Introduction
Anaplastic thyroid carcinoma (ATC) is the most
malignant form of thyroid cancer with the worst prognosis. However,
its incidence is at ~7–8% (1). ATC
undergoes early blood and lymphatic metastasis to distant tissues,
showing characteristics of rapid progression, poor response to
treatment and poor prognosis. The current treatment of ATC is
controversial, and there is a lack of systematic treatment. At
present, clinical treatment of ATC includes surgery, chemotherapy
and radiotherapy, but the three methods only control the disease at
the tumor site; the possibility of a radical cure is very low. For
ATC patients, the average survival time is 3–10 months, the 1-year
survival rate is at ~20%, and the 5-year survival rate is only
5–15% (2–5). Various studies have suggested the use
of 131I to treat ATC, but the degree of differentiation
of ATC is low or it may be undifferentiated, thus, iodine may not
be accumulated, losing the benefit of treatment. In recent years,
various studies have made great progress in the study of
antibodies. Luo et al used antibody phage display technology
to construct an adenocarcinoma lung cancer phage antibody library,
and then, used inoculation of human lung adenocarcinoma cells into
nude mice in vivo and analyzed the biodistribution by
carrying out further imaging (6).
In addition, Qiong et al (7)
completed phage antibody screening of human medullary thyroid
carcinoma, and successfully used this technique for an imaging
study, obtaining good results. With the maturation of phage display
technology and its application, this technique provides a new
method for the treatment of ATC.
Radioimmunoimaging (RII) is a method of imaging
in vitro using a specific antibody conjugated to a
radioactive isotope to make a drug-free conjugate. Its advantage is
the use of the principle of specific binding of antigen and
antibody, combined with imaging examination methods. The early
location and qualitative diagnosis of a tumor is very important
(8). To date, many researchers have
tested RII using radio-pharmaceuticals combined with a
corresponding antibody against tumor markers, and achieved marked
results, such as those reported by Zhao et al (9) who used phage antibody display
technology to construct an anti-ABCG2 single-chain variable
fragment (scFv) antibody against lung adenocarcinoma, and then
inoculated human lung adenocarcinoma cells into nude mice, and
carried out further imaging to confirm specific binding.
In the present study, based on the findings of Xi
et al (10), we completed
the creation of an ATC human phage single-chain variable fragment
antibody (scFv) library and successfully screened specific scFv of
ATC, completed preparation and identification of ATC scFv, and
carried out RII using 131I-labeled scFv against ATC in
tumor-bearing nude mice.
Materials and methods
General procedures
All cell lines were cultured in RPMI-1640 medium
supplemented with 10% fetal bovine serum (both from Gibco, Thermo
Fisher Scientific, Waltham, MA, USA) and 1% penicillin/streptomycin
under a humidified atmosphere of 5% CO2 at 37̊C. Cells
in the logarithmic phase of growth were used for all
experiments.
All experiments using laboratory animals were
carried out in accordance with the National Institutes of Health
Guide for the Care and Use of Laboratory Animals (NIH Publications
no. 8023, revised 1978).
The Symbia T2 single photon emission-computed
tomography/computed tomography (SPECT/CT) detector was obtained
from Siemens (Erlangen, Germany).
Production and purification of
scFv
A few phage clones that reacted with ARO cells
detected from positive phage culture were used to infect E.
coli HB2151 and inoculated onto a SOBAG culture plate and kept
at 37̊C overnight. The cells were then transferred to 2X YT-AI
medium containing isopropyl-β-D-thiogalactoside (IPTG) and
collected using centrifugation at shock times of 4 and 6 h.
Subsequently, the cells were resuspended in phosphate-buffered
saline (PBS) and frozen at −20̊C, then, subjected to five rounds of
rapid thawing at 37̊C followed by re-freezing. The bacteria were
then subjected to ultrasonic disruption, and the supernatant
containing the soluble scFv antibodies was collected and stored at
−20̊C. The soluble scFv was purified using a HiTrap™ Anti E-tag
column. Each tube was monitored at an absorbance of 280 nm, and the
highest peak was identified as the purified soluble scFv antibody,
which was stored at 4̊C.
Sodium dodecyl sulfate polyacrylamide
gel electrophoresis (SDS-PAGE) and western blotting
To examine the expression of scFv, the supernatants
containing scFv induced by IPTG in E. coli HB2151 at 4 and 6
h were run on SDS-PAGE followed by Coomassie brilliant blue
staining, with uninduced E. coli HB2151 as the control
group. Purified scFv was identified by western blotting.
Enzyme-linked immunosorbent assay
(ELISA) detection of phage antibody
The TT, ARO and HepG2 cells were cultured in 96-well
plates at 37̊C for 48 h, washed three times with PBS, and fixed
with 2.5% glutaraldehyde. After fixation the cells were blocked for
1 h in 2% skim milk powder, subsequently they were washed in PBS
and then again in PBS with Tween-20 (PBST). The purified scFv was
added to the wells of a 96-well plate, then, HRP/anti m13k07 was
added and the plate was incubated at 37̊C. PBS was used in place of
purified scFv in ARO cells as the negative control group.
3,3,5,5-Tetramethylbenzidine (TMB) dihydrochloride was added and
allowed to react in the dark for 30 min, then the absorbance was
read at 450 nm.
Radiolabeling, purification and
radiochemical purity test
The scFv was labeled with 131I using the
chloramine T method. An aliquot of scFv (250 µl) was mixed with 100
µl 131I (50 mCi/ml) for 3 min. Then, 250 µl chloramine T
(1 mg/ml) was added, and 1 min later 500 µl sodium metabisulfite (2
mg/ml) was added, followed by 100 µl of 1% potassium iodide to
terminate the labeling process. The 131I-labeled scFv
was purified by gel-filtration on a Sephadex G25M column. The
chlorine-acetic acid method was used to assess the
131I-scFv labeling rate. The paper precipitation method
was used to analyze 131I-scFv radiochemical purity, and
to calculate the specific activity. Purified 131I-scFv
was maintained at room temperature for 1, 6, 12 or 24 h and was
then added into fresh human serum and incubated at 37̊C for 1, 6,
12 or 24 h. Radiation was detected and the stability of the labeled
conjugate at room temperature was analyzed.
Animal models and biodistribution
ARO cells were inoculated subcutaneously into the
right forelimb of 4- to 6-week-old male nude mice (Department of
Laboratory Animal Center at Chongqing Medical University). When the
tumor volume reached >1 cm3, mice were injected via
the tail vein with 100 µl of purified 131I-labeled scFv
(500 µCi/ml, 18.5 MBq/ml). Three mice were sacrificed at 12, 24, 48
and 72 h after injection. The tumor, liver, kidney, spleen, heart,
lung, stomach, intestine, brain, muscle and blood were removed from
each mouse and weighed, and the radioactivity was determined using
a γ counter.
SPECT/CT and RII in tumor-bearing nude
mice
The tumor-bearing mice were provided with water
containing 1% potassium iodide for 3 days prior to imaging to block
the thyroid, and were then injected with 131I-labeled
scFv, as previously described. At 12, 24, 48 and 72 h after
injection the mice were scanned using a single head rotating
scintillation camera of SPECT. When the tumor was clearly visible,
SPECT/CT image fusion was performed in nude mice (high energy
collimator, matrix 256×256, with a peak at 364 keV, and an
acquisition time for each frame of 15 min).
Statistical methods
All experimental data were analyzed using the
statistical software SPSS 22.0 (IBM Corp., Armonk, NY, USA) and
graphically expressed as the mean ± standard deviation (mean ± SD).
A Students t-test was used for the comparison of two sample means,
the mean of variance was compared by single factor analysis, and
the quantitative data were analyzed using the χ2 test. A
value of P<0.05 was considered significant.
Results
Production and purification of scFv. Analysis of
phage antibody specificity by ELISA revealed that of the 12
monoclonal phages, eight reacted positively to ARO cells, giving a
positive rate of 66.7%. Phage-infected E. coli TG1 expressed
the soluble protein. The results of the purification of the soluble
antibody on the Sephadex G25M column and the peak value of 15–21
represents the purified soluble antibody scFv.15-21. The tubes with
the highest readings at A280 nm were considered to be the purified
expression of soluble scFv.
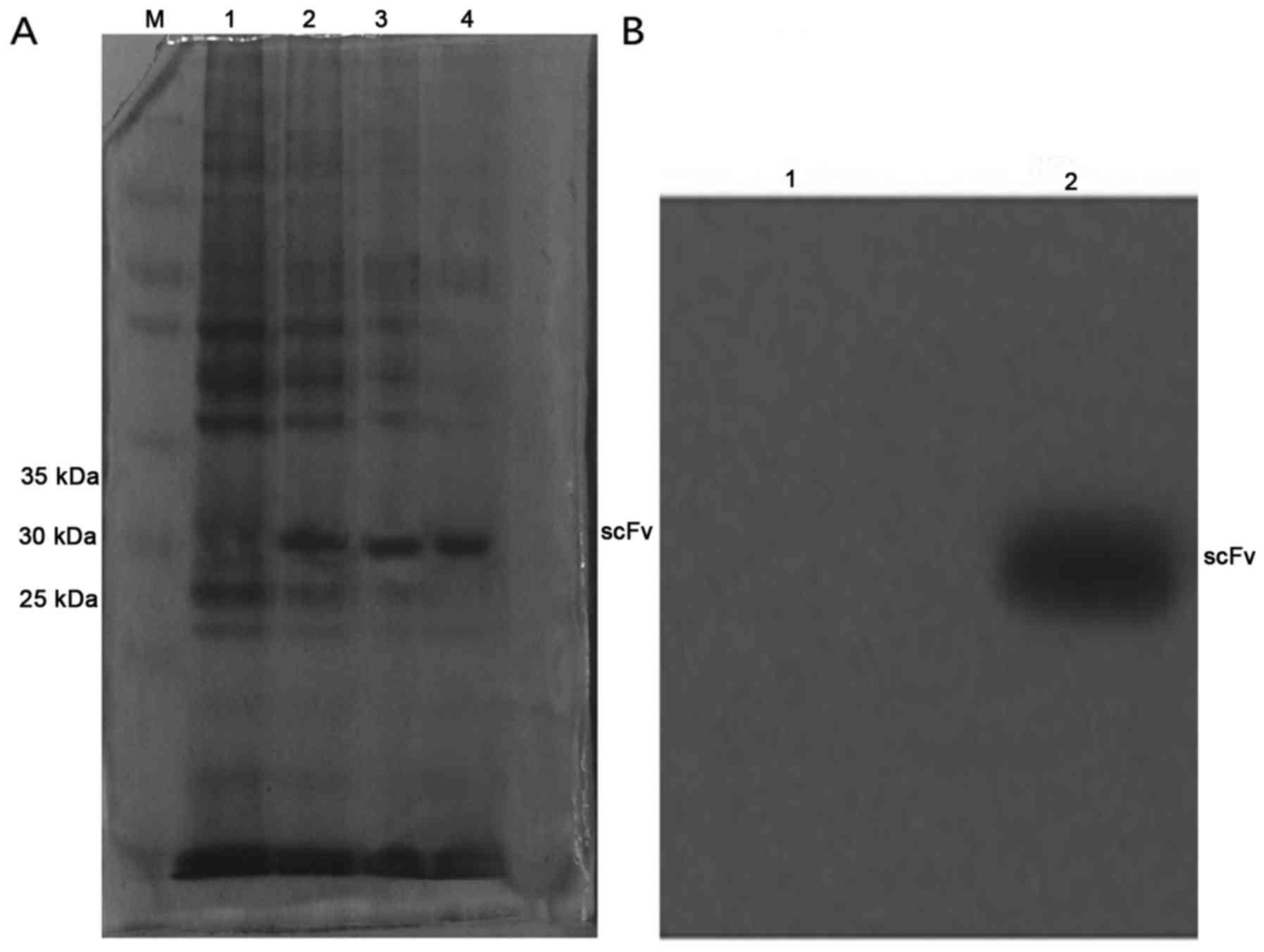
SDS-PAGE and western blotting
The scFv induced by IPTG at 4 and 6 h was run on
SDS-PAGE followed by Coomassie brilliant blue staining, with
uninduced E. coli HB2151 used as the control. Western
blotting of the purified scFv revealed that scFv has a relative
molecular mass of ~29 kDa (Fig.
1).
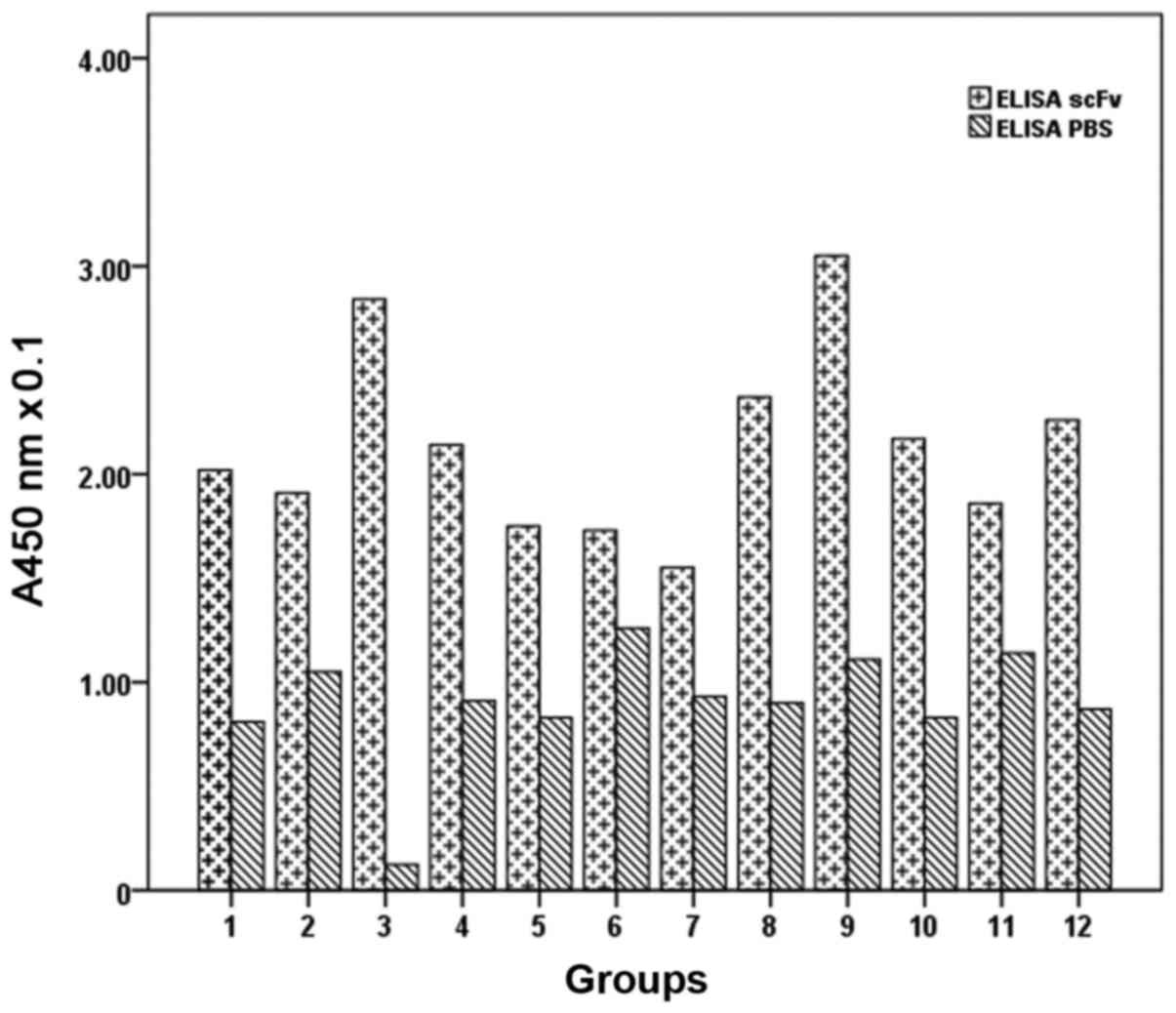
ELISA detection of phage antibody
ELISA results showed that the absorbance of ARO
cells at 450 nm was 0.37±0.03, while the absorbance of
hepatocarcinoma cells was 0.18±0.01, that of TT cells was
0.13±0.02, and the PBS control group was 0.05±0.00. Analysis of
variance showed that the differences were statistically significant
(P<0.05), and confirmed that the specificity of ARO cells was
higher than the other cells (Table
I; Fig. 2).
 | Table I.Detection of scFv. |
Table I.
Detection of scFv.
| A, Detection of
scFv |
|---|
|
|---|
|
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|
|---|
|
| Group | Deviation from
average | Standard error | Lower limit | Upper limit |
|---|
| ARO | HepG2 | 0.191 | 0.0159a | 0.155 | 0.227 |
|
| PBS | 0.318 | 0.0159a | 0.281 | 0.355 |
|
| TT | 0.242 | 0.0159a | 0.205 | 0.279 |
|
| B, Detection of
scFv |
|
| Group |
| Average | No. |
| Standard
deviation |
|
| ARO |
| 0.369 | 3 |
| 0.029 |
| HepG2 |
| 0.178 | 3 |
| 0.015 |
| PBS |
| 0.051 | 3 |
| 0.004 |
| TT |
| 0.127 | 3 |
| 0.021 |
| Total |
| 0.181 | 12 |
| 0.124 |
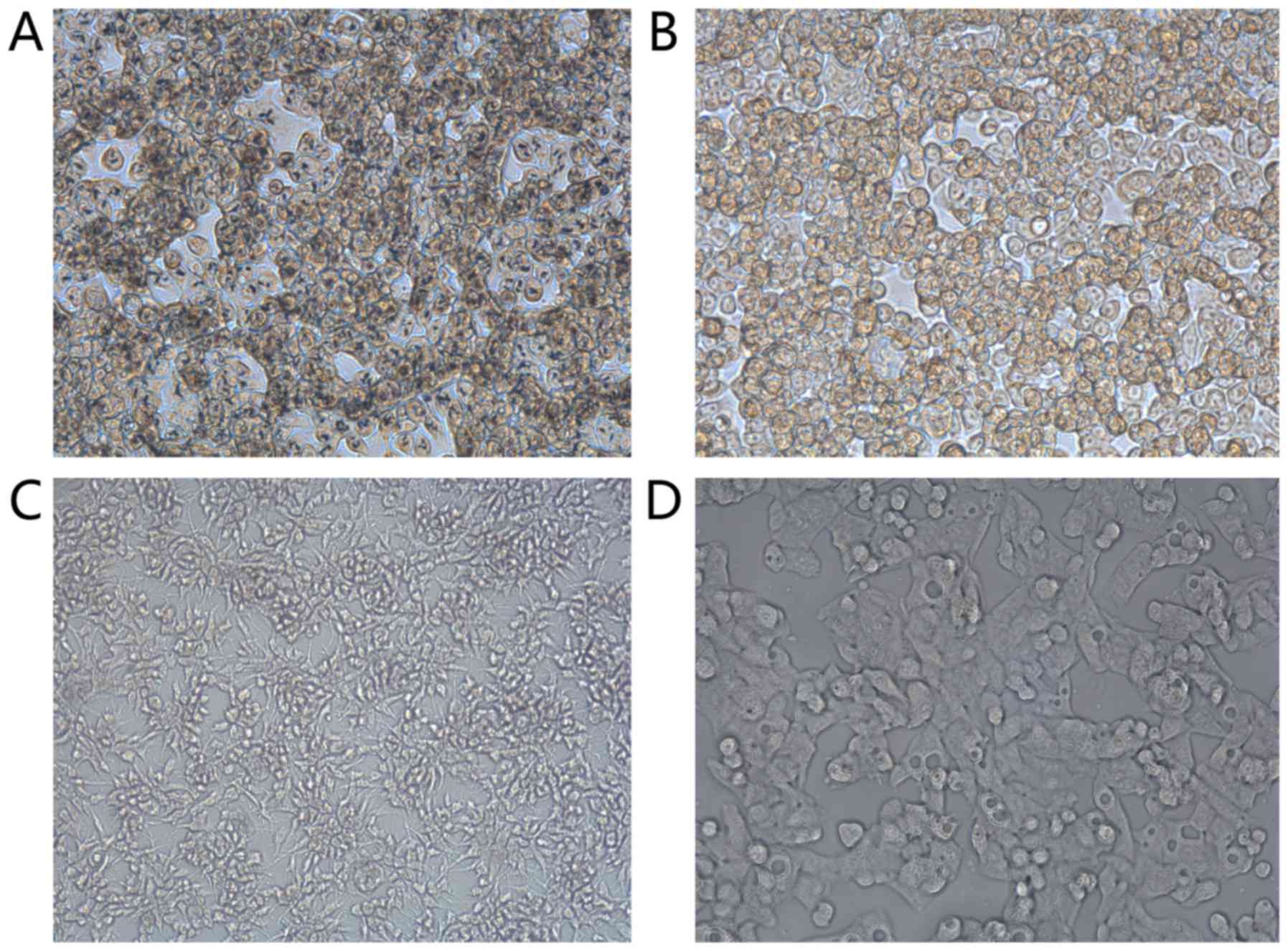
Light microscopy results also showed that scFv had a
high specificity for ARO cells (Fig.
3).
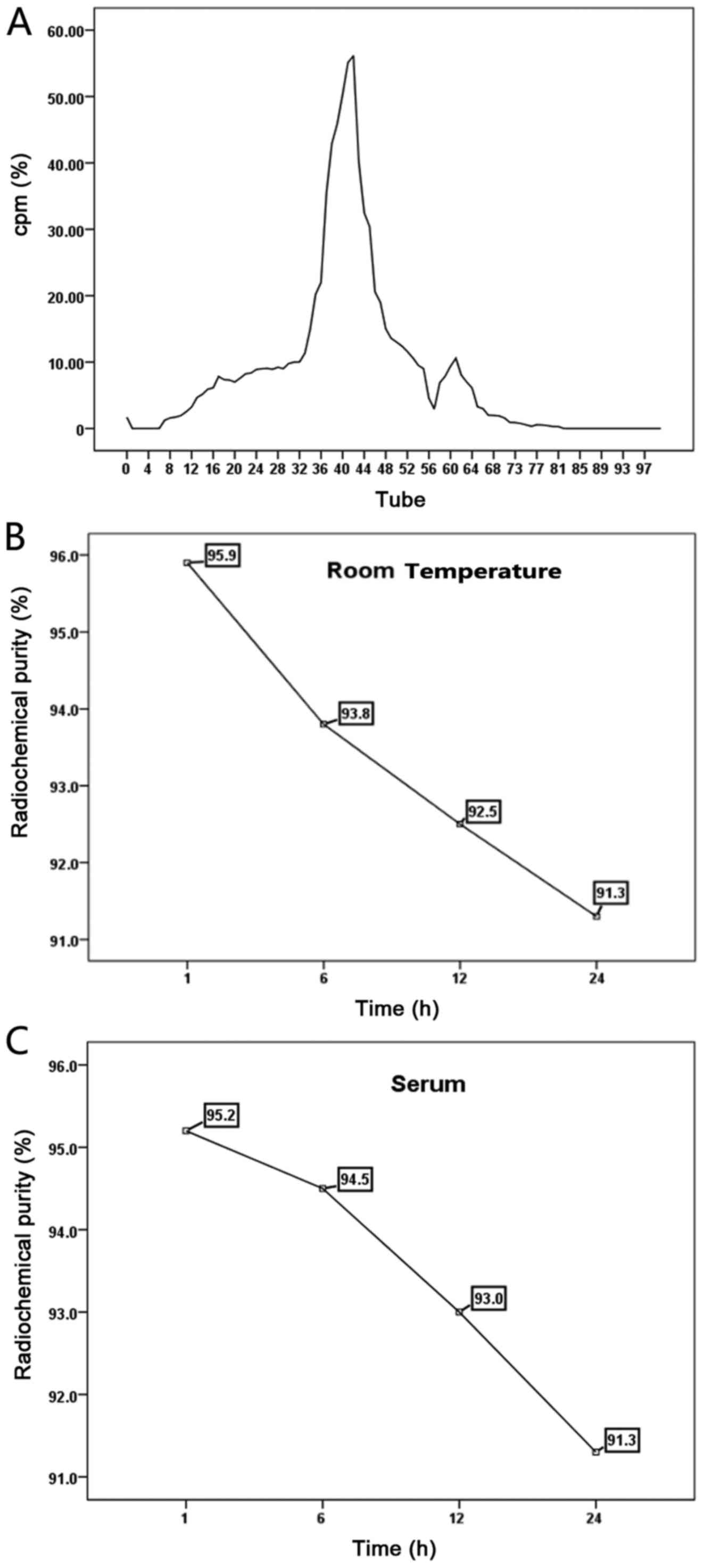
Radiolabeling, purification and
radiochemical purity test
The scFv was successfully labeled with
131I using the chloramine T method, and then purified
using a Sephadex G25M column. Eluent fractions were collected in
100 tubes of 131I-scFv, and by evaluating the
radioactivity in each tube, two emission peaks were revealed, the
first located in tubes 34–49, while the second radioactive peak was
found in tubes 57-64. The eluent of the first radiation peak was
collected, purified and labeled 131I-scFv. The labeling
rate was found to be 91.64% and the radiochemical purity was
93.3±0.32%. Purified 131I-scFv was maintained at room
temperature for 1, 6, 12 or 24 h before its addition to fresh human
serum and incubation at 37̊C for 1, 6, 12 or 24 h. The
radiochemical purity was assessed and the stability of the
131I-scFv after storage at room temperature and in serum
was analyzed at the different time-points (Fig. 4).
 | Figure 4.(A) Purification of
131I-scFv. The assessment of the radioactivity of 100
tubes revealed two emission bands, a first emission peak was
located in tubes 34–49 and the second radioactive peak was found in
tubes 57-64. (B) The room temperature stability test of
131I-scFv. The radiochemical purity was 95.9, 93.8, 92.5
and 91.3% at 1, 6, 12 and 24 h, respectively. (C) The serum
stability test of 131I-scFv. The radiochemical purity
was 95.2, 94.5, 93.0 and 91.3% at 1, 6, 12 and 24 h, respectively.
scFv, single-chain variable fragment. |
Animal models and biodistribution
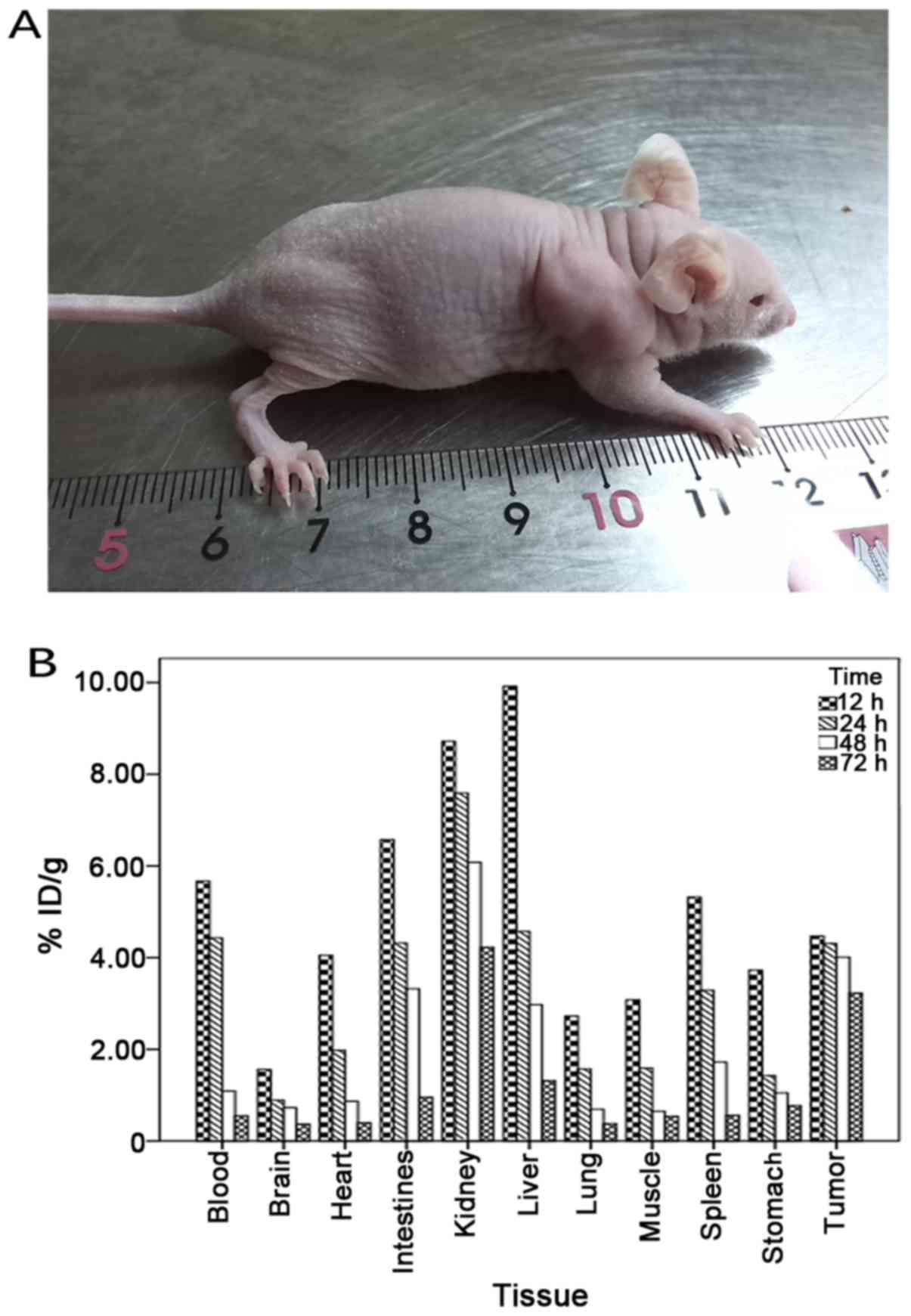
Four weeks after injecting ARO cells, an ATC-bearing
nude mouse model was successfully constructed (Fig. 5). Examination of the in vivo
distribution of 131I-scFv in tumor-bearing nude mice
revealed that 131I-scFv was mainly distributed in tumor
tissue and in liver, kidney, spleen, heart, lung, stomach,
intestine, brain, muscle and blood. The concentrations of
radioactivity in the tumor tissues, liver, kidney, intestine and
blood were all high after injection, but with increasing time, the
levels of radioactivity in other tissues were decreased while those
in the tumor tissues remained high (Table II; Fig.
5). 131I-scFv revealed a long residence time and a
slow rate of clearance in tumor tissue. In addition, the ratio of
radioactivity of tumor:blood and tumor:muscle increased with time,
reaching a peak at 48 h.
 | Table II.Distribution of 131I-scFv
in tumor-bearing nude mice. |
Table II.
Distribution of 131I-scFv
in tumor-bearing nude mice.
| Tissue | 12 h | 24 h | 48 h | 72 h |
|---|
| Tumor | 4.47±0.56 | 4.31±0.72 | 4.01±0.66 | 3.23±0.09 |
| Liver | 9.92±0.31 | 4.57±0.23 | 2.97±0.69 | 1.32±0.33 |
| Kidney | 8.72±0.11 | 7.59±0.47 | 6.08±0.72 | 4.23±0.91 |
| Spleen | 5.32±0.57 | 3.29±0.58 | 1.72±0.37 | 0.56±0.20 |
| Lung | 2.73±0.11 | 1.57±0.52 | 0.69±0.76 | 0.38±0.55 |
| Stomach | 3.73±0.98 | 1.43±0.24 | 1.05±0.63 | 0.77±0.36 |
| Intestine | 6.57±0.72 | 4.32±0.64 | 3.32±0.53 | 0.96±0.32 |
| Brain | 1.56±0.11 | 0.89±0.12 | 0.73±0.26 | 0.37±0.08 |
| Heart | 4.05±0.07 | 1.98±0.20 | 0.87±0.22 | 0.40±0.02 |
| Muscle | 3.08±0.28 | 1.59±0.39 | 0.65±0.32 | 0.54±0.65 |
| Blood | 5.67±0.89 | 4.43±0.25 | 1.09±0.12 | 0.55±0.07 |
| Ratio |
|
|
|
|
| Tumor/brain | 2.86±0.33 | 4.84±0.60 | 5.49±0.31 | 8.72±0.13 |
| Tumor/muscle | 1.45±0.22 | 2.71±0.25 | 6.16±0.02 | 5.98±0.01 |
| Tumor/blood | 0.18±0.03 | 0.97±0.32 | 3.67±0.62 | 5.87±0.11 |
SPECT/CT RII in tumor-bearing nude
mice
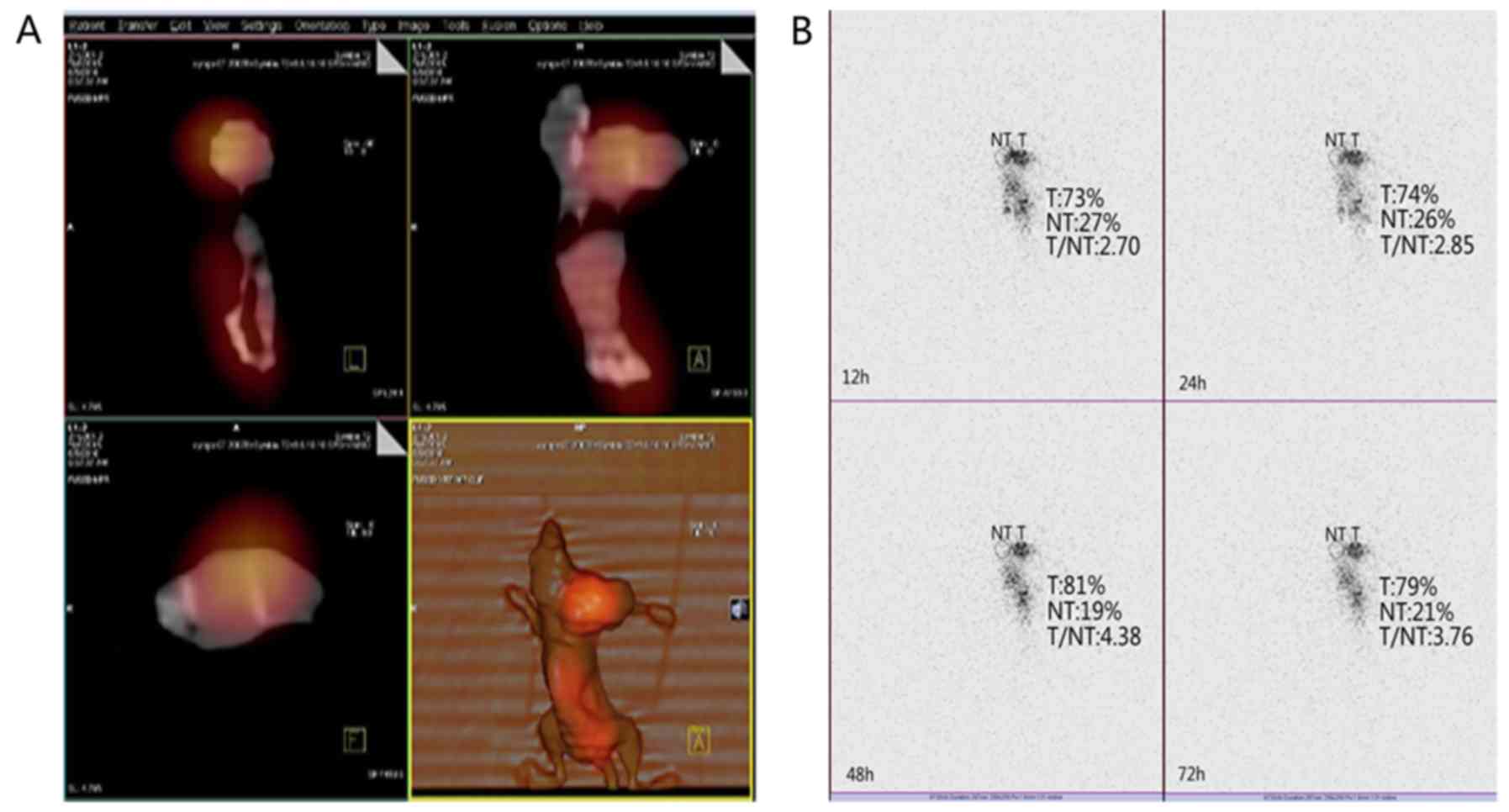
The SPECT/CT imaging results revealed that tumor
tissue in tumor-bearing nude mice was still clearly visible at 48
h, and the highest target/non-target (T/NT) value was 4.38. The
concentrations of radioactivity in other parts of the body were
decreased (Fig. 6).
Discussion
Related studies have shown that the molecular
pathogenesis of ATC involves a series of gene mutations, chiefly of
BRAF, RAS, catenin (cadherin-associated protein) β1, PIK3CA, AXIN1,
TP53, PTEN and APC, and that chromosomal abnormalities are common
(11). Mutations in the p53
tumor-suppressor gene results in the production of inactive p53
proteins that have been found in most cases of ATC, but not in
other thyroid tumors (12).
Mutations in the β-chain protein gene occur in as many as 65% of
ATC cases (13). The evidence for a
high degree of differentiation was provided by a study of
undifferentiated carcinoma and concomitant BRAF mutations in the
region of the p53 mutation in the second step (14,15).
Due to the fact that ATC gene therapy is currently still in the
early stages, most research is still in the exploratory stage.
Induction differentiation therapy has better effects in
vitro, but currently there are no large-scale clinical trials,
pending further research. Coupling of a radioactive nuclide to an
orientation-specific antibody creates a radio-immunoassay drug
treatment method suitable for in vivo use. Known as
radioimmunotherapy (RIT), it has provided a novel approach to ATC
diagnosis and treatment (16,17).
To date, numerous studies (18)
have achieved promising RII results with the use of radioactive
nuclide-labeled antibodies.
Recently, as phage-display technology has matured,
our research group used this technique to establish an ATC phage
antibody library and screen antibodies for ATC specificity. The
biological characteristics of the antibodies were analyzed by
ELISA, SDS-PAGE and western blotting. Results identified an
antibody which exhibited specificity to ARO cells. Then, the
purified scFv was labeled with 131I, the ARO cells were
injected into nude mice in vivo, and a radioimmunoassay was
successfully achieved. Use of the 131I-labeled
polypeptide was feasible in the present study. The labeling rate of
131I-scFv was 91.64%, the radio-chemical purity of
purified 131I-scFv was 93.3±0.32% and the radiochemical
purity of 131I-scFv which was stored at 37̊C in human
blood serum at 24 h was 91.9%. These results demonstrated that
131I-scFv had good stability in vitro, and met
the requirements of in vivo experimental study on peptides
(19). SPECT imaging can directly
observe the dynamic changes in the in vivo distribution of
the agents imaged. Results of 131I-scFv polypeptide
imaging showed that, in nude mice xenografted with ARO cells,
dynamic changes in distribution of the imaging agent in vivo
can be directly observed using SPECT imaging, which is closer to
clinical practice. Concentrations of radioactivity were not found
in the thyroid at any time after injection mainly since the labeled
compound targets the tumor vasculature with high affinity and
specificity and is stable without iodothyronine. This is consistent
with the results from the asssessment of the in vitro
stability of 131I-scFv. SPECT imaging using
131I-scFv revealed a higher tumor uptake in the mice
bearing ARO cells. The experimental results revealed that in
tumor-bearing nude mice at 48 h after intravenous injection of
131I-scFv, the tumor imaging was clear. Concomitantly,
the T/NT reached the highest value of 4.38, suggesting that the
antibody bound specifically to ARO cells. The final objective of
the imaging was to explore methods of treatment which could be used
to target and destroy tumor cells by binding to the specific tumor
antigen. 131I is a good imaging agent which is currently
used in the clinical diagnosis and treatment of thyroid disease
(20). If combined with the
purified scFv, as used for imaging of tumor-bearing nude mice, it
could provide a new way of diagnosing and treating ATC. In future
our research may continue to focus on ATC treatment with
131I-scFv, to confirm the specific effect of
131I-scFv in killing tumor cells.
There are some limitations to this experimental
study. Traditional screening methods were used and ways to increase
antibody concentration to avoid the loss of the antibody under
screening conditions (21) may need
to be explored and further optimized in later experiments. IPTG,
used in this experiment, is toxic; various studies (22) have shown that lactose can also be
utilized in the induction process and is safe and non-toxic, thus
we may consider using lactose induction in our future experiments.
In the early stage of imaging, blood, liver and kidney tissue all
exhibited a high concentration of radioactivity, but with time, the
concentration of radiation in these organs significantly decreased.
The high concentration in the kidney may be related to the
excretion of the labeled antibodies. The high concentration in the
liver may due to reticuloendothelial cells non-specifically taking
up 131I-scFv, or there may be some fragment
crystallizable (Fc) receptors. Consequently, focusing on improving
the process, for example, how to carry out chemical modifications
when designing scFv to decrease the radioactivity uptake of
non-tumor tissue, and to make scFv stay specifically in the tumor
tissue, is warranted.
In conclusion, human single-chain variable fragment
antibodies against ATC were successfully generated.
131I-scFv was successfully prepared, and the imaging of
131I-scFv in a nude mouse model at 48 h after injection
of tumor cells clearly showed it specifically localized in the
tumor tissue. In the future we may continue to investigate the
potential of this construct in anticancer therapy.
Acknowledgements
The authors gratefully acknowledge the assistance of
the Department of Nuclear Medicine, First Affiliated Hospital of
Chongqing Medical University, Institute of Life Sciences and Animal
Experimental Center of Chongqing Medical University. The present
study was funded by the National Natural Science Foundation of
China (no. 81071171), the Project of Science and Technology plan of
Chongqing (CSCT2013JCYJA10072), and supported by the National Key
Clinical Specialties Construction Program of China [National Health
Office of medical letter (2013) no. 544].
References
|
1
|
Amodeo C, Caglià P, Gandolfo L, Veroux M,
Donati M and Immè A: Undifferentiated carcinoma of the thyroid.
Tumori. 89:(Suppl 4). S205–S206. 2003.(In Italian).
|
|
2
|
Denaro N, Nigro CL, Russi EG and Merlano
MC: The role of chemotherapy and latest emerging target therapies
in anaplastic thyroid cancer. Onco Targets Ther. 9:1231–1241. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kihara M, Miyauchi A, Yamauchi A and
Yokomise H: Prognostic factors of anaplastic thyroid carcinoma.
Surg Today. 34:394–398. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xia J, Bi H, Yao Q, Qu S and Zong Y:
Construction of human ScF phage display library against ovarian
tumor. JJ Huazhong Univ Sci Technolog Med Sci. 26:497–499. 2006.
View Article : Google Scholar
|
|
5
|
McFadden DG, Vernon A, Santiago PM,
Martinez-McFaline R, Bhutkar A, Crowley DM, McMahon M, Sadow PM and
Jacks T: p53 constrains progression to anaplastic thyroid carcinoma
in a Braf-mutant mouse model of papillary thyroid cancer.
Proc Natl Acad Sci USA. 111:E1600–E1609. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luo Y, Pang H and Li S, Cao H, Peng Z, Fan
C and Li S: Production and radioimmunoimaging of novel fully human
phage display recombinant antibodies and growth inhibition of lung
adenocarcinoma cell line overexpressing Prx I. Cancer Biol Ther.
8:1369–1377. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qiong L, Hua P, Xi J, Li W and Lu X:
Medullary thyroid carcinoma like humanized phage single chain
antibody screening and identification. Chongqing Yike Daxue Xuebao.
9:12–16. 2015.
|
|
8
|
Duan D, Li SL, Zhu YQ and Wang SB: Effects
of radioimmunoimaging with 99Tcm-EGFR-McAb or
99Tcm-CD44-McAb or combined application of
both on nude mice bearing human lung adenocarcinoma. J Third Mil
Med Univ. 31:1287–1280. 2009.
|
|
9
|
Zhao WS, Luo Y, Li BY, Zhou HJ and Zhang
T: Anti-ABCG2 scFv antibody of lung adenocarcinoma increases
chemosensitivity and induces apoptosis through the activation of
mitochondrial pathway. Am J Cancer Res. 6:1026–1039.
2016.PubMed/NCBI
|
|
10
|
Xi J, Hua P, Sen Z, Qiong L and Li W:
Thyroid without differentiation carcinoma of human scFv antibody
library construction and screening. J Immunol. 31:692–696.
2015.
|
|
11
|
Smallridge RC, Marlow LA and Copland JA:
Anaplastic thyroid cancer: Molecular pathogenesis and emerging
therapies. Endocr Relat Cancer. 16:17–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fagin JA, Matsuo K, Karmakar A, Chen DL,
Tang SH and Koeffler HP: High prevalence of mutations of the p53
gene in poorly differentiated human thyroid carcinomas. J Clin
Invest. 91:179–184. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Garcia-Rostan G, Camp RL, Herrero A,
Carcangiu ML, Rimm DL and Tallini G: β-catenin dysregulation in
thyroid neoplasms: Down-regulation, aberrant nuclear expression,
and CTNNB1 exon 3 mutations are markers for aggressive tumor
phenotypes and poor prognosis. Am J Pathol. 158:987–996. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nikiforova MN, Kimura ET, Gandhi M,
Biddinger PW, Knauf JA, Basolo F, Zhu Z, Giannini R, Salvatore G,
Fusco A, et al: BRAF mutations in thyroid tumors are restricted to
papillary carcinomas and anaplastic or poorly differentiated
carcinomas arising from papillary carcinomas. J Clin Endocrinol
Metab. 88:5399–5404. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Quiros RM, Ding HG, Gattuso P, Prinz RA
and Xu X: Evidence that one subset of anaplastic thyroid carcinomas
are derived from papillary carcinomas due to BRAF and
p53 mutations. Cancer. 103:2261–2268. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gui PL and Cheng MZ: Tumor
radioimmunoimaging and treatment of a predetermined bit technology.
Foreign Medical Oncology. 25:26–29. 1998.
|
|
17
|
Vezzosi D, Bennet A and Caron P: Recent
advance in treatment of medullary thyroid carcinoma. Ann
Endrocrinol. 68:147–153. 2007. View Article : Google Scholar
|
|
18
|
Rubello D, Rampin L, Nanni C, Banti E,
Ferdeghini M, Fanti S, Al-Nahhas A and Gross MD: The role of
18F-FDG PET/CT in detecting metastatic deposits of
recurrent medullary thyroid carcinoma: A prospective study. Eur J
Surg Oncol. 34:581–586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Subedi GP, Satoh T, Hanashima S, Ikeda A,
Nakada H, Sato R, Mizuno M, Yuasa N, Fujita-Yamaguchi Y and
Yamaguchi Y: Overproduction of anti-Tn antibody MLS128 single-chain
Fv fragment in Escherichia coli cytoplasm using a novel
pCold-PDI vector. Protein Expr Purif. 82:197–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Staelens S, Desmet J, Ngo TH, Vauterin S,
Pareyn I, Barbeaux P, Van Rompaey I, Stassen JM, Deckmyn H and
Vanhoorelbeke K: Humanization by variable domain resurfacing and
grafting on a human IgG4, using a new approach for
determination of non-human like surface accessible framework
residues based on homology modelling of variable domains. Mol
Immunol. 43:1243–1257. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vanden Borre P, McFadden DG, Gunda V,
Sadow PM, Varmeh S, Bernasconi M, Jacks T and Parangi S: The next
generation of orthotopic thyroid cancer models: Immunocompetent
orthotopic mouse models of BRAFV600E-positive papillary
and anaplastic thyroid carcinoma. Thyroid. 24:705–714. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang J, Yu C, Liao L and Liao H:
Expression of human insulin like growth factor-1 in Escherichia
coli induced by lactose. Strait Med. 22:248–252. 2010.
|