Introduction
Gastric cancer is the fourth most common cancer with
high morbidity and mortality worldwide (1,2).
Approximately 70% of new gastric cancer cases and gastric
cancer-related deaths occur in developing countries (3). Surgery is regarded as the first line
treatment for solid tumors, such as gastric cancer. Globally, there
are ~234 million individuals undergoing surgery with anesthesia
each year (4). Recently, general
anesthesia was reported to be associated with the risk of malignant
tumor recurrence (5). Therefore,
the role of anesthesia is vital for cancer development.
The inhibitor of growth family member 3 (ING3) is a
member of the ING tumor-suppressor family (6), which is mapped to 7q31.3 and consists
of 12 exons. ING3 was reported to play a significant role in
modulating transcription, cell cycle and apoptosis (7,8). It
could link the function of p53 to histone acetylation (9). ING3 was also reported to be reduced in
human head and neck squamous cell carcinomas (10). ING3 also plays pivotal roles in the
progression of human hepatocellular carcinoma (11) and colorectal adenoma, and may be a
potential target for cancer diagnosis and therapy (12). Although its expression has been
reported in various types of cancer, the role of ING3 in gastric
cancer has not been investigated.
Propofol (2,6-diisopropylphenol) is one of the most
widely used intravenous anesthetic agents during cancer resection
surgery. In addition to its anesthetic properties, propofol also
exhibits antitumor effects (13,14),
which can inhibit proliferation and invasiveness, and induce
apoptosis of cancer cells. Numerous studies have reported that
propofol has an inhibitory effect on cancer cells. For example,
propofol inhibited lung cancer cell proliferation and induced its
apoptosis (15). It has also shown
an inhibitory effect on the invasion and metastasis of cancer cells
such as lung (15), ovarian
(16) and prostate cancer (17), and osteosarcoma (18). There is no doubt that the antitumor
effect of propofol is extremely advantageous for the surgical
treatment of tumor patients. Recent studies have demonstrated that
propofol inhibited the growth of gastric cancer cells (19). However, the molecular mechanisms by
which it acts remain unclear. In the present study, we used gastric
cancer cell lines MGC-803 and SGC-7901 to explore the effects and
the mechanism of action of propofol on gastric cancer cells.
Materials and methods
Cell culture and treatment
Human gastric cancer cell lines (MGC-803 and
SGC-7901) and normal human gastric epithelial cell lines (GES-1 and
HFE145) were obtained from the Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China). The cells were grown
in Dulbecco's μodified Eagles medium (DMEM; Gibco, Rockville, MD,
USA) containing 10% fetal bovine serum (FBS; Invitrogen, Carlsbad,
CA, USA), 100 U/ml penicillin and 100 µg/ml streptomycin (Gibco)
and were maintained in a humidified 5% CO2 atmosphere at
37°C.
Transfection
MGC-803 and SGC-7901 cells were respectively
transfected with ING3 plasmid (pcDNA3-ING3) or ING3 siRNA plasmid
(pcDNA3-siING3) or negative control plasmid (pcDNA3-NC) using
Lipofectamine 2000 reagent (Invitrogen) according to the operating
protocols. The ING3 siRNA sequences (Ambion, Austin, TX, USA) were:
5′-GCUGAUAAUGCUGGAAUUAUU-3′ (sense) and 5′-UAAUUCCAGCAUUAUCAGCUU-3′
(antisense) (20). Twenty-four
hours later, stable transfected cells were selected with G418
(Sigma, St. Louis, MO, USA) and cultured for further study.
Transfection efficiency was monitored by qPCR and western
blotting.
Propofol treatment
To determine the effect of propofol on gastric
cancer, MGC-803 and SGC-7901 cells were exposed to 0, 5, 10 or 20
µM propofol (Sigma) for 72 h before collection for further
analysis. Propofol was diluted with dimethyl sulfoxide (DMSO;
Sigma).
Cell viability
Cell viability of the gastric cancer cells was
measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT; Sigma) assay. Briefly, the cells were seeded on
96-well plates (5×103 cells/well) and treated with
propofol for 72 h. Then, 20 µl of MTT (5 mg/ml) was added into each
well and incubated for another 4 h. The supernatant was aspirated.
DMSO (200 µl) was added to each well to dissolve MTT formazan. The
absorbance at 490 nm was measured using a microplate reader (BMG
Labtech, Inc., Durham, NC, USA). Viability = OD treatment/OD
control × 100%. The experiments were repeated three times
independently.
Migration and invasion assays
The invasion assay was performed using a 24-well
invasion chamber system coated with 50 µl diluted Matrigel (BD
Biosciences, Bedford, MA, USA) according to the manufacturer's
instructions. Cells were seeded in the upper chamber
(1×105 cells/well) in serum-free media. The lower
chamber was filled with media containing 10% FBS. After incubation
for 24 h, the migrating cell number was determined by counting five
random ×100 fields on each membrane under a microscope (Zeiss,
Oberkochen, Germany), and the mean values from three independent
experiments were used. A similar procedure was used for the
invasion assay. The only difference was that the upper chamber was
not coated with Matrigel.
Flow cytometry
Cell apoptosis was detected with Annexin V-FITC and
propidium iodide (PI) staining followed by flow cytometric (Beckman
Coulter Epics XL; Beckman Coulter, Krefeld, Germany) analysis.
Briefly, the cells were harvested and seeded into 96-well plates
and treated with propofol. A total of 3×105 cells were
stained with Annexin V-FITC/PI using the Annexin V-FITC/PI kit (BD
Pharmingen, San Diego, CA, USA).
Quantitative real-time PCR (qPCR)
Total RNA was extracted from gastric cancer MGC-803
and SGC-7901 and gastric epithelial cells GES-1 and HFE145 using
TRIzol reagent (Invitrogen). Reverse transcription was carried out
using the M-MLV Reverse Transcriptase System (Clontech, Palo Alto,
CA, USA), and qPCR was performed using SYBR Premix Ex Taq (Takara,
Dalian, China). GAPDH was used for normalization. The primer
sequences were as follows (12):
ING3 forward, 5′-CTTCACGGAAATGCG-3′ and reverse,
5′-CTCTTCCCTCCACTCA-3′; GAPDH forward,
5′-CTCAGACACCATGGGGAAGGTGA-3′ and reverse,
5′-ATGATCTT2GAGGCTGTTGTCATA-3′. The 2−ΔΔCt method was
used to quantify the relative gene expression. The relative
expression of ING3 was normalized to the expression of GAPDH.
Western blot analysis
Total protein was extracted from cells and separated
on 12% SDS-polyacrylamide gel. After electrophoresis, the proteins
were transferred to nitrocellulose membranes (Amersham, Little
Chalfont, UK), which were then blocked in 5% fat-free milk for 1 h
at 37°C. Subsequently, the membranes were probed with the primary
antibody for ING3 (1:1,000) or GAPDH (1:1,000) (both from Abcam,
Cambridge, UK) at 4°C overnight. Then, they were incubated with
HRP-conjugated anti-rabbit IgG secondary antibody (1:4,000; Abcam)
for 2 h, and then washed with Tris-buffered saline containing
Tween-20 (TBST). The proteins were finally visualized using
chemiluminescent ECL reagent (Santa Cruz Biotechnology, Santa Cruz,
CA, USA) with ImageJ software (Media Cybernetics, Inc., Rockville,
MD, USA).
Statistical analysis
All values are reported as mean ± SD. Statistical
analysis was performed using SPSS 20.0 (SPSS, Inc., Chicago, IL,
USA) by either two-sided Student's unpaired t-test or ANOVA test
with post t-test. Statistical significance was accepted at P-values
<0.05.
Results
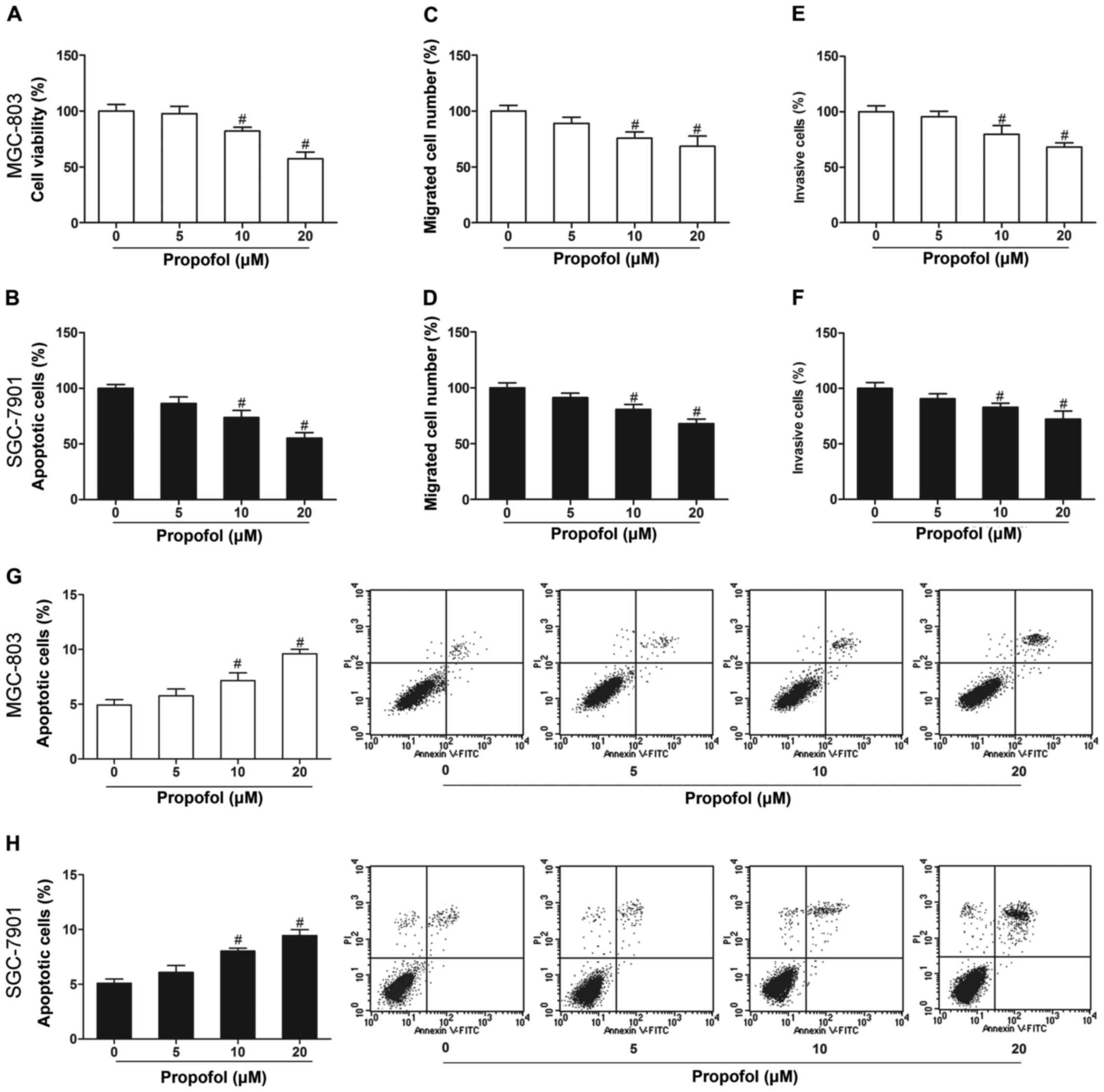
Propofol inhibits gastric cancer cell
growth and induces cell apoptosis
To elucidate the role of propofol in gastric cancer,
we first investigated the effects of propofol on cell
proliferation, invasion, migration and apoptosis. MGC-803 and
SGC-7901 cells treated with propofol (10 and 20 µM) showed markedly
decreased cell proliferation (Fig. 1A
and B), invasion (Fig. 1C and
D) and migration (Fig. 1E and
F), and increased apoptosis (Fig.
1G and H) in comparison with the untreated cells
(P<0.05).
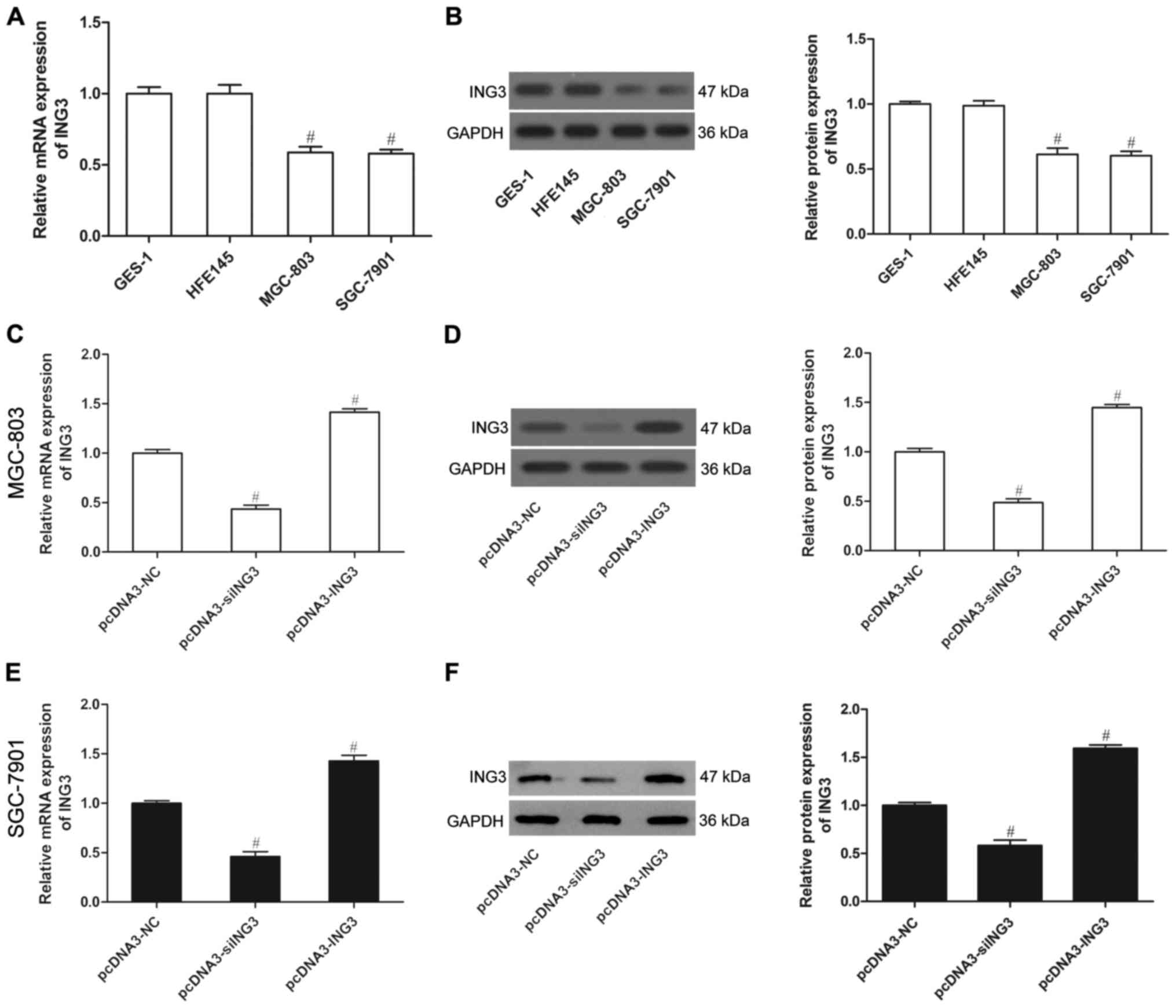
ING3 is expressed at low levels in
human gastric cancer cells
To investigate whether ING3 plays a role in gastric
cancer, we used two gastric cancer cell lines (MGC-803 and
SGC-7901) and two normal gastric epithelial cell lines (CES-1 and
HFE145) to analyze ING3 expression. The results showed that both
the mRNA expression and protein level of ING3 were weak in the
MGC-803 and SGC-7901 cells compared to these levels in the CES-1
and HFE145 cells (P<0.05; Fig. 2A
and B), as demonstrated by qPCR and western blot analysis,
respectively. This indicates that ING3 may play a crucial role in
the development of gastric cancer. To determine the critical role
of ING3 in gastric cancer, we generated MGC-803 and SGC-7901 cells
that stably expressed ING3 or siING3, in which the expression was
significantly upregulated or downregulated (Fig. 2C-F) (P<0.05).
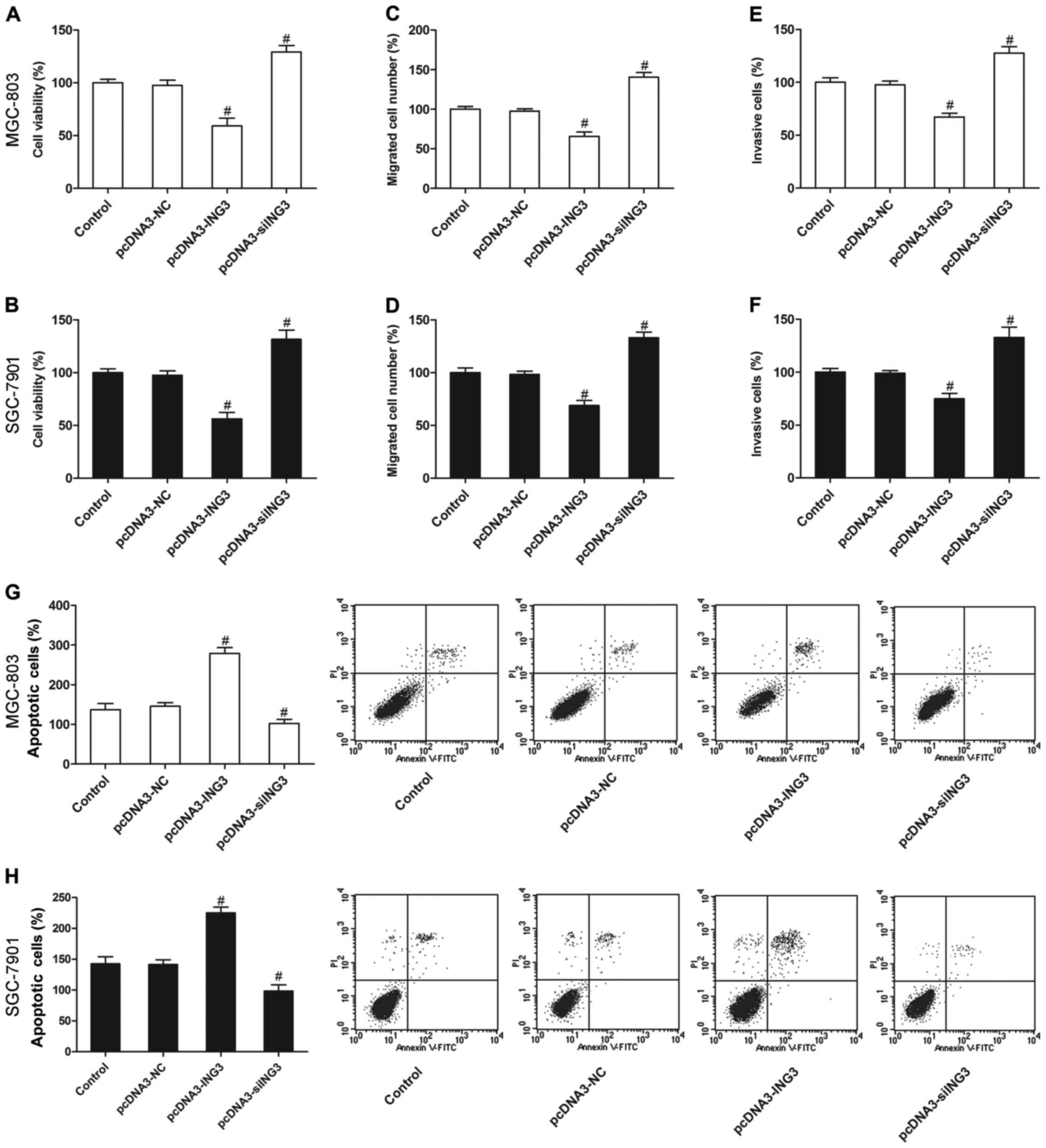
ING3 suppresses the survival and
induces apoptosis of gastric cancer cells
We then evaluated the effect of ING3 on the growth
of gastric cancer cells. The results revealed that ING3 also
significantly inhibited cell proliferation (Fig. 3A and B), invasion (Fig. 3C and D) and migration (Fig. 3E and F), and increased apoptosis
(Fig. 3G and H) compared with the
negative control and control groups (P<0.05). However, siING3
markedly promoted cell proliferation (Fig. 3A and B), invasion (Fig. 3C and D) and migration (Fig. 3E and F), and decreased apoptosis
(Fig. 3G and H) of the MGC-803 and
SGC-7901 cells (P<0.05).
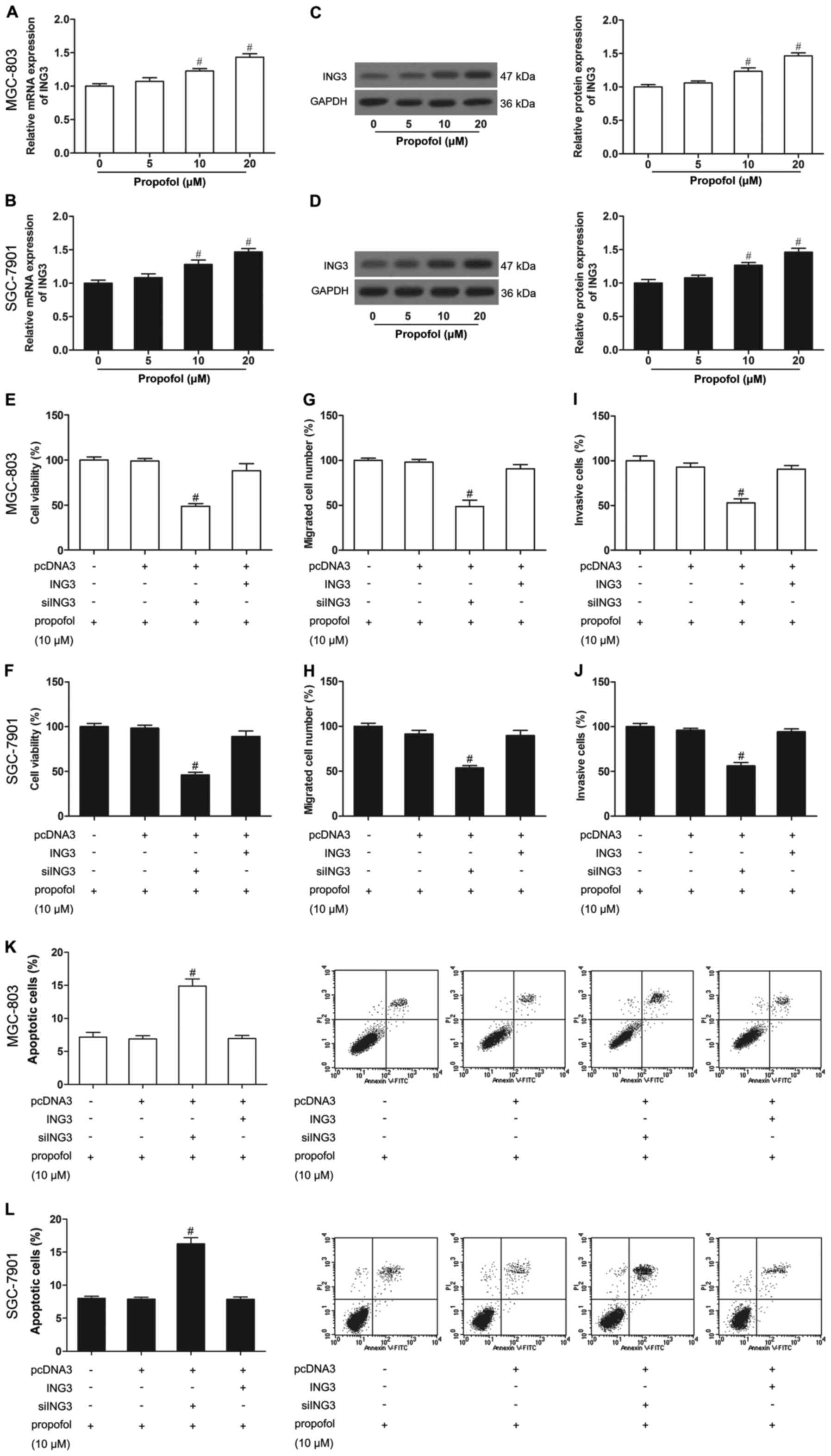
Propofol increases the expression of
ING3 in human gastric cancer cells
To study the correlation of propofol and ING3 in
gastric cancer, we detected ING3 expression in propofol-treated
MGC-803 and SGC-7901 cells. As shown in Fig. 4A-D, propofol-untreated cells induced
low levels of expression of ING3, while propofol-treated (10 and 20
µM) cells showed strong ING3 expression, demonstrating that the
ING3 level was increased by propofol.
ING3 promotes the effect of propofol
on cell growth
To further elucidate whether ING3 was responsible
for the effect of propofol on gastric cancer, we treated cells that
were transfected with ING3 or siING3 with propofol (10 µM). After
administration of propofol (10 µM), ING3-transfected cells showed a
significantly decreased cell proliferation (Fig. 4E and F), invasion (Fig. 4G and H) and migration (Fig. 4I and J), and an increased apoptosis
(Fig. 4K and L) in the MGC-803 and
SGC-7901 cells. In contrast, small interfering RNA (siRNA)-mediated
knockdown of ING3 in the MGC-803 and SGC-7901 cells, even those
treated with propofol (10 µM), exhibited significantly increased
cell proliferation (Fig. 4E and F),
invasion (Fig. 4G and H) and
migration (Fig. 4I and J), and
reduced apoptosis (Fig. 4K and L)
compared with the untreated MGC-803 and SGC-7901 cells. In other
words, siING3 reversed the effects of propofol, while ING3 promoted
the effects of propofol on cell growth.
Discussion
Gastric cancer is the second leading cause of
cancer-related death worldwide (21), with a low 5-year survival rate
(2). Mortality usually results from
recurrence or metastases. Surgical removal of the primary tumor is
of utmost importance for treatment (22). The increasing incidence of cancer
means that anesthesia is important, not only during the
perioperative period, but also during chronic cancer pain
management (23). It was reported
that anesthetic agents influence the pathophysiology of metastasis
in the postoperative period (23).
An appropriate anesthetic strategy could improve the long-term
survival of patients. Anesthetic agents and anesthesia techniques
play pivotal roles in the prevention of tumor metastasis and
recurrence.
In the present study, we found that propofol
suppressed the growth of both MGC-803 and SGC-7901 cells. Lower
ING3 expression was found in the MGC-803 and SGC-7901 cells than
that noted in the CES-1 and HFE145 cells. Propofol also increased
ING3 expression, affecting the biological behavior of gastric
cancer cells. We further found that ING3 was associated with the
inhibitory effect of propofol on MGC-803 and SGC-7901 cells.
Together, our studies provide evidence that propofol is an
important inhibitor of gastric cancer progression in vitro
by upregulating ING3, and suggest that ING3 is a promising
therapeutic target for gastric cancer.
Propofol, a commonly used intravenous anesthetic,
has been reported to exert many non-anesthetic effects (24). Increasing evidence suggests that
propofol suppresses the proliferation (25,26),
adhesion (27,28) and invasion (29,30) of
cancer cells, and induces their apoptosis (31,32).
Therefore, it may be beneficial to use propofol in cancer surgery,
for reasons other than its multiple anesthetic advantages. In the
present study, we verified that propofol was effective at
inhibiting the growth of gastric cancer cells, which was consistent
with previous studies (19).
Moreover, propofol enhanced the expression of ING3.
ING3, a candidate tumor-suppressor of the ING
family, plays an unquestionable role in modulating transcription,
cell cycle and apoptotic induction (33). Its downregulation may contribute to
tumorigenesis. Numerous studies have suggested that ING3 is a
functional tumor-suppressor gene in human cancers (10–12).
In the present study, ING3 expression in gastric cancer cells was
found to be lower than its expression in normal gastric epithelial
cells. These findings indicate that a downregulated ING3 level may
be involved in gastric carcinogenesis. ING3 transfection markedly
promoted the inhibitory effects of propofol on gastric cells,
whereas siING3 transfection reversed the effects of propofol,
suggesting that propofol plays a role in modulating gastric cancer
cells by targeting ING3.
In conclusion, the present study provides new
insights into the effects of propofol on the behavior of gastric
cancer cells and the mechanisms involved. Propofol may have
antitumor potential in gastric cancer, partly due to the
upregulation of ING3.
Acknowledgements
The present study was supported by grants from the
Science and Technology of Tianjin Health Bureau (no.
2015kz124).
References
|
1
|
Yoon H and Kim N: Diagnosis and management
of high risk group for gastric cancer. Gut Liver. 9:5–17. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang S, Chen P, Huang Z, Hu X, Chen M, Hu
S, Hu Y and Cai T: Sirt7 promotes gastric cancer growth and
inhibits apoptosis by epigenetically inhibiting miR-34a. Sci Rep.
5:97872015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xie Z and Xu Z: General anesthetics and
β-amyloid protein. Prog Neuropsychopharmacol Biol Psychiatry.
47:140–146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cakmakkaya OS, Kolodzie K, Apfel CC and
Pace NL: Anaesthetic techniques for risk of malignant tumour
recurrence. Cochrane Database Syst Rev. 11:CD0088772014.
|
|
6
|
Almami A, Hegazy SA, Nabbi A, Alshalalfa
M, Salman A, Abou-Ouf H, Riabowol K and Bismar TA: ING3 is
associated with increased cell invasion and lethal outcome in
ERG-negative prostate cancer patients. Tumour Biol. 37:9731–9738.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
He GH, Helbing CC, Wagner MJ, Sensen CW
and Riabowol K: Phylogenetic analysis of the ING family of PHD
finger proteins. Mol Biol Evol. 22:104–116. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Dai DL, Martinka M and Li G:
Prognostic significance of nuclear ING3 expression in human
cutaneous melanoma. Clin Cancer Res. 13:4111–4116. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Doyon Y, Selleck W, Lane WS, Tan S and
Côté J: Structural and functional conservation of the NuA4 histone
acetyltransferase complex from yeast to humans. Mol Cell Biol.
24:1884–1896. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gunduz M, Ouchida M, Fukushima K, Ito S,
Jitsumori Y, Nakashima T, Nagai N, Nishizaki K and Shimizu K:
Allelic loss and reduced expression of the ING3, a candidate tumor
suppressor gene at 7q31, in human head and neck cancers. Oncogene.
21:4462–4470. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu M, Chen F, Wang Q, Wang K, Pan Q and
Zhang X: Downregulation of inhibitor of growth 3 is correlated with
tumorigenesis and progression of hepatocellular carcinoma. Oncol
Lett. 4:47–52. 2012.PubMed/NCBI
|
|
12
|
Gou WF, Sun HZ, Zhao S, Niu ZF, Mao XY,
Takano Y and Zheng HC: Downregulated inhibitor of growth 3 (ING3)
expression during colorectal carcinogenesis. Indian J Med Res.
139:561–567. 2014.PubMed/NCBI
|
|
13
|
Miyata T, Kodama T, Honma R, Nezu Y,
Harada Y, Yogo T, Hara Y and Tagawa M: Influence of general
anesthesia with isoflurane following propofol-induction on natural
killer cell cytotoxic activities of peripheral blood lymphocytes in
dogs. J Vet Med Sci. 75:917–921. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song J, Shen Y, Zhang J and Lian Q: Mini
profile of potential anticancer properties of propofol. PLoS One.
9:e1144402014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cui WY, Liu Y, Zhu YQ, Song T and Wang QS:
Propofol induces endoplasmic reticulum (ER) stress and apoptosis in
lung cancer cell H460. Tumour Biol. 35:5213–5217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Su Z, Hou XK and Wen QP: Propofol induces
apoptosis of epithelial ovarian cancer cells by upregulation of
microRNA let-7i expression. Eur J Gynaecol Oncol. 35:688–691.
2014.PubMed/NCBI
|
|
17
|
Huang H, Benzonana LL, Zhao H, Watts HR,
Perry NJ, Bevan C, Brown R and Ma D: Prostate cancer cell
malignancy via modulation of HIF-1α pathway with isoflurane and
propofol alone and in combination. Br J Cancer. 111:1338–1349.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ye Z, Jingzhong L, Yangbo L, Lei C and
Jiandong Y: Propofol inhibits proliferation and invasion of
osteosarcoma cells by regulation of microRNA-143 expression. Oncol
Res. 21:201–207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang ZT, Gong HY, Zheng F, Liu DJ and Yue
XQ: Propofol suppresses proliferation and invasion of gastric
cancer cells via downregulation of microRNA-221 expression. Genet
Mol Res. 14:8117–8124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y and Li G: ING3 promotes UV-induced
apoptosis via Fas/caspase-8 pathway in melanoma cells. J Biol Chem.
281:11887–11893. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang G, Zhang Q, Song Y, Wang X, Guo Q,
Zhang J, Li J, Han Y, Miao Z and Li F: PAK1 regulates
RUFY3-mediated gastric cancer cell migration and invasion. Cell
Death Dis. 6:e16822015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Heaney A and Buggy DJ: Can anaesthetic and
analgesic techniques affect cancer recurrence or metastasis? Br J
Anaesth. 109:(Suppl 1). i17–i28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bajwa SJ, Anand S and Kaur G: Anesthesia
and cancer recurrences: The current knowledge and evidence. J
Cancer Res Ther. 11:528–534. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vasileiou I, Xanthos T, Koudouna E, Perrea
D, Klonaris C, Katsargyris A and Papadimitriou L: Propofol: A
review of its non-anaesthetic effects. Eur J Pharmacol. 605:1–8.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang JW, Cheng WW, Xu T and Yang ZY:
Propofol induces apoptosis and inhibits the proliferation of rat
embryonic neural stem cells via gamma-aminobutyric acid type A
receptor. Genet Mol Res. 14:14920–14928. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu YJ, Li SY, Cheng Q, Chen WK, Wang SL,
Ren Y and Miao CH: Effects of anaesthesia on proliferation,
invasion and apoptosis of LoVo colon cancer cells in vitro.
Anaesthesia. 71:147–154. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Yang H, Hu Q, Hou Y, Luo H and Liu
L: Effects of continuous sedation with propofol on peripheral blood
mononuclear cell and intercellular adhesion molecule in beagles
with combined burn-blast injuries. Zhonghua Yi Xue Za Zhi.
94:1573–1576. 2014.(In Chinese). PubMed/NCBI
|
|
28
|
Zhang J, Zhang D, Wu GQ, Feng ZY and Zhu
SM: Propofol inhibits the adhesion of hepatocellular carcinoma
cells by upregulating microRNA-199a and downregulating MMP-9
expression. Hepatobiliary Pancreat Dis Int. 12:305–309. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guo XG, Wang S, Xu YB and Zhuang J:
Propofol suppresses invasion, angiogenesis and survival of EC-1
cells in vitro by regulation of S100A4 expression. Eur Rev Med
Pharmacol Sci. 19:4858–4865. 2015.PubMed/NCBI
|
|
30
|
Wang ZT, Gong HY, Zheng F, Liu DJ and Dong
TL: Propofol suppresses proliferation and invasion of pancreatic
cancer cells by upregulating microRNA-133a expression. Genet Mol
Res. 14:7529–7537. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Man YG, Zhou RG and Zhao B: Efficacy of
rutin in inhibiting neuronal apoptosis and cognitive disturbances
in sevoflurane or propofol exposed neonatal mice. Int J Clin Exp
Med. 8:14397–14409. 2015.PubMed/NCBI
|
|
32
|
Chen J, Chen W, Zhu M, Zhu Y, Xu P and
Miao C: Angiotensin II-induced mouse hippocampal neuronal HT22 cell
apoptosis was inhibited by propofol: Role of neuronal nitric oxide
synthase and metallothinonein-3. Neuroscience. 305:117–127. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen G, Wang Y, Garate M, Zhou J and Li G:
The tumor suppressor ING3 is degraded by
SCFSkp2−mediated ubiquitin-proteasome system. Oncogene.
29:1498–1508. 2010. View Article : Google Scholar : PubMed/NCBI
|