
factor (VEGF), urokinase-type plasminogen activator (uPA),
cyclooxygenase-2 (COX-2), extracellular signal-regulated kinase 1
and 2 (ERK1/2), p-ERK1/2 and GAPDH were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). All secondary antibodies were
obtained from Pierce (Rockford, IL, USA). Notch3 small interfering
RNA (siRNA) and siRNA controls were obtained from Santa Cruz
Biotechnology. Lipofectamine 2000 was purchased from Invitrogen
(Carlsbad, CA, USA). All other chemicals and solutions were
purchased from Sigma-Aldrich unless otherwise indicated.
Immunohistochemistry and evaluation of
staining
The avidin-biotin-peroxidase method was used to
perform immunohistochemistry of all tissues. Xylene was used to
deparaffinise the sections, and a graded alcohol series was used to
dehydrate prior to blocking in endogenous peroxidase activity using
0.5% H2O2 in methanol for 10 min. The
sections were incubated with 10% normal goat serum in
phosphate-buffered saline (PBS) for 1 h at room temperature to
block non-specific binding. Without washing, the sections were
incubated in PBS with an anti-Notch3 antibody (1:50) at 4°C
overnight in a humidified chamber. Then, the sections were
incubated with biotinylated IgG (1:200; Sigma) for 2 h at room
temperature. A streptavidin-peroxidase complex was used for
detection. The brown colour indicative of peroxidase activity was
obtained by incubating with 0.1% 3,3-diaminobenzidine (Sigma) in
PBS with 0.03% H2O2 for 10 min at room
temperature. Using a previously described immunoreactivity scoring
system, two pathologists who were blinded to the
clinicopathological results, and patient outcome independently
scored the tissue specimens (16).
Based on the score, all PDAC specimens were divided into two
subgroups: the low-expression group (score of 0–4) and the
high-expression group (score of 5–12).
Small interfering RNA transfection
Either Notch3 siRNA or control siRNA were
transfected into BXPC-3 and PANC-1 cells using Lipofectamine 2000
according to the manufacturer's protocol. The siRNA-treated cells
were seeded into 6-well cell culture plates at a density of
1×105 cells/well. The cells grew for an additional 24 h,
and were then harvested for further analysis.
Real-time reverse transcription-PCR
Total RNA was extracted and reverse-transcribed. The
primers used for the PCR reaction were as follows: Notch3 forward
primer (5′-aaggacgtggcctctggt-3′), and reverse primer
(5′-tcaggctctcacccttgg-3′); and GAPDH forward primer
(5′-AAATCCCATCACCATCTTCC-3′), and reverse primer
(5′-TCACACCCATGACGAACA-3′). The primer sequences were verified by
running a virtual PCR, and the primer concentrations were optimized
to prevent primer-dimer formation. Additionally, dissociation
curves were evaluated to prevent non-specific amplification.
Real-time PCR amplifications were performed using an Mx4000
Multiplex QPCR System (Stratagene, La Jolla, CA, USA) with 2X
SYBR-Green PCR Master Mix (Applied Biosystems). Data were analysed
according to the comparative Ct method and were normalized to GAPDH
expression in each sample.
Protein extraction and western
blotting
The cells were lysed in lysis buffer [50 mmol/l Tris
(pH 7.5), 100 mmol/l NaCl, 1 mmol/l EDTA, 0.5% NP40, 0.5% Triton
X-100, 2.5 mmol/l sodium orthovanadate, 10 μl/ml protease
inhibitor cocktail and 1 mmol/l PMSF] by incubating for 20 min at
4°C. The protein concentration was determined using the Bio-Rad
assay system (Bio-Rad, Hercules, CA, USA). Total proteins were
fractionated using SDS-PAGE and transferred onto nitrocellulose
membranes. The membranes were blocked with 5% non-fat dried milk or
bovine serum albumin in 1X TBS buffer containing 0.1% Tween-20, and
then incubated with the appropriate primary antibodies. Horseradish
peroxidase-conjugated anti-rabbit or anti-mouse IgG was used as the
secondary antibody, and the protein bands were detected using an
enhanced chemiluminescence detection system (Amersham Pharmacia
Biotech). Quantification of the western blot analyses was performed
using laser densitometry, and the relative protein expression was
then normalized to GAPDH levels.
MTT assay
Treated cells were seeded into 96-well cell culture
plates at a density of 1×104 cells/well and incubated
for up to 48 h. Using
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT) assay (Sigma Chemicals Co.), cell viability was assessed
according to the manufacturer's protocols. Each experiment had six
replications and was repeated three times. The data are summarized
as the means ± SDs.
Migration and invasion assays
Cell migration and invasion were assessed using
non-Matrigel-coated or Matrigel-coated Transwell cell culture
chambers (8-μm pore size) (Millipore, Billerica, MA, USA).
Briefly, treated cells (5×104 cells/well) were
serum-starved for 24 h and plated in the upper insert of a 24-well
chamber in serum-free medium. Medium containing 10% serum as a
chemoattractant was added to the well, and the cells were incubated
for 24 h. Cells on the upper side of the filters were mechanically
removed with a cotton swab. The membrane was fixed with 4%
formaldehyde for 10 min at room temperature and stained with 0.5%
crystal violet for 10 min. Finally, the number of invasive and
migrated cells was counted at a magnification of ×200 in 10
different fields of each filter.
Statistical analysis
Statistical analysis was performed using SPSS 15.0
software (SPSS, Inc., Chicago, IL, USA). Each experiment was
repeated at least three times, and all results were summarized and
presented as the means ± SDs. A t-test was used to statistically
analyse the differences between means. The χ2 test for
proportions was used to analyse the relationship between Notch3
expression and various clinicopathological factors. Survival curves
were calculated using the Kaplan-Meier method and compared using
the log-rank test. Cox proportional hazard analysis was used for
univariate and multivariate analysis to explore the effect of
clinicopathological factors and Notch3 expression. P-values
<0.05 were considered to indicate a statistically significant
result.
Results
Immunohistochemistry of Notch3
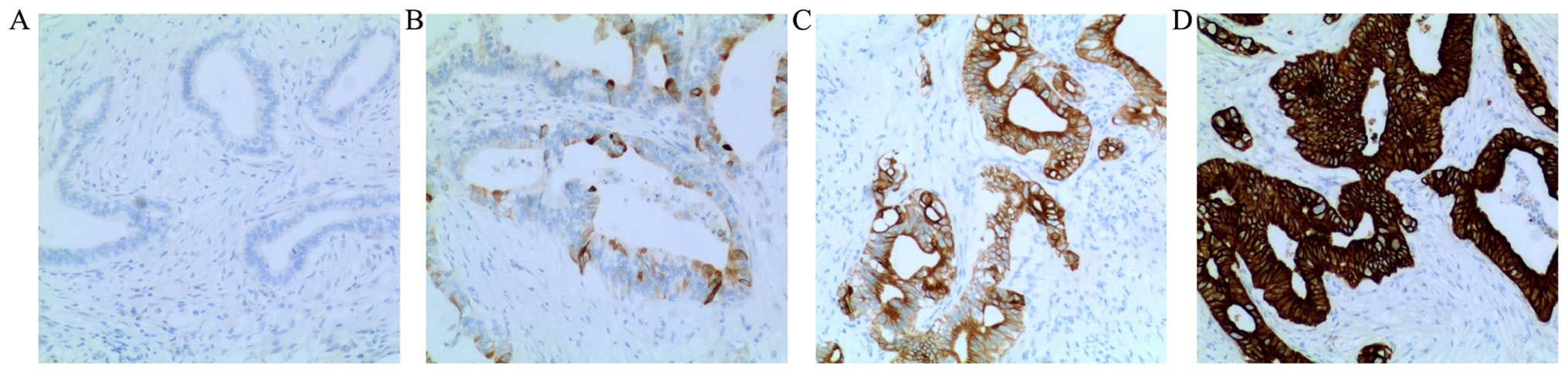
Positive staining for Notch3 was observed mainly in
the cytoplasm and at the cell membrane. In adjacent non-cancerous
tissues, the expression of Notch3 was weak in the cytoplasm and at
the cell membrane. As shown in Fig.
1, the expression of Notch3 was different in PDAC tissue.
Negative staining for Notch3 was observed in 15 cases, weak
positive staining was observed in 28 cases, moderate positive
staining was observed in 23 cases, and strong positive staining was
observed in 35 cases.
Relationships between Notch3 expression
and clinicopathological characteristics
As pathological factors, gender, age, tumour grade,
metastasis and AJCC TNM stage were examined in 101 cases of PDAC.
Vascular invasion was also analysed in patients with metastasis.
For the present study, the 101 patients were divided into two
subgroups: a high-expression group (n=55) and a low-expression
group (n=46). The relationships between the expression of Notch3
and the clinicopathological factors are shown in Table I. The results indicate that a high
expression of Notch3 was strongly correlated with tumour grade
(P=0.003), metastasis (P=0.003), venous invasion (P=0.008) and AJCC
TNM stage (P<0.001). In contrast, a high expression of Notch3
was not correlated with the other pathological factors (P>0.05).
These results suggested that Notch3 may participate in the process
of differentiation, invasion and metastasis in PDAC.
 | Table IAssociation of Notch3 expression with
clinicopathological factors of the PDAC patients. |
Table I
Association of Notch3 expression with
clinicopathological factors of the PDAC patients.
| Tumour
characteristic | n | Notch3
| P-value | χ2 |
|---|
| High (5–12 score,
%) | Low (0–4 score,
%) |
|---|
| All cases | 101 | 55 (54.5) | 46 (45.5) | | |
| Gender |
| Male | 59 | 34 (57.6) | 25 (42.4) | 0.448 | 0.575 |
| Female | 42 | 21 (50.0) | 21 (50.0) | | |
| Age (years) |
| ≤50 | 50 | 30 (60.0) | 20 (40.0) | 0.268 | 1.227 |
| >50 | 51 | 25 (49.0) | 26 (51.0) | | |
| Tumour grade
(differentiation) |
| Well | 33 | 25 (75.8) | 8 (24.2) | 0.003 | 8.968 |
| Moderately or
poorly | 68 | 30 (44.1) | 38 (55.9) | | |
| Metastasis |
| Yes | 38 | 28 (73.7) | 10 (26.3) | 0.003 | 9.082 |
| No | 63 | 27 (42.9) | 36 (57.1) | | |
| Venous
invasion |
| + | 26 | 20 (76.9) | 6 (23.1) | 0.008 | 7.126 |
| − | 75 | 35 (46.7) | 40 (53.3) | | |
| AJCC TNM stage |
| I and II | 19 | 1 (5.3) | 18 (74.7) | <0.001 | 22.834 |
| III and IV | 82 | 54 (65.9) | 28 (34.1) | | |
Correlation between the expression of
Notch3 and the prognosis of PDAC patients
Since the expression of Notch3 was correlated with
tumour grade, metastasis, venous invasion and AJCC TNM stage in
PDAC, we hypothesized that the expression of Notch3 may have a
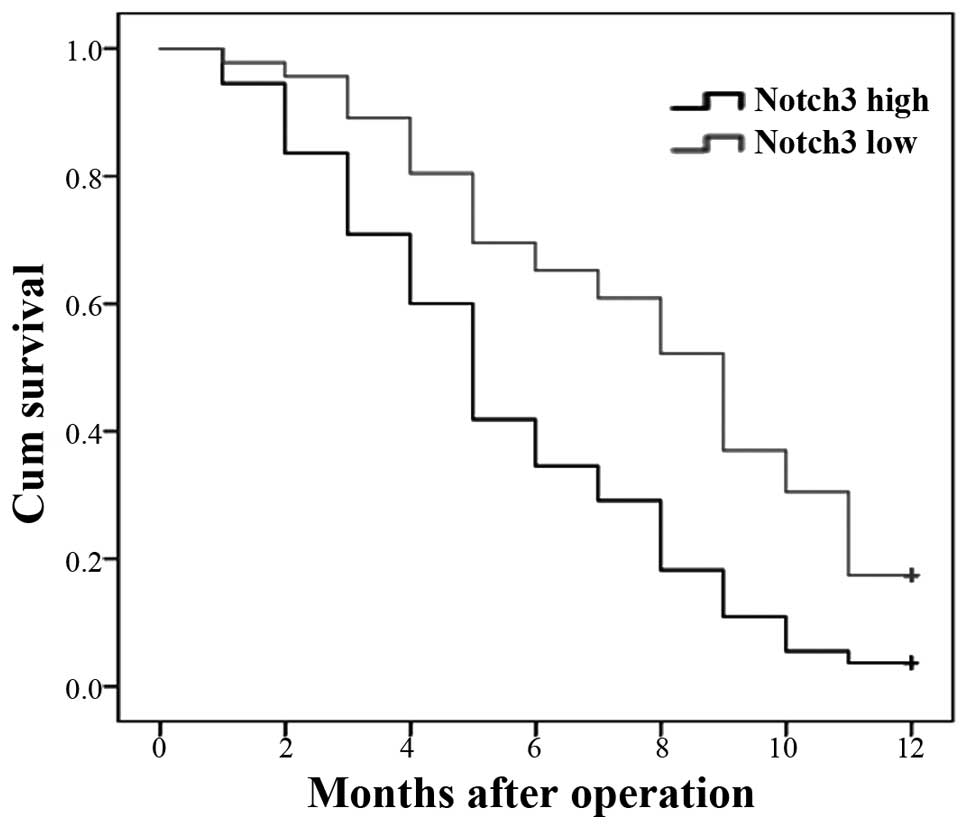
relationship to the prognosis of patients with PDAC. We used
Kaplan-Meier postoperative survival curves to evaluate the
correlation between the overall survival rates of patients with
PDAC and the expression of Notch3. Log-rank tests showed
significantly different survival times between the low and high
Notch3 expression groups (P<0.001). These results suggested that
a low expression of Notch3 increases patient survival, whereas a
high expression of Notch3 reduces patient survival (Fig. 2). The cumulative 1-year survival
rate was 17.6% in patients with a low expression of Notch3, whereas
the rate was only 7.1% in patients with a high expression of
Notch3.
The results of the univariate Cox regression
analysis showed that metastasis, venous invasion, AJCC TNM stage
and the protein expression of Notch3 were strongly correlated with
overall survival (2). We also used
multivariate Cox regression analysis to evaluate whether the high
expression of Notch3 was an independent predictor of overall
survival in patients with PDAC. Data in Table II show that Notch3 expression
predicted overall survival in patients with PDAC.
 | Table IIUnivariate and multivariate analysis
for overall survival of PDAC patients. |
Table II
Univariate and multivariate analysis
for overall survival of PDAC patients.
| Tumour
characteristic | Relative risk (95%
CI) | P-value |
|---|
| Univariate | | |
| Gender | 1.024
(0.674–1.556) | 0.911 |
| Age (years) | 1.075
(0.711–1.625) | 0.732 |
| Tumour grade
(differentiation) | 0.578
(0.370–0.902) | 0.016 |
| Metastasis | 11.292
(6.203–20.557) | <0.001 |
| Venous
invasion | 10.904
(5.925–20.066) | <0.001 |
| AJCC TNM
stage | 3.694
(2.022–6.748) | <0.001 |
| Notch3 | 2.927
(1.876–4.569) | <0.001 |
| Multivariate | | |
| Tumour grade
(differentiation) | 1.194
(0.725–1.965) | 0.486 |
| Metastasis | 5.917
(2.762–12.676) | <0.001 |
| Venous
invasion | 2.516
(1.240–5.108) | 0.11 |
| AJCC TNM
stage | 1.961
(0.981–3.920) | 0.057 |
| Notch3 | 1.960
(1.181–3.252) | 0.009 |
Inhibition of Notch3 decreases PDAC cell
migration and invasion capabilities
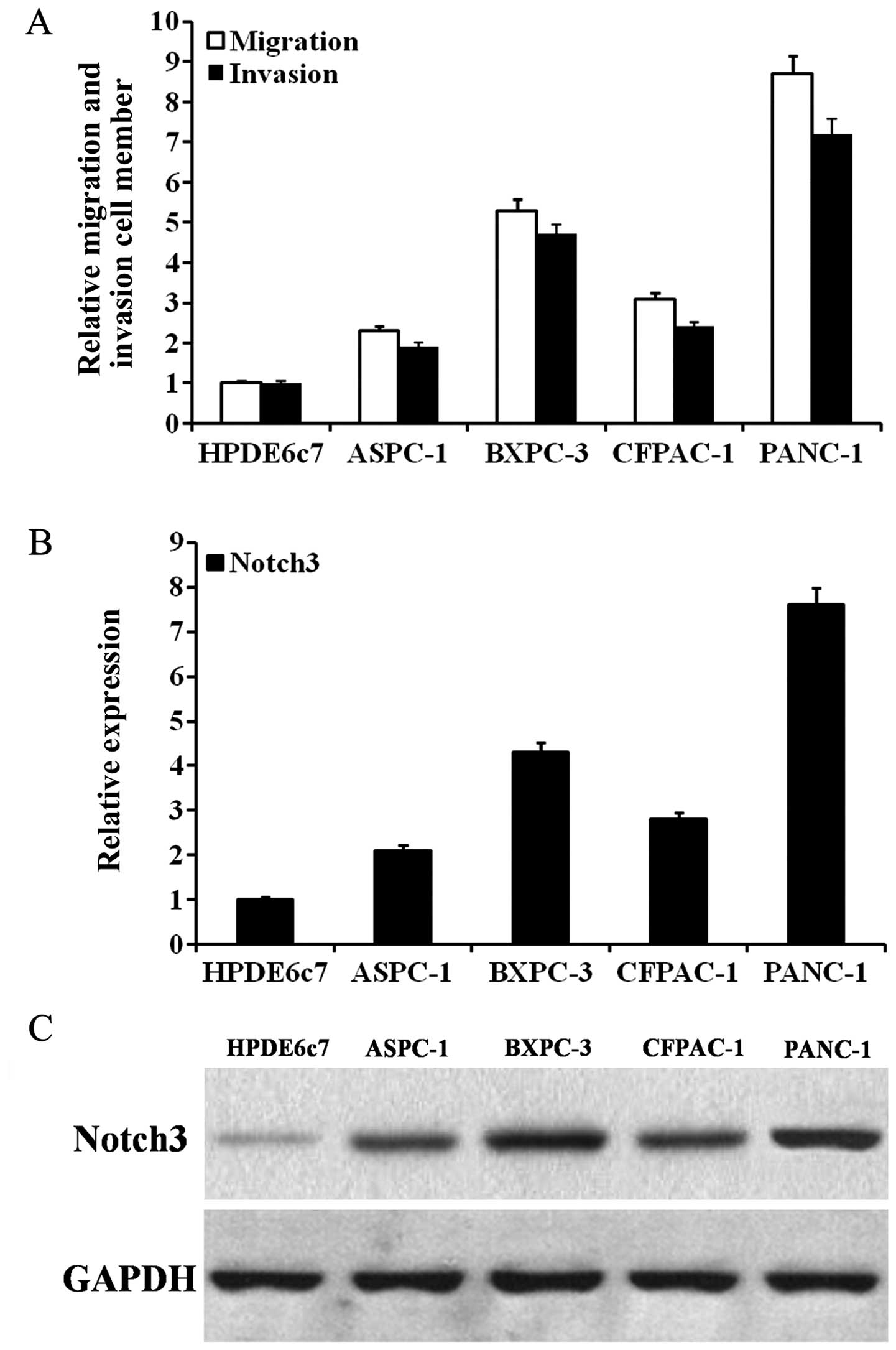
To assess whether Notch3 participates in PDAC cell
invasion and metastasis, we first detected the Notch3 expression in
PDAC cells with different migration and invasion capabilities. As
shown in Fig. 3A, BXPC-3 and PANC-1
cells had high migration and invasion capabilities. As shown in
Fig. 3B and C, as the mRNA and
protein expression of Notch3 increased, the migration and invasion
capabilities of the PDAC cells tended to increase. In PDAC cells,
the expression of Notch3 mRNA and protein could be effectively
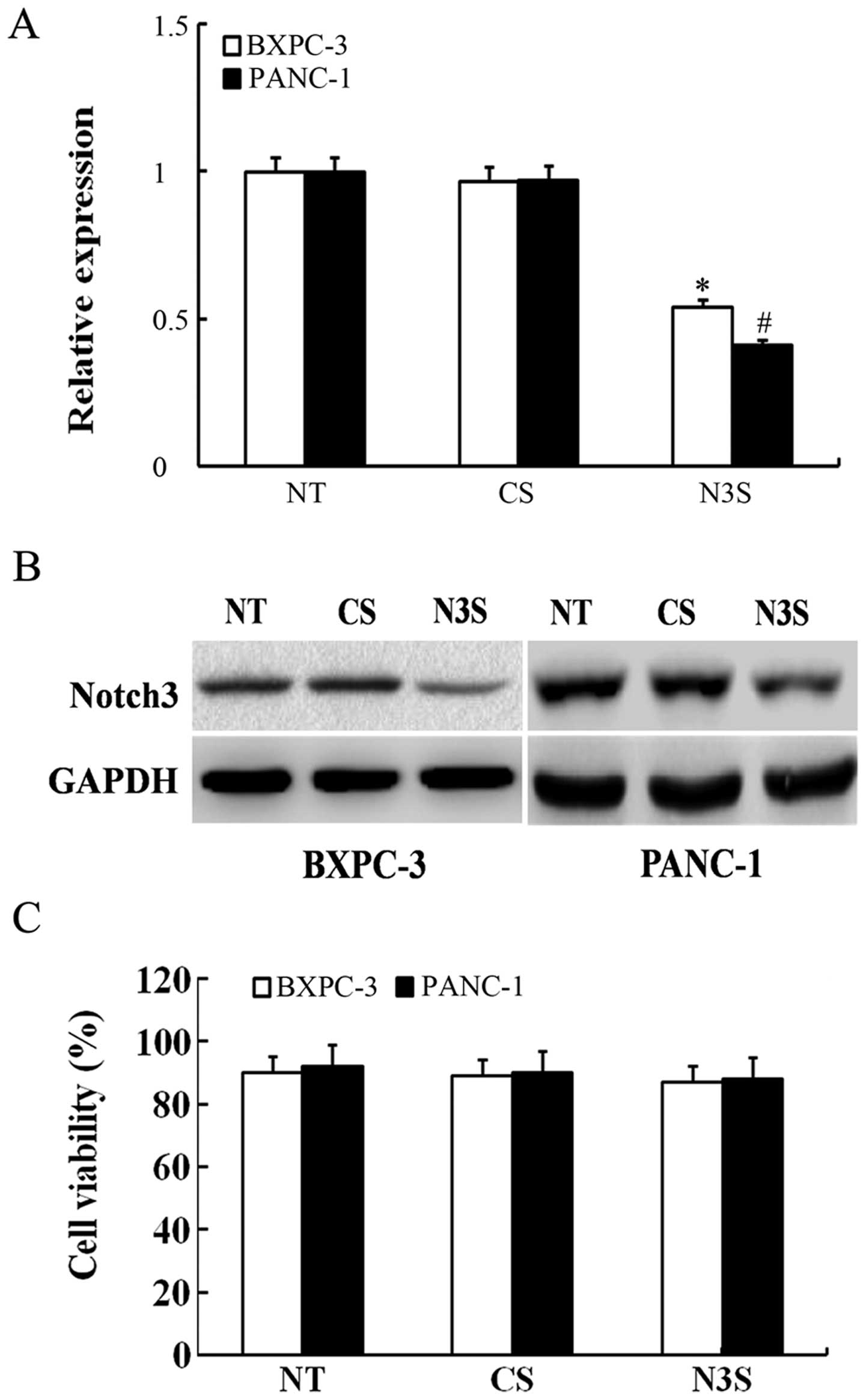
inhibited by siRNA (Fig. 4A and B).
To detect changes in migration and invasion capabilities, we
measured the number of siRNA-transfected PDAC cells using Transwell
cell culture chambers. As shown in Fig.
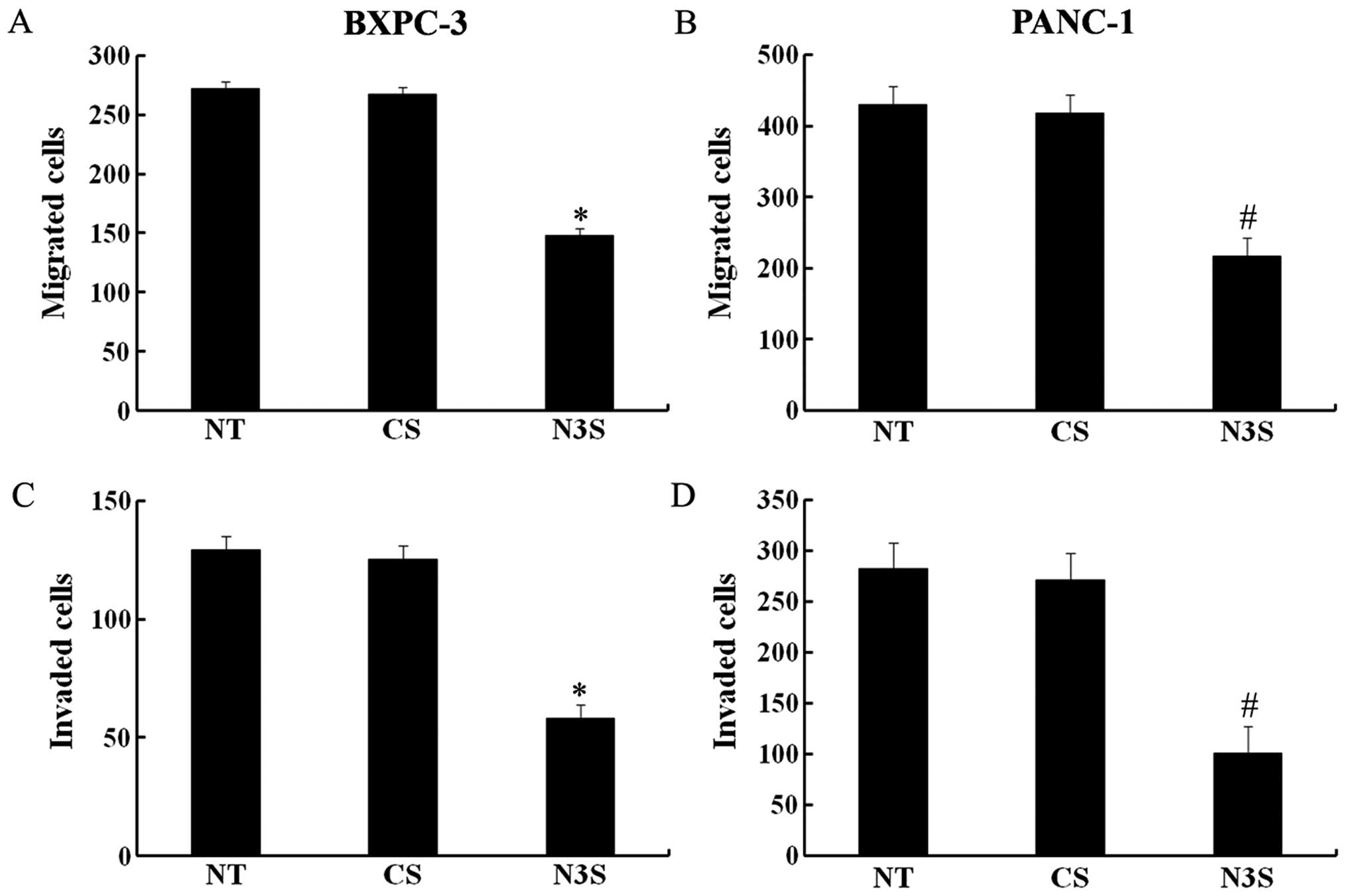
5, the number of BXPC-3 and PANC-1 cells that migrated through
the Transwell was significantly lower among the Notch3-inhibited
cells than among the control siRNA-transfected cells. According to
the MTT assay, inhibition of Notch3 had no effect on cell
viability, which confirmed that the effects of inhibited Notch3 on
cell migration and invasion were independent of apoptosis (Fig. 4C). Thus, based on these results, we
can speculate that the inhibition of Notch3 could decrease the
migration and invasion capabilities of PDAC cells.
Inhibition of Notch3 decreases the
protein expression of CD44v6, MMP-2, MMP-9, VEGF and uPA and
increase the protein expression of E-cadherin
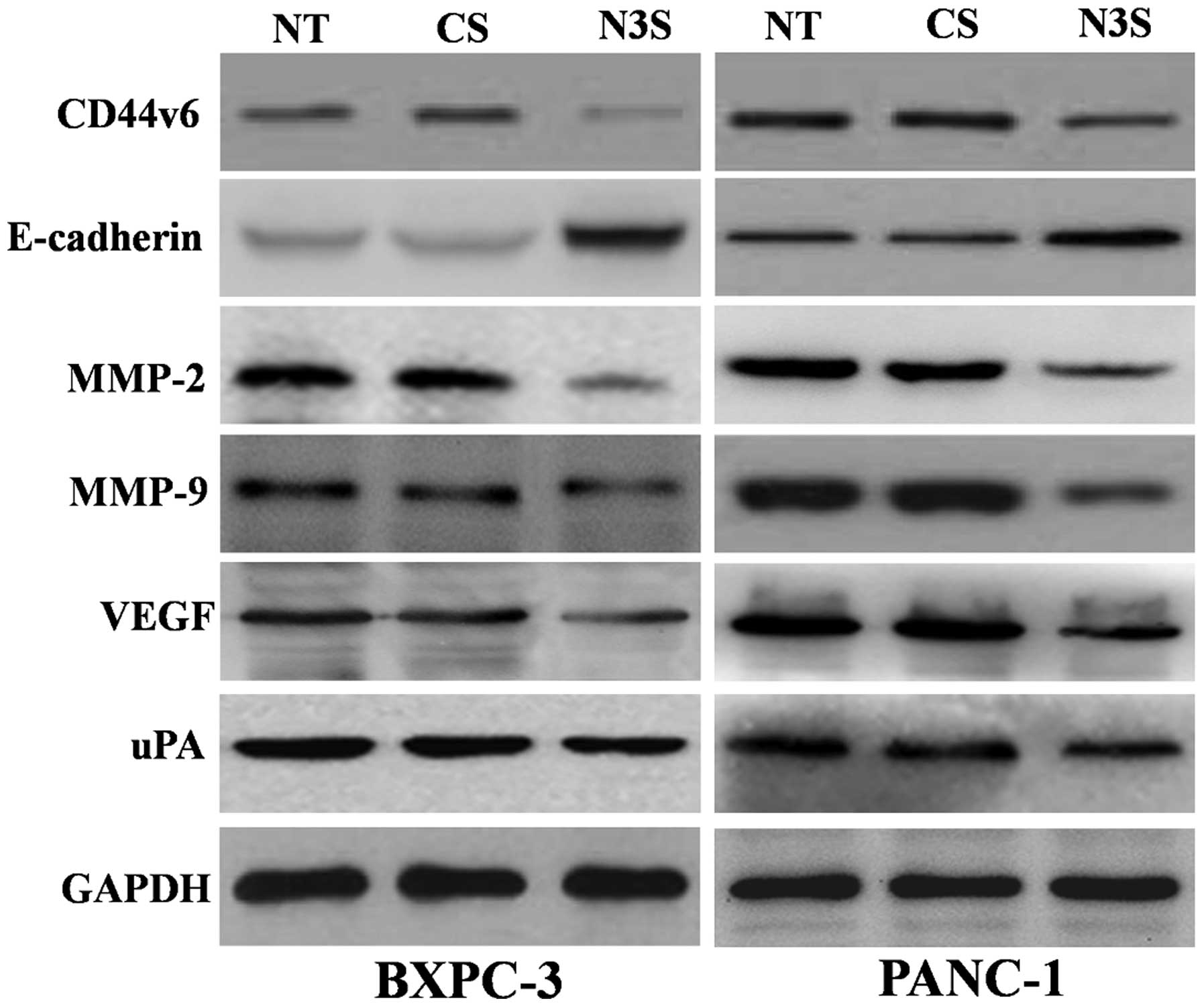
To explore the potential mechanism of action of
Notch3 in PDAC cells, we detected the effect of inhibited Notch3 on
molecules related to metastasis, such as CD44v6, E-cadherin, MMP-2,
MMP-9, VEGF and uPA. We found that Notch3 inhibition could decrease
the protein expression of CD44v6, MMP-2, MMP-9, VEGF and uPA,
whereas the protein expression of E-cadherin increased in PDAC
cells with Notch3 inhibition (Fig.
6). These results suggested that Notch3 may be involved in the
processes of migration and invasion in PDAC cells by regulating
CD44v6, E-cadherin, MMP-2, MMP-9, VEGF and uPA.
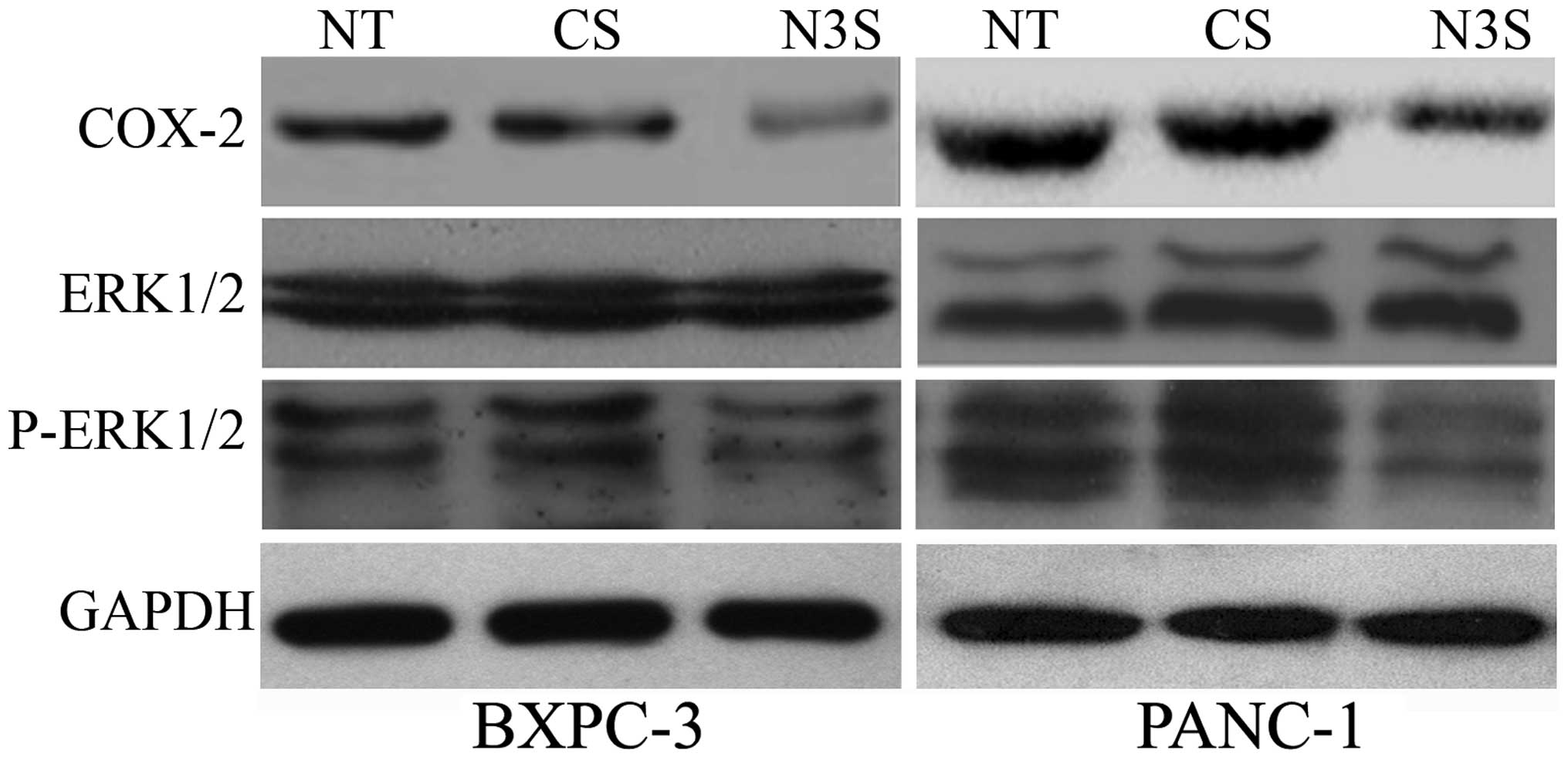
Notch3 regulates the COX-2 and the ERK1/2
pathways
COX-2 is an upstream molecule of CD44v6 and
E-cadherin, and the ERK1/2 pathway includes the up-stream molecules
of MMP-2, MMP-9, VEGF and uPA. Thus, we explored whether inhibition
of Notch3 could affect the COX-2 and ERK1/2 pathways. In
Notch3-inhibited PDAC cells, the expression of COX-2 and p-ERK1/2
decreased, indicating that Notch3 may be up-stream molecule of
COX-2 and the ERK1/2 pathway (Fig.
7).
Discussion
The mortality rate of pancreatic ductal
adenocarcinoma (PDAC) is high, despite the use of surgery,
radiation therapy and chemotherapy to treat PDAC. A lack of
effective therapies is the main cause of mortality. However, it is
difficult to make an early diagnosis since there are no obvious
symptoms and no specific detection methods for the early stage of
PDAC, and most patients have lost the chance to undergo radical
surgery when the diagnosis is made (1).
Numerous previous studies indicated that the Notch
signalling pathway plays an important role in many cellular
development processes and can also regulate tumourigenesis
(17,18). Although various studies showed that
the Notch signalling pathway may play a suppressive role in PDAC in
certain specific conditions (15),
other studies have indicated that abnormal expression of the Notch
signalling pathway may cause tumourigenesis in PDAC (14,19–21).
An abnormal expression of Notch receptors, Notch ligands and Notch
target genes has been observed in PDAC (10,11,20,21).
Furthermore, inhibition of the Notch signalling pathway inhibits
cell growth, migration and invasion in murine pancreatic cancer
cells. However, the role that the Notch pathway plays in PDAC
remains unclear.
In the present study, we used immunohistochemistry
to examine the expression of Notch3 in PDAC tissues. The results
suggested that a high expression of Notch3 was correlated with
tumour grade, metastasis, venous invasion and TNM stage. These
results strongly indicated that Notch3 may play an important role
in the progression of PDAC. An effective prognostic molecular
biomarker may be important in evaluating patient status and
promoting tumour control. In the present study, the results of
survival curves showed that patients with a high expression of
Notch3 had a significantly worse overall survival rate (log-rank
test; P<0.001). Previous studies showed that the increased
expression of Notch3 in pancreatic cancer was statistically
significant for both cytoplasmic and nuclear staining compared to
benign tissue (22). One study also
showed that cytoplasmic expression of Notch3 was upregulated in
tumours in 21/35 patients (60.0%) and nuclear Notch3 was present in
20 resected PDACs (47.6%) and nuclear Notch3 was associated with
the presence of lymph node metastases in resected PDAC specimens
(23). In our results, we found
positive staining for Notch3 was mainly observed in the cytoplasm
and at the cell membrane and nuclear Notch3 was also observed.
However, the expression of Notch3 in nuclear was less than the
expression of Notch3 in cytoplasm and membrane. These results were
similar to previous studies. There may be two reasons for this
result. One reason may be that nuclear staining results were
different using different antibody which had different protein
binding properties and nuclear penetration. Other reason may be
related to the structure of the Notch3. Notch3 are single-pass
transmembrane proteins consisting of extracellular, transmembrane,
and intracellular domains. Upon activation, Notch is cleaved
releasing the Notch intracellular domain (NICD) and NICD is then
ready to be translocated into the nucleus for transcriptional
activation of Notch target genes. Thus, the expression of Notch3 in
nuclear will be less than the expression of Notch3 in cytoplasm and
cell membrane. Nuclear Notch3 is an activator of Notch3 signalling
pathway, so it may play an important role in function of Notch3
signalling pathway such as lymph node metastases. However, this
result does not affect the correlation between Notch3 and
pancreatic cancer. Similar results were also presented in other
tissue. In hepatocellular carcinoma, Notch3 was detected in the
cytoplasm (24). Positive staining
of Notch3 was located in the cytoplasm and high Notch3 expression
had a significantly shorter survival time, compared with those with
no or low expression (25).
Moreover, the results of multivariate analysis indicated the
expression of Notch3 may be an indicator of worse outcome
independent of TNM stage. The above-mentioned results suggest that
a high expression of Notch3 is correlated with a worse patient
outcome and may be an independent prognostic factor for PDAC.
Moreover, in addition to TNM staging, Notch3 expression may be a
useful prognostic biomarker for evaluating PDAC patients. This is
the first report to show that the expression of Notch3 can be used
as a prognostic biomarker for PDAC.
Many cancer patients die from metastasis, yet, the
mechanism of metastasis remains unclear. To escape from the primary
tumour and result in distant metastasis, tumour cells must acquire
the ability to invade and migrate. Tumour cells from the primary
tumour can be degraded and removed from the extracellular matrix
and thus moved into the vicinity of the blood or lymphatic vessels,
which lays the foundation for distant metastasis. Thus, to explore
whether Notch3 plays an important role in PDAC, we focused on
determining whether Notch3 may participate in the migration and
invasion of PDAC in vitro.
For tumour cells to migrate, they first need to
adhere to the blood or lymphatic vessels. Therefore, adhesion is an
essential important process in the migration cascade. Many cell
adhesion molecules, including integrins, cadherins, selectins,
immunoglobulins and proteoglycans, have been implicated in tumour
progression and metastasis. In the present study, we focused on
CD44v6 and E-cadherin, which are two important adhesion receptors.
CD44v6, a member of the CD44 family of cell adhesion molecules,
plays an important role in the progression and metastasis of
tumours (26). Previous research
has shown that in many tumours, the abnormal expression of CD44v6
correlates with a poor prognosis (27,28).
E-cadherin is a main member of the ca-mucoprotein family, which is
associated with differentiation and invasion of tumour cells
(29). Numerous studies have
indicated that E-cadherin plays an inhibitory role in tumour
migration, metastasis and unfavourable prognosis (30–32).
The reduction of E-cadherin expression and the degradation of
E-cadherin adhesion plaques on the cell surface can result in cells
escaping from the primary tumour and moving into the vicinity of
the blood or lymphatic vessels (33). In the present study, it was
interesting that inhibition of Notch3 decreased the migration of
PDAC cells while decreasing the protein expression of CD44v6 and
increasing the protein expression of E-cadherin. This finding
indicated that Notch3 participates in the migration of PDAC cells
and may regulate the expression of CD44v6 and E-cadherin.
However, how Notch3 regulates E-cadherin and CD44v6
is not clear. To further explore the potential mechanism governing
how Notch3 regulates the expression of E-cadherin and CD44v6, we
focused on COX-2, an upstream molecule of E-cadherin and CD44v6
(34,35). COX-2 participates in various
cellular functions of tumours under physiologic and pathologic
conditions (36,37). Abnormal expression of COX-2 also
plays an important role in the progression of carcinogenesis
(34,38). During the progression of
carcinogenesis, COX-2 contributes to the modulation of various
molecules related to metastasis, such as E-cadherin and CD44v6
(34,35). However, there are few studies
concerning the correlation between the Notch signalling pathway and
COX-2. In gastric cancer, N1IC, which is the activating factor of
Notch1, can bind to a COX-2 promoter and thereby regulate the
expression of COX-2 (39). However,
whether Notch3 can regulate the expression of COX-2 is unclear. The
results of the current experiments showed that inhibition of Notch3
reduces the expression of COX-2, indicating that Notch3 may be an
upstream molecule of COX-2. However, the specific mechanisms of
this process should be further explored. Our results indicate that
Notch3 plays an important role in the process of migration in PDAC.
However, many other mechanisms may be involved in the process of
migration, and our results may highlight one of the possible
mechanisms.
During the series of steps involved in tumour
metastasis, tumour cells can degrade the basement membrane and the
stromal extracellular matrix, which leads to tumour cell invasion.
Matrix metalloproteinases (MMPs) and urokinase-type plasminogen
activator (uPA) are important molecules involved in the process of
invasion. MMPs are a family of related enzymes that can degrade the
extracellular matrix (ECM) and cause tumour cells to invade the
vasculature and target organs, leading to metastasis (40). MMPs, such as MMP-2 and MMP-9, can
participate in the invasion and metastasis of the tumour, and they
can degrade type IV collagen, which is the principal component of
the basement membrane (41,42). During cell migration, angiogenesis,
tumour growth and metastasis, the plasminogen activator system
plays an important role. uPA binds to its receptor (uPAR), which
facilitates the conversion of plasminogen to plasmin. Plasmin
participates in the invasion and metastasis of cancer cells by
degrading components of the extracellular matrix, either directly
or indirectly through MMPs (43).
VEGF plays an essential role in tumour cell invasion
and metastasis. VEGF expression is commonly found to increase in
tumours, and there is an association between VEGF expression and
distant metastasis. Abnormal expression of VEGF increases the
migration and invasion of tumour cells (44,45).
In the present study, we found that inhibition of Notch3 decreases
the invasion of PDAC cells and reduce the expression of MMP-2,
MMP-9, VEGF and uPA. Therefore, we can conclude that Notch3
participates in the invasion of PDAC cells by regulating the
expression of MMP-2, MMP-9, VEGF and uPA.
ERK1/2, a member of the family of mitogen-activated
protein kinases (MAPKs), plays an important role in the signalling
pathways related to scattering/motility, invasion, proliferation
and survival (46,47). In addition, activation of ERK1/2
regulates the expression of a variety of important genes in
metastasis, including MMP-2/-9, VEGF and uPA (48,49).
Although in recent years, increased attention was paid to the
interaction between the ERK1/2 pathway with other cell signal
pathways, the relationship between the Notch signalling and the
ERK1/2 pathways is unclear. The results showed that inhibition of
Notch3 reduces the expression of pERK1/2, thereby inactivating the
ERK1/2 pathway and regulating the expression of MMP-2/-9, VEGF and
uPA. Based on the above-mentioned evidence, we suspect that this
may be one of the mechanisms by which Notch3 participates in PDAC
invasion.
In summary, our results strongly indicated that a
high expression of Notch3 significantly correlated with the
progression of PDAC and poor patient prognosis. Thus, Notch3
expression may be used as an adjunct to the TNM staging system to
evaluate the prognosis of patients with PDAC. In vitro,
inhibition of Notch3 can decrease the migration and invasion of
PDAC cells by regulating the expression of CD44v6, E-cadherin,
MMP-2, MMP-9, VEGF and uPA. Therefore, Notch3 may not be only a
novel marker of prognosis for patients with PDAC but may also be a
molecular target for PDAC therapy. However, the underlying
mechanisms involved in these results should be further
explored.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (grants no.
81470843).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stathis A and Moore MJ: Advanced
pancreatic carcinoma: Current treatment and future challenges. Nat
Rev Clin Oncol. 7:163–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: Cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miele L and Osborne B: Arbiter of
differentiation and death: Notch signaling meets apoptosis. J Cell
Physiol. 181:393–409. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ohishi K, Katayama N, Shiku H,
Varnum-Finney B and Bernstein ID: Notch signalling in
hematopoiesis. Semin Cell Dev Biol. 14:143–150. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
You L, Chen G and Zhao YP: Core signaling
pathways and new therapeutic targets in pancreatic cancer. Chin Med
J. 123:1210–1215. 2010.PubMed/NCBI
|
|
9
|
De La O JP and Murtaugh LC: Notch
signaling: Where pancreatic cancer and differentiation meet?
Gastroenterology. 136:1499–1502. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Z, Li Y and Sarkar FH: Notch
signaling proteins: Legitimate targets for cancer therapy. Curr
Protein Pept Sci. 11:398–408. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Z, Zhang Y, Li Y, Banerjee S, Liao J
and Sarkar FH: Down-regulation of Notch-1 contributes to cell
growth inhibition and apoptosis in pancreatic cancer cells. Mol
Cancer Ther. 5:483–493. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Z, Banerjee S, Li Y, Rahman KM, Zhang
Y and Sarkar FH: Down-regulation of notch-1 inhibits invasion by
inactivation of nuclear factor-kappaB, vascular endothelial growth
factor, and matrix metalloproteinase-9 in pancreatic cancer cells.
Cancer Res. 66:2778–2784. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Plentz R, Park JS, Rhim AD, Abravanel D,
Hezel AF, Sharma SV, Gurumurthy S, Deshpande V, Kenific C,
Settleman J, et al: Inhibition of gamma-secretase activity inhibits
tumor progression in a mouse model of pancreatic ductal
adenocarcinoma. Gastroenterology. 136:1741–1749.e6. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ristorcelli E and Lombardo D: Targeting
Notch signaling in pancreatic cancer. Expert Opin Ther Targets.
14:541–552. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hanlon L, Avila JL, Demarest RM, Troutman
S, Allen M, Ratti F, Rustgi AK, Stanger BZ, Radtke F, Adsay V, et
al: Notch1 functions as a tumor suppressor in a model of
K-ras-induced pancreatic ductal adenocarcinoma. Cancer Res.
70:4280–4286. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chu D, Li Y, Wang W, Zhao Q, Li J, Lu Y,
Li M, Dong G, Zhang H, Xie H, et al: High level of Notch1 protein
is associated with poor overall survival in colorectal cancer. Ann
Surg Oncol. 17:1337–1342. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Egan SE, St-Pierre B and Leow CC: Notch
receptors, partners and regulators: From conserved domains to
powerful functions. Curr Top Microbiol Immunol. 228:273–324.
1998.
|
|
18
|
Callahan R and Egan SE: Notch signaling in
mammary development and oncogenesis. J Mammary Gland Biol
Neoplasia. 9:145–163. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mazur PK, Einwächter H, Lee M, Sipos B,
Nakhai H, Rad R, Zimber-Strobl U, Strobl LJ, Radtke F, Klöppel G,
et al: Notch2 is required for progression of pancreatic
intraepithelial neoplasia and development of pancreatic ductal
adenocarcinoma. Proc Natl Acad Sci USA. 107:13438–13443. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yao J and Qian C: Inhibition of Notch3
enhances sensitivity to gemcitabine in pancreatic cancer through an
inactivation of PI3K/Akt-dependent pathway. Med Oncol.
27:1017–1022. 2010. View Article : Google Scholar
|
|
21
|
Mullendore ME, Koorstra JB, Li YM,
Offerhaus GJ, Fan X, Henderson CM, Matsui W, Eberhart CG, Maitra A
and Feldmann G: Ligand-dependent Notch signaling is involved in
tumor initiation and tumor maintenance in pancreatic cancer. Clin
Cancer Res. 15:2291–2301. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Doucas H, Mann CD, Sutton CD, Garcea G,
Neal CP, Berry DP and Manson MM: Expression of nuclear Notch3 in
pancreatic adenocarcinomas is associated with adverse clinical
features, and correlates with the expression of STAT3 and
phosphorylated Akt. J Surg Oncol. 97:63–68. 2008. View Article : Google Scholar
|
|
23
|
Mann CD, Bastianpillai C, Neal CP, Masood
MM, Jones DJ, Teichert F, Singh R, Karpova E, Berry DP and Manson
MM: Notch3 and HEY-1 as prognostic biomarkers in pancreatic
adenocarcinoma. PLoS One. 7:e511192012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gramantieri L, Giovannini C, Lanzi A,
Chieco P, Ravaioli M, Venturi A, Grazi GL and Bolondi L: Aberrant
Notch3 and Notch4 expression in human hepatocellular carcinoma.
Liver Int. 27:997–1007. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu L, Xue F, Shao M, Deng A and Wei G:
Aberrant expression of Notch3 predicts poor survival for
hepatocellular carcinomas. Biosci Trends. 7:152–156.
2013.PubMed/NCBI
|
|
26
|
Günthert U, Hofmann M, Rudy W, Reber S,
Zöller M, Haussmann I, Matzku S, Wenzel A, Ponta H and Herrlich P:
A new variant of glycoprotein CD44 confers metastatic potential to
rat carcinoma cells. Cell. 65:13–24. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jijiwa M, Demir H, Gupta S, Leung C, Joshi
K, Orozco N, Huang T, Yildiz VO, Shibahara I, de Jesus JA, et al:
CD44v6 regulates growth of brain tumor stem cells partially through
the AKT-mediated pathway. PLoS One. 6:e242172011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu YJ, Yan PS, Li J and Jia JF:
Expression and significance of CD44s, CD44v6, and nm23 mRNA in
human cancer. World J Gastroenterol. 11:6601–6606. 2005. View Article : Google Scholar
|
|
29
|
Kemler R: From cadherins to catenins:
Cytoplasmic protein interactions and regulation of cell adhesion.
Trends Genet. 9:317–321. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bremnes RM, Veve R, Gabrielson E, Hirsch
FR, Baron A, Bemis L, Gemmill RM, Drabkin HA and Franklin WA:
High-throughput tissue microarray analysis used to evaluate biology
and prognostic significance of the E-cadherin pathway in
non-small-cell lung cancer. J Clin Oncol. 20:2417–2428. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsujii M and DuBois RN: Alterations in
cellular adhesion and apoptosis in epithelial cells overexpressing
prostaglandin endoperoxide synthase 2. Cell. 83:493–501. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu D, Huang C, Kameyama K, Hayashi E,
Yamauchi A, Kobayashi S and Yokomise: E-cadherin expression
associated with differentiation and prognosis in patients with
non-small cell lung cancer. Ann Thorac Surg. 71:949–954. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wells A, Yates C and Shepard CR:
E-cadherin as an indicator of mesenchymal to epithelial reverting
transitions during the metastatic seeding of disseminated
carcinomas. Clin Exp Metastasis. 25:621–628. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dohadwala M, Batra RK, Luo J, Lin Y,
Krysan K, Pold M, Sharma S and Dubinett SM: Autocrine/paracrine
prostaglandin E2 production by non-small cell lung cancer cells
regulates matrix metalloproteinase-2 and CD44 in
cyclooxygenase-2-dependent invasion. J Biol Chem. 277:50828–50833.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dohadwala M, Yang SC, Luo J, Sharma S,
Batra RK, Huang M, Lin Y, Goodglick L, Krysan K, Fishbein MC, et
al: Cyclooxygenase-2-dependent regulation of E-cadherin:
Prostaglandin E2 induces transcriptional repressors ZEB1
and snail in non-small cell lung cancer. Cancer Res. 66:5338–5345.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dubinett SM, Sharma S, Huang M, Dohadwala
M, Pold M and Mao JT: Cyclooxygenase-2 in lung cancer. Prog Exp
Tumor Res. 37:138–162. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dannenberg AJ and Subbaramaiah K:
Targeting cyclooxy-genase-2 in human neoplasia: Rationale and
promise. Cancer Cell. 4:431–436. 2003. View Article : Google Scholar
|
|
38
|
Park K, Han S, Shin E, Kim HJ and Kim JY:
Cox-2 expression on tissue microarray of breast cancer. Eur J Surg
Oncol. 32:1093–1096. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yeh TS, Wu CW, Hsu KW, Liao WJ, Yang MC,
Li AF, Wang AM, Kuo ML and Chi CW: The activated Notch1 signal
pathway is associated with gastric cancer progression through
cyclooxy-genase-2. Cancer Res. 69:5039–5048. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Itoh Y and Nagase H: Matrix
metalloproteinases in cancer. Essays Biochem. 38:21–36. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zeng ZS, Cohen AM and Guillem JG: Loss of
basement membrane type IV collagen is associated with increased
expression of metalloproteinases 2 and 9 (MMP-2 and MMP-9) during
human colorectal tumorigenesis. Carcinogenesis. 20:749–755. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Komatsu K, Nakanishi Y, Nemoto N, Hori T,
Sawada T and Kobayashi M: Expression and quantitative analysis of
matrix metalloproteinase-2 and -9 in human gliomas. Brain Tumor
Pathol. 21:105–112. 2004. View Article : Google Scholar
|
|
43
|
Shimizu M, Cohen B, Goldvasser P, Berman
H, Virtanen C and Reedijk M: Plasminogen activator uPA is a direct
transcriptional target of the JAG1-Notch receptor signaling pathway
in breast cancer. Cancer Res. 71:277–286. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wey JS, Fan F, Gray MJ, Bauer TW, McCarty
MF, Somcio R, Liu W, Evans DB, Wu Y, Hicklin DJ, et al: Vascular
endothelial growth factor receptor-1 promotes migration and
invasion in pancreatic carcinoma cell lines. Cancer. 104:427–438.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Takahashi Y, Kitadai Y, Bucana CD, Cleary
KR and Ellis LM: Expression of vascular endothelial growth factor
and its receptor, KDR, correlates with vascularity, metastasis, and
proliferation of human colon cancer. Cancer Res. 55:3964–3968.
1995.PubMed/NCBI
|
|
46
|
Chan-Hui PY and Weaver R: Human
mitogen-activated protein kinase kinase kinase mediates the
stress-induced activation of mitogen-activated protein kinase
cascades. Biochem J. 336:599–609. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Trusolino L and Comoglio PM:
Scatter-factor and semaphorin receptors: Cell signalling for
invasive growth. Nat Rev Cancer. 2:289–300. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Arai K, Lee SR and Lo EH: Essential role
for ERK mitogen-activated protein kinase in matrix
metalloproteinase-9 regulation in rat cortical astrocytes. Glia.
43:254–264. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cheng YC, Chen LM, Chang MH, Chen WK, Tsai
FJ, Tsai CH, Lai TY, Kuo WW, Huang CY and Liu CJ:
Lipopolysaccharide upregulates uPA, MMP-2 and MMP-9 via ERK1/2
signaling in H9c2 cardiomyoblast cells. Mol Cell Biochem.
325:15–23. 2009. View Article : Google Scholar : PubMed/NCBI
|