Introduction
Liver cancer is one of the most common malignant
tumors. According to the latest statistics, there were
approximately 782,500 new liver cancer cases and 745,500 deaths
that occurred worldwide during 2012 (1). Chemotherapy remains the primary
therapeutic strategy for malignancies. However, recurrence,
secondary cancer and normal tissue damage resulting from
chemotherapy bring clinical problems for the cancer survivor.
Therefore, it is urgent to develop the new and effective therapies
for cancer.
As initially described by Hayflick and Moorhead,
cellular senescence is an irreversible growth arrest of cells which
occurs in proliferative cells (2).
Cellular senescence can be induced by telomere shortening, DNA
damage and oncogene activation (3).
Previous studies demonstrated that cellular senescence is involved
in protection against cancer and important for tumor inhibition
(4–7). Given the tumor suppressive potential
of senescence, it was suggested that pro-senescence therapy may be
an effective way for anticancer therapy (8,9).
Triptolide (TPL) is diterpenoid extracted from the
plant, Tripterygium wilfordii Hook F (TWHF), which is a traditional
Chinese medicinal herb (10). TPL
has been shown to possess a unique and wide bioactivity, including
immunosuppressive, anti-fertility and anti-cystogenesis activities
(11). Recent studies have revealed
that TPL is effective against a broad range of cancer types,
including lung cancer (12,13), breast cancer (14,15),
colon cancer (16), gastric cancer
(15) and pancreatic cancer
(17).
TPL displays antitumor effect by inhibiting
proliferation, invasion, migration or inducing apoptosis. However,
little is known about the mechanism of TPL on cellular
senescence-associated antitumor effect. We studied the effect of
TPL on HepG2 cell senescence and tumor growth in vitro and
in vivo, as well as the underlying molecular mechanisms,
aiming at providing a promising strategy for cancer treatment and
drug development.
Materials and methods
Cell culture, chemicals and
transfection
HepG2 cells (Institute of Biochemistry and Cell
Biology of the Chinese Academy of Sciences) were cultured in
Dulbecco's modified Eagle's medium contained 10% (v/v) fetal bovine
serum, 100 U/ml penicillin and 100 µg/ml streptomycin. Cells were
treated with triptolide (TPL, Sigma-Aldrich) at the final
concentration of 0–10 nM and/or 1.0 µM MK2206 (Selleck Chemicals)
for the indicated period. Cells were cultured at 37°C in a humid
atmosphere containing 5% CO2. For overexpression of
hTERT in HepG2 cells, HepG2 cells were transfected with the
lentiviral constructs containing pre-hTERT.
Cell proliferation assay
HepG2 cells were seeded on 96-well plates at a
density of 1×105 cell/well and cultured for 24 h. Then
the cells were treated with triptolide for the indicated period.
Cell numbers were counted at the time point indicated in the
relevant figure legend.
TUNEL assay
Apoptotic DNA fragmentation was examined using the
One Step TUNEL Apoptosis Assay kit (C1089, Beyotime Institute of
Biotechnology, Haimen, China) according to the manufacture's
protocol. Briefly, cells were seeded into 24-well plates and
treated with 2.5 nM TPL for 1 day and 3 days, respectively. Then,
cells were fixed in 4% paraformaldehyde for 30 min at 4°C,
permeabilized in 0.1% Triton X-100 for 2 min on ice, followed by
the TUNEL assay for 1 h at 37°C. Cy3 (Cyanine 3)-labeled
TUNEL-positive cells were imaged under a fluorescence
microscope.
Annexin V apoptosis assay
Cell apoptosis quantification was performed by
Annexin V-FITC Apoptosis Detection kit (C1062, Beyotime Institute
of Biotechnology). In brief, cells were treated with TPL and then
washed with PBS. After the addition of 195 µl binding buffer, 5 µl
FITC-labeled Annexin V was added and incubated for 10 min at room
temperature. Then cells were incubated with 10 µl propidium iodide
(PI) for 10 min on ice in the dark and measured by FACS
analysis.
Cell cycle analysis
HepG2 cells (1.0×106) were trypsinized
and fixed in 75% ethanol at −20°C overnight. After washing with
PBS, the cells were resuspended in PBS buffer, supplemented with
100 µg/ml RNase A (Takara) at 4°C for 30 min. Then cells were
stained with PI (100 µg/ml) at 4°C for 30 min. Finally the samples
were analyzed by a flow cytometer (Becton Dickinson).
Senescence-associated β-gal
staining
Senescence-associated β-gal (SA-β-gal) activity was
carried out using SA-β-gal staining kit (Beyotime Biotechnology).
Briefly, after removing medium, HepG2 cells were washed with PBS
and fixed with 2% formaldehyde and 0.2% glutaraldehyde for 10 min.
Then, cells were washed with PBS and incubated with fresh SA-β-gal
stain solution overnight at 37°C (without CO2). After
staining, cells were photographed using a microscope (IX73,
Olympus), and the percentage of senescence cell was determined via
counting five random fields.
Real-time PCR
Total RNA was isolated using TRIzol kit (Invitrogen)
according to the manufacturer's directions. Real-time PCR was
performed using an ABI 7500 Real-Time PCR System (Applied
Biosystems) with the One Step SYBR PrimeScript™ Plus RT-PCR kit
(Takara). The sequences of primers are listed in Table I.
 | Table I.Primers used in RT-PCR. |
Table I.
Primers used in RT-PCR.
| Primer name | Sequence |
|---|
| p53 | F:
5′-GAGGGATGTTTGGGAGATGTAA-3′ |
|
| R:
5′-CCCTGGTTAGTACGGTGAAGTG-3′ |
| p21 | F:
5′-TGTCCGTCAGAACCCATGC-3′ |
|
| R:
5′-AAAGTCGAAGTTCCATCGCTC-3′ |
| cyclin D1 | F:
5′-GAACAAACAGATCATCCGCAAAC-3′ |
|
| R:
5′-GCGGTAGTAGGACAGGAAGTTG-3′ |
| hTERT | F:
5′-GCCTTCAAGAGCCACGTC-3′ |
|
| R:
5′-CCACGAACTGTCGCATGT-3′ |
| β-actin | F:
5′-ACAGAGCCTCGCCTTTGCCGA-3′ |
|
| R:
5′-CACGATGGAGGGGAAGACG-3′ |
Western blotting
Western blots were performed based on the standard
procedures. In brief, cells were lysed using ice-cold lysis buffer
(Takara). The protein concentrations were measured using a BCA
protein assay kit (Takara). For western blotting, protein extracts
were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred to polyvinylidene
difluoride (PVDF) membranes. After incubation with blocking buffer
(5% fat-free milk), the membranes were incubated with primary
antibodies (1:1000) overnight at 4°C and subsequently incubated
with HRP-conjugated secondary antibodies for 1 h at room
temperature. Finally, the membranes were exposed using ECL
Chemiluminescent Substrate Reagent kit (Thermo Scientific). Mouse
anti-human p53 monoclonal antibody (cat. no. ab1101), rabbit
anti-human p21 polyclonal antibody (cat. no. ab7960), rabbit
anti-human cyclin D1 monoclonal antibody (cat. no. ab16663), rabbit
anti-human AKT (phospho S473) polyclonal antibody (cat. no.
ab8932), rabbit anti-humanAKT monoclonal antibody (cat. no.
ab32505) and rabbit anti-human β-actin polyclonal antibody (cat.
no. ab8227) were purchased from Abcam.
Telomerase activity assay
HepG2 cells were lysed with ice-cold lysis buffer
(Takara). After centrifugation at 15,000 × g for 30 min, the
protein concentrations were measured using BCA protein assay kit
(Takara). Telomerase activity assay was performed by using the
telomeric repeat amplification protocol (TRAP) with the TeloTAGGG
Telomerase PCR ELISA kit (Roche) according to the instructions of
the manufacturer.
Establishment of a xenograft model in
nude mice
BALB/C nude mice were purchased from Shanghai Bikai
Lab Animal Co., Ltd. Xenograft was initiated by subcutaneous
injection of 1×107 HepG2 cells in logarithmic phase into
6–8 weeks old nude mice. After 7 days, mice were treated with TPL
(25 mg/kg) by intraperitoneal injection every day. There were 5
mice in each group. Weight of the mice and tumor growth were
monitored every 3 days. The tumor volumes were measured by
calipers. Tumor volumes were calculated according to the formula:
(0.5 × length × width2). All experimental protocols
using mice were approved by the Ethics Committee of Experimental
Animals of Fudan University (Shanghai, China) and all experiments
also conformed to the guidelines of the Chinese Association of
Laboratory Animals. After 16 days, the mice were sacrificed when
tumors reached a volume >2000 mm3, and tumors were
dissected and weighed.
Ki67 staining
After deparaffinized and dehydrated, sections were
immersed in methanol with 0.3% (vol/vol) H2O2
for 30 min and then heated in citrate buffer (10 mM, pH 6.0) at
120°C for 5 min in pressure cooker. Tumor tissue sections were
incubated with primary antibody for Ki67 (polyclonal rabbit
anti-human Ki67 antibody; 1:200, cat. no. ab15580; Abcam) overnight
at 4°C. Sections were incubated with biotinylated secondary
antibody at room temperature for 1 h after washing with PBS buffer.
Peroxidase activity was visualized with diaminobenzidine chromogen.
Slides were mounted in Entellan (Merck KGaA) and observed under
Olympus fluorescence microscopy (Olympus).
Statistical analysis
Data are shown as the mean ± standard deviation (SD)
using GraphPad Prism 5 software (GraphPad Software Inc.).
Comparison between groups was performed using Student t-test.
P<0.05 was considered statistically significant.
Results
TPL inhibits HepG2 cell proliferation
and accelerates cellular senescence and apoptosis
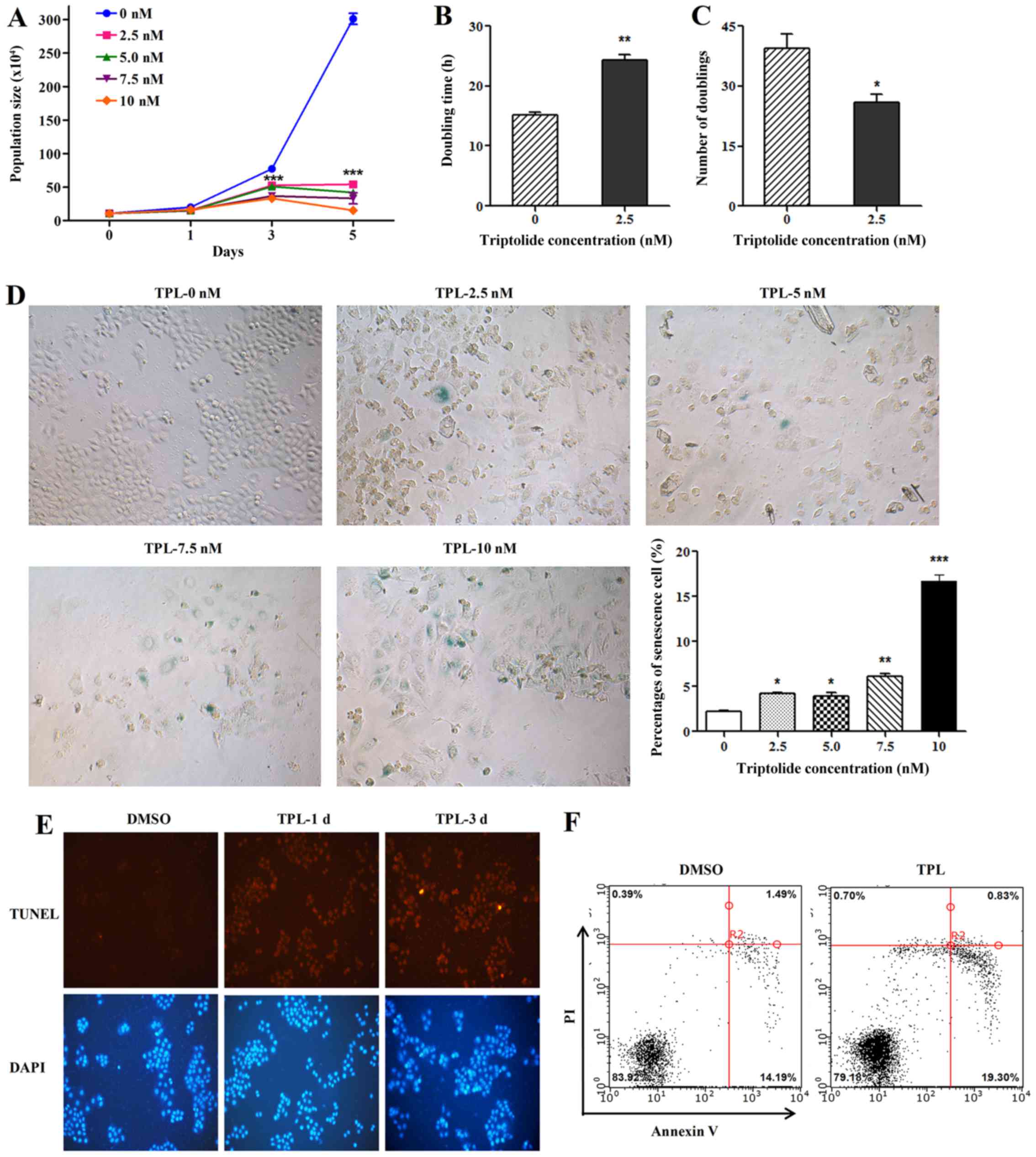
Compared with control cells, treatment of HepG2
cells with 2.5, 5.0, 7.5 and 10 nM TPL reduced the population size
(Fig. 1A). Continuous culturing of
HepG2 cells in a concentration of 2.5 nM TPL, the doubling time of
the cells increased significantly (Fig.
1B) while the number of doublings decreased (Fig. 1C) compared to the untreated
controls. To further determine the effect of TPL on cellular
senescence, the HepG2 cells were treated with 0. 2.5, 5.0, 7.5 and
10 nM TPL, respectively. As shown in Fig. 1D, TPL accelerated cellular
senescence in a dose-dependent manner. To test the effect of TPL on
cell apoptosis, TUNEL assay and flow cytometry were performed to
analyze level of apoptosis. Compared with control (DMSO) group,
apoptosis of cells in TPL treatment groups were elevated (Fig. 1E and F).
TPL affects cell cycle distribution
and related protein expression
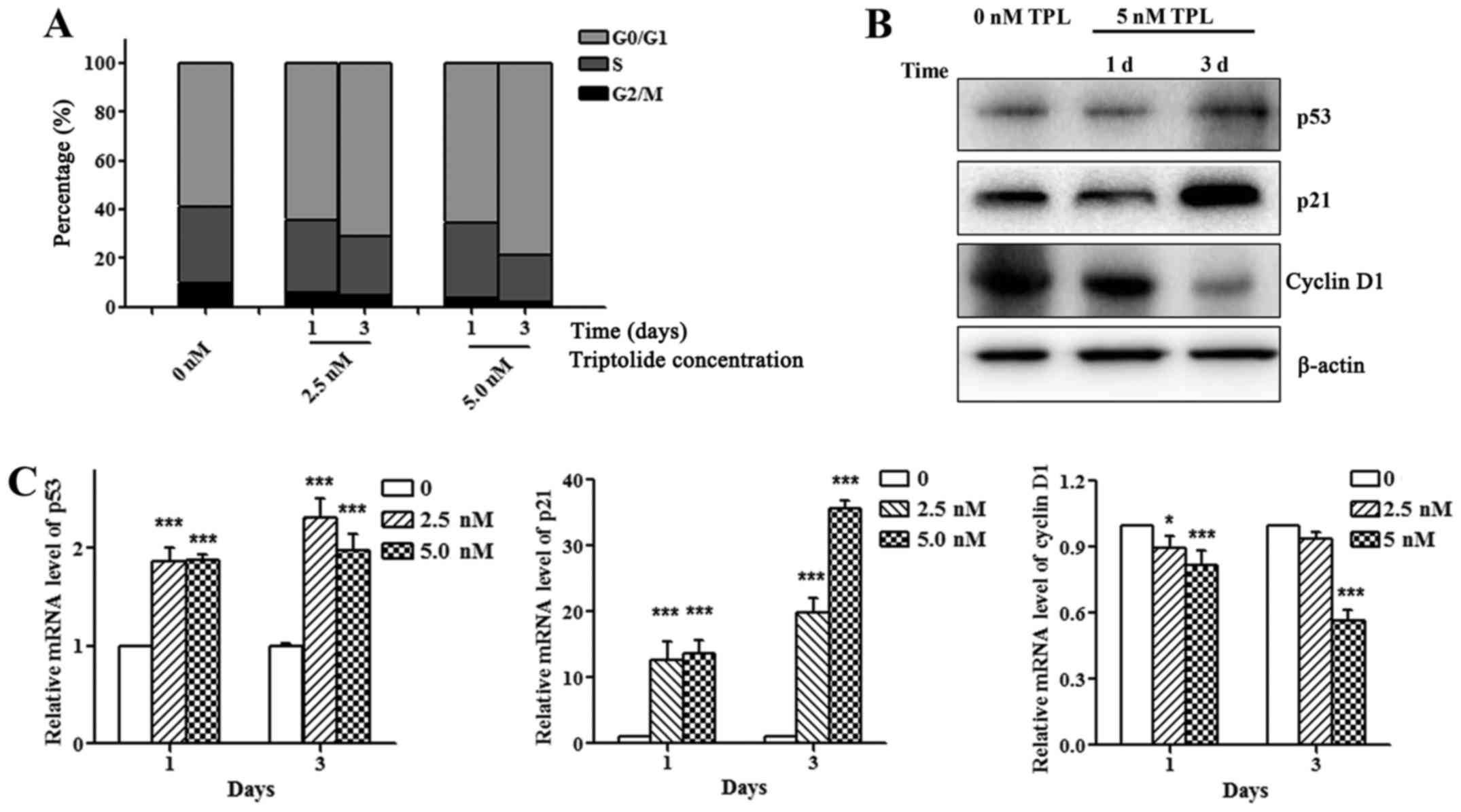
To determine the effect of TPL on cell cycle, HepG2
cells were treated with 0, 2.5, 5.0 nM TPL for 1, or 3 days.
Compared with control group, HepG2 cells treated with TPL had an
increased percentage of cells at G0/G1 phase and decreased
percentage of cells at G2/M phase (Fig.
2A). The p53/p21 signaling pathway is the key regulatory
pathway of the cell cycle. As shown in Fig. 2B, treatment of HepG2 cells with TPL
significantly increased the expression levels of p53 and p21 and
decreased the cyclin D1 expression (Fig. 2B and C), suggesting that TPL arrests
cells at G0/G1 phase by regulating the p53/p21 pathway.
TPL enhances cellular senescence by
activating AKT pathway
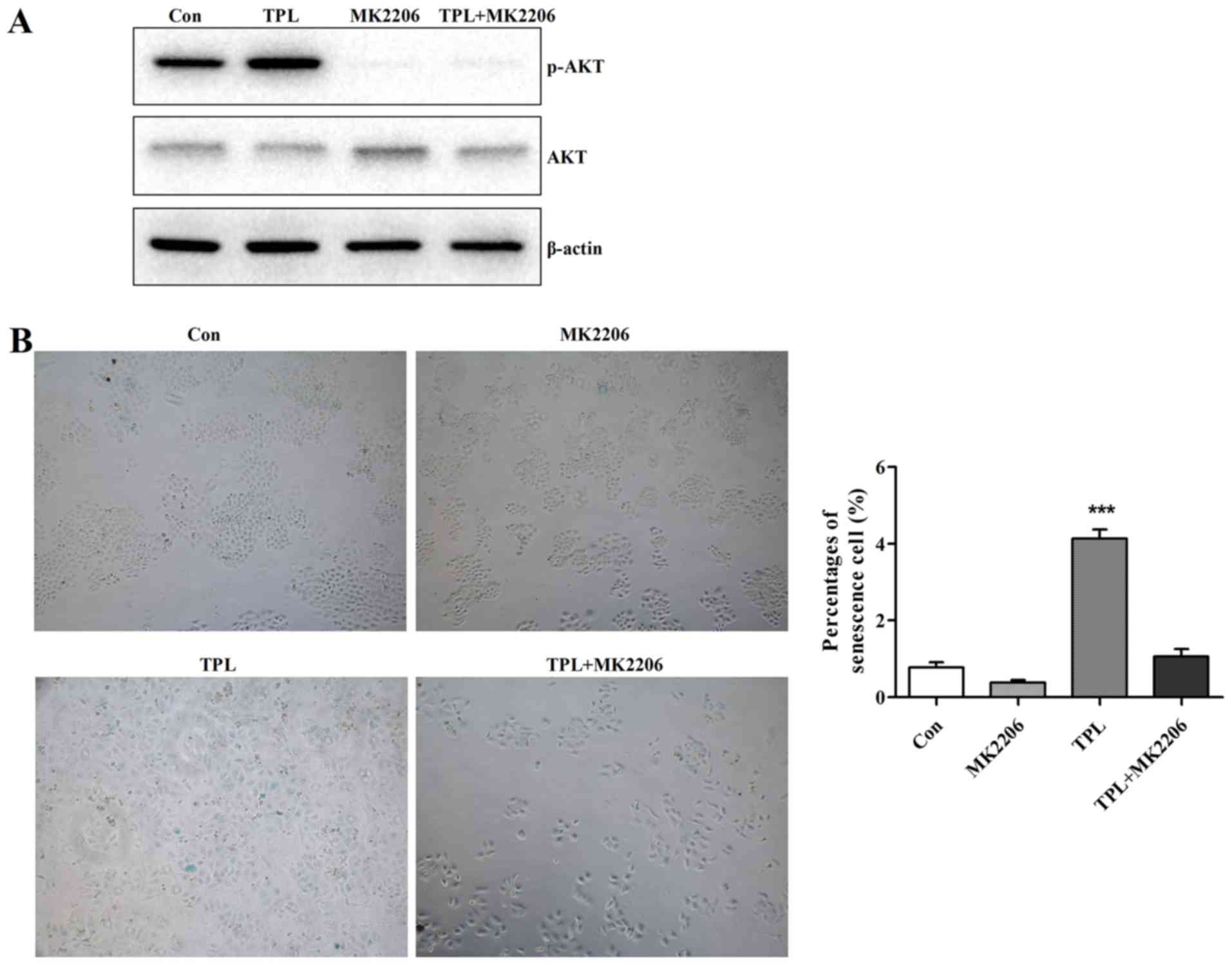
It has been reported that AKT activation is involved
in cell senescence (18,19). To examine whether AKT activity is
involved in TPL-induced HepG2 cell senescence, we detected the
phosphorylated AKT level. Treatment of HepG2 cells with TPL
significantly increased phosphorylated AKT level while
phosphorylated AKT level reduced after treatment with TPL and
MK2206 (AKT inhibitor) (Fig. 3A),
indicating that TPL could enhance phosphorylated AKT level and
activated the AKT pathway. Since AKT pathway was activated in
response to TPL, the MK2206 was used to determine whether
inactivation of AKT could relieve the acceleration of TPL on
cellular senescence. As shown in Fig.
3B, the MK2206 relieved the acceleration of TPL on cellular
senescence to a certain degree after treatment of HepG2 cells with
TPL and MK2206 simultaneously. Taken together, these results showed
that TPL activated the AKT pathway, contributing to acceleration of
HepG2 cell senescence.
TPL enhances cellular senescence by
inhibiting telomerase activity and hTERT expression
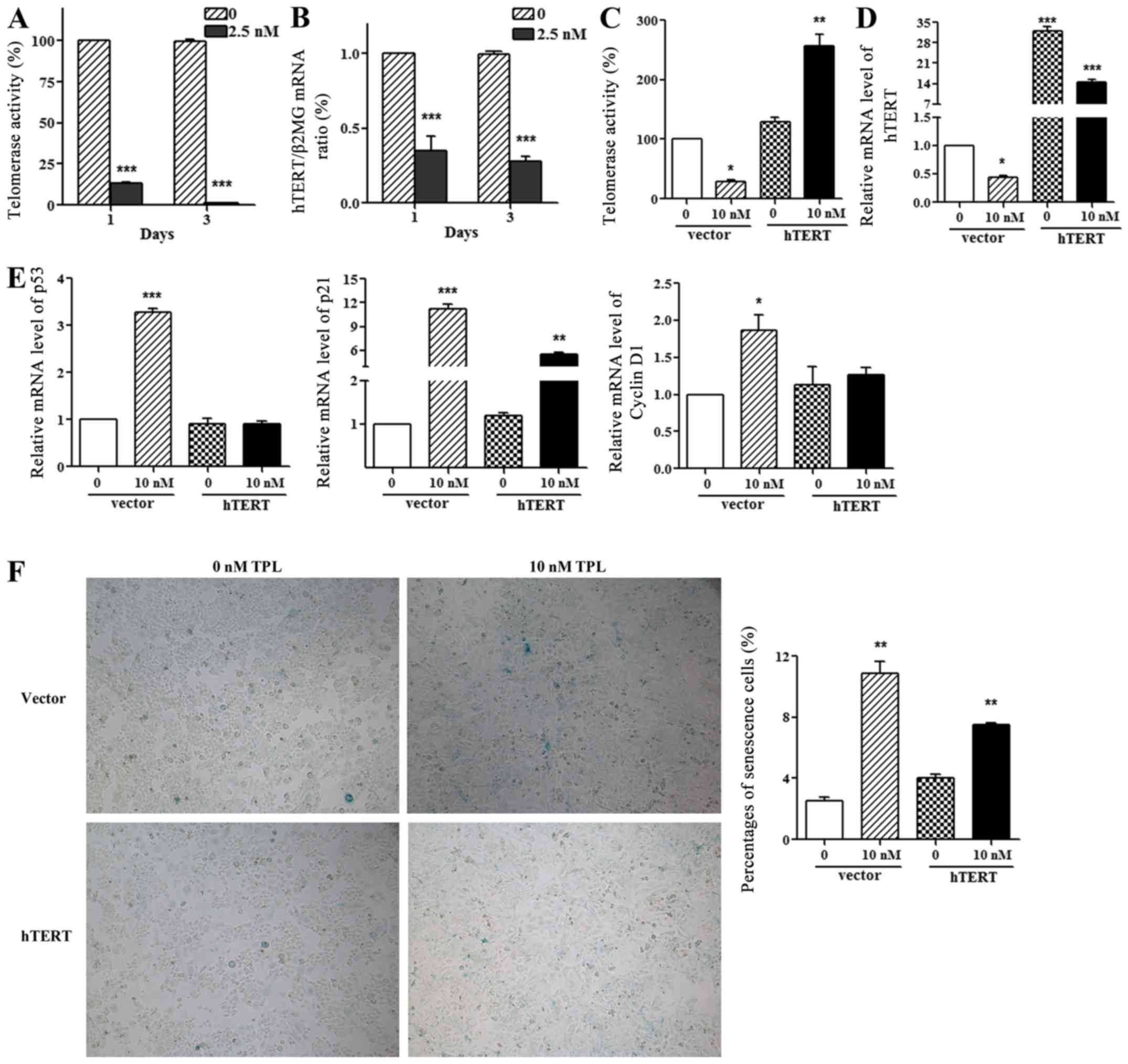
Telomere shortening and dysfunction are related to
cellular senescence. Thus, we measured telomerase activity and
hTERT expression after treating cells with 2.5 nM TPL for 1 day and
3 days. As shown in Fig. 4A and B,
TPL inhibited telomerase activity and hTERT expression in a
time-dependent manner. Since hTERT expression was inhibited by TPL,
the overexpression of hTERT was used to determine whether hTERT
overexpression could reverse the inhibition of TPL on telomerase
activity and hTERT expression. As shown in Fig. 4C and D, hTERT overexpression
reversed the inhibition of TPL on telomerase activity and hTERT
expression. Interestingly, we also found that hTERT overexpression
could relieve the promotion of TPL on p53/p21 pathway (Fig. 4E) and cellular senescence (Fig. 4F). Taken together, these results
indicated that TPL accelerates HepG2 cell senescence by negatively
regulating hTERT signaling pathway.
TPL inhibits tumor growth by
regulating hTERT pathway in vivo
A xenograft model was built to determine the effect
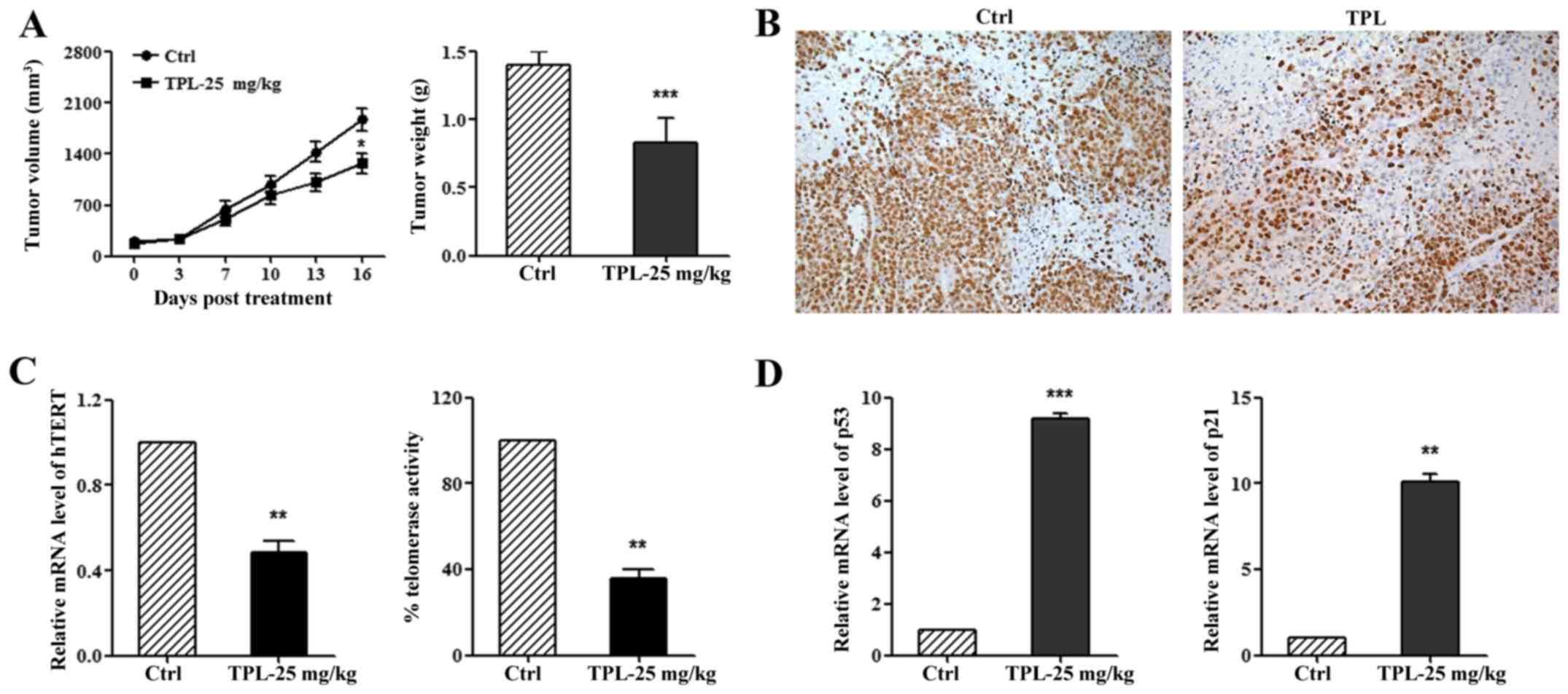
of TPL on tumor growth in vivo. Compared with control group,
tumor volume and weight reduced significantly in the TPL group
(Fig. 5A). Ki67 staining also
demonstrated inhibition of TPL on liver cancer cell proliferation
(Fig. 5B). We further studied the
mechanism of TPL on tumor growth in vivo and found that TPL
could inhibit hTERT expression and telomerase activity (Fig. 5C), moreover, TPL could also increase
p53 and p21 expression (Fig. 5D).
Taken together, these results showed that TPL downregulates the
hTERT pathway, contributing to inhibition of tumor growth.
Discussion
TPL has been shown to decrease cell proliferation
and induce apoptosis and cellular senescence in lung cancer
(20). In this study, we found that
TPL could inhibit HepG2 cell proliferation, accelerate cellular
senescence and cell apoptosis, and arrest cells at G0/G1 phase by
regulating the p53/p21 pathway. Cellular senescence, as a state of
irreversible cell cycle arrest, can be induced by multiple pathways
(21). AKT, a serine/threonine
protein kinase, has a key role in regulation of cell survival,
metabolism and protein synthesis (22). Increasing evidence indicates that
active AKT signaling pathway can also induce cellular senescence
(18,23–25).
However, the effect of AKT on cellular senescence in HepG2 cells is
still unclear. We found that TPL could promote phosphorylated AKT
level and activate AKT pathway, contributing to acceleration of
HepG2 cell senescence. Furthermore, it was reported that berberine
induced AKT activation and inhibited HepG2 cell survival (26), probably due to mTOR-dependent
feedback pathways (27), which were
consistent with our results. Taken together, these findings
suggested that TPL plays an important role in acceleration of HepG2
cell senescence by activating the AKT pathway.
As a special type of RNA nuclear protease,
telomerase maintains the telomere function and is involved in cell
senescence and carcinogenesis. High telomerase activity has been
found in malignant tumors while low telomerase activity is found in
majority of normal tissues and benign tumors (28). As a catalytic subunit of telomerase,
hTERT is the most important regulator of telomerase activity and
telomere length, which is overexpressed in >90% of tumor cells
and promotes tumor cell proliferation (29). Therefore, suppression of telomerase
activity and hTERT expression is one of the promising strategies in
anticancer therapy (30). In the
present study, we found that TPL treatment significantly inhibited
telomerase activity, hTERT expression and promoted cellular
senescence in HepG2 cells while hTERT overexpression reversed the
effect of TPL on telomerase activity, hTERT expression and cellular
senescence.
p53 plays an antitumor role and is closely
associated with differentiation, DNA repair, apoptosis and cell
cycle progression by modulating transcription of many genes
(31). There are two p53 binding
motifs at −1877 and −1240 related to the start of transcription in
hTERT genes. Overexpression of p53 or p21 inhibits the hTERT
promoter (32,33), while silencing of hTERT in HEK 293
cells could promote p53 and p21 transcription and repress cell
proliferation (31), suggesting
that hTERT may be drawn into a feedback loop system. Interestingly,
we also found that hTERT overexpression could relieve the promotion
of TPL on p53/p21 pathway. In addition, we demonstrated the effect
and mechanism of TPL on tumor growth in vivo, and found that
TPL inhibited tumor growth by negatively regulating the hTERT
pathway.
In conclusion, we demonstrated that TPL inhibited
tumor cell proliferation and growth in vitro and in
vivo, induced cellular senescence and cell apoptosis, and
arrested cells at G0/G1 phase. We also demonstrated that TPL
accelerated HepG2 cell senescence through AKT activation. Besides,
TPL could also enhance cellular senescence by inhibiting telomerase
activity and hTERT expression. These findings suggest that TPL
accelerated HepG2 cell senescence by regulating AKT pathway and
blocking hTERT pathway which resulted in inhibition of tumor
growth, providing a promising strategy for cancer treatment and
drug development.
Acknowledgements
This work was supported by the National Natural
Science Foundation of China (no. 81001327).
Glossary
Abbreviations
Abbreviations:
|
Triptolide
|
TPL
|
|
Tripterygium wilfordii Hook F
|
TWHF
|
|
human telomerase reverse transcriptase
|
hTERT
|
|
Senescence-associated β-gal
|
SA-β-gal
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hayflick L and Moorhead PS: The serial
cultivation of human diploid cell strains. Exp Cell Res.
25:585–621. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Collado M and Serrano M: Senescence in
tumours: Evidence from mice and humans. Nat Rev Cancer. 10:51–57.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Braig M, Lee S, Loddenkemper C, Rudolph C,
Peters AH, Schlegelberger B, Stein H, Dörken B, Jenuwein T and
Schmitt CA: Oncogene-induced senescence as an initial barrier in
lymphoma development. Nature. 436:660–665. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartkova J, Rezaei N, Liontos M,
Karakaidos P, Kletsas D, Issaeva N, Vassiliou LV, Kolettas E,
Niforou K, Zoumpourlis VC, et al: Oncogene-induced senescence is
part of the tumorigenesis barrier imposed by DNA damage
checkpoints. Nature. 444:633–637. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rufini A, Tucci P, Celardo I and Melino G:
Senescence and aging: The critical roles of p53. Oncogene.
32:5129–5143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Provinciali M, Cardelli M, Marchegiani F
and Pierpaoli E: Impact of cellular senescence in aging and cancer.
Curr Pharm Des. 19:1699–1709. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nardella C, Clohessy JG, Alimonti A and
Pandolfi PP: Pro-senescence therapy for cancer treatment. Nat Rev
Cancer. 11:503–511. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Acosta JC and Gil J: Senescence: A new
weapon for cancer therapy. Trends Cell Biol. 22:211–219. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kupchan SM, Court WA, Dailey RG Jr,
Gilmore CJ and Bryan RF: Triptolide and tripdiolide, novel
antileukemic diterpenoid triepoxides from Tripterygium wilfordii. J
Am Chem Soc. 94:7194–7195. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun L, Li H, Huang X, Wang T, Zhang S,
Yang J, Huang S, Mei H, Jiang Z and Zhang L: Triptolide alters
barrier function in renal proximal tubular cells in rats. Toxicol
Lett. 223:96–102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reno TA, Kim JY and Raz DJ: Triptolide
inhibits lung cancer cell migration, invasion, and metastasis. Ann
Thorac Surg. 100:1817–1824; discussion 1824–1815. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang XH, Wong BC, Lin MC, Zhu GH, Kung
HF, Jiang SH, Yang D and Lam SK: Functional p53 is required for
triptolide-induced apoptosis and AP-1 and nuclear factor-kappaB
activation in gastric cancer cells. Oncogene. 20:8009–8018. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li C, Xing G, Maeda K, Wu C, Gong L,
Sugiyama Y, Qi X, Ren J and Wang G: The role of breast cancer
resistance protein (Bcrp/Abcg2) in triptolide-induced testis
toxicity. Toxicol Res. 4:1260–1268. 2015. View Article : Google Scholar
|
|
15
|
Yang S, Chen J, Guo Z, Xu XM, Wang L, Pei
XF, Yang J, Underhill CB and Zhang L: Triptolide inhibits the
growth and metastasis of solid tumors. Mol Cancer Ther. 2:65–72.
2003.PubMed/NCBI
|
|
16
|
Wang Z, Jin H, Xu R, Mei Q and Fan D:
Triptolide downregulates Rac1 and the JAK/STAT3 pathway and
inhibits colitis-related colon cancer progression. Exp Mol Med.
41:717–727. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Phillips PA, Dudeja V, McCarroll JA,
Borja-Cacho D, Dawra RK, Grizzle WE, Vickers SM and Saluja AK:
Triptolide induces pancreatic cancer cell death via inhibition of
heat shock protein 70. Cancer Res. 67:9407–9416. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Astle MV, Hannan KM, Ng PY, Lee RS, George
AJ, Hsu AK, Haupt Y, Hannan RD and Pearson RB: AKT induces
senescence in human cells via mTORC1 and p53 in the absence of DNA
damage: Implications for targeting mTOR during malignancy.
Oncogene. 31:1949–1962. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sin S, Kim SY and Kim SS: Chronic
treatment with ginsenoside Rg3 induces Akt-dependent senescence in
human glioma cells. Int J Oncol. 41:1669–1674. 2012.PubMed/NCBI
|
|
20
|
Reno TA, Tong SW, Wu J, Fidler JM, Nelson
R, Kim JY and Raz DJ: The triptolide derivative MRx102 inhibits Wnt
pathway activation and has potent anti-tumor effects in lung
cancer. BMC Cancer. 16:4392016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Collado M, Blasco MA and Serrano M:
Cellular senescence in cancer and aging. Cell. 130:223–233. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dimitrova V and Arcaro A: Targeting the
PI3K/AKT/mTOR signaling pathway in medulloblastoma. Curr Mol Med.
15:82–93. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park JH, Kim JJ and Bae YS: Involvement of
PI3K-AKT-mTOR pathway in protein kinase CKII inhibition-mediated
senescence in human colon cancer cells. Biochem Biophys Res Commun.
433:420–425. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu Y, Li N, Xiang R and Sun P: Emerging
roles of the p38 MAPK and PI3K/AKT/mTOR pathways in
oncogene-induced senescence. Trends Biochem Sci. 39:268–276. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Binet R, Ythier D, Robles AI, Collado M,
Larrieu D, Fonti C, Brambilla E, Brambilla C, Serrano M, Harris CC,
et al: WNT16B is a new marker of cellular senescence that regulates
p53 activity and the phosphoinositide 3-kinase/AKT pathway. Cancer
Res. 69:9183–9191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu R, Zhang ZQ, Wang B, Jiang HX, Cheng L
and Shen LM: Berberine-induced apoptotic and autophagic death of
HepG2 cells requires AMPK activation. Cancer Cell Int. 14:492014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang HH, Lipovsky AI, Dibble CC, Sahin M
and Manning BD: S6K1 regulates GSK3 under conditions of
mTOR-dependent feedback inhibition of Akt. Mol Cell. 24:185–197.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim NW, Piatyszek MA, Prowse KR, Harley
CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL and Shay
JW: Specific association of human telomerase activity with immortal
cells and cancer. Science. 266:2011–2015. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ponnala S, Chetty C, Veeravalli KK, Dinh
DH, Klopfenstein JD and Rao JS: MMP-9 silencing regulates hTERT
expression via β1 integrin-mediated FAK signaling and induces
senescence in glioma xenograft cells. Cell Signal. 23:2065–2075.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Noureini SK and Wink M: Antiproliferative
effect of the isoquinoline alkaloid papaverine in hepatocarcinoma
HepG-2 cells - inhibition of telomerase and induction of
senescence. Molecules. 19:11846–11859. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lai SR, Cunningham AP, Huynh VQ, Andrews
LG and Tollefsbol TO: Evidence of extra-telomeric effects of hTERT
and its regulation involving a feedback loop. Exp Cell Res.
313:322–330. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kanaya T, Kyo S, Hamada K, Takakura M,
Kitagawa Y, Harada H and Inoue M: Adenoviral expression of p53
represses telomerase activity through down-regulation of human
telomerase reverse transcriptase transcription. Clin Cancer Res.
6:1239–1247. 2000.PubMed/NCBI
|
|
33
|
Harada K, Kurisu K, Sadatomo T, Tahara H
and Tahara E, Ide T and Tahara E: Growth inhibition of human glioma
cells by transfection-induced P21 and its effects on telomerase
activity. J Neurooncol. 47:39–46. 2000. View Article : Google Scholar : PubMed/NCBI
|