Introduction
Metastasis is the major cause of cancer death
(1). Although the cancer
dissemination is a complicated process, involving detachment of
cancer cells from primary site, intravasation, extravasation and
seeded in a remote site, the existing of circulating tumor cells
(CTCs) is one of land-marks for metastasis process (2–7). While
the ‘seed-soil’ concept is well-accepted for a successful
metastasis, it is not doubted that the numbers of CTCs reflect
several aspects related with the cancer malignancy and treatment
outcome (8,9). A high numbers of CTCs are likely to
indicate a high potential of metastasis and a possible failure of
cancer treatment with poor prognosis (10). The continuous drop in the CTC number
might suggest a correct regimen of anti-cancer treatment and a good
outcome for patients. Thus, the determination of CTCs has attracted
great attention in past two decades and is called ‘liquid biopsy’
(11).
However, the major obstacle for utilization of this
index raises from the nature of CTCs, which is a very rare event in
whole blood tested, especially in the early stage of cancer, the
best treatment window for cancer. Technically, to capture and
detect a single digit of CTC from 109/ml blood cells is
very challenging. Few assays pass FDA regulation and are used in
clinical practice.
The principles of defining a few of CTCs from huge
numbers of normal blood cells are based on: 1) physical properties
of CTCs: cell diameter, density and charge; 2) biological
characteristics of CTCs: the unique epithelial marker of CTCs (such
as cytokeratin and EpCAM), tumor specific proteins and antigens,
chromosome heteroploid or RNA abnormality. Based on these physical
and biological characteristics of CTCs, several methods have been
developed to accomplish two major steps of determination of CTCs:
enrichment and detection.
For the first step of enrichment, several methods
have been used with their advantages and disadvantages: 1)
Antibody-based method: the best example is the FDA approved
CellSearch method, which uses anti-EpCAM to capture/immobilize the
CTCs as positive selection (12–14).
CTCs chips use the same principle (15). Other immune magnetic separations
utilize anti-CD45 to capture and then discard the WBC after lysis
of RBC as negative selection (13).
The problem encountered by positive selection is that the cancer
cells might have a transition from epithelium to mesenchymal cells
(EMT) and loss of the epithelial marker, resulting in the low
detection rate and false negative. For the negative selection, the
CD45 might not stain well for all non-tumor cells in blood, such as
macronuclei cells (precursor of platelet) or detached endothelium
or transformed WBC, resulting in a false positive. 2) Physics-based
method: the membrane filter method utilizes the fact that the sizes
of CTCs are bigger than most of normal blood cells (16,17);
the centrifugation and /or microfluidic separation methods utilize
the unique density, size and/or charge of CTCs. These methods
require sophisticated techniques and special devices.
For the second step of detection, several methods
are also utilized: 1) nucleotide-based assay: FISH assay is able to
reveal the chromosome heteroploid or RNA abnormality, and the
RT-PCR reflects the overexpression of oncogenes (18); 2) Antibody-based assay: the use of
anti-CK can detect the epithelial origin CTCs by flow cytometry
(19) or immunocytochemistry
staining. The key issues in the detection step are the specificity
of reagents, the sample treatment to expose the target and the
interferences in the amplification process. Although the different
techniques have been tested and used, their specificity and
sensitivity are not ideal (20–22),
and the side-by-side comparison of their characteristics is
needed.
Animal model has been utilized to study tumor
metastasis for a long time, due to 1) the heterogeneity is slight
in a small animal model; 2) the conditions of different types of
animal experiments are easy to control. Similarly, animal models
can be utilized to study the alteration patterns of CTCs varied
with the stage development of metastasis and the different
treatments. However, how to detect mouse CTC alterations and which
is the best method to accurately reflect the CTC alterations are
under studied, since the most attention is paid to detection of
human CTCs, but not in animal models.
In this study, we utilized the dual-gene transfected
4T1 breast cancer cell line, which stably expressed the GFP and
luciferase for accurately tracking and quantitating CTCs, to
perform a side-by-side comparison of three different CTC assays for
their advantages and disadvantages. The same amounts of blood
sample from BABL/c mice with lung metastases as result of
intravenous injection of GFP and luciferase dual-labeled 4T1 cells
were subjected to three different procedures, i.e. after lysis of
RBC, 4T1-CTCs were detected in three formats: 1) smeared blood
samples after lysis of RBC on slides and counted for GFP-4T1; 2)
quantitated luciferase-4T1 with bioluminescence assay; 3) captured
CTCs from blood on filter membrane and counted for GFP-4T1. The
goal of this study was to determine: 1) Which method is more
sensitive in reflecting CTCs; 2) What is unique in each method; and
3) Whether the filter method lose more CTCs. This study will help
us to decide which method should be chosen for different research
purposes.
Materials and methods
Reagents and instruments
Fetal bovine serum (FBS) was purchased from Zhejiang
Tianhang Biotechnology Co; Ltd. D-Luciferin was purchased from
BioVision (Milpitas, CA, USA); DAPI, 4′,6-diamidino-2-phenylindole
dihydrochloride (cat# D8417, Sigma Aldrich Co., USA); Whatman™
Nuclepore™ Polycarbonate Track-Etched Membranes with 8 µm pore size
was purchased from Whatman (cat# 09-300-57, Pittsburgh, PA, USA);
Bioluminescence signal of lung metastases was quantitatively
measured by IVIS Spectrum (PerkinElmer Co., Waltham, MA, USA).
Firefly Luciferase Reporter gene assay kit was purchased from
Beyotime Biotechnology Co., Ltd. Relative light unit (RLU) was
measured by luminometer (Antu, Zhengzhou, China). BX63 fluorescence
microscope was from Olympus, Center Valley, PA, USA.
Cell lines and cell culture
GFP-Luc-4T1 dual labeled cell line was purchased
from Caliper Life Science (Hopkinton, MA, USA) and cultured in
RPMI-1640 medium containing 10% FBS, 100 U/ml of penicillin and
streptomycin.
Experimental animals
Female BALB/c mice were purchased from Slaccas
Experimental Animal LLC (license# SCXK 2012-0002, Shanghai, China).
Animal studies were approved by Fujian Medical University
Institutional Animal Care and Use Committee.
Establishment and evaluation of an
experimental mouse model for breast cancer lung metastasis
GFP-Luc-4T1 cells (106) were freshly
collected, suspended in 300 µl PBS, and then injected into the tail
vein of BALB/c mice. PBS was injected into the tail vein of another
nine BALB/c mice as control group. The growth of pulmonary
metastasis was observed by IVIS Spectrum on day 7, 14, 21, and the
data were analyzed by Spectrum Living Image 4.0 software. Lung
tissue with metastases of GFP-Luc-4T1 were removed and stained by
haematoxylin and eosin (H&E) after the mice were sacrificed on
day 21.
Collection of blood samples for
CTCs
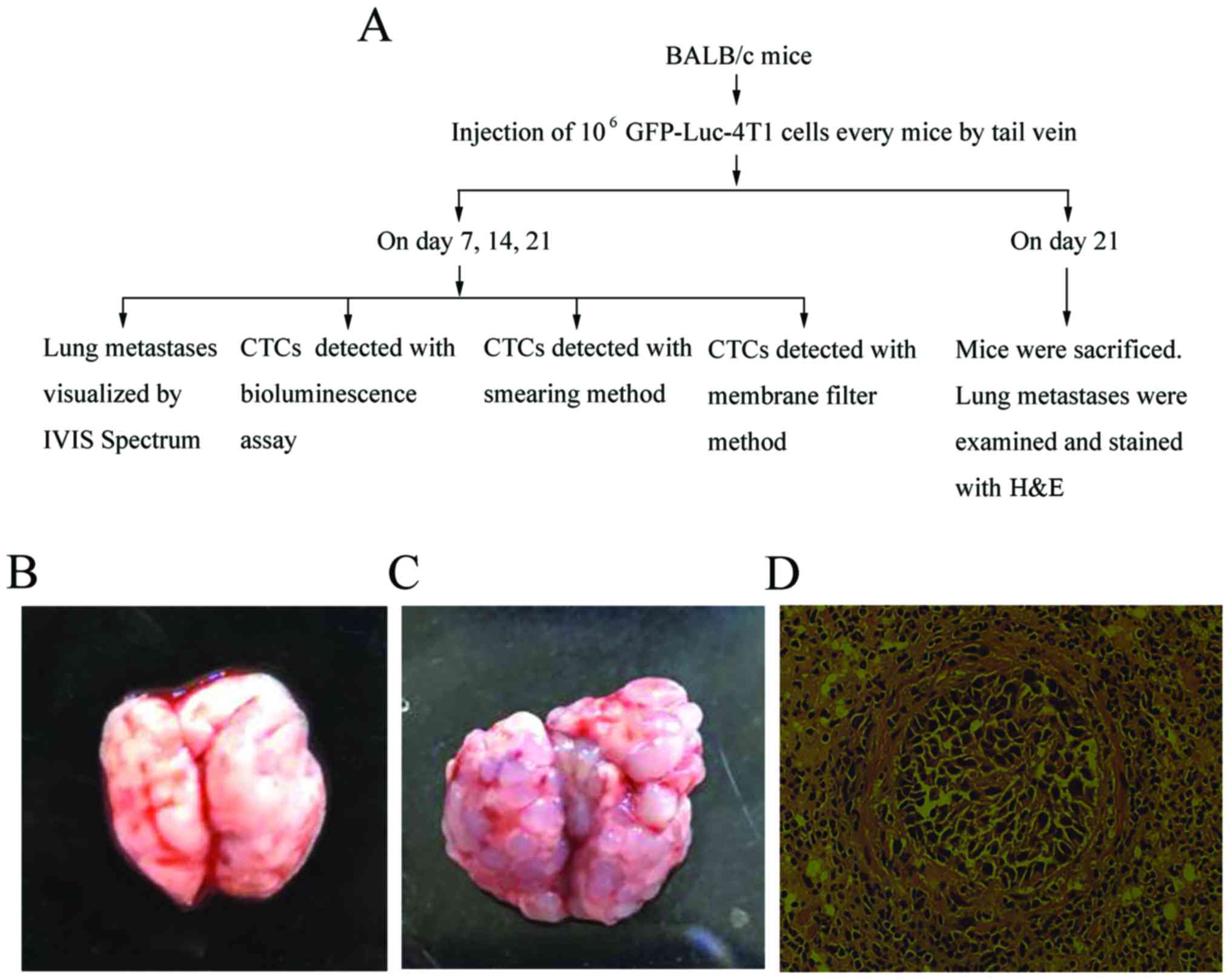
As shown in Fig. 1A,
on day 7, 14, 21 after GFP-Luc-4T1 i.v. injection, 300 µl whole
blood samples were collected by tail vein bleeding into
K3-EDTA-coated tubes, mixed well and then equally divided into
three 2 ml tubes, marked A, B, C, respectively, for assessment of
CTCs with three different methods.
CTCs detected with luciferase
assay
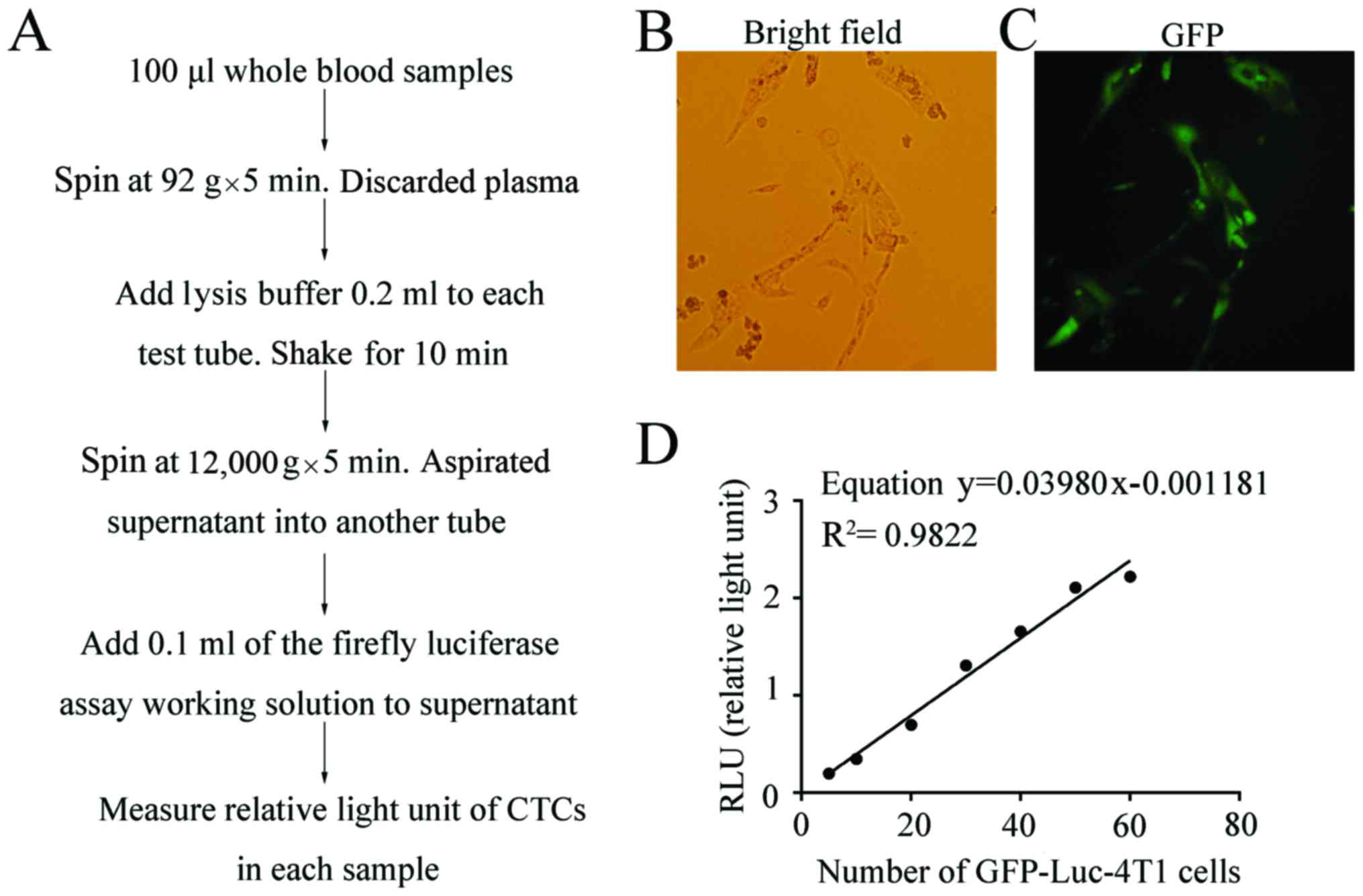
As shown in Fig. 2A,
100-µl blood was centrifuged at 92 × g for 5 min, and plasma was
discarded. Cell lysis buffer (200 µl) (Beyotime Biotechnology Co.,
Ltd.) was added to resuspend cells, incubated at room temperature
for 8–10 min with occasional shaking, then centrifuged at 12,000 ×
g for 5 min. The supernatant was aspirated into another tube and
100 µl of luciferin working solution (Beyotime Biotechnology Co.,
Ltd.) was added to the supernatant, reacting with luciferase of
CTCs of GFP-Luc-4T1. Immediately, the RLU in each tube was assayed
by luminometer (Antu LLC, Henan, China).
To set up standard curve, the freshly harvested
GFP-Luc-4T1 cells were counted, then 0, 5, 10, 20, 30, 40, 50, 60
cells were added to tubes, respectively. RLU was assayed according
to the above method and the derived equation was used as standard
curve to calculate the CTC numbers of GFP-Luc-4T1 cells from mice
with experimental lung metastases.
CTCs detected with counting on the
slide
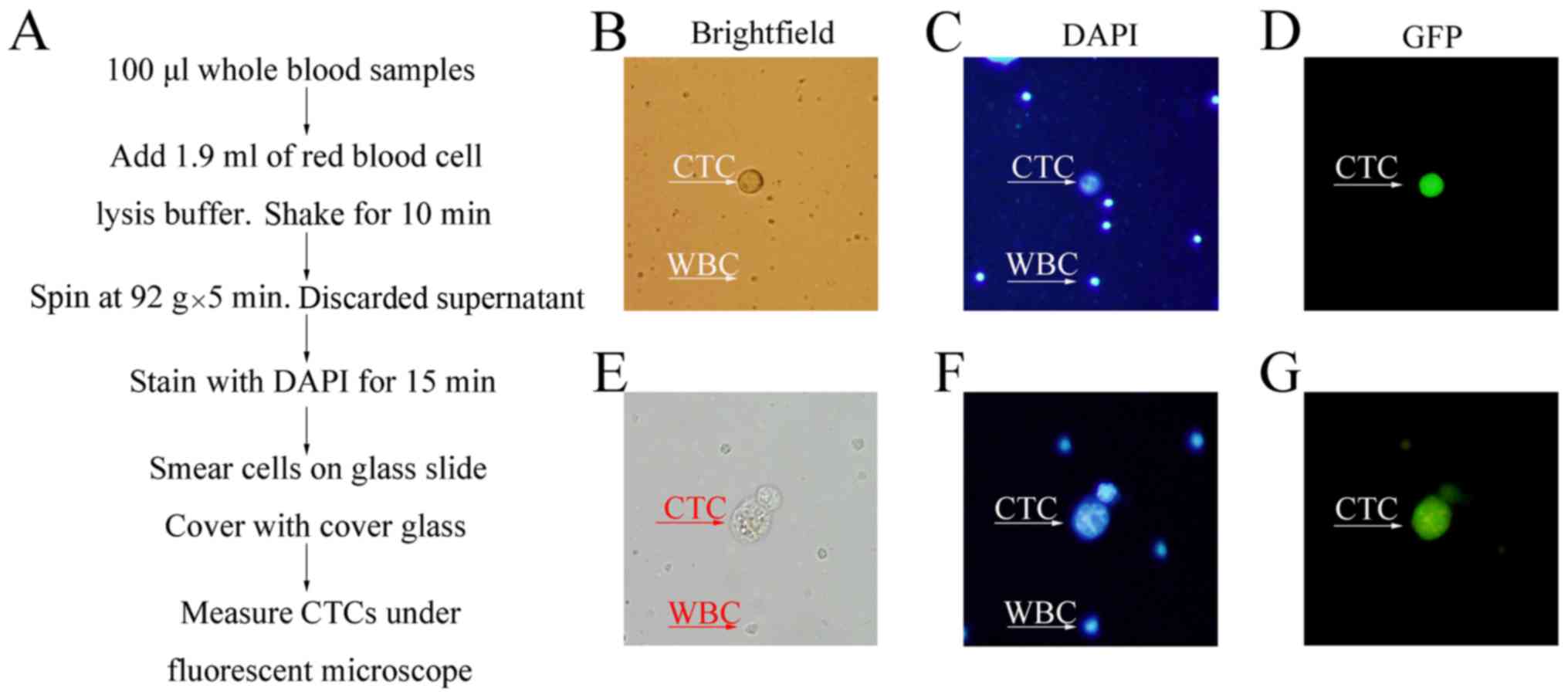
As shown in Fig. 3A,
1.9 ml of RBC lysis buffer was added to 100 µl blood, incubated at
room temperature for 8–10 min with occasional shaking, then
centrifuged at 92 × g for 5 min. After aspiration of supernatant
without disturbing pellet, 200 µl of 3 nM DAPI in PBS was added to
the tube well resuspend cell pellet, smeared on 24×50
mm2 glass slide 15 min later, covered with 0.13–0.17
thick cover glass. The GFP/DAPI positive 4T1 CTCs were pictured and
counted under BX63 Olympus fluorescence microscope. The 3 µM DAPI
stock solution was made in the staining buffer of 100 mM Tris, pH
7.4, 150 mM NaCl, 1 mM CaCl2, 0.5 mM MgCl2,
0.1% Nonidet P-40.
CTCs detected with counting on filter
membrane
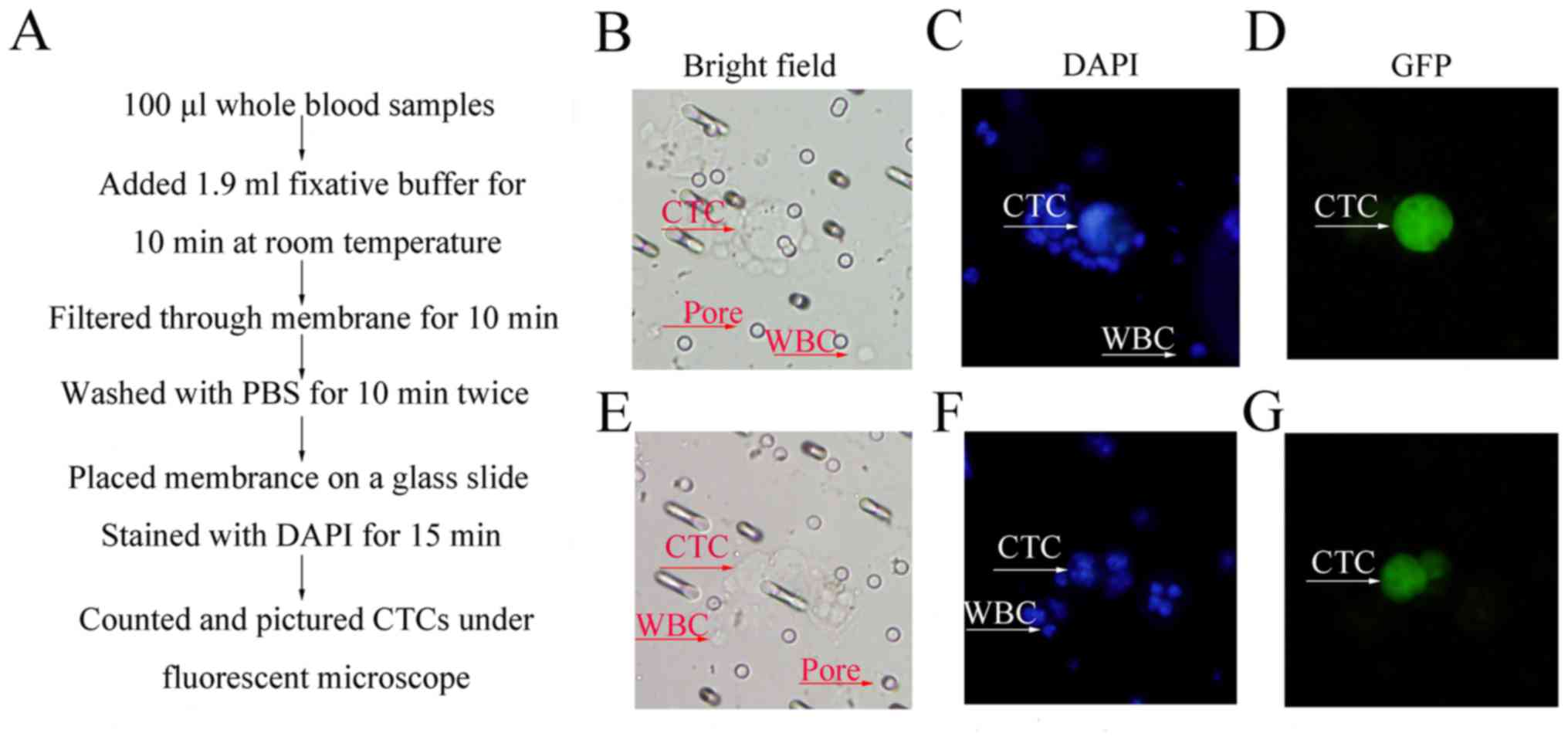
As shown in Fig. 4A,
1.9 ml of fixative buffer (0.2% paraformaldehyde, 0.0372% EDTA, and
0.1% bovine serum albumin) was added to 100 µl blood for 10 min at
room temperature. Then, the 2 ml fixative buffer was placed in a
5-ml syringe connected to a filter supporter with 8 µm pore size of
Polycarbonate Track-Etched Membrane, and passed the filter membrane
in 10 min. After washing with 2 ml of PBS twice at the same speed,
the membrane was placed on a glass slide as support. Of 3 nM DAPI
in PBS, 200 µl was added to the membrane, then covered with cover
glass. The numbers of GFP/DAPI positive 4T1 CTCs were counted, and
images were pictured by BX63 Olympus fluorescent microscope.
Statistical analysis
GraphPad Prism6 software was used for statistical
analysis, and measurement data are expressed as mean ± standard
deviation. An unpaired, t-test was used for comparing two groups of
measurement data, and the difference was statistically significant,
at P<0.05.
Results
Establishment and evaluation of
pulmonary metastasis of 4T1 breast cancer in BALB/c mouse
model
In this study, the 1st key step was to set up the
experimental lung metastasis model. After injection of
1×106 mouse GFP-Luc-4T1 cells into BALB/c syngeneic
mice, the breast cancer cells were able to settle down in lung and
grew with time. In addition, the growth of 4T1 lung metastases was
evidenced by seeing the tumor nodules increase with time. While
none of lung metastases could be seen in the control PBS group
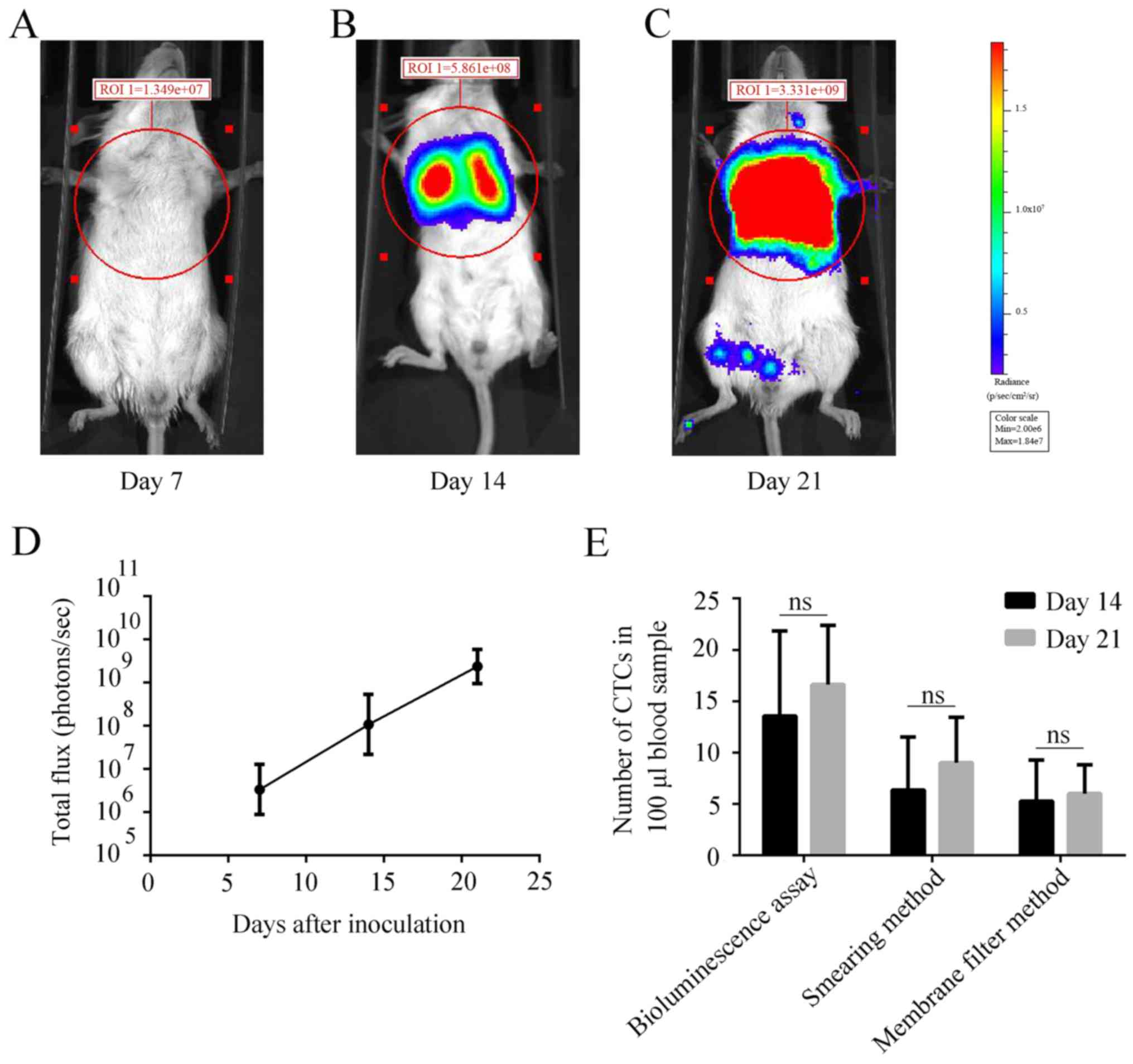
(Fig. 1B), the tumor nodules were
full in the lung of the GFP-Luc-4T1 cells injected mice on day 21
(Fig. 1C), which could cause death
due to respiratory failure of lung cancer. Finally, at the end of
study, the 4T1 metastases could be seen fully occupied in lung
tissues (Fig. 1D). All evidence
supported that this experimental lung metastasis model was
successful, which could provide continuous CTCs for the assessment
with three different CTC assays.
Luc-CTCs quantitatively detected with
luciferase assay
The GFP-Luc-4T1 cells used in this study are stable
and highly expressed in both GFP and luciferase. All living cells
that could be seen under a bright field of regular inverted
microscope (Fig. 2B) were GFP
positive under an inverted fluorescent microscope (Fig. 2C). Since both GFP and luciferase are
driven by a same promoter, the luciferase is also likely to be
expressed by each of 4T1 cells. The RLU efficiency due to the
catalysis of luciferin by luciferase produced by 4T1 cells was
proportional to the cell number, which could well serve as standard
for counting CTCs (Fig. 2D). The
equation was Y = 0.03980x – 0.001181 and the correlation
coefficient R2 was 0.9822. Two trace markers carried by
GFP-Luc-4T1 cells are unique with no background in BALB/c mice,
which provides a clear identity for us to detect and count the CTCs
in a precise fashion.
As shown in Fig. 2A,
we could lyse all the cells in 100 µl of blood, then added
D-luciferin to the lysed CTCs for their luciferase activity. From
the numbers of photo RLU, we were able to dynamically monitor the
alteration of CTCs with time in the mouse model. Due to the
enzymatic reaction having amplification effect, luciferase assay
could be the most sensitive assay in reflecting the existing CTCs
as compared to other two assays with counting GFP positive CTCs one
by one with nude eye under fluorescent microscope, which was
confirmed by Figs. 5 and 6E.
GFP-CTCs visualized on a slide
As shown in Fig. 3A,
after lysis of RBCs, the CTCs were counter-stained with
DAPI/anti-fader to exclude the no-cell green fluorescence debrides,
then the re-suspended cell pellet was smeared on the slide, and
covered with 50% glycerol-PBS for observation under a fluorescent
microscope. This simple process would also allow us to follow-up
the CTC alterations with times. The disadvantage is the high
background of ‘ghost cell’ (membranes of lysed RBCs) debris as
shown in Fig. 3C compared to the
filter membrane method (Fig. 4C).
We found that during the first 14 days, the CTCs were seen mostly
in a single cell (Fig. 3B-D),
however, after 14 days, we could see two cancer cells together as
clusters (Fig. 3E-G), which might
have a special property in the cancer progression.
CTCs assessed on filter membrane
When the fixed CTCs containing blood cells in the
synergies were pushed through 8 µm pore size of Polycarbonate
Track-Etched Membrane at a speed of 2 ml/10 min, the small RBCs and
most of WBCs passed the pores, while the CTCs were retained on the
top of membrane due to their big size. Similar to that seen in
smeared slides, in week 2, CTCs existed mostly as single cells
(Fig. 4B-D), while in week 3, some
CTCs were seen in clusters (Fig.
4E-G).
Comparison of three methods
The sensitivity of CTCs detected by three methods
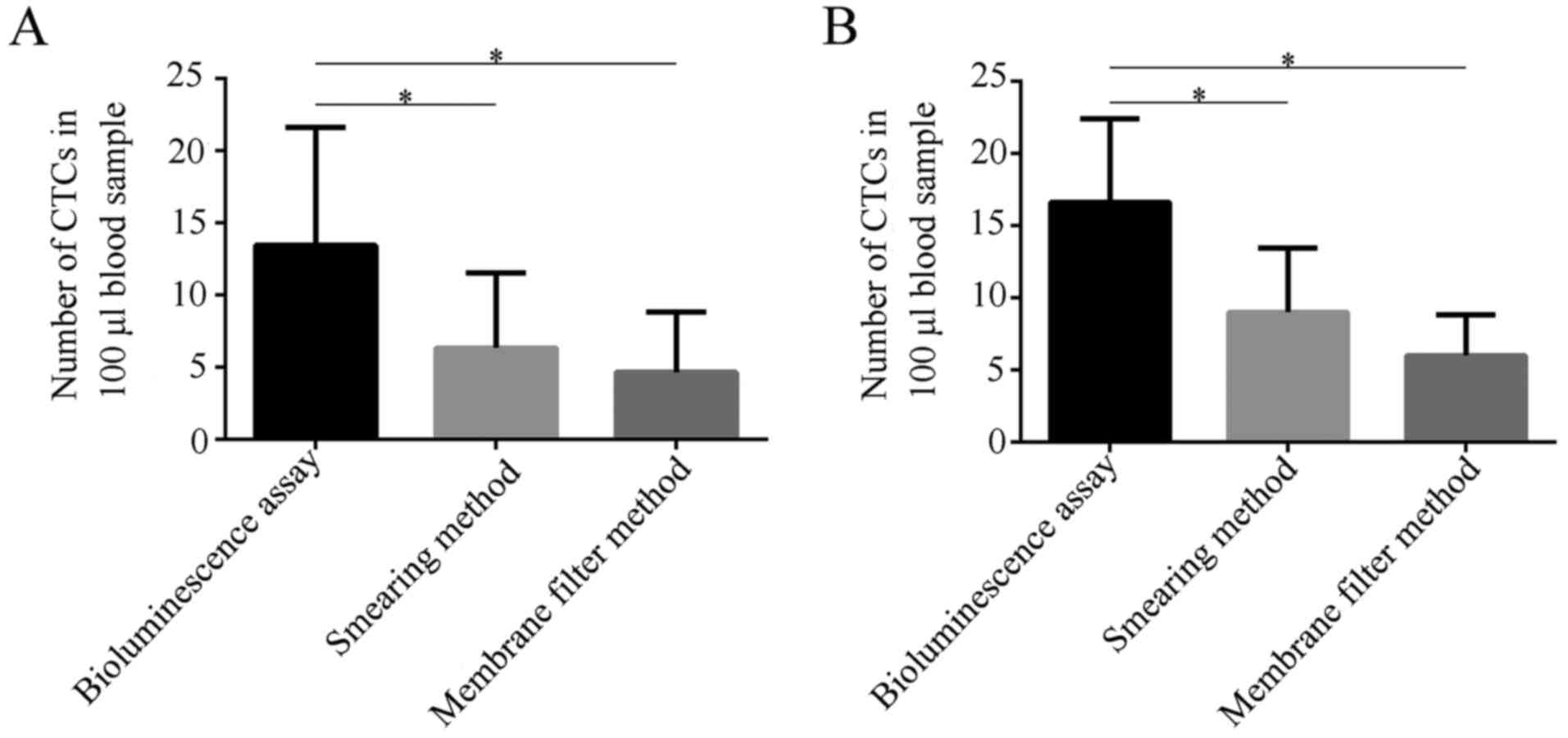
described above was compared at 14 and 21 days after inoculation.
As shown in Fig. 5A, on day 14, the
CTCs in same amount of blood detected by bioluminescence assay had
the highest number as compared to the smear method (P=0.0426) and
membrane filtration method (P=0.0110), while there was not
statistical difference between smear method and membrane filtration
(P=0.4632). A similar pattern was observed on day 21 (Fig. 5B). This set of data indicates that
the bioluminescence assay has a high sensitivity in detecting CTCs,
which might relate with the enzymatic amplification of
luciferase.
The increase of tumor cells in lung
metastatic site was faster than CTCs
To our surprise, when comparing the speed of
increase in lung metastases with that of CTCs with time, the in
vivo bioluminescence imaging signal (measured by IVIS Spectrum)
representing size of lung metastases increased at the log speed
(bioluminescence imaging signal on day 7,
7.507×106±3.885×106; on day 14,
2.615×108±1.001×108; on day 21,
3.198×109±7.959×108; P<0.001, Fig. 6D), while the increase of speed of
CTCs was only a few cells in 7 day intervals even detected by the
sensitive bioluminescence assay (P=0.3748, Fig. 6E). We speculate that this might
relate to a balance between the number of cancer cells entering the
circulation and their clean-up by the immune cells.
Discussion
This study focused on defining good assays for
detecting CTCs in a tumor-bearing mouse model (23), which might be of benefit: 1) to
reveal the process of metastasis; 2) to study how the host
disseminated CTCs; and 3) to search for effective agents against
tumor metastasis.
The CTC assay for experimental mouse model is
different from that for human, since the mouse cancer cells could
be labeled with biomarkers, such as fluorescence protein and/or
luciferase, while human cancer cells exist in an unlabeled native
form. Using the labeled mouse CTCs, we can determine advantages and
disadvantages of the three different assays for their efficacy in
quantification of CTCs.
Utilizing the unique dual labeled GFP-Luc-4T1 cells,
we were able to compare three assays that practically could be used
in monitoring the CTCs in a mouse model. Our new findings are:
First, bioluminescence assay is the most sensitive assay for CTC
detection as compared to other two methods by counting CTCs one by
one with nude eye (Figs. 5 and
6E). This is mainly due to the
luciferase catalytic effect on luciferin, an enzymatic
amplification of signal that can be sensitively measured with a
luminometer. The advantages of this assay are: 1) it is capable of
dealing with relative large volume of blood, increasing the chance
of capturing more CTCs; 2) it requires simple procedures, only
lysis of RBCs and addition of luciferin to cell pellet; 3) its
reading result with luminometer is subjective without human error;
4) it is very specific and a sensitive assay. Sasportas et
al reported that bioluminescence assay could detect 2 CTCs in
100 µl blood (24). Some studies
show that bioluminescence imaging is 3–8-fold more sensitive than
fluorescence imaging (25); 5) it
has very low background, since there is no luciferase background in
living mammals, which yields a high signal/noise ratio, enhancing
its sensitivity. However, the limitation of this method is that the
number of CTCs is calculated from standard curve, therefore, there
are some errors and the accuracy is not high. In addition, this
method could not reflect morphological characteristics of CTCs
since all CTCs are lysed as whole.
Secondly, although GFP-based two assays (CTCs
smeared on slide or captured with filter) are less sensitive
compared to the bioluminescence assay and consume much more human
labor, they allow us to trace GFP marker of CTCs and observe the
morphological characteristics of CTCs. Before day 14, the CTCs
existed mainly as a single cell, however, after 14 days
approximately 10% of CTCs were two together as CTC clusters,
consistent with the report of Choi et al (26). This information is particularly
important, since it is believed that CTC-clusters have 23–50-fold
increased metastatic ability (27).
Besides, detection of the CTC-clusters, they have some more
advantages: 1) they are simple and convenient assays, easy to train
the operators; 2) they do not need other expensive instruments, so
that they can be used in general laboratory. However, the two
assays have some disadvantages: 1) it is time- and
manpower-consuming with human counting errors; 2) during the
long-term growth process of tumor cells, a part of tumor cells
could lose their GFP, which means that the green fluorescence of
some CTCs are weaken with time, which might cause false negative;
3) some unrelated impurities also can emit green fluorescence,
which might generate false positive. To overcome this, we used DAPI
staining in the cell nucleus to eliminate interferences of non-cell
impurities. It should be pointed out that when using ‘smeared CTCs
on slide assay’, the cell drop should be spread thin enough to
avoid crumbles of ghost cells covering the CTCs. When using the
filtering method, the force of pushing blood cells passing the
filter membrane should not be too strong, so that the CTCs could be
kept on the top of membrane. Taken together, the GFP-based assays
could detect the CTCs clusters, which are meaningful in reflecting
the ability of metastasis, while this important information cannot
be achieved by bioluminescence assay.
Thirdly, the increased speed of CTCs is not as fast
as that of original source of lung metastases. The result showed
that while at the 7 day intervals, the CTCs source site of lung
GFP-Luc-4T1 metastases increased at a 10 time-log speed as measured
in vivo with bioluminescence imaging (Fig. 6D), the CTCs increased only by a few
(Fig. 6E). We are puzzled by this
result that the CTCs increasing speed is not proportional to the
fast growing lung metastases. Some studies showed that the number
of CTCs in bloodstream had a peak followed by a decrease (24,28–31);
other researchers observed the number of CTCs in bloodstream had
two peaks (28,29), but that was not observed in this
study. Sasportas et al consider that dynamic change of CTCs
is not correlated with the growth of primary tumor (24), which is consistent with our
observation. We speculate that the mechanism underlying this
phenomenon may be: in early stage, a small amount of tumor cells
have released into bloodstream to be CTCs, and the number of tumor
cells in bloodstream gradually increased along with the growth of
the original tumor. The immune system could be activated by the
increasing CTCs, which in turn could kill CTCs and suppress their
increasing. However, while the newly generated immune cells could
effectively kill a single CTC or cluster, they are not sufficient
to kill the solid tumor or metastases due to the special tumor
microenvironment that blocks the immune cells to penetrate or kill
solid tumor cells.
Taken together, this study compared the sensitivity
of three CTC detection methods side-by-side. Due to each of them
having advantages and disadvantages, researchers should select the
assays based on the needs of the study. For example, if the study
is focused on the effect of certain agent on the suppression of
CTCs, then the bioluminescence assay could be used; if the study is
focused on dissemination mechanism of CTCs, then the GFP-based
assays could be selected and used according to the laboratory
condition and researcher's technical experience. Combination of
both bioluminescence assay and GFP-based assay would certainly
generate more information for the dissemination of cancer cells in
terms of their quantity and malignant quality.
Further investigation of the balance between the
CTCs and immune response might reveal the mechanism of complicated
metastasis process and generate new strategy to control
metastasis.
Acknowledgements
This study was supported in part by grant support
from the China Science and Technology Fund (2015 DFA31770) to J.L.;
Fujian Medical University Affiliated 1st Hospital fund to L.Z.
(2013GXLJRC); Fujian Education Ministry (2013–58), Fujian
Association for international exchange of personnel funds
(W13350000137) to L.Z. and the Key Lab Fund from Fujian Medical
University (0000–081919) to Fujian Key Laboratory of Individualized
Active Immunotherapy and to Key Laboratory of Radiation Biology of
Fujian Province University. We thank Kate Casey-Sawicki and Shimin
Zhang for editing and preparing this manuscript for
publication.
Glossary
Abbreviations
Abbreviations:
|
CTCs
|
circulating tumor cells
|
|
GFP
|
green fluorescence protein
|
|
RBC
|
red blood cells
|
|
WBC
|
white blood cells
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
dihydrochloride
|
|
i.v. injection
|
intravascular injection
|
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sasportas LS and Gambhir SS: Imaging
circulating tumor cells in freely moving awake small animals using
a miniaturized intravital microscope. PLoS One. 9:e867592014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hayashi N, Nakamura S, Tokuda Y, Shimoda
Y, Yagata H, Yoshida A, Ota H, Hortobagyi GN, Cristofanilli M and
Ueno NT: Prognostic value of HER2-positive circulating tumor cells
in patients with metastatic breast cancer. Int J Clin Oncol.
17:96–104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lucci A, Hall CS, Lodhi AK, Bhattacharyya
A, Anderson AE, Xiao L, Bedrosian I, Kuerer HM and Krishnamurthy S:
Circulating tumour cells in non-metastatic breast cancer: A
prospective study. Lancet Oncol. 13:688–695. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rack B, Schindlbeck C, Jückstock J,
Andergassen U, Hepp P, Zwingers T, Friedl TW, Lorenz R, Tesch H,
Fasching PA, et al SUCCESS Study Group, : Circulating tumor cells
predict survival in early average-to-high risk breast cancer
patients. J Natl Cancer Inst. 106:1062014. View Article : Google Scholar
|
|
6
|
Bidard FC, Peeters DJ, Fehm T, Nolé F,
Gisbert-Criado R, Mavroudis D, Grisanti S, Generali D, Garcia-Saenz
JA, Stebbing J, et al: Clinical validity of circulating tumour
cells in patients with metastatic breast cancer: A pooled analysis
of individual patient data. Lancet Oncol. 15:406–414. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scott J, Kuhn P and Anderson AR: Unifying
metastasis - integrating intravasation, circulation and end-organ
colonization. Nat Rev Cancer. 12:445–446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marrinucci D, Bethel K, Bruce RH, Curry
DN, Hsieh B, Humphrey M, Krivacic RT, Kroener J, Kroener L, Ladanyi
A, et al: Case study of the morphologic variation of circulating
tumor cells. Hum Pathol. 38:514–519. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiao LR, Apostolopoulos C, Jacob J, Szydlo
R, Johnson N, Tsim N, Habib NA, Coombes RC and Stebbing J: Unique
localization of circulating tumor cells in patients with hepatic
metastases. J Clin Oncol. 27:6160–6165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alix-Panabières C and Pantel K:
Circulating tumor cells: Liquid biopsy of cancer. Clin Chem.
59:110–118. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kraan J, Sleijfer S, Strijbos MH,
Ignatiadis M, Peeters D, Pierga JY, Farace F, Riethdorf S, Fehm T,
Zorzino L, et al: External quality assurance of circulating tumor
cell enumeration using the CellSearch(®) system: A
feasibility study. Cytometry B Clin Cytom. 80:112–118. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mostert B, Kraan J, Bolt-de Vries J, van
der Spoel P, Sieuwerts AM, Schutte M, Timmermans AM, Foekens R,
Martens JW, Gratama JW, et al: Detection of circulating tumor cells
in breast cancer may improve through enrichment with anti-CD146.
Breast Cancer Res Treat. 127:33–41. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pantel K, Alix-Panabières C and Riethdorf
S: Cancer micrometastases. Nat Rev Clin Oncol. 6:339–351. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stott SL, Hsu CH, Tsukrov DI, Yu M,
Miyamoto DT, Waltman BA, Rothenberg SM, Shah AM, Smas ME, Korir GK,
et al: Isolation of circulating tumor cells using a
microvortex-generating herringbone-chip. Proc Natl Acad Sci USA.
107:pp. 18392–18397. 2010; View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pinzani P, Salvadori B, Simi L, Bianchi S,
Distante V, Cataliotti L, Pazzagli M and Orlando C: Isolation by
size of epithelial tumor cells in peripheral blood of patients with
breast cancer: Correlation with real-time reverse
transcriptase-polymerase chain reaction results and feasibility of
molecular analysis by laser microdissection. Hum Pathol.
37:711–718. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wong NS, Kahn HJ, Zhang L, Oldfield S,
Yang LY, Marks A and Trudeau ME: Prognostic significance of
circulating tumour cells enumerated after filtration enrichment in
early and metastatic breast cancer patients. Breast Cancer Res
Treat. 99:63–69. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zieglschmid V, Hollmann C and Böcher O:
Detection of disseminated tumor cells in peripheral blood. Crit Rev
Clin Lab Sci. 42:155–196. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Basiji DA, Ortyn WE, Liang L,
Venkatachalam V and Morrissey P: Cellular image analysis and
imaging by flow cytometry. Clin Lab Med. 27:653–670, viii. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Van der Auwera I, Peeters D, Benoy IH,
Elst HJ, van Laere SJ, Prové A, Maes H, Huget P, van Dam P,
Vermeulen PB, et al: Circulating tumour cell detection: A direct
comparison between the CellSearch System, the AdnaTest and
CK-19/mammaglobin RT-PCR in patients with metastatic breast cancer.
Br J Cancer. 102:276–284. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Krebs MG, Hou JM, Sloane R, Lancashire L,
Priest L, Nonaka D, Ward TH, Backen A, Clack G, Hughes A, et al:
Analysis of circulating tumor cells in patients with non-small cell
lung cancer using epithelial marker-dependent and -independent
approaches. J Thorac Oncol. 7:306–315. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gervasoni A, Sandri MT, Nascimbeni R,
Zorzino L, Cassatella MC, Baglioni L, Panigara S, Gervasi M, Di
Lorenzo D and Parolini O: Comparison of three distinct methods for
the detection of circulating tumor cells in colorectal cancer
patients. Oncol Rep. 25:1669–1703. 2011.PubMed/NCBI
|
|
23
|
Rashid OM, Nagahashi M, Ramachandran S,
Dumur CI, Schaum JC, Yamada A, Aoyagi T, Milstien S, Spiegel S and
Takabe K: Is tail vein injection a relevant breast cancer lung
metastasis model? J Thorac Dis. 5:385–392. 2013.PubMed/NCBI
|
|
24
|
Sasportas LS, Hori SS, Pratx G and Gambhir
SS: Detection and quantitation of circulating tumor cell dynamics
by bioluminescence imaging in an orthotopic mammary carcinoma
model. PLoS One. 9:e1050792014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
James ML and Gambhir SS: A molecular
imaging primer: Modalities, imaging agents, and applications.
Physiol Rev. 92:897–965. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Choi JW, Kim JK, Yang YJ, Kim P, Yoon KH
and Yun SH: Urokinase exerts antimetastatic effects by dissociating
clusters of circulating tumor cells. Cancer Res. 75:4474–4482.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aceto N, Bardia A, Miyamoto DT, Donaldson
MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, et al:
Circulating tumor cell clusters are oligoclonal precursors of
breast cancer metastasis. Cell. 158:1110–1122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Juratli MA, Sarimollaoglu M, Nedosekin DA,
Melerzanov AV, Zharov VP and Galanzha EI: Dynamic fluctuation of
circulating tumor cells during cancer progression. Cancers (Basel).
6:128–142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bonnomet A, Syne L, Brysse A, Feyereisen
E, Thompson EW, Noël A, Foidart JM, Birembaut P, Polette M and
Gilles C: A dynamic in vivo model of epithelial-to-mesenchymal
transitions in circulating tumor cells and metastases of breast
cancer. Oncogene. 31:3741–3753. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schmidt CM, Settle SL, Keene JL, Westlin
WF, Nickols GA and Griggs DW: Characterization of spontaneous
metastasis in an aggressive breast carcinoma model using flow
cytometry. Clin Exp Metastasis. 17:537–544. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aslakson CJ and Miller FR: Selective
events in the metastatic process defined by analysis of the
sequential dissemination of subpopulations of a mouse mammary
tumor. Cancer Res. 52:1399–1405. 1992.PubMed/NCBI
|