Introduction
Cancer cachexia is a complicated multifactorial
syndrome featuring a progressive loss of skeletal muscle mass (with
or without fat loss) that is associated with significant functional
impairments (1). Cachexia occurs in
~60% of patients with advanced cancer and up to 80% of those in a
terminal stage (2,3). The development of cachexia is divided
into three stages: pre-cachexia, cachexia, and refractory cachexia.
Cachexia significantly and adversely impacts the patient responses
to treatment, quality of life, and survival. Cachexia is
responsible for 20–30% of all cancer-related deaths (2). No treatments effectively alleviating
cachexia have been developed to date, and thus, it is essential to
explore its pathogenesis and underlying mechanisms, and develop
therapeutic approaches targeting these mechanisms.
Muscle wasting is a prominent feature of cachexia
and is closely associated with increased protein degradation.
Studies have identified several mechanisms modulating protein
degradation in muscles. One is the renin-angiotensin system (RAS):
although most commonly associated with vasoconstriction and the
regulation of blood pressure, angiotensin peptides angiotensin I
(Ang I) and II are produced within skeletal muscle and directly or
indirectly inhibit protein synthesis (4), promote protein degradation (5), stimulate cytokine production (6,7), and
generate hydroxyl radicals (•OH) to exacerbate oxidative stress
(8,9). Consistent with its functional
consequences, the RAS is noted to be activated in conditions
involving muscle wasting, including sarcopenia, chronic heart
failure, chronic renal failure, and cancer (10–13).
Our previous study as well as that from Murphy et al
(14), demonstrated the
significance of the RAS in cancer cachexia (15), corroborating the potential of a RAS
inhibitor/antagonist in treating cancer cachexia. A second
mechanism is the autophagy-lysosome pathway (ALP) and
ubiquitin-proteasome pathway (UPP): both are major pathways
responsible for muscular protein degradation (16,17).
Examples of genes critical for regulating these two machineries in
muscles include Bnip3, Beclin1, and LC3, which are involved in
different steps of autophagosome formation, and two muscle-specific
ubiquitin E3 ligases, Atrogin1 and MuRF1 (17).
In this study, we examined the efficacy of a novel
direct renin inhibitor, aliskiren, on cancer cachexia. Previous
studies showed that aliskiren can potently inhibit inflammation,
reduce oxidative stress, and protect against tissue damage
(18–20). However, minimal information is
available on its actions in cancer cachexia. To address this issue,
we established a cancer cachexia mouse model, assessed the
prophylactic as well as therapeutic effects of aliskiren on cancer
cachexia, and explored the molecular mechanisms underlying these
effects.
Materials and methods
Ethics statement
The experimental protocols involving human samples
were established according to the ethical guidelines of the
Helsinki Declaration and approved by the Ethics Committee of
Chongqing Medical University (Chongqing, China). The experimental
protocols involving mice were approved by the Institutional Animal
Care and Use Committee (IACUC) of Chongqing Medical University.
Cells and reagents
C26 mouse colon carcinoma cells were provided by the
Research Center (the First Affiliated Hospital of Chongqing Medical
University, Chongqing, China) and cultured in Dulbecco's Modified
Eagle Medium (DMEM)-F12 supplemented with 10% fetal bovine serum
(FBS; both from Hyclone, Logan, UT, USA).
Aliskiren was purchased from Novartis (San Diego,
CA, USA). The following antibodies were used in this study:
anti-Bnip1 (ab38621), anti-LC3 (ab168831; Abcam, Cambridge, MA,
USA), anti-MuRF1 (sc-32920), anti-Atrogin1 (sc-33782; Santa Cruz
Biotechnology, Santa Cruz, CA, USA), anti-Beclin1 (11306-1-AP),
anti-GAPDH (10494-1-AP), and peroxidase-conjugated anti-rabbit IgG
(SA00001-2; Proteintech, Wuhan, China). A real-time polymerase
chain reaction (PCR) kit was purchased from Takara (Dalian, China),
and a western immunoblot kit was purchased from Beyotime (Jiangsu,
China).
Experimental animals and cachexia
model
Healthy male BALB/c mice between 7 and 9 weeks of
age and with a body weight (BW) of 21–26 g were purchased from the
Experimental Animal Center (Chongqing Medical University). All mice
were housed in a specific-pathogen-free facility at room
temperature of 22±1°C on a 12/12-h light/dark cycle with access to
food and water ad libitum.
To induce cancer cachexia, C26 cells growing in log
phase were detached and resuspended as single cells in PBS at
1×107/ml, and 100 µl C26 cell suspension was
injected subcutaneously into the left axilla of each mouse (N=48).
In healthy controls (HC group), 100 µl PBS was injected into
the mice (n=16). Mice receiving C26 injection were further divided
into three groups (n=16/group): i) cancer-only group (CA): mice
received PBS intragastrically on days 5 and 12 after C26 injection;
ii) aliskiren prevention group (AP): mice received aliskiren (10
mg/kg BW) in PBS intragastrically on day 5 after C26 injection,
when tumor nodules were palpable; and iii) aliskiren treatment
group (AT): mice received aliskiren (10 mg/kg BW) in PBS
intragastrically on day 12 after C26 injection, when cachexia was
well-developed. Following C26 cell injection, all mice were
monitored daily for the appearance of skin and hair, mental state,
BW, and tumor growth until day 20, when eight mice from each group
were sacrificed by CO2 euthanasia followed by cervical
dislocation for sample collection. The tumor volume was calculated
as V = ab2/2, where V is the tumor volume and a and b
are the length and width of the tumor, respectively. The other
eight from each group were continued to be monitored daily and used
to assess whole-body functions (as described below) and survival
time. According to the IACUC guidelines of Chongqing Medical
University, mice were euthanized by CO2 asphyxiation
followed by cervical dislocation when they presented signs of
moribund conditions, including decreased response to stimuli,
inability to remain upright, abnormal feeding behavior, evidence of
severe muscle atrophy, and rough hair coat.
Assessment of grip strength,
coordination, and locomotor activity
Whole body strength and coordination was assessed on
day 20 after C26 cell injection using a TSE Grip Strength Meter and
the rotarod performance test RotaRod Advanced (both from TSE
Systems, Germany) as previously described (21–23).
Briefly, to measure grip strength, the mouse was allowed to hold on
firmly to a grip that was mounted to a high-precision force sensor,
and its tail was pulled backwards with a continuous movement until
the grip was broken. The force value at this point was recorded.
Ten measurements were taken for each animal within 2 min, and the
maximum values were used for statistical analysis. For rotarod
performance test, the mouse was placed on a horizontal rod that
rotated from a starting speed of 4 rpm and accelerated at 1 rpm/8
sec until the mouse fell off the rod. The latency-to-fall was
recorded. The test was repeated three times within 15 min for each
animal, and the longest latency-to-fall was used for statistical
analysis.
Locomotor activity was monitored using an infrared
monitoring system (WV-CF314LCH; Panasonic, Japan) following the
manufacturer's instructions.
Sample collection
On day 20 after C26 inoculation, eight mice from
each group were sacrificed, with blood immediately collected from
the retro-orbital venous sinus. The blood samples were allowed to
sit at 4°C for 1 h, which was followed by centrifugation at 1,006 ×
g for 10 min at 4°C. The serum supernatant was then collected and
stored at −80°C until future use. In addition to the serum sample,
the tumor tissue as well as the bilateral gastrocnemius muscles
were isolated and weighed. The left gastrocnemius muscle was fixed
in 4% paraformaldehyde, and the right one was flash frozen and
stored in liquid nitrogen until further use.
Measurement of cross-sectional area of
gastrocnemius muscle
After 48 h of fixation in paraformaldehyde, the
gastrocnemius muscle was embedded in paraffin, sectioned onto glass
slides, and stained with hematoxylin and eosin (H&E)
(Beyotime). Three slides for each sample were imaged under an
optical microscope (×200 magnification, BX51TF; Olympus, Tokyo,
Japan), with five random fields imaged for each slide, and the
cross-cut area of the gastrocnemius muscle was measured using
Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD,
USA).
Determination of serum Ang I and II
levels and both serum and muscular tumor necrosis factor-α (TNF-α)
and interleukin-6 (IL-6) levels
To prepare muscle lysates, flash frozen muscles were
suspended in buffer containing 10 mM HEPES (pH 7.9), 10 mM KCl, 2
mM MgCl2, 0.1 mM EDTA (pH 8.0), 1.0 mM DTT, 10% NP-40,
and 0.5 mM phenylmethylsulfonyl fluoride (PMSF), and immediately
homogenized on ice. The homogenized muscle tissue was centrifuged
at 16,099 × g for 10 min at 4°C, and the supernatant was collected
for future use. The levels of Ang I and II in serum and those of
TNF-α and IL-6 in serum and in gastrocnemius muscle were determined
using enzyme-linked immunosorbent assay (ELISA) kits (R&D
Systems, Minneapolis, MN, USA) following the manufacturer's
instructions.
Evaluation of oxidative stress in
gastrocnemius muscle
To assess the oxidative stress level in
gastrocnemius muscles, the activities of superoxide dismutase (SOD)
and glutathione peroxidase (GSH-Px) and the levels of •OH and
malondialdehyde (MDA) were determined using the corresponding test
kits (Nanjing Jiancheng Biological Engineering Institute, Nanjing,
China) following the manufacturer's instructions.
Real-time PCR analysis
Total RNA was extracted from flash frozen
gastrocnemius muscles using TRIzol reagent (Takara) according to
the manufacturer's instructions and reversed transcribed into cDNA.
The real-time PCR reaction was set up using SYBR Premix Ex Taq II
kit (Takara) and performed on an ABI Prism 7500 Sequence Detection
System (Applied Biosystems, Foster City, CA, USA). The primer
sequences used for real-time PCR analysis are listed in Table I. GAPDH was used as the internal
control. The quantification of real-time PCR data was performed
using the 2−ΔΔCt method, as previously described
(24).
 | Table IPrimer sequences used in real-time
polymerase chain reaction (PCR) analysis. |
Table I
Primer sequences used in real-time
polymerase chain reaction (PCR) analysis.
| Gene | Sense primer
(5′→3′) | Antisense primer
(5′→3′) |
|---|
| Bnip3 |
5′-GGGTTTTCCCCAAAGGAATA-3′ |
5′-TGACCACCCAAGGTAATGGT-3′ |
| LC3 |
5′-CGGCTTCCTGTACATGGTTT-3′ | 5′-ATGT
GGGTGCCTACGTTCTC-3′ |
| Beclin1 |
5′-ATGGAGGGGTCTAAGGCGTC-3′ |
5′-TGGGCTGTGGTAAGTAATGGA-3′ |
| MuRF1 |
5′-GGAACACGAAGACGAGAAAATC-3′ |
5′-TGGCTATTCTCCTTGGTCACTC-3′ |
| Atrogin1 |
5′-GAAGAGAGCAGTATGGGGTCAC-3′ |
5′-CTTGAGGGGAAAGRGAGACG-3′ |
| Caspase-3 |
5′-TGGGACTGATGAGGAGATGGC-3′ |
5′-TGCTGCAAAGGGACTGGATG-3′ |
| GAPDH |
5′-GGTGAAGGTCGGTGTGAACG-3′ |
5′-CTCGCTCCTGGAAGATGGTG-3′ |
Western blotting
Total proteins from the flash frozen gastrocnemius
muscle samples were extracted using radioimmunoprecipitation assay
(RIPA)/PMSF lysis buffer (Beyotime). Equal amounts of total protein
from different samples were separated by 10% sodium dodecyl sulfate
(SDS)-polyacrylamide gel electrophoresis (PAGE), transferred to
polyvinylidene fluoride (PVDF) membrane (Beyotime), probed with
primary antibodies at 4°C overnight, followed by incubation with
corresponding secondary antibodies. Signal detection and
quantification were achieved using the UVP Bioimaging System (UVP,
Inc., Upland, CA, USA). The relative protein level was expressed as
the ratio of signal density of a target protein to that of the
internal control protein (GAPDH).
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). Quantitative data were
expressed as means ± standard deviation (SD). Differences between
two groups were analyzed using Student-Newman-Keuls q-test, and
those among multiple groups were analyzed using one-way analysis of
variance (ANOVA). Survival analysis was performed using the
log-rank test. P<0.05 was considered statistically
significant.
Results
Aliskiren delays cachexia development,
reduces tumor burden, and prolongs mouse survival
To examine the effects of aliskiren on cancer
cachexia and the underlying mechanisms, we subcutaneously injected
C26 colon cancer cells into isogenic BALB/c mice, a
well-established experimental model of cancer cachexia (25). On day 5 after C26 cell inoculation,
although tumor nodules were palpable through the skin, no
significant differences in BW were noticed among the four groups of
mice. On day 12, mice in the CA and AT groups showed obvious signs
of cachexia, including rough skin, disheveled fur, reduced
motility, and dramatic weight loss (P<0.05, compared with mice
in the HC group). By day 20, mice in the CA group presented the
most dramatic weight loss (P<0.01, compared with mice in the HC
group), followed by those in the AT group (P<0.01, compared with
mice in the HC group; P<0.05, compared with mice in the CA
group), and the least weight loss was observed in mice of the AP
group (P<0.01, compared with mice in the CA group; P<0.05,
compared with mice in the AT group; Fig. 1A). Consistently, the percentage
decrease in tumor-free body mass from day 0 to 20 was most
significant in mice in the CA group (32.67%), and this percentage
was significantly higher than that in the AT group (21.21%;
P<0.05, compared with the CA group) or the AP group (12.42%;
P<0.01, compared with the CA group; P<0.05, compared with the
AT group; Fig. 1B).
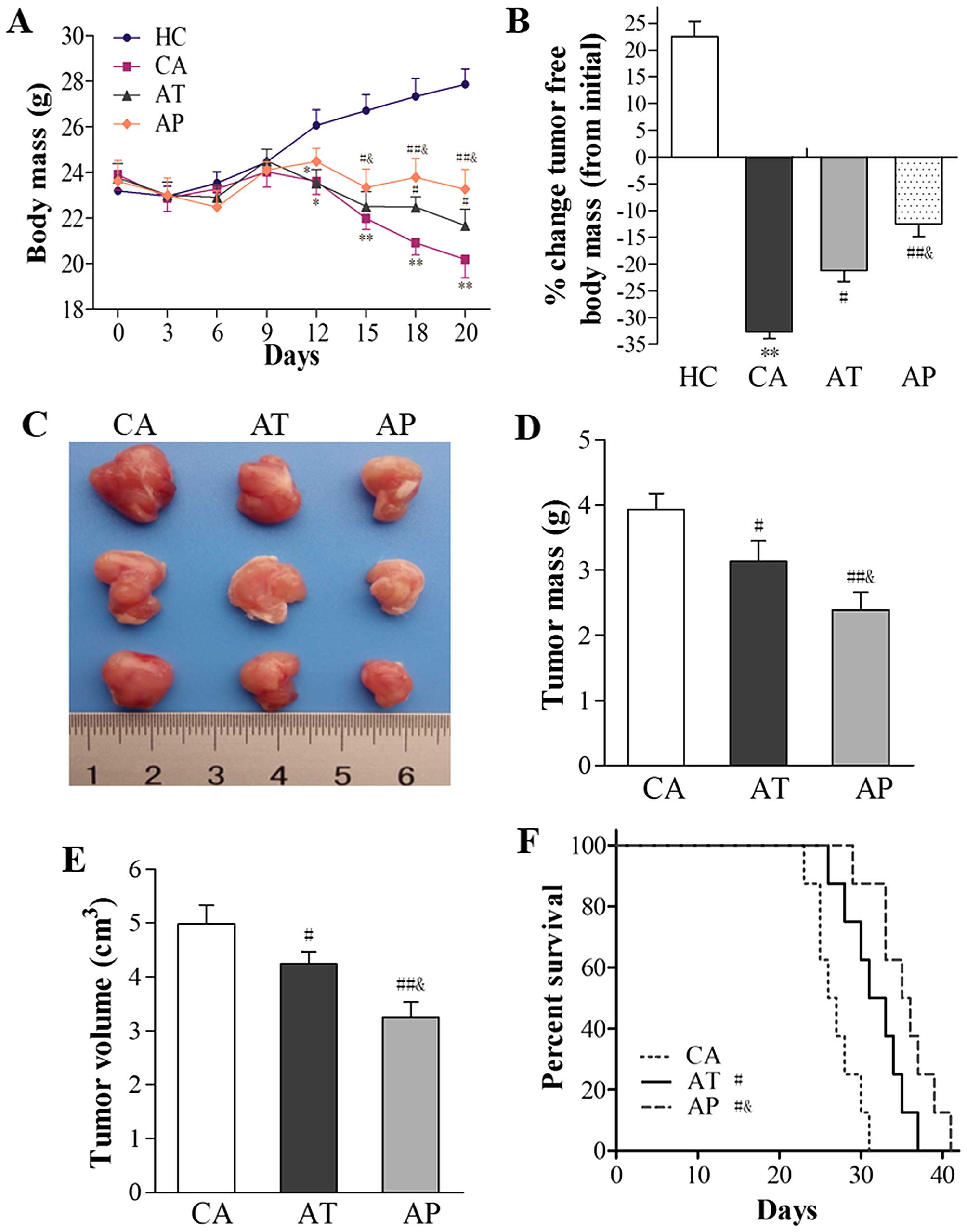 | Figure 1Aliskiren delays cachexia
development, reduces tumor burden, and prolongs mouse survival. C26
cells were subcutaneously inoculated into BALB/c mice without any
interference (n=16; CA group), those that received early
intervention with aliskiren (administered on day 5 after C26
inoculation; n=16; AP group), and those that received aliskiren as
a therapeutic strategy (administered on day 12 after C26
inoculation; n=16; AT group). As healthy control, PBS was injected
subcutaneously into BALB/c mice (n=16; HC group). (A) The body mass
following C26 inoculation was monitored daily and compared among
the four groups. (B) On day 20 after C26 inoculation, eight mice
from each group were sacrificed and tumors were isolated. The
percent change in tumor-free body mass between day 20 and 0 was
compared among all four groups. (C) Representative gross images of
tumors isolated from mice in the CA, AT, and AP groups are shown.
(D) Tumor mass and (E) tumor volume on day 20 were measured and
compared among the CA, AT, and AP groups. (F) Survival rates of
mice in the CA, AT, and AP groups were analyzed and presented in
Kaplan-Meier curves. *P<0.05, **P<0.01,
compared with the HC group; #P<0.05,
##P<0.01, compared with the CA group;
&P<0.05, compared with the AT group. |
With respect to tumor growth, aliskiren as a
treatment agent (as in the AT group) or an early prevention
strategy (as in the AP group) led to reductions in both tumor mass
and tumor volume (P<0.05, compared with those in the CA group),
with the most robust antitumor effects observed in the AP group
(P<0.01, compared with mice in the CA group; P<0.05, compared
with mice in the AT group; Fig.
1C–E).
Functionally, aliskiren resulted in significantly
improved survival in the AT and AP groups (P<0.05, compared with
the CA group), and the best survival was observed in the AP group
(P<0.05, compared to the CA group; P<0.05, compared to the AT
group; Fig. 1F).
Aliskiren improves whole-body strength,
mobility and coordination, enhances locomotor activity, and
inhibits muscle wasting
Whole-body strength was assessed on day 20 using a
grip strength meter and mobility and coordination were assessed
using the rotarod test. As shown in Fig. 3A and B, both grip strength and
rotarod performance were significantly reduced in the CA group
(P<0.01, compared with the HC group). Treatment with aliskiren
on day 12 (AT group) improved grip strength and the latency-to-fall
during the rotarod test by 21.17 and 16.98%, respectively
(P<0.05, compared with the CA group), and prevention using
aliskiren on day 5 (AP group) improved these two parameters by
39.06 and 34.56%, respectively (P<0.01, compared with the CA
group; P<0.05, compared with the AT group). Similar alterations
in locomotor activity also were noticed in different groups; that
is, it was dramatically reduced in the CA group (P<0.01,
compared with the HC group), but was enhanced following aliskiren
interference (P<0.05, comparing the AT with the CA group;
P<0.01, comparing the AP and CA groups), and the best locomotor
activity among tumor-bearing mice was observed in the AP group
(P<0.05, compared with the AT group; Fig. 2C).
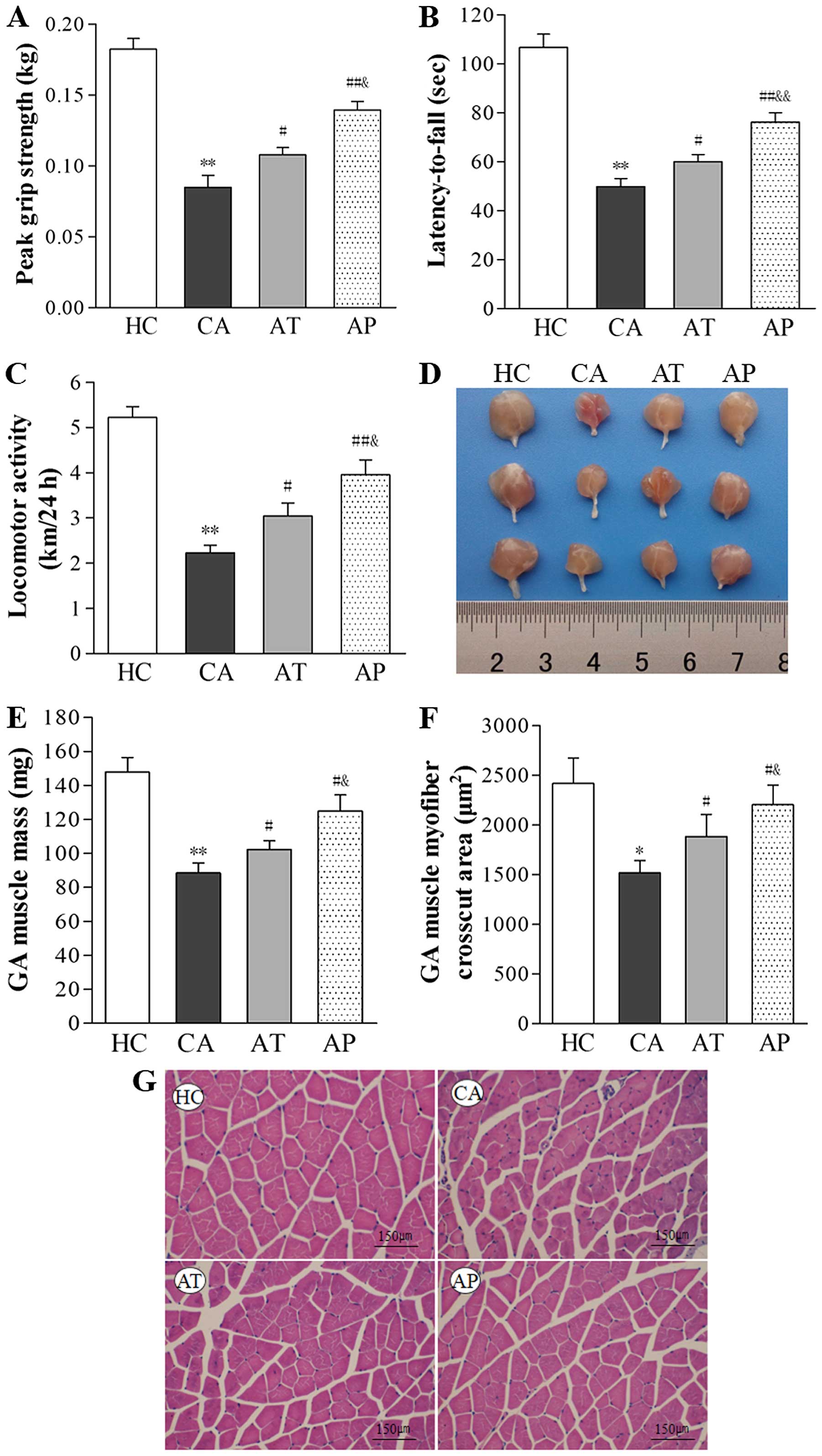 | Figure 2Aliskiren improves whole body
strength, mobility, and coordination, enhances locomotor activity,
and inhibits muscle wasting. (A) Whole-body grip strength was
measured using a grip strength meter, with the maximal value (kg)
of five repeats (peak grip strength) for each mouse recorded for
data analysis. (B) Whole-body mobility and coordination were
assessed using the rotarod performance test, with longest the
latency-to-fall (s) value of three repeats for each mouse recorded
for data analysis. (C) Locomotor activity was measured over 24 h
for each mouse (km/24 h). (D) Representative gross images of
isolated gastrocnemius muscle are shown. (E) The mass of
gastrocnemius muscle on day 20 after C26 inoculation was measured
and compared between the indicated groups. (F) The cross-cut area
of the gasctrocnemius muscle was measured on hematoxylin and eosin
(H&E)-stained sections and compared between the indicated
groups. (G) Representative images of H&E staining (×200
magnification) of gastrocnemius muscle from the indicated groups.
*P<0.05, **P<0.01, compared with the HC
group; #P<0.05, ##P<0.01, compared with
the CA group; &P<0.05,
&&P<0.01, compared with the AT group. |
On the histological level, C26 inoculation resulted
in marked wasting in skeletal muscles, as represented by reductions
in gastrocnemius mass by 40.11% and in the cross-cut area of
gastrocnemius muscle by 36.23% (P<0.05, compared with the HC
group). In contrast, aliskiren increased gastrocnemius mass by
15.47% in the AT group (P<0.05, compared with the CA group) and
41.14% in the AP group (P<0.05, compared with the CA group;
P<0.01, compared with the AT group), respectively. It also
increased the cross-cut area of gastrocnemius muscle by 24.12% in
the AT group (P<0.05, compared with the CA group) and 41.14% in
the AP group (P<0.05, compared with the CA group; P<0.01,
compared with the AT group; Fig.
2D–G).
Aliskiren inhibits the RAS and controls
the upregulation of pro-inflammatory cytokines TNF-α and IL-6 in
both serum and skeletal muscles
To evaluate the effects of aliskiren on the RAS, we
measured the serum levels of both Ang I and II, as shown in
Table II. The development of
cachexia in CA mice was associated with marked RAS activation, as
demonstrated by the dramatic elevation of both Ang I and II in
serum (P<0.01, compared with the HC group). In contrast,
treatment with aliskiren, either before (AP group) or after (AT
group) the development of cachexia, led to serum upregulation of
Ang I and II to a much lower degree, with less upregulation
observed in the AP group than in the AT group (P<0.05).
 | Table IIChanges in Ang I and II levels in
serum and TNF-α and IL-6 levels in serum and gastrocnemius of mice
in the indicated groups (n=8/group, means ± SD). |
Table II
Changes in Ang I and II levels in
serum and TNF-α and IL-6 levels in serum and gastrocnemius of mice
in the indicated groups (n=8/group, means ± SD).
| Group | Ang I (ng/l) | Ang II (ng/l) | Serum (ng/l)
| Gastrocnemius
(pg/mg)
|
|---|
| TNF-α | IL-6 | TNF-α | IL-6 |
|---|
| HC | 9.62±1.08 | 33.62±2.42 | 14.18±2.01 | 2.65±0.54 | 7.24±1.74 | 1.27±0.61 |
| CA | 25.73±1.11b | 78.95±4.15b |
148.42±10.67b | 91.58±4.32b | 52.51±4.66b | 56.13±5.57b |
| AT | 14.70±1.07d | 44.93±3.31d |
116.00±16.08d | 64.27±6.16d | 22.32±2.63d | 30.18±3.01d |
| AP | 13.35±1.15d | 37.65±1.83d,e | 85.85±10.53d,f | 45.52±8.77d,e | 15.82±2.46d,e | 22.95±2.38d,e |
In addition, we also measured the serum and muscular
levels of pro-inflammatory cytokines TNF-α as well as IL-6 in all
four groups. Similar to the changes in serum Ang I and II levels,
TNF-α and IL-6 levels in both the serum and gastrocnemius muscle
were significantly higher in the CA group than in the HC group
(P<0.01). Treatment with aliskiren after cachexia development
(AT group) led to reductions in TNF-α and IL-6 in the serum by
21.84 and 29.82%, respectively, and in the muscle by 30.19 and
46.23%, respectively (P<0.05, compared with the CA group). More
reductions were detected in the AP group: serum TNF-α and IL-6 by
42.16 and 50.29%, respectively, and muscular TNF-α and IL-6 by
69.87 and 59.11%, respectively (P<0.05, compared with both the
CA and AT groups; Table II).
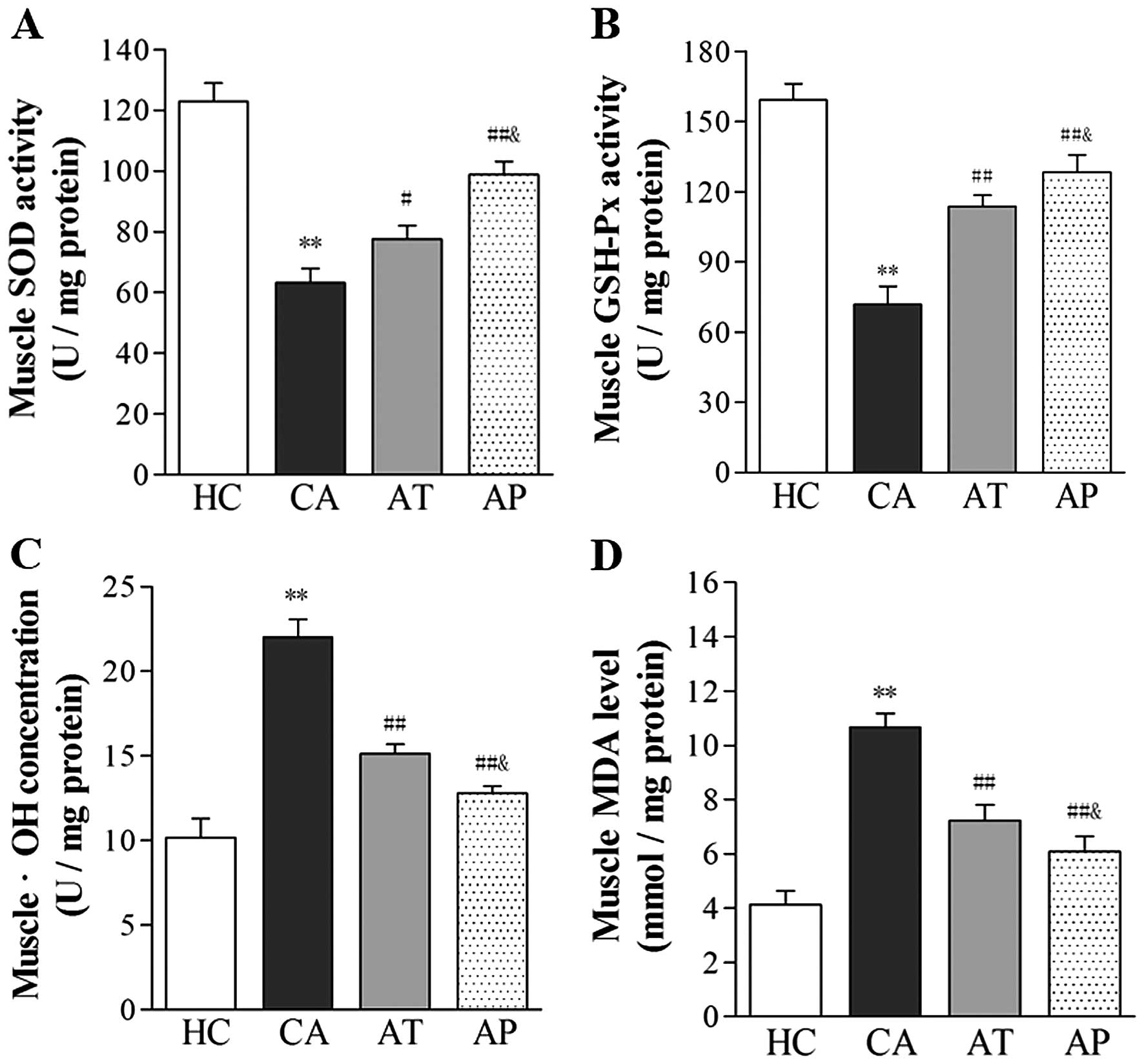
Aliskiren reduces oxidative stress
associated with cancer cachexia
Following the development of cachexia, the level of
oxidative stress significantly increased, as indicated by the
reduced activity of antioxidative enzymes, SOD and GSH-Px, and the
upregulated levels of •OH and MDA in gastrocnemius muscle
(P<0.05, comparing the CA and HC groups). Following aliskiren
treatment, the muscular activities of SOD and GSH-Px increased by
22.91 and 23.92%, respectively, whereas the levels of •OH and MDA
decreased by 31.25 and 32.71%, respectively (P<0.05, compared
with the CA group). Further reductions in oxidative stress were
noted in the AP group, with 56.42 and 37.38% increases in the
muscular activities of SOD and GSH-Px, respectively, and 41.86 and
42.93% decreases in •OH and MDA levels, respectively (P<0.01,
compared with the CA group; P<0.05, compared with the AT
group).
Aliskiren inhibits cachexia-induced ALP
and UPP activation
To assess the status of ALP and UPP signaling in
cancer cachexia, we examined the expression levels of genes related
to these two signaling pathways on both the steady-state mRNA level
and protein level. As shown in Fig.
4A, the mRNA levels of autophagy-related genes Bnip3, LC3, and
Beclin1, as well as ubiquitin-proteasome-related genes MuRF1 and
Atrogin1 in gastrocnemius muscle were significantly upregulated in
mice of the CA group compared with these levels in HC mice
(P<0.01). Upregulation of these genes was dramatically inhibited
in the AT and AP groups, with more robust inhibition achieved in
the AP group (P<0.05, compared with the AT group). Similar
alterations were also observed in the protein levels of these
proteins (Fig. 4B–F). In contrast
to these changes, the steady-state mRNA level of the
apoptosis-related gene caspase-3 was not markedly altered in
tumor-bearing groups (Fig. 4A).
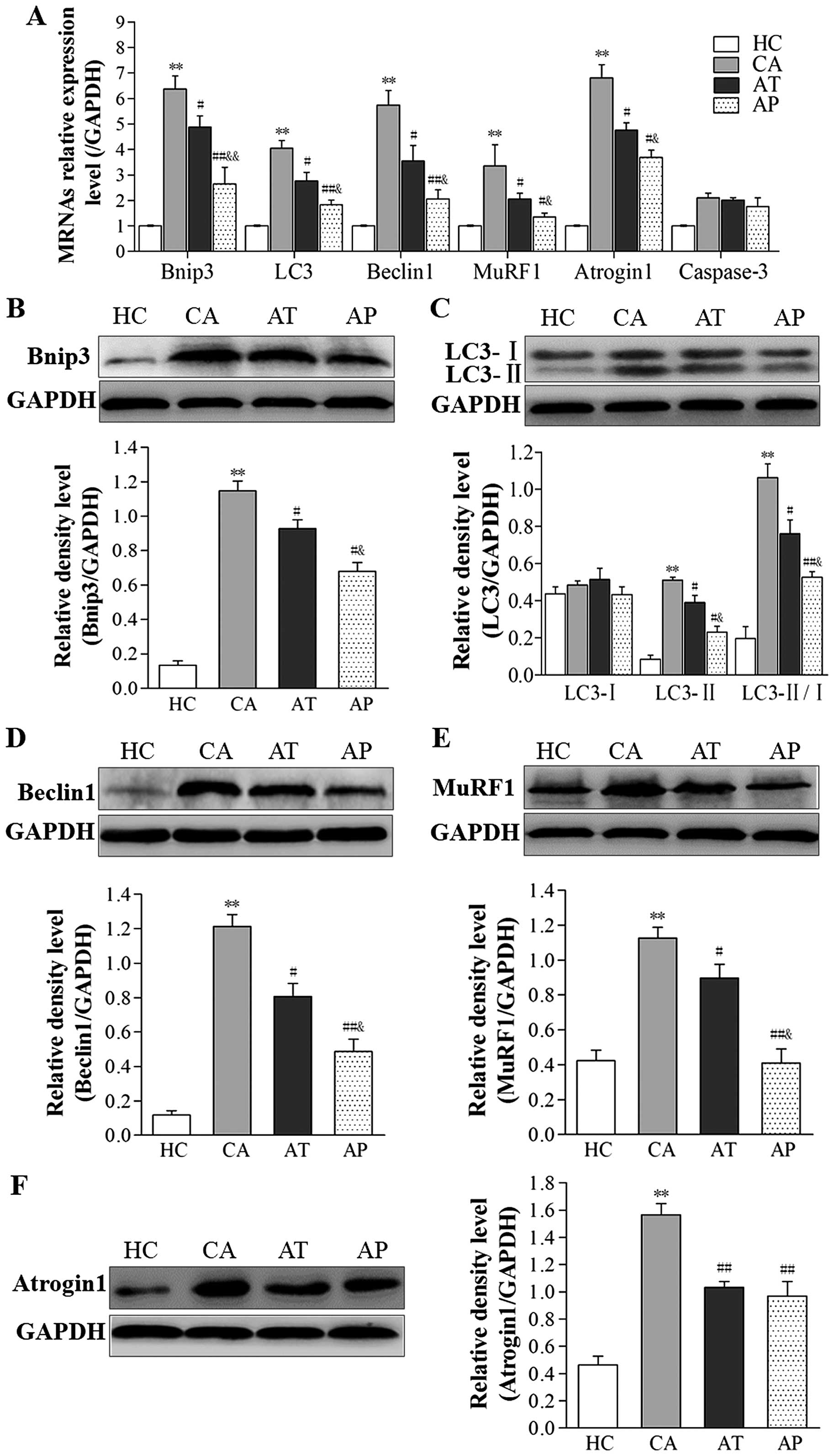 | Figure 4Aliskiren inhibits cachexia-induced
autophagy-lysosome pathway (ALP) and ubiquitin-proteasome pathway
(UPP) signaling. (A) The steady-state mRNA levels of the indicated
genes in gastrocnemius muscle from all four groups were examined by
real-time polymerase chain reaction (PCR) and presented as relative
ratios to the internal control (GAPDH). (B–F) The protein levels of
(B) Bnip3, (C) LC3-I and II, (D) Beclin1, (E) MuRF1, and (F)
Atrogin1 were examined by western immunoblotting, and quantified as
relative density ratio to that of the internal control (GAPDH).
**P<0.01, compared with the HC group;
#P<0.05, ##P<0.01, compared with the CA
group; &P<0.05, &&P<0.01,
compared with the AT group. |
Discussion
Cachexia is a common complication in patients with
malignant tumors and is characterized by increased catabolism,
reduced anabolism, and excessive energy expenditure, which leads to
progressive multi-organ failure and death. Despite extensive
research on cancer cachexia, effective and satisfactory preventive
and therapeutic approaches have yet to be developed. Given the
significance of the RAS in cancer cachexia, we assessed the effects
of a direct renin inhibitor, aliskiren, in this study. Using a
mouse model of cancer cachexia, we showed that aliskiren
effectively alleviated multiple symptoms associated with cachexia,
including weight loss, tumor growth, and muscle wasting.
Consequently, aliskiren treatments significantly improved
whole-body functions and prolonged mouse survival. Mechanistically,
aliskiren antagonizes multiple mechanisms underlying cachexia
development, including RAS activation, upregulated systematic
inflammation, increased oxidative stress, and enhanced ALP and UPP
signaling.
Progressive skeletal muscle wasting is central for
cachexia development and is mostly correlated with functional
defects in these muscles, which increases the risk of postoperative
complications, lowers quality of life, and reduces patient survival
(26). Consistently, we showed that
following cachexia development in C26 cell-inoculated mice, wasting
of skeletal muscles, as demonstrated by loss of gastrocnemius mass
and its cross-cut area, was associated with reduced grip strength,
whole-body coordination, and locomotor activity. The administration
of aliskiren as a therapeutic agent and more robustly as a
preventive agent, significantly ameliorated muscle wasting and
skeletal muscle functions, implying its prophylactic and
therapeutic potential for cancer cachexia.
Chronic inflammation is an important feature of
cancer cachexia. The inflammatory cytokines TNF-α and IL-6 play
critical pathogenic roles in the development of cancer cachexia via
multiple mechanisms, inducing anorexia, promoting muscular protein
degradation, inhibiting protein synthesis, and enhancing energy
expenditure (27–29). Recent studies demonstrated that
aliskiren achieves its anti-inflammatory property by inhibiting the
RAS and reducing the release of inflammatory mediators (18,20).
Consistent with these results, we showed that administration of
aliskiren as a preventive or therapeutic agent significantly
reduced the levels of TNF-α and IL-6 in both serum and skeletal
muscle, suggesting anti-inflammation as a second mechanism for the
anti-cachexia activity of aliskiren.
Oxidative stress is another important mechanism
contributing to the development of anorexia and loss of skeletal
muscles in cancer patients. Several causes have been linked to the
pathogenesis of oxidative stress in cancer cachexia, including: i)
anorexia reduces the intake of antioxidants; ii) alterations in
metabolism reduces the synthesis of reducing agents; iii)
upregulation of pro-inflammatory cytokines, such as TNF-α and IL-6,
increases the production of reactive oxygen species (ROS); and ii)
some anticancer medicines such as alkylating agents boost ROS
generation (30). Of the endogenous
antioxidants, SOD and GSH-Px are two important enzymes. Their
activities reflect the body's capability to clear free radicals and
protect against oxidative damage. MDA results from lipid
degradation by ROS, and together with •OH, indicates the level of
oxidative stress in an organism. In this study, we showed that
cachexia led to significant downregulation of SOD and GSH-Px but
upregulation of MDA and •OH in skeletal muscles, indicating an
increase in oxidative stress. Aliskiren administration
significantly reduced the level of oxidative stress in the mice by
enhancing the activities of SOD and GSH-Px while reducing the
levels of MDA and •OH. Consistently, Patel et al (19) and Thomas et al (31) demonstrated that aliskiren can
inhibit oxidative stress by reducing the generation of free
radicals. Therefore, targeting oxidative stress provides a third
mechanism underlying the anti-cachexia activity of aliskiren.
Protein degradation in skeletal muscles under cancer
cachexia is achieved mainly through two signaling pathways, ALP and
UPP (32,33). Chronic inflammation and oxidative
stress associated with cancer cachexia could upregulate
autophagy-related genes and activate ALP (34,35).
In this study, we found that cachexia development in mice led to
significant upregulation of the ALP-related genes Bnip3, LC3
(increased LC3-II/LC3-I ratio), and Beclin1, indicating ALP
activation. Following aliskiren administration, expression of these
genes was markedly downregulated, implying reduced ALP activity.
This finding is consistent with those of Weng et al and
Zhang et al who reported that aliskiren inhibits ALP
activity (36,37). Atrogin-1 and MuRF1 are two
muscle-specific E3 ligases that critically control UPP activity,
and the inhibition of these two enzymes has been demonstrated to
alleviate the loss of skeletal muscles and ameliorate cancer
cachexia (38,39). Here we showed that aliskiren reduced
the mRNA expression levels of these two enzymes and thus improved
cancer cachexia.
In summary, aliskiren is highly effective at
ameliorating multiple symptoms associated with cancer cachexia. It
inhibits weight loss, wasting of skeletal muscles, and whole-body
dysfunctions, and results in improved locomotor activities as well
as prolonged survival. On the molecular level, aliksiren targets
multiple mechanisms underlying the pathogenesis of cancer cachexia,
including RAS activation, chronic inflammation, increased oxidative
stress, and stimulation of ALP and UPP signaling. Early
administration of aliskiren as a preventive agent (before the
development of cachexia) offers more robust anti-cachexia
activities than later administration as a therapeutic agent (after
the development of cachexia). This study proves the efficacy of
aliskiren as an anti-cachexia agent in an experimental animal
model, and the results support further testing of this agent in
cancer patients in clinical settings.
Abbreviations:
|
RAS
|
renin-angiotensin system
|
|
ALP
|
autophagy-lysosome pathway
|
|
UPP
|
ubiquitin-proteasome pathway
|
|
H&E
|
hematoxylin and eosin
|
|
SOD
|
superoxide dismutase
|
|
GSH-Px
|
glutathione peroxidase
|
|
MDA
|
malondialdehyde
|
|
RIPA
|
radioimmunoprecipitation assay
|
|
PMSF
|
phenylmethylsulfonyl fluoride
|
|
SDS
|
sodium dodecyl sulfate
|
|
PAGE
|
polyacrylamide gel electrophoresis
|
|
PVDF
|
polyvinylidene fluoride
|
References
|
1
|
Fearon K, Strasser F, Anker SD, Bosaeus I,
Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N,
Mantovani G, et al: Definition and classification of cancer
cachexia: An international consensus. Lancet Oncol. 12:489–495.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tan BH and Fearon KC: Cachexia: Prevalence
and impact in medicine. Curr Opin Clin Nutr Metab Care. 11:400–407.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tisdale MJ: Mechanisms of cancer cachexia.
Physiol Rev. 89:381–410. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Russell ST, Sanders PM and Tisdale MJ:
Angiotensin II directly inhibits protein synthesis in murine
myotubes. Cancer Lett. 231:290–294. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sanders PM, Russell ST and Tisdale MJ:
Angiotensin II directly induces muscle protein catabolism through
the ubiquitin-proteasome proteolytic pathway and may play a role in
cancer cachexia. Br J Cancer. 93:425–434. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ruiz-Ortega M, Lorenzo O, Suzuki Y,
Rupérez M and Egido J: Proinflammatory actions of angiotensins.
Curr Opin Nephrol Hypertens. 10:321–329. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang L, Du J, Hu Z, Han G, Delafontaine
P, Garcia G and Mitch WE: IL-6 and serum amyloid A synergy mediates
angiotensin II-induced muscle wasting. J Am Soc Nephrol.
20:604–612. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Russell ST, Eley H and Tisdale MJ: Role of
reactive oxygen species in protein degradation in murine myotubes
induced by proteolysis-inducing factor and angiotensin II. Cell
Signal. 19:1797–1806. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sukhanov S, Semprun-Prieto L, Yoshida T,
Michael Tabony A, Higashi Y, Galvez S and Delafontaine P:
Angiotensin II, oxidative stress and skeletal muscle wasting. Am J
Med Sci. 342:143–147. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dalla Libera L, Ravara B, Angelini A,
Rossini K, Sandri M, Thiene G, Battista Ambrosio G and Vescovo G:
Beneficial effects on skeletal muscle of the angiotensin II type 1
receptor blocker irbesartan in experimental heart failure.
Circulation. 103:2195–2200. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Neo JH, Ager EI, Angus PW, Zhu J, Herath
CB and Christophi C: Changes in the renin angiotensin system during
the development of colorectal cancer liver metastases. BMC Cancer.
10:1342010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun G, Haginoya K, Dai H, Chiba Y, Uematsu
M, Hino-Fukuyo N, Onuma A, Iinuma K and Tsuchiya S: Intramuscular
renin-angio-tensin system is activated in human muscular dystrophy.
J Neurol Sci. 280:40–48. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang XH and Mitch WE: Mechanisms of muscle
wasting in chronic kidney disease. Nat Rev Nephrol. 10:504–516.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Murphy KT, Chee A, Trieu J, Naim T and
Lynch GS: Inhibition of the renin-angiotensin system improves
physiological outcomes in mice with mild or severe cancer cachexia.
Int J Cancer. 133:1234–1246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Q, Li C, Peng X, Kang Q, Deng D,
Zhang L, Zheng Y, Wang C, Qiao Z, Guo D, et al: Combined treatment
of carfilzomib and z-VAD-fmk inhibits skeletal proteolysis and
apoptosis and ameliorates cancer cachexia. Med Oncol. 32:1002015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Acharyya S and Guttridge DC: Cancer
cachexia signaling pathways continue to emerge yet much still
points to the proteasome. Clin Cancer Res. 13:1356–1361. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Penna F, Baccino FM and Costelli P: Coming
back: Autophagy in cachexia. Curr Opin Clin Nutr Metab Care.
17:241–246. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Del Fiorentino A, Cianchetti S, Celi A and
Pedrinelli R: Aliskiren, a renin inhibitor, downregulates
TNF-α-induced tissue factor expression in HUVECS. J Renin
Angiotensin Aldosterone Syst. 11:243–247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Patel RB, Prajapati KD, Sonara BM, Sharma
MM, Patel HM, Pawar VD and Jain MR: Ameliorative potential of
aliskiren in experimental colitis in mice. Eur J Pharmacol.
737:70–76. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schmerbach K, Pfab T, Zhao Y, Culman J,
Mueller S, Villringer A, Muller DN, Hocher B, Unger T and
Thoene-Reineke C: Effects of aliskiren on stroke in rats expressing
human renin and angiotensinogen genes. PLoS One. 5:e150522010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
De Luca A, Pierno S, Liantonio A, Cetrone
M, Camerino C, Fraysse B, Mirabella M, Servidei S, Rüegg UT and
Conte Camerino D: Enhanced dystrophic progression in mdx mice by
exercise and beneficial effects of taurine and insulin-like growth
factor-1. J Pharmacol Exp Ther. 304:453–463. 2003. View Article : Google Scholar
|
|
22
|
Ham DJ, Murphy KT, Chee A, Lynch GS and
Koopman R: Glycine administration attenuates skeletal muscle
wasting in a mouse model of cancer cachexia. Clin Nutr. 33:448–458.
2014. View Article : Google Scholar
|
|
23
|
Murphy KT, Chee A, Trieu J, Naim T and
Lynch GS: Importance of functional and metabolic impairments in the
characterization of the C-26 murine model of cancer cachexia. Dis
Model Mech. 5:533–545. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tanaka Y, Eda H, Tanaka T, Udagawa T,
Ishikawa T, Horii I, Ishitsuka H, Kataoka T and Taguchi T:
Experimental cancer cachexia induced by transplantable colon 26
adenocarcinoma in mice. Cancer Res. 50:2290–2295. 1990.PubMed/NCBI
|
|
26
|
Guo CB, Zhang W, Ma DQ, Zhang KH and Huang
JQ: Hand grip strength: An indicator of nutritional state and the
mix of postoperative complications in patients with oral and
maxillofacial cancers. Br J Oral Maxillofac Surg. 34:325–327. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Carson JA and Baltgalvis KA: Interleukin 6
as a key regulator of muscle mass during cachexia. Exerc Sport Sci
Rev. 38:168–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Deans C and Wigmore SJ: Systemic
inflammation, cachexia and prognosis in patients with cancer. Curr
Opin Clin Nutr Metab Care. 8:265–269. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsoli M and Robertson G: Cancer cachexia:
Malignant inflammation, tumorkines, and metabolic mayhem. Trends
Endocrinol Metab. 24:174–183. 2013. View Article : Google Scholar
|
|
30
|
Vaughan VC, Martin P and Lewandowski PA:
Cancer cachexia: Impact, mechanisms and emerging treatments. J
Cachexia Sarcopenia Muscle. 4:95–109. 2013. View Article : Google Scholar :
|
|
31
|
Thomas CM, Yong QC, Seqqat R, Chandel N,
Feldman DL, Baker KM and Kumar R: Direct renin inhibition prevents
cardiac dysfunction in a diabetic mouse model: Comparison with an
angiotensin receptor antagonist and angiotensin-converting enzyme
inhibitor. Clin Sci (Lond). 124:529–541. 2013. View Article : Google Scholar
|
|
32
|
Penna F, Costamagna D, Pin F, Camperi A,
Fanzani A, Chiarpotto EM, Cavallini G, Bonelli G, Baccino FM and
Costelli P: Autophagic degradation contributes to muscle wasting in
cancer cachexia. Am J Pathol. 182:1367–1378. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tardif N, Klaude M, Lundell L, Thorell A
and Rooyackers O: Autophagic-lysosomal pathway is the main
proteolytic system modified in the skeletal muscle of esophageal
cancer patients. Am J Clin Nutr. 98:1485–1492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
McClung JM, Judge AR, Powers SK and Yan Z:
p38 MAPK links oxidative stress to autophagy-related gene
expression in cachectic muscle wasting. Am J Physiol Cell Physiol.
298:C542–C549. 2010. View Article : Google Scholar :
|
|
35
|
Rahman M, Mofarrahi M, Kristof AS,
Nkengfac B, Harel S and Hussain SN: Reactive oxygen species
regulation of autophagy in skeletal muscles. Antioxid Redox Signal.
20:443–459. 2014. View Article : Google Scholar
|
|
36
|
Weng LQ, Zhang WB, Ye Y, Yin PP, Yuan J,
Wang XX, Kang L, Jiang SS, You JY, Wu J, et al: Aliskiren
ameliorates pressure overload-induced heart hypertrophy and
fibrosis in mice. Acta Pharmacol Sin. 35:1005–1014. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang W, Zhao G, Hu X, Wang M, Li H, Ye Y,
Du Q, Yao J, Bao Z, Hong W, et al: Aliskiren-attenuated myocardium
apoptosis via regulation of autophagy and connexin-43 in aged
spontaneously hypertensive rats. J Cell Mol Med. 18:1247–1256.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Burckart K, Beca S, Urban RJ and
Sheffield-Moore M: Pathogenesis of muscle wasting in cancer
cachexia: Targeted anabolic and anticatabolic therapies. Curr Opin
Clin Nutr Metab Care. 13:410–416. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yuan L, Han J, Meng Q, Xi Q, Zhuang Q,
Jiang Y, Han Y, Zhang B, Fang J and Wu G: Muscle-specific E3
ubiquitin ligases are involved in muscle atrophy of cancer
cachexia: An in vitro and in vivo study. Oncol Rep. 33:2261–2268.
2015.PubMed/NCBI
|