Introduction
Hepatic fibrosis (HF) refers to the abnormal
proliferation of connective tissue in the liver, which is caused by
the long-term existence of various liver injury factors (including
alcohol and viruses). As the pathological characteristics of
chronic liver disease, HF severely damages the morphology and
function of normal liver tissue, and is an important link in the
further development of various chronic liver diseases to full
hepatic cirrhosis. It has been reported that hepatic cirrhosis
resulted in 1.2 million deaths in 2013, up from 0.8 million deaths
in 1990 (1). The essence of HF is
the imbalance between synthesis and degradation of liver tissue ECM
(2–4). After stimulation of various liver
injury factors, the activated HSCs become responsive to both
proliferative and fibrogenic cytokines (3). The role of TGF-β1 in the HF
pathological mechanism has been extensively studied, and it is
considered to be the most central cytokine inducing HF (5,6). HSCs
react to TGF-β1 exposure with a negative feedback regulation
through the induction of Smad7. However, during chronic exposure to
TGF-β1 an epithelial-mesenchymal transition of HSCs into
proliferative myofibroblast cells (MFBs) takes place (7). These cells are activated via autocrine
TGF-β signaling and develop an intrinsic Smad activation without
Smad7 inhibition (8), leading to
copious ECM secretion (6,9).
TSP is a traditional Chinese medicine (TSM) formula
for the treatment of chronic liver disease, which is from 'Golden
Chamber' written long ago by the Eastern Han Dynasty physician
Zhang Zhongjing. It is composed of turtle shell glue, gelatin,
honeycomb, saltpeter and another 23 types of medicinal compounds.
At present, TSP has been approved by the Chinese State Food and
Drug Administration for the clinical prevention and treatment of HF
and early hepatic cirrhosis, also confirming that it has obvious
anti-fibrosis effects (10,11). Moreover, in the TSP formula, turtle
shell, Eupolyphaga sinensis and peach kernel were shown to
inhibit the proliferation of connective tissue, but the specific
mechanism of action has not been studied in depth (12–14).
TSP has serious side-effects that include mental fatigue and weight
loss (15). In addition, some
ingredients in the prescription, such as placenta hominis and
Cordyceps are scarce resources and therefore expensive.
These issues seriously limit its clinical application. In the
present study, we modified the original formula of TSP to produce
NTSD that has significantly less toxicity and gives easier access
to the materials. The outcome rates of both formulas were compared
in an HF rat model. It is suggested that blockade of the
TGF-β1/Smad signaling pathway might be the mechanism underlying the
effectiveness of TCM medication.
Materials and methods
Preparation of traditional Chinese
medicine
Compound turtle shell softening liver pills (TSP)
(batch number: 20090107) were bought from the Inner Mongolia Fu Rui
Medicine Company, dissolved in distilled water to produce a solvent
containing 0.06 g/ml of crude drug. The materials to prepare NTSD
included ground beetle, turtle shell, bupleurum root,
Scutellaria root, Pinellia, Artemisia
capillaris, cassia twig, peach kernel, Poria, Radix Astragali
and white peony root (Chongqing Tongjunge Pharmacy, Chongqing,
China). The above materials were soaked in distilled water for 1 h,
decocted (turtle shell, ground beetle first), filtered and
concentrated, to make the decoction. The different crude drug
dosages of NTSD in distilled water for application were 2.84, 1.42
and 0.71 g/ml, respectively. All the above preparation work was
completed in the Department of Pharmacy, Chongqing Southwest
Hospital, China.
Animals and cell cultures
Healthy male specific pathogen free Sprague-Dawley
(SPF SD rats) were purchased from the Experimental Animal Center of
Daping Hospital, Affiliated to the Third Military Medical
University (Chongqing, China) and were fed in the SPF animal
laboratory at temperatures between 18 and 22°C and a relative
humidity of 50–80%, with free access to a standard diet and sterile
water. HSC-T6 cells were cultured in 5% CO2 saturated
humidity environment at 37°C using DMEM culture medium (containing
10% FBS, 100 U/ml penicillin and 100 U/ml streptomycin). The
present study was approved by the Institutional Review Board of
Southwest Hospital, the Third Military Medical University, and the
methods were carried out in accordance with the approved
guidelines.
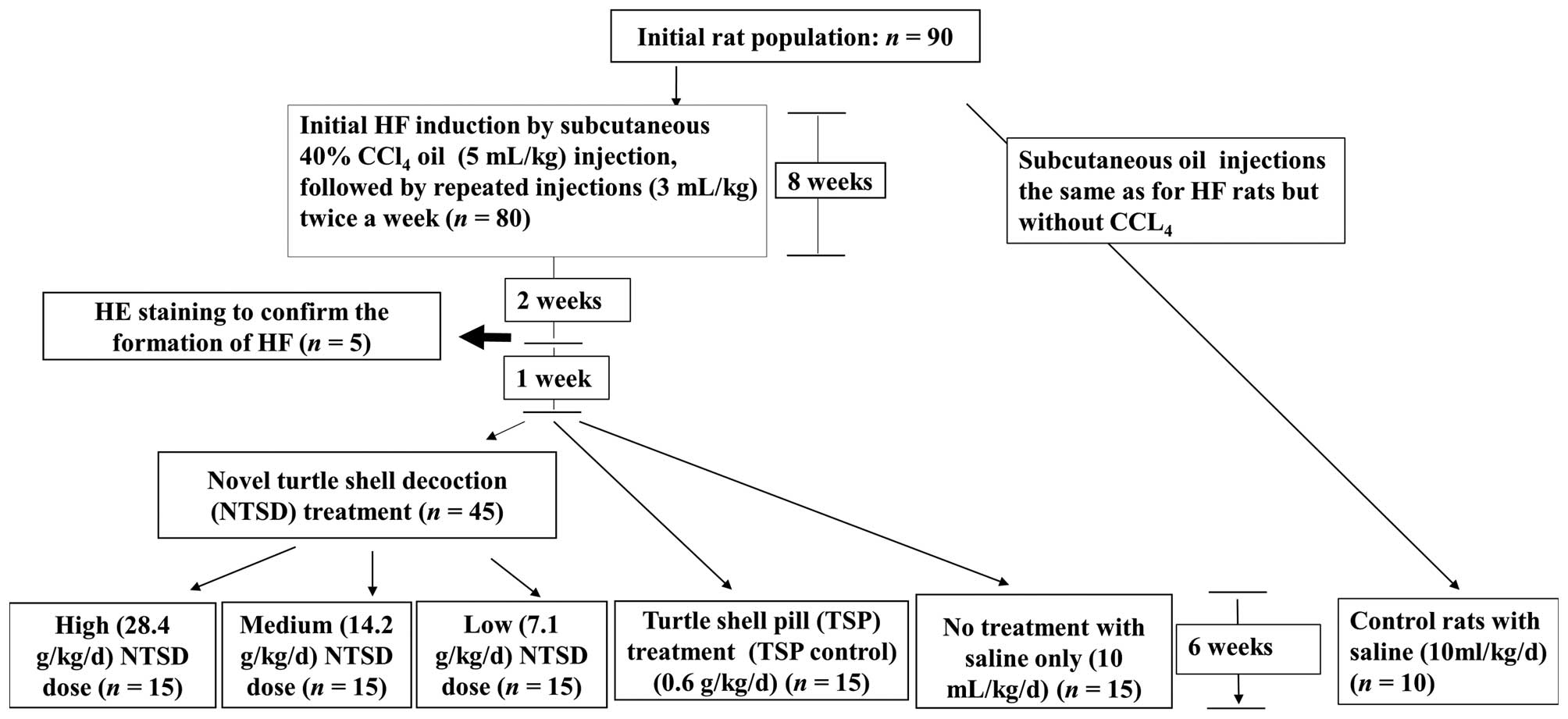
CCl4-induced HF rat model and
treatment
From an initial 90 rats, in 80 rats HF was induced
by CCl4 (Chengdu Kelong Chemical Factory, Chengdu,
Sichuan, China) mixed with olive oil (Sichuan Tianyuan Olive Oil
Co., Sichuan, China) to a final CCl4 concentration of
40%. The solution was injected as 5 ml/kg subcutaneously into the
right hind leg, followed by repeated injections (3 ml/kg), twice a
week for 8 weeks. Two weeks later, we randomly selected 5 HF rats
for H&E staining of liver tissue in order to confirm the
formation of HF. The remaining 75 rats were 1 week later randomly
divided into groups treated with low (7.1 g/kg/day), medium (14.2
g/kg/day) and high (28.4 g/kg/day) doses of NTSD as well as TSP
(0.6 g/kg/day, TSP control) and normal saline (10 ml/kg/day, as the
no treatment control) by gavage for 6 weeks. The 10 control rats
were treated the same way as the HF rats except that they received
only olive oil and saline (Fig.
1).
Evaluation of hepatic fibrosis
After 6 weeks of treatment, rats in each group were
fasted for 24 h and then anesthetized with a 3% sodium
pentobarbital (1 ml/kg) by a single intraperitoneal injection. The
same part of the right liver lobe was dissected from each rat for
fixation in 100 ml/l formaldehyde solution and hematoxylin and
eosin (H&E) staining. The liver cell degeneration, collagen
fiber hyperplasia and tissue morphology changes were investigated
with the aid of a light microscope (BX51; Olympus, Tokyo, Japan).
Quantitative and semi-quantitative methods were adopted to score
the HFs. Zero points indicated no pathological changes in liver
cells, a normal liver or no obvious collagen fiber hyperplasia; 1
point indicated that the proportion of degenerated liver cells were
<25% of the total cells, collagen fiber showed slight
hyperplasia, central vein and portal area exhibited a small amount
of fiber elongation but no septum formation; 2 points indicated
that the proportion of degenerated liver cells was between 25 and
50% of all liver cells with obvious collagen fiber hyperplasia; the
connective tissue around the central vein and portal area were
thickened, which extended fiber chords to form incomplete fiber
septa; 3 points indicated that the proportion of degenerated liver
cells was between 50 and 75% of all liver cells with massive
collagen fiber hyperplasia as well as false lobules, which were
formed by individual complete or incomplete thick fiber septa; 4
points indicated that the proportion of degenerated liver cells was
>75% with thicker complete fiber septa and a large formation of
false lobules.
Biochemical examination of liver
functions
After 6 weeks of treatment, rats in each group that
had been fasted for 24 h were given 3% sodium pentobarbital
anesthesia (1 ml/kg) and blood samples collected from the femoral
artery and subsequently the serum was isolated. The Au2700 full
automatic biochemical instrument (Olympus) was used for the
detection of serum ALT, AST, albumin and globulin content.
Preparation of drug-containing rat blood
serum
Thirty healthy SPF SD rats were randomly divided
into 6 groups (5 rats in each group), which were administered by
gavage low (7.1 g/kg/day), medium (14.2 g/kg/day) or a high (28.4
g/kg/day) NTSD dose as well as TSP (0.6 g/kg/day) and normal saline
as the control. All types of gastric perfusion medicine and liquids
were delivered at a rate of 10 ml/kg/day. Gastric perfusion was
performed twice a day for 3 consecutive days, with the rats having
been fasted for 12 h before each perfusion. Two hours after the
last gastric perfusion, Lumianning II (Jilin Huamu Animal Health
Product, Co., Ltd., Changchun, Jilin, China) was injected
intramuscularly for anesthesia and blood samples collected from the
inferior vena cava. These samples were stored at 4°C overnight,
then centrifuged for 15 min at 1000 × g to isolate the serum. The
sera from the same groups were mixed, filtered through a
0.22-μm filter then inactivated at 56°C for 30 min, in order
to remove possible biologically active substances, and samples
stored at −70°C until required for subsequent analysis.
Cell proliferation assay
HSC-T6 cells were seeded onto 96-well plates (the
volume of each well was 150 μl and contained
3×103 cells), and the culture medium (containing 20% of
different doses of NTSD containing serum) was changed after 24 h,
with equivalent volume TSP-containing serum treated cells as the
positive control, an equivalent volume of normal rat serum-treated
cells as the normal control, and saline-treated HF cells as the
negative control. The 96-well plates 24, 48, 72 and 96 h,
respectively, after incubation started were removed and the cell
growth status observed using an inverted microscope (IX71; Olympus)
and digital images were taken for a permanent record. Subsequently,
we replaced each well with fresh medium, added 10 μl CCK-8
kit solution and removed the plates after 2.5-h incubation,
slightly oscillated them for 25 sec to mix well, and then detected
the light density at 450 nm using a plate reader (Victor X2
Multilabel plate reader; Perkin-Elmer, Waltham, MA, USA). The cell
proliferation curve was plotted with the light density value as the
y-axis and the processing time as the x-axis. Each experiment was
repeated 3 times.
Detection of cell cycle and
apoptosis
HSC-T6 cells with 1×106/ml density were
routinely cultured for 24 h, and replaced by fresh medium
(containing 20% of different doses of normal serum, NTSD or TSP
medicated serum). After 72 h, cells from each group were collected
and the cell number counted to ensure each group contained cell
numbers from 1×104–2×106. Twenty-four to 72 h
after 80% ethanol fixation, cells were stained with propidium
iodide (PI, 4 mg/ml; Sigma-Aldrich), and flow cytometry (BD
FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA) used to
detect and analyze cell cycle changes. Each experiment was repeated
3 times.
For the detection of cell apoptosis, we took 100
μl cell suspensions (5–10 million cells), added 195
μl binding buffer to re-suspend the cells and mixed them
thoroughly with 5 μl Annexin V-FITC (Beyotime Institute of
Biotechnology, Beijing, China). Then the samples were incubated for
10 min in the dark at room temperature (20–25°C), centrifuged for 5
min at 1000 × g, re-suspended in 195 μl binding buffer,
mixed with 10 μl PI, and cell apoptosis was detected by flow
cytometry. Each experiment was repeated 3 times.
Immunohistochemistry
The 4 μm paraffin or 6 μm frozen
sections from liver tissue of rats in all groups were immunostained
with anti-TGF-β1 antibody (1:400; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA), anti-Smad3 antibody (1:400; Santa Cruz
Biotechnology) and anti-Smad7 antibody (1:400; Santa Cruz
Biotechnology) and detection kit (ZSGB-BIO, Beijing, China)
reagents. Pepsin (ZSGB-BIO) digestion was used for antigen
retrieval and a DAB color reagent kit (ZSGB-BIO) was used to
develop the color. Under the microscope, 5 non-overlapping fields
in each section were selected under a high power field (×400).
Image-Pro Plus software (Media Cybernetics, Bethesda, MD, USA) was
used to analyze the mean optical density value.
Western blot analysis
Total protein was extracted by western and IP cell
lysis solution (Beyotime Institute of Biotechnology, Shanghai,
China) according to the manufacturer's instructions from liver
tissues of rats in all groups, and the protein concentration
measured with a BCA protein concentration kit (Beyotime Institute
of Biotechnology). The primary antibodies included anti-TGF-β1
antibody (1:100; Santa Cruz Biotechnology), anti-Smad3 antibody
(1:200; Santa Cruz Biotechnology), anti-Smad7 antibody (1:200;
Santa Cruz Biotechnology) as well as proliferating cell nuclear
antigen (PCNA) antibody (1:800; Millipore, Billerica, MA, USA).
RT-PCR
Both liver tissue and HSC-T6 cell samples were
extracted with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) to
obtain their total RNA, according to the manufacturer's
instructions. Then, we used a PCR kit (Takara, Dalian, China) with
the total RNA as a template to amplify the cDNA of TGF-β1, Smad3,
Smad7 and PCNA genes, and with β-actin gene added as the internal
reference. Reverse transcription reaction conditions were: 30°C for
10 min; 42°C for 30 min, 99°C for 5 min, 5°C for 5 min, 1 cycle.
PCR reaction conditions: 94°C for 1 min; 94°C for 30 sec, 62.8°C
for 30 sec, 72°C for 1 min, 35 cycles; 72°C extension for 5 min.
After the reaction, the PCR reaction product was ran on a 1%
agarose gel electrophoresis and Goldview™ (G8140; Beijing SBS
Genetech, Co., Ltd., Beijing, China) nucleic acid dye staining used
to confirm the product. The sequences of primers of PCNA, TGF-β1,
Smad3 and Smad7 are shown in Table
I.
 | Table IThe sequences of RT-PCR primers of
TGF-β1, Smad3, Smad7, PCNA and β-actin genes. |
Table I
The sequences of RT-PCR primers of
TGF-β1, Smad3, Smad7, PCNA and β-actin genes.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|
| PCNA |
CCTGCTGGGACATCAGTTCG |
GGAGACAGTGGAGTGGCTTT |
| TGF-β1 |
CCGCAACAACGCAATCTATG |
AGCCCTGTATTCCGTCTCCTT |
| Smad3 |
GACTAGGTGTGAGCCCTTTAC |
ATGGTTGACCCACATCCTGGTG |
| Smad7 |
TTTACAACCGCAGCAGTTAC |
AAGATGACCTCCAGCCAGC |
| β-actin |
CCGTGAAAAGATGACCCAGAT |
CATTGCCGATAGTGATGACCT |
Statistical analysis
For statistical analyses SPSS for Windows (version
13.0; SPSS, Inc., Chicago, IL, USA) was used and data shown as the
mean ± SD. Student's t-test was used for comparison between groups
with a normal distribution. P<0.05 was considered to be a
statistically significant difference.
Results
Preparation of NTSD
We first added and omitted several traditional
Chinese medicines from the original TSP formula to prepare NTSD.
The TSP formula contains 8 kinds of traditional Chinese medicines
for resolving hard masses and recovering blood stasis, which have
been maintained in the NTSD compounds, whereas 15 kinds of herbs
inducing loss of vital Qi and pathogenic stagnation factors were
omitted. Instead we added Radix Astragali, Poria, as well as
Artemisia capillaris to the novel NTSD composition.
Hence, the complete formula of NTSD was as follows
(in 142 g): ground beetle 10 g, turtle shell 15 g, bupleurum root
10 g, scutellaria root 12 g, pinellia 15 g,
Artemisia capillaris 15 g, cassia twig 10 g, peach kernel 10
g, Poria 15 g, Radix Astragali 15 g and white peony root 15 g. The
ingredients were treated with soaking, decocting, filtering and
concentration to derive the final NTSD formula.
NTSD improved HF-related biochemical
liver indices induced by CCl4
As shown in Table
II, compared with healthy control rats, the ALT and AST serum
concentrations in the non-treated HF rats were significantly
increased (P<0.05) and albumin concentrations significantly
decreased (P<0.05) compared to the healthy control rats, which
indicated that the liver function of HF rats was affected. However,
TSP treatment significantly reduced the levels of ALT and AST
levels increased in HF rats together with an increased albumin
content, which proved its anti-HF effect. More importantly, NTSD
also showed a significant improvement in liver function regarding
ALT, AST and albumin serum concentrations, which supported its
equal anti-HF efficacy. There was no significant difference between
the effects of high, medium and low doses of NTSD on liver function
suggesting that the effect of NTSD in low dose (7.1 g/kg/day) might
have been saturating already (Table
II).
 | Table IINTSD improves the liver function of
CCl4 induced HF rats. |
Table II
NTSD improves the liver function of
CCl4 induced HF rats.
| Rats | Treatment
(g/kg/day) | N | ALT (IU/l) | AST (IU/l) | Globulin (g/l) | Albumin (g/l) |
|---|
| Control | Saline | 10 | 36.72±7.63 | 36.53±6.79 | 33.85±1.85 | 42.68±4.42 |
| Saline | 15 |
611.21±37.27a |
462.14±28.05a | 33.90±4.75 | 27.05±4.81a |
| NTSD | | | | | |
| 28.4 | 15 |
186.62±23.82b,c |
156.57±24.98b,c | 33.05±3.75 | 37.85±3.08b |
| HF | 14.2 | 15 |
174.73±39.52b,c |
136.93±14.08b,c | 30.45±3.22b | 38.25±1.90b |
| 7.1 | 15 |
153.82±18.44b,c |
167.80±18.62b | 34.21±5.92 | 38.13±1.50b |
| TSP | | | | | |
| 0.6 | 15 |
130.82±18.10b |
180.30±21.65b | 31.30±4.61 | 38.09±3.27b |
NTSD attenuates HF-related
pathomorphological changes induced by CCl4
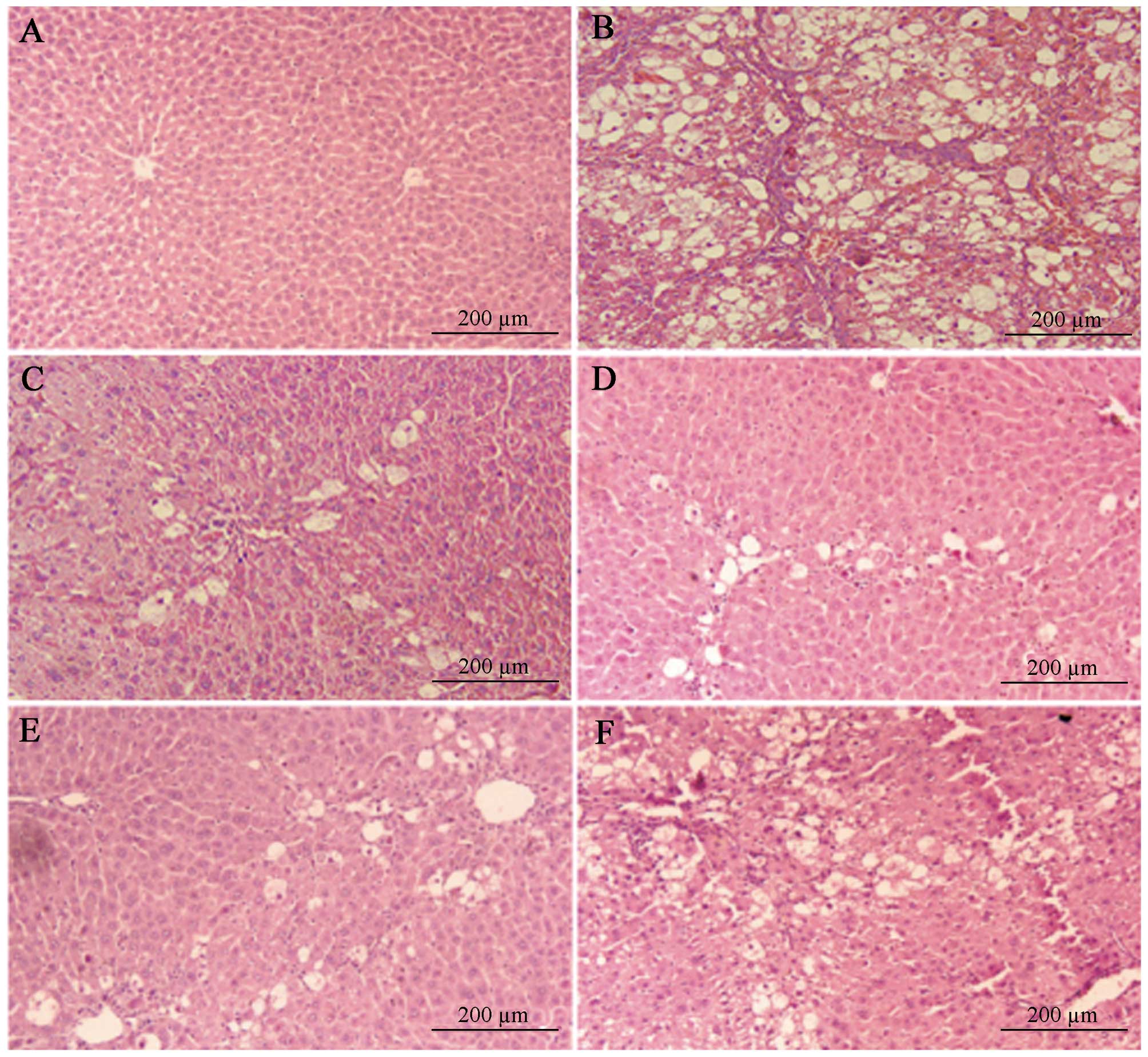
We next investigated the role of NTSD in the
pathological morphology changes in liver tissue in HF rats. As
shown in Fig. 1 and Table III, most of the normal structure
of hepatic lobes in saline treated HF rats (the non-treatment
group) was destroyed or disappeared, large amounts of fat tissue
degenerated and underwent necrosis, which turned into an empty net
with a few liver cells remaining, massive fiber tissue proliferated
around the portal and hepatic necrosis area to form a wide, uneven
thickness and connected fibrous septa, and the HF scores were >3
points, which proved the success of the HF model. Compared with the
non-treatment HF group, NTSD and TSP treatment groups had less
hepatic lobe destruction, reduced fat degeneration, decreased
inflammatory cell infiltrations, mild hyperplasia of collagen
fiber, only thin fiber bundles, no obvious false lobules, and
significantly decreased HF scores (Fig.
2 and Table III). Therefore,
the above results clearly illustrated that both NTSD and TSP could
improve the pathological changes of liver tissue in HF rats, and
the results were consistent with the improvement of liver
function.
 | Table IIIThe effect of NTSD treatment on HF
score in rats. |
Table III
The effect of NTSD treatment on HF
score in rats.
| Rats | Treatment
(g/kg/day) | HF score
|
|---|
| 0 | 1 | 2 | 3 | 4 |
|---|
| Normal | Saline | 10 | 0 | 0 | 0 | 0 |
| Saline | 0 | 0 | 0 | 10 | 5 |
| NTSD | 0 | 8 | 6 | 1 | 0 |
| 28.4 | | | | | |
| HF | 14.2 | 0 | 7 | 7 | 1 | 0 |
| 7.1 | 0 | 8 | 5 | 2 | 0 |
| TSP 0.6 | 0 | 6 | 7 | 2 | 0 |
NTSD-containing serum inhibits the
proliferation of HSC-T6 cells
The activation and proliferation of HSC played a key
role in the occurrence and development of HF (2,4). To
investigate the protection mechanism of NTSD in HF rats, we studied
the effects of NTSD on the growth of HSC-T6 cells in vitro.
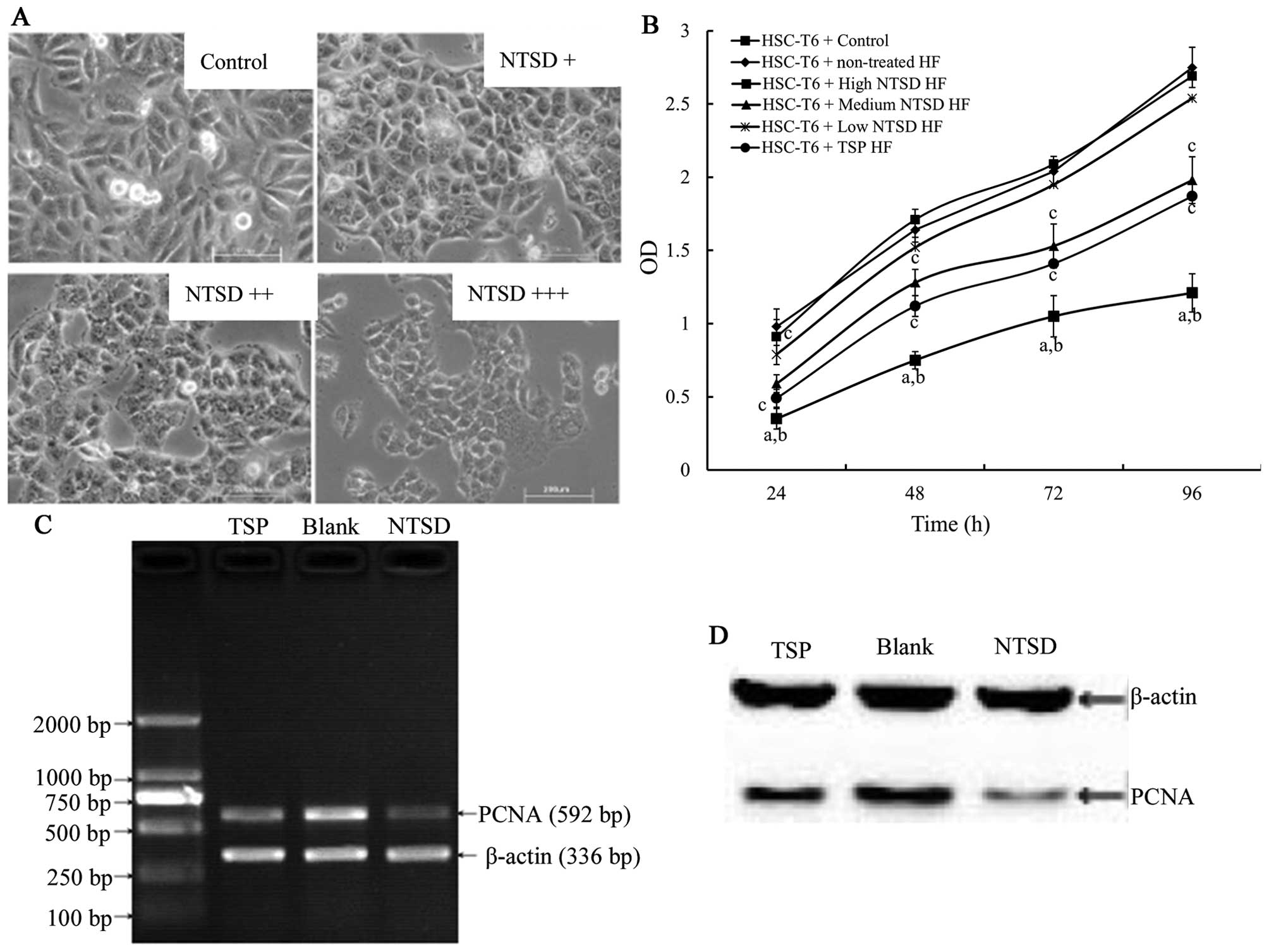
After 72 h of culture in NTSD serum-containing medium, HSC-T6 cells
were slightly smaller and rounder, with a reduced adherent ability,
and this change was dose-dependent (Fig. 3A). CCK-8 assay showed that
NTSD-containing serum inhibited HSC-T6 cell proliferation in a
dose-dependent manner and the inhibitory effect of high dose NTSD
was more effective than TSP-containing serum (Fig. 3B).
RT-PCR and western blotting was used to test for any
PCNA gene expression changes of HSC-T6 after NTSD and
TSP-containing serum treatment. The expression level of PCNA is
closely related to the synthesis of DNA and an indicator reflecting
the state of cell proliferation (16). As shown in Fig. 3C and D, compared with control cells,
both high dose NTSD and TSP-containing serum treated cells showed
decreased transcription levels of mRNA and PCNA protein expression,
with high dose NTSD-containing serum showing the highest effect,
which is consistent with the previous cell proliferation data
(refer to the references cited).
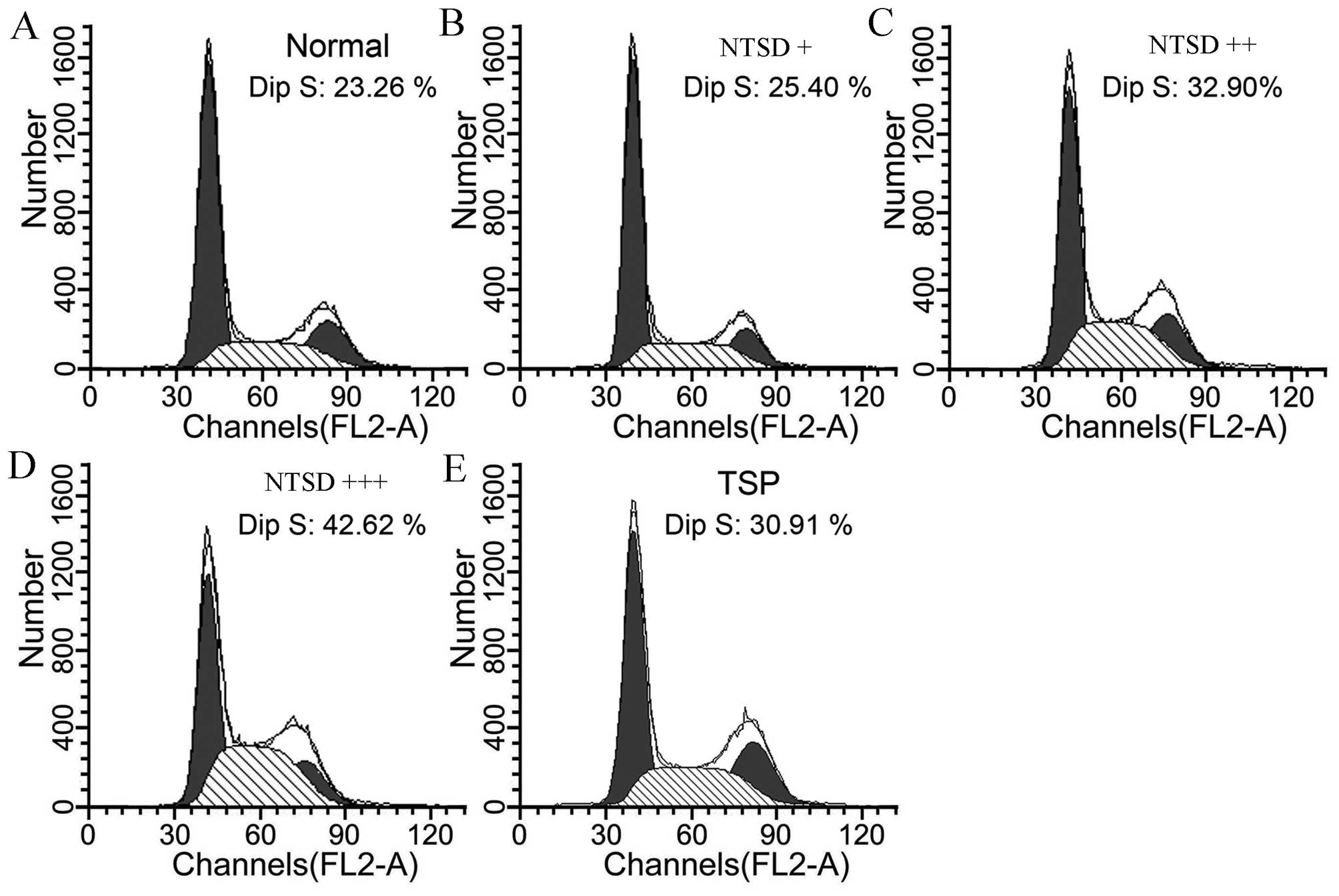
To analyze further the cell proliferation inhibitory
effect of NTSD and TSP, the effects of drugs on the cell cycle of
HSC-T6 was analyzed. Compared with control cells, cells treated by
NTSD and TSP-containing serum for 72 h showed an obvious S-phase
arrest (P<0.05). As shown in Fig.
4, the proportions of S-phase in control cells, low dose NTSD,
medium dose NTSD, high dose NTSD, and TSP-containing serum were
23.26±3.25, 25.40±4.16, 32.90±5.31, 42.62±7.52 and 30.91±4.86%,
respectively. Therefore, both NTSD and TSP effectively induced
S-phase arrest and inhibited the proliferation of HSC-T6 cells
in vitro.
NTSD-containing serum promoted HSC-T6
cell apoptosis
We further studied if the NTSD containing serum
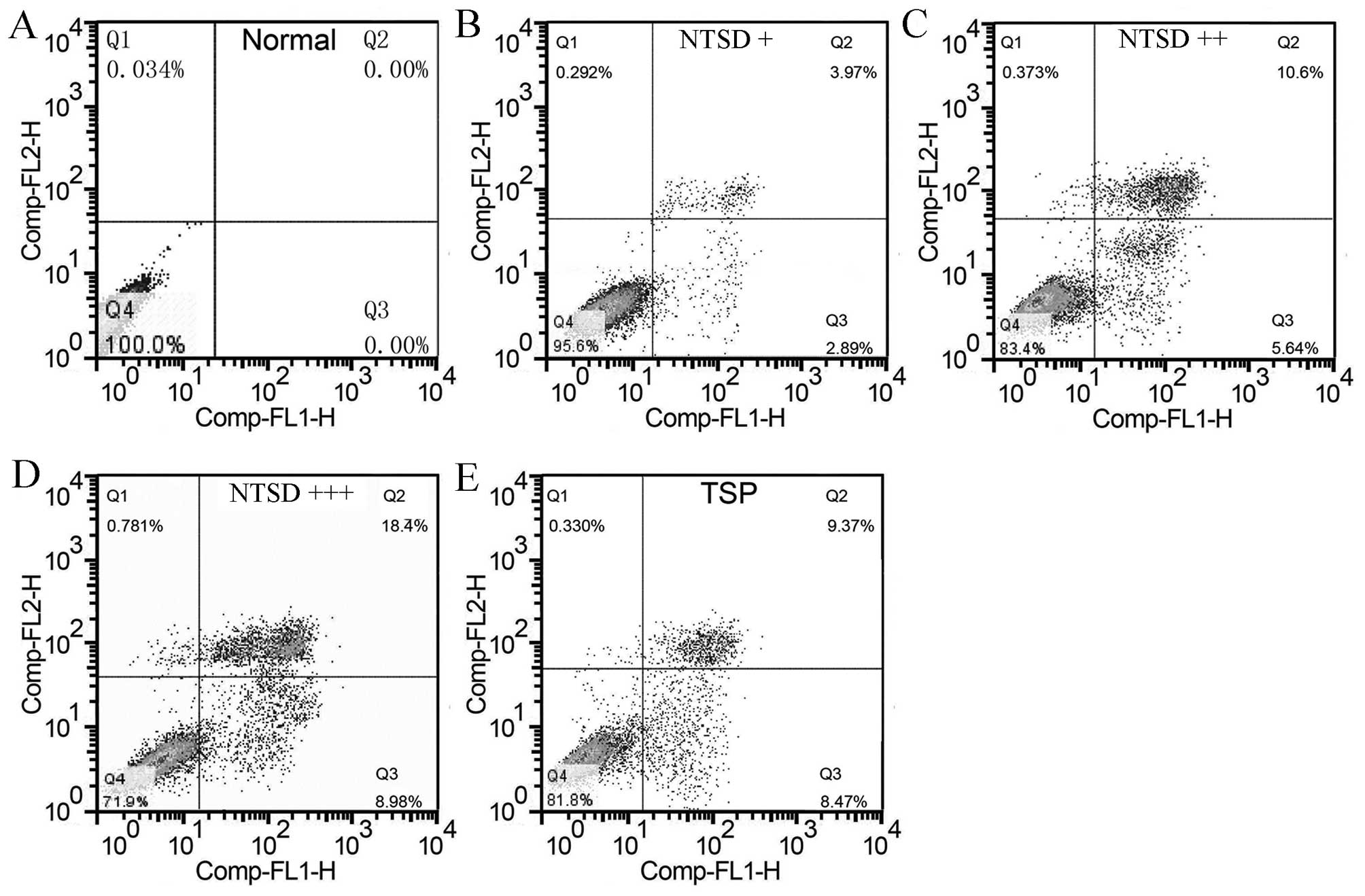
could promote HSC-T6 cell apoptosis. As shown in Fig. 5, 72 h after drug containing serum
treatment, FCM cell apoptosis detection showed that NTSD could
induce HSC-T6 cell apoptosis in a dose-dependent manner, and
percentages of apoptotic cells were 2.89±0.74, 5.64±1.03 and
8.98±1.82%, respectively. Similarly, TSP-containing serum also
significantly promoted apoptosis of HSC-T6 cells, and the apoptosis
percentage was 8.47±1.69% (Fig. 5).
Hence, these data clearly showed that NTSD had the ability to
induce apoptosis of HSC-T6 cells.
NTSD inhibited the TGF-β1/Smad signaling
pathway
The TGF-β1/Smad signaling pathway is one of the most
important signaling pathways that drives the development of HF
(5,17). Therefore, the role of NTSD treatment
on the TGF-β1/Smad signaling pathway of HF rats was investigated.
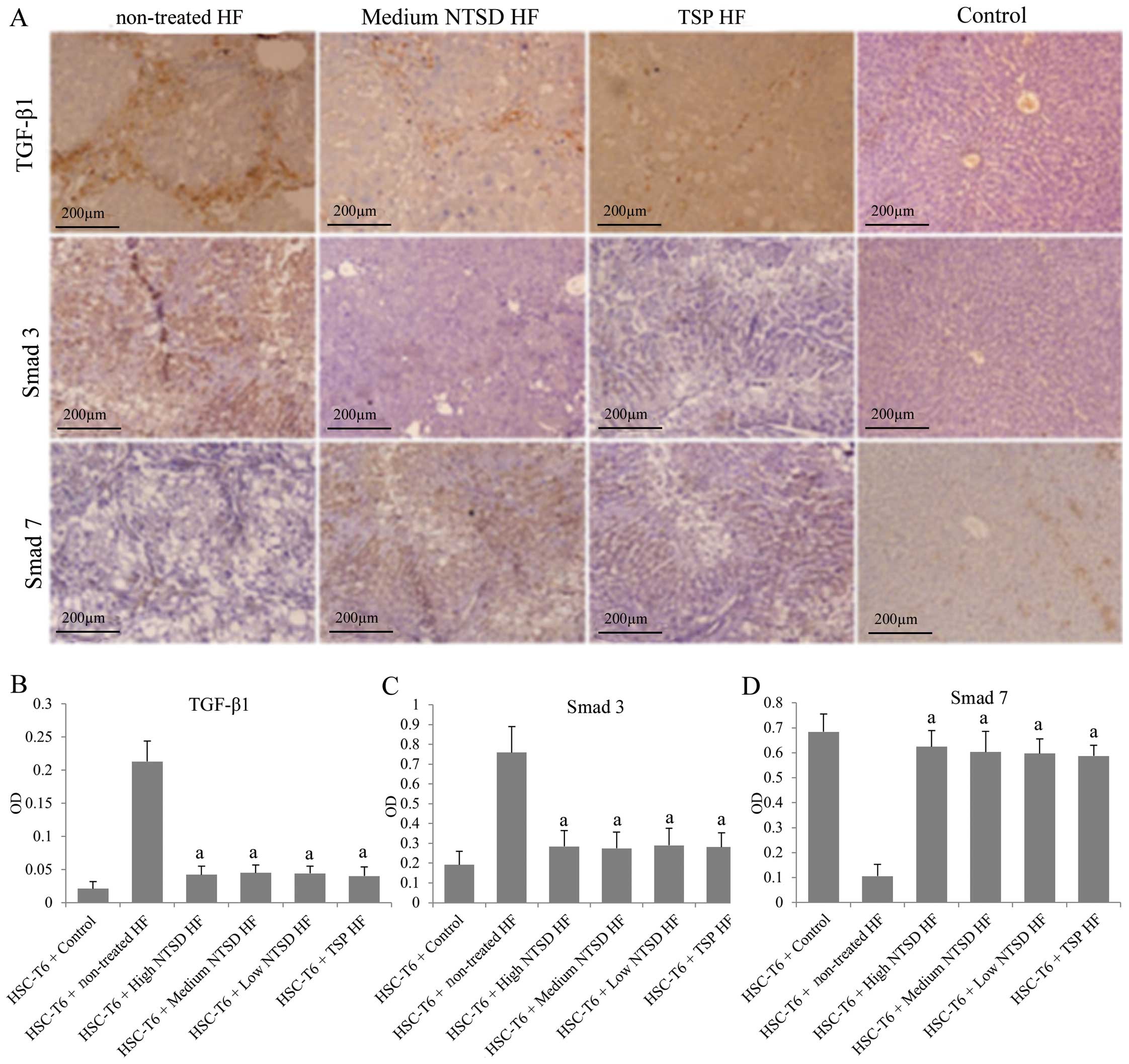
Liver tissue immunohistochemical results showed that compared with
normal rats, the expression of TGF-β1 and Smad3 in the liver of HF
rats was significantly increased, while the expression of Smad7 was
decreased, which supported a role of the TGF-β1/Smad signaling
pathway in promoting the development of HF (Fig. 6). More importantly, low, medium and
high dose NTSD and TSP significantly decreased the TGF-β1 and Smad3
expression levels and increased Smad7 (which is an inhibitory Smad
protein against Smad3) expression, suggesting that NTSD and TSP
could inhibit the TGF-β1/Smad signaling pathway in multiple ways.
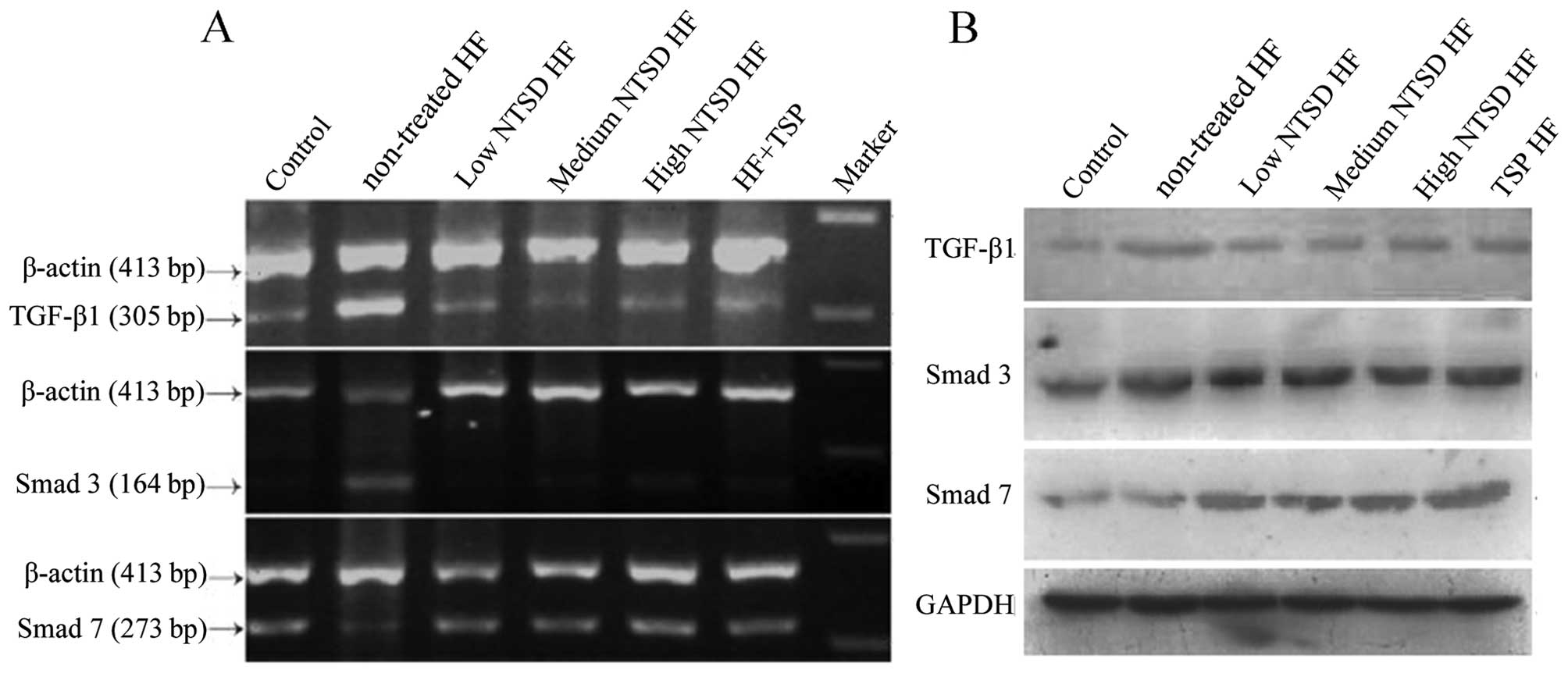
In order to confirm further the changes in the expression of
TGF-β1, Smad3 and Smad7 in the treatment of NTSD and TSP, RT-PCR
and western blotting was used. As shown in Fig. 7, at mRNA and protein levels, changes
were detected in the expression of TGF-β1, Smad3 and Smad7, which
were consistent with the immunohistochemical results. Therefore,
the discovery that NTSD and TSP could significantly inhibit
TGF-β1/Smad signaling pathway, strongly explains their anti-HF
effects.
Discussion
In the present study, the commonly used (in China)
anti-HF drug TSP was modified in order to enhance its availability
as well as to reduce the costs for its production and unwanted
side-effects. The efficacy of the newly developed NTSD was compared
with the conventional TSP medication in a rat HF model and in in
vitro experiments. In the NTSD formula, Radix Astragali, Poria
and Artemisia capillaris were added. We chose these
modifications because Radix Astragali has been shown in previous
studies to downregulate TGF-β/Smad signaling in rat asthma airway
remodeling and HE models (18,19)
and also Poria downregulated TGF-β1 in a rat pulmonary fibrosis
model (20). In addition,
Artemisia capillaris flavonoids were shown to have liver
protection effects in a CCl4 rat model, which was
visible as a reduced serum ALT concentration decrease of 57% in HF
rats (21). Ingredients in both
formulas with previously reported effects on TGF-β1 downregulation
in HF rats and Lx-1 hepatic stellate cells are bupleurum root
(22) and turtle shell (23).
In our first experiment, we found that ALT and AST
serum concentrations in the non-treatment (saline) HF rats were ~17
and 12 times enhanced, respectively compared to the healthy
controls, which is in agreement with previous literature (24,25).
The serum albumin concentrations were reduced, which is also
indicative of HF development as described in a previous study
(26). Serum globulin
concentrations in HF rat sera did not change significantly though
the globulin/albumin ratio was lower (1.26 vs. 0.8) in untreated HF
than in healthy rats, which is in agreement with a previous study
on CCl4-induced HF in rats (27). Treatment with NTSD could alleviate
the HF related ALT increase by 70 and TSP by 77%, and the AST
increase was reduced by 61% by NTSD as well as 53% by TSP. Also the
decrease in serum albumin concentration was significantly lower in
all treated HF rats compared to the non-treated HF animals
(Table II). After liver injury,
HSCs are stimulated by various pathogenic factors from the static
to the proliferation state, which promotes a large amount of ECM
deposition, leading to liver fibrosis (28,29).
Our histopathological findings revealed that pathological liver
cell damage was most obviously visible in non-treated MF rats, but
was essentially reduced in all treated MF rats (Table III and Fig. 2), reflecting the trend of AST and
ALT data. These findings confirmed that NTSD as a treatment for
CCl4-induced HF was as efficient as the conventional TPS
medication.
The abnormal elevation of TGF-β1 expression is
associated with the pathogenesis of many liver diseases, such as
hepatitis virus infection, and TGF-β1 was increased in either the
acute or chronic inflammatory microenvironment (30,31).
NTSD and TSP significantly decreased Smad3 expression levels and
increased Smad7 expression. Smad3 is an important downstream
signaling molecule of TGF-β1, which is closely related to the
activation of HSC (32,33), while Smad7 is a major regulatory
protein that inhibits Smad3, and its overexpression can play a
protective role in HF (8,34). Therefore, the hepatoprotective
function of NTSD and TSP can at least in part be attributed to the
downregulation of TGF-β1/Smad signaling.
Our cell proliferation assay showed that NTSD slowed
down the proliferation of HSC-T6 cells, arrested the cells in the
S-phase as well as induced their apoptosis, which might be
attributed to reduced TGF-β1 expression, since a recent study
reported that TGF-β1 exposed HSC-T6 cells exhibited increased
proliferation and reduced apoptosis due to activation of autophagy
(35).
There are some shortcomings in the present study. We
currently only studied the influence of NTSD on TGF-β1 related
signaling, but not on other mechanisms especially cell protection
factors in HF (e.g. interferon gamma) and NTSD effects on
hepatocytes still needs further research. In addition, potential
reduction in the side-effects of NTSD also requires further
evaluation.
In conclusion, this study provides an improved
anti-HF TCM formula. NTSD could effectively improve the liver
function of CCl4-induced HF in rats and prevent the
destruction of hepatic tissue and the progression of fibrosis. We
found that NTSD promoted HSC apoptosis and inhibited their
proliferation and thus proved our hypothesis that NTSD
downregulated the expression of TGF-β1 and Smad3, as well as
increased the expression of Smad7, thus inhibiting the role of
TGF-β1/Smad signaling pathway in promoting the secretion of ECM by
activated HSCs.
Acknowledgments
The present study was supported by the National
Natural Science Foundation (81273918), the Project of Traditional
Chinese Medicine Science and Technology of Chongqing (2012-2-63)
and the Specific Projiect of Traditional Chinese Medicine of
Chinese PLA (2010ZYZ231), China.
References
|
1
|
Mortality GBD: Global, regional, and
national age-sex specific all-cause and cause-specific mortality
for 240 causes of death, 1990–2013: A systematic analysis for the
Global Burden of Disease Study 2013. Lancet. 385:117–171. 2015.
View Article : Google Scholar
|
|
2
|
Henderson NC and Iredale JP: Liver
fibrosis: Cellular mechanisms of progression and resolution. Clin
Sci (Lond). 112:265–280. 2007. View Article : Google Scholar
|
|
3
|
Parsons CJ, Takashima M and Rippe RA:
Molecular mechanisms of hepatic fibrogenesis. J Gastroenterol
Hepatol. 22(Suppl 1): S79–S84. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsukada S, Parsons CJ and Rippe RA:
Mechanisms of liver fibrosis. Clin Chim Acta. 364:33–60. 2006.
View Article : Google Scholar
|
|
5
|
Cui W, Jin HB and Li ZW: Mechanism of the
transforming growth factor-beta induction of fibronectin expression
in hepatic stem-like cells. Braz J Med Biol Res. 43:36–42. 2010.
View Article : Google Scholar
|
|
6
|
Yoshida K, Murata M, Yamaguchi T and
Matsuzaki K: TGF-β/Smad signaling during hepatic
fibro-carcinogenesis (Review). Int J Oncol. 45:1363–1371.
2014.PubMed/NCBI
|
|
7
|
Kaimori A, Potter J, Kaimori JY, Wang C,
Mezey E and Koteish A: Transforming growth factor-beta1 induces an
epithelial-to-mesenchymal transition state in mouse hepatocytes in
vitro. J Biol Chem. 282:22089–22101. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tahashi Y, Matsuzaki K, Date M, Yoshida K,
Furukawa F, Sugano Y, Matsushita M, Himeno Y, Inagaki Y and Inoue
K: Differential regulation of TGF-beta signal in hepatic stellate
cells between acute and chronic rat liver injury. Hepatology.
35:49–61. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wells RG: Cellular sources of
extracellular matrix in hepatic fibrosis. Clin Liver Dis.
12:759–768. viii2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Q and Jin S: The modern clinical
application and experimental research progress of turtule shell
pills. J Hebei Tradit Chin Med Pharmacol. 21:35–36. 2006.
|
|
11
|
Lin W, Wei N, Gao B, Jiang G and Chang Y:
Systematic evaluation of therapeutic effect of compound turtule
shell pills against hepatic fibrosis. Chin J Gastroenterol Hepatol.
16:69–72. 2007.
|
|
12
|
Gao J, Tao J and Zhao C: The experimental
study of turtle shell pills in prevention and treatment of hepatic
fibrosis. Chin Arch Tradit Chin Med. 11:2462–2471. 2008.
|
|
13
|
Xi Z, Luan X and Li K: The study of
dormant insect on inhibiting immune hepatic fibrosis in rats.
Tradit Chin Med Res. 17:38–40. 2001.
|
|
14
|
Xu L and Liu P: The observation of effects
on anti-hepatic fibrosis of peach extract-the study of
immunohistochemistry and collagen metabollism. Tradit Chin Med Res.
5:14–16. 1993.
|
|
15
|
Lu Y, Ren X and Chen Y: Dynamic
observation of the effects of turtle shell pills on liver collgen
and serum pre-collagen III during the hepatic fibrosis process in
rats. Henan Tradit Chin Med. 21:192001.
|
|
16
|
Moldovan GL, Pfander B and Jentsch S:
PCNA, the maestro of the replication fork. Cell. 129:665–679. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gressner AM, Weiskirchen R, Breitkopf K
and Dooley S: Roles of TGF-beta in hepatic fibrosis. Front Biosci.
7:d793–d807. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dai H, Zhang WX, He XL, Zhao RX, Fang L
and Li CC: Effect of TGF-B1/Smad3 aignal pathway on airway
remodelling in asthma rats with the regulation of Radix Astragali.
Chin Arch Tradit Chin Med. 28:2494–2498. 2010.
|
|
19
|
Huang J, Zhang C, Zhan F and Zhang J:
Effects of astragalan on liver fibrosis rat in TGF-β1/Smads signal
pathway. China J Tradit Chin Med Pharm. 30:2184–2186. 2015.
|
|
20
|
Jiang W, Zhou ZS, Hu HB and Liu BJ: The
Effect of Fuling, Yiyiren and Dongguazi on serum TGF-β1 and TNF-α
levels in rats with pulmonary fibrosis. Med J Qilu. 28:237–240.
2013.
|
|
21
|
Niu SL, Wu ZL and Yao JJ: Hepatoprotective
effect of Artemisia capillaris thumb flavones extract on chronic
liver in-jury induced by carbon tetrachloride in rats. Med J Chin
People's Armed Police Forces. 26:162–166. 2015.
|
|
22
|
Shang LZ, Wang F, Wang Q, et al: Effects
and mechanism of chaihu shugan powder on TGF-β1/Smad signaling
pathways in hepatic fibrosis model rats. Chin J Exp Tradit Med
Formulae. 21:125–128. 2015.
|
|
23
|
Gao JR, Yao HP, Liu YW, et al: Turtle
carapace decoction and drug containing serum on hepatic stellate
cells. Chin Arch Tradit Chin Med. 31:2524–2528. 2013.
|
|
24
|
Zechini B, Pasquazzi C and Aceti A:
Correlation of serum aminotransferases with HCV RNA levels and
histological findings in patients with chronic hepatitis C: The
role of serum aspartate transaminase in the evaluation of disease
progression. Eur J Gastroenterol Hepatol. 16:891–896. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pradat P, Alberti A, Poynard T, Esteban
JI, Weiland O, Marcellin P, Badalamenti S and Trépo C: Predictive
value of ALT levels for histologic findings in chronic hepatitis C:
A European collaborative study. Hepatology. 36:973–977. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Natsume M, Tsuji H, Harada A, Akiyama M,
Yano T, Ishikura H, Nakanishi I, Matsushima K, Kaneko S and Mukaida
N: Attenuated liver fibrosis and depressed serum albumin levels in
carbon tetrachloride-treated IL-6-deficient mice. J Leukoc Biol.
66:601–608. 1999.PubMed/NCBI
|
|
27
|
Hassan EM, El-Kherbawy GM, Ali MAM and
Dewidar OM: The potential effect of special formulas on cirrhotic
rats. Food Nutr Sci. 4:594–603. 2013. View Article : Google Scholar
|
|
28
|
Soon RK Jr and Yee HF Jr: Stellate cell
contraction: Role, regulation, and potential therapeutic target.
Clin Liver Dis. 12:791–803. viii2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Marra F: Hepatic stellate cells and the
regulation of liver inflammation. J Hepatol. 31:1120–1130. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kanzler S, Baumann M, Schirmacher P, Dries
V, Bayer E, Gerken G, Dienes HP and Lohse AW: Prediction of
progressive liver fibrosis in hepatitis C infection by serum and
tissue levels of transforming growth factor-beta. J Viral Hepat.
8:430–437. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Date M, Matsuzaki K, Matsushita M, Tahashi
Y, Furukawa F and Inoue K: Modulation of transforming growth factor
beta function in hepatocytes and hepatic stellate cells in rat
liver injury. Gut. 46:719–724. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Flanders KC: Smad3 as a mediator of the
fibrotic response. Int J Exp Pathol. 85:47–64. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Latella G, Vetuschi A, Sferra R, Catitti
V, D'Angelo A, Zanninelli G, Flanders KC and Gaudio E: Targeted
disruption of Smad3 confers resistance to the development of
dimethylnitrosamine-induced hepatic fibrosis in mice. Liver Int.
29:997–1009. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dooley S, Hamzavi J, Breitkopf K,
Wiercinska E, Said HM, Lorenzen J, Ten Dijke P and Gressner AM:
Smad7 prevents activation of hepatic stellate cells and liver
fibrosis in rats. Gastroenterology. 125:178–191. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fu MY, He YJ, Lv X, Liu ZH, Shen Y, Ye GR,
Deng YM and Shu JC: Transforming growth factor-β1 reduces apoptosis
via autophagy activation in hepatic stellate cells. Mol Med Rep.
10:1282–1288. 2014.PubMed/NCBI
|