Introduction
Among many types of cancer, non-small cell lung
cancer (NSCLC) is associated with the highest morbidity and is the
most common cause of cancer-related deaths worldwide (1). Clinical data have demonstrated that
almost 80% of lung cancers are NSCLC with a considerably low
survival rate (5-year survival rate <15%) (2,3).
Despite the recent improvements in chemotherapy and
molecular-targeted therapy, patients with advanced NSCLC still have
poor prognoses (4–6). Therefore, the challenge in the
treatment of NSCLC is to identify novel targets that can repress
the invasiveness and metastasis of lung cancer cells.
Epithelial-mesenchymal transition (EMT) is a complex
trans-differentiation process in which epithelial cells lose
junctional adhesion and adopt a mesenchymal phenotype and
morphology. During the EMT process, neoplastic cells detach from
the primary epithelial tumor site, invade through the basement
membrane into the circulation, thereby contributing to the
metastatic potential increment and cancer progression (7,8).
Profilins (PFNs) are small proteins (12–15 kDa)
found in eukaryotes and identified as a protein directly
interacting with actin, acidic phospholipids and several proteins
which are key roles of different signaling pathways. The first
isoforms of the PFN family are PFN1 and PFN2 which were discovered
several years ago. There is increasing evidence in recent years to
support the role of PFN2 in increasing tumor progression. Kim et
al showed that PFN2 promotes migration, invasion, and stemness
of HT29 human colorectal cancer stem cells (9). Cui et al demonstrated that PFN2
promotes tumor progression and metastasis in esophageal squamous
cell carcinoma (ESCC) (10).
MicroRNAs (miRNAs) are small endogenous non-coding
interfering RNA molecules. miRNAs often occur abnormal expression
in human tumors (11) and act as
essential modulators for cell proliferation, cell invasion and EMT
(12). miR-30a-5p was previously
reported to be frequently downregulated in various human tumors
including hepatocellular carcinoma (13), non-melanoma skin cancer (14), and lung cancer (15,16).
It has been reported that miR-30a-5p suppresses cell growth, cell
proliferation, whereas induces cell apoptosis in hepatocellular
carcinoma (12,17) and breast cancer (18). These results suggested that
miR-30a-5p acts as a tumor suppressor and may serve as a
therapeutic target for tumor patients. miR-30a-5p can exert its
function by binding to the 3′-untranslated region (3′-UTR) of
target gene mRNA and leading to mRNA cleavage or translational
repression (19). Xiong et
al reported that ubiquitin protein ligase E3C (UBE3C) was a
direct target of miR-30a-5p in breast cancer cells and the
expression of miR-30a-5p was highly negatively correlated with
UBE3C (18). He et al
demonstrated astrocyte elevated gene 1 (AEG-1) was a direct target
of miR-30a-5p in hepatocellular carcinoma cells (17).
In the present study, we used the Target Scan
database predicted that there was a binding site for miR-30a-5p in
the 3′-UTR of PFN2 mRNA, along with the inverse function of PFN2
and miR-30a-5p in NSCLC development and progression as showed in
previous reports. We predicted that PFN2 might be a potential
target for miR-30a-5p in NSCLC. Also, the aim of the present study
was to analyze the association of PFN2 and miR-30a-5p expression in
NSCLC progression.
Materials and methods
Cell lines and cell culture
The human bronchial epithelial cell 16HBE and human
NSCLC cell lines A549, NcI-H520 and 95D were purchased from
American Type Culture Collection (ATCC, Manassas, VA, USA). Cells
were cultured in RPMI-1640 supplemented with 10% fetal bovine serum
(FBS) and 1% streptomycin at 37°C in a humidified 5% CO2
incubator. Cells were maintained in the medium and passaged 2–3
days.
Construction of expression vectors and
cell transfection
Total RNA from cells were isolated using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA), and treated with
RNase-free DNase (Takara, Dalian, China). Then the RNA molecules
were converted to cDNA using the PrimeScript® RT reagent
kit (Takara) with oligo-dT primers, according to the manufacturer's
protocol. The open reading frame of human PFN2 cDNA were cloned and
inserted into the pcDNA3.1 vector (Invitrogen) to construct the
recombinant pcDNA3.1-PFN2 expression vector. The pcDNA3.1 vector
transfected cells were also prepared. Transient transfection was
performed using the FuGENE HD transfection reagent (Roche,
Indianapolis, IN, USA) according to the manufacturer's
instructions, and overexpression was confirmed by western blotting
using the anti-Flag antibody.
siRNA transfection
PFN2 siRNA and control siRNA were purchased from
Cell Signaling (Beverly, MA, USA). The protocol of siRNA
transfection was performed according to the manufacturer's
instructions. Briefly, 5×104 cells were seeded in each
cell of 24-well micro-plates, grown for 24 h to reach 30–50%
confluence, and then incubated with a mixture of siRNA and
Lipofectamine 2000 reagent (Invitrogen) in 100 µl of serum-free
OPTI-MEM according to the manufacturer's instructions. The
transfection efficiency was examined by real-time PCR and western
blotting.
Real-time quantitative PCR
Total RNA containing small RNA was extracted from
cell lines using the mirVana miRNA isolation kit (Ambion, Austin,
TX, USA) according to the manufacturer's protocol. The expression
of miRNA was determined using the miRNA qPCR detection kit (Gene
Copoeia, USA) and the mRNA of different genes were amplified using
a SYBR® Premix Ex Taq™ II kit (Takara) and gene-specific
primers. Real-time quantitative RT-PCR was performed in a
Rotor-Gene RG-3000 Real-Time Thermal Cycler (Corbett Research,
Australia). All reactions were performed in triplicate, and the
relative expression levels for miRNA and mRNAs were normalized
using the 2−∆∆CT method (20) relative to small nuclear RNA U6 (U6
snRNA) and β-actin, respectively.
Western blotting
The RIPA lysis buffer (Beyotime, Nantong, China) was
used to extract proteins from cultured cells. The antibodies are as
follows: anti-PFN2 (1:1,000) anti-β-actin (1:500), HRP-conjugated
secondary (1:500) antibodies (KangChen Bio-tech, Shanghai, China).
ECL reagent (Beyotime) was used for detection. The luminescence was
scanned using a Typhoon scanner (Amersham Biosciences, Piscataway,
NJ, USA), and the protein band density was quantified using the
Quantity One software (version 4.2.2, Bio-Rad Laboratories,
Hercules, CA, USA), and the results were normalized according to
β-actin.
Enforced expression and knockdown of
miR-30a-5p
The miR-30a-5p mimics, negative control miRNA
(control miRNA) and anti-miR-30a-5p inhibitor (anti-miR-30a-5p)
were transfected into 95D cells, using Lipofectamine 2000
(Invitrogen) at a final concentration of 15 or 30 nM. The cells
were then harvested for analysis 24 h post-transfection.
Cell invasion assay
Cell invasion was evaluated using a Transwell
chamber assay (Costar, Pleasanta, CA, USA) according to the
manufacturer's protocol. Serum-free DMEM was added to the upper
chambers and DMEM containing 10% fetal calf serum was added to the
lower chambers. Cells were cultured for 48 h and transferred to the
upper chambers (1×105 cells per Transwell). After 24 h
of incubation, cells that had migrated to the lower surface were
fixed in 90% alcohol and stained with 0.08% crystal violet.
Luciferase reporter assay
A pmirGLO Dual-Luciferase miRNA target expression
vector was used for 3′-UTR luciferase assays (Promega, Madison, WI,
USA). For the luciferase reporter assays, cells cotransfected with
negative control, hsa-miR-30a-5p mimics, or anti-hsa-miR-30a-5p and
PFN2 expression vectors using Lipofectamine 2000. At 18 h
post-transfection, cells were assayed using luciferase assay kits
(Promega) according to the manufacturer's protocol. The results
were normalized with Renilla luciferase.
Statistical analysis
The SPSS version 19.0 software (SPSS, Chicago, IL,
USA) was used to analyze the related data with χ2 test
or t-test. A value of P<0.05 was considered statistically
significant.
Results
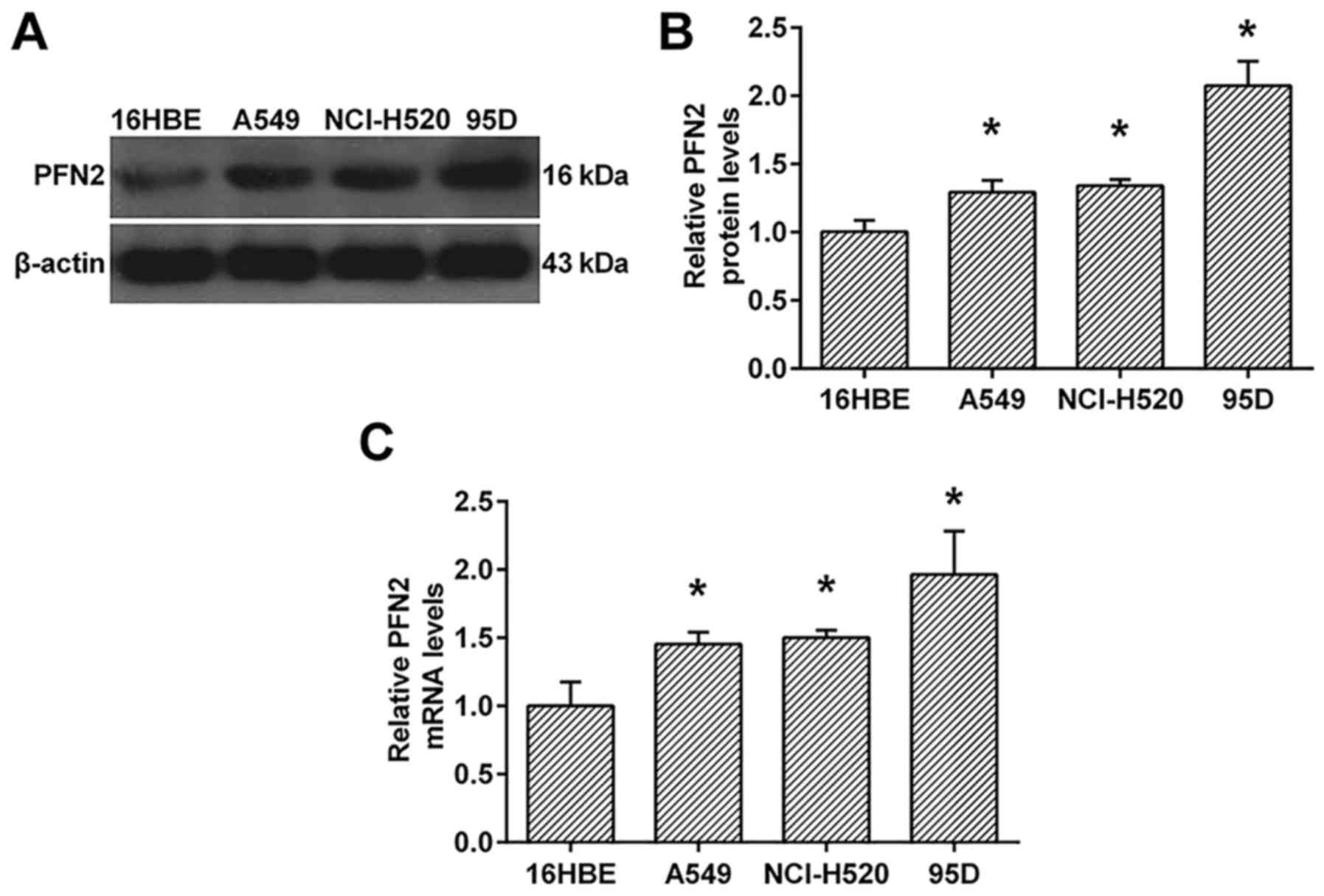
PFN2 is upregulated in NSCLC cell
lines
To investigate the probably role of PFN2 in NSCLC,
we investigate the protein and mRNA levels of PFN2 in NSCLC cell
lines, A549, NcI-H520 and 95D. As shown in Fig. 1, the protein and mRNA levels of PFN2
were all upregulated in NSCLC cell lines when compared to human
bronchial epithelial cell line, 16HBE (Fig. 1). Moreover, the expression of PFN2
in 95D cells was higher than those in A549 and NcI-H520 cells
(Fig. 1).
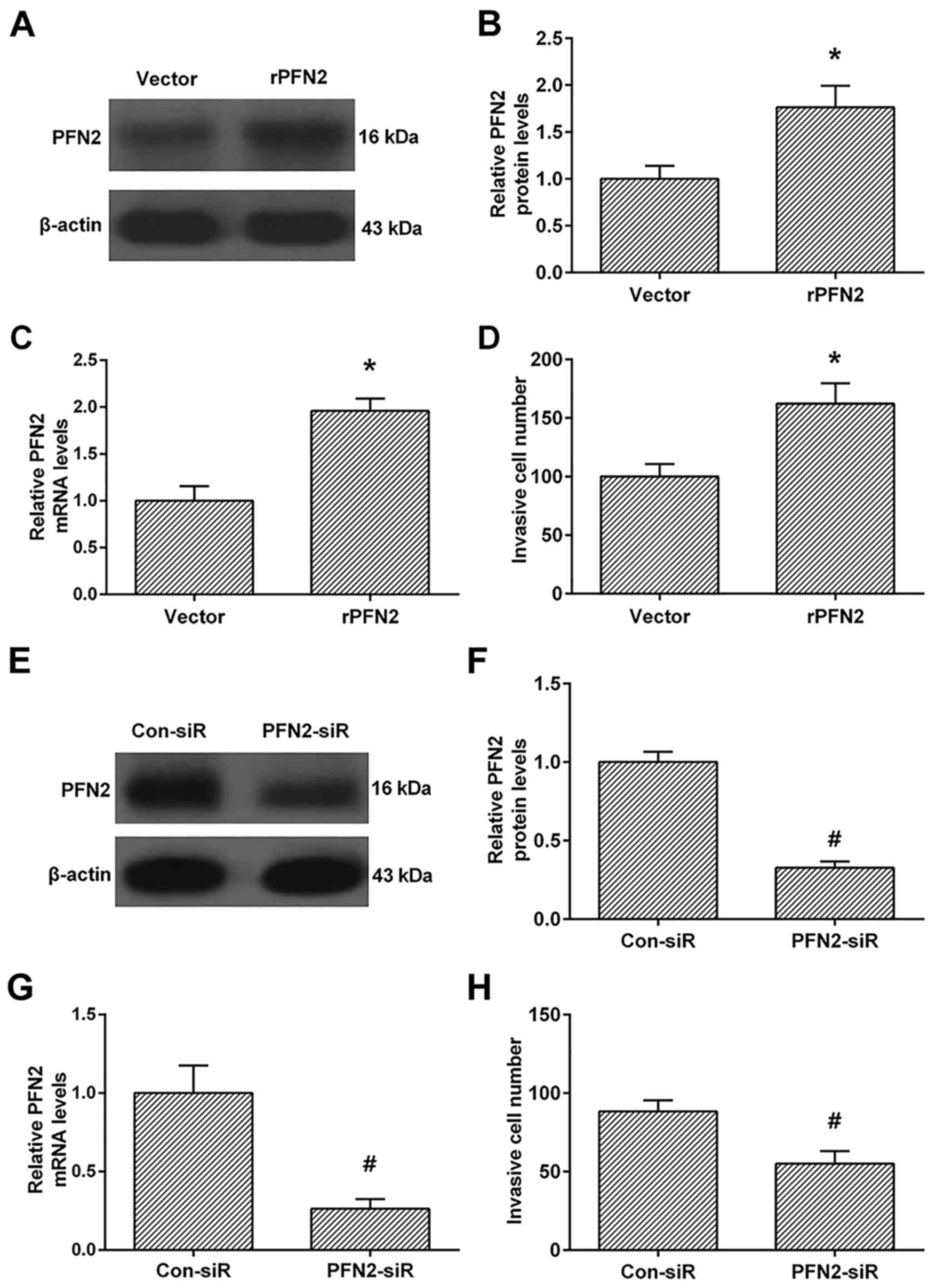
PFN2 enhances invasive ability in high
invasive NSCLC cell line
We then investigated the role of PFN2 in the
invasive ability in high invasive NSCLC cell line 95D after PFN2
upregulation. As shown in Fig.
2A-C, the protein and mRNA levels of PFN2 were significantly
upregulated in PFN2-transfected cells than vecter-transfected cells
(Fig. 2A-C). These results
confirmed that PFN2 overexpressing cells were successfully
established. We then investigated the invasive ability between the
vector and PFN2-transfected cells. The Transwell analysis revealed
that PFN2-transfection enhanced cell invasion when compared to the
vector-transfection group (Fig.
2D). Moreover, we also knocked down the PFN2 levels by PFN2
specific siRNA (Fig. 2E-G), and
demonstrated that PFN2-siRNA inhibits cell invasion compared to the
control group (Fig. 2H).
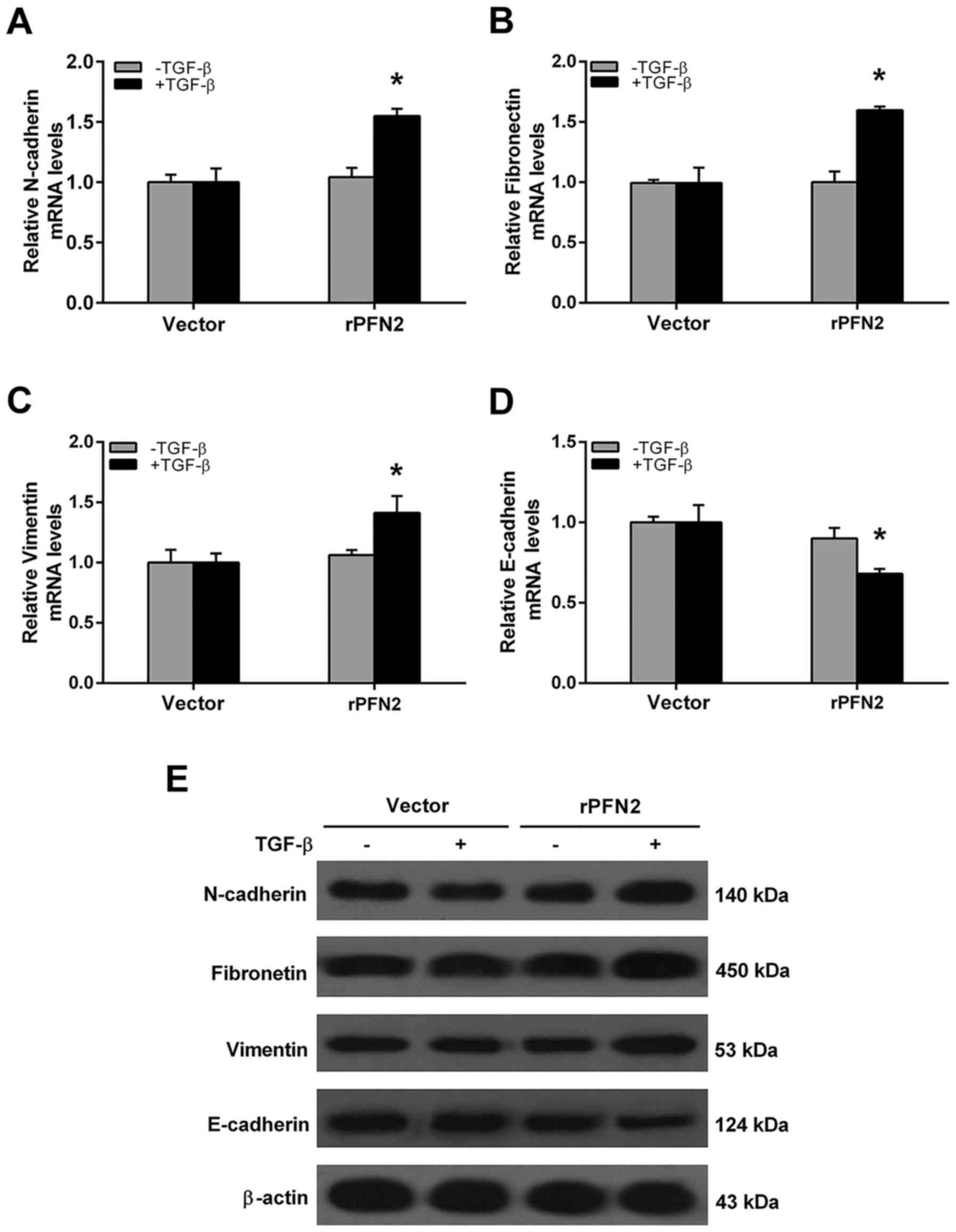
PFN2 promotes TGF-β-induced EMT in
high invasive NSCLC cell line
We evaluated the mRNA levels of EMT markers in 95D
cells treated with TGF-β to investigate the effect of PFN2 on EMT
phenomenon. In the vector group, the mRNA levels of N-cadherin,
fibronectin, vimentin and E-cadherin were all similar with or
without TGF-β treatment (Fig.
3A-D). However, after TGF-β stimulation, PFN2 transfected-95D
cells displayed increased mesenchymal cell marker N-cadherin,
fibronectin, vimentin and reduced epithelial marker E-cadherin
expressions when compared with the vector group (Fig. 3A-D). Moreover, the protein
expression levels of EMT markers were also evaluated by western
blot analysis. As shown in Fig. 3E,
under TGF-β treatment, PFN2 transfected-95D cells possess higher
N-cadherin, fibronectin, vimentin but lower E-cadherin protein
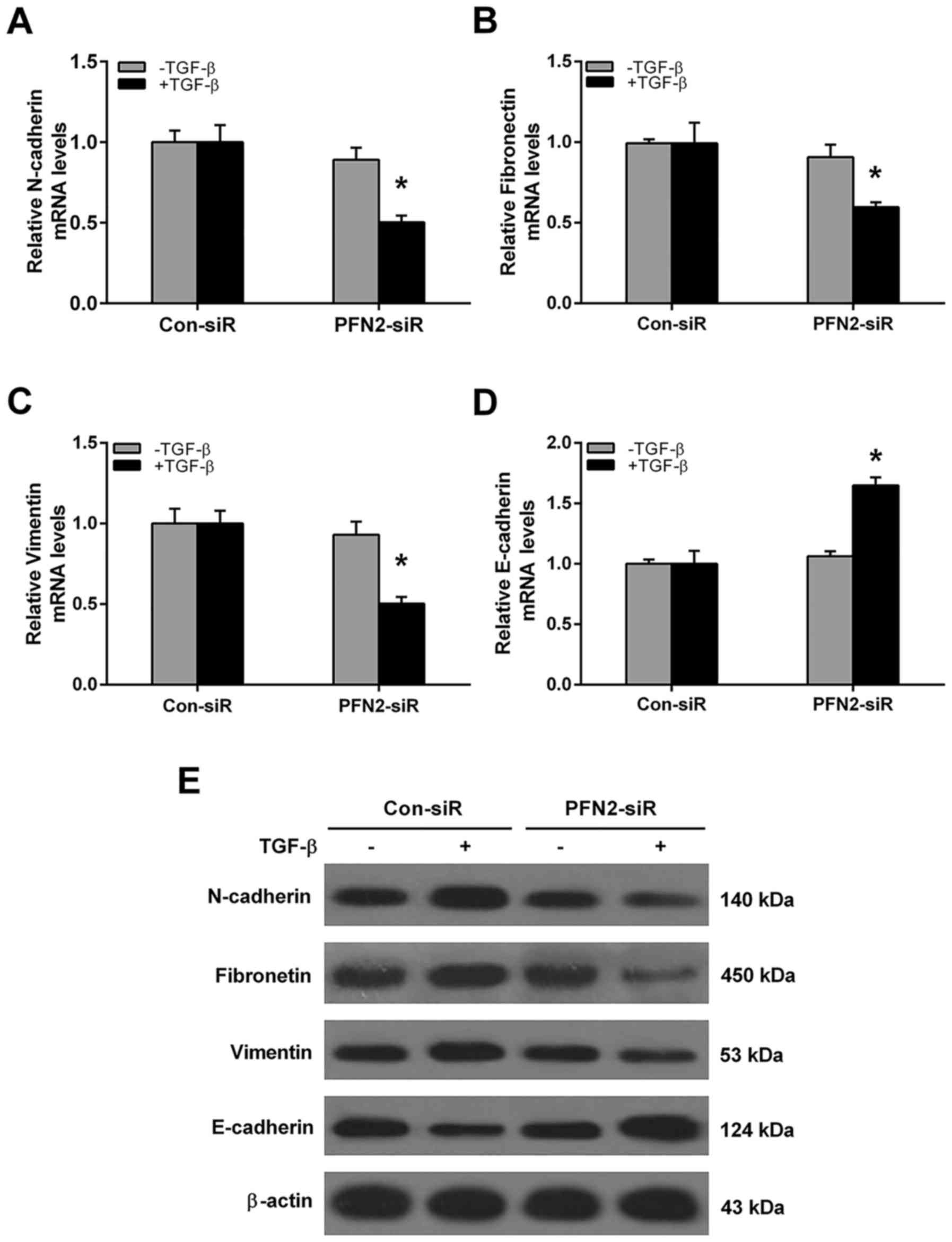
levels compared to the vector group. Conversely, the mRNA and
protein levels of EMT markers were also evaluated after PFN2-siR
treatment. As shown in Fig. 4,
without TGF-β treatment, the mRNA and protein levels of N-cadherin,
fibronectin, vimentin and E-cadherin were all similar between
control siRNA and PFN2-siRNA group. However, the PFN2 siRNA cells
displayed reduced mesenchymal cell marker N-cadherin, fibronectin,
vimentin and increased epithelial marker E-cadherin expressions
when compared to the control siRNA group under TGF-β stimulation
(Fig. 4). These results suggested
that PFN2 positively regulated TGF-β-induced EMT in NSCLC
cells.
Inverse correlation between PFN2 and
miR-30a-5p expression levels
To identify candidate miRNAs that can control 95D
cell invasion and EMT by modulating PFN2 expression, we used the
Target Scan database (http://www.targetscan.org) to search for miRNA binding
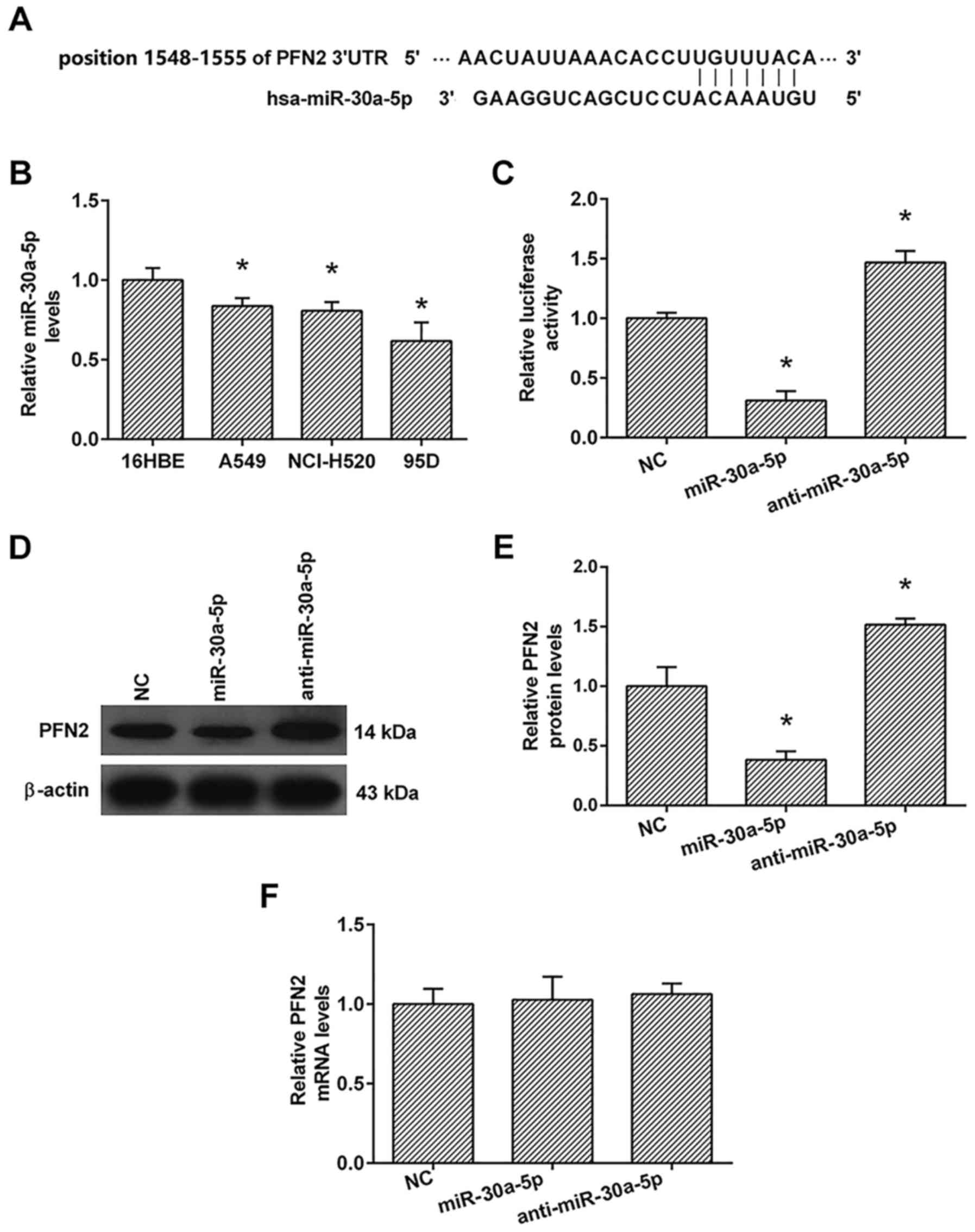
sites in the PFN2 3′-UTR and predicted that the position 1548–1555
of 3′UTR of PFN2 was a binding site for miR-30a-5p (Fig. 5A). Furthermore, we detected the
expression of miR-30a-5p in 16HBE, A549, NcI-H520 and 95D cells
using the real-time PCR analysis. As shown in Fig. 5B, the expression of miR-30a-5p in
A549, NcI-H520 and 95D cells were lower than those in 16HBE
cells.
To confirm whether miR-30a-5p directly targets the
3′-UTR of PFN2 mRNA, we employed vectors encoding a partial
sequence of the 3′UTR of PFN2 mRNA where the predicted miR-30a-5p
target sites were located. We found that the luciferase activity
was significantly increased by co-transfection with anti-miR-30a-5p
and the vector carrying the 3′UTR of PFN2 compared with the
negative control. On the contrary, co-transfection with the
miR-30a-5p mimics and the vector carrying the 3′UTR of PFN2
obviously decreased the luciferase activity (Fig. 5C). These results showed that
miR-30a-5p bound directly to a specific site in the 3′UTR of PFN2
mRNA.
To confirm the role of miR-30a-5p in the regulation
of PFN2 expression, real-time PCR and western blot analyses were
also performed. The real-time PCR analysis showed that the
transfection with miR-30a-5p had no significant effect on the mRNA
levels of PFN2 compared to the negative control (Fig. 5F). The results of western blotting
showed that anti-miR-30a-5p significantly increased the protein
levels of PFN2, whereas miR-30a-5p transfection markedly inhibited
the protein levels of PFN2 compared to the negative control
(Fig. 5D and E).
miR-30a-5p attenuates invasion and
TGF-β-induces EMT in high invasive NSCLC cell lines
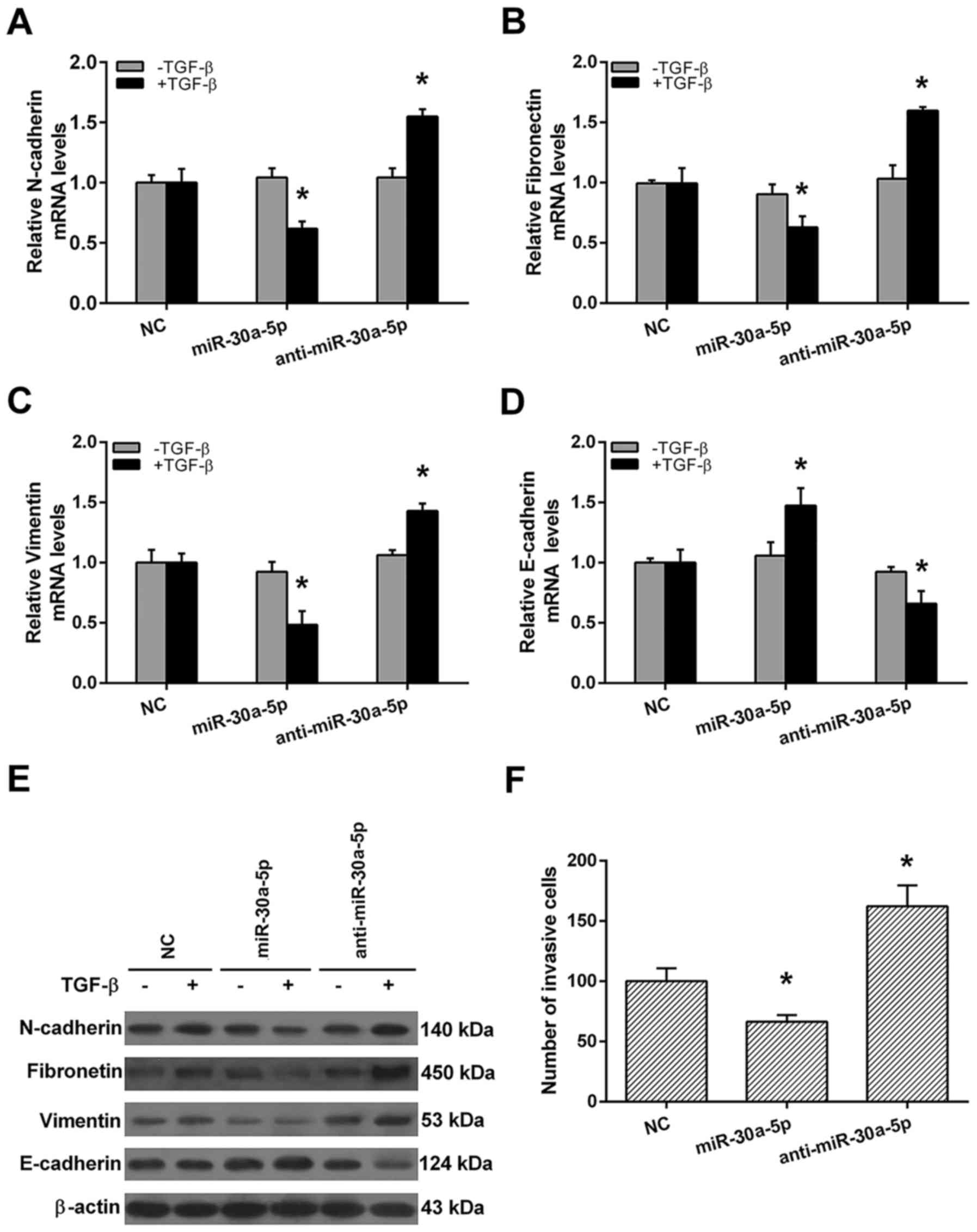
EMT is an initiator of metastasis in cancers. To
investigate whether miR-30a-5p is required for TGF-β-induced EMT in
high invasive NSCL cell line, qRT-PCR and western blot analyses
were performed after miR-30a-5p or anti-miR-30a-5p transfection. As
shown in Fig. 6, without TGF-β
treatment, the mRNA and protein levels of N-cadherin, fibronectin,
vimentin and E-cadherin were all similar among negative control
(NC), miR-30a-5p mimics and anti-miR-30a-5p groups (Fig. 6A-E). With TGF-β treatment,
miR-30a-5p mimics group displayed reduced mesenchymal cell marker
N-cadherin, fibronectin, vimentin and increased epithelial marker
E-cadherin expressions when compared with NC group. Otherwise,
anti-miR-30a-5p group displayed increased mesenchymal cell marker
N-cadherin, fibronectin, vimentin and reduced epithelial marker
E-cadherin expressions when compared with NC group (Fig. 6A-E). Finally, we demonstrated that
miR-30a-5p mimics group possessed weaker, whereas the
anti-miR-30a-5p group possessed stranger invasive ability in 95D
cells when compared to NC group (Fig.
6F). These results indicate that miR-30a-5p may act as a
positive regulator of cell invasion in high invasive NSCLC
cells.
Discussion
PFN2 is an actin-binding protein that regulates the
dynamics of actin polymerization and plays a key role in cell
motility (10). Previous evidence
suggests that PFN2 has crucial roles in conferring the invasive
potential of several human cancers such as colorectal cancers
(9), esophageal squamous cell
carcinoma (10) and even lung
cancer. For example, Kim et al found that PFN2 promotes
migration and invasion in human colorectal cancer stem cells, and
directly regulated the expression of stemness markers (CD133, SOX2,
and β-catenin) and EMT markers (E-cadherin and snail) (9). Cui et al demonstrated that PFN2
protein was overexpressed in ESCC and was positively correlated
with invasion depth and lymph node metastasis, further experiment
proved that PFN2 promotes invasion and migration, as well as
induces an EMT phenotype in ESCC cells in vitro (10). Tang et al reported that PFN2
upregulates Smad2 and Smad3 expressions via an epigenetic
mechanism, and that PFN2 and Smad expression correlate with lung
cancer growth and metastasis (2).
However, the potential contributions of the PFN2 gene to the EMT
phenotypes and invasion in high invasive NSCLC cells required
further investigation.
The present study confirmed that the expression of
PFN2 was higher in three NSCLC cell lines (lung adenocarcinoma cell
line A549, lung squamous cell carcinoma cell line NCI-H520 and
large cell lung cancer cell line 95D) when compared to the
bronchial epithelial cell 16HBE. Interestingly, the high invasive
cell line 95D possesses the highest PFN2 level among the three
NSCLC cell lines. These results suggested that PFN2 may be involved
in the metastatic process of NSCLC. Then the gain-of-function
(overexpression) and loss-of-function (siRNA) experiments showed
that PFN2 promotes cell invasion in high invasive NSCLC cells.
EMT occurs during embryonic development and cancer
metastasis and results in enhanced cell invasion. Further
experiments in our study demonstrated that PFN2 enhanced the mRNA
and protein levels of N-cadherin, fibronectin and vimentin, whereas
inhibited these levels of E-cadherin, which supports a role for
PFN2 as a critical regulator of EMT induction in NSCLC cells.
Therefore, our data provide new evidence to demonstrate that PFN2
has a pivotal role in conferring the EMT potential of NSCLC
cells.
Different miRNAs were shown to play an important
role in the regulation of tumor properties in NSCLC. Multiple
tumor-suppressive miRNAs were downregulated coordinately in NSCLC
cells and tissues, namely, miR-15a/16, miR-34a, miR-126 miR-128
(21), mir-375, mir-133a, mir-33a
(22), and miR-187-5p (23). MicroRNA-30a has been identified as a
tumor suppressor in NSCLC. For example, miR-30a is downregulated in
NSCLC, and it inhibits EMT progression by targeting Snai1 (24). Overexpression of miR-30a in A549
cell line inhibits migration and invasion via targeting EYA2
(25).
In this study, we found that miR-30a-5p
significantly inhibited EMT and cell invasion in NSCLC cells, which
supports the role of miR-30a-5p as a tumor suppressor in NSCLC.
Importantly, we also found that PFN2 was a direct target of
miR-30a-5p in NSCLC cells; miR-30a-5p decreased PFN2 protein but
not mRNA levels, suggesting that miR-30a-5p induces translational
repression of PFN2. Subsequently studies confirmed that increased
miR-30a-5p levels in NSCLC cells coincided with decreased EMT and
cell invasion, whereas decrease in miR-30a-5p levels caused
increased in EMT and cell invasion. To our knowledge this is the
first study analyzing the association of PFN2 and miR-30a-5p
expression with NSCLC progression.
In conclusion, our results provide important
evidence that miR-30a-5p can directly target PFN2, and suggest that
the association of miR-30a-5p and PFN2 may play a role in the
development of NSCLC by modulating EMT and cell invasion.
Therefore, miR-30a-5p activation or PFN2 inhibition may provide a
novel strategy for the treatment of NSCLC.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang YN, Ding WQ, Guo XJ, Yuan XW, Wang DM
and Song JG: Epigenetic regulation of Smad2 and Smad3 by profilin-2
promotes lung cancer growth and metastasis. Nat Commun. 6:82302015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Valenzuela-Iglesias A, Sharma VP, Beaty
BT, Ding Z, Gutierrez-Millan LE, Roy P, Condeelis JS and
Bravo-Cordero JJ: Profilin1 regulates invadopodium maturation in
human breast cancer cells. Eur J Cell Biol. 94:78–89. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coumans JVF, Gau D, Poljak A, Wasinger V,
Roy P and Moens PDJ: Profilin-1 overexpression in MDA-MB-231 breast
cancer cells is associated with alterations in proteomics
biomarkers of cell proliferation, survival, and motility as
revealed by global proteomics analyses. OMICS. 18:778–791. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boyl Pilo P, Di Nardo A, Mulle C,
Sassoè-Pognetto M, Panzanelli P, Mele A, Kneussel M, Costantini V,
Perlas E, Massimi M, et al: Profilin2 contributes to synaptic
vesicle exocytosis, neuronal excitability, and novelty-seeking
behavior. EMBO J. 26:2991–3002. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yue B, Sun B, Liu C, Zhao S, Zhang D, Yu F
and Yan D: Long non-coding RNA Fer-1-like protein 4 suppresses
oncogenesis and exhibits prognostic value by associating with
miR-106a-5p in colon cancer. Cancer Sci. 106:1323–1332. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: An emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yao D, Dai C and Peng S: Mechanism of the
mesenchymal-epithelial transition and its relationship with
metastatic tumor formation. Mol Cancer Res. 9:1608–1620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim MJ, Lee YS, Han GY, Lee HN, Ahn C and
Kim CW: Profilin 2 promotes migration, invasion, and stemness of
HT29 human colorectal cancer stem cells. Biosci Biotechnol Biochem.
79:1438–1446. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cui XB, Zhang SM, Xu YX, Dang HW, Liu CX,
Wang LH, Yang L, Hu JM, Liang WH, Jiang JF, et al: PFN2, a novel
marker of unfavorable prognosis, is a potential therapeutic target
involved in esophageal squamous cell carcinoma. J Transl Med.
14:1372016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li W, Liu C, Zhao C, Zhai L and Lv S:
Downregulation of β3 integrin by miR-30a-5p modulates cell adhesion
and invasion by interrupting Erk/Ets-1 network in triple-negative
breast cancer. Int J Oncol. 48:1155–1164. 2016.PubMed/NCBI
|
|
12
|
Li WF, Dai H, Ou Q, Zuo GQ and Liu CA:
Overexpression of microRNA-30a-5p inhibits liver cancer cell
proliferation and induces apoptosis by targeting MTDH/PTEN/AKT
pathway. Tumour Biol. 37:5885–5895. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oksuz Z, Serin MS, Kaplan E, Dogen A,
Tezcan S, Aslan G, Emekdas G, Sezgin O, Altintas E and Tiftik EN:
Serum microRNAs; miR-30c-5p, miR-223-3p, miR-302c-3p and miR-17-5p
could be used as novel non-invasive biomarkers for HCV-positive
cirrhosis and hepatocellular carcinoma. Mol Biol Rep. 42:713–720.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Balci S, Ayaz L, Gorur A, Yaroglu Yildirim
H, Akbayir S, Dogruer Unal N, Bulut B, Tursen U and Tamer L:
microRNA profiling for early detection of non-melanoma skin cancer.
Clin Exp Dermatol. 41:346–351. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie K, Wang C, Qin N, Yang J, Zhu M, Dai
J, Jin G, Shen H, Ma H and Hu Z: Genetic variants in regulatory
regions of microRNAs are associated with lung cancer risk.
Oncotarget. 7:47966–47974. 2016.PubMed/NCBI
|
|
16
|
Zhu J, Zeng Y, Xu C, Qin H, Lei Z, Shen D,
Liu Z and Huang JA: Expression profile analysis of microRNAs and
downregulated miR-486-5p and miR-30a-5p in non-small cell lung
cancer. Oncol Rep. 34:1779–1786. 2015.PubMed/NCBI
|
|
17
|
He R, Yang L, Lin X, Chen X, Lin X, Wei F,
Liang X, Luo Y, Wu Y, Gan T, et al: MiR-30a-5p suppresses cell
growth and enhances apoptosis of hepatocellular carcinoma cells via
targeting AEG-1. Int J Clin Exp Pathol. 8:15632–15641.
2015.PubMed/NCBI
|
|
18
|
Xiong J, Wei B, Ye Q and Liu W:
MiR-30a-5p/UBE3C axis regulates breast cancer cell proliferation
and migration. Biochem Biophys Res Commun. Mar 18–2016.(Epub ahead
of print). doi: 10.1016/j.bbrc.2016.03.069. View Article : Google Scholar
|
|
19
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tafsiri E, Darbouy M, Shadmehr MB, Cho WC
and Karimipoor M: Abberent expression of oncogenic and
tumor-suppressive microRNAs and their target genes in human
adenocarcinoma alveolar basal epithelial cells. J Cancer Res Ther.
12:395–400. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pastorkova Z, Skarda J and Andel J: The
role of microRNA in metastatic processes of non-small cell lung
carcinoma. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub.
160:343–357. 2016.PubMed/NCBI
|
|
23
|
Mao M, Wu Z and Chen J: MicroRNA-187-5p
suppresses cancer cell progression in non-small cell lung cancer
(NSCLC) through down-regulation of CYP1B1. Biochem Biophys Res
Commun. 478:649–655. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kumarswamy R, Mudduluru G, Ceppi P,
Muppala S, Kozlowski M, Niklinski J, Papotti M and Allgayer H:
MicroRNA-30a inhibits epithelial-to-mesenchymal transition by
targeting Snai1 and is downregulated in non-small cell lung cancer.
Int J Cancer. 130:2044–2053. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yuan Y, Zheng S, Li Q, Xiang X, Gao T, Ran
P, Sun L, Huang Q, Xie F, Du J, et al: Overexpression of miR-30a in
lung adenocarcinoma A549 cell line inhibits migration and invasion
via targeting EYA2. Acta Biochim Biophys Sin (Shanghai).
48:220–228. 2016. View Article : Google Scholar : PubMed/NCBI
|