Introduction
Gastric cancer (GC) is one of the most commonly
diagnosed cancers worldwide and is associated with a poor prognosis
(1). Although there are several
treatments for GC, including surgery, chemotherapy (2) and/or radiation therapy (3), GC is difficult to diagnose in early
stages and therefore difficult to cure. Since GC is a molecular
disease caused by the activation of oncogenes and/or inactivation
of tumor-suppressor genes (such as other tumors) (4), it is beneficial to identify these
important genes to develop efficient prevention and treatment
strategies for GC.
Recent studies indicate that the acidic tumor
microenvironment is closely related to the biological behavior of
tumor cells (5,6). The acidic extracellular environment
and alkaline intracellular environment can promote the
proliferation, invasion and metastasis of tumor cells by
influencing cell metabolism and the activity of
migration-associated genes (6,7).
Several pH-regulated genes (VATP-pase, SLC4A1/4 and NHE) are known
to participate in the progression of tumors such as liver and
breast cancer, and pancreatic carcinoma (8,9).
Na+/H+ exchanger isoform 1 (NHE1) is the most
direct pH regulator and has become a focus of research in recent
years (10). NHE1, the most
abundant isoform of the NHE exchanger family, is a membrane protein
that is present in many mammalian cell types, and is involved in
regulating intracellular pH (pHi) and osmotic homeostasis (11). It has recognized functions in
determining the pHi by catalyzing the electroneutral exchange of
extracellular Na+ and intracellular H+
(12). Previous research has
revealed that NHE1 not only contributes to heart disease and
leukemia (13,14), but is also an important promoter of
cancer in the progression of breast cancer and human small cell
lung cancer cells (15,16). Our previous study demonstrated that
the upregulation of NHE1 enabled liver cancer proliferation,
migration and invasion (8).
Although the expression and function of NHE1 in the
stomach have been demonstrated in healthy humans (17), the function and specific mechanism
of NHE1 in digestive cancer, particularly GC, are not clearly
understood.
In the present study, we showed that the expression
of NHE1 was higher in GC tissues and cell lines than that noted in
normal tissues and cell lines. Loss of function of NHE1 suppressed
GC cell proliferation, migration and invasion in vitro, and
NHE1 inhibition reduced GC tumor growth in nude mice. Moreover,
NHE1 regulated these events through changes in the pHi and the
expression of corresponding genes. Our findings suggest that
modulation of NHE1 and its downstream signaling pathways could be a
novel therapeutic strategy for human GC.
Materials and methods
Human specimens
Tissue samples were obtained under sterile
conditions from 15 patients with primary GC who underwent surgery
at the Department of Surgery, Affiliated Hospital of Zunyi Medical
College (Zunyi, China). Samples from primary GC tissues and their
normal paraneoplastic counterparts were shock frozen in liquid
nitrogen and stored at −80°C. This study was approved by the Ethics
Committee of the Hospital Affiliated to Zunyi Medical College and
all patients provided written in form consent for the use of their
samples.
Cell lines
GES-1, SGC-7901 and MKN-45 cells were obtained from
the Chinese Academy of Sciences (Shanghai, China). GES-1 is an
immortalized human gastric normal epithelial mucosa cell line and
SGC-7901 and MKN-45 are human GC cell lines. These cells are
commonly used in the study of GC. 5-N-ethyl-N-isopropylamiloride
(EIPA) was purchased from Sigma (St. Louis, MO, USA). After
dissolving EIPA in dimethyl sulfoxide (DMSO), it was diluted in
cell culture media to final concentrations below 0.1%.
Cell culture
GES-1, SGC-7901 and MKN-45 cells were maintained in
RPMI-1640 and Iscove's Dulbeccos modified Eagles medium (DMEM)
(Gibco-BRL, Gaithersburg, MD, USA). All cells were grown in medium
supplemented with 10% fetal calf serum (Gibco-BRL) in an incubator
with 5% CO2 at 37°C. The cells were used for experiments
after they reached 70–80% confluence.
Immunohistochemistry
Slides containing human GC tissues were incubated
with an anti-NHE1 monoclonal antibody (1:100 dilution; Abcam,
Cambridge, MA, USA). The primary antibodies were detected with
biotinylated goat anti-mouse IgG (Vector Laboratories, Burlingame,
CA, USA) secondary antibodies. Immunoreactivity was detected using
a horseradish peroxidase (3,3-diaminobenzidine) kit (BioGenex, San
Francisco, CA, USA), followed by counterstaining with hematoxylin,
dehydration and mounting. The slides were then examined with a
Nikon Eclipse 800 Research microscope.
Immunofluorescence staining
The cells were cultured on 8×8 cm coverslips and
fixed with ice-cold methanol/acetone for 20 min, then permeabilized
[0.1% Triton X-100 in phosphate-buffered saline (PBS)] for 25 min,
blocked for 30 min and incubated with primary antibodies against
NHE1 (1:200 dilution; Abcam) overnight at 4°C. The cells were
subsequently incubated with a FITC-IgG fluorescent secondary
antibody (1:500 dilution; ZSGB-BIO, Beijing, China) at 37°C for 1
h. The cells were finally observed through an inverted fluorescence
microscope (Olympus IX70; Olympus, Tokyo, Japan).
Measurement of acid secretion via
digital pHi imaging
Cells were loaded with a 5 µmol/l concentration of
the pH-sensitive dye 2,7-bis-(2-carboxyethyl)-5-(and
6)-carboxyfluorescein, acetomethyl ester (BCECF-AM) (Molecular
Probes, Eugene, OR, USA) for 30 min in low-calcium HEPES-buffered
Ringer's solution (125 mM NaCl, 5 mM KCl, 0.1 mM CaCl2,
32.2 mM HEPES and 5 mM glucose, pH 7.4) at room temperature. After
loading, the cells were excited at 490 and 440 nm, and the emitted
fluorescence was measured at 530 nm. Real-time images were obtained
using a Nikon Eclipse Ti epifluorescence microscope (x40 objective)
and Easy Ratio Pro software (Photon Technology International).
After the establishment of a stable baseline, the cells were
exposed to the solution containing EIPA (20 µmol/l) or the control
vehicle. After 30 min, the cells were exposed to 10 mM
NH4Cl for 4 min. pHi was calculated on the basis of the
fluorescence ratios from an intracellular calibration curve
constructed at the end of each experiment using the
nigericin/high-K+ technique and a linear conversion
formula. The pHi recovery rate was determined on the basis of the
slopes of the linear regression lines of measurements obtained
during the first 3 min of recovery after the removal of
NH4Cl and was expressed as pH units/min.
Quantitative real-time PCR
Total RNA was extracted with RNAiso Plus. The RNA
was reverse transcribed to cDNA using PrimeScript RT-polymerase
(Takara, Tokyo, Japan). Quantitative PCR was performed using cDNA
primers specific for NHE1. The GAPDH gene was used as an internal
control. The real-time RT-PCR assays were performed using
SYBR-Green SuperMix (Bio-Rad, Hercules, CA, USA). The qPCR primer
pair for NHE1 was designed by RiboBio (Guangzhou, China). The
primers were as follows: GAPDH forward, 5-CAGGAGGCATTGCTGATGAT-3
and reverse, 5-GAAGGCTGGGGCTCATTT-3; NHE1 forward, 5-CTC
ATCTGTGCCTGTCTGTCC and reverse, 5-TCTGATGTCA CAGTCTTCGAGCAA-3.
Western blotting
Cells were lysed with lysis buffer [50 mM Tris (pH
7.4), 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1%
SDS and 5 mM EDTA] and centrifuged at 12,000 × g for 15 min to
remove insoluble material. Protein contents were subsequently
normalized. For immunoprecipitation analyses, the lysates were
incubated with an NHE1 antibody for 1 h at 4°C, followed by another
1 h incubation with protein A-agarose at 4°C. Pellets or cell
lysates were resuspended in 2X loading buffer, boiled for 5 min,
and separated via SDS-PAGE (10%). Resolved proteins were
transferred to a PVDF membrane (Millipore Corporation, Billerica,
MA, USA). The membranes were blocked with blocking buffer [1X
Tris-buffered saline with Tween-20 (TBST) with 5% non-fat dry
milk], followed by incubation with monoclonal antibodies against
NHE1 (1:1,000; Abcam), GAPDH (1:5,000; Ambion, Thermo Fisher
Scientific, USA), cyclin D1 (1:1,000), cyclin B1 (1:2,000),
E-cadherin (1:1,000) and vimentin (1:3,000) (all from Abcam). After
washing with TBST, the secondary antibody was applied. The signals
were visualized using enhanced chemiluminescence (ECL; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and recorded on a Gel
Doc 2000 imaging scanner (Bio-Rad).
Cell proliferation assay
Cells were plated in 96-well culture dishes at
2×103 cells/well using DMEM with 5% fetal bovine serum
(FBS) as the culture medium. The cells were transfected with NHE1
shRNA or treated for 72 h with different doses of EIPA (5, 10, 20
or 30 µmol/l). Cell viability was assessed with a Cell Counting
Kit-8 (CCK-8) (Tongren, Shanghai, China). The CCK-8 reagent was
added to each well at 0.5–2 h before the endpoint of incubation.
The optical density (OD) at 450 nm was determined for each well
using a microplate reader. Experiments were repeated at least three
times, in triplicate each time. Extinction measurements were
calculated relative to the negative control at the corresponding
time points.
Cell scratch test
Cells from each group were seeded into 24-well
tissue culture plates at a density that reached 80% confluence as a
monolayer at 24 h. The monolayer was gently scratched across the
center of the well with a 10-µl pipette tip. After scratching, the
well was gently washed with medium to remove the detached cells.
After treatment with EIPA (20 µmol/l) or transfection with NHE1
shRNA, images were obtained at 0 and 24 h using a microscope.
Narrower widths indicate the migration distance. In each group, at
least three parallel wells were utilized for testing.
Transwell invasion assays
A 24-well Transwell chamber (8-µm pores; Corning,
Corning, NY, USA) was used to evaluate the motility and invasive
ability of SGC-7901 or MKN-45 cells exposed to different
treatments. The upper surface of a polycarbonate filter with 8-µm
pores was coated with 100 µg of Matrigel (Collaborative Biomedical,
Bedford, MA, USA). The cells were suspended in serum-free
RPMI-1640/DMEM (1×104 cells/100 µl), and the cells in
the upper chambers were then transfected with NHE1 shRNA or treated
with EIPA (20 µmol/l). The lower chambers were filled with 500 µl
of RPMI-1640 (10% FBS) medium. After 24 h of incubation, the cells
remaining on the upper surface of the filters were removed by
wiping with cotton swabs, and the cells that had migrated onto the
lower surface were stained with crystal violet. The number of cells
on the lower surface of the filters was counted under a microscope
at a magnification of ×200 (Olympus Corporation, Tokyo, Japan), and
the average number of cells in three random selected fields was
taken as the number of cells exhibiting invasion. The experiments
were performed in triplicate, and each sample was assayed in
triplicate.
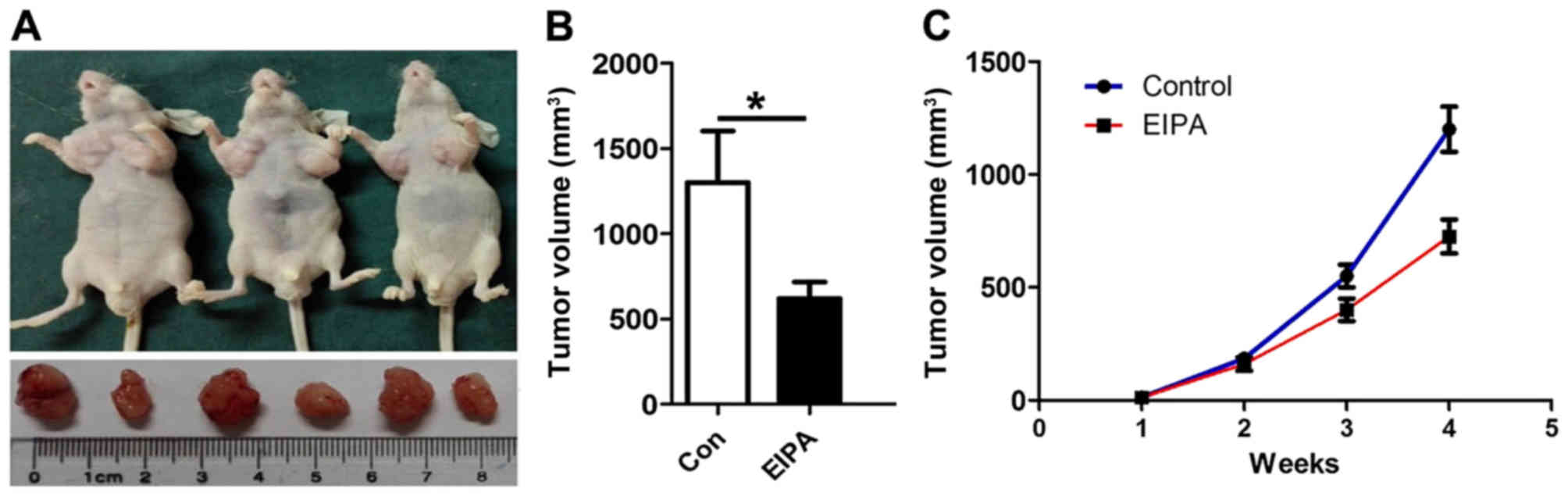
Treatment of subcutaneous (s.c.) GC
xenografts
All animal experiments described in the present
study conformed to the Guidelines of the Animal Care and Use
Committee of Zunyi Medical College. Male nude mice (~4-weeks old)
were purchased from Hua Fu Kang (Beijing, China). Approximately
1×106 SGC-7901 cells from each group were injected into
the armpits of the nude mice. When the tumors reached an average
volume of ~1 mm3, EIPA (30 µmol/l) was injected into the
tumors in the armpits on one side once a day (100 µl) and DMSO
(0.1%) was injected into the tumors in the armpits on the other
side. The volume of the GC xenografts was assessed each week.
Thirty days after the injection of tumor cells, using a digital
caliper, the tumor volume (in mm3) was calculated using
the formula: Volume = (length)2 × width/2.
Cell cycle analysis
Cell cycle analysis was carried out using flow
cytometry. Briefly, cells were fixed in 70% ethanol for >18 h
and incubated with RNase A (0.2 mg/ml) in PBS. Propidium iodide was
then added to the cell suspension. Samples were analyzed by a
FACSCalibur flow cytometer (Becton-Dickinson).
Statistical analysis
All data are expressed as means ± SEM. Data were
analyzed via one-way ANOVA followed by the Student-Newman-Keul post
hoc test or Student's t-test for paired or unpaired samples with
GraphPad Prism 5.0 (GraphPad, San Diego, CA, USA). P<0.05 was
considered to indicate a statistically significant result.
Results
Overexpression of NHE1 in human GC
tissues and cells
NHE1 expression has been reported to be upregulated
in human breast and prostate cancer (8,18). To
study the potential role of NHE1 in GC, we first performed
immunohistochemistry and western blotting to examine and compare
NHE1 protein expression levels in 10 pairs of human GC tissues and
their normal paraneoplastic counterparts. As shown in Fig. 1, NHE1 was detected in native human
GC and normal tissues, but NHE1 expression was significantly
upregulated in GC patient tissues compared with that in the normal
tissues (Fig. 1A-D). Next, we
performed qPCR to compare NHE1 transcripts levels, and the mRNA
level of NHE1 in the GC patients was found to be 4.3-fold higher
than that noted in the normal tissues (Fig. 1E). We also examined the expression
of NHE1 in the human GC cell lines MKN-45 and SGC-7901 and the
gastric normal epithelial mucosa cell line GES-1. Western blot and
qPCR analyses revealed that the expression levels of both the NHE1
protein and transcript were upregulated in the SGC-7901 and MKN-45
cancer cells compared with these levels in the GES-1 cells
(Fig. 1F and G). Finally, the
immunofluorescence staining results showed that the NHE1 protein
was predominately expressed in the plasma membrane and cytoplasm of
the cells, and the intensity of immunofluorescence for endogenous
NHE1 was significant increased in the GC cells (Fig. 1H). These results collectively
demonstrated that NHE1 expression is upregulated in GC, implying a
potential contributing role in GC.
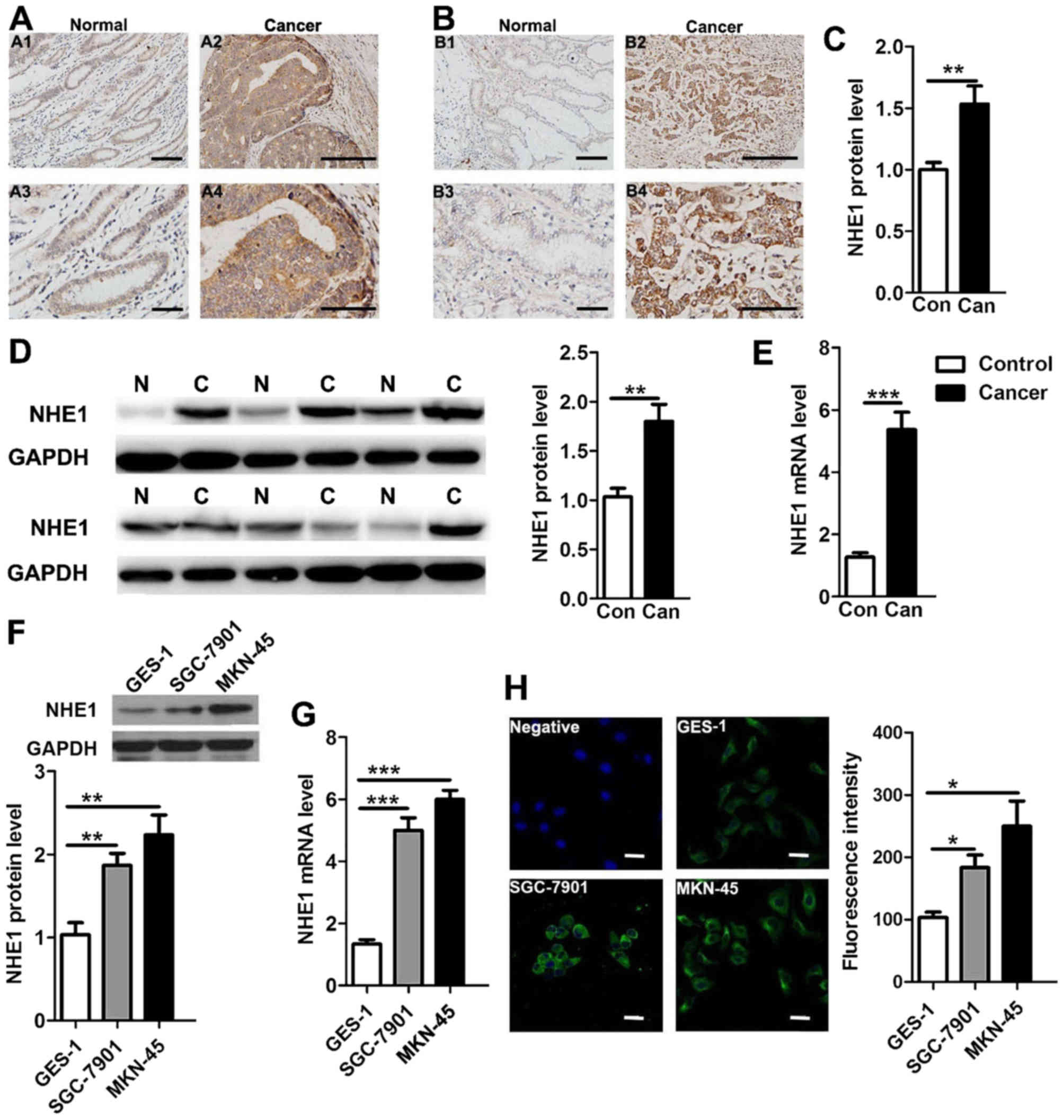 | Figure 1.Enhanced expression of NHE1 in human
gastric cancer (GC) tissues and cell lines. (A-C) Comparison of
NHE1 protein expression through immunohistochemical analysis of
human gastric cancer biopsy tissues (Can) and paraneoplastic normal
tissues (Con) (**P<0.01, n=10 pairs of samples for NHE1
expression analysis). The scale bars in A1, A3, B1 and B3 represent
50 µm. The scale bars in A2, A4, B2 and B4 represent 25 µm. (D)
Western blot analysis of the NHE protein in human GC (C) (six
samples shown) and normal (N) (six samples shown) tissues probed
with anti-NHE1 (91 kDa) antibodies. GAPDH served as the protein
loading control (**P<0.01, n=15). (E) Histogram showing summary
data on NHE1 mRNA expression in human gastric cancer biopsy tissues
(Can) and paraneoplastic normal tissues (Con) (***P<0.001,
n=15). (F and G) Western blot and qPCR analyses of NHE1 protein and
mRNA expression in a human normal gastric epithelial cell line
(GES-1) and gastric cancer cell lines (SGC-7901 and MKN-45)
(**P<0.01, ***P<0.001 n=3 independent experiments). (H)
Representative immunofluorescence imaging of NHE1 protein staining
in GES-1, SGC-7901 and MKN-45 cells and a negative control. The
scale bar is 25 µm (*P<0.05, n=3 independent experiments). |
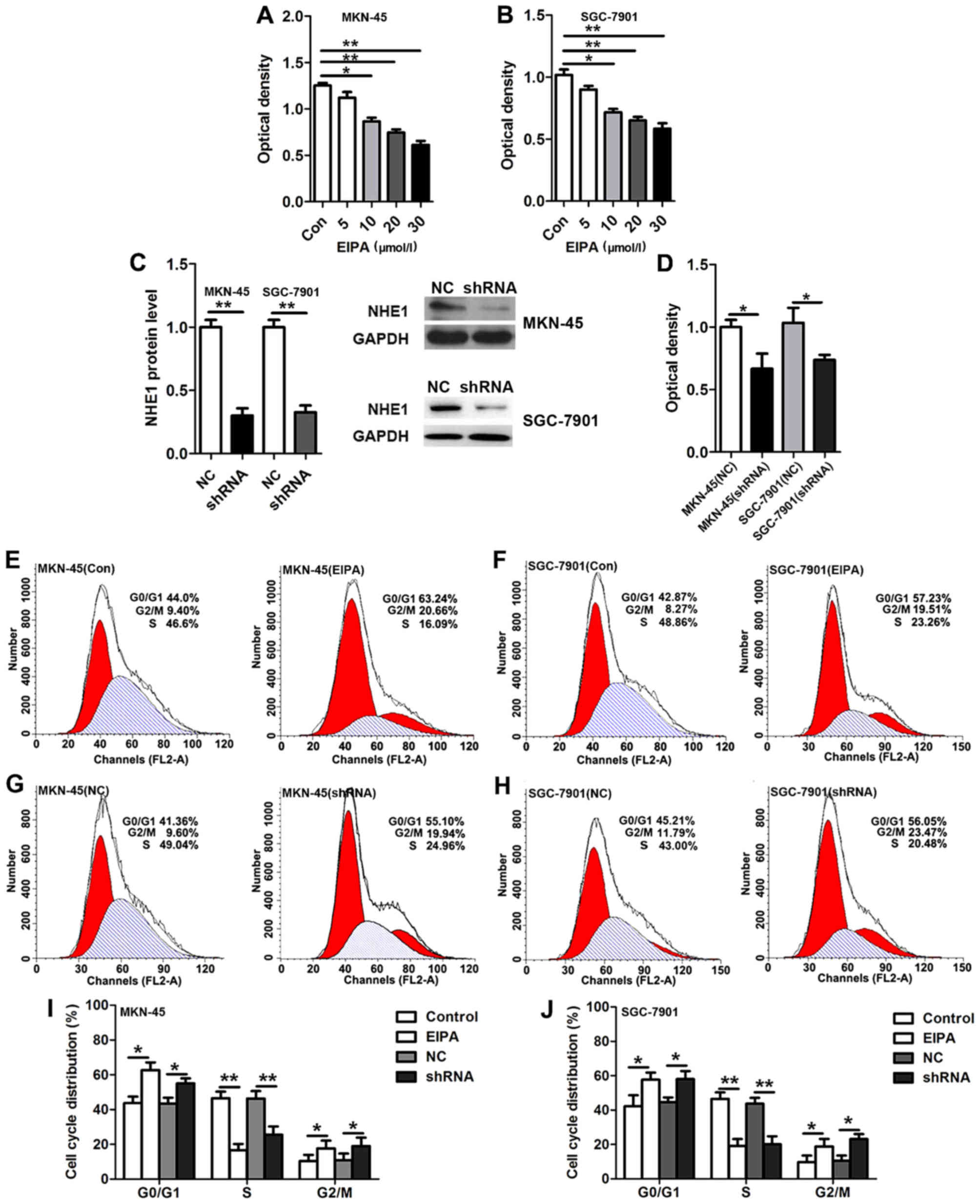
Inhibition of NHE1 suppresses the G1/S
and G2/M cell cycle phase transition and proliferation in GC
cells
The overexpression of NHE1 observed in the primary
human GC resembled the expression profiles detected in breast and
prostate cancer (8). We therefore
hypothesized that in GC, NHE1 probably confers a growth advantage
to the cancer cells. To evaluate this, we performed cell
proliferation assays and found that EIPA (5, 10, 20 or 30 µmol/l),
a selective inhibitor of NHE1, suppressed the proliferation of
MKN-45 and SGC-7901 cancer cells in a dose-dependent manner
(Fig. 2A and B). To verify that
NHE1 specifically mediates GC cell proliferation, an shRNA
targeting NHE1 was constructed and successfully transfected into
the GC cell lines MKN-45 and SGC-7901. Knockdown of NHE1 was found
to significantly inhibit cell proliferation in the MKN-45 and
SGC-7901 cancer cells (Fig. 2C and
D). We next investigated the effect of NHE1 on the cell cycle
distribution via flow cytometric analysis. Compared with the
control (PBS) group, EIPA (20 µmol/l) treatment of the MKN-45 and
SGC-7901 cells resulted in an increase in the proportion of cells
in the G0/G1 and G2/M phases, but a reduction in the proportion of
cells in the S phase (Fig. 2E, F, I and
J). Similarly, knockdown of NHE1 in the MKN-45 and SGC-7901
cells significantly increased the proportion of cells in both the
G0/G1 and G2/M phases, which was associated with a decreased
proportion of cells in the S phase (Fig. 2G-J). These data suggest that
knockdown and inhibition of NHE1 suppressed GC cell proliferation
via regulating cell cycle distribution.
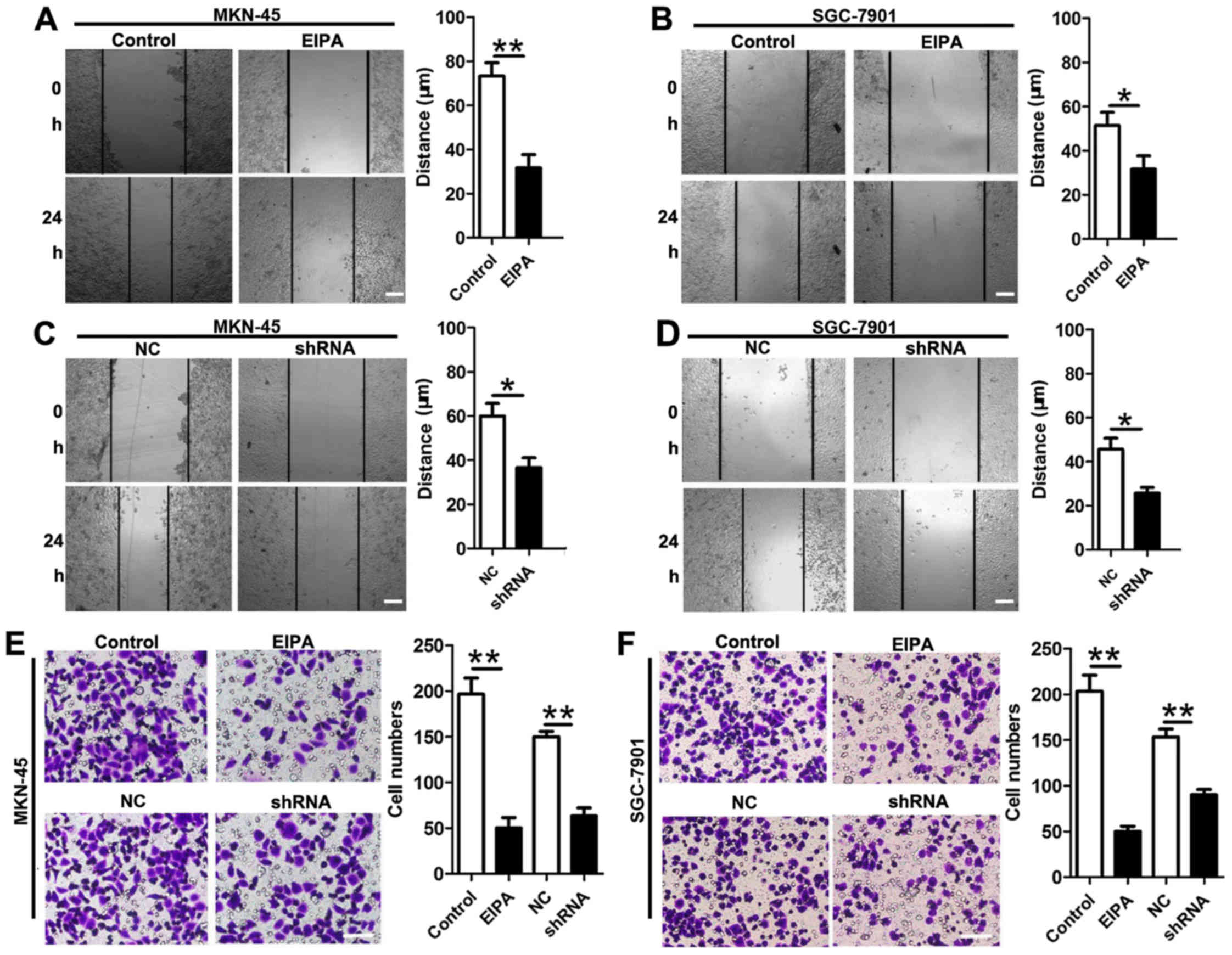
Inhibition of NHE1 suppresses cell
migration and invasion
To further investigate whether NHE1 upregulation is
involved in the progression of GC, we performed assays to test cell
migration and invasion. Wound-healing assays and Transwell
experiments demonstrated that EIPA (20 µmol/l) prevented migration
[MKN-45 control (73.33±6.01 µm) vs. EIPA (32.67±5.01 µm); SGC-7901
control (50.01±5.77 µm) vs. EIPA (20.63±3.48 µm)] and invasion
[MKN-45 control (196±17) vs. EIPA (50±11); SGC-7901 control
(203±17) vs. EIPA (56±5)] (Fig. 3A, B,
E and F). Likewise, knockdown of NHE1 significantly inhibited
cell migration [MKN-45 control (60.13±5.77 µm) vs. EIPA (36.61±4.41
µm); SGC-7901 control (45.11±4.98 µm) vs. EIPA (25.17±2.61 µm)] and
invasion [MKN-45 control (150±5) vs. EIPA (63±8); SGC-7901 control
(153±8) vs. EIPA (90±5)] (Fig.
3C-F). Collectively, these data further support the hypothesis
that NHE1 serves as a tumor promoter in human GC by inducing cell
migration and invasion in vitro.
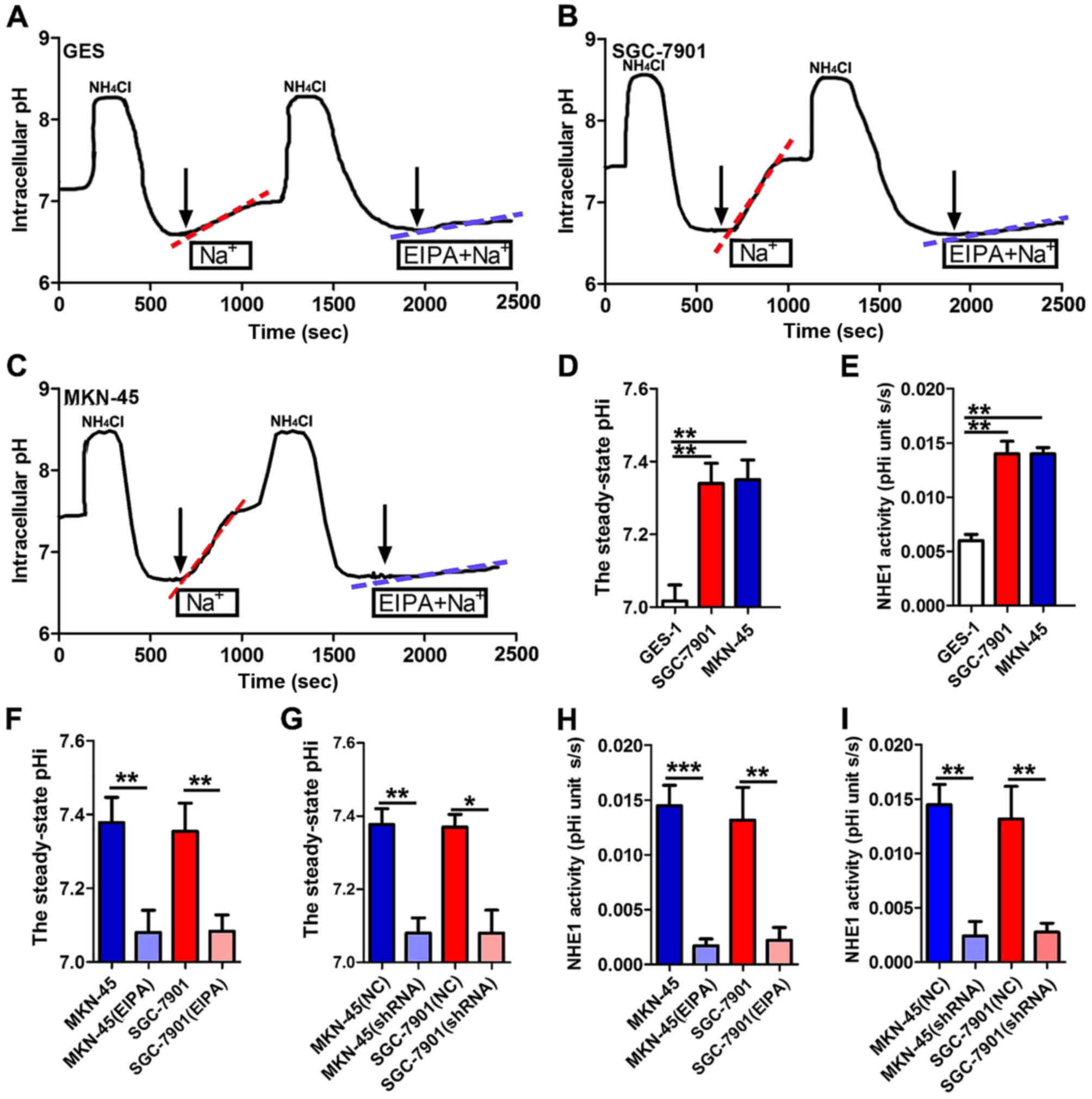
Enhanced NHE1 expression triggers an
elevated pHi in GC cells
As a key pH regulator, NHE1 is known to maintain the
pHi by exchanging one intracellular proton for one extracellular
sodium and has been reported to be involved in the regulation of
pHi in hepatocellular carcinoma and breast cancer development
(8,19,20).
Inhibition of NHE1 function could decrease pHi to suppress cell
proliferation and migration in these cancers. This hypothesis is
consistent with the observation that analkaline intracellular
environments are more suitable for cancer cell growth (21). Therefore, we hypothesized that the
NHE1-mediated elevation of pHi could play a role in GC development.
To test this hypothesis, we first examined the steady-state pHi and
the recovery of pHi following acute intracellular acidification
induced by NH4Cl in GES-1 (Fig. 4A), SGC-7901 (Fig. 4B) and MKN-45 cells (Fig. 4C). The F(440)/F(495)
ratio was converted to pH to obtain the intracellular proton
concentration using the nigericin-based calibration technique, and
the mean ratio values were plotted as a function of pH to produce a
calibration curve (22). As shown
in Fig. 4D, the steady-state pHi of
the MKN-45 and SGC-7901 cancer cell lines was significantly
increased compared with that of the GES-1 cell line. We next
observed the recovery rate of pHi induced by NHE1 activation in the
GES-1, SGC-7901 and MKN-45 cells. The Na+-dependent pHi
recovery rate was faster in the GC cell lines than that noted in
the normal cell line, as shown in Fig.
4E, which may be attributed to the enhanced expression or
function of NHE1 in cancer tissues and cell lines, promoting the
extrusion of intracellular proton (H) ions. Furthermore, the NHE1
inhibitor EIPA (20 µmol/l) effectively reduced the steady-state pHi
and the pHi recovery rate in both the SGC-7901 and MKN-45 cell
lines (Fig. 4B, C, F and H). These
effects may be attributed to the suppression of intracellular
H+ exchange with the extracellular environment by EIPA,
followed by an increase in intracellular H+ and
reduction of pHi. The results of NHE1 knockdown showed the same
effect as EIPA inhibition (Fig. 4G and
I), indicating the specificity of NHE1-mediated pHi signaling.
These results suggested that the regulation of pHi by NHE1 may
contribute to GC development, and the decrease in pHi induced by
NHE1 inhibition is a possible mechanism whereby NHE1 regulates GC
progression.
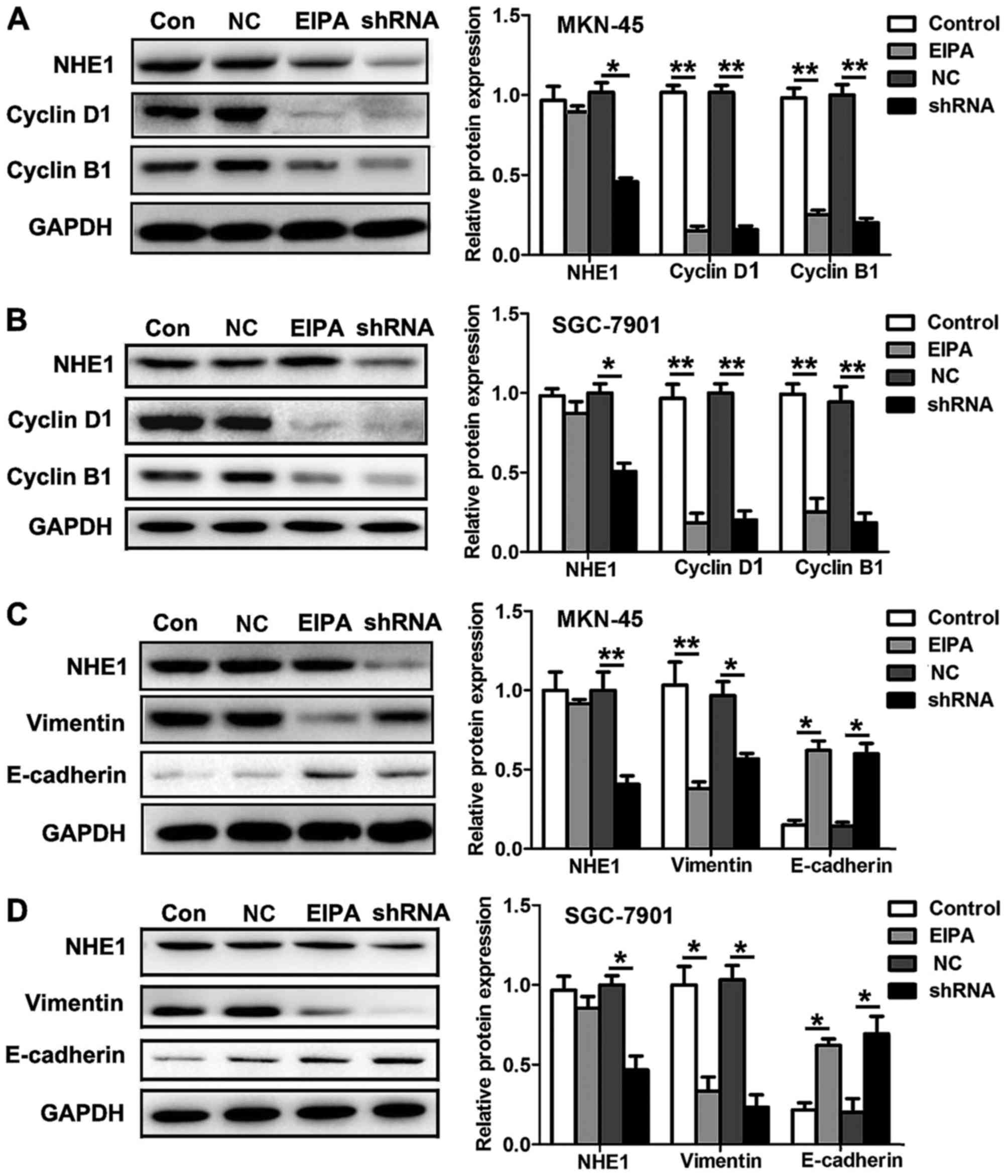
NHE1 mediates GC progression by
depressing the expression of cyclin proteins and
epithelial-mesenchymal transition markers
We further aimed to identify the possible molecular
mechanisms underlying the modulation of pHi by NHE1 in GC
development. Since NHE1 regulates cell cycle distribution, we
examined the expression of important cell cycle-related proteins
after blocking the function and expression of NHE1. The NHE1
antagonist EIPA (20 µmol/l) reduced the expression of the G1/S
phase marker cyclin D1 as well as the G2/M phase marker cyclin B1
in the MKN-45 and SGC-7901 cells. Likewise, knockdown of NHE1 in
the MKN-45 and 7901 cells also decreased the expression of cyclin
D1 and cyclin B1 (Fig. 5A and B).
It is suggested that NHE1 inhibition suppresses both the G1/S and
the G2/M transitions in GC cells. Additionally, we examined some
migration- and invasion-related genes. Studies have indicated that
epithelial-mesenchymal transition (EMT) plays a critical role in
the progression of various types of tumors (23), and we therefore analyzed the changes
in the protein levels of E-cadherin and vimentin, two important
markers of EMT. We found that EIPA (20 µmol/l) significantly
decreased vimentin protein expression and enhanced E-cadherin
protein expression in MKN-45 and SGC-7901 cells. Similar results
were obtained following knockdown of NHE1 in the MKN-45 and
SGC-7901 cells (Fig. 5C and D). The
above results suggested that NHE1 mediates GC cell proliferation,
migration and invasion via cyclin proteins and EMT markers.
Knockdown of NHE1 suppresses GC cell
growth in nude mice
Finally, to obtain evidence that NHE1 is indeed
important for the development of GC in vivo, we established
a subcutaneously xenograft gastric tumor model in nude mice. On day
30 after the implantation of SGC-7901 cancer cells in the armpits
on both sides of the mice (with or without EIPA injection), the
NHE1 antagonist EIPA (30 µmol/l) significantly suppressed GC growth
in the nude mice compared with the control groups (Fig. 6A-C).
Discussion
In the present study, we demonstrated that NHE1
serves as a key promoter in the development and progression of
human gastric cancer (GC). Several lines of evidence support this
hypothesis. First, enhanced expression of NHE1 was found not only
in GC cell lines but also in human primary GC tissues compared with
normal controls. Secondly, knockdown or inhibition of NHE1
significantly suppressed GC cell proliferation, migration and
invasion. Mechanistically, knockdown or inhibition of NHE1 reduced
the steady-state pHi and the recovery rate of pHi in the SGC-7901
and MKN-45 cell lines, further regulating downstream cyclin
proteins and EMT markers involved in GC progression. Finally, the
NHE1 inhibitor EIPA suppressed GC cell growth in nude mice. These
findings indicate that NHE1 may play an important role in the
development and progression of GC.
Since the NHE1 isoform was cloned in 1989 by the
Sardet group (24), nine mammalian
isoforms belonging to the NHE family have been identified. NHE1 is
an integral membrane protein consisting of 12 transmembrane
segments (N-terminal) and a long cytoplasmic tail (C-terminal) that
catalyzes the extrusion of intracellular proton (H) ions in
exchange for extracellular sodium (Na) ions; the maintenance of
this pH gradient from extracellular pH (pHe) to pHi is mainly due
to NHE1 (25,26). Physiologically, NHE1 activation
promotes cell growth, differentiation, regulates sodium flux and
the cell volume after osmotic shrinkage. In the field of cancer
research, NHE1 has been shown to be overexpressed in breast cancer
and cervical cancer, and increased NHE1 activity induced
intracellular alkalization and promoted cell invasion (8,27).
Furthermore, decorin lowered NHE1 activity further inhibiting B16V
melanoma cell migration and invasion by cellular acidification
(28). Moreover, cisplatin-induced
apoptosis was found to involve membrane fluidification via
inhibition of NHE1 in human colon cancer cells (29). In the present study on gastric
cancer (GC), we first examined the expression of NHE1 via
immunohistochemistry and western blot analyses in 15 pairs of GC
and normal samples. Enhanced expression of NHE1 was found in most
of the GC samples compared with that noted in the matched normal
tissues. Likewise, NHE1 protein and transcript levels were
significantly upregulated in the GC cell lines, and knockdown or
inhibition of NHE1 suppressed cell proliferation, the cell cycle,
migration and invasion in GC cells both in vitro and in
vivo. These finding suggest that NHE1 may play a key role in
the advancement of human GC.
However, the understanding of the mechanisms
underlying the role of NHE1 in GC remains insufficient. Both cell
culture experiments and in situ tumor spectroscopic studies
have shown that cancer cells exhibit quite a different acid-base
balance from normal cells; tumor cells have alkaline pHi values of
7.12–7.7 vs. 6.99–7.05 in normal cells while producing acidic pHe
values of 6.2–6.9 vs. 7.3–7.4 in normal cells. As known, the
proteolytic breakdown of ECM proteins is one of the first steps in
invasion in primary cancer lesions, and studies have confirmed that
an acidic pHe can directly or indirectly drive ECM proteolysis and
invasion by increasing protease production and the secretion of the
active forms of cathepsin family members, serine proteases and
matrix metalloproteinases (MMPs) (30–32).
In addition, an increased pHi is necessary for actin
polymerization. Alkaline pHi can stimulate cofilin activity and
de novo actin polymerization to promote the leading edge
membrane of migrating cells (33).
Therefore, an alkaline pHi and acidic pHe not only increase cell
metabolism but also contribute to the promotion of tumor cell
invasion and migration. As an important pH regulator, NHE1 plays an
essential role in the tumor microenvironment. Yang et al
demonstrated that inhibition of NHE1 decreased pHi values and
downregulated MMP-2, MMP-9 and VEGF expression to reduce
hypoxia-induced hepatocellular carcinoma invasion and motility
(34). NHE1 inhibitors are also
reported to reduce the acidic tumor microenvironment, thereby
rendering paclitaxel more effective in breast cancer chemotherapy
(35). In the present study, we
demonstrated that the MKN-45 and 7901 cancer cell lines exhibited a
more alkaline pHi compared with the GES-1 cell line, and
Na+-dependent pHi recovery was also faster in the GC
cell lines than that noted in the normal cell line. These results
may have attributed to the enhanced expression or function of NHE1
in cancer tissues and cell lines, promoting the extrusion of
intracellular proton (H) ions. Furthermore, the NHE1 inhibitor EIPA
and treatment with shRNA targeting NHE1 effectively acidized pHi
and suppressed pHi recovery in the MKN-45 and 7901 cells,
indicating the specificity of NHE1-mediated pHi signaling in GC,
and the suppression of invasion and migration in GC may be due to
the inactivation of NHE1.
Abnormal regulation of the cell cycle is a marked
characteristic of cancer cells, and dysregulation of the cell cycle
by many tumor-suppressor genes and proto-oncogenes is essential for
unlimited proliferation of cancer cells (36). It is known that cyclin D mediates
the G1/S transition by binding to Cdk4. Cyclin D/Cdk4/6
phosphorylates Rb, and E2F is then released to transactivate genes
required for G1/S transition. Furthermore, the G2/M DNA damage
checkpoint prevents the cell from entering mitosis (M phase) if the
genome is damaged, and the cyclin B/Cdk1 complex plays an essential
role in the regulation of the G2/M checkpoint. Putney et al
found that a transient increase in pHi induced by NHE1 promoted the
timing of G2/M in fibroblast cells (37,38),
but the relationship between NHE1 and the cyclin family in cancer
cells is not clearly understood. In the present study, we showed
that, in MKN-45 and SGC-7901 cells, knockdown or inhibition of NHE1
suppressed both G1/S and the G2/M phase transition, along with
decreased expression of cyclin D1 and cyclin B1, suggesting that
NHE1 is an important cell cycle regulator. EMT has been shown to
play a critical role in the early stages of cancer metastasis
(39). In order to investigate the
mechanism whereby NHE1 regulates invasion and migration, we further
examined the expression of various EMT markers. E-cadherin is well
known to increase adhesion and repress migration/invasion at EMT
during cancer progression, and vimentin is a canonical marker of
EMT whose pattern within the cell has important effects on the
formation and function of invadopodia and lamellipodia during
cellular invasion and migration (40). Downregulation of E-cadherin and
upregulation of vimentin have been observed in several types of
cancers, such as breast cancer, GC and colorectal cancers (41,42)
and TNF-α and VEGF can promote EMT by mediating E-cadherin and
vimentin expression in human GC (43,44).
Our research also confirmed that ablation or inhibition of NHE1
significantly increased epithelial marker expression (E-cadherin)
and decreased mesenchymal marker expression (vimentin) to suppress
the invasion and migration of GC cells.
In conclusion, our data suggest that NHE1 is a key
player in the development and progression of GC and that targeting
of NHE1 is a promising therapeutic strategy against human GC.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (no. 81301731 and 81572438),
The Program for Innovative Research Team in Guizhou Province
(QKH-RCTD-20134035), and the Science and Technology Fund of Guizhou
Province (JLKZI2011J46 and KHKHSZ201114).
Glossary
Abbreviations
Abbreviations:
|
NHE1
|
Na+/H+ exchanger
isoform 1
|
|
GC
|
gastric cancer
|
|
pHi
|
intracellular pH
|
|
pHe
|
extracellular pH
|
|
EMT
|
epithelial-mesenchymal transition
|
|
BCECF-AM
|
2,7-bis-(2-carboxy-ethyl)-5-(and
6)-carboxyfluorescein, acetomethyl ester
|
|
MMPs
|
matrix metalloproteinases
|
|
EIPA
|
5-N-ethyl-N-isopropylamiloride
|
References
|
1
|
Ang YL, Yong WP and Tan P: Translating
gastric cancer genomics into targeted therapies. Crit Rev Oncol
Hematol. 100:141–146. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Quéro L, Guillerm S and Hennequin C:
Neoadjuvant or adjuvant therapy for gastric cancer. World J
Gastrointest Oncol. 7:102–110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dehal A, Smith JJ and Nash GM:
Cytoreductive surgery and intraperitoneal chemotherapy: An
evidence-based review-past, present and future. J Gastrointest
Oncol. 7:143–157. 2016.PubMed/NCBI
|
|
4
|
Mattar R, Nonogaki S, Silva C, Alves V and
Gama-Rodrigues JJ: P53 and Rb tumor suppressor gene alterations in
gastric cancer. Rev Hosp Clin Fac Med Sao Paulo. 59:172–180.
2004.PubMed/NCBI
|
|
5
|
Kanamala M, Wilson WR, Yang M, Palmer BD
and Wu Z: Mechanisms and biomaterials in pH-responsive tumour
targeted drug delivery: A review. Biomaterials. 85:152–167. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Parks SK, Cormerais Y, Marchiq I and
Pouyssegur J: Hypoxia optimises tumour growth by controlling
nutrient import and acidic metabolite export. Mol Aspects Med.
47–48, 3–14. 2016.PubMed/NCBI
|
|
7
|
Kato Y, Ozawa S, Miyamoto C, Maehata Y,
Suzuki A, Maeda T and Baba Y: Acidic extracellular microenvironment
and cancer. Cancer Cell Int. 13:892013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Amith SR and Fliegel L: Regulation of the
Na+/H+ Exchanger (NHE1) in Breast Cancer Metastasis. Cancer Res.
73:1259–1564. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu J, Ji B, Wen G, Yang Y, Jin H, Liu X,
Xie R, Song W, Song P, Dong H, et al: Na+/H+ exchanger 1, Na+/Ca2+
exchanger 1 and calmodulin complex regulates interleukin 6-mediated
cellular behavior of human hepatocellular carcinoma.
Carcinogenesis. 37:290–300. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Loo SY, Chang MK, Chua CS, Kumar AP,
Pervaiz S and Clement MV: NHE-1: A promising target for novel
anti-cancer therapeutics. Curr Pharm Des. 18:1372–1382. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reshkin SJ, Cardone RA and Harguindey S:
Na+-H+ exchanger, pH regulation and cancer. Recent Patents
Anticancer Drug Discov. 8:85–99. 2013. View Article : Google Scholar
|
|
12
|
Slepkov E and Fliegel L: Structure and
function of the NHE1 isoform of the Na+/H+ exchanger. Biochem Cell
Biol. 80:499–508. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Odunewu-Aderibigbe A and Fliegel L: The
Na+/H+ exchanger and pH regulation in the heart. IUBMB Life.
66:679–685. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Garden OA, Musk P, Worthington-White DA,
Dewey MJ and Rich IN: Silent polymorphisms within the coding region
of human sodium/hydrogen exchanger isoform-1 cDNA in peripheral
blood mononuclear cells of leukemia patients: A comparison with
healthy controls. Cancer Genet Cytogenet. 120:37–43. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Amith SR, Wilkinson JM and Fliegel L:
Na+/H+ exchanger NHE1 regulation modulates metastatic potential and
epithelial-mesenchymal transition of triple-negative breast cancer
cells. Oncotarget. 7:21091–21113. 2016.PubMed/NCBI
|
|
16
|
Li S, Bao P, Li Z, Ouyang H, Wu C and Qian
G: Inhibition of proliferation and apoptosis induced by a Na+/H+
exchanger-1 (NHE-1) antisense gene on drug-resistant human small
cell lung cancer cells. Oncol Rep. 21:1243–1249. 2009.PubMed/NCBI
|
|
17
|
Konturek SJ, Konturek PC, Pawlik T,
Sliwowski Z, Ochmański W and Hahn EG: Duodenal mucosal protection
by bicarbonate secretion and its mechanisms. J Physiol Pharmacol.
55 Suppl 2:S5–S17. 2004.
|
|
18
|
Steffan JJ, Snider JL, Skalli O, Welbourne
T and Cardelli JA: Na+/H+ exchangers and RhoA regulate acidic
extracellular pH-induced lysosome trafficking in prostate cancer
cells. Traffic. 10:737–753. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fuster DG and Alexander RT: Traditional
and emerging roles for the SLC9 Na+/H+ exchangers. Pflugers Arch.
466:61–76. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Amith SR and Fliegel L: Regulation of the
Na+/H+ exchanger (NHE1) in breast cancer metastasis. Cancer Res.
73:1259–1264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khajah MA, Almohri I, Mathew PM and
Luqmani YA: Extracellular alkaline pH leads to increased metastatic
potential of estrogen receptor silenced endocrine resistant breast
cancer cells. PLoS One. 8:e763272013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boyarsky G, Hanssen C and Clyne LA:
Inadequacy of high K+/nigericin for calibrating BCECF. II.
Intracellular pH dependence of the correction. Am J Physiol.
271:C1146–C1156. 1996.PubMed/NCBI
|
|
23
|
Voulgari A and Pintzas A:
Epithelial-mesenchymal transition in cancer metastasis: Mechanisms,
markers and strategies to overcome drug resistance in the clinic.
Biochim Biophys Acta. 1796:75–90. 2009.PubMed/NCBI
|
|
24
|
Sardet C, Franchi A and Pouysségur J:
Molecular cloning, primary structure, and expression of the human
growth factor-activatable Na+/H+ antiporter. Cell. 56:271–280.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fliegel L: Molecular biology of the
myocardial Na+/H+ exchanger. J Mol Cell Cardiol. 44:228–237. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kemp G, Young H and Fliegel L: Structure
and function of the human Na+/H+ exchanger isoform 1. Channels.
2:329–336. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chiang Y, Chou CY, Hsu KF, Huang YF and
Shen MR: EGF upregulates Na+/H+ exchanger NHE1 by
post-translational regulation that is important for cervical cancer
cell invasiveness. J Cell Physiol. 214:810–819. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stock C, Jungmann O and Seidler DG:
Decorin and chondroitin-6 sulfate inhibit B16V melanoma cell
migration and invasion by cellular acidification. J Cell Physiol.
226:2641–2650. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rebillard A, Tekpli X, Meurette O, Sergent
O, LeMoigne-Muller G, Vernhet L, Gorria M, Chevanne M, Christmann
M, Kaina B, et al: Cisplatin-induced apoptosis involves membrane
fluidification via inhibition of NHE1 in human colon cancer cells.
Cancer Res. 67:7865–7874. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jones EM, Cochrane CA and Percival SL: The
effect of pH on the extracellular matrix and biofilms. Adv Wound
Care. 4:431–439. 2015. View Article : Google Scholar
|
|
31
|
Turk B, Dolenc I, Lenarcic B, Krizaj I,
Turk V, Bieth JG and Björk I: Acidic pH as a physiological
regulator of human cathepsin L activity. Eur J Biochem.
259:926–932. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Greco MR, Antelmi E, Busco G, Guerra L,
Rubino R, Casavola V, Reshkin SJ and Cardone RA: Protease activity
at invadopodial focal digestive areas is dependent on NHE1-driven
acidic pHe. Oncol Rep. 31:940–946. 2014.PubMed/NCBI
|
|
33
|
Yonezawa N, Nishida E and Sakai H: pH
control of actin polymerization by cofilin. J Biol Chem.
260:14410–14412. 1985.PubMed/NCBI
|
|
34
|
Yang X, Wang D, Dong W, Song Z and Dou K:
Inhibition of Na+/H+ exchanger 1 by 5-(N-ethyl-N-isopropyl)
amiloride reduces hypoxia-induced hepatocellular carcinoma invasion
and motility. Cancer Lett. 295:198–204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Weaver BA: How Taxol/paclitaxel kills
cancer cells. Mol Biol Cell. 25:2677–2681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Collins K, Jacks T and Pavletich NP: The
cell cycle and cancer. Proc Natl Acad Sci USA. 94:2776–2778. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Santo L, Siu KT and Raje N: Targeting
cyclin-dependent kinases and cell cycle progression in human
cancers. Semin Oncol. 42:788–800. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Putney LK, Denker SP and Barber DL: The
changing face of the Na+/H+ exchanger, NHE1: Structure, regulation,
and cellular actions. Annu Rev Pharmacol Toxicol. 42:527–552. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Smith BN and Bhowmick NA: Role of EMT in
metastasis and therapy resistance. J Clin Med. 5:E172016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ipekci T, Ozden F, Unal B, Saygin C,
Uzunaslan D and Ates E: Epithelial-mesenchymal transition markers
β-catenin, Snail, and E-cadherin do not predict disease free
survival in prostate adenocarcinoma: A prospective study. Pathol
Oncol Res. 21:1209–1216. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yagasaki R, Noguchi M, Minami M and
Earashi M: Clinical significance of E-cadherin and vimentin
co-expression in breast cancer. Int J Oncol. 9:755–761.
1996.PubMed/NCBI
|
|
42
|
Dorudi S, Sheffield JP, Poulsom R,
Northover JM and Hart IR: E-cadherin expression in colorectal
cancer. An immunocytochemical and in situ hybridization study. Am J
Pathol. 142:981–986. 1993.PubMed/NCBI
|
|
43
|
Li K, Dan Z, Hu X, Gesang L, Ze Y and
Bianba Z: CD14 regulates gastric cancer cell epithelial-mesenchymal
transition and invasion in vitro. Oncol Rep. 30:2725–2732.
2013.PubMed/NCBI
|
|
44
|
Zhou Y, Li G, Wu J, Zhang Z, Wu Z, Fan P,
Hao T, Zhang X, Li M, Zhang F, et al: Clinicopathological
significance of E-cadherin, VEGF, and MMPs in gastric cancer.
Tumour Biol. 31:549–558. 2010. View Article : Google Scholar : PubMed/NCBI
|