Introduction
Pancreatic ductal adenocarcinoma (PDA) is one of the
most difficult to treat of all malignancies (1,2).
Systemic gemcitabine alone or in combination with 5-fluorouracil,
irinotecan and oxaliplatin (Folfirinox) is the current standard of
care for advanced pancreatic cancer, providing short-term
symptomatic improvement with minor impact on survival (3,4). Thus,
there is a dire need for developing novel agents for palliative
care of advanced pancreatic cancer.
Telomeres are nucleoprotein structures present at
the end of chromosomes that are essential for chromosomal stability
and prevention of end-to-end fusion (5). Shortening of telomeres triggers
replicative senescence or apoptosis. Telomerase rebuilds and
maintains telomere length by incorporating hexameric DNA repeats
(TTAGGG) to the 3′ flanking end of the telomeric DNA strands
(6). Human telomerase is comprised
of RNA template (hTERC) and the RNA dependent DNA polymerase
(hTERT) (7,8). hTERC serves as a template for hTERT
mediated telomere extension. In addition, hTERT associates with
several proteins including a six protein complex called shelterin
for proper functioning (9).
Deregulated telomerase activity promotes tumorigenesis (10,11).
hTERT expression and telomerase activity is elevated in PDA
(12–14). Thus, reactivated hTERT/telomerase in
PDA is a potential target for developing novel agents for the
treatment of this malignancy.
Pristimerin (PM) is a quinonemethide triterpenoid
present in various plant species. PM has shown potent
antiproliferative and apoptosis-inducing activity against diverse
types of cancer cells including pancreatic cancer cells (15–19).
Antitumor effects of PM involve induction of apoptosis, generation
of reactive oxygen species (ROS), mitochondrial dysfunction and
inhibition of nuclear factor κB (NF-κB), Akt and MAP kinases
(17–19). In a previous study, we showed that
the inhibition of cell proliferation and induction of apoptosis in
PDA cells by PM was associated with the inhibition of hTERT and its
telomerase activity (20). In the
present study, we investigated the role of epigenetic regulators of
hTERT gene expression in mediating the antitumor activity PM. PM
inhibited hTERT mRNA, native and phospho-hTERT protein and
downregulated transcription factors and transcriptionally active
chromatin markers that regulate hTERT transcription.
Materials and methods
Reagents
Pristimerin (PM) was purchased from Sigma Chemicals
(St. Louis, MO, USA). Antibodies against PARP-1, p-Akt, p-mTOR,
NF-κB (p65), Sp1, c-Myc and β-actin were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Anti-hTERT and p-TERT
(Ser824) antibodies were obtained from Abcam Inc. (Cambridge, MA,
USA). ChIP-validated antibodies anti-acetyl-histone H3 lysine 9,
anti-acetyl-histone H4, anti-tri-methyl histone H3 lysine 9 and
anti-di-methyl histone H3 lysine 4 were from Millipore (Billerica,
MA, USA). Annexin V-FITC apoptosis detection kit II was obtained
from BD Pharmingen (San Diego, CA, USA). CellTiter 96 AQueous One
Solution Proliferation Assay System was from Promega (Madison, WI,
USA). Stock solution of PM (100 mM) was prepared in DMSO and all
test concentrations were prepared by diluting stock solution in
tissue culture medium.
Cell lines
Panc-1 and MiaPaCa-2 PDA cell lines were obtained
from the American Type Culture Collection (ATCC, Rockville, MD,
USA). Both cell lines were grown in DMEM tissue culture medium
supplemented with 10% fetal bovine serum, 1%
penicillin/streptomycin, and 25 mM HEPES buffer. Cells were
incubated at 37°C in a humidified atmosphere consisting of 5%
CO2 and 95% humidity.
MTS assay
Tumor cells (1×104) in 100 µl of tissue
culture medium were seeded into each well of a 96-well plate. After
24 h incubation to allow cells to adhere, cells were treated with
PM at concentrations ranging from 0 to 5 µM. Cultures were
incubated for additional 72 h and cell viability was then
determined by the colorimetric MTS assay using CellTiter 96 AQueous
One Solution Proliferation Assay System. This assay measures the
bioreduction of tetrazolium compound MTS in the presence of
electron-coupling reagent phenazine methosulfate by intracellular
dehydrogenases. MTS and phenazine methosulfate were added to the
culture wells, and cultures were incubated for 2 h at 37°C. The
absorbance, which is directly proportional to the number of viable
cells in the cultures, was measured at 490 nm using a microplate
reader.
Annexin V-FITC binding
Induction of apoptosis was assessed by the binding
of Annexin V-FITC to phosphatidylserine, which is externalized to
the outer leaflet of the plasma membrane early during induction of
apoptosis. Briefly, Panc-1 and MiaPaCa-2 cells treated with PM (0–5
µM) for 24 h were resuspended in the binding buffer and 5 µl of
Annexin V-FITC reagent and 5 µl of PI were added. After incubation
for 30 min at room temperature in the dark, cells were analyzed by
flow cytometry.
Measurement of hTERT expression
hTERT expression was measured by analyzing hTERT
mRNA and hTERT protein. For hTERT mRNA, total cellular RNA was
extracted with TRIzol reagent (Gibco) according to the
manufacturer's recommendations. RNA (1 µg) was then reverse
transcribed by Oligo(dT) primer and high fidelity reverse
transcriptase (Boehringer Mannheim, Germany) to generate cDNAs. One
µl of cDNA was used as the template for polymerase chain reaction
(PCR) using hTERT primers: upper, 5′-TGTTTCTGGATTTGCAGGTG-3′, and
lower, 5′-GTTCTTGGCTTTCAGGATGG-3′; and GAPDH primers: upper, 5′-TCC
CTC AAG, ATT, GTC AGC AA-3′, and lower, 5′-AGA TCC ACA ACG GAT ACA
TT-3′. The PCR conditions used were 33 cycles of denaturation (95°C
for 1 min), annealing (62°C for 30 sec), and polymerization (72°C
for 1 min). The PCR products were separated on 2% agarose gel
electrophoresis and visualized by ethidium bromide staining. Gels
were photographed and band densities were analyzed using the
NIH/Scion image analysis software. The hTERT primers amplified a
DNA fragment of 200 bp and the DNA fragment size amplified by GAPDH
primers was 173 bp.
Western blotting
Cell lysates were prepared by detergent lysis [1%
Triton-X 100 (v/v), 10 mM Tris-HCl (pH 7.5), 5 mM EDTA, 150 mM
NaCl, 10% glycerol, 2 mM sodium vanadate, 5 µg/ml leupeptin, 1
µg/ml aprotinin, 1 µg/ml pepstatin A, and 10 µg/ml
4–2-aminoethyl-benzenesulfinyl fluoride]. Lysates were clarified by
centrifugation at 14,000 × g for 10 min at 4°C, and protein
concentrations were determined by Bradford assay. Samples (50 µg)
were boiled in an equal volume of sample buffer (20% glycerol, 4%
SDS, 0.2% bromophenol blue, 125 mM Tris-HCl (pH 7.5) and 640 mM
2-mercaptoethanol) and separated on 10% SDS-polyacrylamide gels.
Proteins resolved on the gels were transferred to nitrocellulose
membranes. Membranes were blocked with 5% milk in 10 mM Tris-HCl
(pH 8.0), 150 mM NaCl with 0.05% Tween-20 (TPBS) and probed with
protein specific primary antibodies followed by HRP-conjugated
secondary antibody. Immune complexes were visualized with enhanced
chemiluminescence detection system from Amersham Corp. (Arlington
Heights, IL, USA). Protein bands were imaged and band densities
analyzed using NIH/Scion image analysis software. The protein band
densities were normalized to the corresponding β-actin band
densities.
Chomatin immunoprecipitation (ChIP)
assay
ChIP analysis of transcriptionally active chromatin
markers interacting with hTERT promoter was performed using the
EZ-ChIP kit (Upstate Biotechnology) according to the instructions
included in the kit. ChIP-validated antibodies used were:
anti-acetyl-histone H3 lysine 9, anti-acetyl-histone H4,
anti-tri-methyl histone H3 lysine 9 and anti-di-methyl histone H3
lysine 4. ChIP-purified DNA from control cells (untreated) and
cells treated with PM (0–5 µM) for 48 h was amplified by PCR using
hTERT promoter primers: forward, 5′-TCCCCTTCACGTCCGGCATT-3′;
reverse, 5′-AGCGGAGAGAGGTCGAATCG-3′. The PCR products were
separated on 2% agarose gel electrophoresis and visualized by
ethidium bromide staining. The hTERT primers amplified a DNA
fragment of 200 bp.
Statistical analysis
Data are presented as means ± SD. The differences
between control and treatment groups were analyzed using Student's
t-test and differences with p<0.05 were considered statistically
significant.
Results
Pristimerin reduces viability and
induces apoptosis in PDA cells
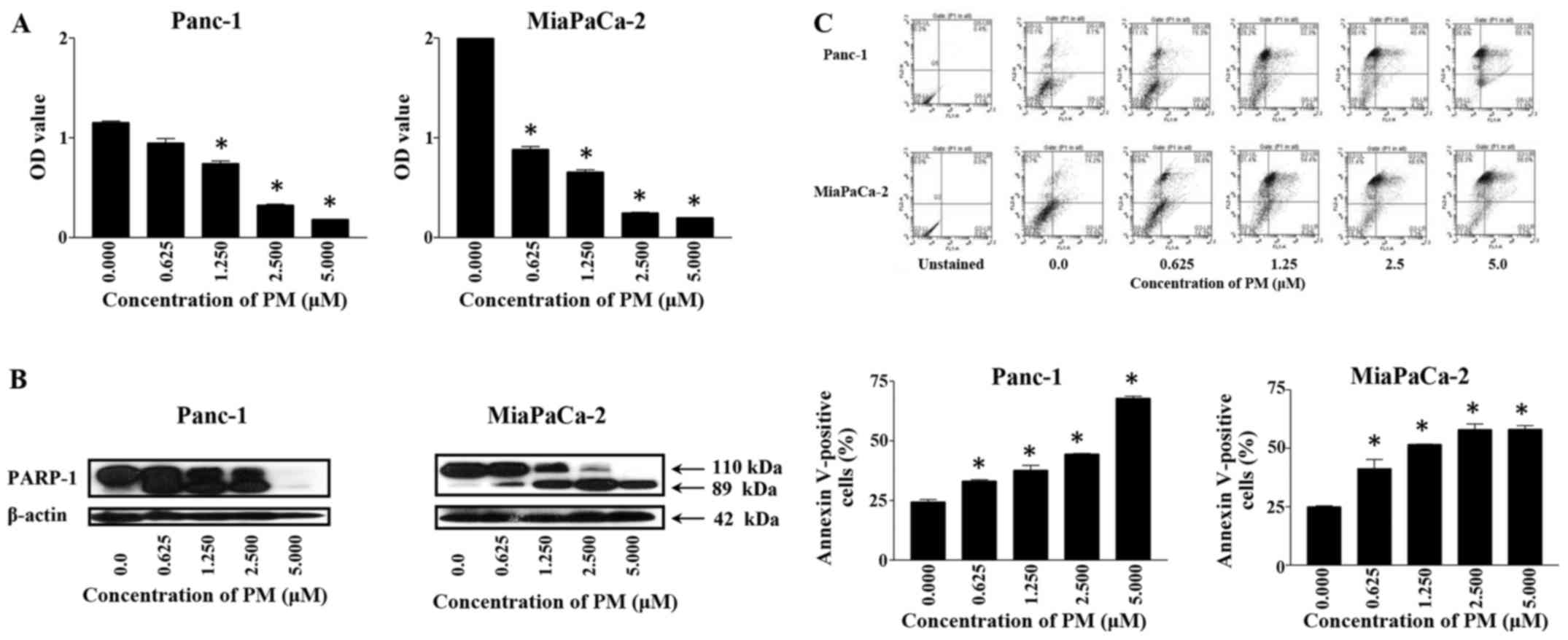
To measure the effect of PM on viability of PDA
cells, Panc-1 and MiaPaCa-2 cells were treated with PM for 72 h at
concentrations ranging from 0.0 to 5.0 µM. At the end of the
treatment, viability of cultures was determined by MTS assay. As
shown in Fig. 1A, treatment with PM
significantly reduced the viability of both cell lines (p<0.05).
In the case of Panc-1 cells, the reduction in viability ranged from
18 to 84% (e.g., 18, 36, 72 and 84% at 0.0625, 1.25, 2.5 and 5 µM,
respectively). PM reduced the viability of MiaPaCa-2 cells more
potently at lower concentrations than in Panc-1 cells. For example,
viability of MiaPaCa-2 was inhibited 58 and 68% at 0.0625 and 1.25
µM PM, which increased to 88–90% inhibition at 2.5–5 µM
(p<0.05).
Whether PM induces apoptosis in PDA
cells was investigated next
Induction of apoptosis was measured by the cleavage
PARP-1 and Annexin V-FITC binding by western blotting and flow
cytometry, respectively. As shown in Fig. 1B, treatment with PM for 24 h caused
the cleavage of PARP-1. The cleavage of PARP-1 was detectable at
0.625 µM PM by the appearance of the 89 kDa split product in both
cell lines. The cleavage of PARP-1 was more pronounced at higher
concentrations of PM, especially in MiaPaCa-2 cells. The induction
of apoptosis was confirmed by the increased binding of Annexin
V-FITC after treatment of cells with PM for 24 h. As shown in
Fig. 1C (upper and bottom panels),
25–20% of untreated Panc-1 and MiaPaCa-2 cells bound Annexin
V-FITC. After treatment with PM, the percentage of Annexin
V-FITC-binding Panc-1 cells ranged from 33 to 68% at 0.625–5 µM PM.
The percentage of Annexin V-FITC-binding also increased in
MiaPaCa-2 cells from 45% at 0.0625 µM to 60% at 5 µM PM. Together,
the cleavage of PARP-1 and an increase in Annexin V-FITC-binding
demonstrated induction of apoptosis by PM in PDA cells.
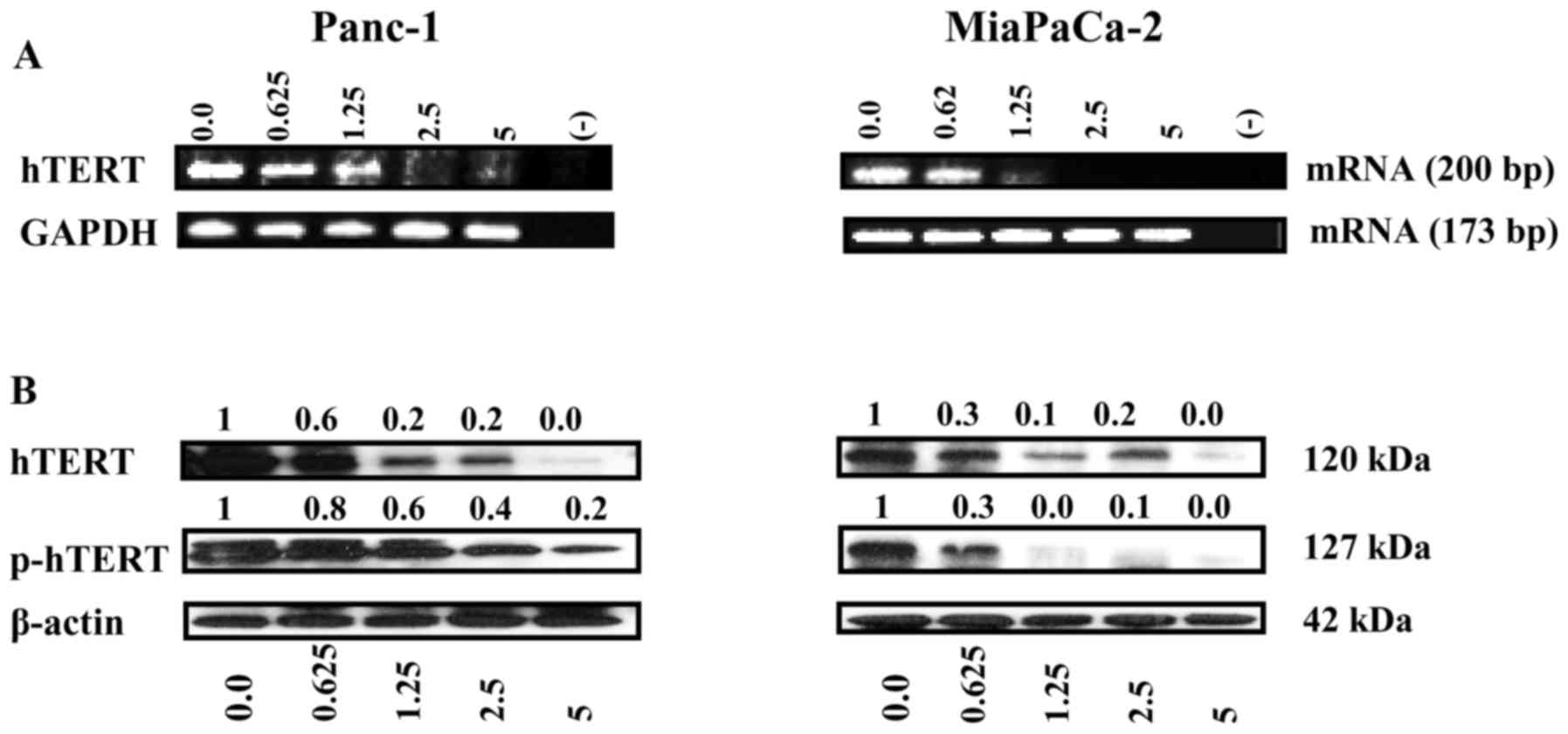
PM inhibits hTERT gene expression
The inhibition of hTERT/telomerase leads to cellular
senescence and/or apoptosis. We thus determined the effect of PM on
the expression hTERT mRNA and hTERT protein. The effect on hTERT
gene expression was measured by analyzing hTERT mRNA by RT-PCR.
Treatment with PM resulted in significant to complete inhibition of
hTERT mRNA in both cell lines at 1.25–5 µM PM without affecting the
expression of GAPDH (Fig. 2A). As
shown in Fig. 2B, PM also reduced
both the native and phosphorylated hTERT (p-hTERT) levels in both
cell lines. Together, these data showed inhibition of hTERT
expression in PDA cells by PM.
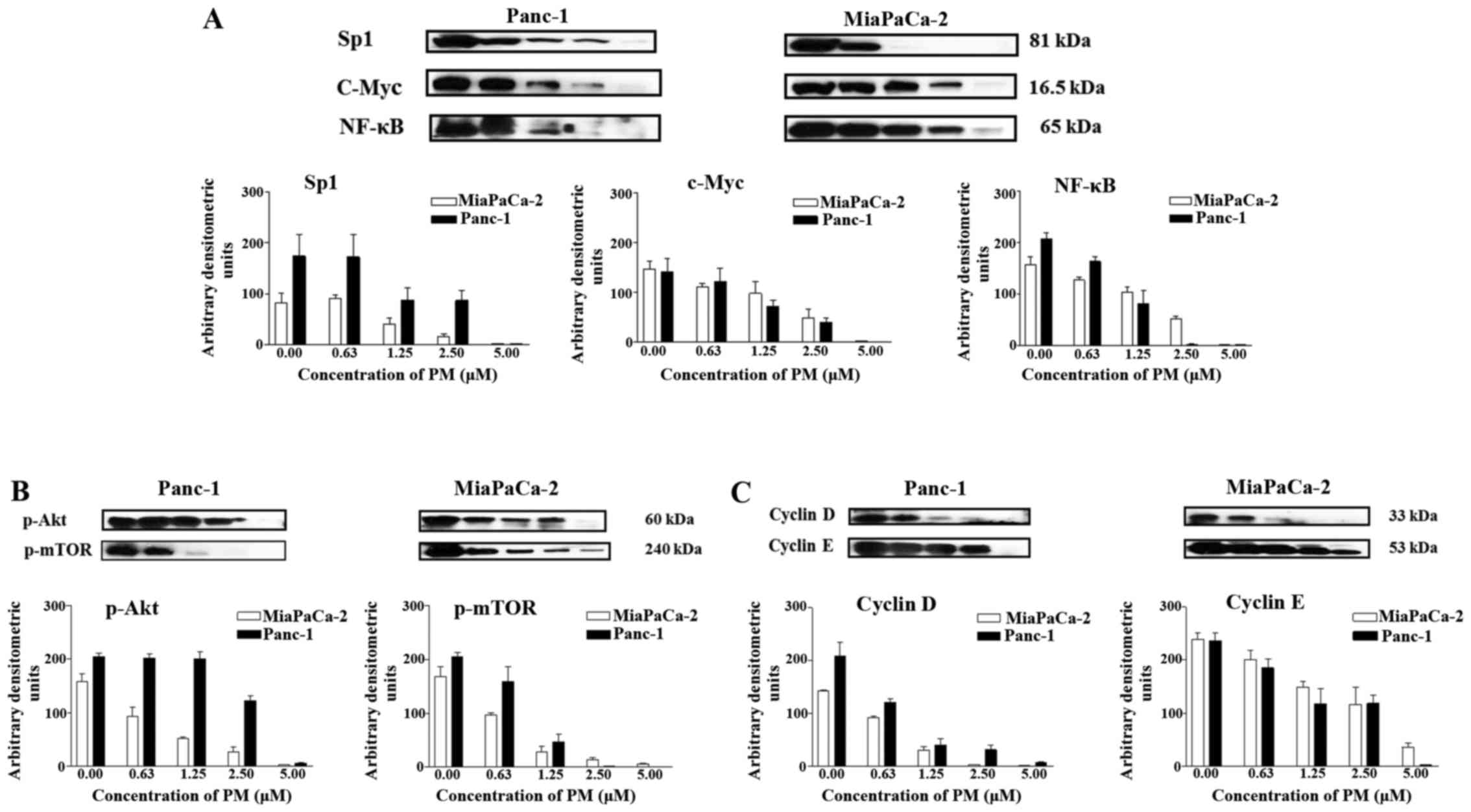
PM inhibits cellular proteins that
regulate hTERT expression
hTERT plays a major role in cell proliferation and
inhibition of apoptosis by maintaining telomere length. Thus, we
examined the effect of PM on proteins that regulate hTERT gene
transcription, post-translational modification of hTERT and cell
division. PM inhibited the transcription factors Sp1, c-Myc, and
NF-κB (p65) which control hTERT gene expression in a dose-dependent
manner with complete inhibition occurring at 5 µM PM (Fig. 3A). PM also inhibited p-Akt and
p-mTOR that modify hTERT post-translationally in both cell lines
(Fig. 3B). In addition, depending
on the concentration, treatment with PM (0 to 5 µM) partially to
completely inhibited cyclin D1 and cyclin E (Fig. 3C). Overall, these data showed that
PM inhibits proteins that regulate hTERT expression,
post-translational modifications of hTERT and cell cycle
progression.
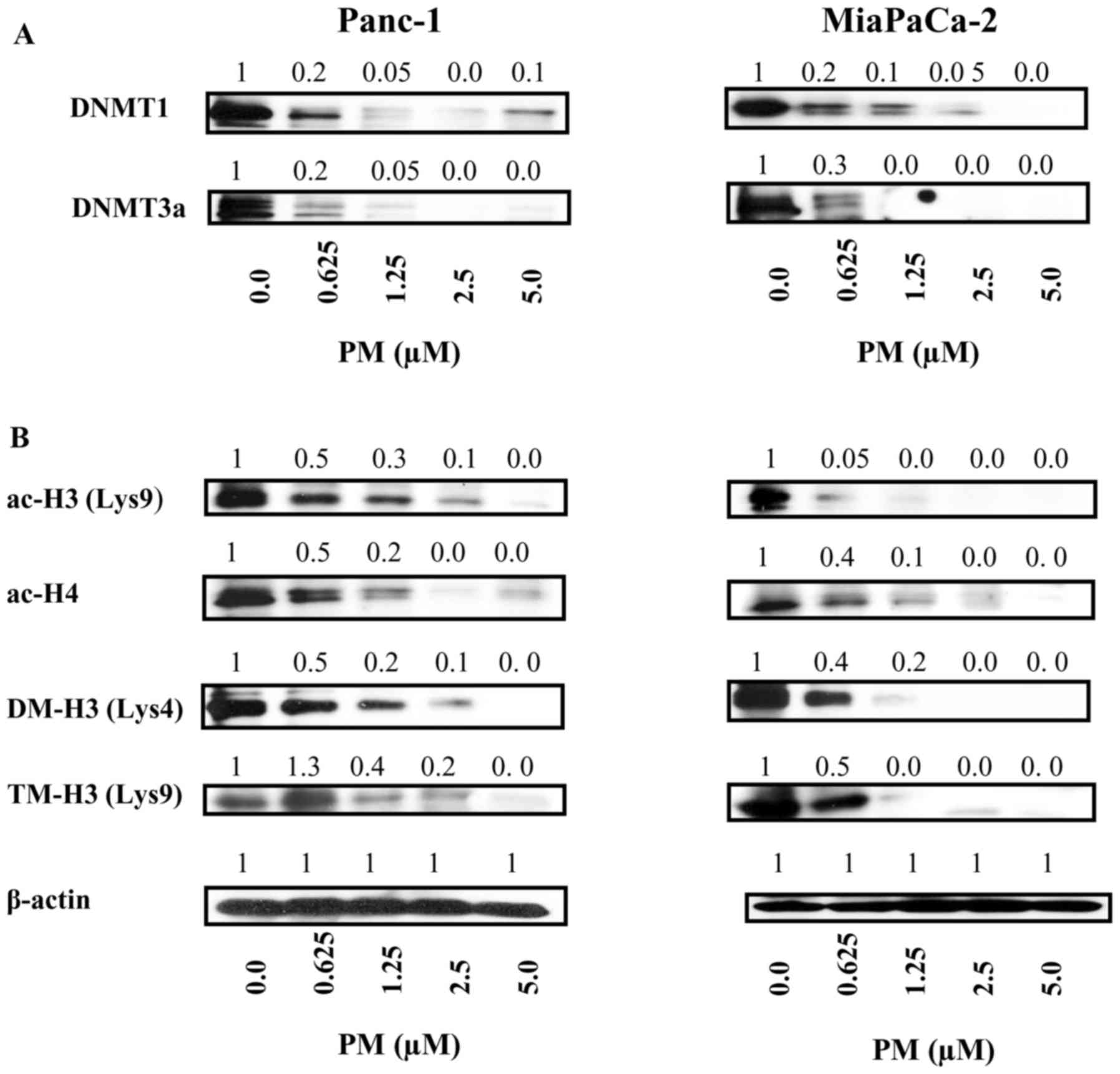
PM inhibits epigenetic regulators of
hTERT expression
Promoter methylation and histone modifications play
a critical role in hTERT expression. Whether PM targets effectors
of epigenetic pathways of hTERT gene expression was evaluated.
First, we analyzed the effect of PM on DNA methyltransferases
responsible for DNA methylation. PM caused significant decrease in
DNA methyltransferases DNMT1 and DNTM3α in both cell lines at the
lowest concentration of 0.625 µM with complete inhibition at higher
concentrations (Fig. 4A).
In addition to DNA methylation, histone
modifications (e.g., histone acetylation and histone methylation)
play pivotal roles in hTERT transcription, therefore, we determined
the effect of PM on histone acetylation and methylation. For
histone acetylation, effect of PM on cellular levels of
transcriptionally active acetylated histone H3 at lysine 9
(ac-H3K9) and acetylated histone H4 (ac-H4) was analyzed. Treatment
with PM significantly to completely inhibited ac-H3K9 and ac-H4 in
both cell lines (Fig. 4B). PM also
affected histone methylation as histone markers dimethyl-H3 lysine
4 (di-me-H3K4) and trimethy-H3 lysine 9 (tri-me-H3K9) were
drastically reduced in cells treated with PM (Fig. 4B).
The preceding findings demonstrated the inhibition
of transcription factors and transcriptionally active histone
markers by PM. Whether PM impacts transcription factors and histone
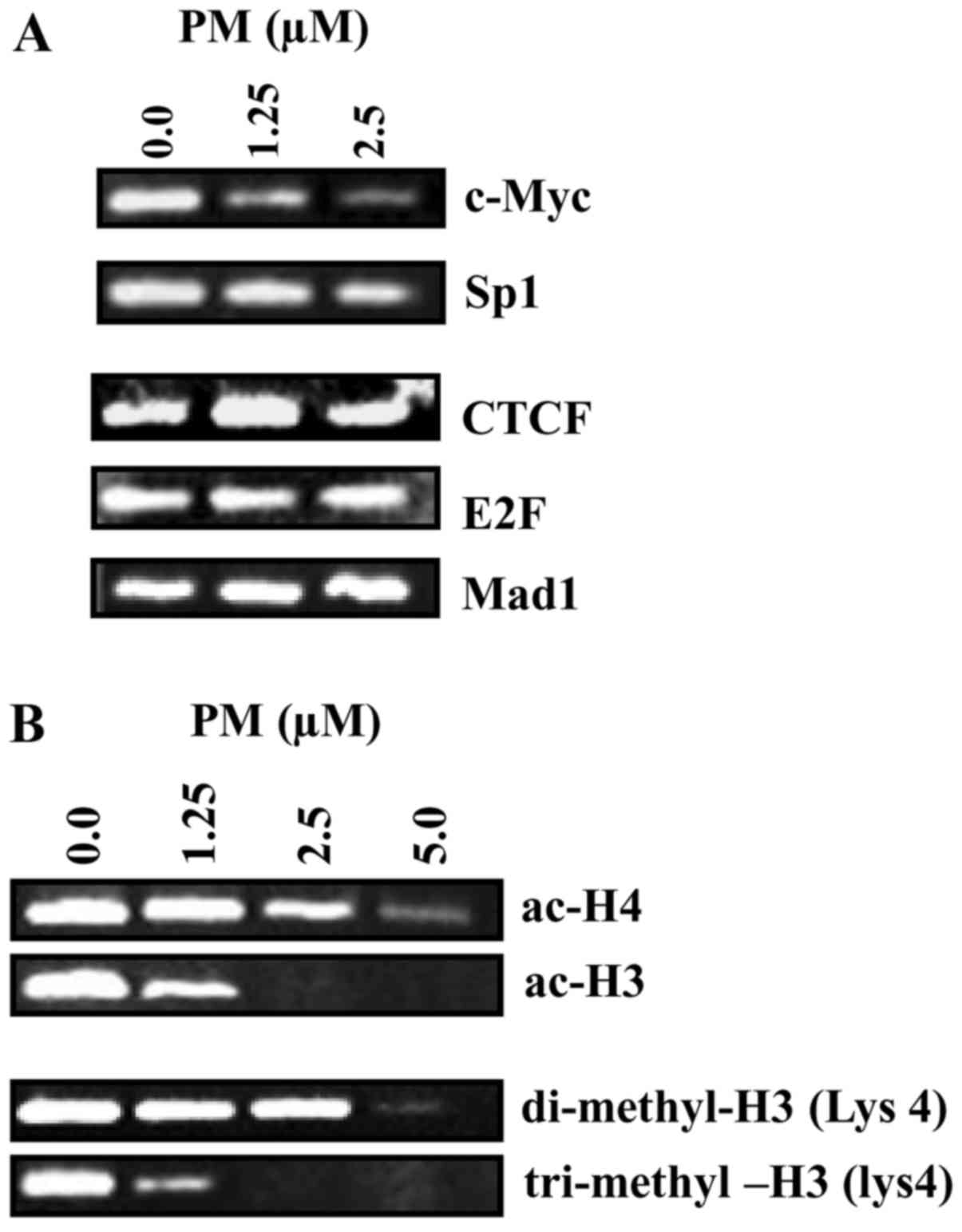
modifications at hTERT promoter was analyzed next. For this, we
analyzed changes in levels of positive (c-Myc and Sp1) and
repressive transcription factors (CTCF, E2F and Mad1) and
transcriptionally active histones (ac-H4, ac-H4, DM-H3 and TM-H3)
in the regulatory region of hTERT promoter by ChIP assay after
treatment with PM. As shown in Fig.
5A, treatment with PM partially to significantly reduced the
level of c-Myc and Sp1 in hTERT promoter. In contrast, repressive
factors CTCF, E2F and Mad1 were not affected by PM. Furthermore,
ChIP analysis of histone modifications at hTERT promoter showed
decrease in ac-H3 and ac-H4 at 2.5–5 µM PM and significant to
complete reduction in DM-H3 and TM-H3 at 1.25–5 µM PM (Fig. 5B). These data indicated that
inhibition of hTERT expression by PM involves inhibition of the
transcription factors and transcriptionally active chromatin
markers that upregulate hTERT gene expression.
Discussion
Although the antiproliferative and
apoptosis-inducing activity of pristimerin (PM) has been shown in
tumor cell lines, including pancreatic cancer cell lines (14–19),
the molecular mechanism of the anticancer effects of PM has not
been fully delineated. Telomerase, the enzyme that rebuilds and
maintains telomere length, plays a vital role in cell proliferation
and prevention of apoptosis. Deregulated telomerase activity
promotes tumorigenesis and provides unlimited proliferative
advantage to the cancer cells. On the other hand, inhibition of
hTERT, the gene that codes for the catalytic subunit of telomerase
results in lack of telomerase activity and consequently the
inhibition of cell proliferation, cellular senescence or apoptotic
cell death.
In a previous study, we showed that inhibition of
cell proliferation and induction of apoptosis by PM correlated with
the inhibition of hTERT and its telomerase activity in PDA cells,
suggesting that inhibition of telomerase is part of the mechanism
by which PM inhibits proliferation of PDA cells (20). Since hTERT gene expression is
heavily regulated epigenetically, the present study was undertaken
to determine the effect of PM on the epigenetic regulators of hTERT
gene transcription. First though, we reevaluated the effect of PM
on the viability and expression of hTERT in PDA cells. Indeed, new
data confirmed our previous findings that inhibition of
proliferation and induction of apoptosis in PDA cells by PM is
associated with the inhibition of hTERT mRNA as well as production
and phosphorylation of hTERT protein. These findings are in
agreement with other reports showing that the inhibition of hTERT
telomerase activity is necessary for the antiproliferative and
apoptosis-inducing activity of natural compounds (21). However, whether PM binds and
degrades RNA template or causes shortening of telomeres remains to
be determined.
A number of factors and molecules that regulate
hTERT transcription have been identified. The hTERT core promoter
contains binding sites for transcription factors, such as Sp1,
c-Myc, NF-κB and STAT-3 (22,23).
Inhibition of these transcription factors would likely impact
transcription of hTERT gene. PM inhibited Sp1, c-Myc and NF-κB in
PDA cells, indicating that diminished hTERT expression and protein
production by PM is at least partly attributed to the inhibition of
these transcription factors. Post-translationally, phosphorylation
of hTERT by protein kinase B/Akt and mTOR is required for nuclear
import and activation of hTERT telomerase activity (20,24).
PM inhibited both p-Akt and p-mTOR, indicating that inhibition of
post-translational modifications by PM also contributes to the
inhibition of hTERT/telomerase.
As stated before hTERT gene is heavily regulated
through the epigenetic mechanisms. Contrary to the prevalent view
that hypermethylation of gene promoters typically inhibits their
transcription; hypermethylation of hTERT promoter is associated
with increased hTERT expression (25,26).
Epigenetic processes that regulate gene expression include DNA
methylation, chromatin remodeling and modulation of the activity of
enzymes and factors associated with these processes.
Promoter DNA methylation catalyzed by DNMTs plays an
important role in hTERT transcription. DNMT1, a maintenance
methyltransferase, maintains hypermethylation of hTERT promoter,
whereas DNMT3a and DNMT3b are responsible for de novo
activity (27). PM inhibited DNMT1
and DNMT3a in Panc-1 and MiaPaCa-2 cells, thereby accounting for
demethylation of hTERT promoter and inhibition of hTERT expression.
Besides DNA methylation, histone acetylation and methylation also
play critical roles in the transcription of hTERT gene. The histone
modifications result in the loosening of chromatin which allows
binding of the activators and/or repressors of gene transcription
at gene promoters (28). PM
inhibited cellular levels of transcriptionally active acetylated
histones ac-H3 and ac-H4. PM also inhibited the active di-methyl-H3
lysine 4 and inactive chromatin marker trimethyl-H3K9. The decrease
in transcription factors and transcriptionally active chromatin
markers suggested that PM may also alter the levels of
transcription factors and chromatin structures at the regulatory
region of hTERT promoter.
ChIP analysis showed decrease in c-Myc and Sp1
transcription factors that upregulate the expression of hTERT
without affecting the repressive factors CTCF, E2F and Mad1. PM
also reduced the levels of transcriptionally active chromatin
markers ac-H3 and ac-H4, DM-H3 and TM-H3 in hTERT promoter. These
data demonstrated that downregulation of transcription factors and
active chromatin markers plays a role in the inhibition of hTERT
expression by PM in pancreatic cancer cells.
Acknowledgements
This work was supported by an Institutional grant
A10176.
References
|
1
|
National Cancer Institute. Pancreatic
Cancer, . U.S. National Institutes of Health. https://www.cancer.gov/cancertopics/types/pancreaticJune
4–2010.
|
|
2
|
Li D, Xie K, Wolff R and Abbruzzese JL:
Pancreatic cancer. Lancet. 363:1049–1057. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pino SM, Xiong HQ, McConkey D and
Abbruzzese JL: Novel therapies for pancreatic adenocarcinoma. Curr
Oncol Rep. 6:199–206. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vaccaro V, Sperduti I and Milella M:
FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N
Engl J Med. 365:768–769. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Greider CW: Chromosome first aid. Cell.
67:645–647. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blackburn EH: Structure and function of
telomeres. Nature. 350:569–573. 1991. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kilian A, Bowtell DD, Abud HE, Hime GR,
Venter DJ, Keese PK, Duncan EL, Reddel RR and Jefferson RA:
Isolation of a candidate human telomerase catalytic subunit gene,
which reveals complex splicing patterns in different cell types.
Hum Mol Genet. 6:2011–2019. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feng J, Funk WD, Wang SS, Weinrich SL,
Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu J, et al:
The RNA component of human telomerase. Science. 269:1236–1241.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Palm W and de Lange T: How shelterin
protects mammalian telomeres. Annu Rev Genet. 42:301–334. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blasco MA and Hahn WC: Evolving views of
telomerase and cancer. Trends Cell Biol. 13:289–294. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Janknecht R: On the road to immortality:
hTERT upregulation in cancer cells. FEBS Lett. 564:9–13. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohuchida K, Mizumoto K, Yamada D,
Yamaguchi H, Konomi H, Nagai E, Yamaguchi K, Tsuneyoshi M and
Tanaka M: Quantitative analysis of human telomerase reverse
transcriptase in pancreatic cancer. Clin Cancer Res. 12:2066–2069.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grochola LF, Greither T, Taubert HW,
Möller P, Knippschild U, Udelnow A, Henne-Bruns D and Würl P:
Prognostic relevance of hTERT mRNA expression in ductal
adenocarcinoma of the pancreas. Neoplasia. 10:973–976. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hashimoto Y, Murakami Y, Uemura K,
Hayashidani Y, Sudo T, Ohge H, Fukuda E, Sueda T and Hiyama E:
Detection of human telomerase reverse transcriptase (hTERT)
expression in tissue and pancreatic juice from pancreatic cancer.
Surgery. 143:113–125. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yan YY, Bai JP, Xie Y, Yu JZ and Ma CG:
The triterpenoid pristimerin induces U87 glioma cell apoptosis
through reactive oxygen species-mediated mitochondrial dysfunction.
Oncol Lett. 5:242–248. 2013.PubMed/NCBI
|
|
16
|
Wu CC, Chan ML, Chen WY, Tsai CY, Chang FR
and Wu YC: Pristimerin induces caspase-dependent apoptosis in
MDA-MB-231 cells via direct effects on mitochondria. Mol Cancer
Ther. 4:1277–1285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Zhou Y, Zhou H, Jia G, Liu J, Han
B, Cheng Z, Jiang H, Pan S and Sun B: Pristimerin causes G1 arrest,
induces apoptosis, and enhances the chemosensitivity to gemcitabine
in pancreatic cancer cells. PLoS One. 7:e438262012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu Z, Jin Y, Chen C, Li J, Cao Q and Pan
J: Pristimerin induces apoptosis in imatinib-resistant chronic
myelogenous leukemia cells harboring T315I mutation by blocking
NF-kappaB signaling and depleting Bcr-Abl. Mol Cancer. 9:1122010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deeb D, Gao X, Liu YB, Pindolia K and
Gautam SC: Pristimerin, a quinonemethide triterpenoid, induces
apoptosis in pancreatic cancer cells through the inhibition of
pro-survival Akt/NF-κB/mTOR signaling proteins and anti-apoptotic
Bcl-2. Int J Oncol. 44:1707–1715. 2014.PubMed/NCBI
|
|
20
|
Deeb D, Gao X, Liu Y, Pindolia K and
Gautam SC: Inhibition of hTERT/telomerase contributes to the
antitumor activity of pristimerin in pancreatic ductal
adenocarcinoma cells. Oncol Rep. 34:518–524. 2015.PubMed/NCBI
|
|
21
|
Meeran SM, Ahmed A and Tollefsbol TO:
Epigenetic targets of bioactive dietary components for cancer
prevention and therapy. Clin Epigenetics. 1:101–116. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kyo S, Takakura M, Taira T, Kanaya T, Itoh
H, Yutsudo M, Ariga H and Inoue M: Sp1 cooperates with c-Myc to
activate transcription of the human telomerase reverse
transcriptase gene (hTERT). Nucleic Acids Res. 28:669–677. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Konnikova L, Simeone MC, Kruger MM,
Kotecki M and Cochran BH: Signal transducer and activator of
transcription 3 (STAT3) regulates human telomerase reverse
transcriptase (hTERT) expression in human cancer and primary cells.
Cancer Res. 65:6516–6520. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chung J, Khadka P and Chung IK: Nuclear
import of hTERT requires a bipartite nuclear localization signal
and Akt-mediated phosphorylation. J Cell Sci. 125:2684–2697. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Renaud S, Loukinov D, Abdullaev Z,
Guilleret I, Bosman FT, Lobanenkov V and Benhattar J: Dual role of
DNA methylation inside and outside of CTCF-binding regions in the
transcriptional regulation of the telomerase hTERT gene. Nucleic
Acids Res. 35:1245–1256. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Renaud S, Loukinov D, Bosman FT,
Lobanenkov V and Benhattar J: CTCF binds the proximal exonic region
of hTERT and inhibits its transcription. Nucleic Acids Res.
33:6850–6860. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bestor TH: The DNA methyltransferases of
mammals. Hum Mol Genet. 9:2395–2402. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu L, Lai S, Andrews LG and Tollefsbol
TO: Genetic and epigenetic modulation of telomerase activity in
development and disease. Gene. 340:1–10. 2004. View Article : Google Scholar : PubMed/NCBI
|