Introduction
The inflammation-associated tumor microenvironment
has been demonstrated to promote the aggressiveness of many types
of cancers. However, the underlying mechanisms have not been well
described. Previous studies have shown a correlation between the
inflammatory microenviroment and tumor hypoxia which confers a
worse prognosis as well as chemotherapy resistance (1,2). In
lung cancer, hypoxia is positively correlated with an increased
risk of tumor relapse (3).
Lipopolysaccharides (LPS), an integral constituent of the outer
cell membrane of gram-negative bacteria, induces systemic
inflammation that results in the increase of a variety of
inflammatory factors and production of reactive oxygen species
(ROS) (4,5). In addition, other studies have
reported that LPS displays potent effects on tumor invasion and
metastasis (6). In the present
study, we investigated the roles of an important hypoxia-activated
modulator, hypoxia-inducible factor 1α (HIF-1α), in LPS-induced
hypoxia of non-small cell lung cancer (NSCLC).
HIF-1 is a heterodimer composed of 2 subunits,
HIF-1α and HIF-1β. HIF-1β is constitutively expressed while the
level of HIF-1α protein depends on different physiologic conditions
(7). Therefore, the HIF-1α level
determines the transcriptional activation and functions of HIF-1α
in response to different oxygen levels. In normoxia, degradation of
HIF-1α by ubiquitination and proteasome leads to suppression of the
conformation of the HIF-1 complex. However, under hypoxic
conditions, degradation of HIF-1α is inhibited and nuclear
accumulation of HIF-1α results in the formation of active HIF-1
transcription factors with HIF-1β (8). Abnormal expression or activation of
HIF-1α has been reported in many solid tumors including breast and
colorectal cancer, and hepatocarcinoma (9–11).
However, the role of HIF-1α in the tumor progression of lung cancer
is still controversial.
Propofol (2,6-diisopropylphenol) is one of the most
commonly used intravenous anesthetic agents during surgery.
Notably, evidence suggests that propofol possesses antioxidant
properties and suppresses the inflammatory process both in
vitro and in vivo (12,13).
However, other studies have shown that propofol has an influence on
the proliferation, motility and invasiveness of cancer cells
(14,15). More and more studies have indicated
a potential antitumor property of propofol. Previous studies have
shown that propofol can induce the apoptosis of human leukemia
cells and inhibit pulmonary metastasis of osteosarcoma cells
(14,16). Consistently, propofol also
suppresses the invasion and migration of lung cancer cells
(17). Nevertheless, the mechanisms
underlying the antitumor effects of propofol are not yet
available.
In the present study, we examined the effects of
propofol on LPS-induced migration and invasion of NSCLC cells.
Moreover, we found that propofol inhibited the aggressive
capabilities of NSCLC cells partly through decreasing the
expression of HIF-1α which is induced by inflammatory hypoxia.
Materials and methods
Cell culture
Human lung adenocarcinoma cell line A549 was
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA) and cultured in RPMI-1640 medium suppplemented
with 10% fetal bovine serum (FBS; Gibco, Carlsbad, CA, USA), 100
mg/ml streptomycin and 100 IU/ml penicillin in a 5% CO2
atmosphere at 37°C.
Chemicals and reagents
LPS (from Escherichia coli 0111:B4) was
purchased from Sigma (St. Louis, MO, USA) and stored in a stock
solution of 1 mg/ml. Various concentrations of LPS in the
experiments were diluted with serum-free culture medium. Propofol
was obtained from Sigma-Aldrich (St. Louis, MO, USA) and diluted in
dimethyl sulfoxide (DMSO) for in vitro experiments.
Subsequent concentrations of propofol were diluted with culture
medium when used. The following antibodies were used in the western
blotting or immunohistochemistry (IHC). Antibodies to E-cadherin,
vimentin and GAPDH were purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). Antibodies to MMP2 and MMP9 were
purchased from Cell Signaling Technology (CST; Beverly, MA, USA).
Antibody to HIF-1α was purchased from Abcam (Cambridge, UK).
Plasmids and siRNAs
pGL3-HRE plasmids containing 3 repeated hypoxic
response elements (HREs) in the promoter region were constructed
from pGL3-basic plasmids. HIF-1α siRNAs were purchased from Santa
Cruz Biotechnology, Inc. PcDNA3.1-HIF-1α and HIF-1α siRNAs were
transfected using Lipofectamine 2000 transfection reagent
(Invitrogen, Carlsbad, CA, USA) according to the manufacturer's
instructions.
Quantitative real-time PCR
Extraction of total RNA was performed with RNAiso™
Plus reagent and further reverse-transcribed using a PrimeScript™
RT reagent kit (both from Takara, Tokyo, Japan). SYBR-Green mix
(Roche) was used to carry out quantitative PCR according to the
manufacturer's instructions. β-actin served as a loading
control.
Western blotting
The whole cell protein was obtained with cold cell
lysis buffer and the total protein concentration was measured using
the Bradford protein assay (Bio-Rad, Hercules, CA, USA). Equal
amount of protein was separated on 8–12% SDS-PAGE gel and
transferred to a nitrocellulose membrane. The membrane was blocked
with 5% milk and then incubated with primary antibodies at 4°C
overnight. Next, the membranes were incubated with appropriate
secondary antibodies at room temperature for 1 h. IRDye®
800CW- or IRDye® 680-conjugated secondary antibodies
were used for staining and then the proteins were detected using an
Odyssey® infrared imaging system (both from LI-COR,
Lincoln, NE, USA).
Immunofluorescence microscopy
The cells were washed with cold phosphate-buffered
saline (PBS) and fixed in 4% paraformaldehyde. After incubating
with primary antibodies, the cells were stained with the
fluorescein isothiocyanate (FITC) or tetramethylrhodamine
(TRITC)-conjugated secondary antibodies.
4,6-Diamidino-2-phenylindole (DAPI) was used for nuclear staining.
At least six randomly chosen fields of view were counted, with each
experiment performed in triplicate, and at least 100 total cells
were examined in each experiment.
Luciferase reporter assay
A549 cells were plated onto 24-well plates. The next
day, the cells were co-transfected with 0.5 µg firefly luciferase
reporter constructs and 0.01 µg pRL-SV40 Renilla luciferase
reporter plasmids (Promega, Madison, WI, USA). The pRL-SV40 plasmid
was used to normalize the transfection efficiency. Two days after
transfection, the cells were lysed and the luciferase activities
were measured using a Dual-Luciferase Reporter Assay System
(Promega) and a luminometer (LB9507; Berthold, Bad Wildbad,
Germany). All experiments were carried out in triplicate.
Wound healing assay
A549 cells were plated at a density of
1×106 cells in a 35-mm culture dish and incubated at
37°C. The next day, a scratch in the form of a lane was made
through the confluent monolayers with a sterile white pipette tip.
Thereafter, the A549 cells were treated with LPS or propofol.
Images of the views for assessment of the cell migration ability at
24 h after scratch were captured. The wound was evaluated using
ImageJ.
Transwell migration assay
A549 cells were seeded on Matrigel-coated membrane
inserts with a pore size of 8-µm (BD Biosciences, San Diego, CA,
USA) in the presence of serum-free medium. The complete medium with
or without LPS and propofol was placed in the lower wells of the
chamber system. After incubation for 48 h, the undersurface
adherent cells that had invaded through the Matrigel were fixed in
methanol and stained with 0.5% crystal violet. The air-dried filter
membrane was viewed under a microscope and 4 random fields were
selected for cell counting.
Detection of ROS generation
Nicotinamide adenine dinucleotide phosphate
oxidase-dependent ROS generation was analyzed by a luminometric
assay as previously described (18).
IHC
NSCLC tissue specimens (n=187) were collected from
2005 to 2010 from the First Affiliated Hospital of Soochow
University (Suzhou, China) (n=127) and the First Affiliated
Hospital of Wenzhou Medical University (Wenzhou, China) (n=60).
Informed consent was obtained from all patients, and the study was
approved by the Institutional Review Board of the First Affiliated
Hospital of Soochow University and the First Affiliated Hospital of
Wenzhou Medical University. All specimens were fixed in 10% neutral
formalin, embedded in paraffin and cut into 4-µm sections for
immunohistochemical staining. The EnVision™ two-step method was
used (Dako, Hamburg, Germany), as well as the antibody against
HIF-1α. To estimate the score for each slide, at least 10
individual fields at ×200 were chosen, and 100 cancer cells were
counted in each field. The immunostaining intensity was divided
into 4 grades: 0, no expression; 1, mildly positive; 2, moderately
positive; and 3, markedly positive. The proportion of
positive-staining cells was divided into 5 grades: 0, <10%; 1,
11–25%; 2, 26–50%; 3, 51–75%; and 4, >75%. The staining results
were assessed and confirmed by 2 independent investigators blinded
to the clinical data. The percentage of positivity of the tumor
cells and the staining intensities were then multiplied in order to
generate the IHC score, and were graded as 0–3, negative (−); 4–6,
positive (+); 7–9, strongly positive (++); and 10–12, very strongly
positive (+++). Cases with a discrepancy in scores were discussed
to obtain a consensus.
Statistical analysis
The software SPSS 13.0 and GraphPad Prism 5 were
used in the statistical analyses. Group distributions were
performed with the Student's t-test or one-way analysis of
variance. A value of P<0.05 was considered to indicate a
statistically significant result.
Results
Aberrant high expression of HIF-1α in
clinical NSCLC tissues indicates a poor prognosis
To examine the levels of HIF-1α mRNA in clinical
specimens, we investigated lung cancer microarray data sets on
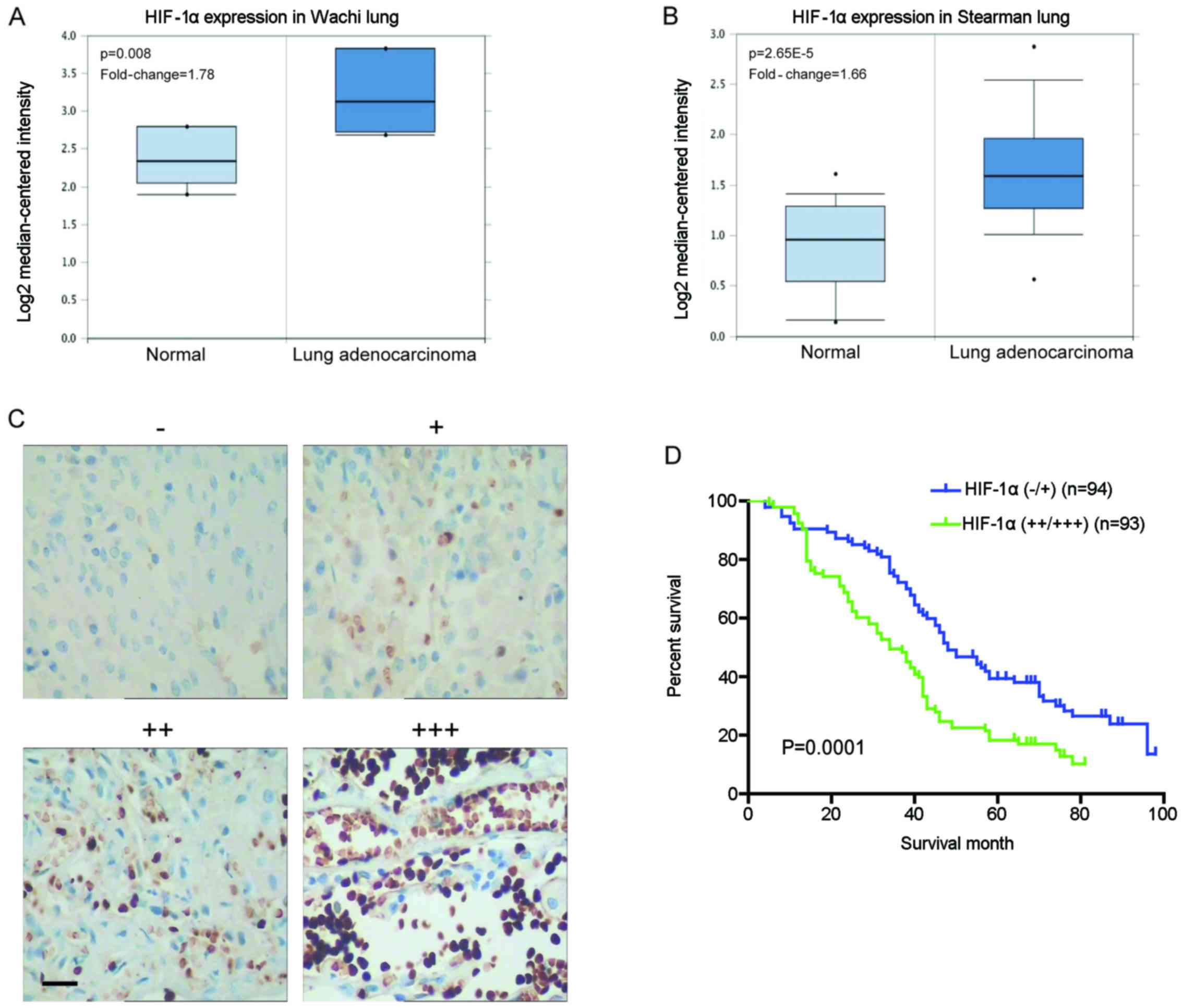
Oncomine and found higher HIF-1α mRNA levels in lung adenocarcinoma
when compared with normal lung tissues (Fig. 1A and B). Immunohistochemical
staining was carried out to analyze the clinical relevance of
HIF-1α expression in 187 human NSCLC specimens. The expression
level of HIF-1α was increased in the tumor compared with the normal
lung tissues (Fig. 1C). Further
analyses showed that the HIF-1α staining was positively correlated
with tumor size, lymph node metastasis, differentiation status and
tumor-node-metastasis (TNM) stage (P<0.05) but not with gender,
age and histological type (P>0.05) (Table I). Moreover, the 5-year overall
survival (OS) rate of the HIF-1α high expression group was
significantly lower than that of the HIF-1α low expression group
(18.28 vs. 34.04%; P=0.0001) (Fig.
1D). These data suggest that HIF-1α exhibits aberrant high
expression in NSCLC tissues, which is involved in cancer
progression and metastasis, and indicative of a poor prognosis.
 | Table I.Correlation of the expression of
HIF-1α with clinicopathological features of the NSCLC cases. |
Table I.
Correlation of the expression of
HIF-1α with clinicopathological features of the NSCLC cases.
|
|
| HIF1α expression |
|
|---|
|
|
|
|
|
|---|
| Characteristics | Total | − | + | ++ | +++ | P-value |
|---|
| Total | 187 | 21 | 73 | 67 | 26 |
|
| Gender |
|
|
|
|
| 0.5164 |
| Male | 136 | 15 | 52 | 43 | 16 |
|
|
Female | 51 | 6 | 21 | 24 | 10 |
|
| Age (years) |
|
|
|
|
| 0.6943 |
|
<57 | 92 | 13 | 34 | 34 | 11 |
|
|
≥57 | 95 | 8 | 39 | 33 | 15 |
|
| Tumor size
(cm) |
|
|
|
|
| 0.0452a |
| ≤3 | 86 | 9 | 46 | 24 | 7 |
|
|
>3 | 101 | 12 | 27 | 43 | 19 |
|
| Histological
type |
|
|
|
|
| 0.3427 |
|
Squamous cell carcinoma | 75 | 9 | 24 | 27 | 15 |
|
|
Adenocarcinoma | 112 | 12 | 49 | 40 | 11 |
|
| Lymph node
metastasis |
|
|
|
|
|
0.0049b |
| No | 85 | 13 | 41 | 25 | 6 |
|
|
Yes | 102 | 8 | 32 | 42 | 20 |
|
| Differentiation
status |
|
|
|
|
| 0.0012a |
|
Well | 43 | 12 | 23 | 6 | 2 |
|
|
Moderate | 81 | 6 | 37 | 29 | 9 |
|
|
Poor | 63 | 3 | 13 | 32 | 15 |
|
| TNM stage |
|
|
|
|
| 0.0003a |
|
I/II | 146 | 19 | 66 | 50 | 11 |
|
|
III/IV | 41 | 2 | 7 | 17 | 15 |
|
Expression of HIF-1α is upregulated by
LPS but downregulated by propofol in NSCLC cells
Considering that a close relationship between
expression of HIF-1α and severe inflammation is observed in
colorectal carcinoma cells, we aimed to ascertain whether LPS
stimulation leads to induction of this transcriptional factor in
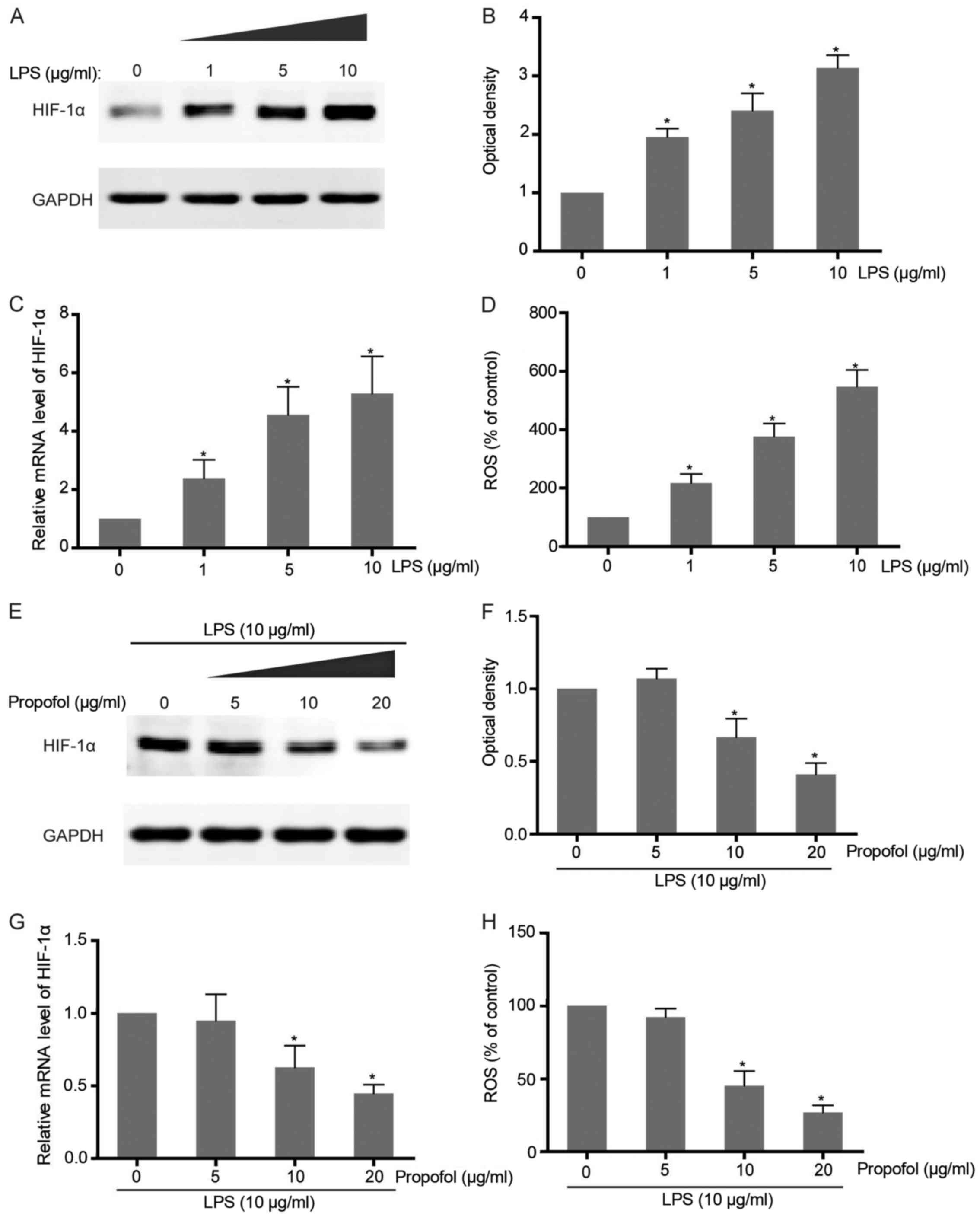
NSCLC cells. Exposure of A549 cells to different concentrations of
LPS caused a dose-dependent accumulation of HIF-1α at both the mRNA
and protein levels, which confirms that LPS-induced
pro-inflammation triggers the upregulation of HIF-1α expression
(Fig. 2A-C). As an inflammatory
indicator, LPS-induced ROS production in NSCLC cells was also found
to be observantly increased along with LPS addition (Fig. 2D). To further examine the effect of
propofol on LPS-activated HIF-1α expression, co-intervention
experiments were carried out with increasing concentrations of
propofol and HIF-1α inducer, LPS. The results showed that HIF-1α
expression promoted by LPS in the NSCLC cells was attenuated
following treatment with propofol in a dose-dependent manner
(Fig. 2E-G). Consistently,
LPS-induced ROS generation was also abrogated by propofol which
suggests its function as an antioxidant agent and inflammatory
suppressor (Fig. 2H).
LPS-induced protein stability and
nuclear accumulation of HIF-1α is attenuated by propofol
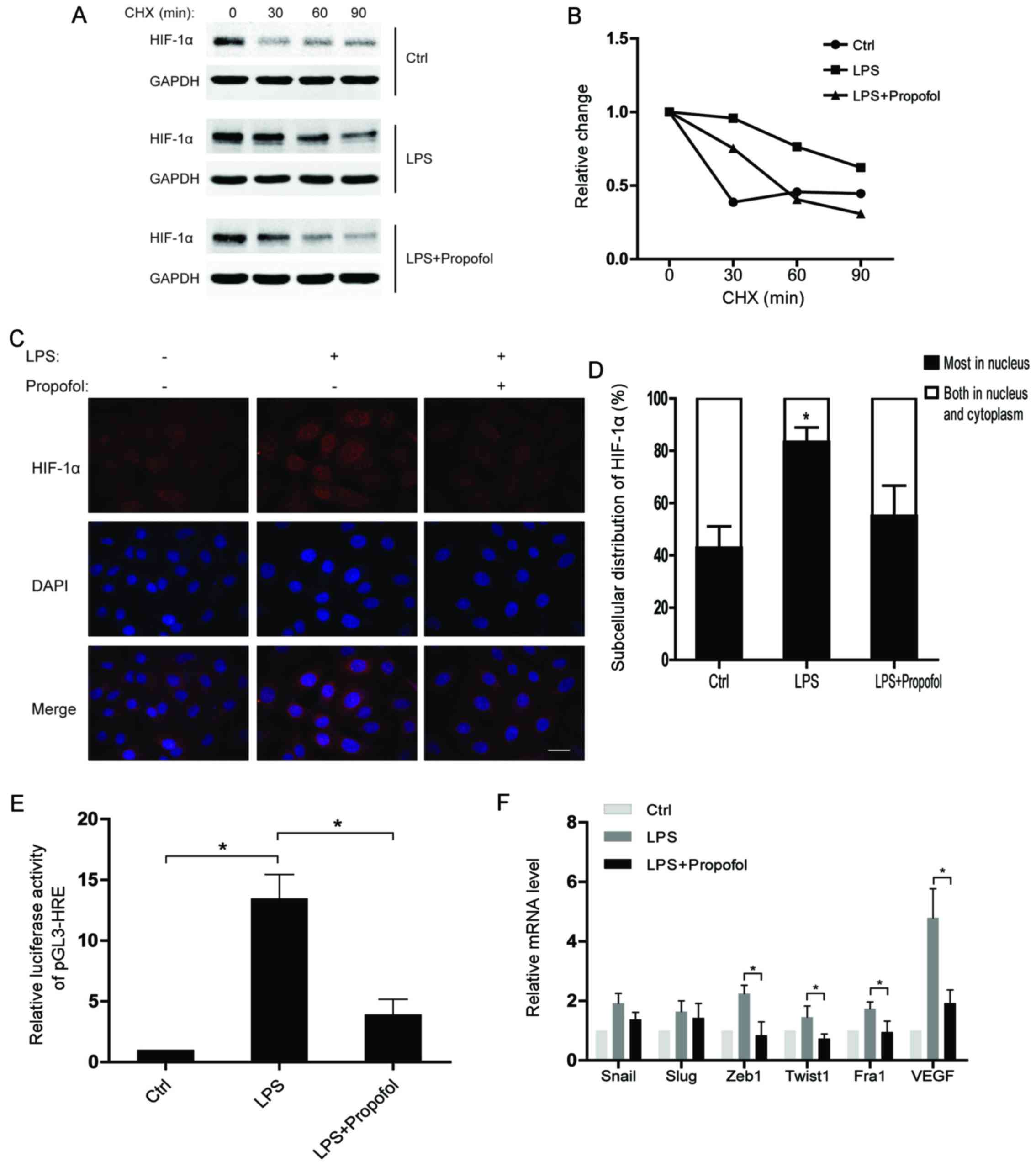
To understand the regulatory mechanisms of HIF-1α
under inflammatory conditions, we assessed the half-life of the
HIF-1α protein after the indicated treatments. The results showed
that the HIF-1α protein in the LPS-treated group had a much shorter
half-life than that noted in the control group, while the half-life
of HIF-1α in the LPS and propofol double-treated group was
decreased (Fig. 3A and B).
Moreover, translocation of HIF-1α from the cytoplasm to the nucleus
was observed in the NSCLC cells after LPS stimulation. As expected,
propofol significantly weakened the effect of LPS on subcellular
distribution of HIF-1α (Fig. 3C and
D). Consequently, propofol suppressed the LPS-induced
transcriptional activity of HIF-1α in the NSCLC cells (Fig. 3E). Notably, we found that the
expression of EMT-transcription factors (EMT-TFs) were upregulated
by LPS, whereas propofol suppressed the upregulation of the EMT-TFs
(Fig. 3F). In conclusion, propofol
inhibits the expression of HIF-1α-targeting genes which are
promoted by LPS via regulating the protein stability and
subcellular distribution of HIF-1α in NSCLC cells.
Propofol impairs LPS-induced migration
of NSCLC cells by decreasing HIF-1α expression
Based on previous studies indicating that LPS
induces EMT in cancer cells (19,20),
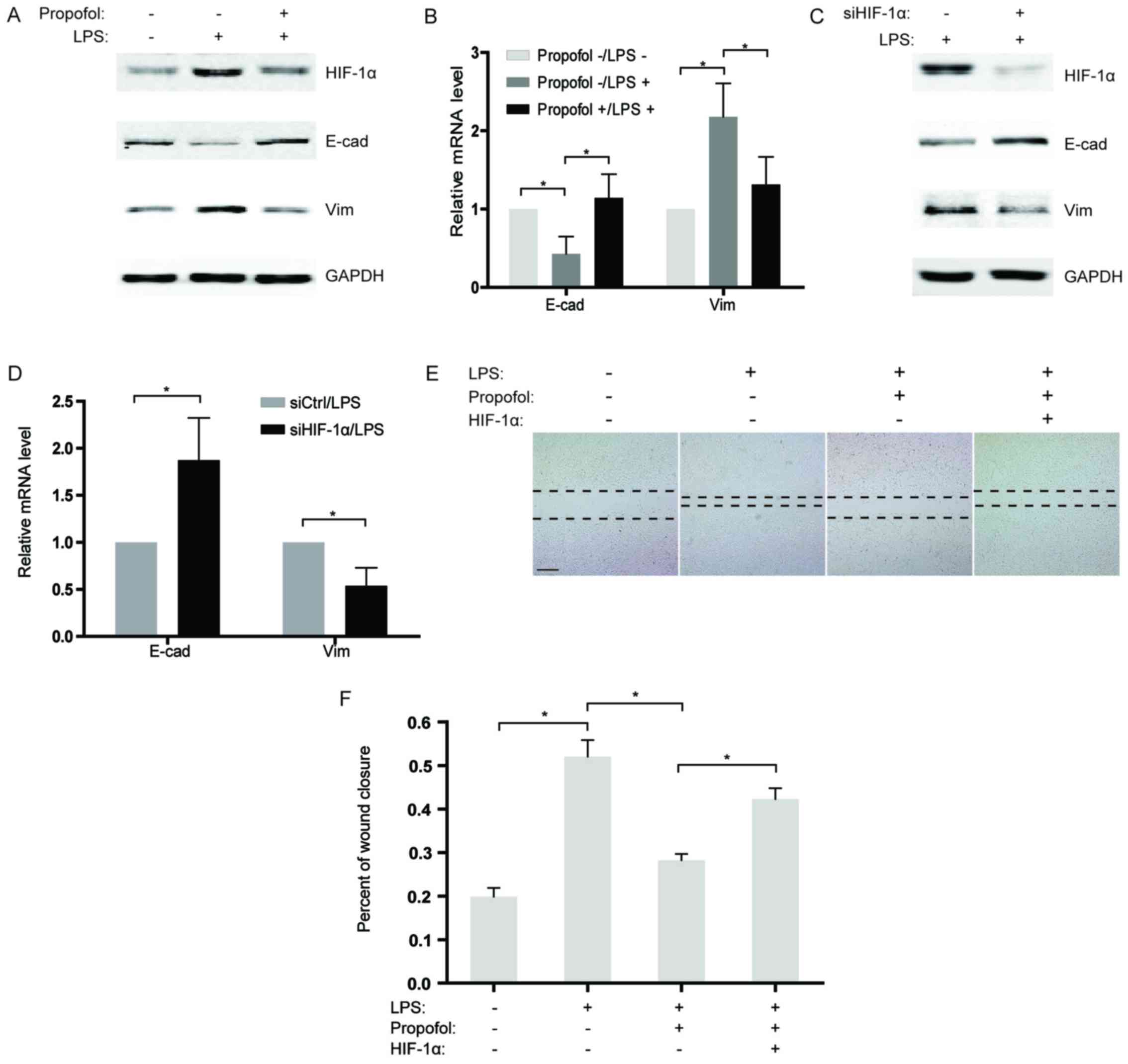
we sequentially analyzed the expression of two EMT markers,
E-cadherin and vimentin in the NSCLC cells after LPS stimulation.
Our findings revealed that the expression of E-cadherin was
significantly downregulated in the LPS-treated group compared to
that noted in the control, while vimentin expression was
substantially increased (Fig. 4A and
B). To further confirm the effects of propofol on LPS-induced
EMT, LPS-stimulated NSCLC cells were co-treated with propofol and
the expression of EMT markers was measured. As expected, propofol
reversed LPS-induced EMT, causing re-induction of E-cadherin and
suppression of vimentin expression (Fig. 4A and B). Considering that propofol
reduces the expression of HIF-1α induced by LPS, knockdown of
HIF-1α by siRNAs in NSCLC cells was performed under LPS
stimulation. The results showed that, similar to propofol,
knockdown of HIF-1α significantly blocked EMT activated by LPS
(Fig. 4C and D). Consistently, in a
wound healing assay, propofol weakened the LPS-induced capability
of migration in the NSCLC cells (Fig.
4E and F). Notably, overexpression of HIF-1α counteracted the
inhibitory effect of propofol on LPS-activated migration in the
NSCLC cells (Fig. 4E and F), which
demonstrated that propofol suppressed the LPS-promoted migration of
NSCLC cells by decreasing HIF-1α expression.
Propofol suppresses LPS-induced
invasion of NSCLC cells by diminishing HIF-1α expression
Although several studies have found that LPS could
enhance cell invasive capacity in human cancer cells (21), the underlying mechanisms have not
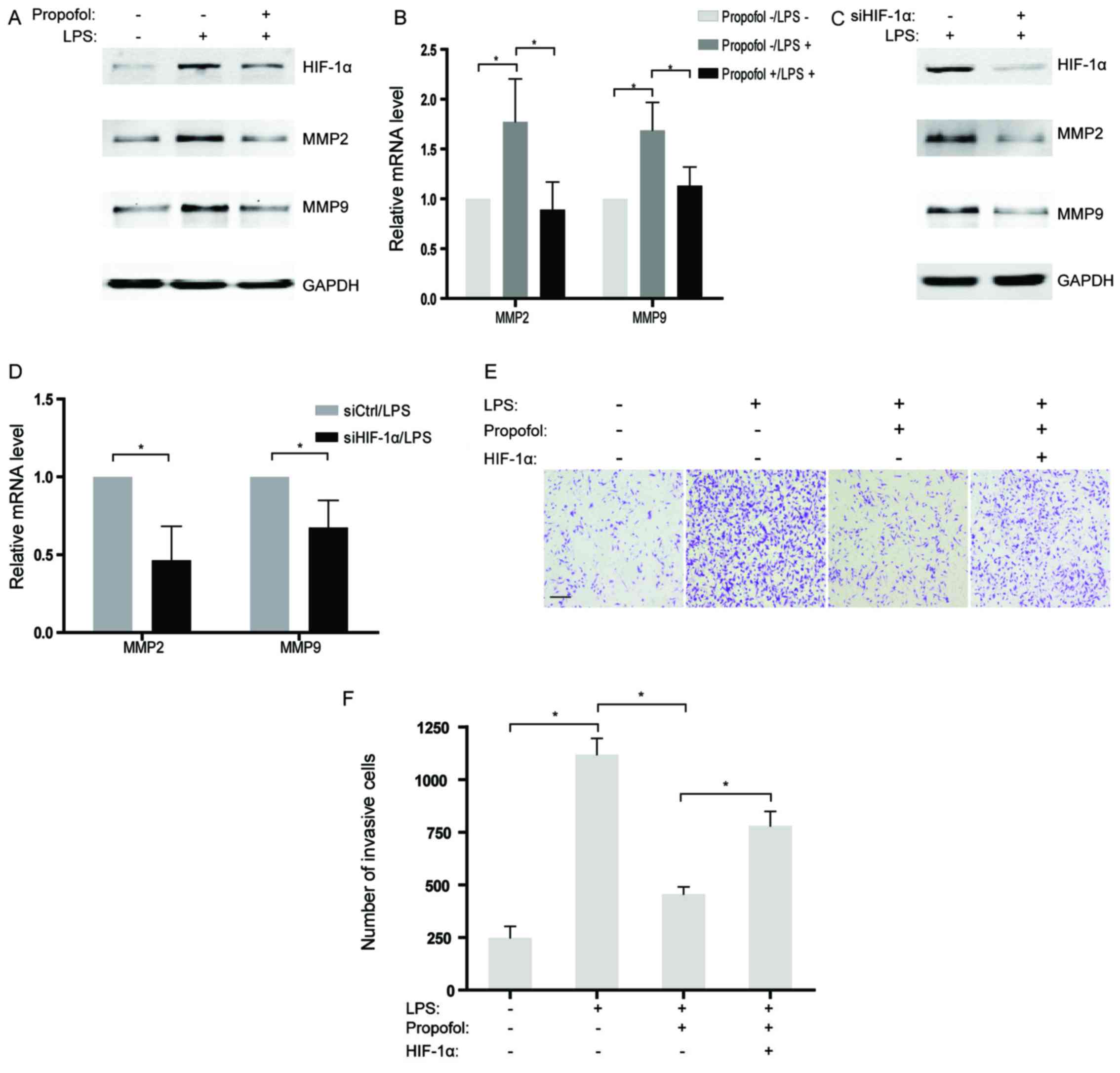
been well illustrated. In the present study, MMP2 and MMP9, two
genes related to invasion, were observed to be increased in the
NSCLC cells treated with LPS at both the mRNA and protein levels
(Fig. 5A and B). However, the
promotive influence of LPS on expression of MMP2 and MMP9 were
largely reversed by co-incubation with propofol in NSCLC cells
(Fig. 5A and B). Further studies
indicated that abrogation of HIF-1α by siRNAs also attenuated the
expression of MMP2 and MMP9 activated by LPS treatment in the NSCLC
cells (Fig. 5C and D), which
suggests that HIF-1α may play a role as a pivotal target of
propofol for inhibiting invasion-related genes. Moreover, the
interplay of LPS and propofol on the invasion of NSCLC cells in
vitro was evaluated by Transwell assays. The results showed
that the acquired ability of invasion in the NSCLC cells stimulated
by LPS was partly eliminated by treatment with propofol (Fig. 5E and F). Notably, overexpression of
HIF-1α rescued LPS-induced cell invasion which was suppressed by
propofol (Fig. 5E and F).
Therefore, it is reasonable to hypothesize that propofol suppressed
LPS-activated cell invasion by decreasing HIF-1α expression in the
LPS-treated NSCLC cells.
Discussion
Hypoxia-inducible factor 1α (HIF-1α) activation has
been demonstrated in cancer progression. Abnormal expression of
HIF-1α has been found in a variety of human cancers and is
associated with tumor growth, metastasis and poor prognosis
(8,22). As a hypoxia-dependent transcription
factor, HIF-1α regulates the expression of numerous genes involved
in angiogenesis, metabolism and proliferation (23,24).
Insufficient blood supply or inflammation can be one of the reasons
for the hypoxic environment during cancer development. Therefore,
HIF-1α is an important microenvironment regulator in tumor
progression (25). In the present
study, clinical analysis indicated a significant upregulation of
HIF-1α at both the mRNA and protein levels in lung cancer specimens
compared with levels noted in the normal tissues. Further
investigation of overall survival suggested that patients with high
HIF-1α expression had a relatively poor prognosis, which confirmed
the tumor-promoting role of HIF-1α in lung cancer.
More recent evidence suggests that inflammatory
stimuli accompanied by hypoxia can activate HIF-1α during the
history of malignant tumors (26).
Thus, to explore the potential mechanisms underlying the elevated
expression of HIF-1α in lung cancer, LPS was used to induce an
inflammatory response in NSCLC cells. Our findings indicated that
LPS treatment notably increased HIF-1α expression as well as the
level of ROS in the NSCLC cells. Notably, hypoxia has been shown in
previous studies to produce ROS which contributes to stabilizing
and activating HIF1 (27). However,
the mRNA level of HIF-1α is also induced by LPS, which implies a
complicated regulatory process for HIF-1α in response to LPS. To
further study the relationship between LPS-induced inflammation and
HIF-1α expression, propofol, an anti-inflammatory agent, was
applied to inhibit the cellular response to LPS. As expected,
propofol suppressed the upregulation of HIF-1α caused by LPS
stimulation in the NSCLC cells. Simultaneously, ROS production
generated after LPS addition was also decreased by propofol.
Although propofol has been verified to possess antioxidant property
(28,29), whether propofol inhibits HIF-1α
expression by eliminating ROS needs to be further investigated.
In addition to transcriptional regulation, the
functions of HIF-1α depend on its protein stability and subcellular
localization. Under a hypoxic condition, ubiquitination of HIF-1α
is suppressed and increased HIF-1α binds to HIF-1β to form the
HIF-1 transcription complex (30).
HIF1 then translocates to the nucleus, where it binds to the
hypoxic response element (HRE) within the promoters of its target
genes (31,32). In the present study, LPS was found
to enhance the stability of HIF-1α as well as its nuclear
localization. Meanwhile, propofol impaired the effects of LPS on
HIF-1α regulation. With regard to the promotive effect of ROS on
activating HIF-1α, our findings suggested that propofol may disrupt
the functions of HIF-1α via antagonizing LPS-induced ROS.
As an anesthetic, propofol is widely used in
relieving the pain of patients with chronic cancer as an adjuvant
therapy. In addition, our findings suggest that propofol suppresses
LPS-activated migration and invasion of NSCLC cells. Momentously,
overexpression of HIF-1α restored the capacities of migration and
invasion suppressed by propofol in NSCLC cells, which indicates
that HIF-1α is a pivotal regulator involved in the antitumor
characteristics of propofol. Notably, in breast cancer, propofol
was found to reduce MMP expression by inhibiting NF-κB activity
(33). Other evidence revealed that
phosphorylated ERK1/2 is markedly reduced after propofol treatment,
which leads to apoptosis in lung cancer (34). Therefore, the effects of propofol on
different cancer types involve various signaling pathways and
biological processes.
In conclusion, our findings demonstrated that LPS-
stimulated inflammation enhanced the functions of HIF-1α by
increasing the mRNA levels, protein stability and nuclear
localization of HIF-1α in NSCLC cells, which led to concomitant
increases in markers and mediators of migration and invasion.
Meanwhile, propofol treatment suppressed all of those effects and
appeared to do so by suppressing HIF-1α. As such, the present study
establishes a new mechanistic explanation for the effects of
propofol on lung cancer. However, in consideration of the aberrant
HIF-1α expression in lung cancer tissues, the present study
provides further evidence for the application of propofol in
treating patients with lung cancer.
References
|
1
|
Giatromanolaki A, Koukourakis MI, Sivridis
E, Pastorek J, Wykoff CC, Gatter KC and Harris AL: Expression of
hypoxia-inducible carbonic anhydrase-9 relates to angiogenic
pathways and independently to poor outcome in non-small cell lung
cancer. Cancer Res. 61:7992–7998. 2001.PubMed/NCBI
|
|
2
|
Giatromanolaki A, Koukourakis MI, Sivridis
E, Turley H, Talks K, Pezzella F, Gatter KC and Harris AL: Relation
of hypoxia inducible factor 1 alpha and 2 alpha in operable
non-small cell lung cancer to angiogenic/molecular profile of
tumours and survival. Br J Cancer. 85:881–890. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Le QT, Chen E, Salim A, Cao H, Kong CS,
Whyte R, Donington J, Cannon W, Wakelee H, Tibshirani R, et al: An
evaluation of tumor oxygenation and gene expression in patients
with early stage non-small cell lung cancers. Clin Cancer Res.
12:1507–1514. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beutler B: Inferences, questions and
possibilities in Toll-like receptor signalling. Nature.
430:257–263. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pchejetski D, Nunes J, Coughlan K, Lall H,
Pitson SM, Waxman J and Sumbayev VV: The involvement of sphingosine
kinase 1 in LPS-induced Toll-like receptor 4-mediated accumulation
of HIF-1α protein, activation of ASK1 and production of the
pro-inflammatory cytokine IL-6. Immunol Cell Biol. 89:268–274.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu WT, Jing YY, Yan F, Han ZP, Lai FB,
Zeng JX, Yu GF, Fan QM, Li R, Zhao QD, et al: LPS-induced
CXCR4-dependent migratory properties and a mesenchymal-like
phenotype of colorectal cancer cells. Cell Adh Migr. 0:1–11.
2016.
|
|
7
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl
Acad Sci USA. 92:5510–5514. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rankin EB and Giaccia AJ: Hypoxic control
of metastasis. Science. 352:175–180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang T, Gilkes DM, Takano N, Xiang L, Luo
W, Bishop CJ, Chaturvedi P, Green JJ and Semenza GL:
Hypoxia-inducible factors and RAB22A mediate formation of
microvesicles that stimulate breast cancer invasion and metastasis.
Proc Natl Acad Sci USA. 111:E3234–E3242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagaraju GP, Bramhachari PV, Raghu G and
El-Rayes BF: Hypoxia inducible factor-1α: Its role in colorectal
carcinogenesis and metastasis. Cancer Lett. 366:11–18. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tian H, Huang P, Zhao Z, Tang W and Xia J:
HIF-1α plays a role in the chemotactic migration of hepatocarcinoma
cells through the modulation of CXCL6 expression. Cell Physiol
Biochem. 34:1536–1546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen J, Gu Y, Shao Z, Luo J and Tan Z:
Propofol protects against hydrogen peroxide-induced oxidative
stress and cell dysfunction in human umbilical vein endothelial
cells. Mol Cell Biochem. 339:43–54. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kobayashi K, Yoshino F, Takahashi SS,
Todoki K, Maehata Y, Komatsu T, Yoshida K and Lee MC: Direct
assessments of the antioxidant effects of propofol medium chain
triglyceride/long chain triglyceride on the brain of stroke-prone
spontaneously hypertensive rats using electron spin resonance
spectroscopy. Anesthesiology. 109:426–435. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mammoto T, Mukai M, Mammoto A, Yamanaka Y,
Hayashi Y, Mashimo T, Kishi Y and Nakamura H: Intravenous
anesthetic, propofol inhibits invasion of cancer cells. Cancer
Lett. 184:165–170. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Altenburg JD, Harvey KA, McCray S, Xu Z
and Siddiqui RA: A novel 2,6-diisopropylphenyl-docosahexaenoamide
conjugate induces apoptosis in T cell acute lymphoblastic leukemia
cell lines. Biochem Biophys Res Commun. 411:427–432. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsuchiya M, Asada A, Arita K, Utsumi T,
Yoshida T, Sato EF, Utsumi K and Inoue M: Induction and mechanism
of apoptotic cell death by propofol in HL-60 cells. Acta
Anaesthesiol Scand. 46:1068–1074. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu KC, Yang ST, Hsia TC, Yang JS, Chiou
SM, Lu CC, Wu RS and Chung JG: Suppression of cell invasion and
migration by propofol are involved in down-regulating matrix
metalloproteinase-2 and p38 MAPK signaling in A549 human lung
adenocarcinoma epithelial cells. Anticancer Res. 32:4833–4842.
2012.PubMed/NCBI
|
|
18
|
Kapiszewska M, Cierniak A, Elas M and
Lankoff A: Lifespan of etoposide-treated human neutrophils is
affected by antioxidant ability of quercetin. Toxicol In Vitro.
21:1020–1030. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen MC, Chang WW, Kuan YD, Lin ST, Hsu HC
and Lee CH: Resveratrol inhibits LPS-induced epithelial-mesenchymal
transition in mouse melanoma model. Innate Immun. 18:685–693. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang T, Chen Z and Fang L: Curcumin
inhibits LPS-induced EMT through downregulation of NF-κB-Snail
signaling in breast cancer cells. Oncol Rep. 29:117–124.
2013.PubMed/NCBI
|
|
21
|
Yang H, Wang B, Wang T, Xu L, He C, Wen H,
Yan J, Su H and Zhu X: Toll-like receptor 4 prompts human breast
cancer cells invasiveness via lipopolysaccharide stimulation and is
overexpressed in patients with lymph node metastasis. PLoS One.
9:e1099802014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen S, Zhang M, Xing L, Wang Y, Xiao Y
and Wu Y: HIF-1α contributes to proliferation and invasiveness of
neuroblastoma cells via SHH signaling. PLoS One. 10:e01211152015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wilde BR and Ayer DE: Interactions between
Myc and MondoA transcription factors in metabolism and
tumourigenesis. Br J Cancer. 113:1529–1533. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wan J, Che Y, Kang N and Wu W: SOCS3
blocks HIF-1α expression to inhibit proliferation and angiogenesis
of human small cell lung cancer by downregulating activation of
Akt, but not STAT3. Mol Med Rep. 12:83–92. 2015.PubMed/NCBI
|
|
25
|
Ward C, Langdon SP, Mullen P, Harris AL,
Harrison DJ, Supuran CT and Kunkler IH: New strategies for
targeting the hypoxic tumour microenvironment in breast cancer.
Cancer Treat Rev. 39:171–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bredholt G, Mannelqvist M, Stefansson IM,
Birkeland E, Bø TH, Øyan AM, Trovik J, Kalland KH, Jonassen I,
Salvesen HB, et al: Tumor necrosis is an important hallmark of
aggressive endometrial cancer and associates with hypoxia,
angiogenesis and inflammation responses. Oncotarget. 6:39676–39691.
2015.PubMed/NCBI
|
|
27
|
Gatenby RA and Gillies RJ: Why do cancers
have high aerobic glycolysis? Nat Rev Cancer. 4:891–899. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Adaramoye OA, Akinwonmi O and Akanni O:
Effects of propofol, a sedative-hypnotic drug, on the lipid
profile, antioxidant indices, and cardiovascular marker enzymes in
wistar rats. ISRN Pharmacol. 2013:2302612013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Braz MG, Braz LG, Freire CM, Lucio LM,
Braz JR, Tang G, Salvadori DM and Yeum KJ: Isoflurane and propofol
contribute to increasing the antioxidant status of patients during
minor elective surgery: A randomized clinical study. Medicine.
94:e12662015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cockman ME, Masson N, Mole DR, Jaakkola P,
Chang GW, Clifford SC, Maher ER, Pugh CW, Ratcliffe PJ and Maxwell
PH: Hypoxia inducible factor-alpha binding and ubiquitylation by
the von Hippel-Lindau tumor suppressor protein. J Biol Chem.
275:25733–25741. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Semenza GL, Agani F, Booth G, Forsythe J,
Iyer N, Jiang BH, Leung S, Roe R, Wiener C and Yu A: Structural and
functional analysis of hypoxia-inducible factor 1. Kidney Int.
51:553–555. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chachami G, Paraskeva E, Mingot JM,
Braliou GG, Görlich D and Simos G: Transport of hypoxia-inducible
factor HIF-1alpha into the nucleus involves importins 4 and 7.
Biochem Biophys Res Commun. 390:235–240. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Q, Zhang L, Han Y, Jiang Z and Wang Q:
Propofol reduces MMPs expression by inhibiting NF-κB activity in
human MDA-MB-231 cells. Biomed Pharmacother. 66:52–56. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Song J, Shen Y, Zhang J and Lian Q: Mini
profile of potential anticancer properties of propofol. PLoS One.
9:e1144402014. View Article : Google Scholar : PubMed/NCBI
|