Introduction
Human colorectal cancer (CRC) is one of the most
common cancers and lethal malignancies in both Taiwan and
worldwide. Approximately 1.4 million new cases of CRC are diagnosed
annually, and approximately half of all CRC patients will develop
metastatic cancer (1). Indeed, the
major cause of death among CRC patients is metastasis (2). Thus, research concerning
anti-metastatic agents is important for cancer prevention and
therapy.
During metastasis, multistage progression and a
series of regulatory events occur, with involvement of specific
regulatory molecules. Major metastatic processes include migration
and invasion, intravasation, circulation and adhesion,
extravasation, and colonization (3). In the early stage of metastasis,
migration and intravasation occur when the structure responsible
for cell-cell contact, i.e., the extracellular matrix (ECM), is
degraded by matrix metalloproteinases (MMPs) (4). After migration and intravasation,
cancer cells leave the original tumor organ and enter the
circulation. For extravasation to a metastatic organ, circulating
cancer cells first need to adhere to endothelial cells, and
adhesion molecules [e.g., integrin-1 in tumor cells and E-selectin
and intracellular adhesion molecule 1 (ICAM-1) in endothelium
cells] play a key role in regulating adhesion of tumor cells to
endothelial cells (5,6). Thus, inhibition of cancer cell
migration, invasion and adhesion and/or regulators of expression
may be an effective strategy for suppressing CRC metastasis.
The root of Panax notoginseng (P.
notoginseng) is used as a traditional Chinese medicine and
functional food. Previous studies have shown that P.
notoginseng suppressed liver metastasis when B16 melanoma cells
were transplanted into the spleen of C57BL/6 mice (7). In addition,
20(S)-25-methoxyldammarane-3b, 12b, 20-triol
(25-OCH3-PPD), a ginsenoside from P. notoginseng,
was active against the proliferation and migration of breast cancer
cells via downregulation of mouse double minute 2 (MDM2) (8). Recently, we demonstrated that a P.
notoginseng ethanol extract exerted a suppressive effect on
metastasis by inhibiting migration, invasion and adhesion through
alterations in the expression of associated regulatory molecules
(9). P. notoginseng contains
additional active ingredients, mainly consisting of dammarane-type
saponins (10). The total saponin
content of P. notoginseng is ~12%, with notoginsenoside,
ginseng saponin and gynostemma glycosides being the three major
types (10). Notoginsenoside R1
(NGR1), a unique major saponin of P. notoginseng, has been
shown to effectively prevent cardiovascular disease,
cerebrovascular disease, neurotoxicity and osteoporosis (11–14).
Regarding cancer, some studies have demonstrated that NGR1 is
beneficial for the prevention and treatment of colon cancer and
leukaemia (15–17). However, the effects of NGR1 on CRC
metastasis and the related regulatory mechanisms remain
unclear.
The aims of this study were to investigate the
possible anti-metastatic effects of NGR1 and to identify whether
these effects are involved in regulating human CRC cell migration,
invasion or adhesion. Wound healing and invasion assays and MMP-2
and MMP-9 expression analyses were performed using HCT-116 cells
treated with NGR1. In addition, the effects of NGR1 on the adhesion
of HCT-116 cells to EA.hy926 human endothelial cells were
investigated, and the expression levels of integrin-1, E-selectin
and ICAM-1 in EA.hy926 human endothelial cells were examined. To
determine the effects of NGR1 on intravasation and extravasation,
the cell shape and transepithelial electrical resistance (TEER) of
EA.hy926 cells were evaluated. The results of this study will help
to elucidate the effects of NGR1 on migration, invasion and
adhesion of human CRC cells via regulation of metastasis-associated
molecules.
Materials and methods
Reagents
Notoginsenoside R1 (NGR1)
(3β,6α,12β)-20-(β-D-glucopyranosyloxy)-3,12-dihydroxydammar-24-en-6-yl
2-O-β-D-xylopyranosyl-β-D-glucopyranoside) was purchased
from Tianjin Zhongxin Pharmaceutical Co. (Tianjin, China). Antisera
against integrin-1, E-selectin and ICAM-1 were purchased from Abcam
(Cambridge, MA, USA). Antisera against MMP-2 and MMP-9 were
obtained from GeneTex, Inc. (Hsinchu, Taiwan). In addition,
2′,7′-bis-(2-carboxyethyl)-5- (and-6)-carboxyfluorescein (BCECF)
was purchased from Life Technologies GmbH (Darmstadt, Germany).
Lipopolysaccharide (LPS) was purchased from Sigma-Aldrich (St.
Louis, MO, USA).
Cell cultures and treatment
The human CRC cell line HCT-116 was used as a model
for CRC intravasation, migration and adhesion (18), and the human umbilical vein
endothelial cell line EA.hy926 was used as an extravasation model
for cancer cell adhesion to endothelial cells (19). HCT-116 and EA.hy926 cells were
purchased from Bioresource Collection and Research Center (Hsinchu,
Taiwan) and cultured in RPMI-1640 supplemented with 10% fetal
bovine serum (FBS) and 1% penicillin/streptomycin at 37°C in a
humidified 5% CO2 atmosphere.
For various biochemical analyses, the cells were
plated and incubated for 24 h before treatment with 75, 150, 300 or
500 µM NGR1 for the indicated times. NGR1 was diluted in ethanol,
and cells treated with ethanol alone served as the control
group.
Cell viability analysis
Cell viability was evaluated using the
3-(4,5-dimethyazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
reduction assay as described by Denizot and Lang (20). HCT-116 cells were incubated in 3-cm
plates (1×106 cells) for 24 h and treated with 75, 150,
300, or 500 µM NGR1 for 12, 24 or 48 h. MTT (5 mg/ml) was then
added, and the optical density (OD) was measured at 570 nm.
Wound healing assay
To determine whether the migration of HCT-116 cells
was suppressed by NGR1, a monolayer wound healing assay was
conducted. The protocol for the wound healing assay was a modified
version of that described by Ang et al (21). HCT-116 cells were cultured in 3-cm
plates (1×106 cells) for 24 h, and a micropipette tip
was then used to make a uniform scratch in the center of the
monolayer. After washing with phosphate-buffered saline (PBS), 75,
150 or 300 µM NGR1 was added for 0, 12, 24, or 48 h. HCT-116 cell
morphology was subsequently observed under an inverted fluorescence
microscope (Olympus IX51 microscope; Olympus, Tokyo, Japan), and
the width of the wound area was measured using ImageJ software to
determine the cell migration distance (22).
Invasion assay
An invasion assay was performed to assess
intravasation and extravasation abilities. Matrigel solution (50
µl) was added to the wells of a 24-well Transwell plate, which was
then incubated at 37°C for 30 min. In the upper chamber, HCT-116
cells were resuspended in serum-free RPMI-1640 medium
(5×104 cells/3-cm plate) in the absence or presence of
NGR1 (75, 150 or 300 µM). RPMI-1640 medium (500 µl) containing 10%
FBS was added to the lower chamber. After incubation for 24 h, the
invading cells that migrated to the lower surface of the filter
membrane were stained with 0.2% crystal violet for 15 min. The
number of invading cells on the lower surface of the membrane was
determined under an inverted fluorescence microscope (Olympus IX51;
Olympus) using NIH ImageJ software (22).
Adhesion assay
The adhesion assay protocol used was a modification
of that described by Braut-Boucher et al (23). EA.hy926 cells (1×106
cells) were cultured in 3-cm plates and exposed to 75, 150 or 300
µM NGR1 for 6 h. The cells were then washed with PBS and
co-cultured for 1 h with HCT-116 cells labelled with 10 µM BCECF.
After washing with PBS, the morphology of the BCECF-stained HCT-116
cells was evaluated under an inverted fluorescence microscope
(Olympus IX51; Olympus). The BCECF-stained cells were collected and
measured fluorometrically using an ELISA reader at an OD of 580
nm.
Scanning electron microscopy (SEM)
examination
SEM was used to examine morphological changes in
cell shape and to examine protruding surface structures (24). HCT-116 cells were treated with 1
µg/ml LPS, an invasion inducer (25), or 300 µM NGR1 combined with 1 µg/ml
LPS for 24 h. The cells were then collected, washed with PBS, and
fixed for 30 min in 10% glutaraldehyde solution (Sigma Chemical
Co., St. Louis, MO, USA; 25%). Dehydration was achieved in a graded
ethanol series (2×15 min in 50 vol% ethanol, 2×15 min in 70 vol%
ethanol, 2×15 min in 80 vol% ethanol, 2×15 min in 90 vol% ethanol,
2×20 min, and 60 min overnight in absolute ethanol). After
dehydration, the cells were prepared on gold substrates and
platinum-coated conductive substrates (JEOL JFC-1600; Jeol, Ltd.,
Tokyo, Japan). SEM images were obtained using a JEOL JSM-7000F SEM
microscope at an accelerating voltage of 5 kV.
Measurement of TEER
TEER is a quantitative measurement of the barrier
integrity of a monolayer that is used to examine cell-cell
integrity and permeability (26).
For TEER measurements, EA.hy926 cells (5×104 cells/well)
were cultured for 24 h in RPMI-1640 medium containing 75, 150 or
300 µM NGR1. TEER values were obtained by subtracting the TEER
measurement for the cell culture dish groove from the measurement
obtained in the presence of a cell layer. These measurements were
collected using a Millicell-ERS voltohmmeter (Millipore-Continental
Water Systems, Bedford, MA, USA).
Analysis of expression of regulatory
proteins involved in migration, adhesion and invasion
Approximately 5×105 HCT-116 cells/3-cm
plate were used for MMP-2, MMP-9, and integrin-1 expression
analyses; 5×105 EA.hy926 cells/3-cm plate were used for
E-selectin and ICAM-1 expression analyses. The cells were incubated
in a 12-well plate with 75, 150 or 300 µM NGR1 for 24 h, washed
twice in cold PBS and harvested using 200 µl of lysis buffer
containing 10 mM Tris-HCl, 5 mM EDTA, 0.2 mM phenylmethylsulphonyl
fluoride (PMSF), and 20 µg/ml aprotinin at pH 7.4. The levels of
cellular protein were determined following the method described by
Lowry et al (27).
For each sample, 10–20 mg of cellular protein was
applied to 10% sodium dodecyl sulphate (SDS) polyacrylamide gels
(28). After electrophoresis, the
proteins were transferred to polyvinylidene difluoride membranes
(29). The membranes were then
incubated with an anti-MMP-2, anti-MMP-9, anti-integrin-1,
anti-E-selectin or anti-ICAM-1 antibody at 37°C for 1 h and
subsequently with a peroxidase-conjugated secondary antibody. Bands
were visualized using hydrogen peroxide/tetrahydrochloride
diaminobenzidine or an enhanced chemiluminescence detection kit
(Amersham Life Science, Buckinghamshire, UK) and quantified using
an AlphaImager 2000 (Alpha Innotech, San Leandro, CA, USA).
Statistical analysis
Data were analysed using the statistical analysis
software SPSS for Windows, version 20.0 (SPSS, Inc., Chicago, IL,
USA). One-way analysis of variance (ANOVA) and Duncans multiple
range tests were employed to evaluate the significance of
differences between two mean values. A P-value of <0.05 or 0.01
was considered to indicate a statistically significant result.
Results
NGR1 suppresses the viability of human
CRC cells
According to the MTT assay results, HCT-116 cell
viability after treatment with 75, 150 or 300 µM NGR1 did not
differ significantly from that of the control during the 48-h
incubation period (data not shown). However, the viability of cells
treated with 500 µM NGR1 for 48 h was significantly reduced
(58±7.26%) compared with the control cells (100%) (p<0.05).
Therefore, we used an NGR1 concentration of 75, 150 or 300 µM for
all subsequent experiments.
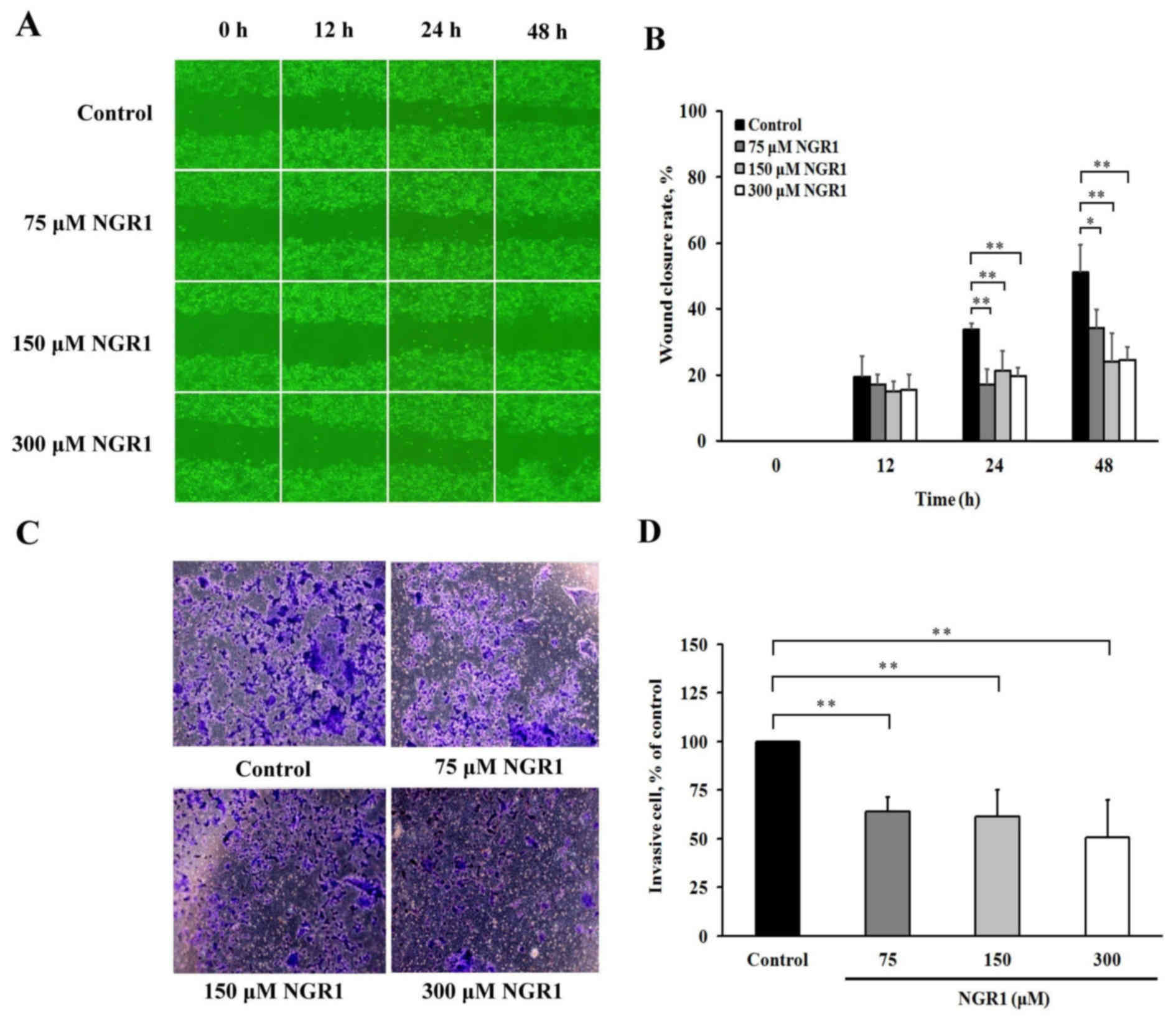
NGR1 inhibits the migratory and invasive abilities
of human CRC cells. The effects of NGR1 on the migratory abilities
are presented in Fig. 1. When
HCT-116 cells were incubated with various concentrations of NGR1
for 12, 24 or 48 h, the wound healing areas varied as shown in
Fig. 1A. The results showed that
the HCT-116 cells treated with 75, 150 or 300 µM NGR1 for 24 or 48
h exhibited reduced wound closure rates compared with the control
group (17–21 and 24–34%, respectively) (Fig. 1B). These results indicate that NGR1
significantly suppressed HCT-116 cell migration. The results of the
Transwell Matrigel invasion assay indicated that when HCT-116 cells
were incubated in various concentrations of NGR1 for 24 h (Fig. 1C), the level of HCT-116 cell
invasion was significantly decreased by 64±8, 61±14 or 51±19% after
treatment with 75, 150 or 300 µM NGR1 for 24 h, respectively,
compared with the control group (100%) (p<0.01) (Fig. 1D). This result suggests that NGR1
inhibits the inter-colon migratory and invasive abilities of colon
cancer cells.
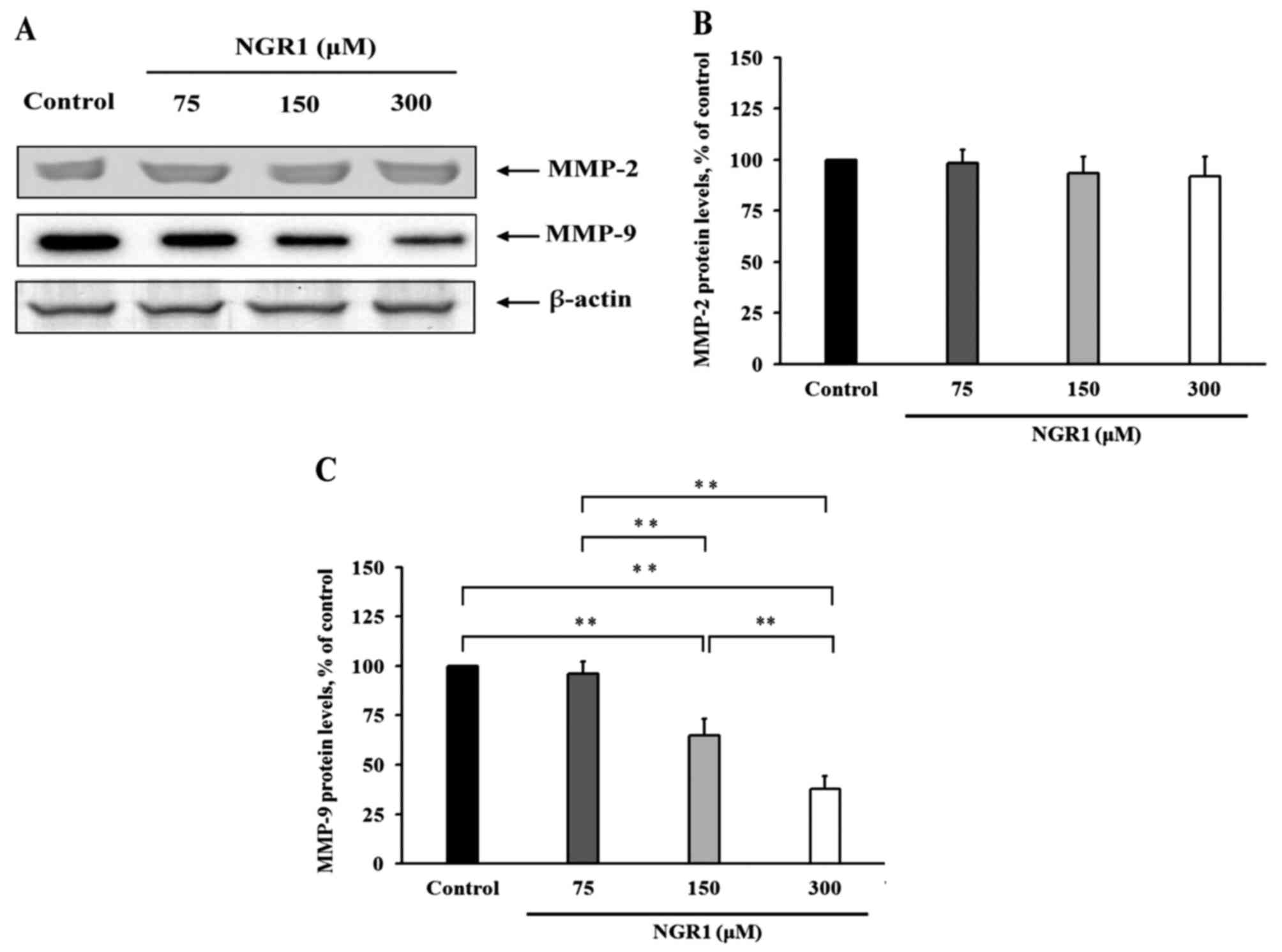
The effects of NGR1 on the levels of MMP-2 and MMP-9
are presented in Fig. 2.
Immunoblotting assays showing the expression of MMP-2 and MMP-9 in
HCT-116 cells after NGR1 treatment are presented in Fig. 2A. NGR1 did not regulate MMP-2
expression in the HCT-116 cells (Fig.
2B). However, when HCT-116 cells were treated with 150 or 300
µM NGR1 for 24 h, the MMP-9 protein expression was significantly
decreased by 35 and 68%, respectively, compared with the control
group (100%) (p<0.01) (Fig. 2C).
These results indicate that NGR1 regulates migration and
intravasation by regulating MMP-9 expression.
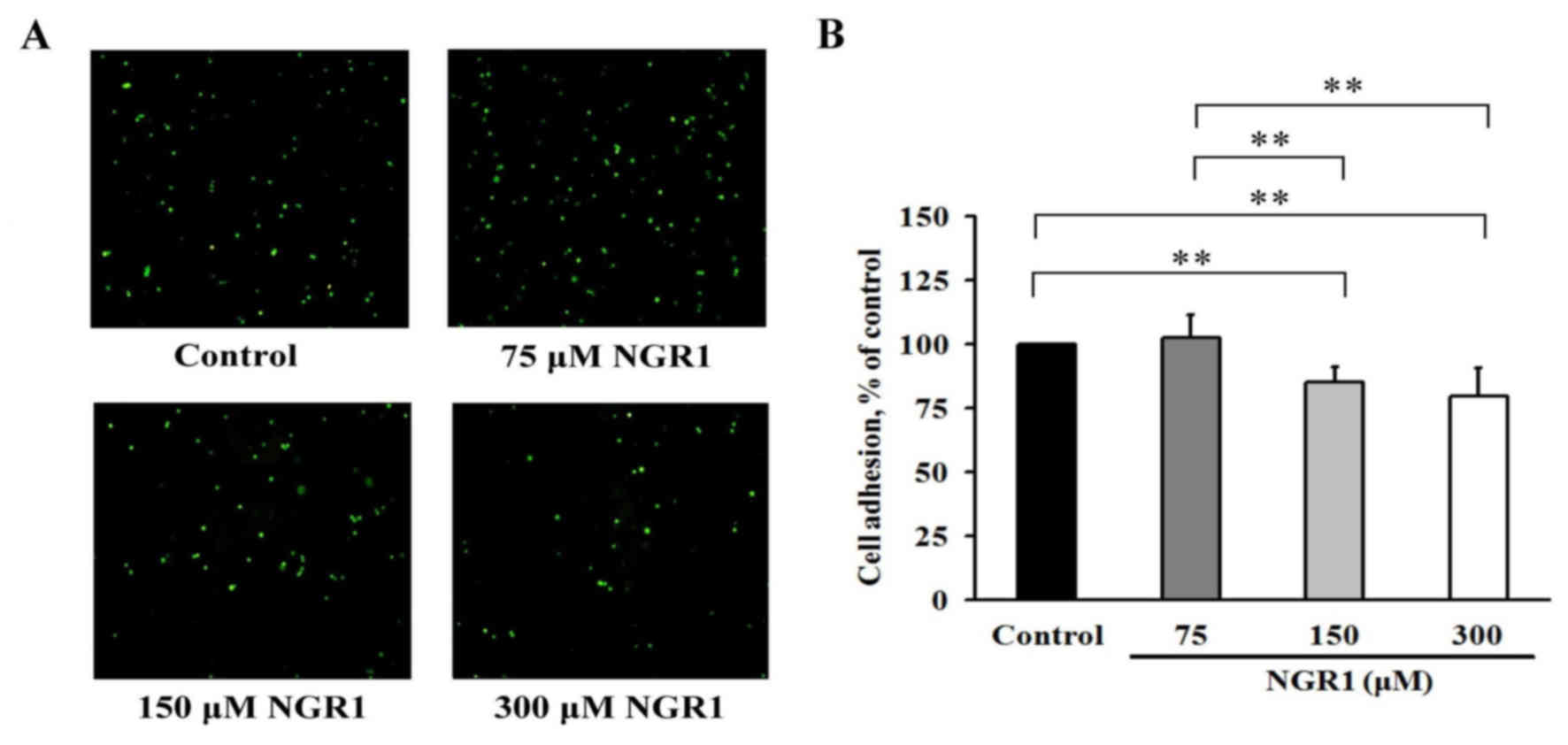
NGR1 reduces adhesion of human CRC
cells
Fluorescence microscopic examination revealed that
treatment of EA.hy926 cells with various concentrations of NGR1
decreased the number of adherent cells detected after co-culture
with the HCT-116 cells (Fig. 3A).
After EA.hy926 cells were incubated with 150 or 300 µM NGR1 for 24
h (Fig. 3B), cell adhesion was
significantly decreased by 18 to 20% in a dose-dependent manner.
Thus, the adhesion levels of these cells were significantly reduced
compared with that of the control group (100%) (p<0.01) after 24
h. These results demonstrated that NGR1 decreased the ability of
HCT-116 cells to adhere to EA.hy926 cells and may be able to reduce
cancer cell adhesion to a metastasis target organ.
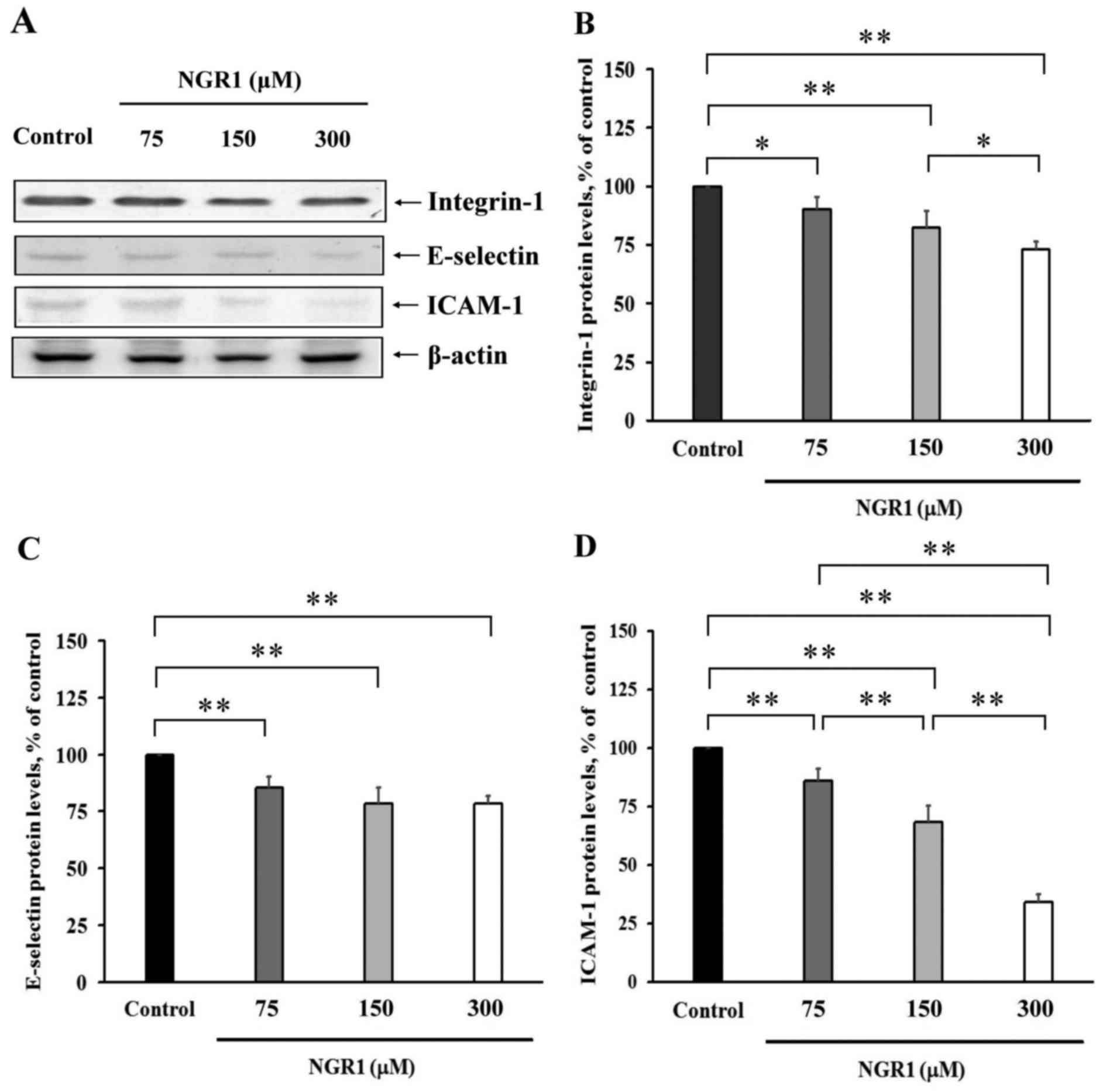
The protein levels of integrin-1 in HCT-116 cells,
and E-selectin and ICAM-1 in the EA.hy926 cells were analysed
(Fig. 4). The expression levels of
integrin-1, E-selectin and ICAM-1 are presented in Fig. 4A. Treatment of HCT-116 cells with
75, 150 or 300 µM NGR1 for 24 h significantly decreased integrin-1
protein levels by 10 to 27% compared with the control group (100%)
(Fig. 4B). As shown in Fig. 4C, after treatment with 75, 150 or
300 µM NGR1 for 24 h, the levels of E-selectin protein in the
EA.hy926 cells were 85±5, 78±7 and 78±4%, respectively, compared
with the control values (100%); and these values were significantly
lower than levels in the control group (p<0.01). The levels of
ICAM-1 protein in cells incubated with 75, 150 or 300 µM NGR1 were
significantly reduced (86±3, 68±3 and 34±2%, respectively)
(Fig. 4D) compared with these
levels in control cells (100%). These results indicate that
expression of these adhesion molecules was suppressed by NGR1 and
NGR1 may exert an important anti-adhesion effect during
metastasis.
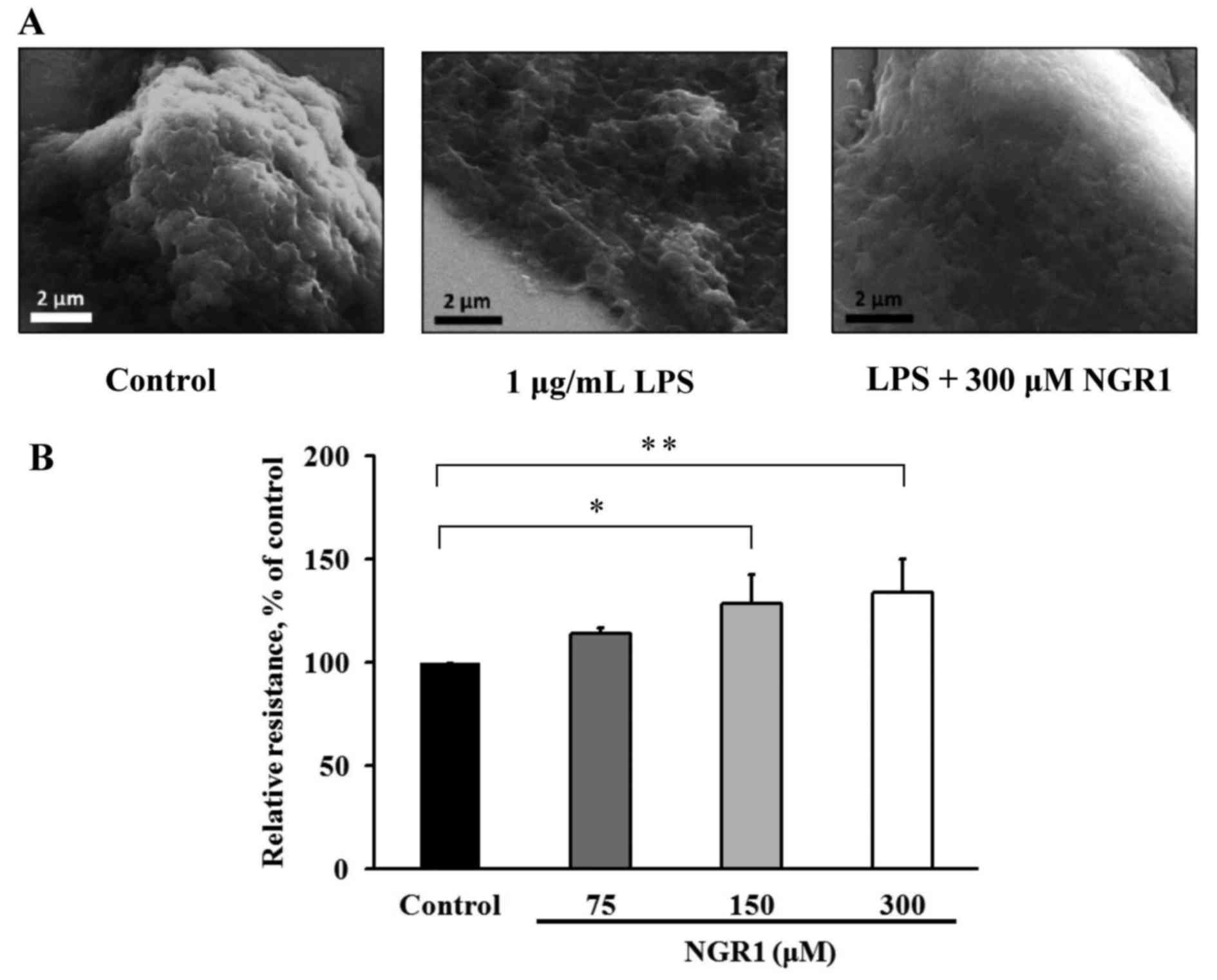
NGR1 inhibits extravasation of human
CRC cells
To investigate the effects of NGR1 on extravasation,
changes in HCT-116 cell shape were examined using SEM technology.
The LPS-treated HCT-116 cells exhibited a flattened and partially
collapsed shape compared with the shape of the control cells,
whereas HCT-116 cells treated with LPS combined with 300 µM NGR1
exhibited a less flattened shape compared with the cells treated
with LPS alone (Fig. 5A). The
effect of NGR1 on the TEER of EA.hy926 cells was also examined.
After treatment with 75, 150 or 300 µM NGR1, the TEER values were
113±3, 128±14 and 134±16%, respectively (Fig. 5B), representing significant
increases compared with the control group (100%) (p<0.05). This
result shows that NGR1 may increase inter-epithelial cell TEER,
thereby decreasing cell permeability and inhibiting the invasive
ability of endothelial cells.
Discussion
This study demonstrated that notoginsenoside R1
(NGR1), an important component of P. notoginseng,
significantly inhibited HCT-116 cell metastasis by suppressing
migration, invasion and adhesion through regulation of MMP-9,
integrin-1, E-selectin and ICAM-1 expression. In addition, our
previous findings indicated that a P. notoginseng ethanol
extract significantly suppressed metastasis of CRC (9). We conclude that NGR1 may be an
important component of P. notoginseng, exerting an
anti-metastasis effect. This finding is important in regard to the
use of P. notoginseng and NGR1 as anti-metastatic agents, as
40 to 70% of colon cancers metastasize to the liver, and the
prognosis is quite poor once metastasis has occurred (2). Indeed, anti-metastasis has become an
important field of study for cancer prevention and therapy, as have
investigations of the use of P. notoginseng or other active
principle components.
P. notoginseng contains ~12% total saponins.
The three major types of saponins are ginsenosides (e.g., Rb1, Rg1,
Rg3), notoginsenosides (e.g., R1, R2, R3) and jiaogulan glucosides
(e.g., RVI) (30–33). The structures of both
notoginsenosides and ginsenosides exhibit a tetracyclic gonane
steroid core. Gonane, the simplest steroid, and its metabolites
typically act as signaling molecules that are associated with
various physiological effects (31). These saponins are also active
principle components of P. notoginseng, and NGR1 is the most
abundant of the notoginsenosides (33). Previous anticancer studies have
indicated that NGR1 suppresses human colon cancer cell
proliferation (16,17,34).
However, we are the first to report that NGR1 significantly
suppressed CRC metastasis. Among saponins with a gonane structure,
ginsenoside Rb1 was found to exhibit anti-metastatic and
anti-angiogenic effects by suppressing the formation of endothelial
tube-like structures on human umbilical vein endothelial cells
(HUVECs) (35). Ginsenoside Rg1
also suppressed transforming growth factor β1 (TGF β1)-induced
invasion and migration in HepG2 liver cancer cells (36). In addition, by inhibiting MMP-13
expression, ginsenoside Rg3 significantly suppressed the migration,
invasion, wound healing, and colony-forming abilities of B16F10
cells in a dose-dependent manner (37), and by inhibiting wound healing and
MMP-9 expression via suppression of NF-κB phosphorylation and DNA
binding activity, ginsenoside Rg1 suppressed phorbol myristate
acetate-induced invasion and migration in MCF-7 breast cancer cells
(38). The above studies indicate
that saponins can reduce the activities of MMPs to inhibit cancer
metastasis.
MMPs can degrade collagen in the ECM and basement
membrane components such as collagen type IV, elastin and
fibronectin (39). MMP-2 and MMP-9
are major gelatinases that catalyse degradation of type IV collagen
(39). Our previous study showed
that a P. notoginseng ethanol extract inhibited HCT-116 cell
migration by significantly decreasing MMP-9 but had no effect on
MMP-2 expression (9). Ginsenoside
Rg1 also suppressed PMA-induced MMP-9 expression but did not affect
MMP-2 expression (38). In the
present study, NGR1 was found to regulate MMP-9 expression in the
HCT-116 cells. Levi et al demonstrated that MMP-2 and MMP-9
have different specificities for growth factor receptors in
metastatic regulation (40).
Nonetheless, the MMP signalling mechanism of NGR1 requires further
study.
In the present study, NGR1 suppressed adhesion of a
colon cancer cell line to an endothelial cell line of the blood
vasculature of metastatic target organs by inhibiting integrin-1
levels in HCT-116 cells and reducing E-selectin and ICAM-1 levels
in EA.hy926 cells. This important finding indicates the potential
anti-metastatic effect of P. notoginseng and its active
principle components. During the metastatic process, cancer cells
leave the primary tumor site and enter the blood or lymphatic
circulation via migration and intravasation. These circulating
tumor cells migrate along the endothelial cell surface and invade
metastatic target organs, which is referred to as adhesion and
extravasation (41,42). Once the adhesion reaction is
triggered, various adhesion molecules, such as integrin-1,
E-selectin and ICAM-1, play key roles in regulating the adhesion of
circulating tumor cells to endothelial cells (43,44). A
previous study indicated that tumor necrosis factor-α (TNF-α)
induced ICAM-1 expression in human A549 alveolar epithelial cells
and adhesion of U937 cells to these cells reduced their metastatic
ability (45).
Bisdemethoxycurcumin, a major active compound of curcuminoids,
significantly inhibited the adhesion, migration, invasion and
metastasis of SKOV-3 cells by inhibiting MMP-9 and ICAM-1
expression (46). Among these
adhesion-regulating molecules, integrins, a major class of cancer
cell surface receptors of primary cancer cells, were responsible
for the adhesion of cells to ECM proteins (47), and as demonstrated previously,
E-selectin-mediated adhesion of tumor cells to the vascular
endothelium is crucial for extravasation and metastasis (48). ICAM-1 plays a central role in
cell-cell contacts, and cells overexpressing ICAM-1 may become
targets for T cell infiltration. Indeed, ICAM-1 can enhance immune
cell adhesion and migration (49).
These integrins mediate strong adhesion between cancer cells and
endothelial cells (50), and this
process promotes the transmigration of cancer cells through the
endothelium to metastatic sites (51,52).
Our previous study showed that P. notoginseng exerts
anti-metastasis effects in HCT-116 cells by decreasing adhesion
activity through reduction in the levels of such adhesion
molecules, including integrin-1, E-selectin and ICAM-1 (9). The suppressive effect of NGR1 on
adhesion may be an important factor that contributes to the
observed anti-migration effect.
In addition to their use in our analysis of the
adhesion reaction, EA.hy926 cells were used in this study as a
blood model to investigate the effects of NGR1 on extravasation in
the vascular endothelial monolayer of the circulatory system.
Extravasation is triggered when tumor cells adhere to endothelial
cells and penetrate the endothelial cell layer. These metastatic
cells bind to the monolayer cells, leading to disruption of
endothelial cell-cell interactions and retraction of metastatic
cells (52–54). The results of SEM examination
indicated that HCT-116 cells became flattened and retracted after
LPS induction. However, this flattening was significantly reduced
by treatment with both NGR1 and LPS. Monolayer endothelium
cell-cell permeability and integrity also play important roles in
regulating the extravasation of tumor cells to endothelial cells
(5,6). The TEER value is used as an indicator
of the integrity of cellular barriers before they are evaluated for
the transport of organisms (e.g., cells, microorganisms), drugs or
chemicals, and cell-cell permeability and barrier integrity are
vital for the physiological activities of tissues (55). Thus, TEER is a widely accepted
quantitative technique for measuring the integrity of endothelial
and epithelial monolayers in cell culture models. Our results
indicate that NGR1 inhibits HCT-116 cell extravasation. EA.hy926
cells treated with 150 or 300 µg/ml NGR1 exhibited significantly
reduced monolayer endothelial cell permeability, resulting in the
inhibition of extravasation.
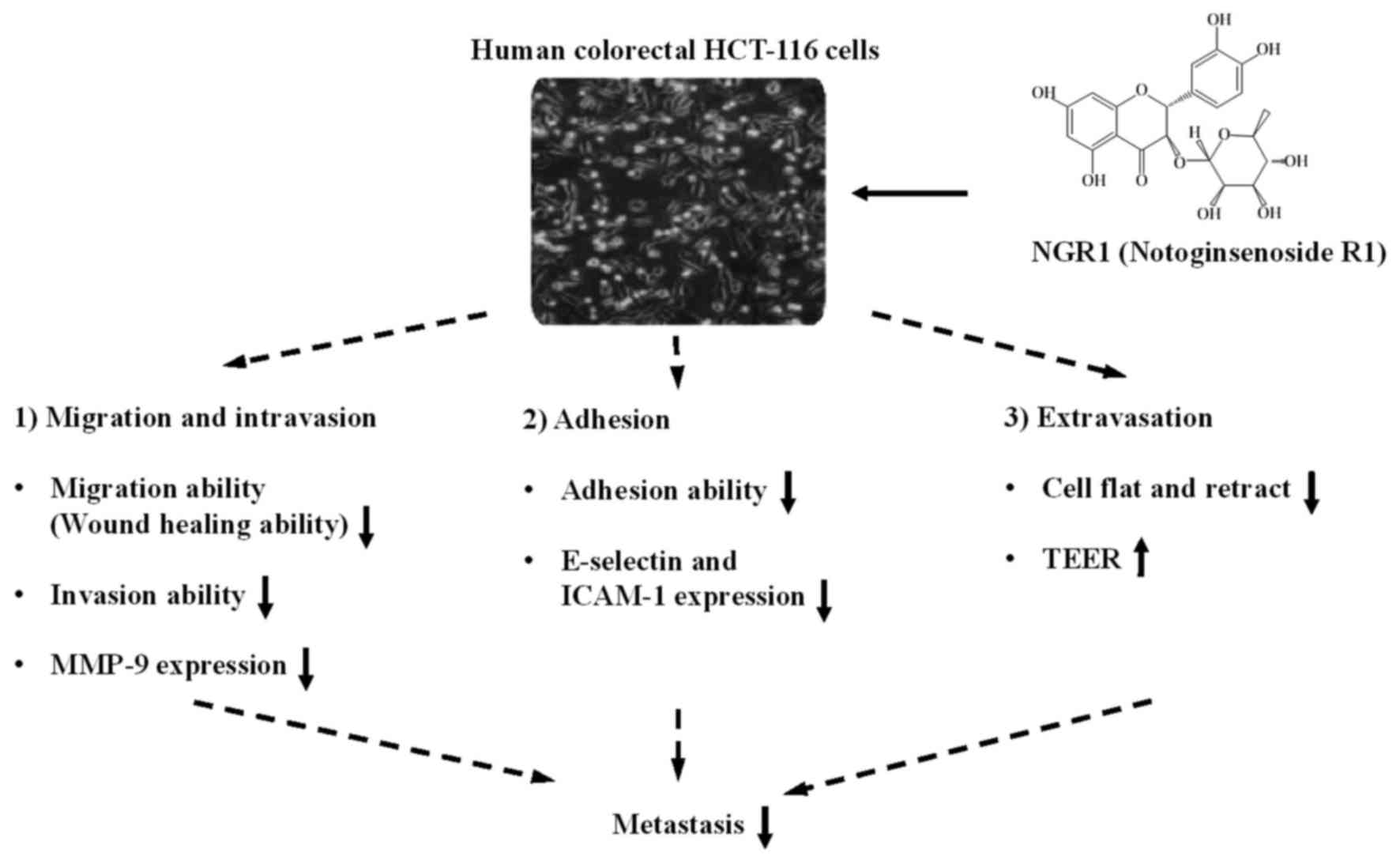
As shown in Fig. 6,
our results illustrate a model in which NGR1 suppresses CRC cell
metastasis. NGR1 significantly suppresses migration-regulated
molecules (e.g., MMP-9), reduces adhesion trigger molecules (e.g.,
integrin-1, E-selectin and ICAM-1), and increases cell-cell
permeability, resulting in inhibition of HCT-116 migration,
invasion and adhesion. Hence, NGR1 may serve as a potential
anti-metastatic agent.
Acknowledgements
This study was supported by E-Da Hospital
(EDAHP103032) in Kaohsiung, Taiwan.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics for Hispanics/Latinos, 2012. CA Cancer J Clin.
62:283–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leber MF and Efferth T: Molecular
principles of cancer invasion and metastasis (Review). Int J Oncol.
34:881–895. 2009.PubMed/NCBI
|
|
4
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okegawa T, Li Y, Pong RC and Hsieh JT:
Cell adhesion proteins as tumor suppressors. J Urol. 167:1836–1843.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yates CM, McGettrick HM, Nash GB and
Rainger GE: Adhesion of tumor cells to matrices and endothelium.
Methods Mol Biol. 1070:57–75. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen PF, Liu LM, Chen Z, Lin SY, Song WX
and Xu YF: Effects of ethanol extracts of Panax notoginseng on
liver metastasis of B16 melanoma grafted in mice. Zhong Xi Yi Jie
He Xue Bao. 4:500–503. 2006.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang W, Zhang X, Qin JJ, Voruganti S, Nag
SA, Wang MH, Wang H and Zhang R: Natural product ginsenoside
25-OCH3-PPD inhibits breast cancer growth and metastasis
through down-regulating MDM2. PLoS One. 7:e415862012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hsieh SL, Hsieh S, Kuo YH, Wang JJ, Wang
JC and Wu CC: Effects of Panax notoginseng on the metastasis of
human colorectal cancer cells. Am J Chin Med. 44:851–870. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang X, Xiong X, Wang H and Wang J:
Protective effects of Panax notoginseng saponins on cardiovascular
diseases: A comprehensive overview of experimental studies. Evid
Based Complement Alternat Med. 2014:2048402014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jia C, Xiong M, Wang P, Cui J, Du X, Yang
Q, Wang W, Chen Y and Zhang T: Notoginsenoside R1 attenuates
atherosclerotic lesions in ApoE deficient mouse model. PLoS One.
9:e998492014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan S, Li Z, Li H, Arancio O and Zhang W:
Notoginsenoside R1 increases neuronal excitability and ameliorates
synaptic and memory dysfunction following amyloid elevation. Sci
Rep. 4:63522014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang T, Wan D, Shao L, Dai J and Jiang C:
Notoginsenoside R1 stimulates osteogenic function in primary
osteoblasts via estrogen receptor signaling. Biochem Biophys Res
Commun. 466:232–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu Y, Sun G, Luo Y, Wang M, Chen R, Zhang
J, Ai Q, Xing N and Sun X: Cardioprotective effects of
Notoginsenoside R1 against ischemia/reperfusion injuries by
regulating oxidative stress- and endoplasmic reticulum
stress-related signalingpathways. Sci Rep. 6:217302016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu L, Wang B and Gao J: Preliminary
studies of notoginsenoside R1-induced differentiation of HL-60 cell
lines in vitro. Hua Xi Yi Ke Da Xue Xue Bao. 22:124–127. 1991.(In
Chinese). PubMed/NCBI
|
|
16
|
Wang CZ, Xie JT, Zhang B, Ni M, Fishbein
A, Aung HH, Mehendale SR, Du W, He TC and Yuan CS: Chemopreventive
effects of Panax notoginseng and its major constituents on SW480
human colorectal cancer cells. Int J Oncol. 31:1149–1156.
2007.PubMed/NCBI
|
|
17
|
Wang CZ, Xie JT, Fishbein A, Aung HH, He
H, Mehendale SR, He TC, Du W and Yuan CS: Antiproliferative effects
of different plant parts of Panax notoginseng on SW480 human
colorectal cancer cells. Phytother Res. 23:6–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang X, Wang Q, Ives KL and Evers BM:
Curcumin inhibits neurotensin-mediated interleukin-8 production and
migration of HCT116 human colon cancer cells. Clin Cancer Res.
12:5346–5355. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang XM, Lv YG, Chen GB, Zou Y, Lin CW,
Yang L, Guo P and Lin MP: Effect of mild hypothermia on breast
cancer cells adhesion and migration. Biosci Trends. 6:313–324.
2012.PubMed/NCBI
|
|
20
|
Denizot F and Lang R: Rapid colorimetric
assay for cell growth and survival. Modifications to the
tetrazolium dye procedure giving improved sensitivity and
reliability. J Immunol Methods. 89:271–277. 1986.
|
|
21
|
Ang SF, Zhao ZS, Lim L and Manser E: DAAM1
is a formin required for centrosome re-orientation during cell
migration. PLoS One. 5:e130642010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vincent C, Siddiqui TA and Schlichter LC:
Podosomes in migrating microglia: components and matrix
degradation. J Neuroinflammation. 9:1902012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Braut-Boucher F, Pichon J, Rat P, Adolphe
M, Aubery M and Font J: A non-isotopic, highly sensitive,
fluorimetric, cell-cell adhesion microplate assay using calcein
AM-labeled lymphocytes. J Immunol Methods. 178:41–51. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kung ML, Hsieh SL, Wu CC, Chu TH, Lin YC,
Yeh BW and Hsieh S: Enhanced reactive oxygen species overexpression
by CuO nanoparticles in poorly differentiated hepatocellular
carcinoma cells. Nanoscale. 7:1820–1829. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harmey JH, Bucana CD, Lu W, Byrne AM,
McDonnell S, Lynch C, Bouchier-Hayes D and Dong Z:
Lipopolysaccharide-induced metastatic growth is associated with
increased angiogenesis, vascular permeability and tumor cell
invasion. Int J Cancer. 101:415–422. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gopal PK, Prasad J, Smart J and Gill HS:
In vitro adherence properties of Lactobacillus rhamnosus DR20 and
Bifidobacterium lactis DR10 strains and their antagonistic activity
against an enterotoxigenic Escherichia coli. Int J Food Microbiol.
67:207–216. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
28
|
Towbin H, Staehelin T and Gordon J:
Electrophoretic transfer of proteins from polyacrylamide gels to
nitrocellulose sheets: Procedure and some applications. Proc Natl
Acad Sci USA. 76:4350–4354. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lau AJ, Woo SO and Koh HL: Analysis of
saponins in raw and steamed Panax notoginseng using
high-performance liquid chromatography with diode array detection.
J Chromatogr A. 1011:77–87. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoshikawa M, Morikawa T, Kashima Y,
Ninomiya K and Matsuda H: Structures of new dammarane-type
triterpene saponins from the flower buds of Panax notoginseng and
hepatoprotective effects of principal ginseng saponins. J Nat Prod.
66:922–927. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li L, Zhang JL, Sheng YX, Guo DA, Wang Q
and Guo HZ: Simultaneous quantification of six major active
saponins of Panax notoginseng by high-performance liquid
chromatography-UV method. J Pharm Biomed Anal. 38:45–51. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun S, Wang C, Tong R, Li X, Fishbein A,
Wang Q, He T, Du W and Yuan C: Effects of steaming the root of
Panax notoginseng on chemical composition and anticancer
activities. Food Chem. 118:307–314. 2010. View Article : Google Scholar
|
|
34
|
He NW, Zhao Y, Guo L, Shang J and Yang XB:
Antioxidant, antiproliferative, and pro-apoptotic activities of a
saponin extract derived from the roots of Panax notoginseng (Burk.)
F.H. Chen. J MedFood. 15:350–359. 2012.
|
|
35
|
Leung KW, Cheung LW, Pon YL, Wong RN, Mak
NK, Fan TP, Au SC, Tombran-Tink J and Wong AS: Ginsenoside Rb1
inhibits tube-like structure formation of endothelial cells by
regulating pigment epithelium-derived factor through the oestrogen
β receptor. Br J Pharmacol. 152:207–215. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu M, Yu X, Guo D, Yu B, Li L, Liao Q and
Xing R: Ginsenoside Rg1 attenuates invasion and migration by
inhibiting transforming growth factor-β1-induced epithelial to
mesenchymal transition in HepG2 cells. Mol Med Rep. 11:3167–3173.
2015.PubMed/NCBI
|
|
37
|
Lee SG, Kang YJ and Nam JO:
Anti-metastasis effects of Ginsenoside Rg3 in B16F10 cells. J
Microbiol Biotechnol. 25:1997–2006. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li L, Wang Y, Qi B, Yuan D, Dong S, Guo D,
Zhang C and Yu M: Suppression of PMA-induced tumor cell invasion
and migration by ginsenoside Rg1 via the inhibition of
NF-κB-dependent MMP-9 expression. Oncol Rep. 32:1779–1786.
2014.PubMed/NCBI
|
|
39
|
Woessner JF Jr: Matrix metalloproteinases
and their inhibitors in connective tissue remodeling. FASEB J.
5:2145–2154. 1991.PubMed/NCBI
|
|
40
|
Levi E, Fridman R, Miao HQ, Ma YS, Yayon A
and Vlodavsky I: Matrix metalloproteinase 2 releases active soluble
ectodomain of fibroblast growth factor receptor 1. Proc Natl Acad
Sci USA. 93:7069–7074. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fidler IJ: Critical determinants of
metastasis. Semin Cancer Biol. 12:89–96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jacobs PP and Sackstein R: CD44 and HCELL:
Preventing hematogenous metastasis at step 1. FEBS Lett.
585:3148–3158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dimitroff CJ, Lechpammer M, Long-Woodward
D and Kutok JL: Rolling of human bone-metastatic prostate tumor
cells on human bone marrow endothelium under shear flow is mediated
by E-selectin. Cancer Res. 64:5261–5269. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kobayashi H, Boelte KC and Lin PC:
Endothelial cell adhesion molecules and cancer progression. Curr
Med Chem. 14:377–386. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Huang WC, Chan ST, Yang TL, Tzeng CC and
Chen CC: Inhibition of ICAM-1 gene expression, monocyte adhesion
and cancer cell invasion by targeting IKK complex: Molecular and
functional study of novel α-methylene-γ-butyrolactone derivatives.
Carcinogenesis. 25:1925–1934. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pei H, Yang Y, Cui L, Yang J, Li X, Yang Y
and Duan H: Bisdemethoxycurcumin inhibits ovarian cancer via
reducing oxidative stress mediated MMPs expressions. Sci Rep.
6:287732016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xiong J, Balcioglu HE and Danen EH:
Integrin signaling in control of tumor growth and progression. Int
J Biochem Cell Biol. 45:1012–1015. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Köhler S, Ullrich S, Richter U and
Schumacher U: E-/P-selectins and colon carcinoma metastasis: First
in vivo evidence for their crucial role in a clinically relevant
model of spontaneous metastasis formation in the lung. Br J Cancer.
102:602–609. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhu XW and Gong JP: Expression and role of
ICAM-1 in the occurrence and development of hepatocellular
carcinoma. Asian Pac J Cancer Prev. 14:1579–1583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Barthel SR, Hays DL, Yazawa EM, Opperman
M, Walley KC, Nimrichter L, Burdick MM, Gillard BM, Moser MT,
Pantel K, et al: Definition of molecular determinants of prostate
cancer cell bone extravasation. Cancer Res. 73:942–952. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sreeramkumar V, Leiva M, Stadtmann A,
Pitava C, Ortega-Rodríguez I, Wild MK, Lee B, Zarbock A and Hidalgo
A: Coordinated and unique functions of the E-selectin ligand ESL-1
during inflammatory and hematopoietic recruitment in mice. Blood.
122:3993–4001. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wood S Jr: Pathogenesis of metastasis
formation observed in vivo in the rabbit ear chamber. AMA Arch
Pathol. 66:550–568. 1958.PubMed/NCBI
|
|
53
|
Kramer RH and Nicolson GL: Interactions of
tumor cells with vascular endothelial cell monolayers: A model for
metastatic invasion. Proc Natl Acad Sci USA. 76:5704–5708. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kramer RH, Gonzalez R and Nicolson GL:
Metastatic tumor cells adhere preferentially to the extracellular
matrix underlying vascular endothelial cells. Int J Cancer.
26:639–645. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Srinivasan B, Kolli AR, Esch MB, Abaci HE,
Shuler ML and Hickman JJ: TEER measurement techniques for in vitro
barrier model systems. J Lab Autom. 20:107–126. 2015. View Article : Google Scholar : PubMed/NCBI
|