Introduction
Lung cancer is the second most common lung tumor in
humans and is characterized by uncontrolled cell growth in tissues
of the lung and has been reported to be the major cause of
cancer-related mortality in China (1). Non-small cell lung cancer (NSCLC) is a
type of epithelial lung cancer and accounts for approximately 85%
of all lung cancers (2). NSCLC
patients undergoing complete resection have a 40–70% 5-year overall
survival and chemotherapy administered after complete resection
improves overall survival at 5 years by approximately 5% (3). Therefore, studies concerning the
molecular mechanisms underlying the occurrence and progression of
NSCLC may have a significant impact on the systematic treatment of
this disease.
C-C chemokine receptor type 7 (CCR7), a G
protein-coupled receptor, is mainly expressed on immune cells and
mediates leukocyte adhesion and chemotaxis from peripheral sites of
inflammation through lymphatic channels to secondary lymphoid
organs (4,5). Recently, the role of CCR7 in
tumorigenesis has attracted attention in oncology research, as
aberrant CCR7 expression has been identified in certain tumor types
and has been linked to pro-survival and invasive pathways. Hong
et al reported that CCR7 is highly expressed in gallbladder
cancer and mediates the TNF-α-induced lymphatic metastasis of
gallbladder cancer (6). In NSCLC,
CCR7 activation by its specific ligand, exogenous chemokine ligand
21 (CCL21), prevented apoptosis by upregulating the expression of
Bcl-2 and inhibiting the expression of Bax and caspase-3 in NSCLC
A549 and H460 cells (7). Thus, CCR7
may serve as a novel prognostic biomarker and therapeutic target
for NSCLC. However the effect of CCR7 downregulation in NSCLC has
not been well characterized. In this study, we investigated the
effects of the upregulation and silencing of CCR7 on cell apoptosis
and the potential signaling mechanism in human lung cancer cell
line A549. In addition, transforming growth factor β1 (TGF-β1) was
used to induce epithelial-mesenchymal transition (EMT) in A549
cells and the function of CCR7 in EMT was also investigated.
Materials and methods
Reagents
Anti-GAPDH antibody (ab8245), anti-PCNA antibody
(ab29), anti-Akt antibody (ab8805), anti-p-Akt antibody (ab131443),
anti-IκBα antibody (ab32518), anti-NF-κB p65 antibody (ab86299),
anti-CCR7 antibody (ab32527), anti-p53 antibody [DO-1] (ab1101),
anti-Bax antibody [E63] (ab32503), anti-Bcl-2 antibody [E17]
(ab32124), anti-caspase-3 antibody (ab13847), anti-vimentin
antibody [RV202] (ab8978), anti-N-cadherin antibody (ab18203),
anti-E-cadherin antibody [HECD-1] (ab1416), and anti-keratin
antibody [C-11] (ab118817) were obtained from Abcam (Cambridge,
UK). Pyrrolidine dithiocarbamate (PDTC) was purchased from
Calbiochem (San Diego, CA, USA).
Cell culture
Human lung cancer A549 cells were cultured in
DMEM-F12 supplemented with 10% fetal bovine serum (FBS) (Gibco,
Gaithersburg, MD, USA) and 1% penicillin in a humidified 5%
CO2 atmosphere at 37°C. Cells were seeded at
1×104 cells/well into 96-well plates and allowed to
attach overnight. Cell viability was assessed by the CKK-8 assay
(Sigma-Aldrich, St. Louis, MO, USA). Briefly, cells were treated
with 1, 10, 20, 50, 100 and 200 nM CCL21 (Peprotech, Rocky Hill,
NJ, USA) for 24 h and then assayed.
CCR7-siRNA tranfection
Human CCR7-siRNA was obtained from Guangzhou RiboBio
Co. Ltd., (Guangzhou, China) and the sequences were in accordance
with a previous study (7). Cells
were cultured in 6-well plates and grown to 30–50% confluence
before transfection. The duplexes were diluted to give a final
concentration of 30 nM. The siRNA was transfected into cells using
Lipofectamine RNAiMAX reagent according to the manufacturer's
instructions (Invitrogen Life Technologies, Carlsbad, CA, USA).
Western blotting
Total proteins from 10-cm dishes
(106-107 cells) were extracted using protein
extraction reagents (Thermo Fisher Scientific Inc., Waltham, MA,
USA). The nuclear proteins from 10-cm dishes
(106-107 cells) were extracted using a
CelLytic™ NuCLEAR™ extraction kit (Sigma-Aldrich). Proteins samples
were quantified using a BCA protein assay kit (Beyotime Institute
of Biotechnology, Jiangsu, China) and denaturated with SDS-PAGE
sample loading buffer (Beyotime). Proteins (30–50 µg) were
separated by reducing SDS-PAGE electrophoresis. Then the proteins
were transferred onto a PVDF membrane (Millipore, Billerica, MA,
USA) and blocked with 5% non-fat milk in Tris-Tween buffered saline
buffer for 1.5 h. The primary antibody was incubated overnight at
4°C and the HRP-conjugated secondary antibodies were subsequently
incubated for 2 h at room temperature. Then the blots were
developed on the membrane using Alpha Imager 2200 software (Alpha
Innotech Corporation, San Leandro, CA, USA). We digitally
quantified the resultant signals and normalized the data to GAPDH
or PCNA abundance.
Real-time PCR
Inflammatory cytokines were quantitatively detected
by real-time PCR. Approximately 1×106 cells/ml from each
group were collected from 6-well plates for real-time PCR
detection. The gene sequences were used to design primers and were
synthesized by Invitrogen Life Technologies (Table I). Double-distilled water was used
instead of a template as a negative control. The number of β-actin
transcripts was used as a reference of endogenous RNA, and the
quantification of test genes for each sample was standardized
relative to the number of β-actin transcripts. The
2−∆∆Ct cycle threshold formula was used to calculate the
relative abundance of the transcripts.
 | Table I.Primers used in this study. |
Table I.
Primers used in this study.
| Gene | Accession no. | Nucleotide sequence
of primers (5′-3′) | Size (bp) |
|---|
| β-actin | NM_007393.3 | F:
GTCCACCTTCCAGCAGATGT | 117 |
|
|
| R:
GAAAGGGTGTAAAACGCAGC |
|
| IL-1β | NM_008361.3 | F:
CTGTGACTCGTGGGATGATG |
|
|
|
| R:
GGGATTTTGTCGTTGCTTGT | 213 |
| IL-6 | NM_031168.1 | F:
TGCAAGAGACTTCCATCCAGT |
|
|
|
| R:
GTGAAGTAGGGAAGGCCG | 116 |
| IL-10 | NM_010548.2 | F:
ACAGCCGGGAAGACAATAAC |
|
|
|
| R:
CAGCTGGTCCTTTGTTTGAAAG | 116 |
| IL-17 | NM_010552.3 | F:
TACCTCAACCGTTCCACGTC |
|
|
|
| R:
TTTCCCTCCGCATTGACAC | 119 |
| TNF-α | NM_013693.2 | F:
AGGCACTCCCCCAAAAGAT |
|
|
|
| R:
TGAGGGTCTGGGCCATAGAA | 192 |
Statistical analysis
All data were analyzed by SPSS 17.0 software.
Differences were assessed by Ducan's multiple comparison test. Data
are expressed as the mean ± SEM. Values in the same row with
different superscripts are significant (P<0.05).
Results
Effects of CCR7 on cell apoptosis in
A549 cells
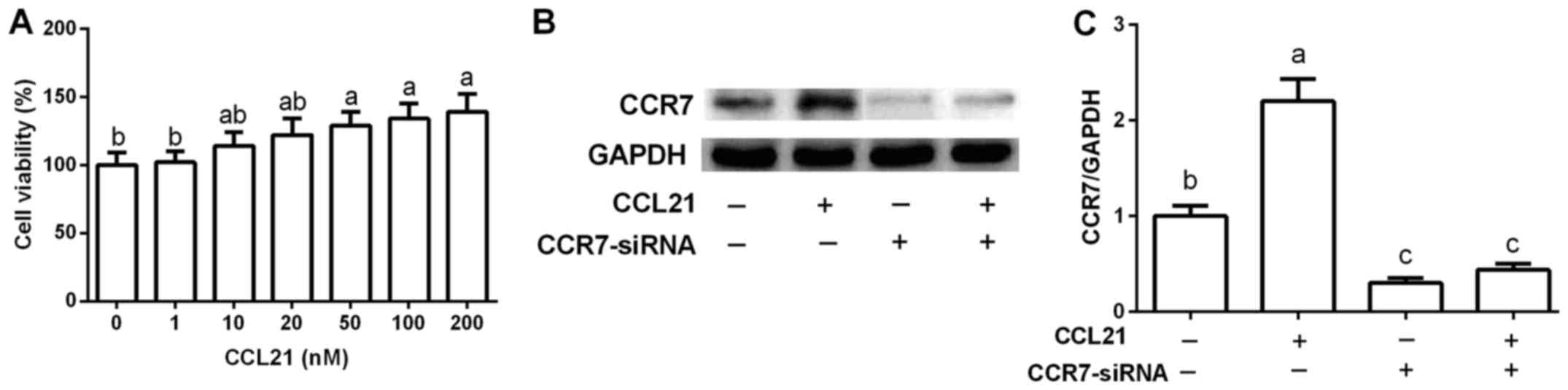
CCL21 is a ligand of CCR7 and has been suggested to
upregulate CCR7 expression (7). As
shown in Fig. 1A, CCL21 increased
cell viability in a dose-dependent manner. After exposure to 50,
100 and 200 nM of CCL21, cell proliferation was markedly promoted
compared with that noted in the control group (P<0.05). A
significant difference was observed at 50 nM, and was thus used to
upregulate CCR7 expression (P<0.05) for the following analysis
(Fig. 1B and C). A549 cells were
transfected with CCR7-siRNA to inhibit CCR7 expression. The results
revealed that CCL21 treatment markedly enhanced CCR7 expression,
while CCR7-siRNA suppressed its expression (Fig. 1B and C).
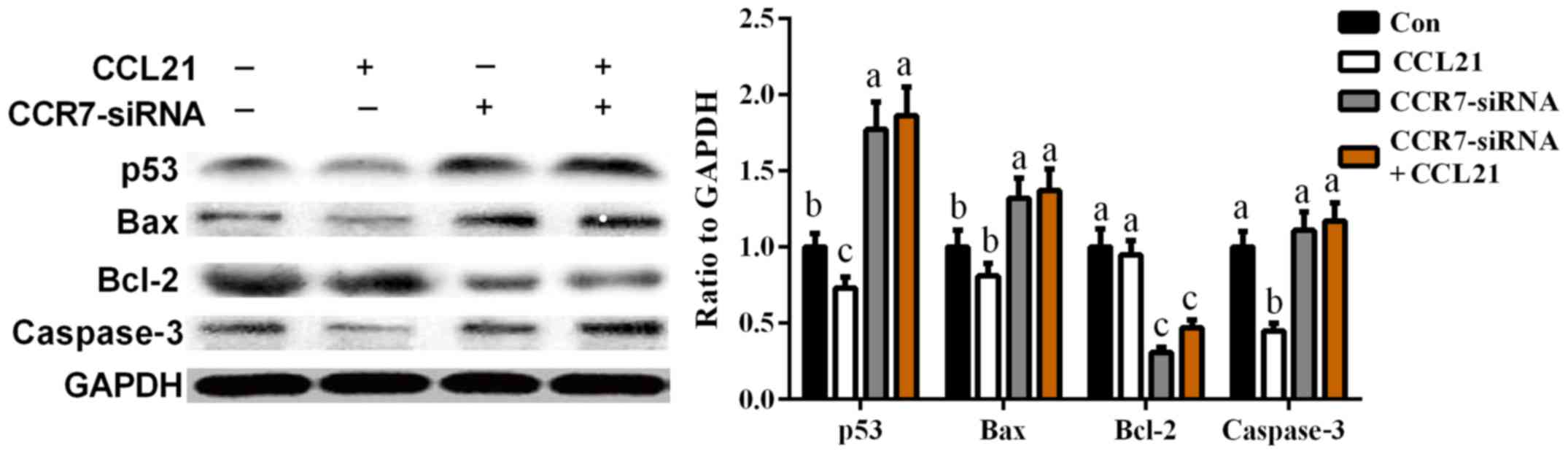
Effects of CCR7 on apoptosis-related
proteins in A549 cells
To further investigate the mechanism of
CCR7-mediated cell apoptosis, four apoptosis-related proteins (p53,
Bax, Bcl-2 and caspase-3) were analyzed after CCR7 overexpression
and inhibition in A549 cells. As shown in Fig. 2, CCR7 overexpression markedly
inhibited p53 and caspase-3 expression (P<0.05), while
CCR7-siRNA enhanced cellular abundance of p53, Bax, and caspase-3
(P<0.05). Meanwhile, the expression of Bcl-2, an anti-apoptotic
protein, was significantly decreased after CCR7-siRNA transfection
when compared with that in the control group (P<0.05).
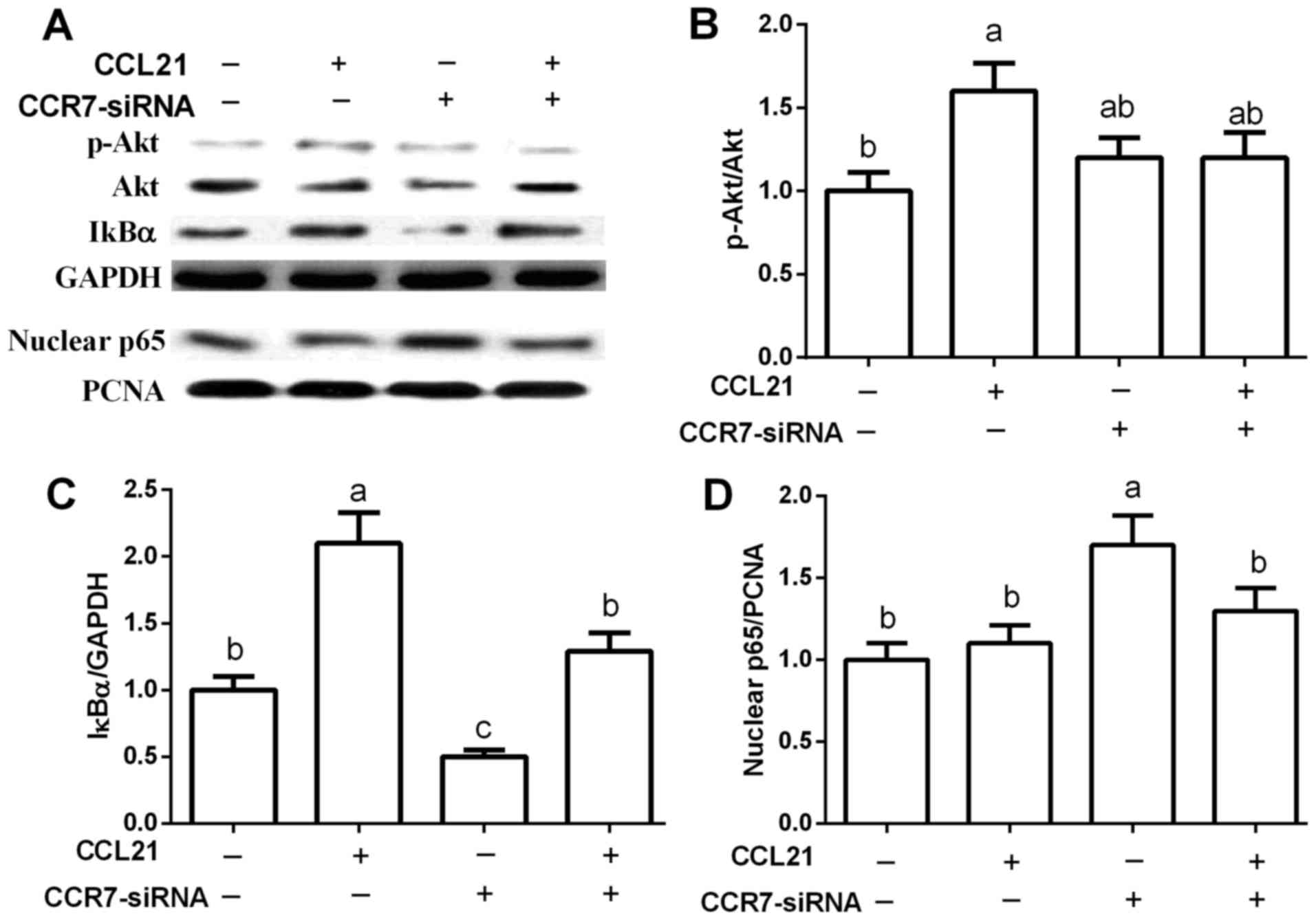
Effects of CCR7 on Akt and NF-κB
signals in A549 cells
Akt and NF-κB are widely associated with apoptosis.
In this study, we found that CCR7 upregulation markedly activated
Akt signaling as evidenced by the increased Akt phosphorylation
(p<0.05) (Fig. 3A and B), while
CCR7-siRNA failed to influence Akt (P>0.05).
Furthermore, CCR7 upregulation enhanced IκBα
expression while CCR7-siRNA inhibited the expression of IκBα
(P<0.05) (Fig. 3A and C), an
inhibitory protein of the NF-κB pathway. Thus, we further
determined nuclear NF-κB p65 abundance and the results revealed
that CCR7-siRNA markedly increased the expression level of nuclear
p65 (P<0.05) (Fig. 3A and
D).
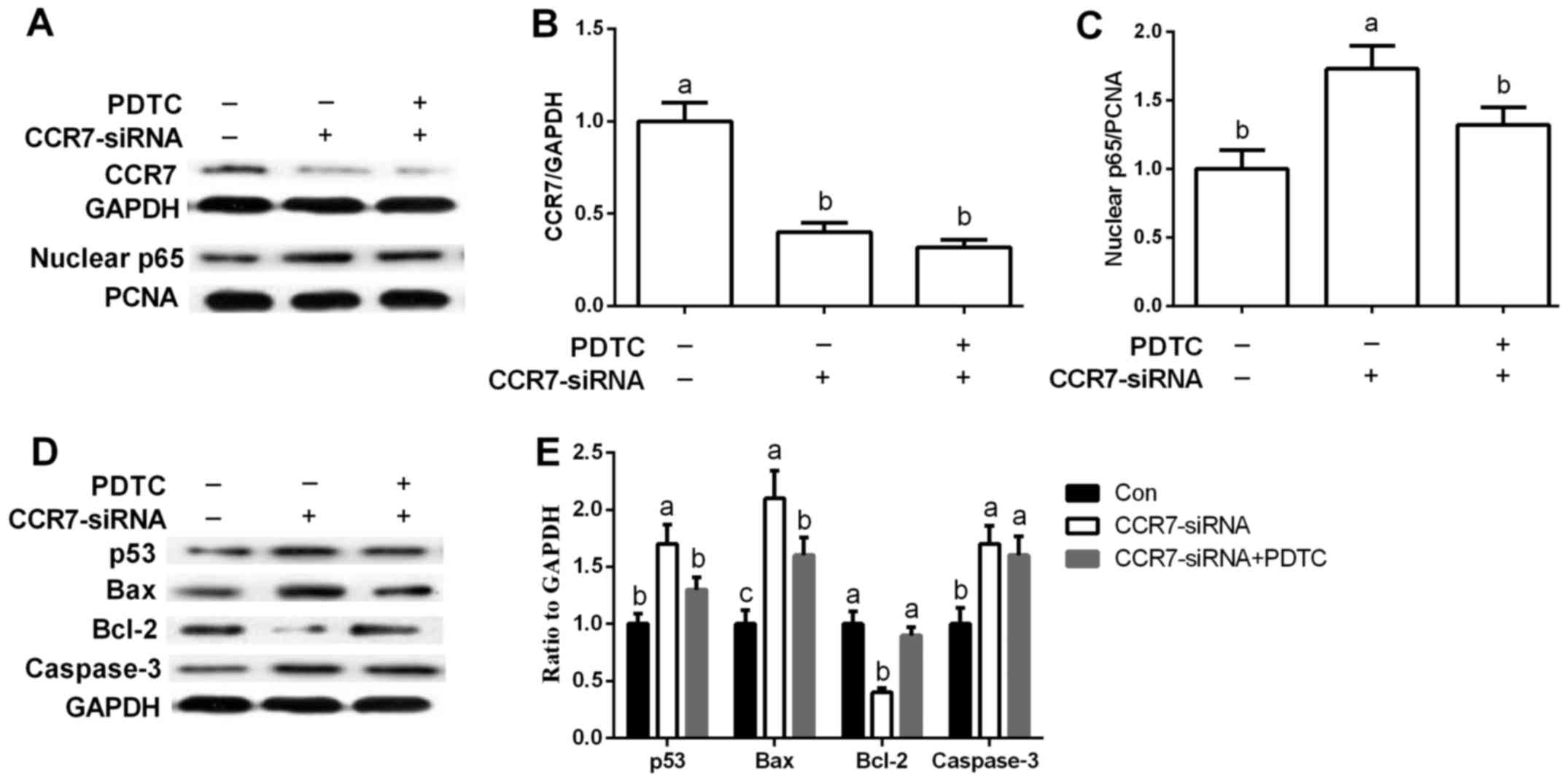
Effect of NF-κB signaling on
CCR7-mediated apoptosis in A549 cells
PDTC was used to inhibit NF-κB signaling in A549
cells. CCR7-siRNA markedly inhibited CCR7 expression and enhanced
nuclear p65 abundance (P<0.05), while PDTC, a special antagonist
of NF-κB, suppressed NF-κB activation induced by CCR7 inhibition
(P<0.05) (Fig. 4A-C).
Inhibition of NF-κB via PDTC treatment markedly
downregulated p53 and Bax when compared with the levels noted
following CCR7-siRNA treatment (P<0.05) (Fig. 4D and E). Meanwhile, Bcl-2 abundance
was significantly higher after PTDC exposure than that in the
CCR7-siRNA group (P<0.05) (Fig. 4D
and E).
Effects of CCR7 on inflammatory
cytokines in A549 cells
NF-κB signaling has been widely demonstrated to
regulate inflammatory cytokines. Thus cellular expression of IL-1β,
IL-6, IL-10, IL-17 and TNF-α was determined via RT-PCR. The results
revealed that CCL21 treatment markedly upregulated IL-1β and IL-10
expression (P<0.05) (Table II),
suggesting that overexpression of CCR7 promoted the inflammatory
response. However, CCR7-siRNA treatment significantly suppressed
IL-1β and IL-10 expression (P<0.05) (Table II).
 | Table II.Effects of CCR7 on the expression of
proinflammatory cytokines via RT-PCR. |
Table II.
Effects of CCR7 on the expression of
proinflammatory cytokines via RT-PCR.
| Item | Con | CCL21 | CCR7-siRNA | CCR7-siRNA+CCL21 |
|---|
| IL-1β |
1.00±0.12b |
1.62±0.17a |
0.86±0.09b |
1.04±0.13b |
| IL-6 | 1.00±0.15 | 1.20±0.16 | 1.34±0.25 | 1.23±0.13 |
| IL-10 |
1.00±0.11b |
1.40±0.14a |
1.09±0.09b |
1.12±0.15b |
| IL-17 | 1.00±0.19 | 1.31±0.23 | 0.93±0.12 | 1.12±0.21 |
| TNF-α | 1.00±0.23 | 0.95±0.21 | 1.12±0.16 | 1.17±0.24 |
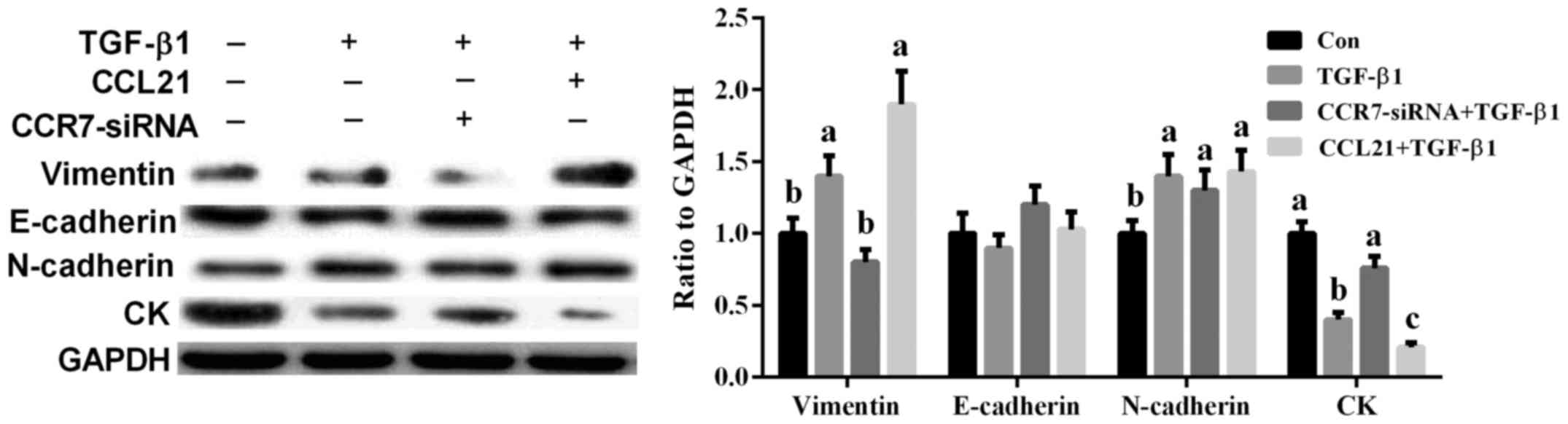
Effects of CCR7 on TGF-β1-induced EMT
in A549 cells
TGF-β1 (20 ng/ml) was used to induce EMT in A549
cells according to a previous study (8). Vimentin (a mesenchymal cell marker),
CK (an epithelial cell marker), N-cadherin, and E-cadherin have
been widely used to evaluate EMT (8,9). The
results showed that TGF-β1 markedly induced cell EMT as evidenced
by the increased vimentin and N-cadherin and decreased CK
(P<0.05) (Fig. 5). Meanwhile,
CCR7-siRNA treatment significantly alleviated TGF-β1-induced EMT in
A549 cells via mediating vimentin and CK expression (P<0.05)
(Fig. 5).
Inflammatory response in
TGF-β1-induced EMT in A549 cells
TGF-β1 treatment induced a cell inflammatory
response via upregulation of IL-1β, IL-17, and TNF-α (P<0.05)
(Table III), while CCR7-siRNA
treatment significantly alleviated IL-1β and TNF-α expression after
TGF-β1 exposure (P<0.05) (Table
III).
 | Table III.Effects of TGF-β1 and CCR7 on the
expression of proinflammatory cytokines via RT-PCR. |
Table III.
Effects of TGF-β1 and CCR7 on the
expression of proinflammatory cytokines via RT-PCR.
| Item | Con | TGF-β1 |
TGF-β1+CCR7-siRNA | TGF-β1+CCL21 |
|---|
| IL-1β |
1.00±0.11b |
1.93±0.21a |
1.44±0.16b |
2.33±0.25a |
| IL-6 | 1.00±0.17 | 1.33±0.21 | 1.23±0.17 | 1.38±0.25 |
| IL-10 | 1.00±0.14 | 1.13±0.16 | 1.17±0.21 | 1.32±0.19 |
| IL-17 |
1.00±0.12b |
1.56±0.19a |
1.23±0.16a,b |
1.67±0.22a |
| TNF-α |
1.00±0.14b |
1.46±0.14a |
0.96±0.09b |
1.77±0.28a |
Discussion
Although previous studies suggest that CCR7 is
involved in carcinogenesis (10,11),
the functions and underlying mechanisms of CCR7 in NSCLC are still
largely obscure. In this study, we investigated the effects of CCR7
activation and silencing on apoptosis and the signaling mechanism
in A549 cells. The results revealed that CCR7 upregulation by CCL21
promoted cell proliferation, while CCR7 inhibition through siRNA
treatment accelerated cell apoptosis and the signaling mechanism
may be associated with the NF-κB pathway. To our knowledge, this is
the first study demonstrating the effect of CCR7 on apoptosis in an
NSCLC cell model, which may serve as a potential tumor marker or a
therapeutic target for NSCLC.
CCR7 has been widely investigated in the immune
response and previous studies suggest that CCR7 is involved in
T-cell homeostasis, activation and polarization (12,13).
Recent studies indicate an effect of CCR7 in carcinogenesis
(10,11). In this study, we confirmed that CCR7
is involved in cell apoptosis in A549 cells. CCL21 is a ligand of
CCR7 and has been suggested to upregulate CCR7 expression (7,14).
CCL21 treatment markedly enhanced CCR7 expression, which further
inhibits cell apoptosis. Mo et al reported that
CCL21-mediated CCR7 expression exhibited an antiapoptotic activity
in human bladder cancer T24 cells via regulation of Bcl-2 and Bax
proteins (15), while inhibition of
CCR7 via RNA interference led to a significant inhibition of
prostate cancer cell proliferation, migration and invasion
(16). In NSCLC, CCR7 activation
promoted G2/M phase progression and upregulated vascular
endothelial growth factor-D expression via the ERK pathway
(17–19). However, little is known concerning
the effect of CCR7 inhibition on NSCLC. In this study, A549 cells
transfected with CCR7-siRNA showed marked cell apoptosis and
CCR7-siRNA transfection influenced apoptosis-related genes, such as
p53, Bax, Bcl-2 and caspase-3.
Akt has been demonstrated to be involved in cell
growth, proliferation, apoptosis and neoangiogenesis (20,21).
In this study, we found that upregulation of CCR7 activated Akt
signaling as evidenced by the increased Akt phosphorylation, which
is similar with previous studies which reported that CCL21/CCR7 is
associated with Akt signaling (22,23).
However, CCR7-siRNA treatment failed to influence Akt, thus we
speculated that inhibition of CCR7-mediated cell apoptosis may not
be associated with Akt signaling.
To elucidate the underlying mechanisms involved in
CCR7-mediated cell apoptosis, NF-κB signaling was further
investigated after CCR7 upregulation and silencing. The results
revealed that CCR7-siRNA markedly activated the NF-κB pathway.
Thus, PDTC, a specific antagonist of NF-κB, was used to inhibit
NF-κB after CCR7-siRNA treatment, which suggested that
CCR7-mediated cell apoptosis in A549 cells may be associated with
NF-κB signaling. Similarly, Kuwabara et al reported that
CCR7 ligands upregulate IL-23 via the NF-κB pathway in dendritic
cells (24). NF-κB signaling has
been widely demonstrated to regulate inflammatory cytokines
(25,26) and the present data indicated that
NF-κB was involved in CCR7-mediated apoptosis. Thus, inflammatory
cytokines were determined after CCR7 upregulation and inhibition
and the results revealed that inhibition of CCR7 suppressed CCL21
and TGF-β1-induced inflammation, indicating that CCR7 mediates
inflammation-associated tumor progression. These results are
similar with previous studies (4,27).
EMT has been considered to be a key process
promoting tumor metastasis in epithelial cancers (28,29).
In this study, TGF-β1 was used to induce EMT and inhibition of CCR7
suppressed TGF-β1-induced EMT in A549 cells via the mediation of
vimentin and CK expression. Similarly, Li et al reported
that the CCL21/CCR7 axis is involved in the EMT process during
chemotaxis of breast carcinoma cells and knockdown of CCR7 by shRNA
suppressed tumor cell invasion, migration and the EMT phenotype
(30). In gastric cancer, CCR7
promoted Snail expression to induce EMT, resulting in cell cycle
progression, migration, and invasion in gastric cancer (11). Thus, the CCR7-EMT pathway may
provide a potential regimen for cancer therapy, especially in
NSCLC.
In conclusion, upregulation of CCR7 promotes cell
proliferation and inflammation in A549 cells. However, silencing of
CCR7 via siRNA treatment promoted cell apoptosis and suppressed the
inflammatory response and TGF-β1-induced EMT, which may be
associated with NF-κB signaling.
References
|
1
|
Chen D, Guo W, Qiu Z, Wang Q, Li Y, Liang
L, Liu L, Huang S, Zhao Y and He X: MicroRNA-30d-5p inhibits tumour
cell proliferation and motility by directly targeting CCNE2 in
non-small cell lung cancer. Cancer Lett. 362:208–217. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Facchinetti F, Marabelle A, Rossi G, Soria
JC, Besse B and Tiseo M: Moving immune checkpoint blockade in
thoracic tumors beyond non-small cell lung cancer. J Thorac Oncol.
11:1819–1836. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Naylor EC: Adjuvant therapy for stage I
and II non-small cell lung cancer. Surg Oncol Clin N Am.
25:585–599. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mburu YK, Wang J, Wood MA, Walker WH and
Ferris RL: CCR7 mediates inflammation-associated tumor progression.
Immunol Res. 36:61–72. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Worbs T and Förster R: A key role for CCR7
in establishing central and peripheral tolerance. Trends Immunol.
28:274–280. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hong H, He C, Zhu S, Zhang Y, Wang X, She
F and Chen Y: CCR7 mediates the TNF-α-induced lymphatic metastasis
of gallbladder cancer through the ‘ERK1/2 - AP-1’ and ‘JNK - AP-1’
pathways. J Exp Clin Cancer Res. 35:512016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu Y, Liu L, Qiu X, Liu Z, Li H, Li Z, Luo
W and Wang E: CCL21/CCR7 prevents apoptosis via the ERK pathway in
human non-small cell lung cancer cells. PLoS One. 7:e332622012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma H, Gao L, Li S, Qin J, Chen L, Liu X,
Xu P, Wang F, Xiao H, Zhou S, et al: CCR7 enhances TGF-β1-induced
epithelial-mesenchymal transition and is associated with lymph node
metastasis and poor overall survival in gastric cancer. Oncotarget.
6:24348–24360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kallergi G, Papadaki MA, Politaki E,
Mavroudis D, Georgoulias V and Agelaki S: Epithelial to mesenchymal
transition markers expressed in circulating tumour cells of early
and metastatic breast cancer patients. Breast Cancer Res.
13:R592011. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tutunea-Fatan E, Majumder M, Xin X and
Lala PK: The role of CCL21/CCR7 chemokine axis in breast
cancer-induced lymphangiogenesis. Mol Cancer. 14:352015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang J, Zhou Y and Yang Y: CCR7 pathway
induces epithelial-mesenchymal transition through up-regulation of
Snail signaling in gastric cancer. Med Oncol. 32:4672015.PubMed/NCBI
|
|
12
|
Moschovakis GL and Förster R: Multifaceted
activities of CCR7 regulate T-cell homeostasis in health and
disease. Eur J Immunol. 42:1949–1955. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saban DR: The chemokine receptor CCR7
expressed by dendritic cells: A key player in corneal and ocular
surface inflammation. Ocul Surf. 12:87–99. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cai W, Tao J, Zhang X, Tian X, Liu T, Feng
X, Bai J, Yan C and Han Y: Contribution of homeostatic chemokines
CCL19 and CCL21 and their receptor CCR7 to coronary artery disease.
Arterioscler Thromb Vasc Biol. 34:1933–1941. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mo M, Zhou M, Wang L, Qi L, Zhou K, Liu
LF, Chen Z and Zu XB: CCL21/CCR7 enhances the proliferation,
migration, and invasion of human bladder cancer T24 cells. PLoS
One. 10:e01195062015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chi BJ, Du CL, Fu YF, Zhang YN and Wang
RW: Silencing of CCR7 inhibits the growth, invasion and migration
of prostate cancer cells induced by VEGFC. Int J Clin Exp Pathol.
8:12533–12540. 2015.PubMed/NCBI
|
|
17
|
Xu Y, Liu L, Qiu X, Jiang L, Huang B, Li
H, Li Z, Luo W and Wang E: CCL21/CCR7 promotes G2/M phase
progression via the ERK pathway in human non-small cell lung cancer
cells. PLoS One. 6:e211192011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun L, Zhang Q, Li Y, Tang N and Qiu X:
CCL21/CCR7 up-regulate vascular endothelial growth factor-D
expression via ERK pathway in human non-small cell lung cancer
cells. Int J Clin Exp Pathol. 8:15729–15738. 2015.PubMed/NCBI
|
|
19
|
Wang M, Jing Y, Hu L, Gao J, Ding L and
Zhang J: Recent advances on the circadian gene PER2 and metabolic
rhythm of lactation of mammary gland. Anim Nutr. 1:257–261.
2015.doi: 10.1016/j.aninu.2015.11.008. View Article : Google Scholar
|
|
20
|
Nitulescu GM, Margina D, Juzenas P, Peng
Q, Olaru OT, Saloustros E, Fenga C, Spandidos DΑ, Libra M and
Tsatsakis AM: Akt inhibitors in cancer treatment: The long journey
from drug discovery to clinical use (Review). Int J Oncol.
48:869–885. 2016.PubMed/NCBI
|
|
21
|
Jansen VM, Mayer IA and Arteaga CL: Is
there a future for AKT inhibitors in the treatment of cancer? Clin
Cancer Res. 22:2599–2601. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang W, Tu G, Lv C, Long J, Cong L and
Han Y: Matrix metalloproteinase-9 is up-regulated by CCL19/CCR7
interaction via PI3K/Akt pathway and is involved in CCL19-driven
BMSCs migration. Biochem Biophys Res Commun. 451:222–228. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chuang CW, Pan MR, Hou MF and Hung WC:
Cyclooxygenase-2 up-regulates CCR7 expression via AKT-mediated
phosphorylation and activation of Sp1 in breast cancer cells. J
Cell Physiol. 228:341–348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kuwabara T, Tanaka Y, Ishikawa F, Kondo M,
Sekiya H and Kakiuchi T: CCR7 ligands up-regulate IL-23 through
PI3-kinase and NF-κB pathway in dendritic cells. J Leukoc Biol.
92:309–318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hollenbach M, Thonig A, Pohl S, Michel M,
Ripoll C, Greinert RA, Michl P and Zipprich A: Inflammation and
hypoxia regulate RAGE, NF-κB und pERK via glyoxalase I and
methylglyoxal in sinusoidal endothelial cells: A possible mechanism
in development of cirrhosis. Hepatology. 62:951a. 2015.
|
|
26
|
Zhao W, Sun Z, Wang S, Li Z and Zheng L:
Wnt1 participates in inflammation induced by lipopolysaccharide
through upregulating scavenger receptor A and NF-κB. Inflammation.
38:1700–1706. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cupovic J, Gil-Cruz C, Onder L,
Perez-Shibayama C, Chai Q and Ludewig B: Extra-lymphatic
CCR7-ligand expression controls virus-induced CNS inflammation.
Immunology. 140:35. 2013.
|
|
28
|
Zhou X, Liu S, Cai G, Kong L, Zhang T, Ren
Y, Wu Y, Mei M, Zhang L and Wang X: Long non coding RNA MALAT1
promotes tumor growth and metastasis by inducing
epithelial-mesenchymal transition in oral squamous cell carcinoma.
Sci Rep. 5:159722015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu J, Yang D, Zhang H, Liu W, Zhao Y, Lu
H, Meng Q, Pang H, Chen X, Liu Y, et al: USP22 promotes tumor
progression and induces epithelial-mesenchymal transition in lung
adenocarcinoma. Lung Cancer. 88:239–245. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li F, Zou Z, Suo N, Zhang Z, Wan F, Zhong
G, Qu Y, Ntaka KS and Tian H: CCL21/CCR7 axis activating chemotaxis
accompanied with epithelial-mesenchymal transition in human breast
carcinoma. Med Oncol. 31:1802014. View Article : Google Scholar : PubMed/NCBI
|