Introduction
Lung cancer has the highest mortality rate of all
carcers worldwide (1,2), and its incidence rate is still
increasing in Taiwan (3,4). Lung adenocarcinoma accounts for ~30%
of primary lung tumors in male smokers and 40% in female smokers
(5). However, males and females
suffer from adenocarcinoma approaching 60 and 80%, respectively
among non-smokers (6). Although
there is a rapid development in the treatment by targeted
therapies, the 5-year survival rate is still low and <16% all
over the world (7). In addition,
75–80% of lung cancer is non-small cell lung cancer (NSCLC)
(8). Radical resection is the
treatment of choice for NSCLC (9).
Over 75% of NSCLC patients are potential candidates for
chemotherapy, but chemotherapy usually produces rather poor
response rates in NSCLC patients with rare complete remissions
(10,11). Thus, it is imperative to develop new
treatment to improve the overall disease-free survival of NSCLC
patients.
Our previous study showed that a series of
carboxamide derivatives were designed and synthesized as novel
anticancer agents (12), and these
novel compounds exhibited potent cytotoxic effect on multiple
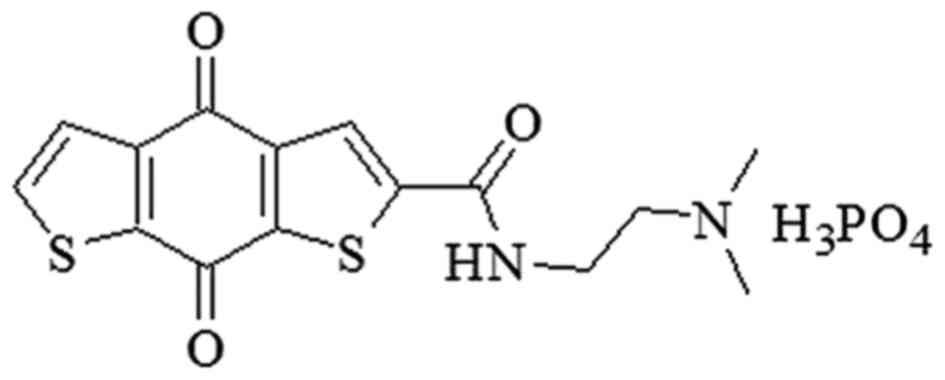
cancer cell lines (12–14). Among them, ITR-284
[N-(2-dimethylaminoethyl)-4,8-dihydrobenzo (1,2-b;4,5-b')
dithio-phene-2-carboxamide phosphoric acid salt] (Fig. 1) is one of the most potent agents
(12,13). It has been demonstrated that ITR-284
significantly inhibited leukemia cell proliferation (HL-60 and
WEHI-3) and possessed low cytotoxicity to normal cells. In
addition, ITR-284 had a greater growth inhibition than that of
other compounds in human hepatocellular carcinoma cell lines
(HepG2, Hep3B, SK-HEP-1 and J5) and colorectal cancer cell lines
(HT 29, COLO 205, HCT 116 and SW 620 (14). Therefore, ITR-284 has the potential
for treating multiple cancers, but it might be needed for further
investigations.
Tumor cells possess metastasis and invasion ability
to become malignant tumor cells (15). Cancer cell migration and invasion
are a complex cell spreading process from primary locations to
secondary site in the body, in which cell adhesion molecules and
signal transduction factors are involved (16,17).
During migration and invasion, several phenotypic changes of tumor
cells, including cell-cell adhesion and remodeling of cell-matrix
adhesion sites have been characterized. This process is known as
epithelial-mesenchymal transition (EMT) (18,19).
Loss of E-cadherin expression to lead to reduction of cell-cell
adhesion is a symbol of occurrence of EMT, while increase of
vimentin expression, close relationship with differentiation and
metastasis of cancer cell is another distinctive event in EMT
(20,21). In this study, we focused on
exploring the anti-proliferative effects, cell cycle arrest,
apoptosis and anti-migration ability of ITR-284 on human lung
adenocarcinoma A549 cells in vitro.
Materials and methods
Materials and reagents
Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS), thiazolyl blue tetrazolium bromide (MTT),
trypsin-EDTA, propidium iodide (PI), RNase A, sodium citrate and
anti-actin antibody were purchased from Sigma-Aldrich (St. Louis,
MO, USA). Primary antibodies against p53, p-p53 (Ser15), Bax,
Bcl-2, caspase-3, PARP, E-cadherin and vimentin were obtained from
Santa Cruz Biotechnology (Santa Cruz, CA, USA). Secondary
antibodies conjugated with infrared fluorescently-labeled dye were
purchased from LI-COR Biosciences (Lincoln, NE, USA).
Cell culture
Human adenocarcinoma cell line A549 was obtained
from the Bioresource Collection and Research Center (BCRC, Hsinchu,
Taiwan). Cells were grown in DMEM supplemented with 10% FBS, 100
U/ml penicillin and 100 µg/ml streptomycin at a humidified
environment with 95% air and 5% CO2 at 37°C. Cells in
the logarithmic phase of growth were used for the following
experiments.
Cell viability assay
A549 cells (7×103 cells/well) were seeded
into 96-well plates (Costar, Corning Inc., Coring, NY, USA),
allowed to attach overnight, and then exposed to various
concentrations (1, 5, 10, 15 and 20 nM) of ITR-284 for 24 h. Cell
viability was determined by the MTT assay, as previously described
(22). The absorbance was recorded
by using an enzyme-linked immunosorbent assay (ELISA) reader and
control absorbance was normalized to 100%. Six replicated wells
were included in each group. At least three independent experiments
were done.
DNA content analysis
Cell cycle phase and sub-G1 population (apoptosis)
of A549 cells were detected by flow cytometry as previously
described (23,24). Cells were treated with or without 1,
5, 10, 15 and 20 nM of ITR-284 for 24 h, harvested and washed with
phosphate-buffered saline (PBS). Cells were fixed in 70% ice-cold
ethanol/PBS for 20 min on ice and then incubated in PI solution
containing 69 mM PI, 388 mM sodium citrate, 100 µg/ml RNase A for
15 min. Cells were immediately detected with FACScan flow cytometry
(BD Biosciences, San Jose, CA, USA).
Annexin V/PI double staining
A549 cells (2.5×105 cells/ml) were seeded
in 6-well plates and cultured overnight. Culture medium was removed
and replaced with 1, 5, 10, 15 and 20 nM of ITR-284. After
incubation for 24 h, cells were washed with PBS and collected in a
tube before centrifugation at 1,200 rpm for 5 min. Cell pellet was
washed once with PBS, re-suspended with the 0.1 ml binding buffer
(1X) and mixed with 5 µl fluorescein isothiocyanate (FITC)-Annexin
V and 10 µl PI staining reagent as recommended in the
manufacturer's protocol (FITC Annexin V Apoptosis Detection kit I,
BD Pharmingen, San Diego, CA, USA). Following staining and mixing
carefully, apoptotic cell populations were detected using a flow
cytometer.
Measurement of intracellular reactive
oxygen species (ROS) production
ROS generation inside living cells was measured by
Muse Oxidative Stress kit (Millipore, Billerica, MA, USA), and the
assays were performed according to the manufacturer's instructions.
Dihydroethidium (DHE), an oxidation-sensitive probe, displays blue
fluorescence in the cell cytoplasm and forms a red fluorescent
product, 2-hydroxyethidium upon oxidation by ROS. Briefly,
untreated or treated A549 cells were stained with 0.5 µM DHE for 30
min at 37°C in the dark and subsequently assayed by flow
cytometry.
Western blotting
A549 cells (1×107 cells/dish) were
incubated in a 10-cm dish for 24 h. Culture medium was removed and
replaced with the indicated concentrations of ITR-284. After
incubation for 24 h, cells were collected, treated with cell lysis
buffer, disrupted by sonication, and then centrifuged at 12,000 rpm
for 30 min. The protein-containing supernatant was collected and
stored at −80°C before use. Quantitation of protein was carried out
by using the Bio-Rad protein assay reagent (Bio-Rad Laboratories,
Hercules, CA, USA) according to the manufacturer's instructions. A
total of 50 µg sample was prepared and mixed with sample buffer,
boiled in a 95°C water bath for 5 min, and cooled on ice. Samples
were separated on 10% SDS-polyacrylamide gel under 100 V and
transferred onto a polyvinylidene fluoride (PVDF) membrane
(Immobilon-P Transfer Membrane, Millipore), which was presoaked in
methanol for 30 sec for activation and then immersed in transfer
buffer. After transferring at 4°C under 100 V for 2 h, the PVDF
membrane was soaked in blocking buffer containing 5% skim milk for
1 h. The primary antibodies dissolved in the Tris-buffered saline
with Tween-20 (TBST) buffer solution containing 0.1% Tween-20, 150
mM NaCl, and 10 mM Tris-HCl (pH 8.0) were diluted with an
appropriate ratio, including p53 (1:1,000), p-p53 (1:1,000), Bax
(1:1,000), Bcl-2 (1:1,000), caspase-3 (1:1,000), PARP (1:1,000),
E-cadherin (1:1,000), vimentin (1:1,000) and actin (1:1,000). After
probed at 4°C overnight, PVDF membranes were washed with the TBST
buffer three times and incubated with the infrared
fluorescently-labeled dye-conjugated secondary antibody (IgG) at
room temperature for 1 h. PVDF membranes were washed with the TBST
buffer three times and scanned by the Odyssey infrared imager
(LI-COR Biosciences) for measurement of protein expression
intensity.
Wound healing assay
Cells were seeded in 6-well plates and incubated for
24 h to reach ~80–90% confluency. A linear wound was created by
dragging a 200-µl pipette tip through the monolayer. Cellular
debris was removed by gentle washes with culture medium. Cells were
treated with 1, 2, 3, 4 and 5 nM of ITR-284 for 24 h. The healing
process was photographed after the wounding using a microscope
(Olympus 600 auto-biochemical analyzer, Tokyo, Japan). Migration
distance of A549 cells was measured from 5 field images, and the
gap size was determined using Image-Pro Plus 6.0 software (Media
Cybernetics, Silver Spring, MD, USA).
Statistical analysis
All data are presented as the mean ± standard
deviation (SD). One way analysis of variance (ANOVA) with Student's
t-test was used for multiple comparisons among groups. A value of
p<0.05 was considered statistically significant.
Results
ITR-284 inhibits the proliferation of
A549 cells
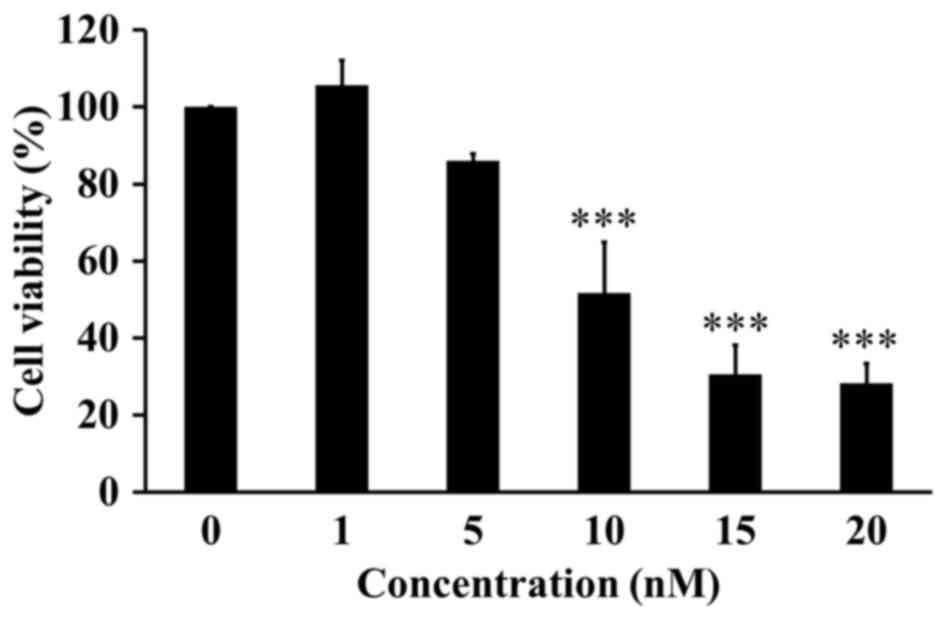
MTT assay was performed to investigate the cytotoxic
effect of ITR-284 on the cell proliferation and viability in A549
cells. We found that ITR-284 obviously inhibited the cell viability
of A549 cells in a dose-dependent manner (Fig. 2). The IC50 (50%
inhibitory concentration) of ITR-284 was 10 nM after a 24-h
treatment in A549 cells.
ITR-284 induces S phase arrest in A549
cells
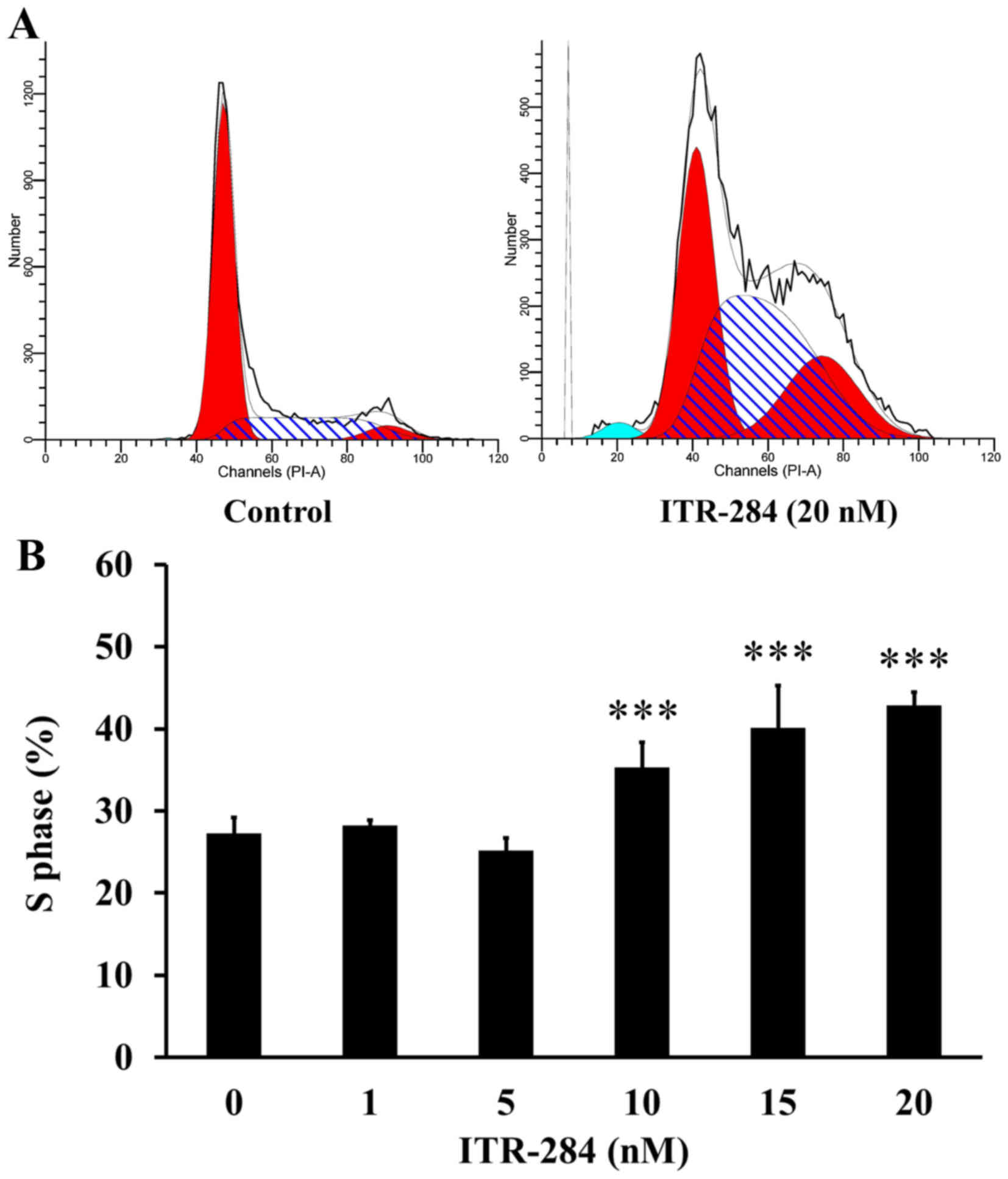
It is well known that cell cycle is a reflection of
cell growth and division, which is easily disturbed by drug
treatment (25). We examined the
cell cycle distribution in ITR-284-treated A549 cells. The cells
were treated with different concentrations of ITR-284 for 24 h and
analyzed by flow cytometry after PI staining. The data demonstrated
that the percentage of A549 cells in S phase was increased in a
dose-dependent manner after exposure to 10, 15 and 20 nM of ITR-284
(Fig. 3A). The proportion of cells
at S phase was 42.4±1.78% (p<0.05) in 20 nM ITR-284-treated A549
cells for 24 h (Fig. 3B).
ITR-284 triggers apoptosis in A549
cells
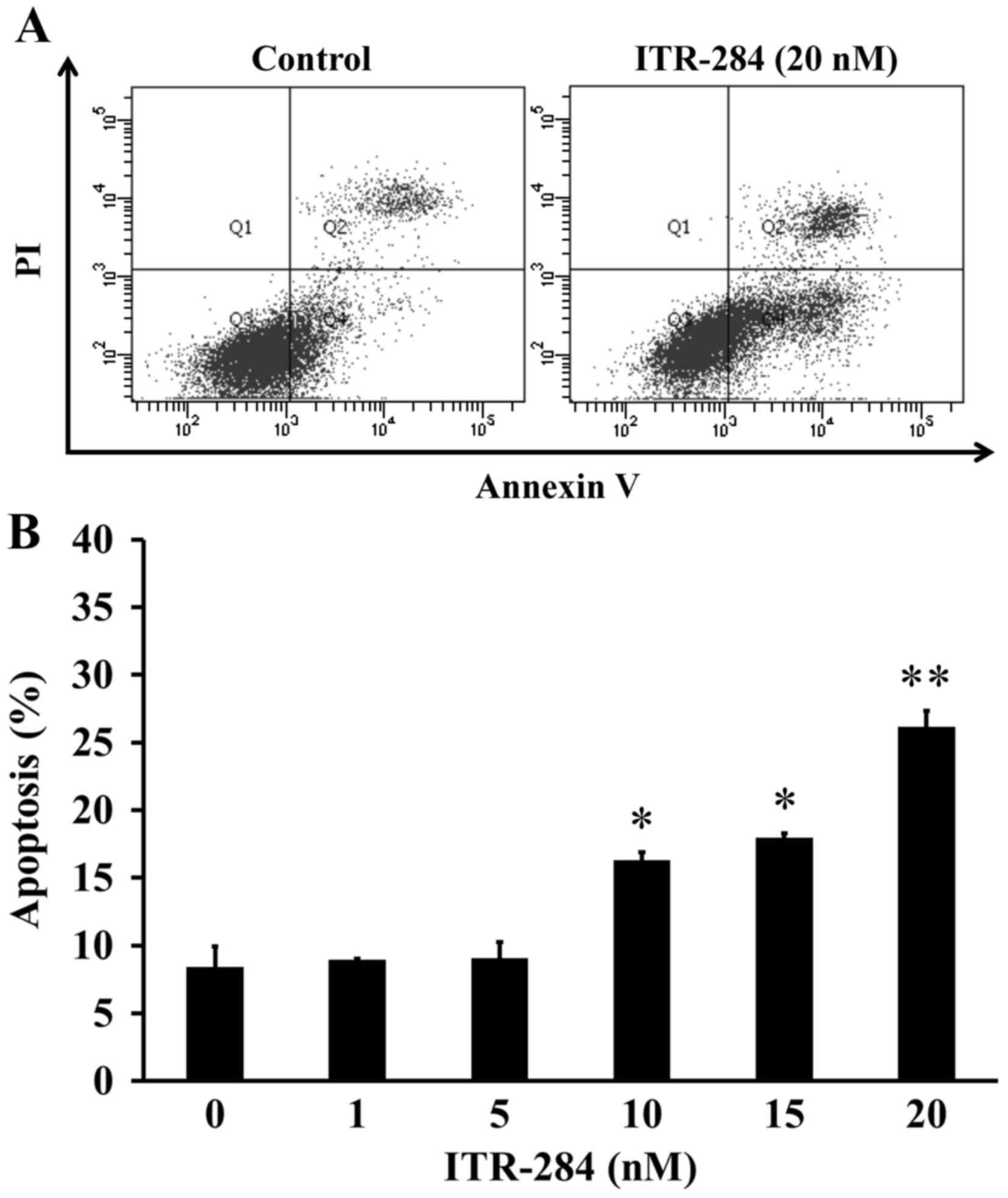
ITR-284 possesses inhibitory ability via inducing
apoptosis when leukemia and colon cancer cells were treated with
ITR-284, as previous studies reported (13,14).
To investigate the effect of ITR-284 on apoptosis in A549 cells,
Annexin V/PI double staining assay was conducted. After treatment
with 0, 1, 5, 10, 15 and 20 nM of ITR-284 for 24 h in A549 cells,
the profiles from flow cytometric analysis were performed (Fig. 4A) and the percentage of A549
apoptotic cells (Annexin V+/PI−)
significantly increased (Fig. 4B)
prior to 10–20 nM ITR-284 exposure for 24 h. Thus, ITR-284 showed
apoptotic evidence in A549 cells after challenge.
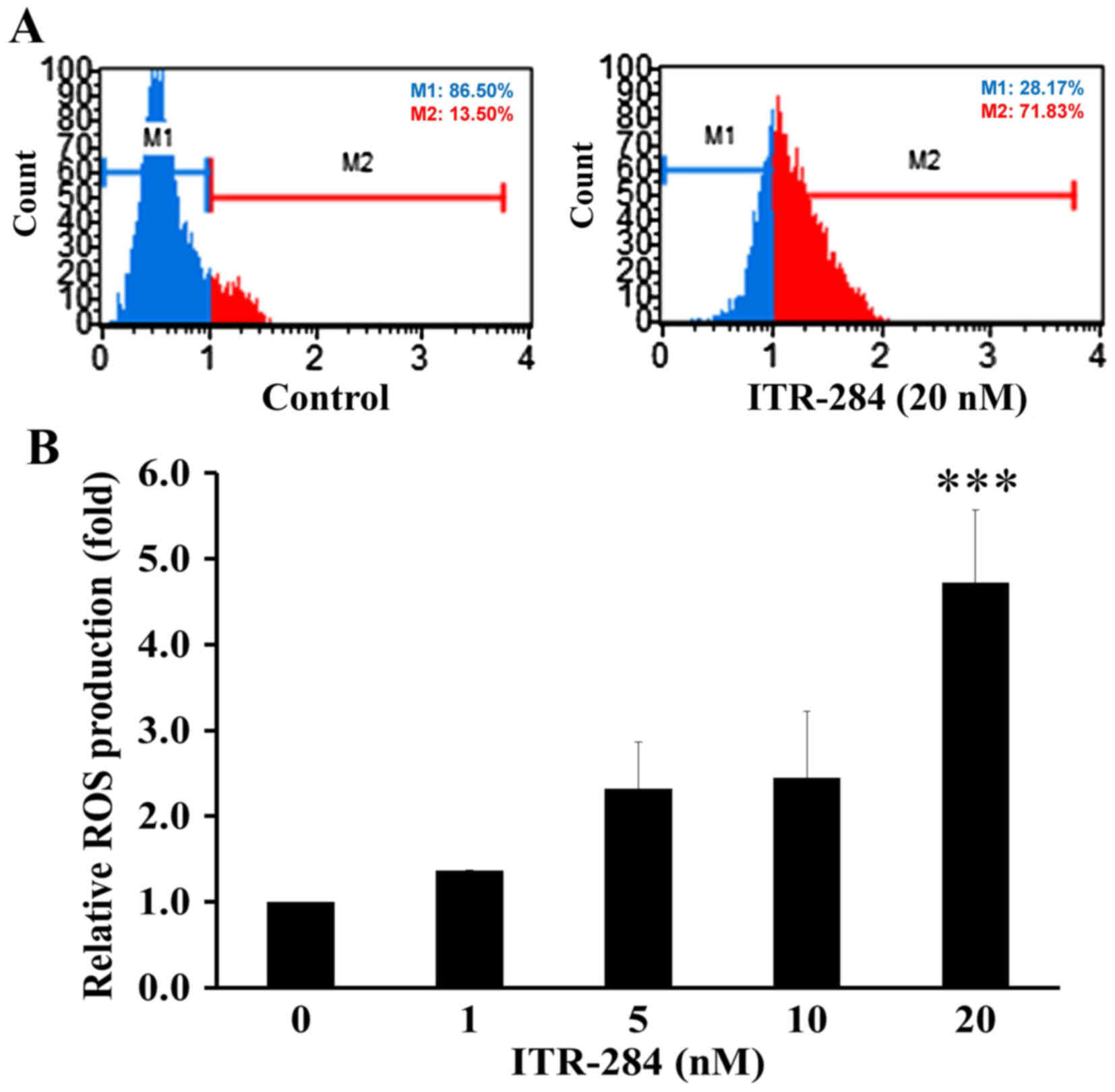
ITR-284 promotes ROS production in
A549 cells
Reactive oxygen species (ROS) generation plays an
important role in mitochondria-mediated apoptotic pathway (26,27).
ITR-284 has been shown to induce apoptosis through ROS generation
in other cancer cells (13,14). This study indicated that ROS
production was elevated in A549 cells after 20 nM ITR-284 challenge
(Fig. 5A and B) consistent with
previous findings (13).
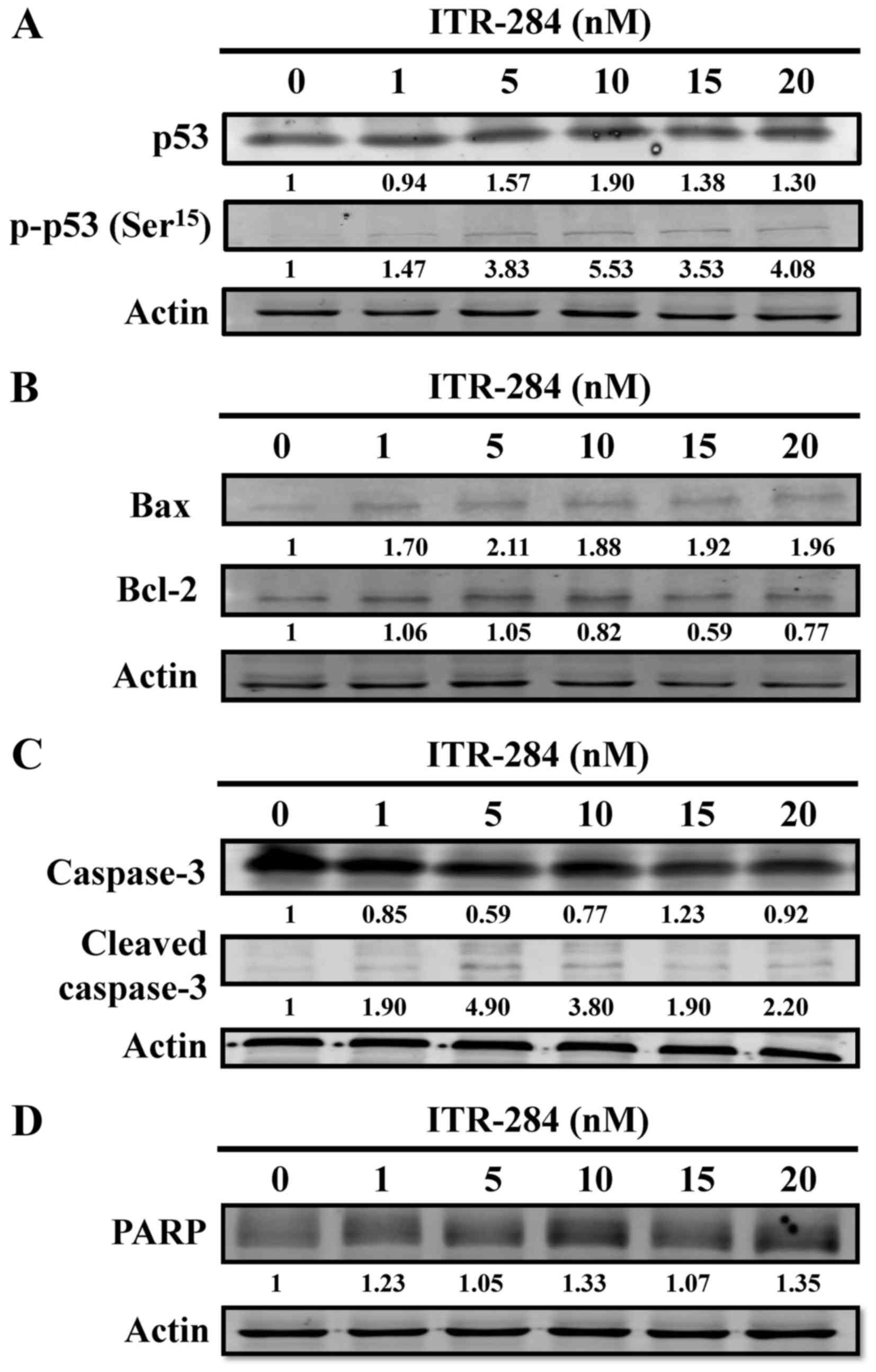
ITR-284 alters protein levels of p53,
Bcl-2 family and caspase-3 in A549 cells
To confirm the results of ITR-284-induced S phase
arrest and apoptosis in A549, we investigated whether increased p53
phosphorylation, Bcl-2 family protein and cleaved caspase-3 were
observed in A549 cells after ITR-284 treatment by western blotting.
A dose-dependent increase in p53 and p-p53 protein levels was found
(Fig. 6A). When A549 cells were
treated with ITR-284, we observed that there was a decrease of the
expression level of Bcl-2 and an increase of Bax expression; these
effects were dose-dependent (Fig.
6B). The expression levels of cleaved caspase-3 (Fig. 6C) and cleaved PARP (Fig. 6D) were increased in a dose-dependent
manner in ITR-284-treated A549 cells. Thus, ITR-284 induced A549
cell apoptosis through mitochondria-dependent pathway.
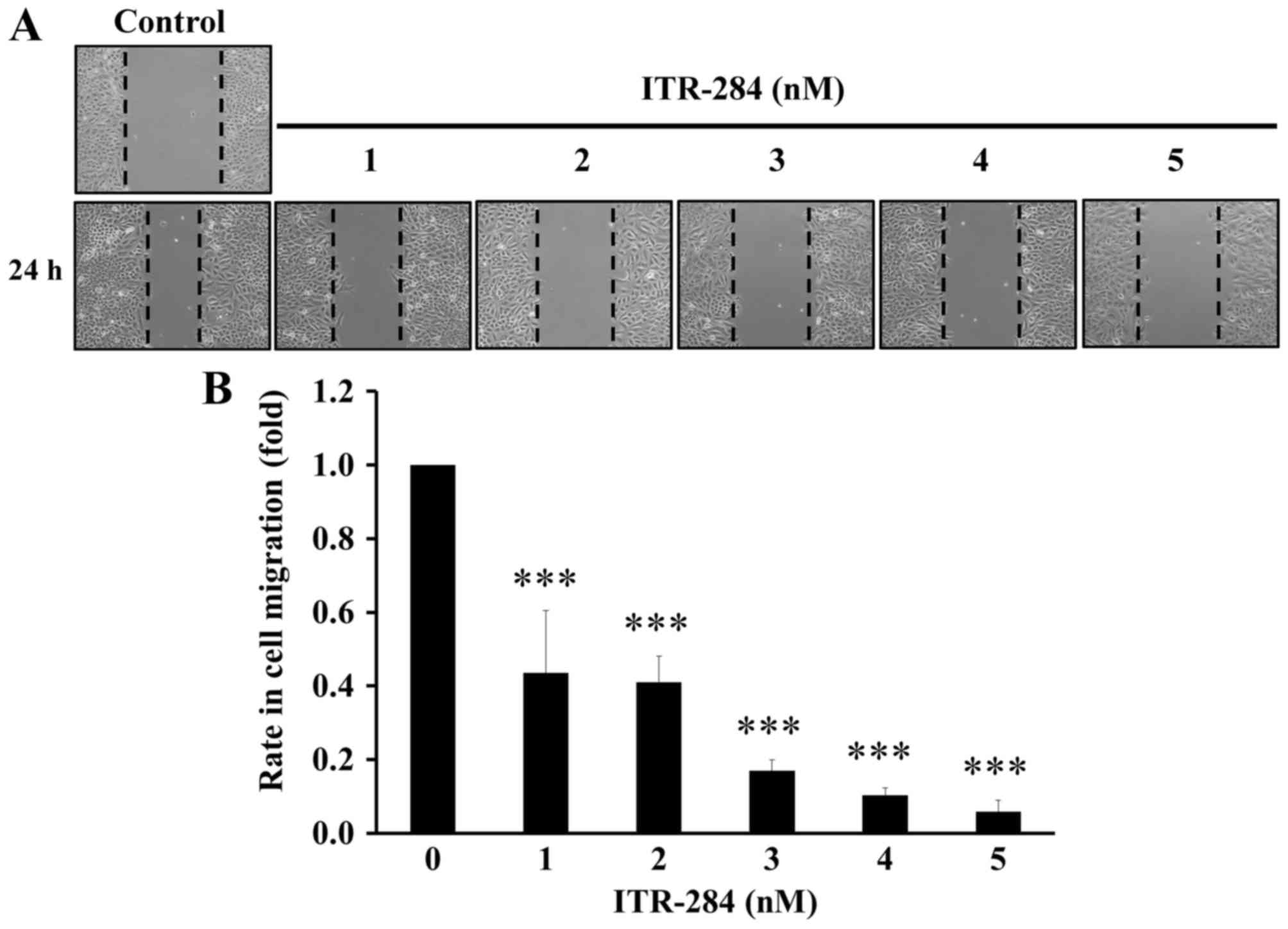
ITR-284 inhibits cell migration in
A549 cells
To examine the anticancer ability of ITR-284, we
further investigated whether ITR-284 suppresses A549 cell migration
by a ‘scratch wound healing’ assay. Cells were seeded for near
confluency and scratched with a pipette tip. Cells were treated
with low concentrations (1, 2, 3, 4 and 5 nM) of ITR-284 for 24 h.
Motility of cells at different concentrations after generation of
the wound was monitored under a microscope. Closure of the wound
was inhibited within 24 h compared to the untreated control A549
cells. In contrast, ITR-284-treated A549 cells migrated toward the
wound at a much slower rate (Fig.
7A). The quantitative data show that ITR-284 dose-dependently
suppressed cell migration in A549 cells (Fig. 7B). Thus, the inhibitory effect of
ITR-284 on A549 cell migration was performed in vitro.
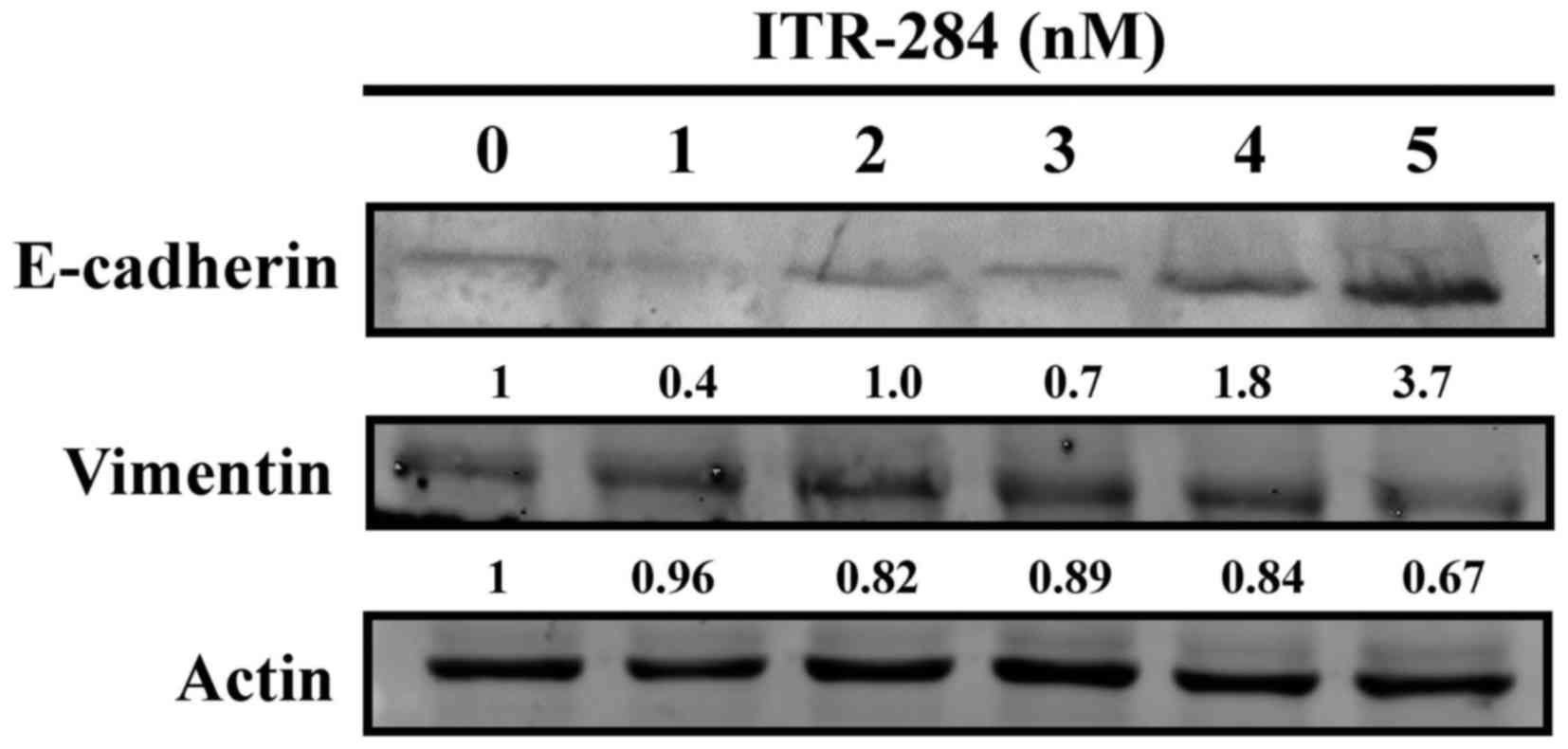
ITR-284 modulates E-cadherin
expression and vimentin expression in A549 cells
To evaluate the anti-cell migration ability of
ITR-284, we investigated effects of ITR-284 on expression of
E-cadherin and vimentin in ITR-284-treated A549 cells. Results
indicated that E-cadherin protein level was dose-dependently
increased (Fig. 8). We also
observed that ITR-284 induced a decrease in the protein level of
vimentin in A549 cells dose-dependently (Fig. 8). Therefore, ITR-284-suppressed cell
migration might be mediated through modulating E-cadherin and
vimentin signaling in A549 cells.
Discussion
In this study, we examined the effects of ITR-284 on
the proliferation, cell cycle, apoptosis and migration of A549
cells in vitro. We found that ITR-284 inhibited cell
proliferation in a dose-dependent manner and changed the morphology
of A549 cells in vitro (Fig.
2). Furthermore, ITR-284 treatment significantly reduced the
levels of Bcl-2 expression and induced the levels of Bax, cleaved
caspase-3 and cleaved PARP expression in A549 cells (Fig. 6B and C). In addition, ITR-284
induced cell cycle arrest at S phase and triggered cell apoptosis
in A549 cells (Fig. 3). Cell cycle
arrest is associated with inhibition of tumor cell proliferation,
and cell cycle progression is positively regulated by a series of
cyclins and CDKs, but negatively regulated by CDK inhibitors of
p21, p16 and p27 (28,29). We found that treatment with ITR-284
significantly induced the levels of p53 and phosphorylated p53
expression in A549 cells (Fig. 6A).
Given that p53 is an important regulator of p21 expression
(30). It is possible that ITR-284
treatment may upregulate p53 expression (Fig. 6A) and in turn elevate the expression
of p21 to inhibit the cyclin1/CDK complex, leading to cell cycle
arrest at S phase in A549 cells. To the best of our knowledge, this
was the first study to reveal potent antitumor activity of ITR-284
in A549 cells. Our novel findings may aid in the design of new
therapy for intervention of human NSCLC.
Once cell cycle distribution is disrupted, cells
trigger cell cycle arrest to examine the status of cells (28). Induction of cell cycle S phase
arrest is associated with triggering cell apoptosis (31). To understand the molecular
mechanisms underlying the action of ITR-284 in inhibiting the
proliferation of A549 cells, we examined the effect of ITR-284 on
the survival of A549 cells. We found that treatment with ITR-284
triggered apoptosis of A549 cells by Annexin V/PI staining
(Fig. 4). There are two distinct
apoptotic signaling pathways (32,33).
Intrinsic apoptotic pathway is mitochondria associated, which
induces the formation of cytochrome c complex, which
activates caspase-9 and in turn activates caspase-3, leading to
cell apoptosis. Cell apoptosis can be regulated positively by the
pro-apoptotic Bcl-2 family members, such as Bax, Bad and others,
and negatively regulated by anti-apoptotic Bcl-2 family members,
such as Bcl-2 and Bcl-xL. The balance of pro-apoptotic and
anti-apoptotic factors is crucial for the development of apoptosis
(32,33). In this study, we found that ITR-284
treatment significantly increased the levels of Bax, cleaved
caspase-3 but decreased the levels of Bcl-2 expression in A549
cells (Fig. 6). Therefore, ITR-284
induced A549 cell apoptosis through activating the mitochondrial
pathway.
Cancer cells can migrate and invade the adjacent and
distant tissues, which increases the risk for poor outcome of
patients with NSCLC (15,17). Tumor cell migration is regulated by
many factors, such as chemokines, their receptors, the EMT process,
matrix degrading enzymes and antioxidant enzymes, such as MMPs,
which mediate the degradation of extracellular matrix (ECM)
proteins (18,19). EMT is accompanied by profound
changes in cell characteristics that enable the epithelial cells to
detach from tight junctions, change the cell's shape and polarity,
delaminate, and migrate (19).
During tumor metastasis transition, EMT-associated molecules are
changed accordingly, such as increasing the expression of
mesenchymal cells specific proteins (vimentin, fibronectin, and
decreasing the expression of epithelial cell adhesion protein
(E-cadherin) (20,21). We found that ITR-284 treatment
significantly inhibited the migration of A549 cells, accompanied by
significantly attenuating the expression of vimentin and increased
E-cadherin levels (Fig. 8). We
observed the changes of EMT phenotype and found that vimentin and
E-cadherin expression contributed to regulate EMT in A549 cells
caused by ITR-284, and ITR-284 affected the invasion and metastasis
of tumor cells, leading to cell functional changes.
In conclusion, our results indicate that ITR-284
treatment significantly inhibits the A549 cell proliferation by
inducing their cell cycle arrest at S phase through upregulating
p53 and phosphorylation of p53 expression. ITR-284 triggers
apoptosis of A549 cells by activating the caspase-3-dependent
pathway. Furthermore, ITR-284 significantly inhibited the migration
of A549 cells by increasing E-cadherin expression and reducing
vimentin expression. Therefore, our data suggest that ITR-84 may be
a promising candidate for intervention of NSCLC, and our findings
may provide a new insight into understanding the pharmacological
mechanisms underlying antitumor activity of ITR-284.
Acknowledgements
This study was supported by research grant (no.
CMU100- TC-08) from China Medical University, Taichung, Taiwan.
References
|
1
|
Hagedoorn P, Vandenheede H, Willaert D,
Vanthomme K and Gadeyne S: Regional inequalities in lung cancer
mortality in Belgium at the beginning of the 21st century: The
contribution of individual and area-level socioeconomic status and
industrial exposure. PLoS One. 11:e01470992016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang JY, Jian ZH, Nfor ON, Ku WY, Ko PC,
Lung CC, Ho CC, Pan HH, Huang CY, Liang YC, et al: The effects of
pulmonary diseases on histologic types of lung cancer in both
sexes: A population-based study in Taiwan. BMC Cancer. 15:8342015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang WS, Wang SC, Chuang CL, Ji HX, Hsiao
CL, Hsu CM, Tsai CW, Liu SP, Hsu PC, Lo YL, et al: Contribution of
interleukin-4 genotypes to lung cancer risk in Taiwan. Anticancer
Res. 35:6297–6301. 2015.PubMed/NCBI
|
|
5
|
Cruz CS Dela, Tanoue LT and Matthay RA:
Lung cancer: Epidemiology, etiology, and prevention. Clin Chest
Med. 32:605–644. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu Y, Liu H, Zheng S, Ding Z, Chen Z, Jin
W, Wang L, Wang Z, Fei Y, Zhang S, et al: Gender susceptibility for
cigarette smoking-attributable lung cancer: A systematic review and
meta-analysis. Lung Cancer. 85:351–360. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Norén A, Eriksson HG and Olsson LI:
Selection for surgery and survival of synchronous colorectal liver
metastases; a nationwide study. Eur J Cancer. 53:105–114. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Villaruz LC and Socinski MA: Is there a
role of nab-paclitaxel in the treatment of advanced non-small cell
lung cancer? The data suggest yes. Eur J Cancer. 56:162–171. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Van Schil PE, Balduyck B, De Waele M,
Hendriks JM, Hertoghs M and Lauwers P: Surgical treatment of
early-stage non-small-cell lung cancer. EJC (Suppl). 11:110–122.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang W, Chen P, Tang M, Li J, Pei Y, Cai
S, Zhou X and Chen S: Tumstatin 185–191 increases the sensitivity
of non-small cell lung carcinoma cells to cisplatin by blocking
proliferation, promoting apoptosis and inhibiting Akt activation.
Am J Transl Res. 7:1332–1344. 2015.PubMed/NCBI
|
|
11
|
Stahel R, Peters S, Baas P, Brambilla E,
Cappuzzo F, De Ruysscher D, Eberhardt WE, Felip E, Fennell D,
Marchetti A, et al: Strategies for improving outcomes in NSCLC: A
look to the future. Lung Cancer. 82:375–382. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wen YF, Lee KH, Huang PT, Chen MH, Shin
WC, Huang LJ, Hsu MH, Chen CJ and Kuo SC: Cell differentiation
enhancement by hydrophilic derivatives of
4,8-dihydrobenzo[1,2-b:5,4-b']dithiophene-4,8-diones in HL-60
leukemia cells. Bioorg Med Chem Lett. 17:2908–2912. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wen YF, Yang JS, Kuo SC, Hwang CS, Chung
JG, Wu HC, Huang WW, Jhan JH, Lin CM and Chen HJ: Investigation of
anti-leukemia molecular mechanism of ITR-284, a carboxamide analog,
in leukemia cells and its effects in WEHI-3 leukemia mice. Biochem
Pharmacol. 79:389–398. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liao YR, Lu CC, Lai KC, Yang JS, Kuo SC,
Wen YF, Fushiya S and Wu TS: The novel carboxamide analog ITR-284
induces caspase-dependent apoptotic cell death in human
hepatocellular and colorectal cancer cells. Mol Med Rep.
7:1539–1544. 2013.PubMed/NCBI
|
|
15
|
Joosse SA, Gorges TM and Pantel K:
Biology, detection, and clinical implications of circulating tumor
cells. EMBO Mol Med. 7:1–11. 2014. View Article : Google Scholar :
|
|
16
|
Kamińska K, Szczylik C, Bielecka ZF,
Bartnik E, Porta C, Lian F and Czarnecka AM: The role of the
cell-cell interactions in cancer progression. J Cell Mol Med.
19:283–296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Farahani E, Patra HK, Jangamreddy JR,
Rashedi I, Kawalec M, Rao Pariti RK, Batakis P and Wiechec E: Cell
adhesion molecules and their relation to (cancer) cell stemness.
Carcinogenesis. 35:747–759. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang J, Wang H, Wang X, Zhao Y, Zhao D,
Wang C, Li Y, Yang Z, Lu S, Zeng Q, et al: Molecular mechanisms of
Polyphyllin I-induced apoptosis and reversal of the
epithelial-mesenchymal transition in human osteosarcoma cells. J
Ethnopharmacol. 170:117–127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sui H, Zhu L, Deng W and Li Q:
Epithelial-mesenchymal transition and drug resistance: Role,
molecular mechanisms, and therapeutic strategies. Oncol Res Treat.
37:584–589. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Foroni C, Broggini M, Generali D and Damia
G: Epithelial-mesenchymal transition and breast cancer: Role,
molecular mechanisms and clinical impact. Cancer Treat Rev.
38:689–697. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu CC, Huang BR, Liao PJ and Yen GC:
Ursolic acid triggers nonprogrammed death (necrosis) in human
glioblastoma multiforme DBTRG-05MG cells through MPT pore opening
and ATP decline. Mol Nutr Food Res. 58:2146–2156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin CC, Chuang YJ, Yu CC, Yang JS, Lu CC,
Chiang JH, Lin JP, Tang NY, Huang AC and Chung JG: Apigenin induces
apoptosis through mitochondrial dysfunction in U-2 OS human
osteosarcoma cells and inhibits osteosarcoma xenograft tumor growth
in vivo. J Agric Food Chem. 60:11395–11402. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chiang JH, Yang JS, Lu CC, Hour MJ, Chang
SJ, Lee TH and Chung JG: Newly synthesized quinazolinone HMJ-38
suppresses angiogenetic responses and triggers human umbilical vein
endothelial cell apoptosis through p53-modulated Fas/death receptor
signaling. Toxicol Appl Pharmacol. 269:150–162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shapiro GI and Harper JW: Anticancer drug
targets: Cell cycle and checkpoint control. J Clin Invest.
104:1645–1653. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang BB, Wang DG, Guo FF and Xuan C:
Mitochondrial membrane potential and reactive oxygen species in
cancer stem cells. Fam Cancer. 14:19–23. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hwang KE, Kim YS, Hwang YR, Kwon SJ, Park
DS, Cha BK, Kim BR, Yoon KH, Jeong ET and Kim HR: Enhanced
apoptosis by pemetrexed and simvastatin in malignant mesothelioma
and lung cancer cells by reactive oxygen species-dependent
mitochondrial dysfunction and Bim induction. Int J Oncol.
45:1769–1777. 2014.PubMed/NCBI
|
|
28
|
Lim S and Kaldis P: Cdks, cyclins and
CKIs: Roles beyond cell cycle regulation. Development.
140:3079–3093. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Harashima H, Dissmeyer N and Schnittger A:
Cell cycle control across the eukaryotic kingdom. Trends Cell Biol.
23:345–356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kreis NN, Louwen F and Yuan J: Less
understood issues: p21(Cip1) in mitosis and its therapeutic
potential. Oncogene. 34:1758–1767. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Song X, Li L, Shi Q, Lehmler HJ, Fu J, Su
C, Xia X, Song E and Song Y: Polychlorinated biphenyl quinone
metabolite promotes p53-dependent DNA damage checkpoint activation,
S-phase cycle arrest and extrinsic apoptosis in human liver
hepatocellular carcinoma HepG2 cells. Chem Res Toxicol.
28:2160–2169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Akl H, Vervloessem T, Kiviluoto S,
Bittremieux M, Parys JB, De Smedt H and Bultynck G: A dual role for
the anti-apoptotic Bcl-2 protein in cancer: Mitochondria versus
endoplasmic reticulum. Biochim Biophys Acta. 1843:2240–2252. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
van Vliet AR, Verfaillie T and Agostinis
P: New functions of mitochondria associated membranes in cellular
signaling. Biochim Biophys Acta. 1843:2253–2262. 2014. View Article : Google Scholar : PubMed/NCBI
|