Introduction
Breast cancer (BC) is a complex and biologically
heterogeneous disease, presenting diverse histopathological,
clinical and molecular characteristics whose importance must be
highlighted when determining its prognosis and treatment outcome.
Through immunochemistry, BC is classified as hormone receptor
(ER+, PR+)-positive, human epidermal growth
factor receptor-2 (HER2+)-positive and triple-negative
(ER−, PR− and HER2−).
Approximately 21–27% of BC tumours possess a mutated p53
gene (1–4).
P53 plays an important role in cell growth arrest,
cell death, DNA repair, invasion and metastasis (5). P53 is a tumour suppressor gene whose
protein possesses three domains: trans-activating, DNA binding and
oligomerization (6). To date,
~4,250 p53 gene mutations have been reported in BC tumours;
~90% of these mutations are found within the DNA binding domain of
p53, and these are associated with worse prognosis and poor
response to anthracycline treatment [http://wwwp53.iarc.fr/] (1,7).
Previous studies have demonstrated that p53 mutants favour
cancer development; e.g., p53wt acts as a
transcriptional repressor of the TOP2α gene. However, p53
mutants fail to inhibit the expression of TOP2α, thus,
promoting the carcinogenic process (8,9).
Overexpression of the topoisomerase 2α (TOP2α) protein is
considered a predictive factor with respect to anthracycline
sensitivity, whereas its deletion status is associated with
increased resistance to the same drug (10–13).
Conversely, underexpression of the TOP2α protein is considered to
be a good prognosis factor (14,15).
Despite its potential usefulness through its
utilization as a predictive and prognostic factor for p53, and
TOP2α is inconsistent in several studies. Unfortunately, in Mexican
women with BC, these markers not have been studied. In the present
study population, we found that p53 protein overexpression could be
considered a factor for poor prognosis in Mexican women with
BC.
Materials and methods
Patients
A total of 102 biopsies were collected de
novo (adjacent and tumour tissues) from patients with BC
between September 2011 and December 2012 at the Instituto Estatal
de Cancerología (IECan) Dr. Arturo Beltrán Ortega, a Tertiary-Level
Hospital (Acapulco, Guerrero, Mexico). Inclusion criteria were the
following: patients who voluntarily accepted to be part of the
study and who signed an informed consent letter; patients residing
in the state of Guerrero; patients of any age at the time of
diagnosis of invasive ductal carcinoma (range 31–85 years);
clinical stage I, II and III, patients not currently under
neoadjuvant treatment, and diagnosis of BC confirmed by the
hospital's pathology department. Medical records were checked
thoroughly to obtain the patients' clinic-pathological and
gynaecological characteristics. The exclusion criteria were as
follows: presence of cancer antecedents, recurrence of cancer
and/or pregnancy. The elimination criteria were as follows:
insufficient biopsy material and incomplete clinical history. The
project was evaluated and approved by the IECan Ethics and Research
Committee.
Classification of genetic
alterations
We investigated the genetic alterations of
p53 and TOP2α, which were classified as: small-scale
mutations (point mutations and deletions) for p53 gene and
large-scale mutations (amplifications or deletions) for
TOP2α gene.
Genomic DNA extraction
Genomic DNA was extracted from breast tissue
biopsies (adjacent and tumour tissues) using the DNeasy Blood &
Tissue kit (Qiagen GmbH, Hilden, Germany) according to the
manufacturer's instructions. The genomic DNA obtained was
quantified, and its integrity was verified by GAPDH end-point
PCR.
Protein extraction
Total protein was extracted from breast tissue
biopsies (adjacent and tumour tissues) using an Ambion PARIS kit
(Applied Biosystems, Carlsbad, CA, USA) according to the
manufacturer's instructions. The proteins were quantified using a
Pierce BCA protein assay kit (Thermo Fisher Scientific, Rockford,
IL USA), and their integrity was verified by resolving the proteins
in 8% sodium dodecyl sulphate-polyacrylamide gel electrophoresis
(SDS-PAGE) and Coomassie staining.
End-point PCR and sequencing
To amplify the DNA binding domain of p53 (exons
5–8), two sets of primers were designed using Primer Express ver.
3.0 software (Applied Biosystems, Foster City, CA, USA). One set of
primers amplifies a 459 bp fragment from exons 5–6 (F,
5′-TTCCTCTTCCTACAGTAC-3′ and R, 5′-AGTTGCAAACCAGACCTCA-3′); the
second set amplifies a 510-bp fragment from exons 7–8 (F,
5′-GTGTTATCTCCTAGGTTG-3′ and R, 5′-TCCTCCACCGCTTCTTGT-3′). PCR
products were purified using a QIAquick PCR Purification kit
(Qiagen GmbH) according to the manufacturer's instructions. The
amplicons obtained were quantified and sequenced in a Genetic
Analyzer 3130 (Applied Biosystems). The sequences were analysed
using MEGA6 software.
TOP2α copy number evaluation
(amplification and deletion) through qPCR
Copy number of the TOP2α gene were analysed
by performing duplex reactions in triplicate using 20 ng of genomic
DNA (obtained from adjacent or tumour tissues), a TOP2α
TaqMan Copy Number Assay probe labelled with FAM, a RNase P
TaqMan Copy Number Reference probe labelled with VIC, and TaqMan
Genotyping Master Mix (all from Applied Biosystems) according to
the manufacturer's instructions. The Ct values obtained were
analysed using CopyCaller ver. 2.0 software (Applied Biosystems).
The method was validated by performing standard curves generated by
serial dilutions of the genomic DNA (1,000-0.1 ng) in triplicate.
As a diploid copy number control, genomic DNA was extracted from
pooled peripheral-blood samples of healthy women (n=5). As a
positive amplification control, genomic DNA from the breast-cancer
cell line SKBR3 was utilized.
Proximity ligation assay and qPCR
(PLA-qPCR)
To perform the proximity ligation assay (PLA), 200
nM aliquots of the probes 3′ Prox-Oligo and 5′ Prox-Oligo (Applied
Biosystems) were separately incubated with 200 nM biotinylated
polyclonal p53 antibody (R&D Systems, Minneapolis, MN, USA) for
60 min at room temperature. Afterward, Assay Probe Storage Buffer
was added and incubated for 30 min at room temperature. One
microgram of total protein extract (from adjacent or tumour tissue)
was incubated overnight with 250 pmol of the probes 3′ and 5′
Prox-Oligo antibody conjugated at 4̊C. Next, the ligation mix was
added to each tissue lysate containing total protein. The ligation
reaction was incubated for 10 min at 37̊C and after this, at 4̊C
for an additional 10 min. Finally, the reaction mix was
supplemented with protease, incubated for 10 min at 37̊C,
inactivated at 95̊C for 5 min and maintained overnight at 4̊C
(Applied Biosystems). The qPCR assay was performed using the
TaqMan® Protein Assays Fast Master Mix (Applied
Biosystems) and 9 µl of the protein lysate obtained from the PLA
assay. PCR conditions were as follows: 2 min at 95̊C and 40 cycles
of 15 sec at 95̊C and 1 min at 60̊C. Ct values were imported into
ProteinAssist™ software v1.0 (Applied Biosystems), in which the
relative quantification analysis was performed. Prior to analysis
of the protein lysates, a dynamic range was elaborated, and the
reaction efficiency was verified with a serial dilution standard
curve in triplicate. The cut-off limit for the relative expression
of the p53 protein was <1.0 for underexpression and >1.0 for
overexpression.
Western blotting
Thirty micrograms of total protein (from adjacent
and tumour tissues) were resolved in a 10% SDS-PAGE gel. The
proteins were transferred onto a nitrocellulose membrane (GE
Healthcare, Buckinghamshire, UK) and blocked with 5% non-fat milk
for 1 h at room temperature. The membrane was then incubated
overnight with polyclonal rabbit anti-human TOP2α antibody (Abcam,
Cambridge, UK) or monoclonal mouse anti-human p53-HRP antibody
(Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4̊C. The
membranes were washed three times with TBS/Tween-20 0.1%.
Additionally, the membrane employed for TOP2α detection was
incubated for an additional 1 h with a secondary horseradish
peroxidase (HRP)-conjugated anti-rabbit antibody (Sigma Chemical
Co., St. Louis, MO, USA). Protein detection was performed using the
reagent SuperSignal West Pico Chemiluminescent Substrate (Thermo
Scientific, Rockford, IL, USA), and the chemiluminescent signal was
detected using a VersaDoc Imaging System (Bio-Rad, Hercules, CA,
USA). The monoclonal mouse anti-human β-tubulin antibody (Sigma
Chemical Co.) was employed as a loading control. The densitometric
analysis was performed using ImageJ software. TOP2α protein
expression was normalized against the loading control (β-tubulin);
then, TOP2α expression in the tumour tissue was normalized against
the adjacent tissue. P53 protein expression was analysed in all
samples (adjacent and tumour tissues), but detection was only
identified in the tumour samples. In this case, the p53 protein was
normalized only against the loading control (β-tubulin), since the
half-life of the p53 protein is short, and it was not possible to
detect its expression in the adjacent tissue.
Immunohistochemistry
To determine the presence of the estrogen,
progesterone and HER2 receptors, 5-µm-thick sections were obtained
from paraffin-embedded tissue. The primary antibodies used were the
monoclonal mouse anti-human estrogen receptor clone 1D5 (Biocare
Medical, Concord, CA, USA), the monoclonal mouse anti-human
progesterone receptor clone PgR 636, and the polyclonal rabbit
anti-human c-erbB-2 oncoprotein (both from Dako, Carpinteria, CA,
USA). The detection system utilized was the mouse/rabbit UnoVue
HRP/DAB detection system (Diagnostic BioSystems, Pleasanton, CA,
USA). For each biopsy, a previously selected positive control was
also analysed by the pathologist as an internal quality control.
Data interpretation was performed using the Allred system, and for
HER2, cells were defined as 3+ positive, 2+ intermediate and 0–1+
negative. HER2 biopsies with intermediate values (2+) were
outsourced to an immunohistochemistry and molecular pathology
laboratory (INMUNO Q, México, D.F.) for validation by means of a
Silver In Situ Hybridization (SISH) assay.
Statistical analysis
Patients were subdivided into two groups: non-TNBC
patients, expressing at least one of the receptors (RE, RP or HER2)
and TNBC, lacking all of these receptors. To compare clinical and
pathological variables, point mutation or deletion in p53
gene, amplification or deletion in TOP2α gene, and SNPs in
p53 gene by groups, we used an χ2 test. Age at
the time of diagnosis was compared with a non-linear Gaussian
adjustment for accumulated relative frequencies. The relative
expression of the TOP2α and p53 proteins determined through western
blotting and/or PLA-qPCR, were analysed by the multiple Students
t-test. Analysis of variance (ANOVA) was used to compare the
relative expression of the p53 and TOP2α proteins vs. genetic
alterations of these genes. In all cases, p<0.05 was considered
to be significant.
In addition, a multiple correspondence analysis
(MCA) was used (16), which is a
descriptive method to determine the relationship between specific
groups. MCA uses an χ2-calculated distance to assess the
association between clinicopathological variables (clinical groups,
SBR histological grade), large-scale mutations in the TOP2α
(amplification or deletion) and small-scale mutations in the
p53 (point mutation or deletion) genes, and the relative
expression of the TOP2α and p53 proteins. Based on these distances,
the correspondence analysis registers two sets of combinations,
generating a score calculated for each group. The first combination
(dimension 1) defined a scale of variation that allowed the highest
discrimination between groups. The second combination (dimension 2)
defined the maximum dispersion between uncorrelated groups and the
relative expression of the TOP2α and p53 proteins. To compare
relative expression values of the TOP2α and p53 proteins, the
results were divided in percentiles starting from 33 and 66%. Three
sets of each variable were generated: underexpression <33%
(<0.9), basal expression (normal) 33–66% (0.9–1.1) and
overexpression >66% (>1.1) (16).
Finally, survival curves were determined using the
Kaplan-Meier method and verified by a log-rank test (Mantel-Cox)
with a follow-up period of 26 months, as patients continue in
follow-up on the present study.
Results
Clinicopathological features of
patients with BC
The analysed population consisted of 102 patients
with BC, of whom 71.5% were diagnosed as non-TNBC and 28.5% were
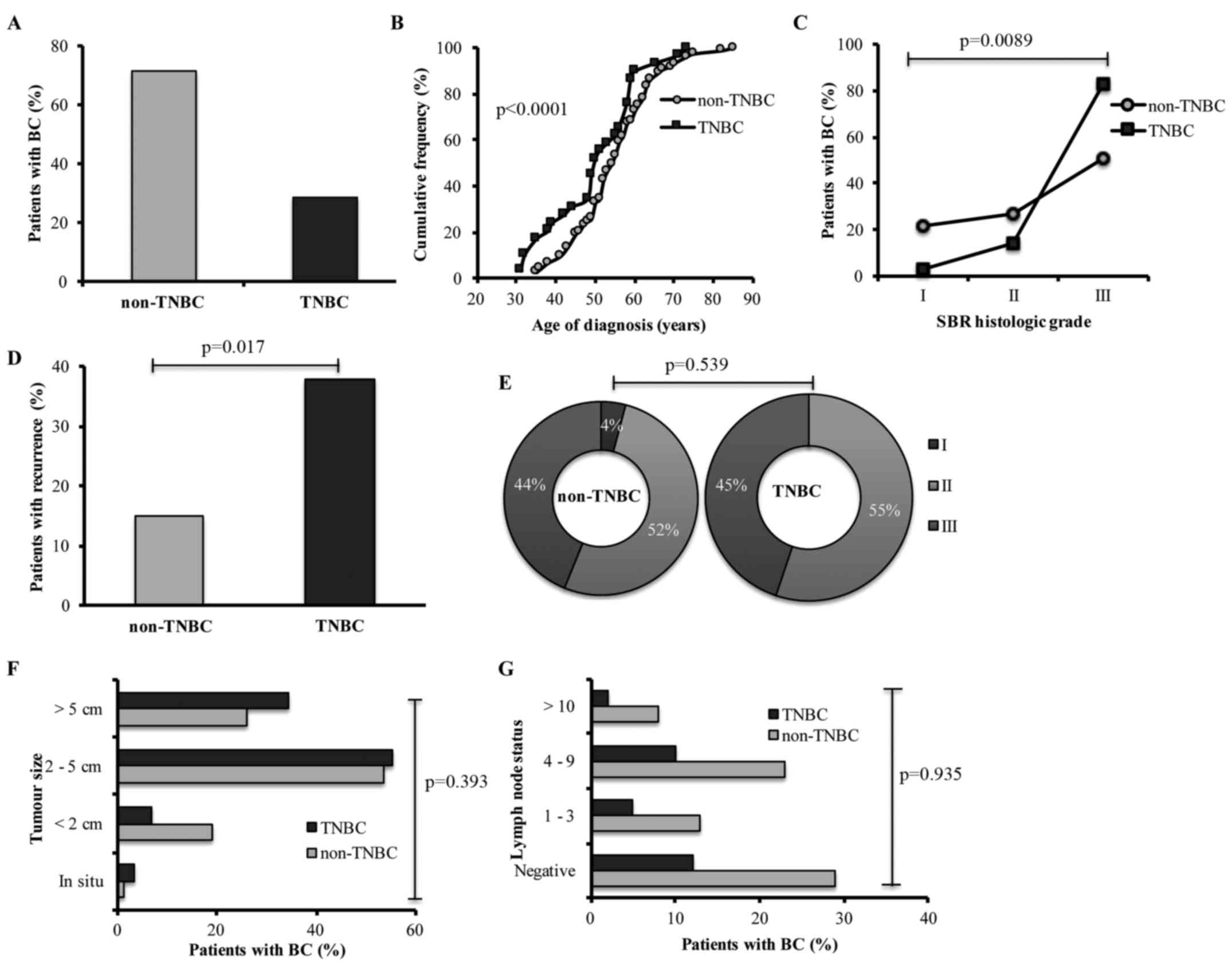
diagnosed as TNBC (Fig. 1A). The
age of TNBC patients at time of diagnosis was estimated as 50.2
(±11.3) years of age on average, whereas the age of non-TNBC
patients was estimated as 55.01 (±10.3) years of age on average.
The accumulated relative frequencies of both groups were
significant (p<0.0001) (Fig.
1B). TNBC patients exhibited 82.7% of SBR histological grade
III (p=0.0089) (Fig. 1C) and 37.9%
recurrence (p=0.017) (Fig. 1D) when
compared with non-TNBC patients (50.6 and 15.1%, respectively).
Furthermore, TNBC patients presented only stages II
and III (p=0.539) (Fig. 1E), larger
tumour size (p=0.393) (Fig. 1F),
and a higher frequency of positive lymph nodes in N2 status
(p=0.935) (Fig. 1G); we did not
observe significant differences between non-TNBC and TNBC
patients.
Genetic alterations of the p53 gene
and p53 protein expression
Since the tumour suppressor gene p53 is
mutated in ~50% of all types of cancer, we evaluated small-scale
mutations (point mutations, deletions) in this gene through DNA
sequencing of exons 5–8, which are frequently mutated. We found
genetic alterations in the p53 gene in 27.5% of patients
with BC, of which 9.8% were point mutations (missense) and 17.7%
were specific deletions in exons 5–6 (Table I).
 | Table I.TP53 and TOP2α status
in patients with BC. |
Table I.
TP53 and TOP2α status
in patients with BC.
| Gene status | Position |
Characteristics | Frequency
(n=102) |
|---|
| Wild-type
TP53 gene |
|
| 72.5% |
| Alteration of the
TP53 gene Subcategorization of the alteration of the
TP53 gene |
|
| 27.5% |
|
Missense mutation | Exons
5-8a | Protein change by
one amino acid or truncated | 9.8% |
|
Deletion | Exons
5-6a | Truncating
mutation | 17.7% |
| SNPs in the
TP53 gene |
|
|
|
| SNP
rs12947788 | Intron 7 | No change in
expression of the protein | 25.5% |
| SNP
rs12951053 | Intron 7 | No change in
expression of the protein | 25.5% |
| Wild-type
TOP2α gene |
|
| 67.6% |
| Alteration of the
TOP2α gene Subcategorization of the alteration of the
TOP2α gene |
|
| 32.4% |
|
Deletion | Gene | No protein
expression | 20.6% |
|
Amplification | Gene | Modified protein
expression (underexpression, overexpression) | 11.8% |
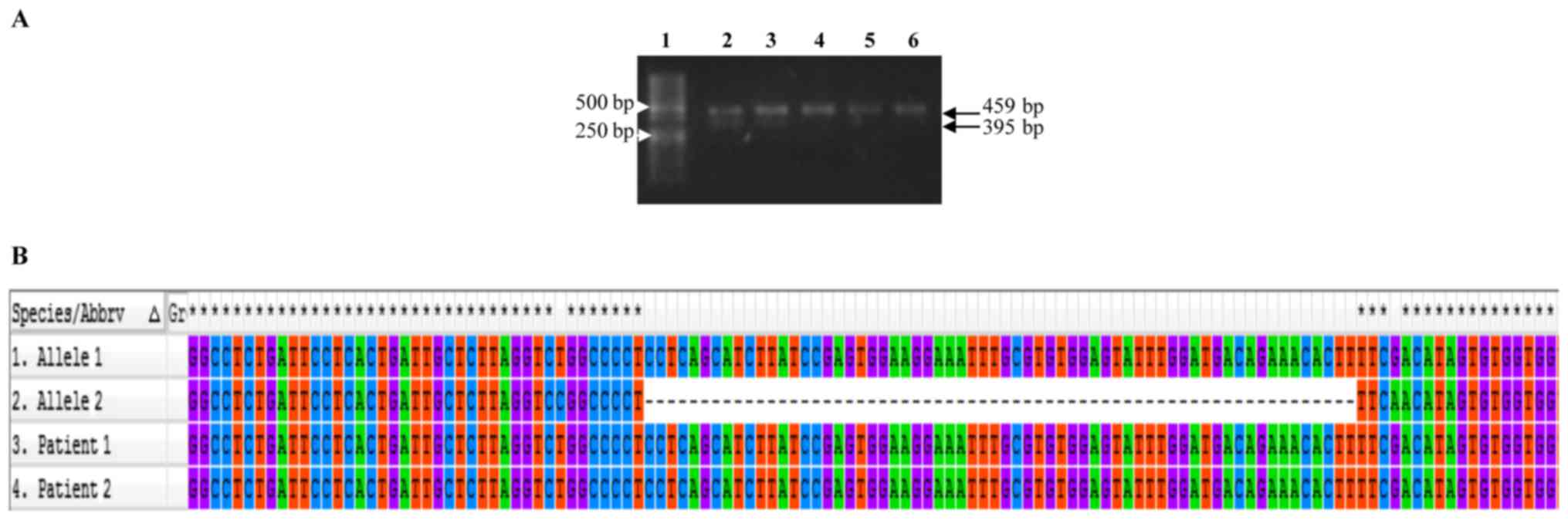
The majority of PCR amplicons spanning exons 5–6
exhibited a similar migration pattern by electrophoresis (459 bp,
expected molecular weight); however, in some cases, we observed an
amplicon of ~395 bp, suggesting that the latter represents an
allele carrying a deletion (Fig.
2A). We were able to identify an ~64 bp deletion corresponding
to codons 191–216 (exon 6) (Fig.
2B).
In addition, it was through DNA sequencing that we
identified single nucleotide polymorphisms (SNPs) rs1294778 and
rs12951053 localized inside intron 7 of p53, both of which
had a frequency of 25.5% in the study population (Table I). Both SNPs (homozygotes or
heterozygotes, according to the case) were identified in the same
patients. No significant differences were found between TNBC and
non-TNBC patients regarding either deletions or mutations
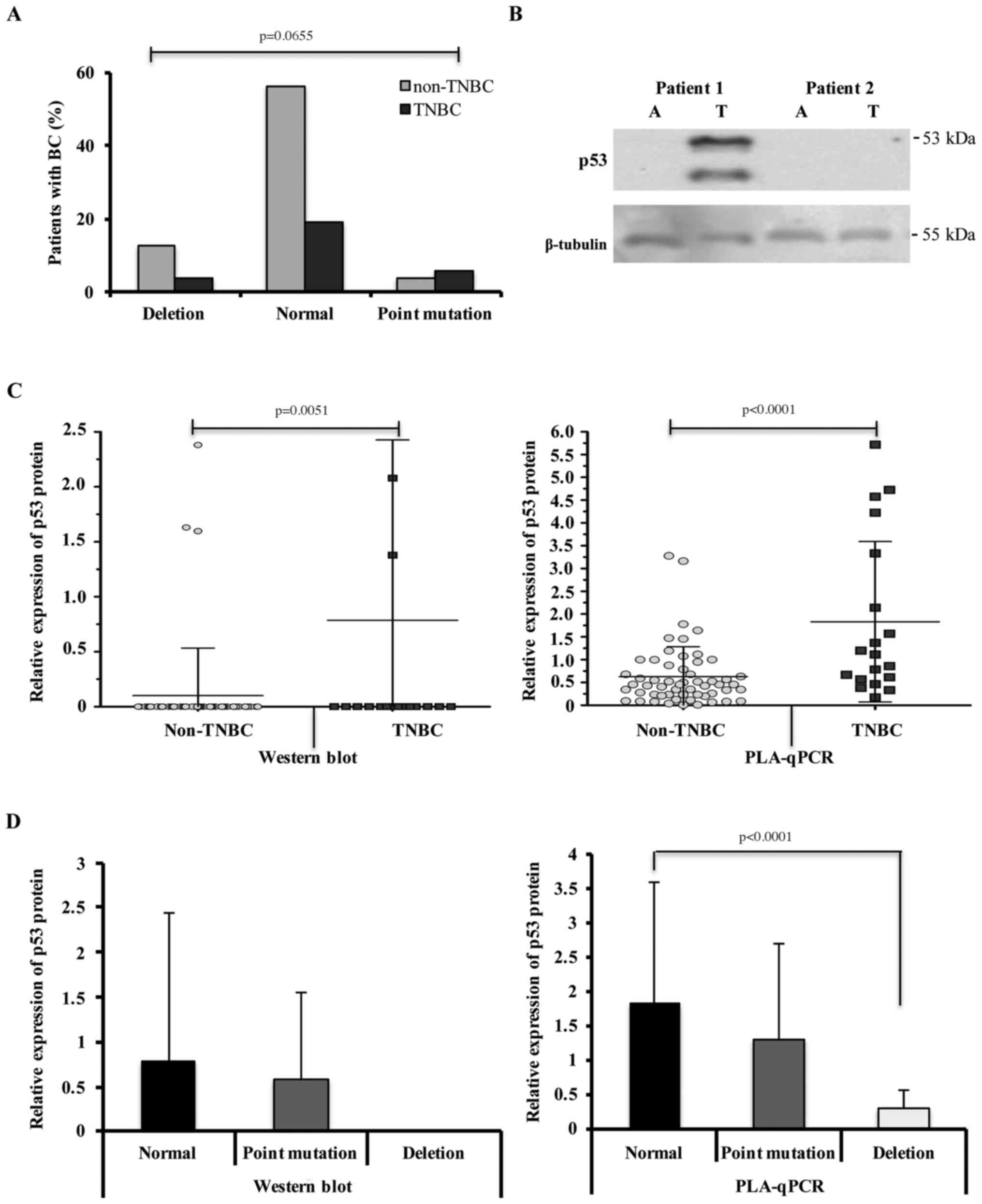
(p=0.0655) (Fig. 3A), and SNPs
(rs1294778 and rs12951053) (data not shown) identified for
p53.
To determine whether point mutations or deletions
found in the p53 gene compromise its protein expression, all
of the BC samples were analysed by western blotting. A
representative image of western blotting of the p53 protein is
shown in Fig. 3B. When comparing
p53 protein expression levels, we observed a statistically
significant average of 0.78 (±1.63) in the TNBC group (p=0.005)
compared with the non-TNBC group (mean, 0.10±0.43) (Fig. 3C). We normalized the relative
expression of the p53 protein using the loading control β-tubulin
since normal adjacent tissue lack or present low levels of p53
protein. In contrast, p53 mutant proteins have a characteristically
long half-life (4,17).
Next, we validated the results of p53 protein
expression using PLA-qPCR, a technique more sensitive than western
blotting. We found an average p53 protein expression of 1.83
(±1.75) and 0.62 (±0.65) in the TNBC and non-TNBC groups,
respectively. The difference among these results was statistically
significant (p<0.0001) (Fig.
3C).
Furthermore, we analysed an association among
p53 gene alterations with p53 protein expression. We
observed that patients carrying deletions in the p53 gene
present underexpression or null expression of the p53 protein, as
shown by PLA-qPCR or western blotting analysis, respectively
(Fig. 3D). Patients with different
mutations in the p53 gene present either underexpression or
overexpression of the p53 protein, with both situations observed
through western blotting and PLA-qPCR (Fig. 3D). In some cases, patients showing
no alterations (point mutations or deletions) in exons 5–8 also
showed expression of the p53 protein. This may be due to mutations
localized in the unexamined regions of the p53 gene
(Fig. 3D).
Genetic alterations of the TOP2α gene
and protein expression
Similar to p53, TOP2α is altered in cancer. Thus, we
evaluated large-scale mutations (amplifications or deletions) for
the TOP2α gene by qPCR. We found that 32.4% of all patients
analysed showed genetic alterations in this gene, among whom 20.6%
presented deletions and 11.8% showed amplifications of the
TOP2α gene (Table I).
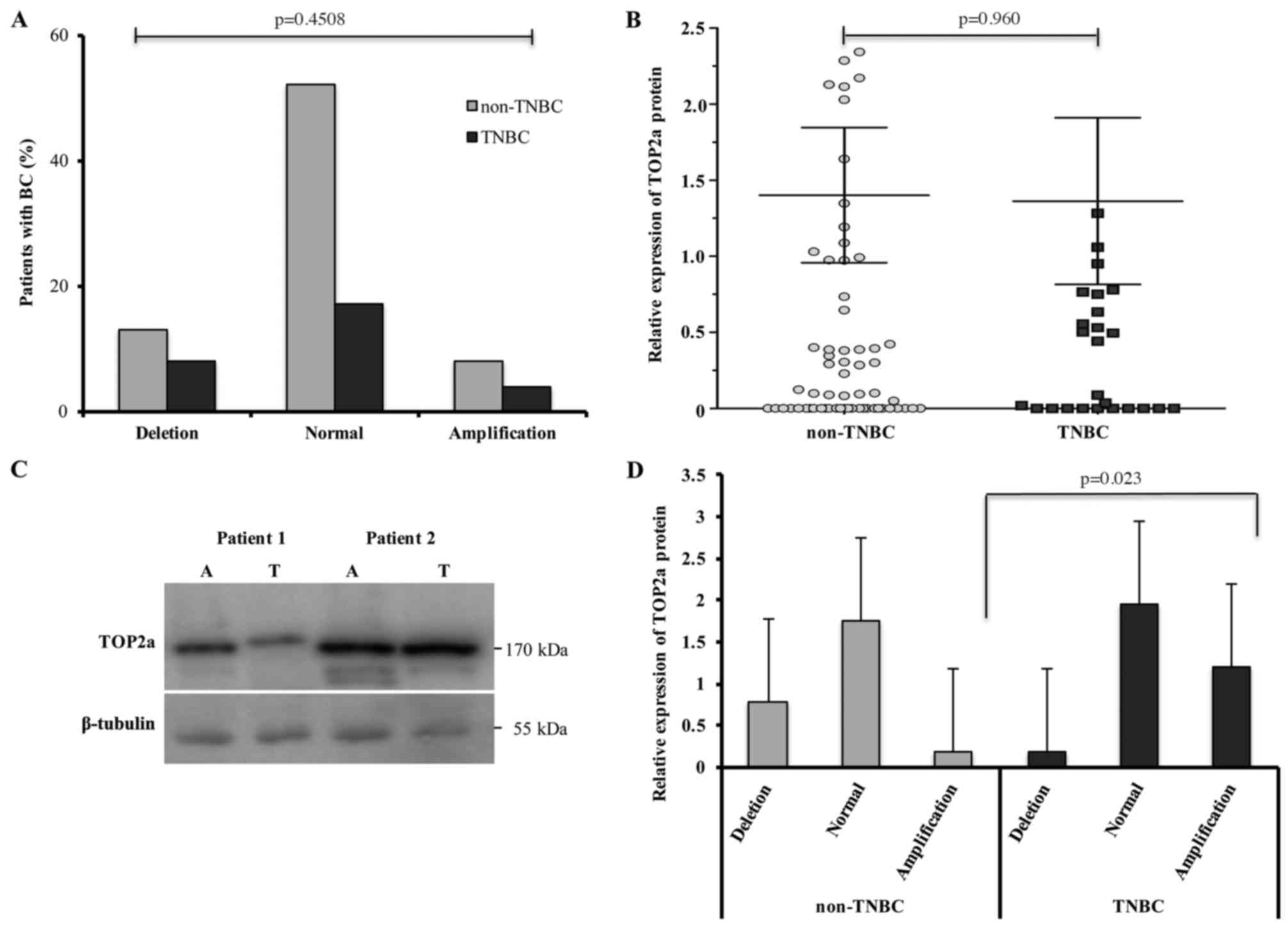
However, there were no significant differences in either
TOP2α gene deletions or amplifications between the TNBC and
non-TNBC patient groups (p=0.4508) (Fig. 4A). To determine whether such
amplifications or deletions exert any effect on TOP2α protein
expression, the BC samples were evaluated by western blot analysis
(adjacent and tumour tissues). We observed that the average TOP2α
protein expression was 1.36 (±2.95) in TNBC and 1.40 (±3.79) in the
non-TNBC group. The patients showed similar expression levels of
TOP2α protein between the groups without a significant difference
(p=0.960) (Fig. 4B). We show a
representative image of the western blotting of proteins obtained
of two patients with BC for TOP2α protein. β-tubulin was used as
the loading control (Fig. 4C). When
comparing the amplifications or deletions and protein expression of
TOP2α, we observed a higher expression of the TOP2α protein in
patients lacking any TOP2α genetic alterations and decreased
TOP2α protein levels in patients carrying TOP2α deletions.
Individual group evaluations revealed higher TOP2α protein
expression in TNBC patients carrying gene amplifications when
compared with non-TNBC patients (p=0.023) (Fig. 4D).
Associated characteristics in patients
with BC
To determine the association between the clinical
and pathological characteristics (clinical group, histological
grade) of patients with genetic alterations (previously described)
in the TOP2α and p53 genes and the expression level
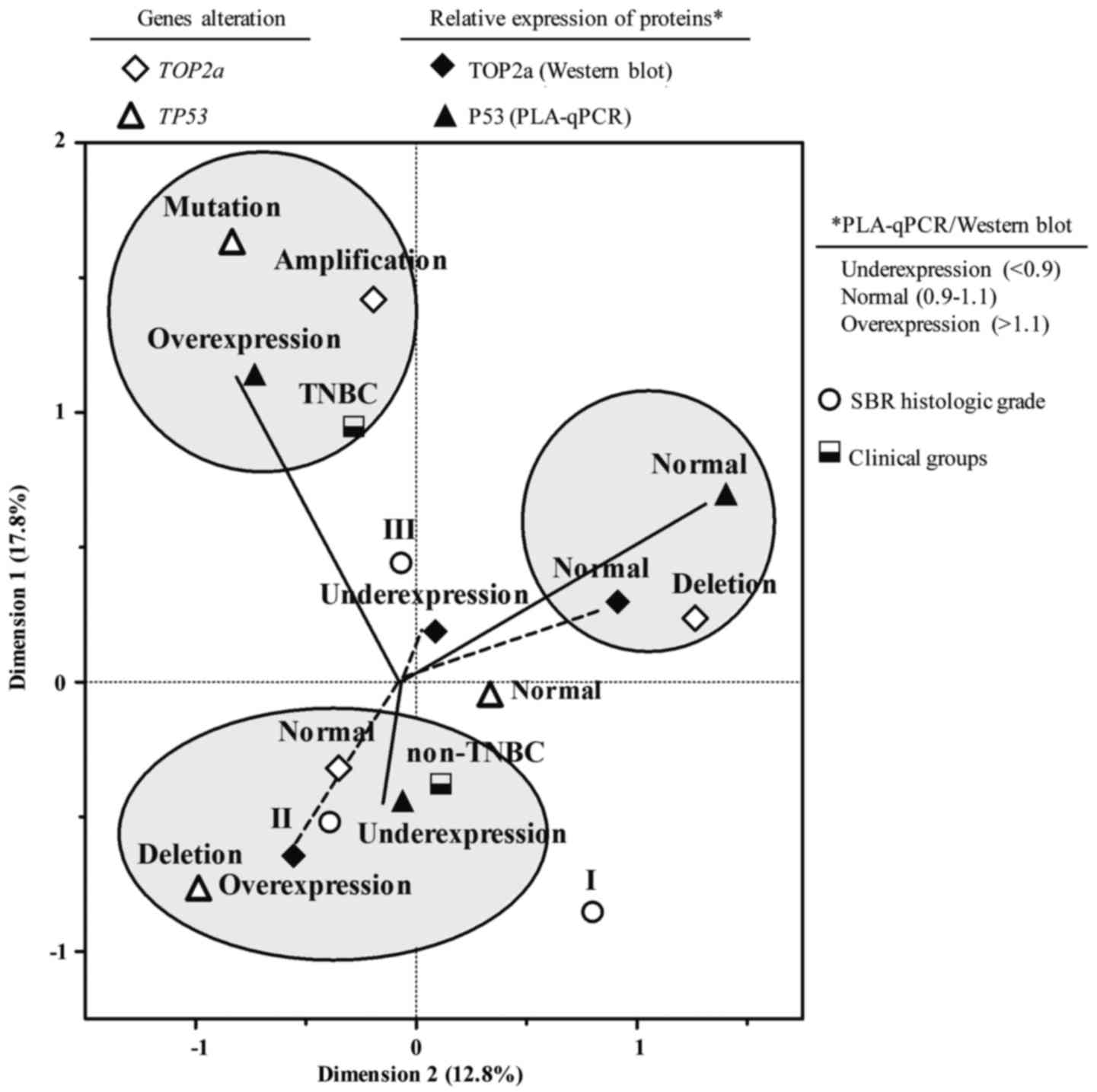
of proteins, we carried out an MCA. We identified three groups by
means of an MCA as follows: the first group of TNBC patients was
associated with a point mutation in the p53 gene and p53
protein overexpression, due to a increase in the p53 protein's
half-life. Additionally, we detected the amplification of the
TOP2α gene in this group despite the fact that TOP2α protein
expression was not associated with any clinical group. The second
group of non-TNBC patients was associated with a p53 gene
deletion, underexpression of the p53 protein, a non-altered
TOP2α gene (although overexpressed protein), and SBR
histological grade II. The third group represented by patients of
any group (TNBC or non-TNBC) presented with a TOP2α gene
deletion and a baseline expression level of both the p53 and TOP2α
proteins (Fig. 5).
Survival analysis of patients with
BC
The follow-up period for patients studied with BC
was set at 26 months, and the patients are undergoing continued
follow-up. We observed a general survival of 91.2% in the study
population. In the groups, we observed a 79.3% survival for the
TNBC group and 95.8% for the non-TNBC group (p=0.015). In relation
to time of survival for the study population, the mean was 25.36
months, with 24.1 months for the TNBC group and 25.8 months for the
non-TNBC group (p=0.002) (data not shown).
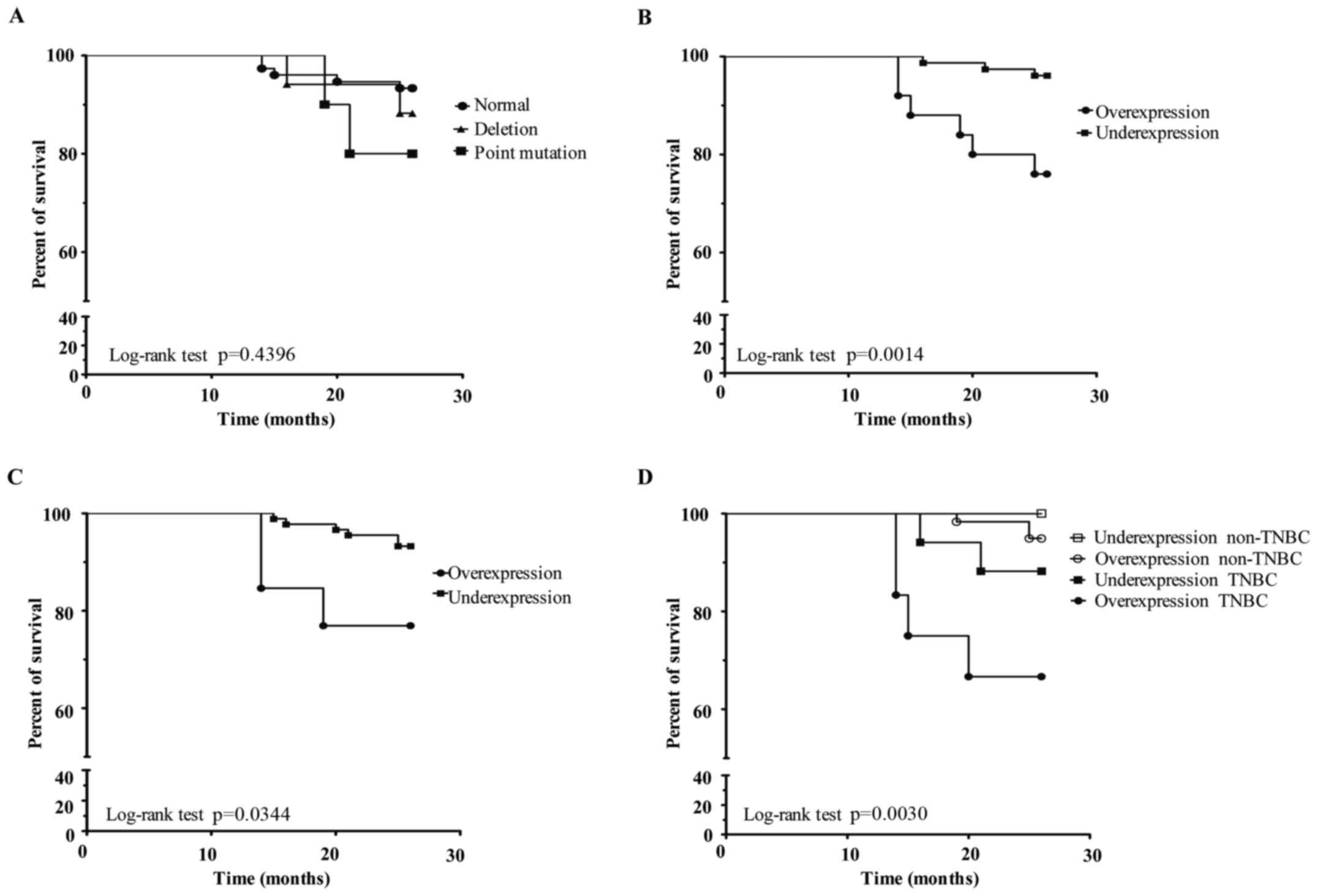
After that, we analysed the prognostic factor of
point mutations or deletions in the p53 gene. In patients
with BC that presented a deletion in the p53 gene, the
survival was 88.2%; in patients who had a point mutation, the
survival was 80%. In neither case was the survival statistically
significant (p=0.4396) (Fig. 6A).
In contrast, when we analysed p53 protein expression through
PLA-qPCR (Fig. 6B) in the same
group of patients, we found that p53 overexpression was associated
with a lower survival (76%) compared with p53 underexpression
(96%), suggesting a poor prognosis factor (p=0.0014). Similarly,
using western blotting we found an association between p53
overexpression and a lower survival (76.9%, p=0.0344) (Fig. 6C). In addition, when we analysed p53
protein expression and its association with a clinical group, we
observed that TNBC patients with p53 overexpression presented a
lower survival (p=0.0030) (Fig.
6D).
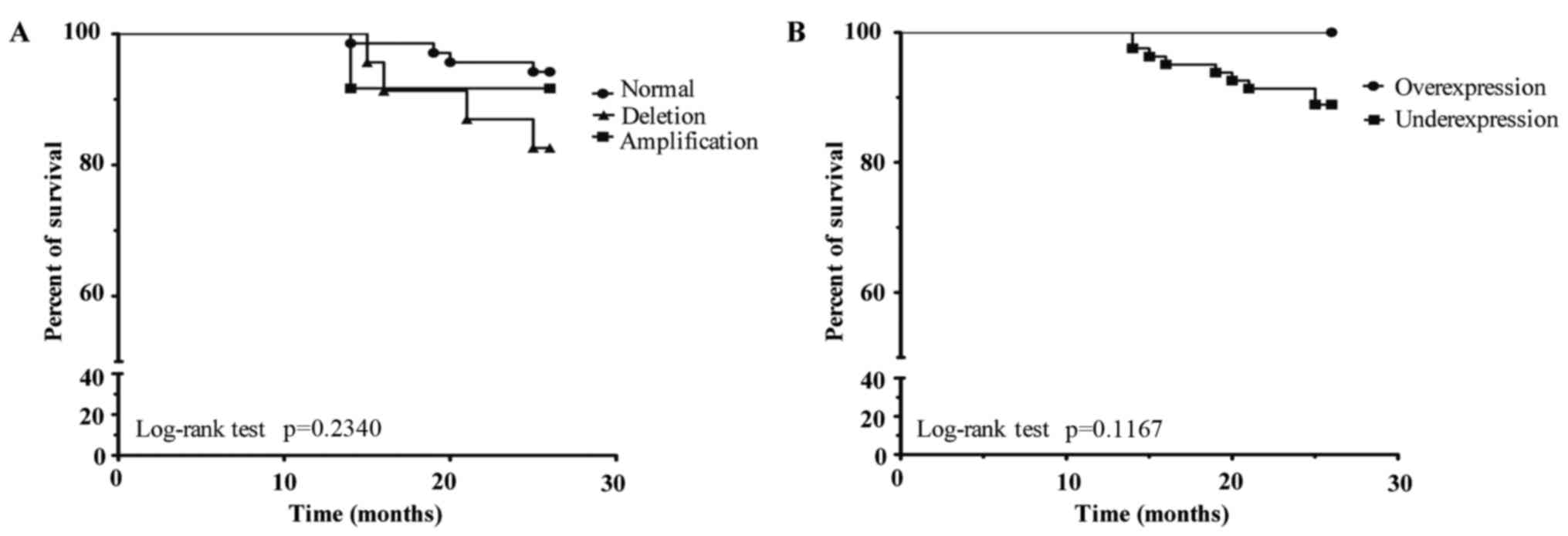
Finally, we analysed whether amplifications or
deletions in the TOP2α gene are a prognostic factor. To
perform this analysis, two groups were generated as follows: i) a
group without genetic alterations of the TOP2α gene (normal)
presented with a survival of 94.2%; ii) a group with amplification
presented with a survival of 91.6%; and iii) a group with deletion
presented with a survival of 82.6% (p=0.2340) (Fig. 7A). Although patients with TOP2α
underexpression had a survival of 88.8%, this result was not
statistically significant (p=0.1167) (Fig. 7B). However, neither TOP2α
amplifications or deletions nor protein expression was a factor for
poor prognosis in patients with BC.
Discussion
We found that overexpression of the p53 protein was
associated with lower survival and could be considered to be a
factor for poor prognosis in Mexican women with BC.
In the present study, TNBC patients were
characterized as being younger at the time of diagnosis, with SBR
histological grade III, and presented recurrence, which have been
previously reported (18,19). Our findings agree with past studies
performed on Mexican TNBC patients or Hispanic women residing in
the US (18–20).
We did not find any correlation between
amplifications or deletions and protein expression of TOP2α, as has
been previously reported (11).
Brase et al solely identified an association in the
borderline limit between TOP2α mRNA and protein levels
(11). Similarly, TOP2α
amplifications or deletions and protein expression are not
considered to be factors for poor prognosis in the present study
population. Our results are in agreement with studies in which
these variables were also analysed without finding a difference in
prognosis (15,21). Nevertheless, there are other studies
where TOP2α overexpression is considered to be a factor for poor
prognosis (11,14,22).
Point mutations (missense) in the p53 gene,
localized mainly in the DNA binding domain (exons 5–8), are
associated with worse prognosis and are considered to be predictive
factors of anthracycline treatment unresponsiveness (1,7). We
found genetic alterations (point mutations or deletions) for the
p53 gene similar to previously reported frequencies
(3,4,23). The
alterations most frequently found in the present study were
deletions (17%), despite the fact that point mutations are reported
as the most common. Similar to the present study, several studies
(in different tumours) also have found p53 gene deletions
ranging from 8 to 25% (24–26). We observed deletions in only one
allele, localized in exons 5–6 of p53 gene. One sample was
characterized by the deletion of 65 bp in codons 191–216 (exon 6),
which to date has not been reported in the tumour mutation database
for p53 gene [http://wwwp53.iarc.fr/]. Unfortunately, it was not
possible to characterize the deletions detected in all samples due
to insufficient sample material. Similar to what has been reported,
we found that point mutations were more frequent in TNBC patients
(4,17,27).
It is known that some p53 point mutations can increase the
half-life of the protein, thus favouring its overexpression
(4,17). In agreement with these studies, we
observed p53 overexpression in TNBC patients. However, we did not
find that point mutations or deletions of the p53 gene were
associated with survival. We observed that patients with point
mutations in the p53 gene have a survival of <80%, which
was not statistically significant (p=0.4396). It is possible that
with a larger sample size, point mutations in the p53 gene
may be shown to be a poor prognostic factor. Nevertheless,
overexpression of the p53 protein was significantly associated with
a lower survival (p=0.0014), and we suggest in the present study
that it could be a factor for poor prognosis in Mexican patients
with BC. This result was observed using both analysis techniques,
PLA-qPCR and western blotting. In addition, overexpression of the
p53 protein was a worse prognostic indicator for patients with
TNCB. Our results coincide with others studies that report the
overexpression of p53 protein is a factor of poor prognosis in BC,
and particularly in the TNBC subtype (28–30).
Additionally, we identified a high frequency of Mexican patients
with TNBC. Other studies, have also reported a high frequency of
the TNBC subtype in Mexican patients with BC (18,20).
Therefore, in the future the identification of TNBC patients (which
on its own is a poor prognosis) who present overexpression of the
p53 protein (as a prognostic biomarker) should be carried out to
establish health policies and suggest a treatment more personalized
for this group.
In addition, p53 mutations are considered to
be predictive factors for unresponsiveness to anthracycline
treatment schemes (7,25,31).
The overexpression of the TOP2α protein is also considered to be a
predictive factor for anthracycline sensitivity, whereas deletion
status is associated with increased resistance to the same drug
(10–13). We cannot determine the predictive
value of these molecules with the anthracycline scheme since they
were not administered with neoadjuvant chemotherapy in the study
population. However, it is possible that at the end of five years
of follow-up of the patients, we may be able to establish an
association between recurrence and genetic alterations, and p53 and
TOP2α protein expression. We suggest that recurrence is the result
of a negative response to the anthracycline scheme since 94.1%
received adjuvant chemotherapy containing anthracycline, adriamycin
and cyclophosphamide (A/C), and 5-fluorouracil, adriamycin and
cyclophosphamide (FAC). At this time, only 21.5% of the patients
have presented with recurrence, and 18.1% present with deletion of
the TOP2α gene, which has been reported to be associated
with resistance to anthracyclines (10–13).
Furthermore, 18.1% of patients had TOP2α overexpression, which is
considered to be a predictive factor for drug sensitivity (10–13),
and 27.2% had mutations in the p53 gene, which are
associated with poor response to anthracycline treatment (7,23,31).
Finally, using MCA, we identified three groups that
defined the present study population. The first group comprised
TNBC patients presenting with p53 mutations, p53 protein
overexpression, and TOP2α amplification. This group meets
the characteristics from previous studies describing genetic
alterations of the p53 gene, or its protein overexpression,
in TNBC patients. The second group comprised non-TNBC patients
demonstrating p53 gene deletions, underexpression of the p53
protein, and an unaltered TOP2α gene, but overexpression of
the TOP2α protein and SBR histological grade II. This group is
composed of patients with BC presenting a 65-bp deletion in the
p53 gene, thus explaining its protein underexpression.
Conversely, p53 mutants could induce TOP2α expression;
however, we did not observe any correlation between p53
point mutations (overexpression of p53) and TOP2α protein
overexpression (data not shown), suggesting that mechanisms other
than p53 mutations are able to induce or regulate TOP2α
expression (32,33). The third group included both
non-TNBC and TNBC patients carrying a deletion in the TOP2α
gene and with baseline expression of the TOP2α and p53 proteins.
This analysis allowed us to identify well-defined groups of
patients associated with the characteristics analysed who exhibited
tumour heterogeneity. Furthermore, through this analysis, we
identified a specific group containing TNBC patients and p53
overexpression, both characteristics that are associated with poor
prognosis. Similarly, MAC has been used to associate
clinicopathological features of patients with cancer (34). The limitations of the present study
included a short follow-up time period for the patients,
insufficient DNA material for identifying deletions in all samples
with genetic alterations of the p53 gene, and only two
schemes of available adjuvant chemotherapy (with or without
anthracyclines) for comparison. It is necessary to carry out
studies with a larger sample size to validate our results.
The present study population, is characterized by a
high frequency of TNBC cases found mainly among the younger
patients exhibiting SBR grade III, p53 gene mutations, and
protein overexpression. P53 overexpression was associated with a
lower survival in patients with BC, suggesting that it is a factor
for poor prognosis in our population.
Acknowledgements
Este estudio representa parte de los requisitos para
obtener el grado a Doctor de Jisela Dimas González estudiante del
Programa de Doctorado en Ciencias Biomédicas de la Universidad
Nacional Autónoma de México (UNAM). JDG agradece el apoyo de
CONACYT (no. Becario 175068). The authors wish to thank Enrique
Garcia Villa (CINVESTAV-1PN) and Rubén Arturo Cortés González
(INSP) for their technical assistance, and the Red Temática
Farmoquímicos (CONACYT, #271520) for help in to carry out the
English language editing of the present study. This study was
supported by grants from CONACyT (#168896) and (#261875) to J.
Díaz-Chávez.
References
|
1
|
Olivier M, Langerød A, Carrieri P, Bergh
J, Klaar S, Eyfjord J, Theillet C, Rodriguez C, Lidereau R, Bièche
I, et al: The clinical value of somatic TP53 gene mutations in
1,794 patients with breast cancer. Clin Cancer Res. 12:1157–1167.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Olivier M, Hollstein M and Hainaut P: TP53
mutations in human cancers: Origins, consequences, and clinical
use. Cold Spring Harb Perspect Biol. 2:a0010082010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Banerji S, Cibulskis K, Rangel-Escareno C,
Brown KK, Carter SL, Frederick AM, Lawrence MS, Sivachenko AY,
Sougnez C, Zou L, et al: Sequence analysis of mutations and
translocations across breast cancer subtypes. Nature. 486:405–409.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fountzilas G, Giannoulatou E, Alexopoulou
Z, Zagouri F, Timotheadou E, Papadopoulou K, Lakis S, Bobos M,
Poulios C, Sotiropoulou M, et al: TP53 mutations and protein
immunopositivity may predict for poor outcome but also for
trastuzumab benefit in patients with early breast cancer treated in
the adjuvant setting. Oncotarget. 7:32731–32753. 2016.PubMed/NCBI
|
|
5
|
Bieging KT, Mello SS and Attardi LD:
Unravelling mechanisms of p53-mediated tumour suppression. Nat Rev
Cancer. 14:359–370. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cho Y, Gorina S, Jeffrey PD and Pavletich
NP: Crystal structure of a p53 tumor suppressor-DNA complex:
Understanding tumorigenic mutations. Science. 265:346–355. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fernández-Cuesta L, Oakman C,
Falagan-Lotsch P, Smoth KS, Quinaux E, Buyse M, Dolci MS, Azambuja
ED, Hainaut P, Dell'órto P, et al: Prognostic and predictive value
of TP53 mutations in node-positive breast cancer patients
treated with anthracycline- or anthracycline/taxane-based adjuvant
therapy: Results from the BIG 02–98 phase III trial. Breast Cancer.
14:R702012. View
Article : Google Scholar
|
|
8
|
Wang Q, Zambetti GP and Suttle DP:
Inhibition of DNA topoisomerase IIα gene expression by the p53
tumor suppressor. Mol Cell Biol. 17:389–397. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Joshi AA, Wu Z, Reed RF and Suttle DP:
Nuclear factor-Y binding to the topoisomerase IIalpha promoter is
inhibited by both the p53 tumor suppressor and anticancer drugs.
Mol Pharmacol. 63:359–367. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bartlett JM, Munro AF, Dunn JA, McConkey
C, Jordan S, Twelves CJ, Cameron DA, Thomas J, Campbell FM, Rea DW,
et al: Predictive markers of anthracycline benefit: A prospectively
planned analysis of the UK National Epirubicin Adjuvant Trial
(NEAT/BR9601). Lancet Oncol. 11:266–274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brase JC, Schmidt M, Fischbach T, Sültmann
H, Bojar H, Koelbl H, Hellwig B, Rahnenführer J, Hengstler JG and
Gehrmann MC: ERBB2 and TOP2A in breast cancer: A comprehensive
analysis of gene amplification, RNA levels, and protein expression
and their influence on prognosis and prediction. Clin Cancer Res.
16:2391–2401. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Di Leo A, Desmedt C, Bartlett JM, Piette
F, Ejlertsen B, Pritchard KI, Larsimont D, Poole C, Isola J, Earl
H, et al: HER2/TOP2Α Meta-analysis Study Group: HER2 and
TOP2A as predictive markers for anthracycline-containing
chemotherapy regimens as adjuvant treatment of breast cancer: A
meta-analysis of individual patient data. Lancet Oncol.
12:1134–1142. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Won HS, Lee KE, Sung SH, Choi MY, Jo JY,
Nam EM, Mun YC, Seong CM and Lee SN: Topoisomerase II alpha and
microtubule-associated protein-tau as a predictive marker in
axillary lymph node positive breast cancer. Tumori. 100:80–86.
2014.PubMed/NCBI
|
|
14
|
Györffy B, Lanczky A, Eklund AC, Denkert
C, Budczies J, Li Q and Szallasi Z: An online survival analysis
tool to rapidly assess the effect of 22,277 genes on breast cancer
prognosis using microarray data of 1,809 patients. Breast Cancer
Res Treat. 123:725–731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qiao JH, Jiao DC, Lu ZD, Yang S and Liu
ZZ: Clinical significance of topoisomerase 2A expression and gene
change in operable invasive breast cancer. Tumour Biol.
36:6833–6838. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hill MO: Correspondence analysis: A
neglected multivariate method. Appl Stat. 3:340–354. 1974.
View Article : Google Scholar
|
|
17
|
Kim JY, Park K, Jung HH, Lee E, Cho EY,
Lee KH, Bae SY, Lee SK, Kim SW, Lee JE, et al: Association between
mutation and expression of TP53 as a potential
prognostic marker of triple-negative breast cancer. Cancer Res
Treat. 48:1338–1350. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lara-Medina F, Pérez-Sánchez V,
Saavedra-Pérez D, Blake-Cerda M, Arce C, Motola-Kuba D,
Villarreal-Garza C, González-Angulo AM, Bargalló E, Aguilar JL, et
al: Triple-negative breast cancer in Hispanic patients: High
prevalence, poor prognosis, and association with menopausal status,
body mass index, and parity. Cancer. 117:3658–3669. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liedtke C, Mazouni C, Hess KR, André F,
Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B,
Green M, et al: Response to neoadjuvant therapy and long-term
survival in patients with triple-negative breast cancer. J Clin
Oncol. 26:1275–1281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Banegas MP, Tao L, Altekruse S, Anderson
WF, John EM, Clarke CA and Gomez SL: Heterogeneity of breast cancer
subtypes and survival among Hispanic women with invasive breast
cancer in California. Breast Cancer Res Treat. 144:625–634. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fountzilas G, Christodoulou C, Bobos M,
Kotoula V, Eleftheraki AG, Xanthakis I, Batistatou A,
Pentheroudakis G, Xiros N, Papaspirou I, et al: Topoisomerase II
alpha gene amplification is a favorable prognostic factor in
patients with HER2-positive metastatic breast cancer treated with
trastuzumab. J Transl Med. 10:2122012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hua W, Sa KD, Zhang X, Jia LT, Zhao J,
Yang AG, Zhang R, Fan J and Bian K: MicroRNA-139 suppresses
proliferation in luminal type breast cancer cells by targeting
Topoisomerase II alpha. Biochem Biophys Res Commun. 463:1077–1083.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dobes P, Podhorec J, Coufal O, Jureckova
A, Petrakova K, Vojtesek B and Hrstka R: Influence of mutation type
on prognostic and predictive values of TP53 status in
primary breast cancer patients. Oncol Rep. 32:1695–1702.
2014.PubMed/NCBI
|
|
24
|
Bazrafshani MR, Nowshadi PA, Shirian S,
Daneshbod Y, Nabipour F, Mokhtari M, Hosseini F, Dehghan S,
Saeedzadeh A and Mosayebi Z: Deletion/duplication mutation
screening of TP53 gene in patients with transitional cell
carcinoma of urinary bladder using multiplex ligation-dependent
probe amplification. Cancer Med. 5:145–152. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Delfau-Larue MH, Klapper W, Berger F,
Jardin F, Briere J, Salles G, Casasnovas O, Feugier P, Haioun C,
Ribrag V, et al: European Mantle Cell Lymphoma Network: High-dose
cytarabine does not overcome the adverse prognostic value of
CDKN2A and TP53 deletions in mantle cell lymphoma.
Blood. 126:604–611. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dufour A, Palermo G, Zellmeier E, Mellert
G, Duchateau-Nguyen G, Schneider S, Benthaus T, Kakadia PM,
Spiekermann K, Hiddemann W, et al: Inactivation of TP53 correlates
with disease progression and low miR-34a expression in previously
treated chronic lymphocytic leukemia patients. Blood.
121:3650–3657. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Millis SZ, Gatalica Z, Winkler J, Vranic
S, Kimbrough J, Reddy S and O'Shaughnessy JA: Predictive biomarker
profiling of > 6000 breast cancer patients shows heterogeneity
in TNBC, with treatment implications. Clin Breast Cancer.
15:473–481.e3. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang P, Du CW, Kwan M, Liang SX and Zhang
GJ: The impact of p53 in predicting clinical outcome of breast
cancer patients with visceral metastasis. Sci Rep. 3:22462013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ren J, Chen QC, Jin F, Wu HZ, He M, Zhao
L, Yu ZJ, Yao WF, Mi XY, Wang EH, et al: Overexpression of Rsf-1
correlates with pathological type, p53 status and survival in
primary breast cancer. Int J Clin Exp Pathol. 7:5595–5608.
2014.PubMed/NCBI
|
|
30
|
Maeda T, Nakanishi Y, Hirotani Y,
Fuchinoue F, Enomoto K, Sakurai K, Amano S and Nemoto N:
Immunohistochemical co-expression status of cytokeratin 5/6,
androgen receptor, and p53 as prognostic factors of adjuvant
chemotherapy for triple negative breast cancer. Med Mol Morphol.
49:11–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lehmann BD, Ding Y, Viox DJ, Jiang M,
Zheng Y, Liao W, Chen X, Xiang W and Yi Y: Evaluation of public
cancer datasets and signatures identifies TP53 mutant signatures
with robust prognostic and predictive value. BMC Cancer.
15:1792015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Srikantan S, Abdelmohsen K, Lee EK,
Tominaga K, Subaran SS, Kuwano Y, Kulshrestha R, Panchakshari R,
Kim HH, Yang X, et al: Translational control of TOP2A influences
doxorubicin efficacy. Mol Cell Biol. 31:3790–3801. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tamaichi H, Sato M, Porter AC, Shimizu T,
Mizutani S and Takagi M: Ataxia telangiectasia mutated-dependent
regulation of topoisomerase II alpha expression and sensitivity to
topoisomerase II inhibitor. Cancer Sci. 104:178–184. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rodriguez-Lara V, Peña-Mirabal E,
Baez-Saldaña R, Esparza-Silva AL, García-Zepeda E, Cervantes Cerbon
MA, Diaz D and Fortoul TI: Estrogen receptor beta and CXCR4/CXCL12
expression: Differences by sex and hormonal status in lung
adenocarcinoma. Arch Med Res. 45:158–169. 2014. View Article : Google Scholar : PubMed/NCBI
|