Introduction
Angiogenesis is the process of the development of
the intrinsic vascular network, and it is an essential event in the
progression and metastatic spread of solid tumors such as
neuroblastoma, where new capillaries spread from preexisting
vessels and the transition from avascular to vascular phase occurs
via neovascularization (1).
Angiogenesis is mediated by multiple regulatory factors such as
growth factors, adhesion molecules and matrix degrading enzymes.
The balance between angiogenic activators and inhibitors maintains
endothelial cells in an angiogenic or quiescent stage (2). Malignant neuroblastoma is a highly
vascularized solid tumor that requires access to blood vessels for
growth, invasion and metastasis, being highly dependent on
angiogenesis (1). Therefore,
anti-angiogenic strategies can be effective in inhibiting tumor
cell dissemination and metastasis in highly vascular neuroblastoma
(3,4). Vascular endothelial growth factor
(VEGF) is the most active endogenous pro-angiogenic factor, and it
can induce angiogenesis by directly acting on the endothelium in
vivo as well as by increasing the microvascular permeability
(5). VEGF is widely known to play
an important role in tumor biology and more specifically, in the
process of tumor angiogenesis, as VEGF expression plays a crucial
role in the pathogenesis and neovascularization of neuroblastoma
(1). VEGF signaling plays a
regulatory role in neuroblastoma angiogenesis via a paracrine
mechanism through two specific tyrosine kinase VEGF receptors,
VEGFR-1 and VEGFR-2, located at the surface of endothelial cells.
Tumor-induced VEGF secretion may function in both paracrine and
autocrine manners to allow tumor expansion and growth (6). Treatment of neuroblastoma in both
in vitro and in vivo models with an anti-VEGF agent
results in antitumor activity with a decrease in both tumor
vascularity and in the number of intratumoral vessels and a
decrease in VEGF expression suggesting a diversified role of VEGF
in the progression of advanced stage neuroblastoma (7,8).
Melatonin, the main secretory product of the pineal
gland, is widely known to decrease the growth and development of
estrogen-responsive breast cancers (9–12).
Melatonin exerts its oncostatic properties in hormone-dependent
breast cancer by interfering at different levels with the estrogen
signaling pathways (13–15). Melatonin downregulates circulating
levels of gonadal estrogens, interferes with the activation of the
estrogen receptor and regulates the activity and expression of
several enzymes involved in the biosynthesis of estrogens in
tumoral and peritumoral tissues (16–19).
In vitro studies demonstrated that melatonin plays a role in
the paracrine interactions between malignant epithelial and
proximal endothelial cells, through the downregulation of VEGF
expression in human breast cancer cells, decreasing the levels of
VEGF around endothelial cells and decreasing angiogenesis. VEGF
secreted by breast cancer cells interacts with VEGF receptors in
endothelial cells and stimulates downstream signaling molecules to
promote proliferation, growth, survival and migration of
endothelial cells (20–22). Therefore, inhibition of VEGF
secretion by tumor cells, as well as VEGF-regulated signaling in
endothelial cells, could be important in decreasing tumor
angiogenesis and growth.
Since melatonin has anti-angiogenic effects in other
tumor cell lines (23), in the
present study, we investigated the anti-angiogenic activity of
melatonin at different steps of the angiogenic process in SH-SY5Y
neuroblastoma cells. We studied whether melatonin decreases the
pro-angiogenic effects of VEGF, as well as the conditioned media
from SH-SY5Y neuroblastoma cells. To accomplish this, we used
cultures of neuroblastoma cells (SH-SY5Y) and co-cultures of
SH-SY5Y cells with human umbilical vein endothelial cells
(HUVECs).
Materials and methods
Cells and culture conditions
HUVECs were purchased from the American Type Culture
Collection (ATCC; Rockville, MD, USA). They were maintained as
monolayer cultures in 75 cm2 plastic culture flasks
containing Vascular Cell Basal Medium (VCBM) supplemented with
endothelial cell growth kit-BBE (both from ATCC) which consisted of
2% fetal bovine serum (FBS) (PAA Laboratories, Pasching, Austria),
0.2% bovine brain extract, 5 ng/ml rhEGF, 10 mM L-glutamine, 0.75
U/ml heparin sulfate, 1 µg/ml hydrocortisone hemisuccinate, 50
µg/ml ascorbic acid, penicillin (20 U/ml) and streptomycin (20
µg/ml) (Sigma-Aldrich, Madrid, Spain), at 37̊C in a humid
atmosphere containing 5% CO2. To avoid genetic mutation
and low viability, no more than 6 passages of HUVECs were used for
the experiments.
SH-SY5Y neuroblastoma cells were a kind gift from Dr
María Elsa Valdizán, from the Pharmacology Division of the
Department of Physiology and Pharmacology of the University of
Cantabria (Spain). They were maintained as monolayer cultures in 75
cm2 plastic culture flasks in Dulbecco's modified
Eagle's medium (DMEM)/Ham's Nutrient Mixture F-12 (Sigma-Aldrich),
supplemented with 10% FBS, penicillin (20 U/ml) and streptomycin
(20 µg/ml) at 37̊C in a humid atmosphere containing 5%
CO2.
Conditioned media collection for
experimentation
SH-SY5Y cells were seeded into 24-multiwell plates
(25×104 cells/well) in DMEM/Ham's Nutrient Mixture F-12
supplemented with 10% FBS and incubated at 37̊C. When a homogenous
monolayer of pre-confluent SH-SY5Y cells was reached (48 h), the
cells were washed twice with PBS and placed in serum-free VCBM.
After 24 h of incubation, the conditioned medium was collected,
filtered through a 0.2-µm pore size membrane to remove particles
and stored at −80̊C.
Cell proliferation assay
SH-SY5Y were seeded into 96-multiwell plates at a
density of 25×103 cells/well in DMEM/Ham's Nutrient
Mixture F-12 supplemented with 2% FBS and incubated at 37̊C. After
24 h of incubation, the media were aspirated and replaced by fresh
media supplemented with 5% charcoal-stripped FBS (sFBS) containing
either 1 mM or 1 nM melatonin (Sigma-Aldrich) or vehicle (ethanol
at a final concentration of <0.0001%). Cells were cultured for 6
days and proliferation was assessed using the
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]
(MTT) method (24), with a reading
absorbance at 570 nm on a microplate reader (Labsystems Multiskan
RC 351; Vienna, VA, USA). MTT was obtained from Molecular Probes,
Inc. (Eugene, OR, USA).
Paracrine effects of SH-SY5Y
conditioned medium on endothelial cell proliferation
HUVECs were seeded at 8×103 cells/well in
a 96-well multiplate containing VCBM supplemented with 2% FBS
overnight. Cells were washed twice with PBS and the media were
replaced with serum-free VCBM containing SH-SY5Y conditioned media
alone or supplemented with 1 mM melatonin or 150 ng/ml anti-VEGF
(R&D Systems Europe Ltd., Abingdon, UK). The cells were then
cultured for 24 h and cellular proliferation was assessed using the
MTT method.
Co-culture of HUVECs and SH-SY5Y
cells
HUVECs were co-cultured along with SH-SY5Y cells
using Falcon 24-multiwell plates and Falcon cell culture inserts.
HUVECs were plated (24×103 cells/well) on the bottom
wells containing VCBM supplemented with 2% FBS and incubated
overnight. Subsequently, SH-SY5Y cells were seeded
(35×103 cells) on the permeable membrane (0.45-µm) of
the tissue-culture inserts containing VCBM supplemented with 2% FBS
for 24 h. The media were then changed to serum-free VCBM for 72 h
containing melatonin (1 mM) or vehicle (ethanol). At the end of the
experiment, the media were collected, centrifuged to remove
particles and subjected to the assessment of the protein levels of
VEGF. Cells in the bottom plate were evaluated for proliferative
indices by MTT method.
Assessment of VEGF mRNA
expression
Analysis of the VEGF expression in SH-SY5Y cells was
carried out by RT-PCR after incubation of cells with either 1 mM, 1
µM and 1 nM of melatonin and/or 100 µM CoCl2
(Sigma-Aldrich) and/or vehicle (ethanol) for 4 h. Total RNA was
isolated from SH-SY5Y cells and purified with the Nucleospin RNA II
kit (Machenery-Nagel, Düren, Germany) following the manufacturer's
instructions. The absorbance ratio A260 nm/A280
nm was >1.8. For cDNA synthesis 0.5 µg of total RNA was
denatured at 65̊C for 10 min and reverse-transcribed for 50 min at
45̊C with a cDNA Synthesis kit (BioLine, London, UK) in a final
volume of 20 µl in the presence of 500 ng of oligo(dT) (12–18
primers). Quantitative real-time (qRT-PCR) was performed using the
following set of human VEGF 165 specific primers:
[5′-ACCAAGGCCAGCACATAGG-3′ (forward) and 5-ACGCTCCAGGACTTATACCG-3′
(reverse)] (Sigma Genosys Ltd., Cambridge, UK). As a quantification
control, s14 mRNA was also subjected to qRT-PCR using a set of
specific primers: [5′-TCCTGCGAGTGCTGTCAGAG-3′ (forward) and
5′-TCACCGCCCTACACATCAAA-3′ (reverse)] (Sigma Genosys Ltd.).
qRT-PCRs were performed on an MX3005P system (Stratagene, La Jolla,
CA, USA) using Brilliant® SYBR®-Green PCR
Master Mix (Applied Biosystems, Madrid, Spain) following the
manufacturer's instructions. Amplifications were performed for 40
cycles using the following temperature profile: 60̊C, 45 sec
(annealing); 72̊C, 30 sec (extension) and 95̊C, 30 sec
(denaturation).
Assessment of the protein levels of
VEGF
For the determination of the concentration of VEGF
in the SH-SY5Y cell cultures and the HUVEC/SH-SY5Y cell co-culture
media, a Human VEGF Immunoassay kit (R&D Systems Europe Ltd.)
was used. The samples (in triplicate) were processed according to
the manufacturers instructions. Briefly, SH-SY5Y cells were seeded
into 96-multiwell plates at a density of 8×103
cells/well in DMEM/Ham's Nutrient Mixture F-12 supplemented with
10% FBS and incubated at 37̊C for 24 h. Next, the media were
aspirated and replaced by fresh media supplemented with 0.5% sFBS
and containing either 1 mM or 1 nM of melatonin or vehicle
(ethanol). After 24 h of incubation, the media were collected,
centrifuged to remove particles and subjected to ELISA according to
the manufacturers instructions. For the determination of the
protein levels of VEGF in the cell co-culture media, samples were
collected, centrifuged and immediately processed. At the end of the
procedure, the absorbance was determined at a wavelength of 450 nm,
with the correction wavelength set at 570 nm.
Endothelial cell migration assay:
Wound healing assay
HUVECs were seeded onto 6-multiwell plates (Falcon)
containing VCBM supplemented with 2% FBS and were allowed to reach
full confluency. A line of HUVECs was then scraped away in each
plate using a pipette tip. Subsequently, the cells were washed
twice with PBS to remove detached cells and SH-SY5Y conditioned
media alone or supplemented with 1 mM melatonin or 150 ng/ml
anti-VEGF were added to each plate. Thereafter, three randomly
selected views along the scraped line in each plate were
photographed using an ORCA-R2 camera (Hamamatsu Photonics, Massy
Cedex, France) attached to a Nikon Eclipse-Ti microscope set
(Werfen Group, Barcelona, Spain) at a magnification of ×10.
Photomicrographs, with an exposure time of 5 msec, were captured
every 10 min during the course of the experiment, which was
terminated as soon as the wound was completely filled in the
vehicle-treated controls (after 8 h). Initial and final wound sizes
were determined using the NIS-Elements v.3.8 software (Nikon,
Tokyo, Japan), and the difference between the two wound sizes was
used to determine the migratory distance using the following
formula: Initial wound size minus final wound size divided by two.
Three independent experiments were carried out.
Endothelial cell differentiation
assay: Endothelial cell capillary-like tube formation assay
To study the effects of SH-SY5Y conditioned media on
the formation of tubular structures in HUVECs we used the In
vitro Angiogenesis Assay Tube Formation kit (Cultrex; Trevigen
Inc., Gaithersburg, MD, USA) according to the manufacturer's
instructions. First, reduced growth factor basement membrane
extract (BME) was pippetted into a 24-well plate and polymerized
for 1 h at 37̊C. HUVECs (1×105/well) were then seeded in
VCBM supplemented with 2% FBS containing SH-SY5Y conditioned media
alone or supplemented with 150 ng/ml anti-VEGF or melatonin 1 mM.
After 5 h of incubation, 4 random fields were selected/well and
tubular structures were photographed using a Nikon Sight DS-SML1
camera (Sendai Nikon Corporation, Miyagi, Japan) attached to a
Nikon Eclipse Ti-S fluorescence microscope at a magnification of
×10. The formation of the tubular network was assessed using ImageJ
1.45S software. Each assay was performed three times.
Statistical analysis
The data are expressed as the mean ± standard errors
of the mean (SEM). Statistical differences between groups were
analyzed using one way analysis of variance (ANOVA), followed by
the Student-Newman-Keuls test. Results were considered as
statistically significant at p<0.05.
Results
Effects of melatonin on SH-SY5Y
neuroblastoma cell proliferation
To investigate the influence of melatonin on the
proliferation of SH-SY5Y cells we studied the effects of
physiological (1 nM) and pharmacological (1 mM) concentrations of
melatonin on the proliferation of neuroblastoma cells. Only
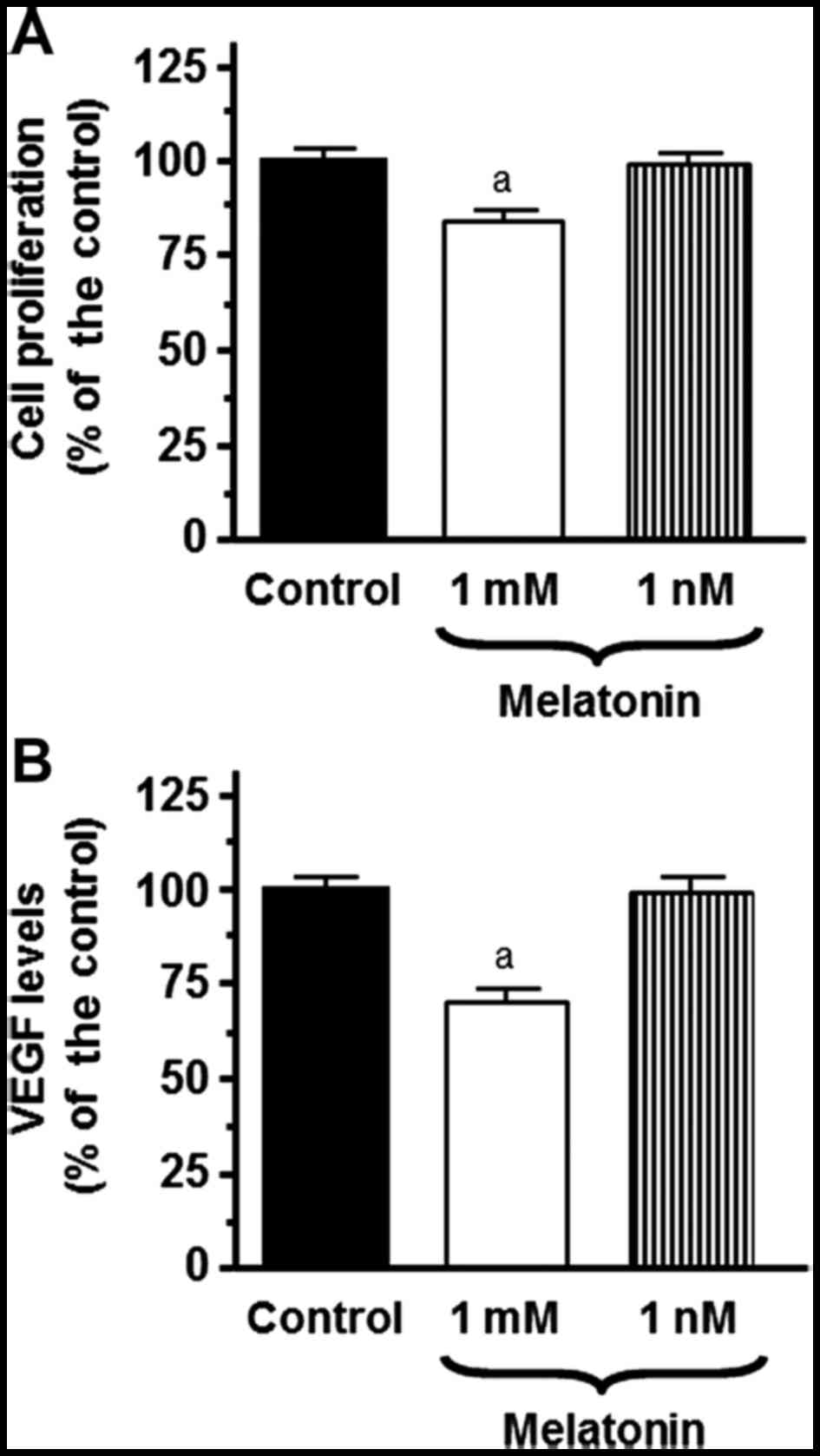
pharmacological concentrations of melatonin (1 mM) had a
significant inhibitory effect on cell proliferation (Fig. 1A).
Effects of melatonin on secretion and
VEGF mRNA expression in SH-SY5Y cells
First, we studied the effect of melatonin on VEGF
synthesis by SH-SY5Y cells. For this purpose, we assessed the VEGF
concentration in the SH-SY5Y-cell culture media. The levels of VEGF
were significantly (p<0.001) decreased by the addition of
melatonin at a 1 mM concentration (Fig.
1B).
With the aim of determining whether this inhibitory
effect of melatonin on VEGF production was due to a downregulation
of VEGF mRNA expression, we incubated SH-SY5Y cells with melatonin
(1 mM, 1 µM and 1 nM) or vehicle for 4 h, and total RNA was
isolated to perform RT-PCR with specific primers for human VEGF,
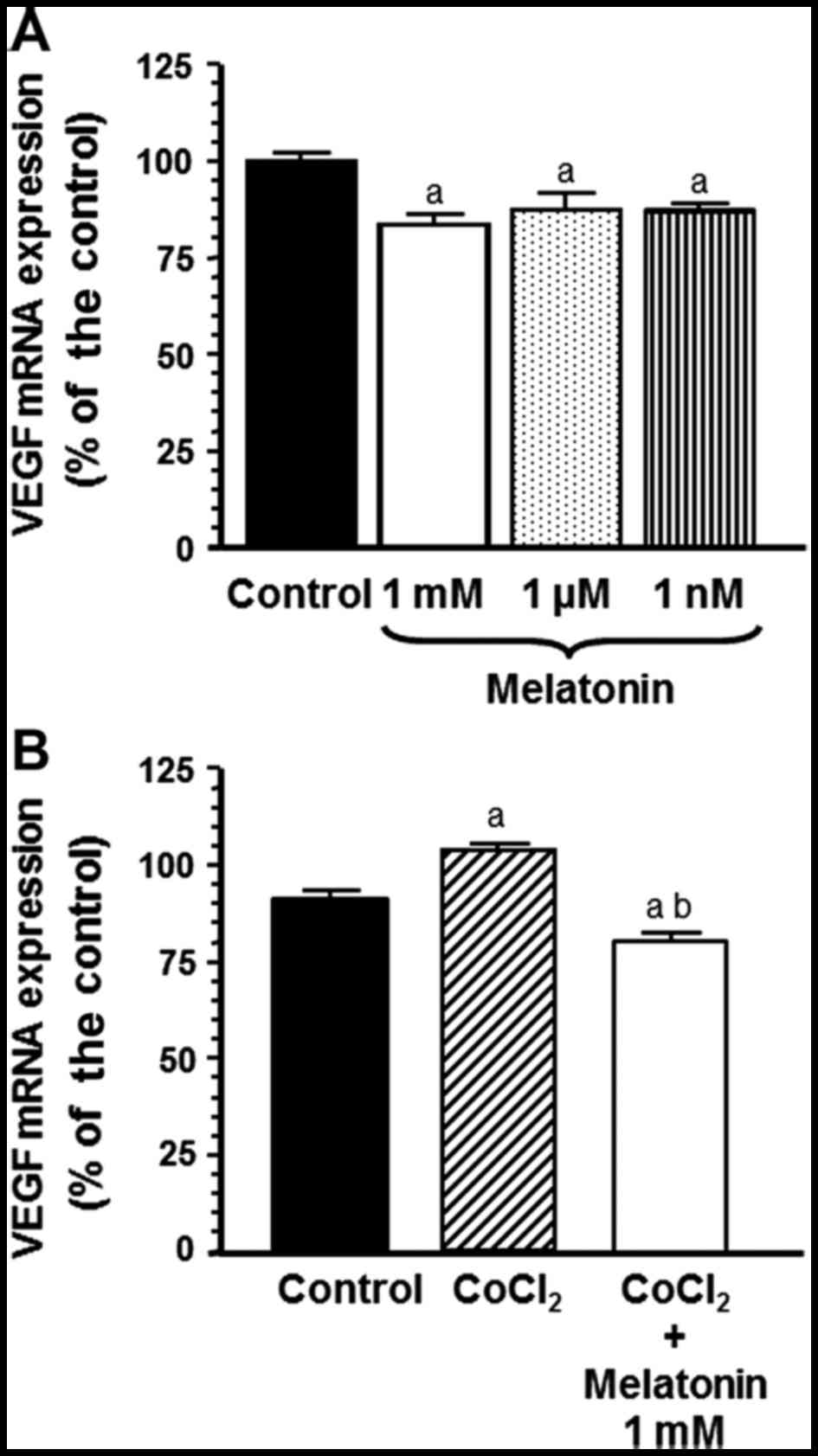
using s14 as a control housekeeping gene. Melatonin at different
concentrations decreased VEGF mRNA expression (Fig. 2A). To mimick hypoxia, a well-known
inducer of VEGF mRNA expression, SH-SY5Y cells were exposed to 100
µM CoCl2 and the addition of melatonin at the
pharmacological (1 mM) concentration, significantly counteracted
the stimulatory effect of CoCl2 on VEGF mRNA gene
expression (p<0.001) (Fig.
2B).
We developed co-cultures of HUVECs and SH-SY5Y cells
to first investigate whether the presence of neuroblastoma cells in
the cultures affects the growth of the endothelial cells.
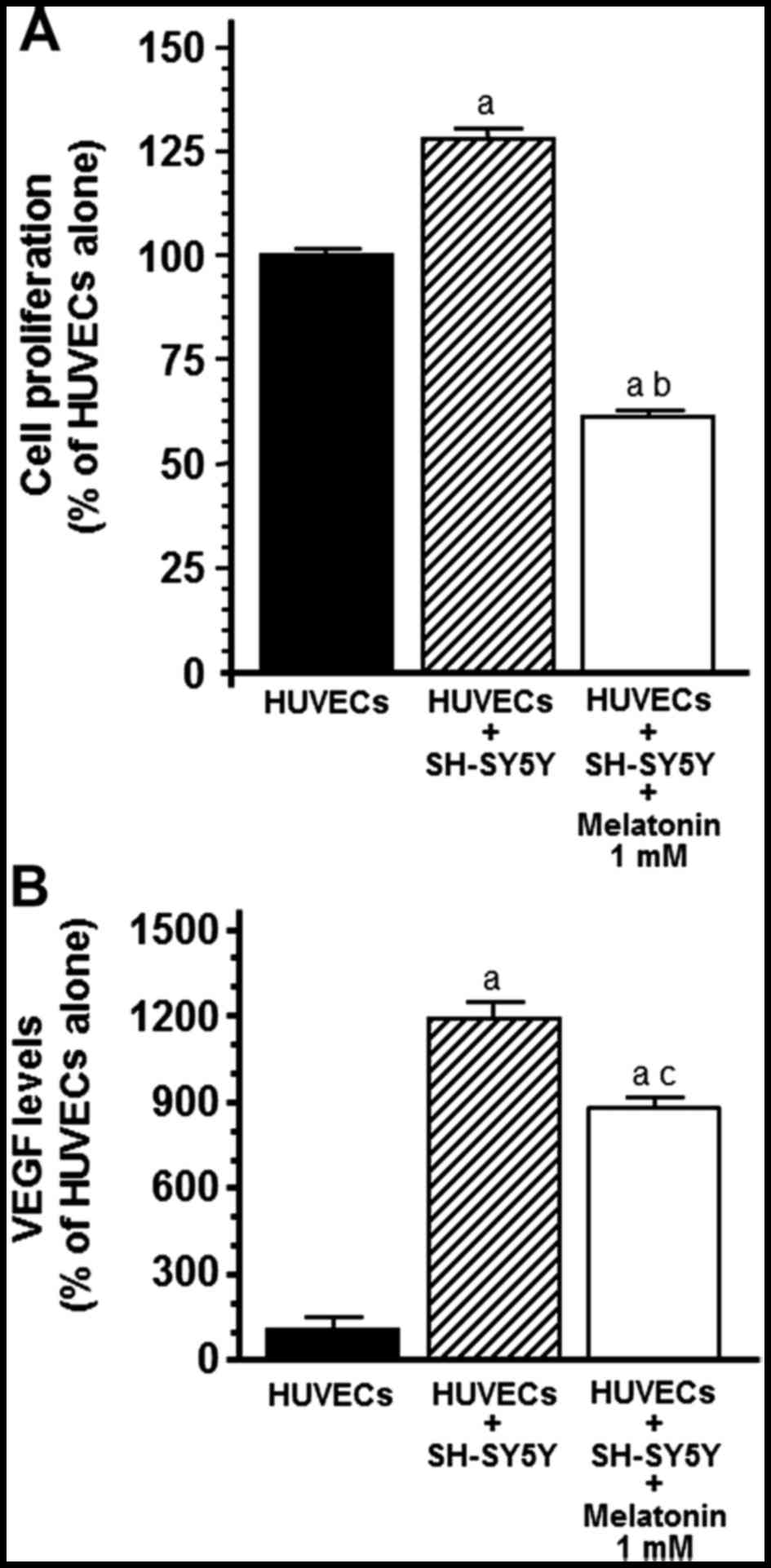
Undoubtedly, we observed that the presence of SH-SY5Y cells in the
co-cultures promoted an increase in HUVEC proliferation
(p<0.001) and 1 mM melatonin counteracted this stimulatory
effect (Fig. 3A). To study whether
the increase in HUVEC proliferation could be due to the release of
VEGF by SH-SY5Y cells, the co-culture media were subjected to ELISA
to determine VEGF protein levels. The presence of SH-SY5Y cells
significantly increased the protein levels of VEGF in the cell
co-culture media (p<0.001) whereas the addition of 1 mM
melatonin decreased the concentration levels of VEGF and
counteracted the stimulatory effect induced by the presence of
tumoral cells (p<0.05) (Fig.
3B).
Effects of melatonin on the paracrine
activity of VEGF produced by SH-SY5Y cells on HUVEC
proliferation
To demonstrate that VEGF produced by tumor cells is
angiogenically active and stimulates endothelial cell
proliferation, serum-free conditioned media from SH-SY5Y cells were
collected and subsequently added to HUVECs to assess their
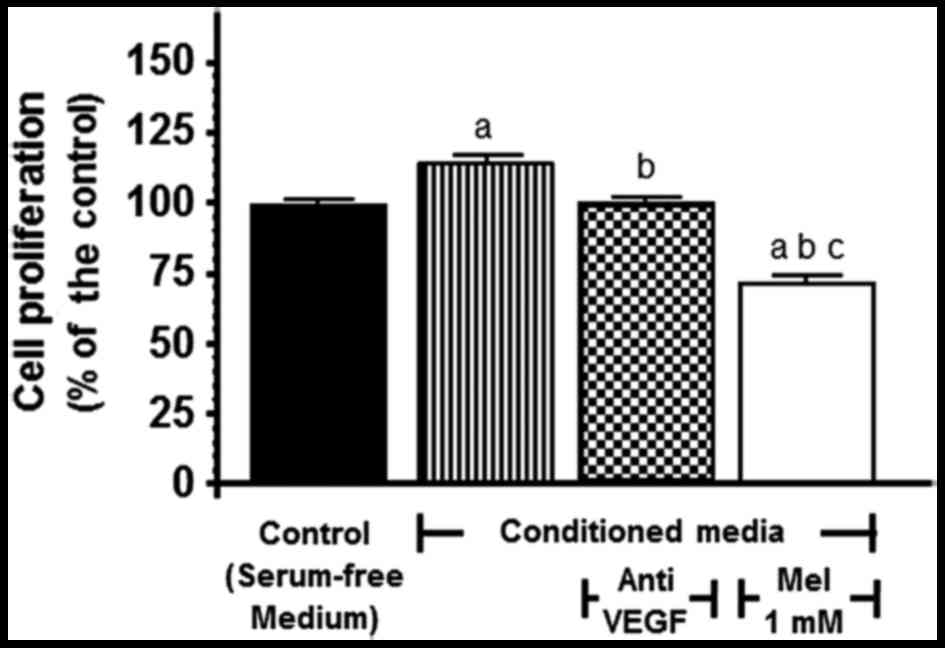
proliferative response. When the conditioned media from the SH-SY5Y
cells were added to the cultures, HUVEC growth was markedly
stimulated (p<0.001) and this effect was significantly
(p<0.001) counteracted by the addition of either the anti-VEGF
antibody (150 ng/ml) or 1 mM melatonin (Fig. 4).
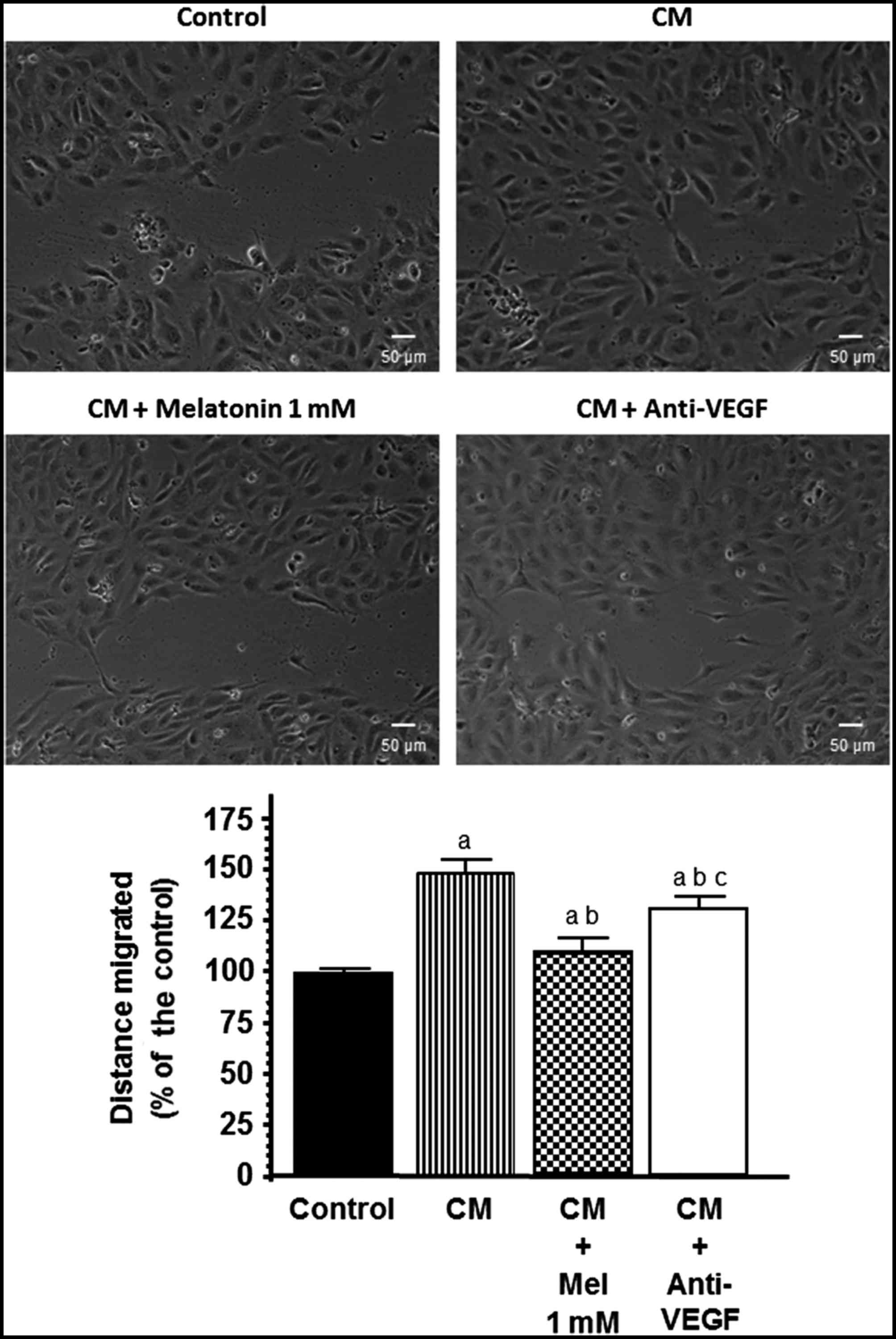
Effects of melatonin on endothelial
cell migration
Since endothelial cell migration is essential for
the formation of new blood vessels during neo-angiogenesis, we next
studied the effects of melatonin treatment on the migratory
properties of endothelial cells using wound-healing assays. We
studied whether VEGF contained in the conditioned media collected
from the SH-SY5Y cells was angiogenically active and able to
increase the migration of HUVECs and whether melatonin was able to
counteract this effect. Clearly, when conditioned media from the
SH-SY5Y cells were added to the cultures the migration of HUVECs
was markedly stimulated (p<0.001) and this effect was
significantly counteracted by the addition of either anti-VEGF (150
ng/ml) or melatonin 1 mM (Fig.
5).
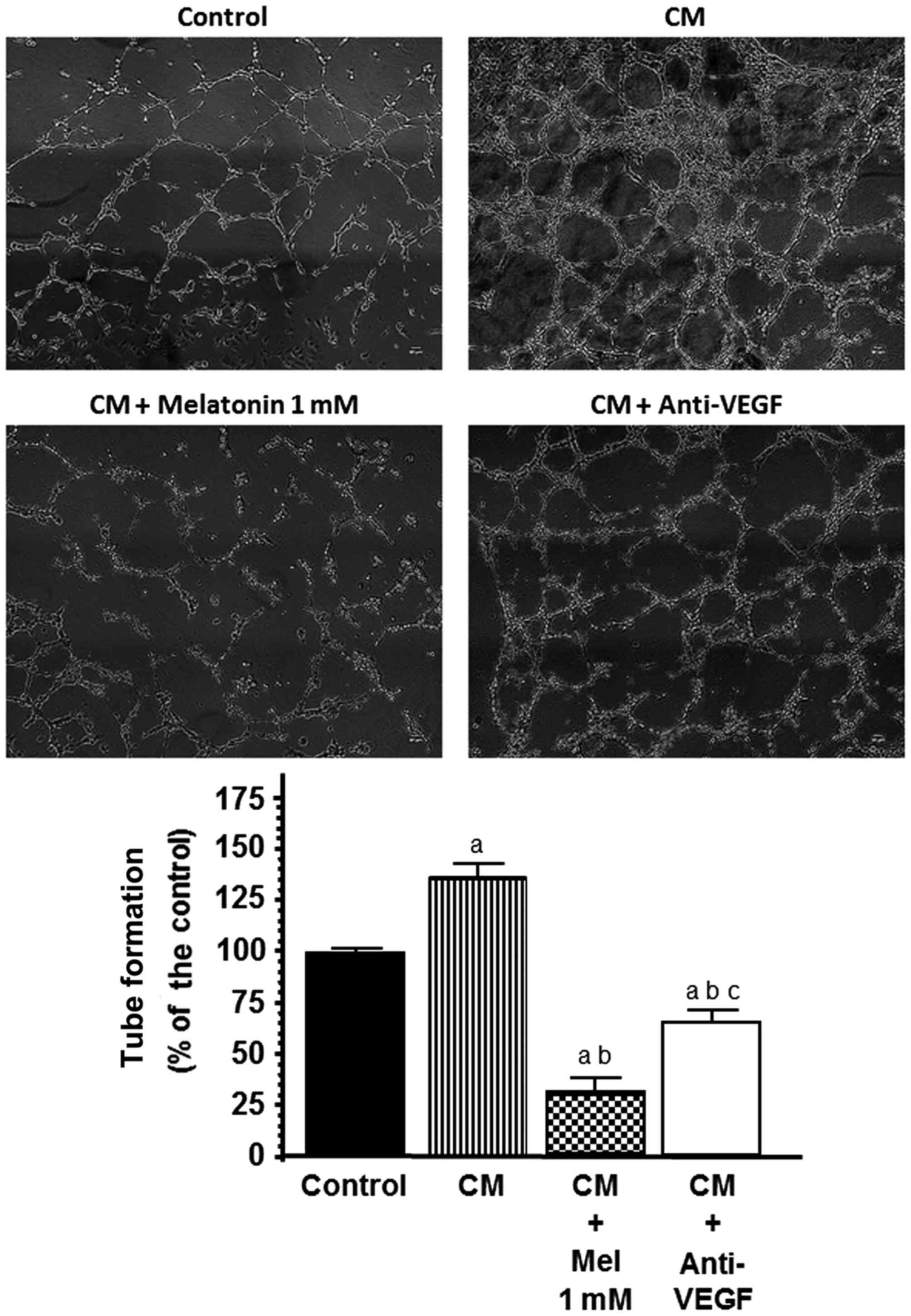
Effects of melatonin on HUVEC
capillary structure formation
To study the effects of melatonin on the formation
of tubular structures by endothelial cells, forming a complex
network of vessels and capillaries, we used an in vitro
angiogenesis tube formation assay where we assessed: i) whether
VEGF contained in the conditioned media collected from the SH-SY5Y
cells was angiogenically active and able to increase the tube
formation of HUVECs; and ii) whether melatonin was able to
counteract this effect. As shown in Fig. 6, HUVECs plated on reduced growth
factor-BME, formed a massive network of tubes after 5 h. When the
conditioned media from the SH-SY5Y cells were added to the
cultures, tube formation was markedly stimulated and this effect
was significantly (p<0.001) counteracted by the addition of
either the anti-VEGF antibody (150 ng/ml) or melatonin 1 mM
(Fig. 6).
Discussion
Neuroblastoma is the most common pediatric
extracranial and heterogeneous solid tumor in children. It is a
highly vascular and aggressive tumor that originates from the
neural crest. Angiogenesis, genetic abnormalities and oncogene
amplification are mainly responsible for the malignant phenotype of
this tumor (1). New blood vessel
formation, which supplies vital nutrients to the growing tumor, is
regulated by different growth factors, and VEGF is one of the most
effective. VEGF is a critical mitogen that regulates growth,
neovascularization and migration of endothelial cells in addition,
it is expressed and secreted by many neuroblastoma cell lines and
primary tumors that contribute to the growth of endothelium in
vitro and angiogenesis in vivo leading to poor prognosis
in high-risk neuroblastoma (1,25).
SH-SY5Y is a human cell line, cloned from a bone marrow
biopsy-derived line obtained from a four-year old female with
neuroblastoma, called SK-N-SH and first reported in 1973 (26). It has been demonstrated that VEGF
produced from tumor cells is essential for the progression of
experimental neuroblastoma and may function in both paracrine and
autocrine manners to allow tumor expansion and growth in the
SH-SY5Y neuroblastoma cell line (25). At present, the role of melatonin in
the regulation of cancer cell growth, more particularly in
estrogen-dependent tumors, is well established (9–11,14,16,17,27).
Lissoni et al (28) were the
first to suggest that melatonin could exert anti-angiogenic effects
in cancer and they also demonstrated a decrease in the serum levels
of VEGF in cancer patients treated with melatonin. The effects of
melatonin on angiogenesis have been studied in pancreatic, colon,
breast, lung, hepatocellular and cervical cancer (23,29–32).
In the present study, our objective was to demonstrate the
usefulness of melatonin against the pro-angiogenic effects of
either VEGF or the conditioned media from human neuroblastoma cells
(SH-SY5Y).
The present study demonstrated that melatonin
treatment has anti-angiogenic activity at different steps of the
angiogenic process in SH-SY5Y cells. Melatonin inhibited the
pro-angiogenic effects of SH-SY5Y cells and decreased the
pro-angiogenic effects of the conditioned media from the SH-SY5Y
cells stimulating proliferation, migration and tubular network
formation by endothelial cells. After disruption of the vessel
wall, endothelial cells must adhere to the extracellular matrix in
the stromal space, migrate out from the original vascular
structure, proliferate and organize into new vascular structures.
Each of these processes was simulated in part by one of the in
vitro assays used. Melatonin at a concentration of 1 mM had an
inhibitory effect on cell proliferation of the neuroblastoma cell
line. SH-SY5Y cells express melatonin MT1 receptor and different
aspects related with their growth are modulated by this indolamine;
thus, melatonin induces apoptosis (33), histone hyperacetylation (34) or anti-neuroinflammatory effects
(35) in this neuroblastoma cell
line. The effects of melatonin on cancer cells depend on different
factors, such as its concentration, duration of exposure and the
characteristics of the cells assessed. In the present study, only
pharmacological concentrations of melatonin were effective. In
previous studies (10–12,17,36), a
strong inhibitory effect induced by nanomolar concentrations of
melatonin on human breast cancer cells has been described. However,
high concentrations of melatonin are necessary to obtain oncostatic
effects in many other types of normal and cancer cells (31,33,37–39).
Melatonin is a highly lipid-soluble indolamine which may easily
cross the blood-brain barrier, and there is evidence that melatonin
concentration in the cerebrospinal fluid is higher than in blood
(40). Melatonin is also three
orders of magnitude more concentrated in neoplastic and adipose
tissue of the breast (41). These
high concentrations of melatonin in some tissues may explain why
high levels of melatonin are necessary to achieve oncostatic
effects.
Since endothelial cell migration/invasion is
essential for the formation of new blood vessels during
neo-angiogenesis, we next studied the regulatory effects of
melatonin on the conditioned media collected from the SH-SY5Y
cells, which are angiogenically active and able to increase the
migration of endothelial cells. Thus, the conditioned media from
SH-SY5Y cells stimulated the migration of endothelial cells and
this effect was significantly counteracted by the addition of
either anti-VEGF or 1 mM of melatonin. These results suggest that
VEGF produced by tumor cells could be responsible for inducing a
proliferative response of endothelial cells.
Another important step during neo-angiogenesis is
the formation of tubes by endothelial cells forming a complex
network of vessels and capillaries (2,42,43).
To study the effects of melatonin on this biological event we used
an endothelial cell capillary-like tube formation assay.
Endothelial cells plated on a basement membrane extract formed a
massive network of tubes. Conditioned medium collected from the
neuroblastoma cells was angiogenically active and stimulated tube
formation. This effect was significantly counteracted by the
addition of either the anti-VEGF antibody or melatonin, which
suggests that melatonin-decreased capillary structure formation
stimulated by the conditioned media from the SH-SY5Y cells, may
occur through the inhibition of VEGF. Our results support the
hypothesis that melatonin decreases the proliferation, migration
and tube formation of endothelial cells by regulating the
production of VEGF by neuroblastoma cells. We found that the same
concentration of melatonin that inhibits the proliferation of
SH-SY5Y cells also decreases the levels of VEGF in neuroblastoma
cell-culture media. Previous studies have described a decrease in
the production of VEGF protein induced by melatonin in human
pancreatic carcinoma cells (PANC-1), human alveolar adenocarcinoma
cells (A549) and human breast cancer cells (MCF-7) (29,31,32).
With the aim of determining whether this inhibitory effect of
melatonin on VEGF production was due to a downregulation of the
VEGF mRNA expression in SH-SY5Y cells, we then incubated SH-SY5Y
cells with different melatonin concentrations. The results obtained
demonstrate that melatonin did in fact downregulate VEGF mRNA
expression. SH-SY5Y cells were also exposed to cobalt chloride
(CoCl2) to mimick hypoxia, a well-known inducer of VEGF
mRNA expression, and the addition of 1 mM of melatonin
significantly counteracted the stimulatory effect of
CoCl2 on VEGF mRNA gene expression. It has been shown
that the pharmacological concentrations of melatonin also suppress
the VEGF mRNA expression induced by cobalt chloride in other tumor
cells, such as human pancreatic carcinoma (PANC-1), human breast
cancer (MCF-7) and human alveolar adenocarcinoma cells (A549)
(29,31,32).
In these tumor cells, melatonin also decreases hypoxia-inducible
factor (HIF)-1α protein levels, suggesting a role for transcription
factor HIF-1α in the suppression of VEGF expression (29,30).
In the present study, we developed a co-culture system of HUVECs
and SH-SY5Y cells to investigate whether neuroblastoma cells affect
the growth of the endothelial cells. We found that HUVECs exhibited
increased cell proliferation when they were co-cultured with
neuroblastoma cells and this process was prevented when melatonin
was added to the culture medium. These results suggested that the
local VEGF produced by tumor cells could be responsible for the
proliferative response of endothelial cells. Undoubtedly, the
presence of neuroblastoma cells increased the protein levels of
VEGF in the cell co-culture media and the addition of melatonin
decreased the concentration levels of VEGF and counteracted the
stimulatory effect induced by the presence of neuroblastoma cells.
In addition, to demonstrate the paracrine effects of
neuroblastoma-secreted VEGF on HUVEC growth, we studied whether
VEGF within the conditioned medium collected from the neuroblastoma
cell cultures was able to increase the proliferation of HUVECs.
When the conditioned medium was added to the HUVECs in the culture,
their growth was stimulated. The proliferative response of the
HUVECs was abolished in the presence of an anti-VEGF antibody,
indicating that VEGF produced by the neuroblastoma cells was the
major factor that induces cellular proliferation in endothelial
cells. When melatonin was added to the HUVEC cultures in the
presence of the conditioned medium collected from the neuroblastoma
cells, the stimulatory effect of the conditioned medium was also
counteracted by this indolamine.
The present study, describes the anti-angiogenic
effects of melatonin in relation to neuroblastoma. The
anti-angiogenic effects of melatonin through its inhibitory effects
on VEGF have also been described in human pancreatic carcinoma
cells (PANC-1), human alveolar adenocarcinoma (A549), human colon
cancer (HCT116), human breast cancer (MCF-7), cervical cancer cells
(HeLa) and adenocarcinomic human alveolar basal epithelial cells
(29–32). All these findings support a model in
which neuroblastoma cells produce VEGF that functions in a
paracrine manner to increase proliferation, invasion, migration and
tube formation of endothelial cells. Melatonin may play a role in
the paracrine interactions between neuroblastoma cells and proximal
endothelial cells through the downregulation of the expression of
VEGF in SH-SY5Y cells, thus decreasing the levels of VEGF around
endothelial cells. Lower levels of VEGF could be important in
decreasing endothelial cell proliferation, invasion, migration and
tube formation. Collectively, our findings suggest that the
anti-angiogenic effects of melatonin provide a promising
therapeutic approach for the treatment of neuroblastoma.
Acknowledgements
We are grateful to Dr María Elsa Valdizán from the
Pharmacology Division of the Department of Physiology and
Pharmacology of the University of Cantabria (Spain), for the
neuroblastoma cells (SH-SY5Y) which were a kind gift. The present
study was supported by grants from the Spanish Ministry of Science,
Technology and Innovation (SAF2013-42012-P) and from the Instituto
de Investigación Sanitaria Valdecilla (IDIVAL) (APG/12).
References
|
1
|
Roy Choudhury S, Karmakar S, Banik NL and
Ray SK: Targeting angiogenesis for controlling neuroblastoma. J
Oncol. 2012:7820202012.PubMed/NCBI
|
|
2
|
Bareschino MA, Schettino C, Colantuoni G,
Rossi E, Rossi A, Maione P, Ciardiello F and Gridelli C: The role
of antiangiogenetic agents in the treatment of breast cancer. Curr
Med Chem. 18:5022–5032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lyons J III, Anthony CT and Woltering EA:
The role of angiogenesis in neuroendocrine tumors. Endocrinol Metab
Clin North Am. 39:839–852. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rössler J, Monnet Y, Farace F, Opolon P,
Daudigeos-Dubus E, Bourredjem A, Vassal G and Geoerger B: The
selective VEGFR1-3 inhibitor axitinib (AG-013736) shows antitumor
activity in human neuroblastoma xenografts. Int J Cancer.
128:2748–2758. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cook KM and Figg WD: Angiogenesis
inhibitors: Current strategies and future prospects. CA Cancer J
Clin. 60:222–243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liang Y and Hyder SM: Proliferation of
endothelial and tumor epithelial cells by progestin-induced
vascular endothelial growth factor from human breast cancer cells:
Paracrine and autocrine effects. Endocrinology. 146:3632–3641.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brignole C, Marimpietri D, Pastorino F,
Nico B, Di Paolo D, Cioni M, Piccardi F, Cilli M, Pezzolo A,
Corrias MV, et al: Effect of bortezomib on human neuroblastoma cell
growth, apoptosis, and angiogenesis. J Natl Cancer Inst.
98:1142–1157. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Segerström L, Fuchs D, Bäckman U,
Holmquist K, Christofferson R and Azarbayjani F: The anti-VEGF
antibody bevacizumab potently reduces the growth rate of high-risk
neuroblastoma xenografts. Pediatr Res. 60:576–581. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Blask DE, Sauer LA and Dauchy RT:
Melatonin as a chronobiotic/anticancer agent: Cellular,
biochemical, and molecular mechanisms of action and their
implications for circadian-based cancer therapy. Curr Top Med Chem.
2:113–132. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cos S and Sánchez-Barceló EJ: Melatonin
and mammary pathological growth. Front Neuroendocrinol. 21:133–170.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cos S and Sánchez-Barceló EJ: Melatonin,
experimental basis for a possible application in breast cancer
prevention and treatment. Histol Histopathol. 15:637–647.
2000.PubMed/NCBI
|
|
12
|
Hill SM and Blask DE: Effects of the
pineal hormone melatonin on the proliferation and morphological
characteristics of human breast cancer cells (MCF-7) in culture.
Cancer Res. 48:6121–6126. 1988.PubMed/NCBI
|
|
13
|
Cos S, Fernández R, Güézmes A and
Sánchez-Barceló EJ: Influence of melatonin on invasive and
metastatic properties of MCF-7 human breast cancer cells. Cancer
Res. 58:4383–4390. 1998.PubMed/NCBI
|
|
14
|
Cos S, González A, Martínez-Campa C,
Mediavilla MD, Alonso-González C and Sánchez-Barceló EJ:
Estrogen-signaling pathway: A link between breast cancer and
melatonin oncostatic actions. Cancer Detect Prev. 30:118–128. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alonso-González C, González A, Mazarrasa
O, Güezmes A, Sánchez-Mateos S, Martínez-Campa C, Cos S,
Sánchez-Barceló EJ and Mediavilla MD: Melatonin prevents the
estrogenic effects of sub-chronic administration of cadmium on mice
mammary glands and uterus. J Pineal Res. 42:403–410. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cos S, González A, Güezmes A, Mediavilla
MD, Martínez-Campa C, Alonso-González C and Sánchez-Barceló EJ:
Melatonin inhibits the growth of DMBA-induced mammary tumors by
decreasing the local biosynthesis of estrogens through the
modulation of aromatase activity. Int J Cancer. 118:274–278. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cos S, González A, Martínez-Campa C,
Mediavilla MD, Alonso-González C and Sánchez-Barceló EJ: Melatonin
as a selective estrogen enzyme modulator. Curr Cancer Drug Targets.
8:691–702. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
González A, Cos S, Martínez-Campa C,
Alonso-González C, Sánchez-Mateos S, Mediavilla MD and
Sánchez-Barceló EJ: Selective estrogen enzyme modulator actions of
melatonin in human breast cancer cells. J Pineal Res. 45:86–92.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Martínez-Campa C, González A, Mediavilla
MD, Alonso-González C, Alvarez-García V, Sánchez-Barceló EJ and Cos
S: Melatonin inhibits aromatase promoter expression by regulating
cyclooxygenases expression and activity in breast cancer cells. Br
J Cancer. 101:1613–1619. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gerber HP, McMurtrey A, Kowalski J, Yan M,
Keyt BA, Dixit V and Ferrara N: Vascular endothelial growth factor
regulates endothelial cell survival through the
phosphatidylinositol 3-kinase/Akt signal transduction pathway.
Requirement for Flk-1/KDR activation. J Biol Chem. 273:30336–30343.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gingis-Velitski S, Zetser A, Flugelman MY,
Vlodavsky I and Ilan N: Heparanase induces endothelial cell
migration via protein kinase B/Akt activation. J Biol Chem.
279:23536–23541. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gu Q, Wang D, Wang X, Peng R, Liu J, Jiang
T, Wang Z, Wang S and Deng H: Basic fibroblast growth factor
inhibits radiation-induced apoptosis of HUVECs. I. The PI3K/AKT
pathway and induction of phosphorylation of BAD. Radiat Res.
161:692–702. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alvarez-García V, González A,
Alonso-González C, Martínez-Campa C and Cos S: Antiangiogenic
effects of melatonin in endothelial cell cultures. Microvasc Res.
87:25–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bäckman U, Svensson A and Christofferson
R: Importance of vascular endothelial growth factor A in the
progression of experimental neuroblastoma. Angiogenesis. 5:267–274.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Biedler JL, Helson L and Spengler BA:
Morphology and growth, tumorigenicity, and cytogenetics of human
neuroblastoma cells in continuous culture. Cancer Res.
33:2643–2652. 1973.PubMed/NCBI
|
|
27
|
Sánchez-Barceló EJ, Cos S, Mediavilla D,
Martínez-Campa C, González A and Alonso-González C:
Melatonin-estrogen interactions in breast cancer. J Pineal Res.
38:217–222. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lissoni P, Rovelli F, Malugani F, Bucovec
R, Conti A and Maestroni GJ: Anti-angiogenic activity of melatonin
in advanced cancer patients. Neuro Endocrinol Lett. 22:45–47.
2001.PubMed/NCBI
|
|
29
|
Dai M, Cui P, Yu M, Han J, Li H and Xiu R:
Melatonin modulates the expression of VEGF and HIF-1α induced by
CoCl2 in cultured cancer cells. J Pineal Res.
44:121–126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park SY, Jang WJ, Yi EY, Jang JY, Jung Y,
Jeong JW and Kim YJ: Melatonin suppresses tumor angiogenesis by
inhibiting HIF-1α stabilization under hypoxia. J Pineal Res.
48:178–184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cui P, Yu M, Peng X, Dong L and Yang Z:
Melatonin prevents human pancreatic carcinoma cell PANC-1-induced
human umbilical vein endothelial cell proliferation and migration
by inhibiting vascular endothelial growth factor expression. J
Pineal Res. 52:236–243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Alvarez-García V, González A,
Alonso-González C, Martínez-Campa C and Cos S: Regulation of
vascular endothelial growth factor by melatonin in human breast
cancer cells. J Pineal Res. 54:373–380. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
García-Santos G, Antolín I, Herrera F,
Martín V, Rodríguez-Blanco J, del Pilar Carrera M and Rodríguez C:
Melatonin induces apoptosis in human neuroblastoma cancer cells. J
Pineal Res. 41:130–135. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pan Y and Niles LP: Epigenetic mechanisms
of melatonin action in human SH-SY5Y neuroblastoma cells. Mol Cell
Endocrinol. 402:57–63. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wongprayoon P and Govitrapong P: Melatonin
attenuates methamphetamine-induced neuroinflammation through the
melatonin receptor in the SH-SY5Y cell line. Neurotoxicology.
50:122–130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cos S, Martínez-Campa C, Mediavilla MD and
Sánchez-Barceló EJ: Melatonin modulates aromatase activity in MCF-7
human breast cancer cells. J Pineal Res. 38:136–142. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sainz RM, Mayo JC, Tan DX, León J,
Manchester L and Reiter RJ: Melatonin reduces prostate cancer cell
growth leading to neuroendocrine differentiation via a receptor and
PKA independent mechanism. Prostate. 63:29–43. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Alvarez-García V, González A,
Alonso-González C, Martínez-Campa C and Cos S: Melatonin interferes
in the desmoplastic reaction in breast cancer by regulating
cytokine production. J Pineal Res. 52:282–290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
González A, Alvarez-García V,
Martínez-Campa C, Alonso-González C and Cos S: Melatonin promotes
differentiation of 3T3-L1 fibroblasts. J Pineal Res. 52:12–20.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Longatti P, Perin A, Rizzo V, Comai S,
Giusti P and Costa CV: Ventricular cerebrospinal fluid melatonin
concentrations investigated with an endoscopic technique. J Pineal
Res. 42:113–118. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Maestroni GJ and Conti A: Melatonin in
human breast cancer tissue: Association with nuclear grade and
estrogen receptor status. Lab Invest. 75:557–561. 1996.PubMed/NCBI
|
|
42
|
Dvorak HF, Weaver VM, Tlsty TD and Bergers
G: Tumor microenvironment and progression. J Surg Oncol.
103:468–474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Staton CA, Stribbling SM, Tazzyman S,
Hughes R, Brown NJ and Lewis CE: Current methods for assaying
angiogenesis in vitro and in vivo. Int J Exp Pathol. 85:233–248.
2004. View Article : Google Scholar : PubMed/NCBI
|