Introduction
Prostate carcinoma is one of the most prevalent
malignant tumors in males. In 2012, 241,740 cases of prostate
carcinoma were diagnosed in the US, and this was the most common
type of newly diagnosed tumor among males, accounting for 29% of
new diagnoses. It also accounted for ~28,170 mortalities, ranking
as the second most common cause of cancer-related mortality in
males (1). It is estimated that
220,800 new cases of prostate carcinoma were diagnosed in 2015, and
27,540 mortalities were attributed to prostate carcinoma (2). Despite numerous studies, the
pathogenesis of prostate carcinoma has not been fully elucidated
(3–5). Novel diagnostic markers and improved
treatment strategies for prostate carcinoma must be further
explored.
Orr et al (6)
demonstrated that the stanniocalcin 1 (STC1) expression pattern was
varied in prostate carcinomas. The results indicated that STC1 may
be crucial during prostate carcinoma progression and development.
Numerous experiments need to be conducted to further explore the
role of STC1 in prostate carcinogenesis and its potential as a
novel cancer biomarker
STC1 is a peptide hormone that was initially
identified in teleost fish and is widely expressed in mammalian
tissues (7,8). Its function and mechanism are complex.
STC1 is involved in the regulation of calcium and phosphorus,
inflammatory reactions and vascular sclerosis (9,10). Law
and Wong (11) identified a
hypoxia-inducible factor-1α (HIF-1α) binding motif in the promoter
region of the STC1 gene, indicating that STC1 expression may be
responsive to hypoxia in human tumors. The present study
demonstrates that STC1 is overexpressed in the prostate carcinoma
cell lines DU145 and LNCaP2. A series of experiments were performed
to study the function of STC1 in prostate carcinoma, and to improve
the understanding of prostate cancer pathogenesis.
Materials and methods
Cell culture
LNCaP2 and DU145 prostate carcinoma and normal
prostate RWPE-1 cells were cultured in Gibco RPMI-1640 medium,
supplemented with 10% fetal bovine serum (FBS) (both from Gibco,
Thermo Fisher Scientific, Inc., Waltham, MA, USA). The cells were
incubated at 37̊C in an atmosphere of 5% CO2.
Reverse transcription-polymerase chain
reaction (RT-PCR)
mRNA was isolated from two prostate carcinoma and
one normal prostate cell lines, and reverse transcribed and
amplified using a One-Step RT-PCR System (Fermentas, Vilnius,
Lithuania). The following primer sequences were used for PCR: GAPDH
antisense, 5′-CCTGCTTCACCACCTTCTTG-3′ and sense,
5′-AATCCCATCACCATCTTCCA-3′; STC1 sense, 5′-TTCTGGTGCTGGTGATCAGTG-3′
and antisense, 5′-TTTGGGCACAGTGGTCTGTCT-3′. Samples were initially
heated to 95̊C for 1 min, then subjected to 30 cycles (GAPDH, 28
cycles) of 95̊C for 30 sec, 56̊C for 30 sec and 72̊C for 90 sec; a
final 10-min extension step at 72̊C was also included. All reaction
products were purified on 1% agarose gels containing ethidium
bromide. The relative expression levels of mRNA were analyzed by a
Phosphor-Imager.
Western blot analysis
The sample cells were washed with cold
phosphate-buffered saline (PBS), and then lysed in Laemmli buffer
[62.5 mM Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, 50 mM
dithiothreitol and 0.01% bromphenol blue] for 5 min at 98̊C. Cell
lysate samples were separated by SDS-PAGE, and the proteins were
electrophoretically transferred to polyvinylidene difluoride
membranes. The blots were subsequently blocked for 1 h with non-fat
milk and probed with the specific primary antibodies followed by a
secondary detection step. The immunoreactive proteins were revealed
by an enhanced chemiluminescence kit. The following antibodies were
used in the western blotting: rabbit anti-STC1 (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), rabbit anti-cyclin D1
(Cell Signaling Technology, Inc., Danvers, MA, USA), rabbit
anti-cyclin E1 (Abcam, Cambridge, MA, USA), rabbit
anti-cyclin-dependent kinase 4 (CDK4), and rabbit anti-CDK2 (both
from Santa Cruz Biotechnology, Inc.).
Vector construction and cell
transfection
To knock down STC1 expression, a pRNAT-U6.1/Neo
vector encoding a small interfering RNA (siRNA) directed against
the target gene, STC1, in prostate cells was utilized (si-STC1).
The target sequence for STC1 was, 5′-TTAGTCCAGGAAGCAATAGTA-3′. An
empty pRNAT-U6.1/Neo vector was used as a negative control (NC).
For transfection, prostate carcinoma cells were cultured to 70%
confluency, transfected with a recombinant plasmid, and harvested
after 48 h for further experiments.
Methyl thiazolyl tetrazolium (MTT) and
colony formation assays
For the MTT assay, the cells were seeded in 96-well
plates at a density of 103 cells/well (n=6) and cultured
for 12, 24, 48 or 72 h. Subsequently, the cells were incubated with
10 µl MTT (50 µg/well; Sigma-Aldrich, St. Louis, MO, USA) for 4 h.
The generated formazan was assessed at 490 nm to detect the cell
viability.
Additionally, a colony formation assay was conducted
as previously described (12).
Flow cytometric analysis
Cells were cultured in RPMI-1640 medium containing
1% FBS for the first 24 h and 10% FBS for the subsequent 24 h
(n=3). The cells were then harvested and resuspended in fixation
fluid at a density of 106 cells/ml. Propidium iodide
(PI) solution (1,500 µl) was then added, and the cell cycle was
analyzed using a FACSCalibur flow cytometer (BD Biosciences, San
Diego, CA, USA).
Tumor formation in nude mice
To evaluate tumor growth in vivo, cells
transfected with the si-STC1 or NC vectors (DU145/si-STC1,
DU145/NC, LNCaP2/si-STC1 and LNCaP2/NC; 5×106
cells/mouse) were subcutaneously injected into 4-week-old BALB/c
nude mice (n=3/group; Shanghai Laboratory Animal Center, Shanghai,
China). The experimental pairs (DU145/si-STC1 and DU145/NC; and
LNCaP2/si-STC1 and LNCaP2/NC) were established in different mice.
The development and growth of solid tumors were monitored by
measuring tumor size using a Vernier caliper in a blinded manner
every 5 days for a 40-day period, and the following formula was
used to calculate tumor volume: Tumor volume = width2 ×
length × 0.5. All nude mice were sacrificed and individual tumor
weights were gauged at the end of the experiment. The animal
experimental protocols were approved by the Ethics Committee of
Xiangya Hospital of Central South University.
Statistical analysis
All experiments were repeated and data are expressed
as the mean ± standard deviations. Differences among >2 groups
were assessed by ANOVA, and differences between 2 groups were
analyzed using a Student's t-test. Analyses were performed with
GraphPad Prism software version 5.0 (GraphPad Software, Inc., San
Diego, CA, USA). Statistical significance was indicated by
P<0.05.
Results
mRNA and protein expression of STC1 in
prostate carcinoma and normal prostate cell lines
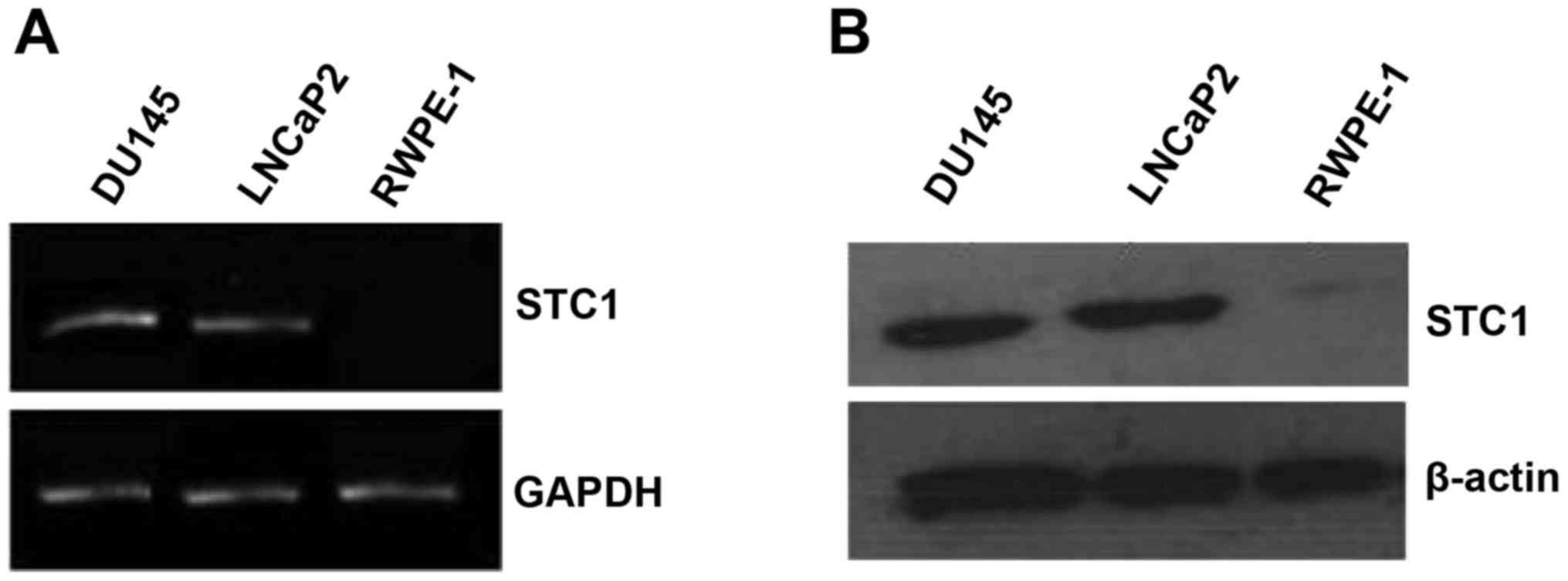
The mRNA and protein expression levels of STC1 were
assessed by RT-PCR and western blotting in the normal prostate
(RWPE-1) and prostate carcinoma (DU145 and LNCaP2) cell lines. The
results revealed that STC1 mRNA and protein levels were markedly
higher in the DU145 and LNCaP2 cells compared with the RWPE-1 cells
(Fig. 1A and B).
Effect of STC1 knockdown on prostate
cancer cell proliferation
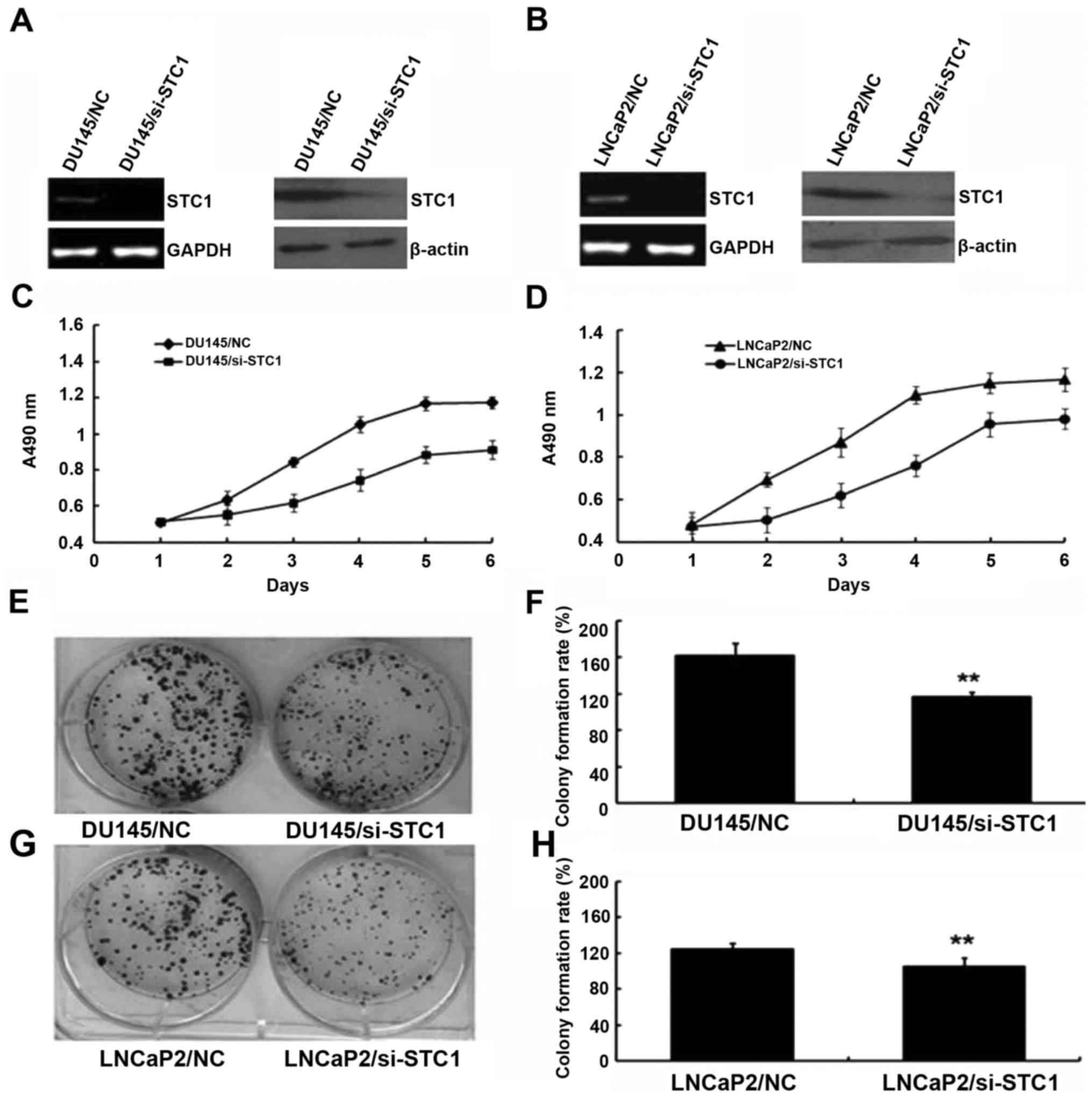
To examine the biological function of STC1,
STC1-knockdown DU145 and LNCaP2 cells were established. As shown in
Fig. 2A and B, cells stably
transfected with si-STC1 had markedly decreased levels of STC1 mRNA
and protein compared with the control group cells (DU145/NC and
LNCaP2/NC).
The effect of STC1 knockdown on the proliferation of
prostate cancer cells (DU145 and LNCaP2) was determined using MTT
analysis. During a 6-day period, the proliferation results
suggested that DU145/si-STC1 and LNCaP2/si-STC1 cells proliferated
more slowly compared with DU145/NC and LNCaP2/NC cells (Fig. 2C and D). Additionally, colony
formation assays were used to investigate the effect of decreased
STC1 expression in DU145 and LNCaP2 cells (Fig. 2E-H), also indicating that
downregulation of STC1 expression inhibited cell proliferation
in vivo.
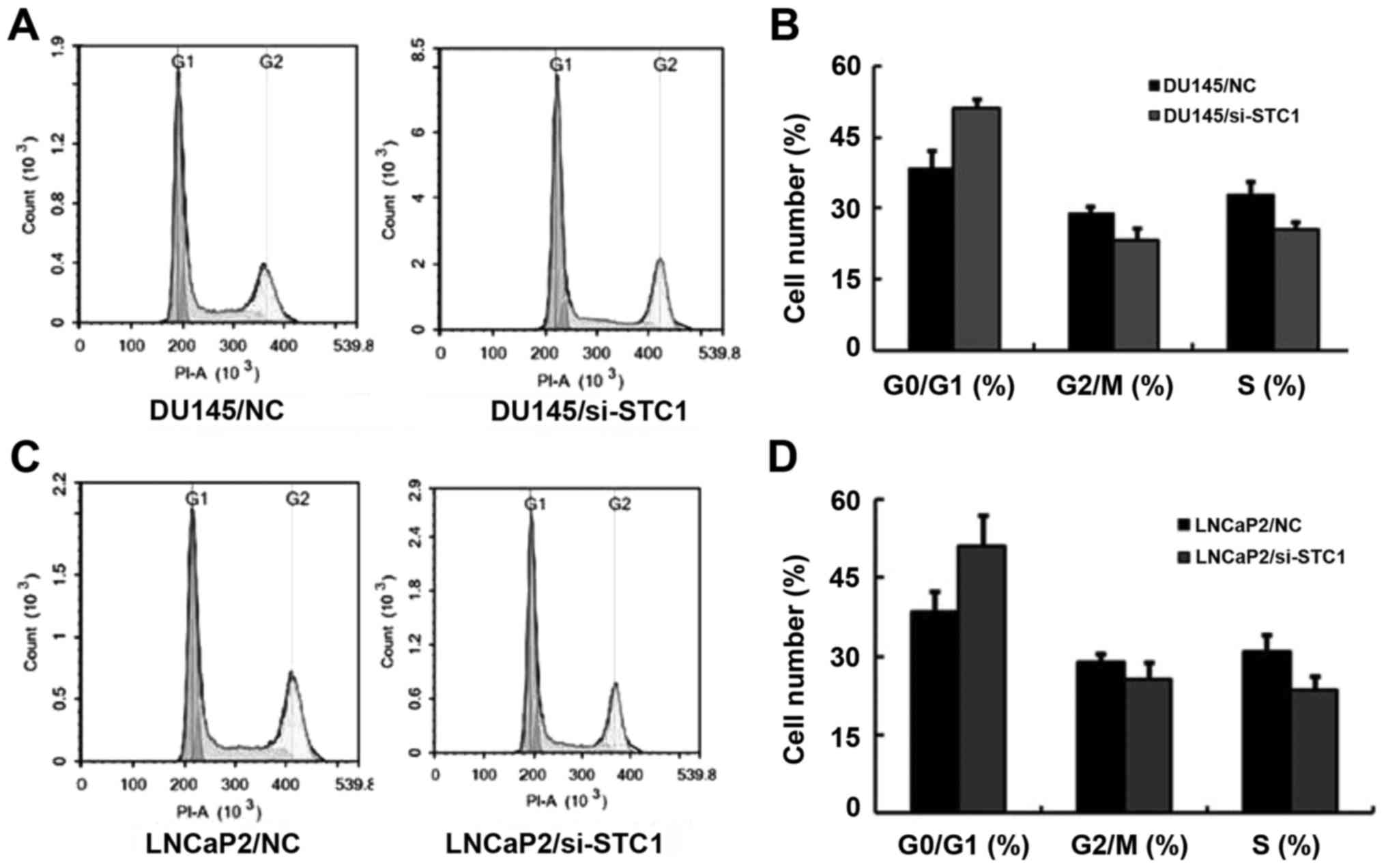
Flow cytometric analysis was also used to assess
cell proliferation. The results demonstrated that transfection with
si-STC1 led to increased G1 phase cell cycle arrest in DU145 and
LNCaP2 cells (Fig. 3A-D). This
result was in agreement with the previous analyses.
Overexpression of STC1 promotes RWPE-1
cell proliferation
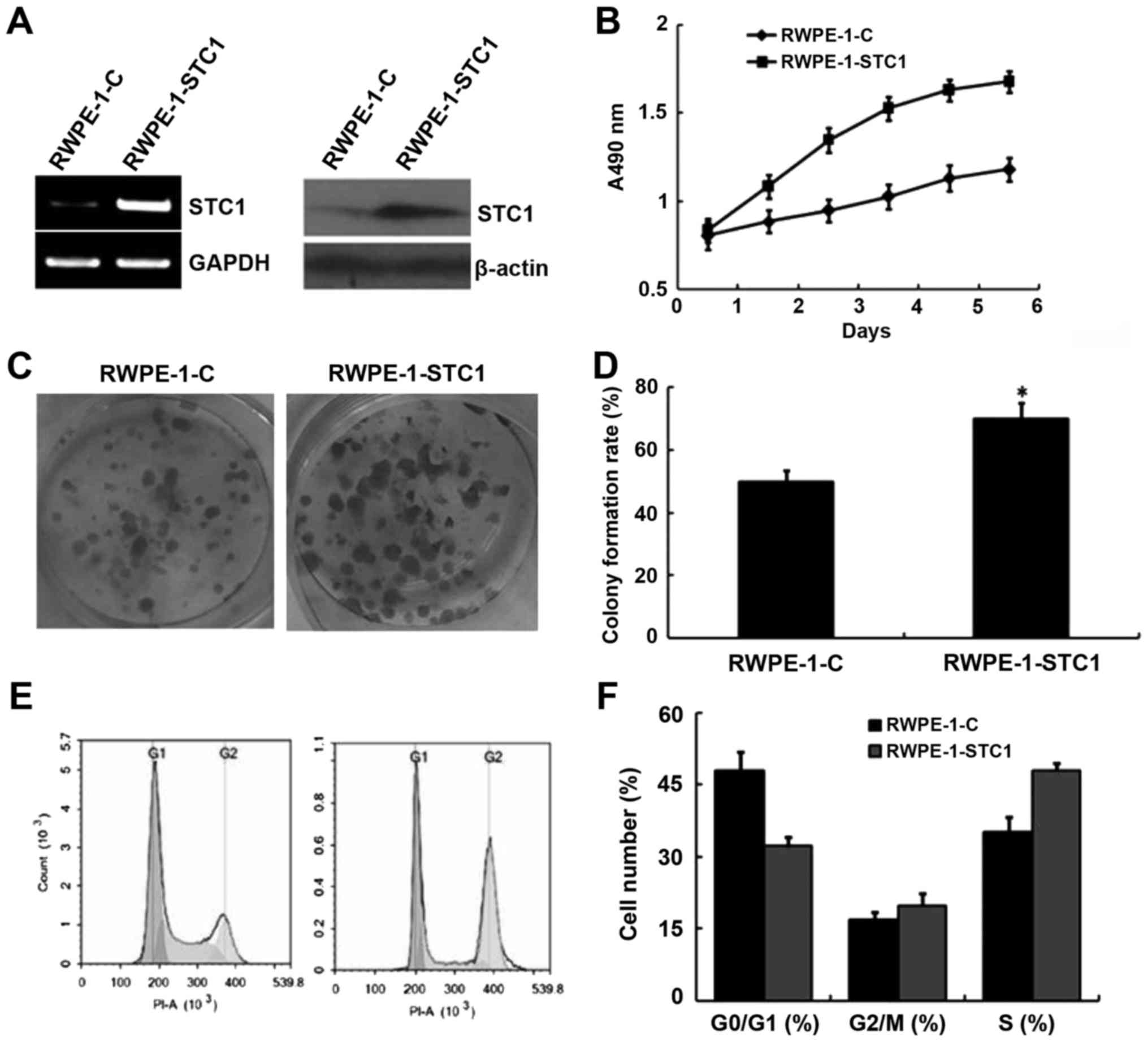
Given the aforementioned results, we hypothesized
that STC1 overexpression may promote RWPE-1 cell proliferation. To
confirm this, cells were transfected with a plasmid encoding STC1
(RWPE-1-STC1), and these cells exhibited increased levels of STC1
mRNA and protein compared with the control group (RWPE-1-C;
Fig. 4A). An MTT proliferation
analysis indicated that RWPE-1-STC1 cells had a higher rate of
proliferation compared with RWPE-1-C cells (Fig. 4B). Similarly, a colony formation
assay revealed that STC1 overexpression promoted the growth of
RWPE-1-STC1 cells compared with the control cells. These results
indicated that upregulation of STC1 expression promoted cell
proliferation in vivo (Fig. 4C
and D).
Furthermore, flow cytometric analysis demonstrated
that in RWPE-1-STC1 cells a smaller proportion of cells were
arrested in the G1 phase, while the percentage of cells in the S
phase was increased compared with that in the RWPE-1-C cells
(Fig. 4E and F). This result was
also consistent with the aforementioned analyses.
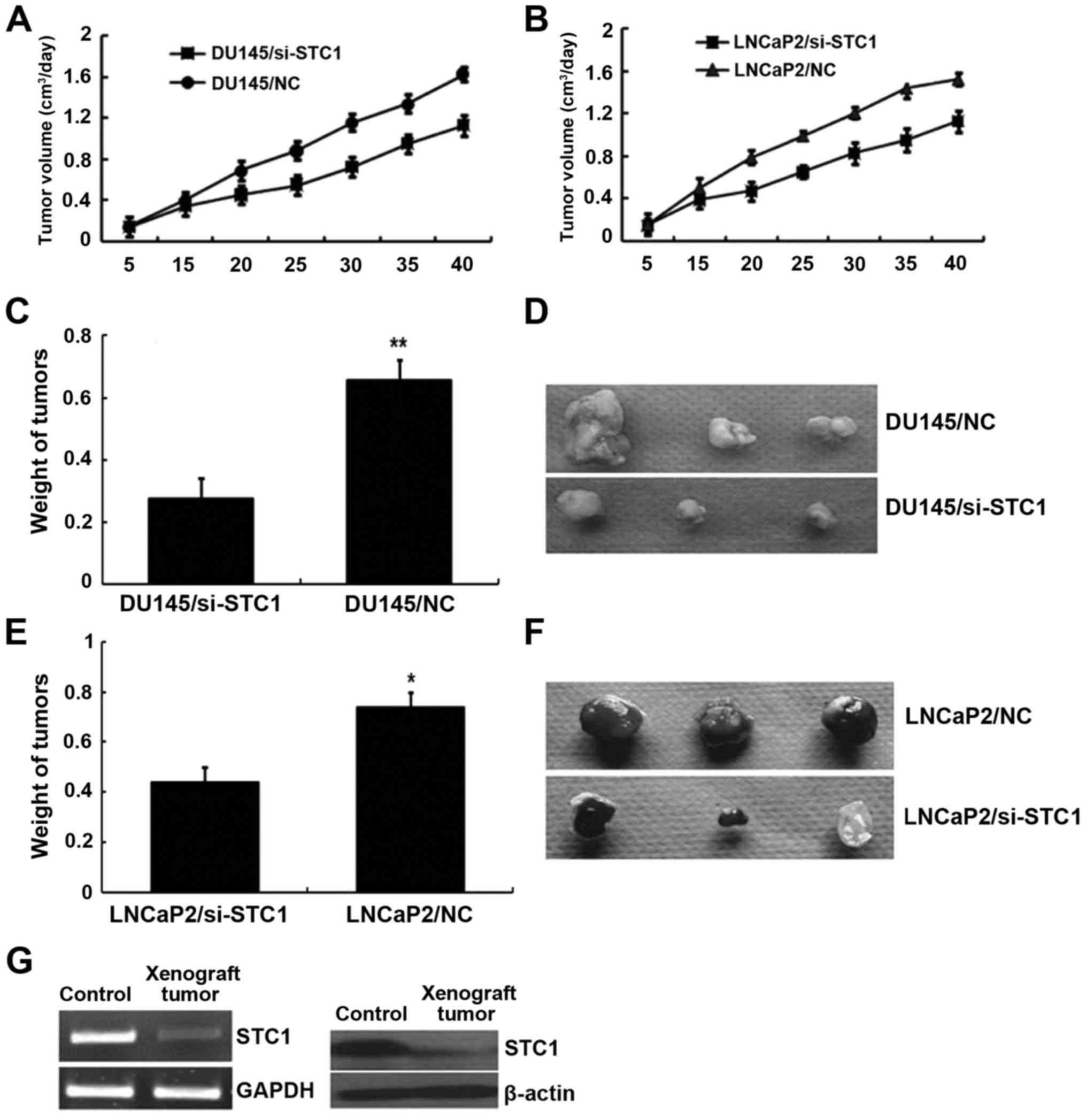
Tumor formation in nude mice
To further determine the effects of STC1 on tumor
growth and development in vivo, DU145/si-STC1 and
LNCaP2/si-STC1 or DU145/NC and LNCaP2/NC cells were subcutaneously
implanted into 4-week-old nude mice. After 40 days of growth, the
nude mice were sacrificed and the tumor weight was assessed. As
shown in Fig. 5A and B,
STC1-knockdown tumors emerged later and grew more slowly compared
with the control tumors. After 40 days, tumors developed from
STC1-knockdown DU145 and LNCaP2 cells (0.276±0.065 and 0.441±0.057
g, respectively) weighed less than the DU145 and LNCaP2 control
group tumors (0.658±0.098 and 0.739±0.072 g, respectively)
(Fig. 5C-F). The STC1 mRNA and
protein expression in tumor tissue removed from the nude mice was
also monitored by RT-PCR and western blotting (Fig. 5G). These results suggested that STC1
promoted xenograft tumor development in vivo.
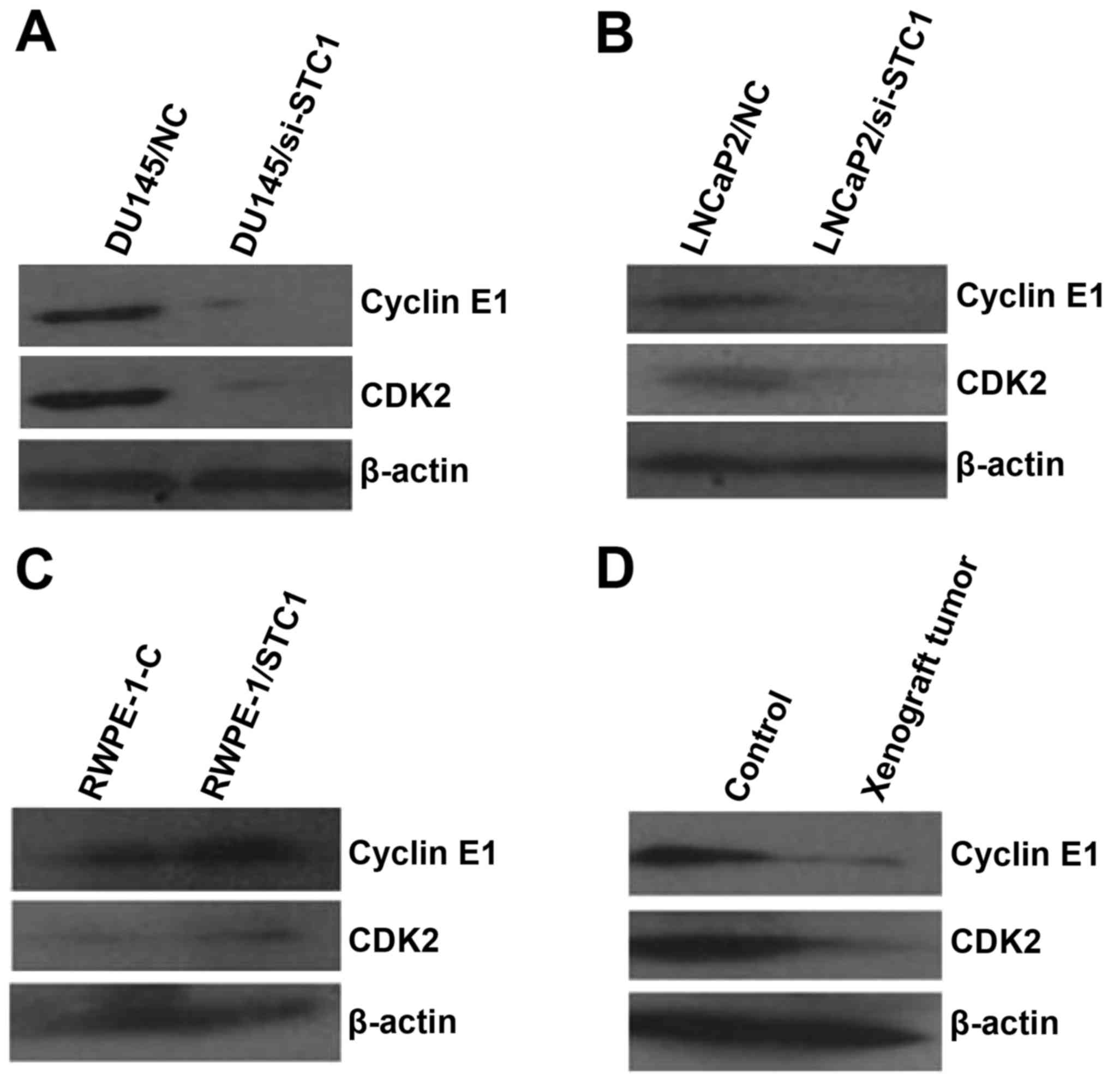
STC1 affects the expression of cell
cycle-related proteins in prostate carcinoma cells, normal prostate
cells and xenograft tumors
Previous studies revealed that cyclin D/CDK4 and
cyclin E/CDK2 have vital roles in cell cycle progression and are
often overexpressed in cancer cells (12). To confirm the association between
STC1 expression and cell cycle-related proteins in prostate
carcinoma and normal prostate cells and xenograft tumors, the
expression levels of cyclin D1/CDK4 and cyclin E1/CDK2 were
evaluated using western blotting. The results revealed that STC1
knockdown had no significant effect on cyclin D1/CDK4 protein
levels (data not shown). However, the protein levels of cyclin E1
and CDK2 were decreased by the downregulation of endogenous STC1 in
prostate carcinoma cell lines (Fig. 6A
and B). Consistent results were also obtained in RWPE-1-STC1
cells and xenograft tumors by western blot analyses (Fig. 6C and D).
Discussion
Extensive evidence suggests that the STC1
participates in various types of carcinoma, including colorectal
cancer (13), renal cell (14) and laryngeal squamous cell carcinoma
(15), ovarian (16) and non-small cell lung cancer
(17), and breast carcinoma
(18). Previous research has also
shown that STC1 is overexpressed in prostate carcinoma (6), suggesting that STC1 may play a
significant role in this type of cancer. In the present study, STC1
was detected in a normal prostate cell line and two prostate
carcinoma cell lines. The results illustrated that STC1 may
regulate the growth and metastasis of prostate carcinoma, as its
expression levels were markedly increased in the prostate carcinoma
cell lines (DU145 and LNCaP2) compared with the normal prostate
cell line. To ascertain how STC1 regulates prostate cell
proliferation, STC1 was knocked down in prostate carcinoma cells
and overexpressed in normal prostate cells. The results revealed
that knockdown of STC1 induced a decrease in cell proliferation,
while overexpression of STC1 in RWPE-1 cells promoted cell
growth.
Recent studies (19)
have demonstrated that STC1 participates in cancer progression and
metastasis, which prompted our investigation into the role of STC1
in prostate cancer development and progression. Certain authors
(20) have suggested that STC1 can
regulate the calcium concentration in cells and activate a series
of intracellular signals, which may result in tumor cell
proliferation and invasion, and provide the necessary conditions
for migration. In addition, STC1 can increase the phosphorus
concentration in the cell. Therefore, the overexpression of STC1 in
tumor cells may be associated with adaptation to a hypoxic
environment (21).
In the present study, a flow cytometric analysis was
performed to assess cell cycle distribution. Cell proliferation is
controlled by cell cycle progression, which is regulated by
numerous cell proliferation signaling pathways (22–24).
In contrast to normal cells, the cell cycle is unregulated in
cancer cells. Studies have demonstrated that the cell cycle is
controlled via various cyclins and CDKs (12). Cyclins are essential for the
regulation of the cell cycle and the activation of CDKs (25–27).
In the present study, prostate carcinoma cells transfected with
si-STC1 exhibited cell cycle arrest in the G1 phase, and decreased
proliferative and tumorigenic abilities. Previous studies have
indicated that cyclin E1/CDK2 is vital in various cancer-associated
processes, including tumor formation, invasion and metastasis
(28,29). The results of the present study
supported that the function of cyclin E1/CDK2 is associated with
prostate carcinoma cell growth, as noted in multiple previous
experiments (30). It is
established that cyclin E1/CDK2 activation promotes cell
proliferation and replication; however, the precise effects of
cyclin E1/CDK2 require further study.
In conclusion, to the best of our knowledge, the
present study is the first to suggest STC1 as a potential biomarker
associated with the development and metastasis of prostate
carcinoma. The results indicate a novel mechanistic role for STC1
in the regulation of prostate carcinoma cell proliferation via
cyclin E1/CDK2. This novel biomarker may aid in clinical treatment
and prediction of prognosis in prostate carcinoma. Further research
is necessary to explore the regulatory mechanism of STC1.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Hunan Province (14JJ7004), and the
Independent Innovation Foundation of Central South University
(2016zzts516) (Changsha, China).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics for Hispanics/Latinos, 2012. CA Cancer J Clin.
62:283–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Fedewa SA, Miller KD,
Goding-Sauer A, Pinheiro PS, Martinez-Tyson D and Jemal A: Cancer
statistics for Hispanics/Latinos, 2015. CA Cancer J Clin.
65:457–480. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hoyne G, Rudnicka C, Sang QX, Roycik M,
Howarth S, Leedman P, Schlaich M, Candy P and Matthews V: Genetic
and cellular studies highlight that A Disintegrin and
Metalloproteinase 19 is a protective biomarker in human prostate
cancer. BMC Cancer. 16:1512016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marzec J, Mao X, Li M, Wang M, Feng N, Gou
X, Wang G, Sun Z, Xu J, Xu H, et al: A genetic study and
meta-analysis of the genetic predisposition of prostate cancer in a
Chinese population. Oncotarget. 7:21393–21403. 2016.PubMed/NCBI
|
|
5
|
Zhou C, Dai X, Chen Y, Shen Y, Lei S, Xiao
T, Bartfai T, Ding J and Wang MW: G protein-coupled receptor GPR160
is associated with apoptosis and cell cycle arrest of prostate
cancer cells. Oncotarget. 7:12823–12839. 2016.PubMed/NCBI
|
|
6
|
Orr B, Riddick AC, Stewart GD, Anderson
RA, Franco OE, Hayward SW and Thomson AA: Identification of
stromally expressed molecules in the prostate by tag-profiling of
cancer-associated fibroblasts, normal fibroblasts and fetal
prostate. Oncogene. 31:1130–1142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ono M, Ohkouchi S, Kanehira M, Tode N,
Kobayashi M, Ebina M, Nukiwa T, Irokawa T, Ogawa H, Akaike T, et
al: Mesenchymal stem cells correct inappropriate
epithelial-mesenchyme relation in pulmonary fibrosis using
stanniocalcin-1. Mol Ther. 23:549–560. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohkouchi S, Ono M, Kobayashi M, Hirano T,
Tojo Y, Hisata S, Ichinose M, Irokawa T, Ogawa H and Kurosawa H:
Myriad functions of stanniocalcin-1 (STC1) cover multiple
therapeutic targets in the complicated pathogenesis of idiopathic
pulmonary fibrosis (IPF). Clin Med Insights Circ Respir Pulm Med.
9:(Suppl 1). S91–S96. 2015.
|
|
9
|
Lee S, Naesens M, Li L and Sarwal M:
Stanniocalcin supports the functional adaptation of adult-sized
kidneys transplanted into the pediatric recipients.
Transplantation. 93:1130–1135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang SE, Wu CP, Wu SY, Peng CK, Perng WC,
Kang BH, Chu SJ and Huang KL: Stanniocalcin-1 ameliorates
lipopolysaccharide-induced pulmonary oxidative stress,
inflammation, and apoptosis in mice. Free Radic Biol Med.
71:321–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Law AY and Wong CK: Stanniocalcin-2 is a
HIF-1 target gene that promotes cell proliferation in hypoxia. Exp
Cell Res. 316:466–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Y, Yang XH, Fang SJ, Qin CF, Sun RL,
Liu ZY, Jiang BY, Wu X and Li G: HOXA7 stimulates human
hepatocellular carcinoma proliferation through cyclin E1/CDK2.
Oncol Rep. 33:990–996. 2015.PubMed/NCBI
|
|
13
|
Peña C, Céspedes MV, Lindh MB, Kiflemariam
S, Mezheyeuski A, Edqvist PH, Hägglöf C, Birgisson H, Bojmar L,
Jirström K, et al: STC1 expression by cancer-associated fibroblasts
drives metastasis of colorectal cancer. Cancer Res. 73:1287–1297.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma X, Gu L, Li H, Gao Y, Li X, Shen D,
Gong H, Li S, Niu S, Zhang Y, et al: Hypoxia-induced overexpression
of stanniocalcin-1 is associated with the metastasis of early stage
clear cell renal cell carcinoma. J Transl Med. 13:562015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou H, Li YY, Zhang WQ, Lin D, Zhang WM
and Dong WD: Expression of stanniocalcin-1 and stanniocalcin-2 in
laryngeal squamous cell carcinoma and correlations with clinical
and pathological parameters. PLoS One. 9:e954662014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu G, Yang G, Chang B, Mercado-Uribe I,
Huang M, Zheng J, Bast RC, Lin SH and Liu J: Stanniocalcin 1 and
ovarian tumorigenesis. J Natl Cancer Inst. 102:812–827. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Du YZ, Gu XH, Li L and Gao F: The
diagnostic value of circulating stanniocalcin-1 mRNA in non-small
cell lung cancer. J Surg Oncol. 104:836–840. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang AC, Doherty J, Huschtscha LI,
Redvers R, Restall C, Reddel RR and Anderson RL: STC1 expression is
associated with tumor growth and metastasis in breast cancer. Clin
Exp Metastasis. 32:15–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park WY, Hong BJ, Lee J, Choi C and Kim
MY: H3K27 demethylase JMJD3 employs the NF-κB and BMP signaling
pathways to modulate the tumor microenvironment and promote
melanoma progression and metastasis. Cancer Res. 76:161–170. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gu J, Law AY, Yeung BH and Wong CK:
Activation of gill Ca2+-sensing receptor as a protective
pathway to reduce Ca2+-induced cytotoxicity. J Mol
Endocrinol. 53:155–164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi X, Wang J and Qin Y: Recombinant
adeno-associated virus-delivered hypoxia-inducible stanniocalcin-1
expression effectively inhibits hypoxia-induced cell apoptosis in
cardiomyocytes. J Cardiovasc Pharmacol. 64:522–529. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nagappan A, Lee HJ, Saralamma VV, Park HS,
Hong GE, Yumnam S, Raha S, Charles SN, Shin SC, Kim EH, et al:
Flavonoids isolated from Citrus platymamma induced G2/M cell
cycle arrest and apoptosis in A549 human lung cancer cells. Oncol
Lett. 12:1394–1402. 2016.PubMed/NCBI
|
|
23
|
Huang Y, Zhao S, Zhang Y, Zhang C and Li
X: Downregulation of coding transmembrane protein 35 gene inhibits
cell proliferation, migration and cell cycle arrest in osteosarcoma
cells. Exp Ther Med. 12:581–588. 2016.PubMed/NCBI
|
|
24
|
Tsai TC, Huang HP, Chang KT, Wang CJ and
Chang YC: Anthocyanins from roselle extract arrest cell cycle G2/M
phase transition via ATM/Chk pathway in p53-deficient leukemia
HL-60 cells. Environ Toxicol. Jul 22–2016.(Epub ahead of print).
doi: 10.1002/tox.22324.
|
|
25
|
Huang MY, Xuan F, Liu W and Cui HJ: MINA
controls proliferation and tumorigenesis of glioblastoma by
epigenetically regulating cyclins and CDKs via H3K9me3
demethylation. Oncogene. Jun 13–2016.(Epub ahead of print). doi:
10.1038/onc.2016.208.
|
|
26
|
Hydbring P, Malumbres M and Sicinski P:
Non-canonical functions of cell cycle cyclins and cyclin-dependent
kinases. Nat Rev Mol Cell Biol. 17:280–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de Azevedo WF: Opinion paper: Targeting
multiple cyclin-dependent kinases (CDKs): A new strategy for
molecular docking studies. Curr Drug Targets. 17:22016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu QX, Wang XF, Ikeo K, Hirose S, Gehring
WJ and Gojobori T: Evolutionarily conserved transcription factor
Apontic controls the G1/S progression by inducing cyclin E
during eye development. Proc Natl Acad Sci USA. 111:9497–9502.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gladden AB and Diehl JA: Cell cycle
progression without cyclin E/CDK2: Breaking down the walls of
dogma. Cancer Cell. 4:160–162. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rath SL and Senapati S: Why are the
truncated cyclin Es more effective CDK2 activators than the
full-length isoforms? Biochemistry. 53:4612–4624. 2014. View Article : Google Scholar : PubMed/NCBI
|