Introduction
Primary central nervous system lymphoma (PCNSL) is a
distinct type of non-Hodgkin's lymphoma located in the brain,
leptomeninges, spinal cord, cerebrospinal fluid (CSF) and
intraocular structures, accounting for 2–3% of all brain tumors and
is associated with a dismal prognosis (1,2).
Approximately 95% of PCNSL cases are histologically characterized
as diffuse large B-cell lymphoma (DLBCL) (3). Although PCNSL shares some common
characteristics with systemic DLBCL, it has unique features based
on the transcription profiles and therapeutic protocols (4–8). In
clinical practice, patients with PCNSL have greatly benefitted from
well-established routine chemotherapy and radiotherapy strategies.
However, treating refractory and relapsed PCNSL is still a huge
challenge. Novel therapeutical strategies including immunotherapy
and targeted therapy by clinically available epigenetic modifiers
such as 5-aza-2′-deoxycytidine have been adopted as potential
options for these B-cell malignancies, which may shed light on the
potential clinical management of PCNSL.
The development of functional B cells is dependent
on the maturation of progenitor cells in the bone marrow, and
transcription factor networks play a fundamental role in B cell
differentiation. Recent evidence has revealed that most PCNSL cells
demonstrate an activated B-cell-like phenotype with constitutive
activation of the JAK/STAT and NF-κB signaling pathways (4,9,10).
Cytokines including IL-4 and IL-10 were found to stimulate B cell
proliferation and survival through upregulation of JAK1 and
constitutive activation of STAT3 and STAT6 in PCNSL (11–13).
In addition, oncostatin M (OSM) was found to trigger activation of
the JAK family, which in turn activated the STAT family in PCNSL,
while downregulation of SOCS1 and SOCS3 led to activation of the
JAK/STAT signaling pathway (9,14).
Molecules involved in the JAK/STAT signaling pathway may be
regulating factors contributing to the pathogenesis of PCNSL.
The Src homology region 2 domain-containing
phosphatase-1 (SHP1) is an important negative regulator involved in
the JAK/STAT signaling pathway through cytokine/growth factors
(15). SHP1 is a non-transmembrane
protein tyrosine phosphatase predominantly expressed in
hematopoietic cells (16–19), participating in various pathways
which negatively regulate molecular signals involved in cell
activation, proliferation, differentiation, and migration through
three categories of receptors (20). These receptors include growth factor
receptors with an intrinsic tyrosine kinase activity such as c-kit,
EGF, and CSF-1; cytokine receptors such as IL-2R, IL-3R, and Epo-R;
and receptor complexes involved in immune response such as TCR and
CD5 (21–23). A previous study reported that SHP1
expression in PCNSL reflects the origin of PCNSL from a late
germinal center to an early postgerminal center stage (24). It has previously been shown that
DLBCL expresses a very low level of SHP1 protein, suggesting that
loss of SHP1 may result in the development of DLBCL (25). In hematologic malignancies, the SHP1
gene silenced by aberrant CpG promoter methylation was found to
commonly induce a marked decrease in the SHP1 protein level,
indicating that SHP1 expression is predominantly regulated by DNA
methylation. Nevertheless, the impact of SHP1 methylation on the
development of PCNSL has not been comprehensively
characterized.
In this study, we applied immunohistochemistry and
pyrosequencing analysis to 33 PCNSL samples to investigate the SHP1
protein expression profile and promoter methylation status. By
evaluating molecular alterations in the JAK/STAT signaling pathway
through western blot analysis, we aimed to clarify the influence of
SHP1 and SHP1 promoter methylation on the pathogenesis of
PCNSL.
Patients and methods
Patients
Clinical data and tumor specimens from 33
immunocompetent patients with PCNSL were analyzed retrospectively.
Diagnosis of DLBCL was carried out by histologic review for all
specimens according to the Revised European-American Lymphoma and
WHO classification (26). For this
study, patients were selected on the basis of the availability of
paraffin-embedded tumor tissues and fresh-frozen tumor tissues. All
patients received chemotherapy regimens based on high-dose
methotrexate (HD-MTX). Patients who did not achieve complete
remission to HDMTX-based chemotherapy were given rescue whole brain
radiotherapy (WBRT). Informed consent from all the patients was
obtained. This study was approved by the Beijing Tiantan Hospital
Ethics Committee, Capital Medical University.
Tumor specimens
Tumor specimens were obtained during stereotactic
biopsy or surgery. Formalin-fixed and paraffin-embedded tumor
specimens were used for immunohistochemistry, whereas fresh-frozen
tumor specimens were stored at −80°C until extraction of DNA for
pyrosequencing analysis. Of the 33 fresh-frozen samples, only 8
samples for protein extraction were used for further western blot
analysis due to the quantity of tissue available after
pyrosequencing analysis. For normal control samples, we used 11
paraffin-embedded reactive hyperplasia of lymph nodes for
immunohistochemistry, peripheral blood from the 33 PCNSL patients
for pyrosequencing analysis and 1 fresh-frozen normal lymph node
for western blot analysis.
Immunohistochemical analysis
A panel of immunohistochemical staining was
performed on 4-µm thick sections (EnVision method). Sections were
deparaffinized in xylene and dehydrated with ethanol. Antigen
retrieval was carried out in a microwave oven for 15 min. And then
sections were incubated with a working dilution of each monoclonal
antibody: mouse anti-SHP1 monoclonal antibody (Sc-7289, 1:500
dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA),
monoclonal antibodies against CD10 (ZM-0283, 1:100 dilution), BCL-6
(ZM-0011, 1:50 dilution), MUM1 (ZM-0399; 1:50 dilution) and BCL-2
(ZA-0536, 1:100 dilution) from Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd. (Beijing, China). Reactive hyperplasia of
lymph nodes was used as positive controls.
Two pathologists assessed the immunohistochemical
staining independently. Fifteen to 20 fields at ×400 magnification
were analyzed per specimen. SHP1 immunohistochemical staining was
evaluated semiquantitatively by estimating the fraction of positive
cells according to the cut-off point as published by Sugita et
al (24). Tumor tissues with
more than 60% positively stained cells were considered (3+), 30–60%
(2+), 0–30% (1+), and no staining was considered (−). Only
cytoplasmic staining of cells was considered for grading. Staining
was considered positive for CD10, BCL-6, and MUM1 when more than
30% of tumor cells were positively stained (27). For BCL-2, staining was considered
positive when more than 50% of tumor cells expressed the BCL-2
protein according to a previously published method for systemic
DLBCL (28).
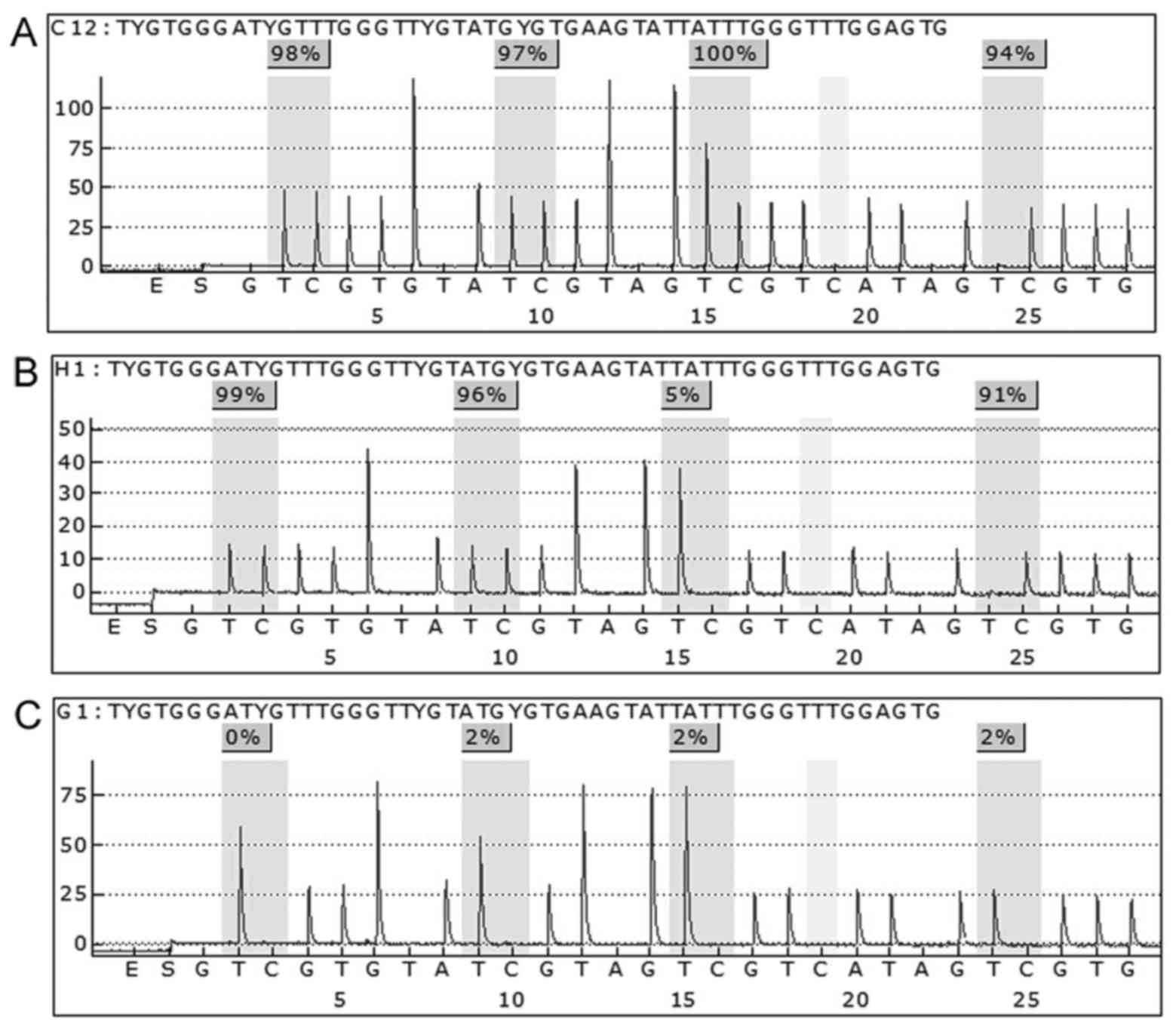
Pyrosequencing detection
Genomic DNA was extracted from 33 PCNSL frozen tumor
tissues and paired peripheral blood by using the QIAamp DNA Mini
kit (Qiagen). Pyrosequencing analysis was carried out by Gene-Tech
Co., Ltd. (Shanghai, China). For SHP1, 4 CpG sites were studied.
PCR primer sequences and sequencing primer are listed as follows:
GGTTGTGGTGAGAAATTAATTAGA (forward primer) and
CTCCAAACCCAAATAATACTTCA (reverse primer); and GGAGGAGGGAGAGATG
(sequencing primer). The annealing temperature was 54°C. A
bisulfite-treated sample (2 µl) was amplified in 40 µl of reaction
mixture, containing primers and 2.5 U of Takara Hot Start Taq
(Takara Biotechnology Co., Ltd.). The PCR product was purified to
obtain single-stranded DNA mixed with 40 µl sequencing buffer,
containing 0.5 µM sequencing primers. Sequencing analysis used the
PyroMark ID technique. Methylation data are presented as the
percentage of average methylation in all observed CpG sites.
Positive rate using the mean methylation level plus two times the
standard deviation (SD) of the control samples as a cut-off point
(29).
Western blot analysis
The 8 fresh-frozen tumor tissues and 1 normal lymph
node were ground and lysed in lysis buffer and proteinase inhibitor
(50:1). After incubation for 30 min on ice, the lysates were
subjected to centrifugation at 15,000 rpm for 15 min at 4°C. The
supernatants were collected after centrifugation. Proteins were
quantified using the Bicinchoninic Acid Protein Assay kit
(Beyotime, Nantong, China). A total of 40 µg protein was loaded
onto each lane of 12% polyacrylamide gel followed by
electrotransfer onto polyvinylidene fluoride membranes (Thermo
Fisher Scientific, Waltham, MA, USA). The membranes were then
blocked with skimmed milk powder in phosphate-buffered saline with
0.05% Tween-20 (PBST) for 2 h and incubated with the following
primary antibodies at 4°C overnight: mouse monoclonal antibody
against SHP1 (Sc-7289) was purchased from Santa Cruz Biotechnology,
Inc. and used at a dilution of 1:500; rabbit monoclonal antibody
against SHP1 phosphorylation (pSHP1) was purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA) and used at a
dilution of 1:1,000; mouse monoclonal antibody against STAT3 was
purchased from Cell Signaling Technology, Inc. and used at a
dilution of 1:1,000; antibody against GADPH was purchased from
TransGen Biotech Co., Ltd. (Beijing, China) and used at a dilution
of 1:2,000. Membranes were rinsed in PBST three times and incubated
with the secondary antibody at a dilution of 1:2,000 (horseradish
peroxidase-conjugated monoclonal goat anti-mouse IgG or horseradish
peroxidase-conjugated monoclonal goat anti-rabbit IgG; TransGen
Biotech Co., Ltd.) at room temperature for 1 h. Membranes were
rinsed in PBST three times again and were developed using an
enhanced chemiluminescence detection system (GE Healthcare,
Piscataway, NJ, USA).
Statistical analysis
Distribution of patient characteristics was applied
by the Chi-squared (χ2) test. The relationship between
SHP1 protein expression and the clinicopathologic variables was
evaluated by the Fisher's exact test and χ2 test. Two
group comparison was carried out using the Student's t-test or
Wilcoxon signed-rank test. All statistical analyses were performed
using the SPSS 17.0 software package. A value of p<0.05 was
considered statistically significant.
Results
Patient characteristics
The general information of the PCNSL patients
involved in this study is described in Table I. All PCNSLs were proven to be DLBCL
by pathological analysis and 29 (87.9%) specimens were obtained
from stereotactic biopsies. The median age was 57 years (range,
28–83 years) with a gender ratio of male-to-female of 1.75. Ten
patients (30.3%) were scored 0 or 1 by the Eastern Cooperative
Oncology Group (ECOG) performance status and the other 23 patients
(69.7%) scored 2–4 by the same scoring system. Multiple brain
lesions were observed in 63.6% of the patients (21/33) and deep
brain structures were compromised in 72.7% of the patients (24/33).
Among routine biochemical markers, an elevated serum lactate
dehydrogenase (LDH) level was observed in 13 of the 33 patients
(39.4%). For all samples from these patients, CD10, BCL-6, MUM1 and
BCL-2 positive expression was noted in 12.12% (4/33), 45.46%
(15/33), 87.88% (29/33) and 78.57% (22/28) of the cases,
respectively. According to the Hans classification criteria
(30), 4 cases (12.12%) were
classified as the germinal center B-cell-like (GCB) subgroup, and
the other 29 cases (87.88%) were non-GCB.
 | Table I.Clinical characteristics of the PCNSL
patients. |
Table I.
Clinical characteristics of the PCNSL
patients.
|
Characteristics | Patients, n
(%) |
|---|
| Age (years) |
|
|
>60 | 12 (36.4) |
|
≤60 | 21 (63.6) |
| Gender |
|
|
Male | 22 (66.7) |
|
Female | 11 (33.3) |
| LDH |
|
|
Elevated | 13 (39.4) |
|
Normal | 20 (60.6) |
|
Immunophenotype |
|
|
GCB | 4 (12.1) |
|
Non-GCB | 29 (87.9) |
| No. of lesions |
|
| 1 | 12 (36.4) |
| At
least 2 | 21 (63.6) |
| ECOG performance
status |
|
| 0 to
1 | 10 (30.3) |
| At
least 2 | 23 (69.7) |
| Deep structure
involvement |
|
|
Presence | 24 (72.7) |
|
Absence | 9 (27.3) |
|
Stereotactic biopsy | 29 (87.9) |
|
Surgery | 4 (12.1) |
| Treatment |
|
| HD-MTX
based chemotherapy | 33 (100) |
| Pathology |
|
|
DLBCL | 33 (100) |
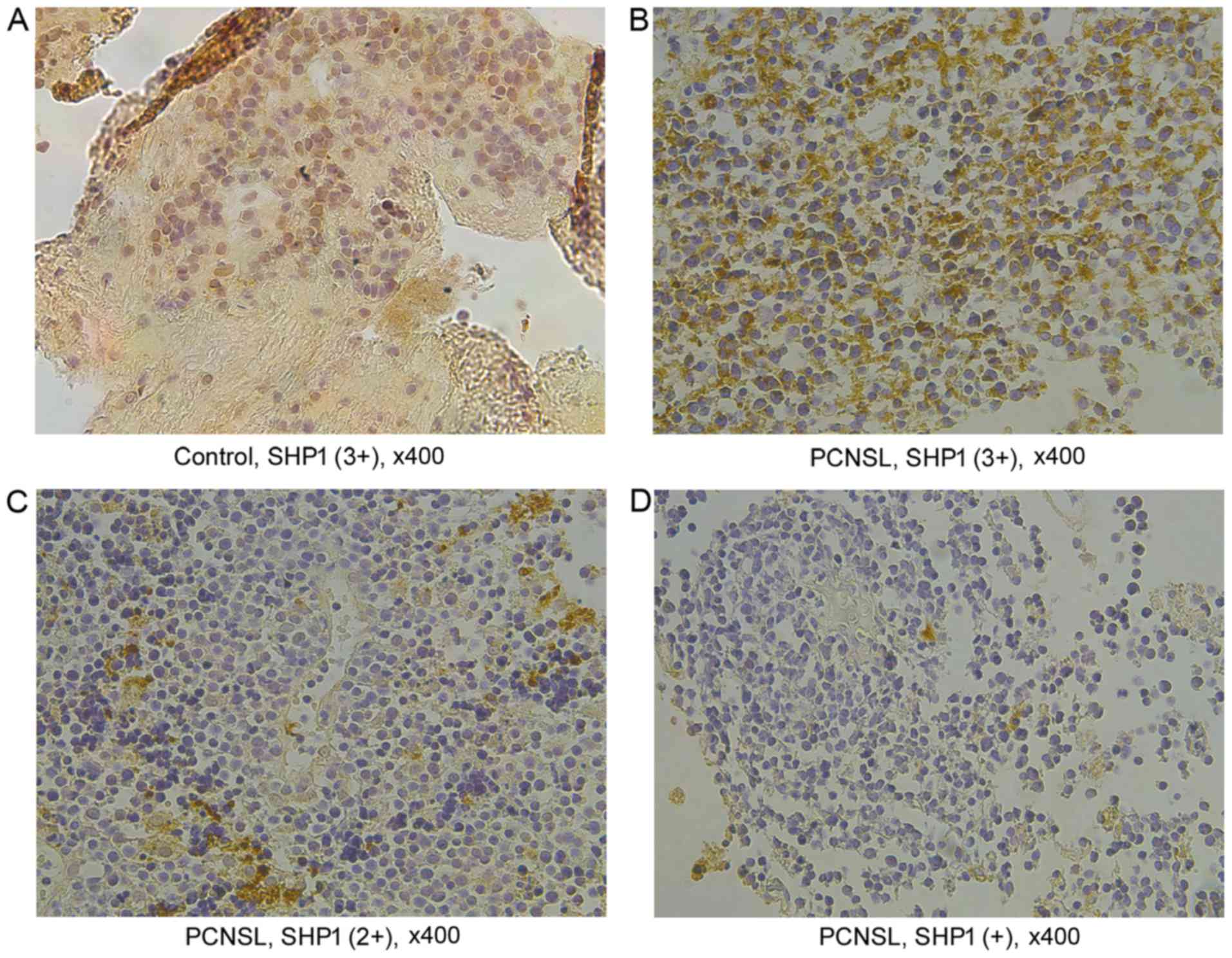
SHP1 protein expression was confirmed
by immunohistochemical staining
Immunohistochemical staining was conducted for SHP1
protein expression. Immunoreactivity for SHP1 was strong in
reactive hyperplasia of lymph node as a positive control and scored
as (3+) (Fig. 1A). SHP1-positive
immunostaining was detected in 30 PCNSL tissues (90.9%), of which,
17 positive cases showing a high SHP1 expression (over 60% positive
cells) were scored as (3+) (Fig.
1B); while the remaining 13 cases showed a low SHP1 expression
with less than 60% of positive cells in the tissues, including 9
cases of (2+) and 4 cases of (+), respectively (Fig. 1C and D).
SHP1 protein expression is correlated
with promoter methylation status in PCNSL patients
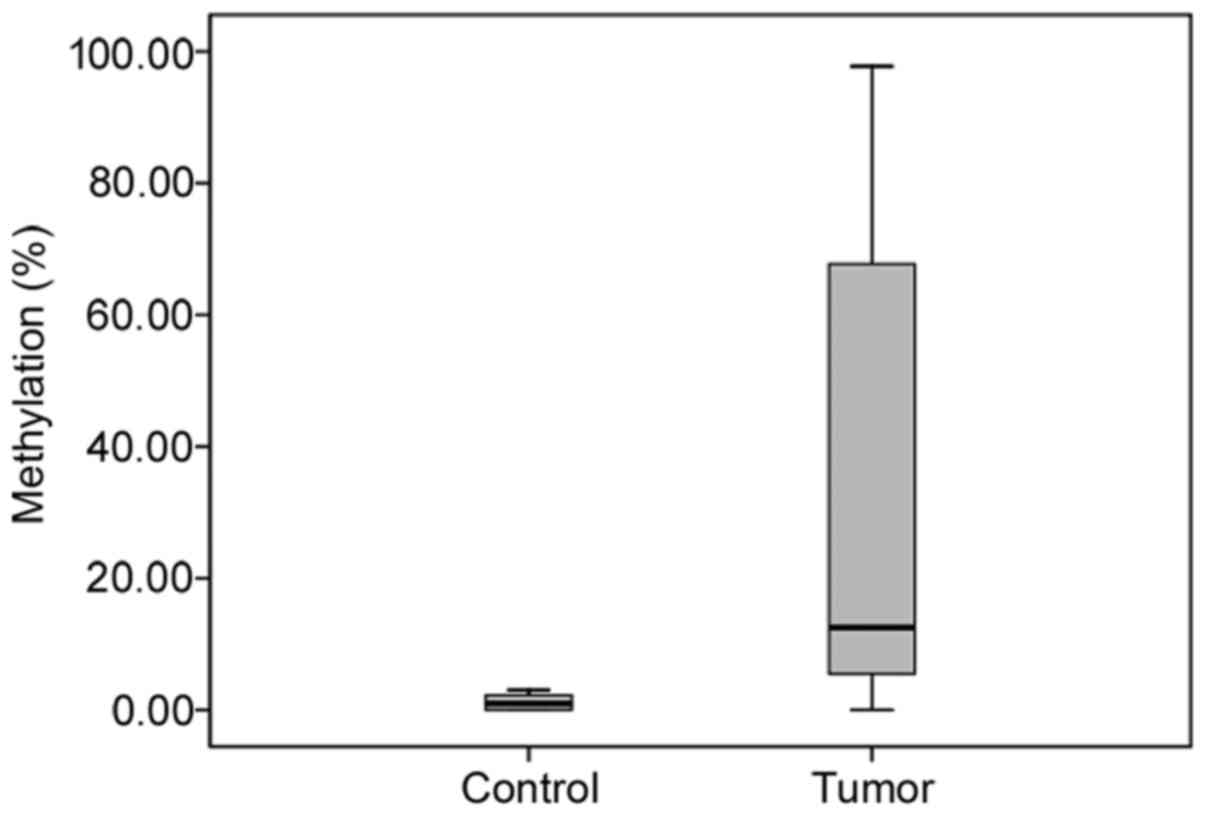
The SHP1 mean methylation level in the control
samples was 1.2±1.1%. SHP1 promoter methylation was detected in
29/33 of the tumor tissues (87.9%) with a mean methylation level of
31.7±36.5%. In this study, PCNSL tissues with a mean methylation
level of >3.4% (mean ± 2SD of the control samples) were
considered to have a bona fide methylation state, otherwise they
were considered unmethylated (Fig.
2). Compared with the methylation level of the PCNSL samples,
no significant methylation was observed in the control samples
(p<0.001) (Fig. 3).
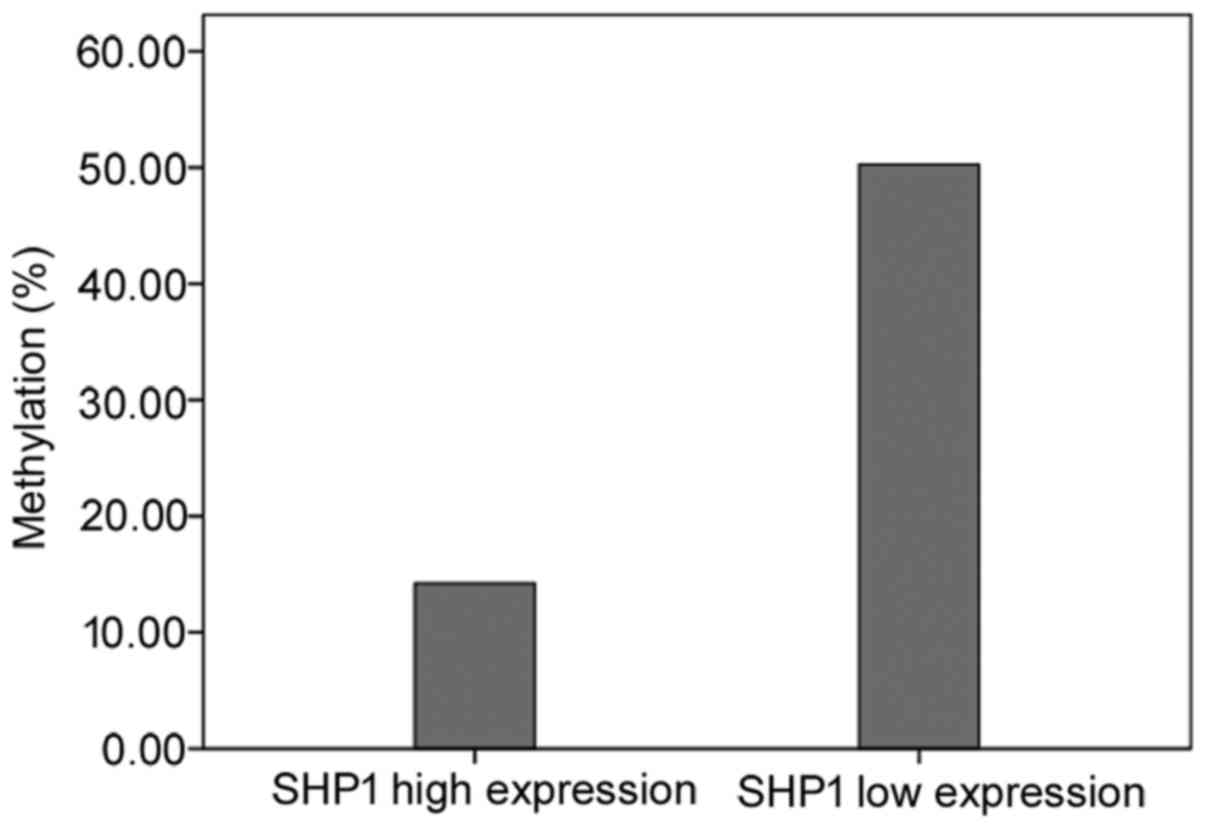
In 15 patients, the tumor tissues showed a low level
of SHP1 expression and promoter methylation, and 3 unmethylated
patients showed a high SHP1 expression. However, one unmethylated
patient had a decreased SHP1 protein expression. The methylation
levels in patients with a low SHP1 protein expression were higher
than those with a high SHP1 protein expression (50.3±38.9 vs.
14.2±24.0%, p=0.004) (Fig. 4).
Low SHP1 expression was present more frequently in
PCNSL patients with elevated LDH than those with a normal LDH level
(76.92 vs. 35.00%, p=0.03) (Table
II), while SHP1 protein expression was not related to the
indicated clinicopathologic variables routinely detected in
PCNSL.
 | Table II.Clinical characteristics of the
patients with PCNSL in relation to SHP1 protein expression
(Fisher's exact test). |
Table II.
Clinical characteristics of the
patients with PCNSL in relation to SHP1 protein expression
(Fisher's exact test).
|
| SHP1 protein
expression (n=33), n (%) |
|
|---|
|
|
|
|
|---|
| Variables | Low (n=16) | High (n=17) | P-value |
|---|
| Age (years) |
|
|
|
|
>60 | 7 (58.33) | 5 (41.67) | 0.48 |
|
≤60 | 9 (42.86) | 12 (57.14) |
|
| Gender |
|
|
|
|
Male | 11 (50.00) | 11 (50.00) | 1.00 |
|
Female | 5 (45.45) | 6 (54.55) |
|
| LDH |
|
|
|
|
Elevated | 10 (76.92) | 3 (23.08) | 0.03 |
|
Normal | 7 (35.00) | 13 (65.00) |
|
|
Immunophenotype |
|
|
|
|
GCB | 3 (75.00) | 1 (25.00) | 0.34 |
|
non-GCB | 13 (44.83) | 16 (55.17) |
|
| No. of lesions |
|
|
|
| 1 | 5 (41.67) | 7 (58.33) | 0.72 |
| At
least 2 | 11 (52.38) | 10 (47.62) |
|
| ECOG performance
status |
|
|
|
| 0 to
1 | 5 (50.00) | 5 (50.00) | 1.00 |
| At
least 2 | 11 (47.83) | 12 (52.17) |
|
| Deep structure
involvement |
|
|
|
|
Presence | 11 (45.83) | 13 (54.17) | 0.71 |
|
Absence | 5 (55.56) | 4 (44.44) |
|
Promoter methylation downregulates
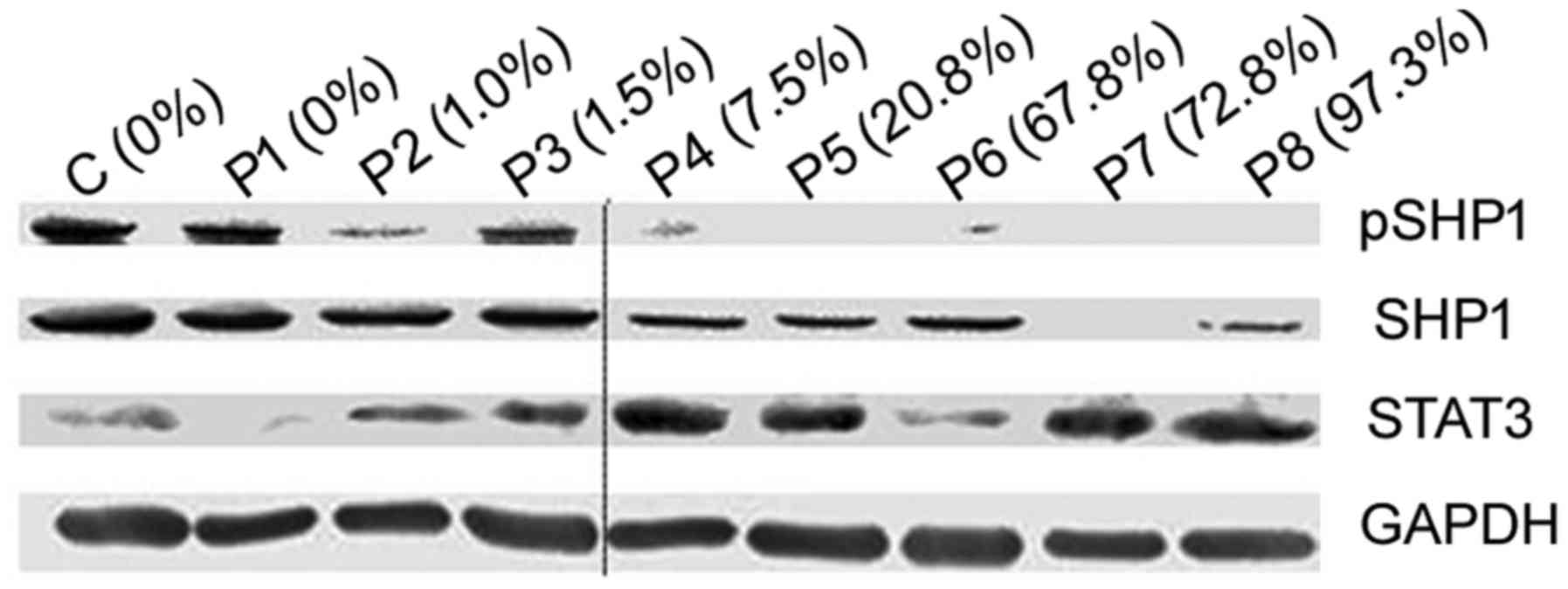
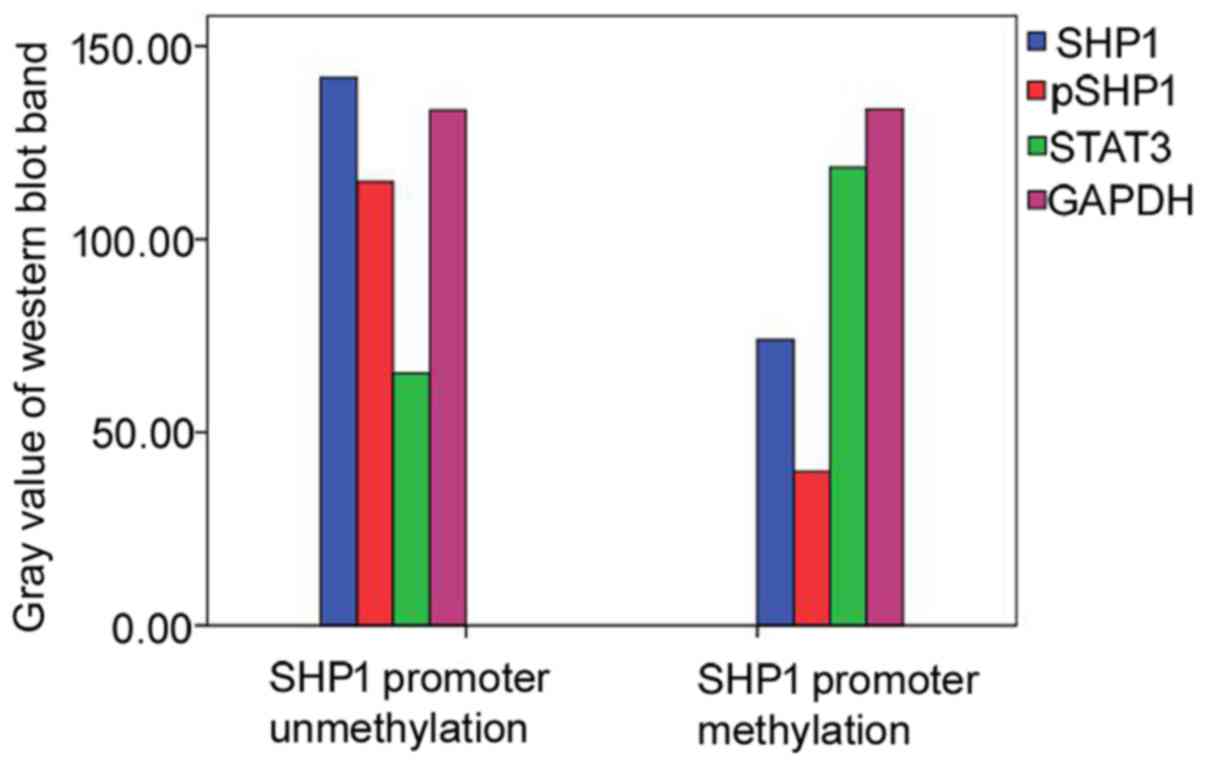
SHP1 expression and phosphorylation levels in PCNSL tissues
For the samples used for western blot analysis,
expression levels of SHP1, pSHP1 and STAT3 were detected and
analyzed. Compared with samples with unmethylation of the SHP1
promoter and controls, decreased SHP1 and pSHP1 expression was
observed in samples with SHP1 promoter methylation. Contrarily, the
STAT3 expression was substantially elevated, showing that SHP1
promoter methylation was associated with a decrease in SHP1
(p=0.001) and pSHP1 (p=0.005), and an increase in STAT3 (p=0.020)
in PCNSL. The experiments were performed three times on different
samples and a representative result is shown in Figs. 5 and 6.
Discussion
PCNSL is a rare form of extranodal lymphoma confined
to the central nervous system. Largely due to the low incidence and
obstacles to obtain tumor tissues, researchers are facing great
challenges for a complete recognition of PCNSL via genomic
analysis. Although the underlying genetic basis still remains
unclear, existing evidence has uncovered the constitutive
activation of JAK1, STAT3 and STAT6 in PCNSL. The constitutive
activation of the JAK/STAT signaling pathway can induce sustained
survival. Defining the key molecular pathways in PCNSL development
would provide clues for targeted therapies against these
malignancies.
In the present study, we confirmed that SHP1 protein
was expressed in PCNSL tissues. The majority of PCNSL patients
showed strong SHP1 protein expression, in line with results
reported by one previous study on PCNSL (24). Sugita et al showed that SHP1
expression in PCNSL reflects the origin of PCNSL from a late
germinal center to an early postgerminal center stage (24). Kossev et al showed that
normal B cells in the mantle and marginal zones as well as plasma
cells uniformly express SHP1. However, specific areas of the
germinal centers showed no SHP1 expression (31). These results collectively suggest
that SHP1 is downregulated at antigen-dependent stages of B-cell
proliferation and differentiation. Moreover, this pattern of SHP1
protein expression was also observed in small B cell lymphomas,
including mantle cell lymphoma, marginal zone lymphoma and chronic
lymphocytic leukemia/small lymphocytic lymphoma whereas follicle
center cell lymphoma did not. In this study, SHP1 expression was
positive in the majority of PCNSL cases with lower expression of
germinal center marker CD10, confirming their post-germinal center
origin. Notably, we found that 17 of 30 SHP1-positive cases were
scored as 3+, showing a percentage less than the result reported by
Sugita et al who found that all SHP1-positive cases strongly
expressed and were scored as 3+ (24). By comparing the results vis-à-vis,
we believe that the alteration in the SHP1 expression profile in
PCNSL per se and experimental variations may contribute to the
discrepancy in expression intensity interpretations.
Our results indicate that SHP1 expression and
phosphorylation are suppressed by SHP1 promoter methylation. SHP1
gene methylation associated with loss of SHP1 protein has been
demonstrated in many hematologic cancers. To clarify the difference
in SHP1 protein expression intensity, we performed pyrosequencing
analysis and western blot analysis. We found that SHP1 promoter
methylation occurred in 29 of 33 PCNSL cases, and aberrant promoter
SHP1 promoter methylation decreased the SHP1 protein expression, in
accordance with the results noted in extranodal lymphomas (32,33).
SHP1 plays a critical role in the regulation of the JAK/STAT
pathway (15,34,35).
The biological features of SHP1 were emphasized by the ‘moth-eaten’
(mev/mev) mouse model, which showed markedly decreased SHP1
expression and defects in myeloid cell function with a tendency for
developing lymphomas (36,37). SHP1 gene methylation associated with
loss of SHP1 protein has been demonstrated in many hematologic
cancers. In view of the normal biological significance of SHP1,
SHP1 likely plays an important role in the pathogenesis of these
cancers. Our data indicate that abnormal SHP1 expression
contributes to the constitutive activation of the JAK/STAT
signaling pathway in the pathogenesis of PCNSL.
In addition, our study demonstrated that SHP1
promoter methylation did not correlate with a complete absence of
SHP1 protein expression. Several studies have also indicated this
phenomenon and have suggested that monoallelic promoter methylation
may not sufficiently cause complete loss of gene expression or
promoter methylation, only in a small portion of tumor cells
(38,39), presumably due to the fact that
promoter methylation may be one of the multiple causative
mechanisms in tumor cells. Notably, this phenomenon was not limited
to the SHP1 gene (38–41). In this study, the SHP1 promoter
methylation did not cause a complete loss but a decrease in SHP1
protein expression, and the phosphorylation level reflecting the
biological activities of SHP1 protein was synchronously decreased.
Notably, our further analysis of the downstream signals indicated
that the absence of or reduction in the phosphatase activity of
SHP1 contributed to STAT3 activation in PCNSL. These results
support the notion that promoter methylation contributes to
decreased SHP1 protein expression and function, eventually
affecting the pathogenesis of PCNSL.
Additionally, in our study, a low-level expression
of SHP1 was associated with an elevated LDH level, which was
similar to previous reports (42,43).
Our preliminary study showed that a high LDH concentration was
associated with a poor outcome in PCNSL (44). Currently, the prognostic value of
SHP1 protein expression and SHP1 promoter methylation has not been
well acknowledged in PCNSL. Nevertheless, in other tumor types
including DLBCL, anaplastic large cell lymphoma (ALCL), prostate
cancer and high-grade glioma, SHP1 has been proposed as a
prognostic factor (42,45,46).
In these studies, the SHP1 promoter methylation status and loss of
SHP1 protein expression commonly indicate a poor prognosis.
Given that PCNSL increasingly occurs in elderly
populations, in which a large proportion of patients are not
eligible for routine intensive therapies such as HD-MTX
chemotherapy, novel therapies which target key survival molecular
points in signaling pathways of PCNSL would enhance the overall
patient outcomes. In view of the fact that SHP1 promoter
methylation and constitutive activation of the JAK/STAT pathway are
co-existent in PCNSL, epigenetic therapeutic strategies by
clinically available DNA demethylation agent 5-aza-2′-deoxycytidine
may be an effective approach. Most importantly, the demethylation
anchor drug 5-aza-2′-deoxycytidine penetrates the blood-brain
barrier, providing further merit for the potential clinical
application. Currently, how to achieve an effective and safe
concentration of such agents in the central nervous system is a
critical challenge. Thus, further prospective studies are needed to
validate these results and to develop optimal therapies using
epigenetic modifiers for PCNSL treatment.
In conclusion, we report in the present study that
aberrant methylation of the SHP1 promoter is correlated with
decreased SHP1 expression and phosphorylation. Attenuation of the
biological functions of SHP1 contributes to the activation of
transcription factor STAT3, which may affect the pathogenesis of
PCNSL. Epigenetic modifiers such as 5-aza-2′-deoxycytidine may be
of significance for the development of methylation-targeted
therapeutic regimens.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81272842 awarded to Y.L.
and no. 81500157 awarded to Q.C.). We thank Dr Young Whang for
review of the manuscript.
References
|
1
|
Batchelor T and Loeffler JS: Primary CNS
lymphoma. J Clin Oncol. 24:1281–1288. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang CC, Carnevale J and Rubenstein JL:
Progress in central nervous system lymphomas. Br J Haematol.
166:311–325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Campo E, Swerdlow SH, Harris NL, Pileri S,
Stein H and Jaffe ES: The 2008 WHO classification of lymphoid
neoplasms and beyond: Evolving concepts and practical applications.
Blood. 117:5019–5032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rubenstein JL, Fridlyand J, Shen A, Aldape
K, Ginzinger D, Batchelor T, Treseler P, Berger M, McDermott M,
Prados M, et al: Gene expression and angiotropism in primary CNS
lymphoma. Blood. 107:3716–3723. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ponzoni M, Issa S, Batchelor TT and
Rubenstein JL: Beyond high-dose methotrexate and brain
radiotherapy: Novel targets and agents for primary CNS lymphoma.
Ann Oncol. 25:316–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jordanova ES, Riemersma SA, Philippo K,
Giphart-Gassler M, Schuuring E and Kluin PM: Hemizygous deletions
in the HLA region account for loss of heterozygosity in the
majority of diffuse large B-cell lymphomas of the testis and the
central nervous system. Genes Chromosomes Cancer. 35:38–48. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tun HW, Personett D, Baskerville KA, Menke
DM, Jaeckle KA, Kreinest P, Edenfield B, Zubair AC, O'Neill BP, Lai
WR, et al: Pathway analysis of primary central nervous system
lymphoma. Blood. 111:3200–3210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Booman M, Szuhai K, Rosenwald A, Hartmann
E, Kluin-Nelemans H, de Jong D, Schuuring E and Kluin P: Genomic
alterations and gene expression in primary diffuse large B-cell
lymphomas of immune-privileged sites: The importance of apoptosis
and immunomodulatory pathways. J Pathol. 216:209–217. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sung CO, Kim SC, Karnan S, Karube K, Shin
HJ, Nam DH, Suh YL, Kim SH, Kim JY, Kim SJ, et al: Genomic
profiling combined with gene expression profiling in primary
central nervous system lymphoma. Blood. 117:1291–1300. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deckert M, Montesinos-Rongen M, Brunn A
and Siebert R: Systems biology of primary CNS lymphoma: From
genetic aberrations to modeling in mice. Acta Neuropathol.
127:175–188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Komohara Y, Horlad H, Ohnishi K, Ohta K,
Makino K, Hondo H, Yamanaka R, Kajiwara K, Saito T, Kuratsu J, et
al: M2 macrophage/microglial cells induce activation of Stat3 in
primary central nervous system lymphoma. J Clin Exp Hematop.
51:93–99. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang SH, Lee KS, Kim IS, Hong JT, Sung JH,
Son BC, Lee SW and Hong YK: Long-term survival in primary CNS
lymphoma treated by high-dose methotrexate monochemotherapy: Role
of STAT6 activation as prognostic determinant. J Neurooncol.
92:65–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rubenstein JL, Wong VS, Kadoch C, Gao HX,
Barajas R, Chen L, Josephson SA, Scott B, Douglas V, Maiti M, et
al: CXCL13 plus interleukin 10 is highly specific for the diagnosis
of CNS lymphoma. Blood. 121:4740–4748. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu BM, Ishida Y, Robinson GW,
Pacher-Zavisin M, Yoshimura A, Murphy PM and Hennighausen L: SOCS3
negatively regulates the gp130-STAT3 pathway in mouse skin wound
healing. J Invest Dermatol. 128:1821–1829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakata K, Suzuki Y, Inoue T, Ra C, Yakura
H and Mizuno K: Deficiency of SHP1 leads to sustained and increased
ERK activation in mast cells, thereby inhibiting IL-3-dependent
proliferation and cell death. Mol Immunol. 48:472–480. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yi TL, Cleveland JL and Ihle JN: Protein
tyrosine phosphatase containing SH2 domains: Characterization,
preferential expression in hematopoietic cells, and localization to
human chromosome 12p12-p13. Mol Cell Biol. 12:836–846. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dixon JE: Protein tyrosine phosphatases:
their roles in signal transduction. Recent Prog Horm Res.
51:405–415. 1996.PubMed/NCBI
|
|
18
|
Healy JI and Goodnow CC: Positive versus
negative signaling by lymphocyte antigen receptors. Annu Rev
Immunol. 16:645–670. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu C, Sun M, Liu L and Zhou GW: The
function of the protein tyrosine phosphatase SHP-1 in cancer. Gene.
306:1–12. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chim CS, Wong AS and Kwong YL: Epigenetic
dysregulation of the Jak/STAT pathway by frequent aberrant
methylation of SHP1 but not SOCS1 in acute leukaemias. Ann Hematol.
83:527–532. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen HE, Chang S, Trub T and Neel BG:
Regulation of colony-stimulating factor 1 receptor signaling by the
SH2 domain-containing tyrosine phosphatase SHPTP1. Mol Cell Biol.
16:3685–3697. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Law CL, Sidorenko SP, Chandran KA, Zhao Z,
Shen SH, Fischer EH and Clark EA: CD22 associates with protein
tyrosine phosphatase 1C, Syk, and phospholipase C-gamma(1) upon B
cell activation. J Exp Med. 183:547–560. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yi T, Zhang J, Miura O and Ihle JN:
Hematopoietic cell phosphatase associates with erythropoietin (Epo)
receptor after Epo-induced receptor tyrosine phosphorylation:
Identification of potential binding sites. Blood. 85:87–95.
1995.PubMed/NCBI
|
|
24
|
Sugita Y, Tokunaga O, Nakashima A and
Shigemori M: SHP-1 expression in primary central nervous system
B-cell lymphomas in immunocompetent patients reflects maturation
stage of normal B cell counterparts. Pathol Int. 54:659–666. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oka T, Ouchida M, Koyama M, Ogama Y,
Takada S, Nakatani Y, Tanaka T, Yoshino T, Hayashi K, Ohara N, et
al: Gene silencing of the tyrosine phosphatase SHP1 gene by
aberrant methylation in leukemias/lymphomas. Cancer Res.
62:6390–6394. 2002.PubMed/NCBI
|
|
26
|
Harris NL, Jaffe ES, Stein H, Banks PM,
Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter
KC, et al: A revised European-American classification of lymphoid
neoplasms: A proposal from the International Lymphoma Study Group.
Blood. 84:1361–1392. 1994.PubMed/NCBI
|
|
27
|
Patel B, Chacko G, Nair S, Anandan J,
Chacko AG, Rajshekhar V and Turel M: Clinicopathological correlates
of primary central nervous system lymphoma: Experience from a
tertiary care center in South India. Neurol India. 63:77–82. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mounier N, Briere J, Gisselbrecht C, Emile
JF, Lederlin P, Sebban C, Berger F, Bosly A, Morel P, Tilly H, et
al: Rituximab plus CHOP (R-CHOP) overcomes bcl-2--associated
resistance to chemotherapy in elderly patients with diffuse large
B-cell lymphoma (DLBCL). Blood. 101:4279–4284. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Feng W, Shen L, Wen S, Rosen DG, Jelinek
J, Hu X, Huan S, Huang M, Liu J, Sahin AA, et al: Correlation
between CpG methylation profiles and hormone receptor status in
breast cancers. Breast Cancer Res. 9:R572007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hans CP, Weisenburger DD, Greiner TC,
Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E,
Braziel RM, Jaffe ES, et al: Confirmation of the molecular
classification of diffuse large B-cell lymphoma by
immunohistochemistry using a tissue microarray. Blood. 103:275–282.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kossev PM, Raghunath PN, Bagg A, Schuster
S, Tomaszewski JE and Wasik MA: SHP-1 expression by malignant small
B-cell lymphomas reflects the maturation stage of their normal
B-cell counterparts. Am J Surg Pathol. 25:949–955. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Koyama M, Oka T, Ouchida M, Nakatani Y,
Nishiuchi R, Yoshino T, Hayashi K, Akagi T and Seino Y: Activated
proliferation of B-cell lymphomas/leukemias with the SHP1 gene
silencing by aberrant CpG methylation. Lab Invest. 83:1849–1858.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Han Y, Amin HM, Frantz C, Franko B, Lee J,
Lin Q and Lai R: Restoration of shp1 expression by
5-AZA-2′-deoxycytidine is associated with downregulation of
JAK3/STAT3 signaling in ALK-positive anaplastic large cell
lymphoma. Leukemia. 20:1602–1609. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bittorf T, Seiler J, Zhang Z, Jaster R and
Brock J: SHP1 protein tyrosine phosphatase negatively modulates
erythroid differentiation and suppression of apoptosis in J2E
erythroleukemic cells. Biol Chem. 380:1201–1209. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jiao H, Berrada K, Yang W, Tabrizi M,
Platanias LC and Yi T: Direct association with and
dephosphorylation of Jak2 kinase by the SH2-domain-containing
protein tyrosine phosphatase SHP-1. Mol Cell Biol. 16:6985–6992.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kozlowski M, Mlinaric-Rascan I, Feng GS,
Shen R, Pawson T and Siminovitch KA: Expression and catalytic
activity of the tyrosine phosphatase PTP1C is severely impaired in
motheaten and viable motheaten mice. J Exp Med. 178:2157–2163.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma XZ, Jin T, Sakac D, Fahim S, Zhang X,
Katsman Y, Bali M and Branch DR: Abnormal splicing of SHP-1 protein
tyrosine phosphatase in human T cells. Implications for
lymphomagenesis. Exp Hematol. 31:131–142. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Brell M, Tortosa A, Verger E, Gil JM,
Viñolas N, Villá S, Acebes JJ, Caral L, Pujol T, Ferrer I, et al:
Prognostic significance of O6-methylguanine-DNA
methyltransferase determined by promoter hypermethylation and
immunohistochemical expression in anaplastic gliomas. Clin Cancer
Res. 11:5167–5174. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Baeza N, Weller M, Yonekawa Y, Kleihues P
and Ohgaki H: PTEN methylation and expression in glioblastomas.
Acta Neuropathol. 106:479–485. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cameron EE, Baylin SB and Herman JG:
p15(INK4B) CpG island methylation in primary acute leukemia is
heterogeneous and suggests density as a critical factor for
transcriptional silencing. Blood. 94:2445–2451. 1999.PubMed/NCBI
|
|
41
|
Lenz G, Hutter G, Hiddemann W and Dreyling
M: Promoter methylation and expression of DNA repair genes hMLH1
and MGMT in acute myeloid leukemia. Ann Hematol. 83:628–633. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Amara K, Trimeche M, Ziadi S, Laatiri A,
Hachana M and Korbi S: Prognostic significance of aberrant promoter
hypermethylation of CpG islands in patients with diffuse large
B-cell lymphomas. Ann Oncol. 19:1774–1786. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Khoury JD, Rassidakis GZ, Medeiros LJ,
Amin HM and Lai R: Methylation of SHP1 gene and loss of SHP1
protein expression are frequent in systemic anaplastic large cell
lymphoma. Blood. 104:1580–1581. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu J, Sun XF, Qian J, Bai XY, Zhu H, Cui
QU, Li XY, Chen YD, Wang YM and Liu YB: Immunochemotherapy for
primary central nervous system lymphoma with rituximab,
methotrexate, cytarabine and dexamethasone: Retrospective analysis
of 18 cases. Mol Clin Oncol. 3:949–953. 2015.PubMed/NCBI
|
|
45
|
Tassidis H, Brokken LJ, Jirström K,
Ehrnström R, Pontén F, Ulmert D, Bjartell A, Härkönen P and Wingren
AG: Immuno-histochemical detection of tyrosine phosphatase SHP-1
predicts outcome after radical prostatectomy for localized prostate
cancer. Int J Cancer. 126:2296–2307. 2010.PubMed/NCBI
|
|
46
|
Zhang L, Wang M, Wang W and Mo J:
Incidence and prognostic value of multiple gene promoter
methylations in gliomas. J Neurooncol. 116:349–356. 2014.
View Article : Google Scholar : PubMed/NCBI
|