Introduction
Lung cancer is the most frequently diagnosed cancer
and the leading cause of cancer death, resulting in more than 1.5
million deaths worldwide in 2012 (1). The morbidity of lung cancer has
continued to increase worldwide, especially in less developed
countries, in part because of air pollution. Non-small cell lung
cancer (NSCLC) accounts for almost 85% of all lung cancer cases.
Despite significant progress in the diagnosis and treatment of
NSCLC, the 5-year overall survival rate of patients with NSCLC
remains very low (2).
Cisplatin-based, two-drug combination chemotherapy has been
recommended as the first-line treatment for NSCLC, but intrinsic
and acquired cisplatin resistance limits its efficacy in lung
cancer treatment. Thus, the identification of new therapeutic
targets and new methods to enhance the sensitivity of cisplatin for
NSCLC would be of great value.
Autophagy is a process of phagocytosis of
unnecessary or dysfunctional components, fusion with lysosomes and
degradation of the contents of the lysosomes to maintain cellular
homeostasis (3). As one of the
important mechanisms of essential material recycling, autophagy is
thought to participate in a variety of diseases, including lung
cancer (4). Many tumors survive via
autophagy, which suggests that targeting autophagy may be a
universal method for cancer therapy (5). Dysregulation of autophagy is often
observed in lung cancer (6).
Inhibition of autophagy sensitizes NSCLC cells to chemotherapy
(7,8). Kinase inhibitors have proven
successful in the clinic. As the only conserved serine/threonine
kinase in the autophagy cascade, uncoordinated (Unc) 51-like kinase
1 (Ulk1) is a very attractive cancer drug target (5). Ulk1 plays a key role in the initial
stages of autophagy by forming a complex with autophagy-related 13
(ATG13) and RB1-inducible coiled-coil 1 (RB1CC1) (9,10).
Under nutrient-rich conditions, both Ulk1 and ATG12 are
phosphorylated by target of rapamycin (TOR), which represses Ulk1
kinase activity and thus results in inhibition of autophagy.
Conversely, upon nutrient deprivation, activated AMP-activated
protein kinase (AMPK) activates Ulk1, which subsequently leads to
initiation of autophagy (11). Pike
et al showed that Ulk1 promotes cell survival of several
cancers (12). Moreover,
overexpression of Ulk1 has been shown to be negatively correlated
with the prognosis of a variety of cancers, such as colorectal
cancer, breast cancer, human nasopharyngeal carcinoma and
esophageal squamous cell carcinoma (12–16).
Egan et al reported that the compound SBI0206965, a highly
selective kinase inhibitor of Ulk1 both in vitro and in
vivo, suppresses Ulk1-mediated phosphorylation events in cells
and prevents Ulk1-dependent cell survival by suppressing autophagy
(5). However, the roles of Ulk1 and
SBI0206965 in NSCLC are largely unknown.
We report that Ulk1 was upregulated in NSCLC cell
lines and was negatively correlated with prognosis in NSCLC
patients. Knockdown of Ulk1 inhibited cell growth and sensitized
NSCLC cells to cisplatin. Inhibition of Ulk1 by SBI0206965 reduced
the proliferation of NSCLC cells and induces cell apoptosis by
inhibiting autophagy and destabilizing Bcl2/Bclxl. In summary, our
results show that SBI0206965 suppresses NSCLC cell growth and
sensitizes NSCLCL cells to cisplatin by modulating both autophagy
and apoptosis pathways and that Ulk1 is a promising therapeutic
target for NSCLC treatment.
Materials and methods
Cell culture
The NSCLC cell lines A549, H1299, H292, H460, and
HCC827 and the normal human lung cell line BEAS-2B were obtained
from the American Type Culture Collection (Manassas, VA, USA). The
cell lines were grown in RPMI-1640 medium (HyClone, South Logan,
VT, USA), supplemented with 10% (v/v) fetal bovine serum (Gibco,
Grand Island, NY, USA) and 100 IU/ml penicillin and 100 µg/ml
streptomycin (Beyotime Biotechnology, Shanghai, China). All of the
cells were cultured at 37°C in a humidified incubator with 5%
CO2.
Reagents and antibodies
Cisplatin and Z-VAD-FMK were purchased from Selleck
(Houston, TX, USA). SBI0206965 was obtained from DC Chemicals
(Shanghai, China). The anti-Ulk1 (8054) and anti-LC3 (3868)
antibodies were obtained from Cell Signaling Technology (Danvers,
MA, USA). The anti-Bcl2 (12789-1-AP) and Bclxl (10783-1-AP)
antibodies were purchased from Proteintech (Chicago, IL, USA). The
anti-p62 (ab109012) antibody was purchased from Abcam (Cambridge,
UK). The mouse anti-β-actin monoclonal antibody (AM1021B) was from
Abgent (San Diego, CA, USA).
Cell proliferation and EdU
incorporation assay
A total of 1×104 cells in 100 µl of
supplemented culture medium per well were cultured in 96-well
plates overnight, followed by treatment with the indicated
compounds in quintuplicate for 72 h. Cell viability was assessed at
450 nm using a microplate reader (Bio-Rad, Hercules, CA, USA), 1 h
after adding 10 µl of CCK-8 reagent (Cell Counting Kit-8, Dojindo
Molecular Technologies, Inc., Kumamoto, Japan) to each well. Cell
proliferation was also assessed using a Cell-Light EdU DNA cell
proliferation kit (RiboBio, Guangzhou, China), according to the
manufacturer's instructions. All analyses were performed three
times.
Colony formation assay
H460 and A549 cells were seeded at 800 or 500 cells
per well in 2 ml of medium in 6-well plates. The cells were treated
as required the next day, and were allowed to grow for 10–14 days
in the constant temperature and humidity incubator until colonies
formed. The cells were then washed twice with PBS, stained with a
solution of 0.5% crystal violet and 70% ethanol, washed with PBS
twice and dried. Colonies with >50 cells were counted, and each
assay was conducted in triplicate and three separate assays were
performed.
Annexin V assay of cell apoptosis
Cells were cultured in six-well plates to 70–80%
confluence and were then treated with the indicated compounds.
After exposure for 48 or 72 h, the cells were harvested by trypsin,
washed twice with PBS, resuspended in 400 µl of Annexin-binding
buffer, and stained with 5 µl of Annexin V-PE (FITC) at room
temperature in the dark for 15 min and with 10 µl of 7-AAD (PI) for
5 min according to the manufacturer's instructions for the Annexin
V-FITC Apoptosis assay kit (BestBio, Shanghai, China). Both
apoptotic cells and live cells were detected by flow cytometry (BD
Biosciences, San Jose, CA, USA).
Plasmids and transfection
The shRNA vector pLKO.1 and GFP-LC3 plasmids were
obtained from Addgene, Inc. (Cambridge, MA, USA). pLKO.1 puro was a
gift from Bob Weinberg (Addgene plasmid #8453) (17). GFP-LC3 was a gift from Jayanta
Debnath (Addgene plasmid #22405) (18). Endogenous Ulk1 was knocked down
using pLKO.1-shRNA-Ulk1-1 (Mission TRC shRNA TRCN0000000838) and
pLKO.1-shRNA-Ulk1-2 (Mission TRC shRNA TRCN0000000839). The control
shRNA sequence was CAACAAGATGAAGAGCACCAA. Cells were transfected
with the indicated plasmids according to the manufacturer's
instructions for TurboFect DNA transfection reagent (Thermo Fisher
Scientific, Waltham, MA, USA).
Western blotting
Cells were lysed on ice with cell lysis buffer
(Beyotime, Shanghai, China), and the protein concentrations were
measured using the Pierce BCA Protein assay kit (Thermo Fisher
Scientific). The samples were subjected to 10 or 12% SDS-PAGE with
a power supply (Bio-Rad) and transferred to polyvinylidene fluoride
membranes (Millipore, Bedford, MA, USA). The membranes were blocked
in 5% skimmed milk (Difco Laboratories, Detroit, MI, USA) in TBST
for 1.5 h and then incubated with primary antibodies overnight at
4°C, followed by incubation with the corresponding horseradish
peroxidase (HRP)-conjugated secondary antibody (Proteintech) for
1.5 h. Finally, the bands were visualized with the ECL system
(Beyotime) by exposure to X-ray film. Actin was used as a loading
control. All experiments were performed three times.
Total RNA isolation, reverse
transcription and quantitative real-time PCR (qRT-PCR)
Total RNA from NSCLC cells was isolated using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA). The quantity of isolated
RNA was measured by NanoPhotometer (Implen, Munich, Germany).
First-strand cDNA was synthesized using SuperScript First-Strand
Synthesis System for RT-PCR (Invitrogen) using 2.5 µg of total RNA
isolated from NSCLC cells. Each reaction of real-time polymerase
chain reactions (PCR) was conducted with SYBR® Premix Ex
Taq™ (Takara, Dalian, China) in a final volume of 20 µl using 1 µg
of cDNA. β-actin was used as endogenous control transcripts for
normalization of the target transcripts. All primers were tested
for optimal annealing temperatures and PCR conditions were
optimized with gradient PCRs on an iCycler (Bio-Rad). The PCR
primer sequences for β-actin, Bcl2 and Bclxl are as follows (5′ to
3′, sense and antisense, respectively): Human β-actin sense: TGG
CACCCAGCACAATGAA, and antisense: CTAAGTCATAG TCCGCCTAGAAGCA; human
Ulk1 sense: GGCAAGTTCGA GTTCTCCCG, and antisense:
CGACCTCCAAATCGTGCT TCT; human Bcl2 sense: GGTGGGGTCATGTGTGTGG, and
antisense: CGGTTCAGGTACTCAGTCATCC; human Bclxl sense:
GACTGAATCGGAGATGGAGACC, and antisense: GCAGTTCAAACTCGTCGCCT.
The PCR reaction was carried out as follows: step 1:
95°C 5 min; step 2: 39 cycles at 95°C for 30 sec, 60°C for 30 sec,
72°C for 30 sec; step 3: 95°C for 10 sec, 65°C for 10 sec. Each
reaction tube contained 10 µl 2X SYBR Premix Ex Taq + 7 µl
nuclease-free water + 2 µl 0.1 µg/μl primer (pair) + 1 µl cDNA (0.2
µg/µl). Genes were amplified in triplicate. The average cycle
threshold (Ct) value for each group was determined and normalized
by the endogenous control Ct value. Relative gene expression was
analyzed using the 2-∆∆Ct method.
Statistical analysis
Statistical software including SPSS 17.0 and
GraphPad Prism 5.0 was used for data analysis. All values are
presented as mean ± standard deviation (SD) of triplicate
measurements and repeated three times with similar results. All
data were subjected to Student's t-test. Differences were
considered statistically significant at P<0.05.
Results
Ulk1 is overexpressed in NSCLC cell
lines
It has been reported that in several cancers, such
as breast cancer, colorectal cancer, esophageal squamous cell
carcinoma and human nasopharyngeal carcinoma, Ulk1 is upregulated
and is negatively correlated with prognosis, but the role of Ulk1
in NSCLC remains unclear (12–16).
In the current study, the expression of Ulk1 was tested in five
NSCLC cell lines (A549, H292, H460, H1299 and HCC827) and one
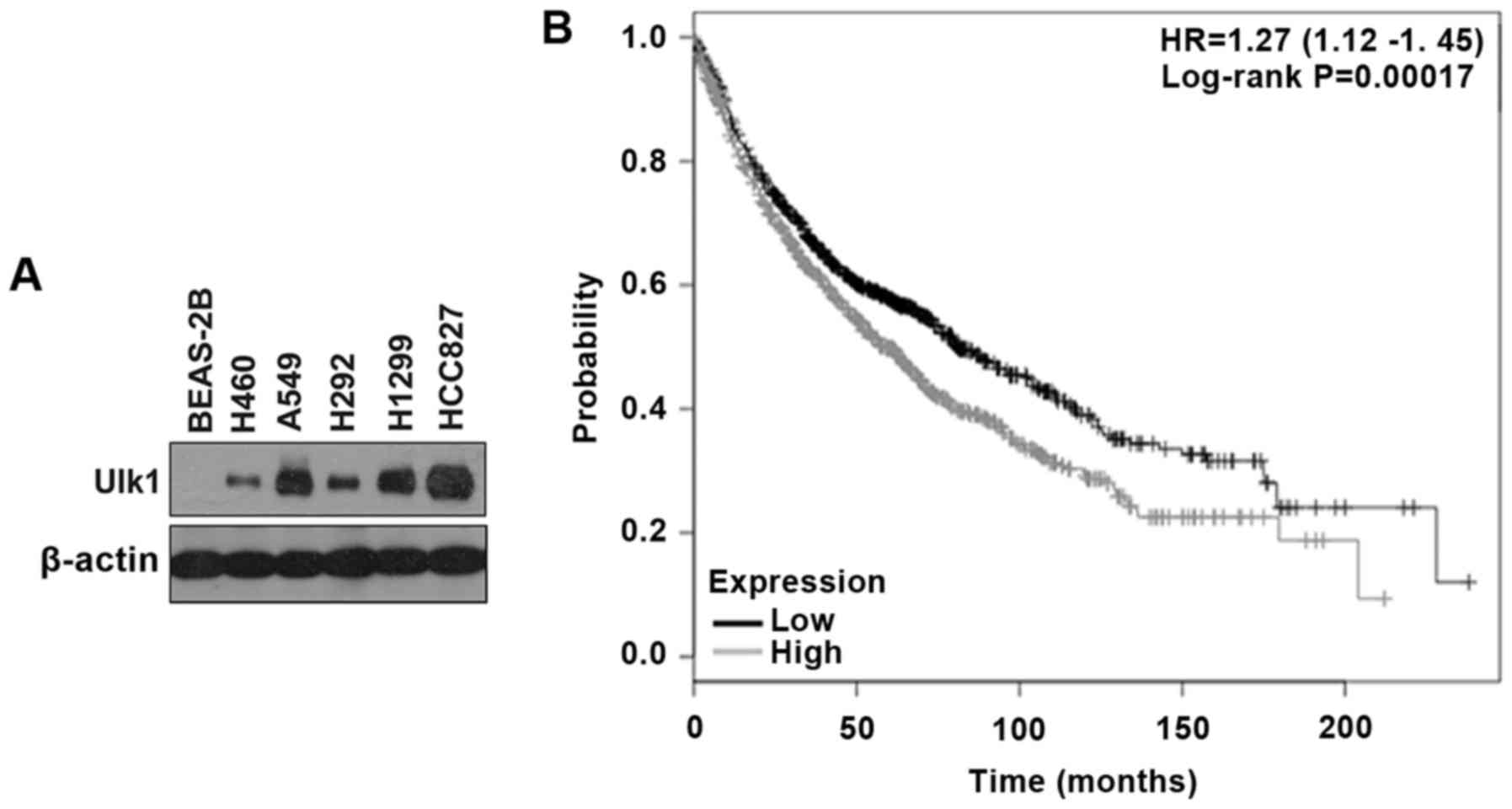
normal human lung cell line (BEAS-2B). As shown in Fig. 1A, the NSCLC cell lines exhibited
higher expression of Ulk1 compared with BEAS-2B cells. Moreover, by
searching the online Kaplan-Meier plotter database for the analysis
of the prognostic value of biomarkers using transcriptomic data in
NSCLC (19), we found that high
expression of Ulk1 is negatively correlated with prognosis in
patients with lung cancer (P<0.01) (Fig. 1B). Tyrosine kinases inhibitors
(TKIs) are considered as first-line treatment for advanced or
metastatic NSCLC patients with epidermal growth factor receptor
(EGFR) activating mutations in the clinic. HCC827 is an
adenocarcinoma line with the E746-A750 EGFR deletion mutation and
is sensitive to TKIs (20). In our
preliminary experiment, we found that HCC827 cells showed striking
growth inhibition with gefitinib treatment and that inhibition of
Ulk1 did not increase the efficacy of gefitinib in HCC827 cells.
Therefore, we used A549 and H460 cells in the following study
instead of HCC827 cells, though HCC827 cells showed a higher
expression of Ulk1 than the other cell lines (Fig. 1A).
Ulk1 promotes the proliferation of
NSCLC cells
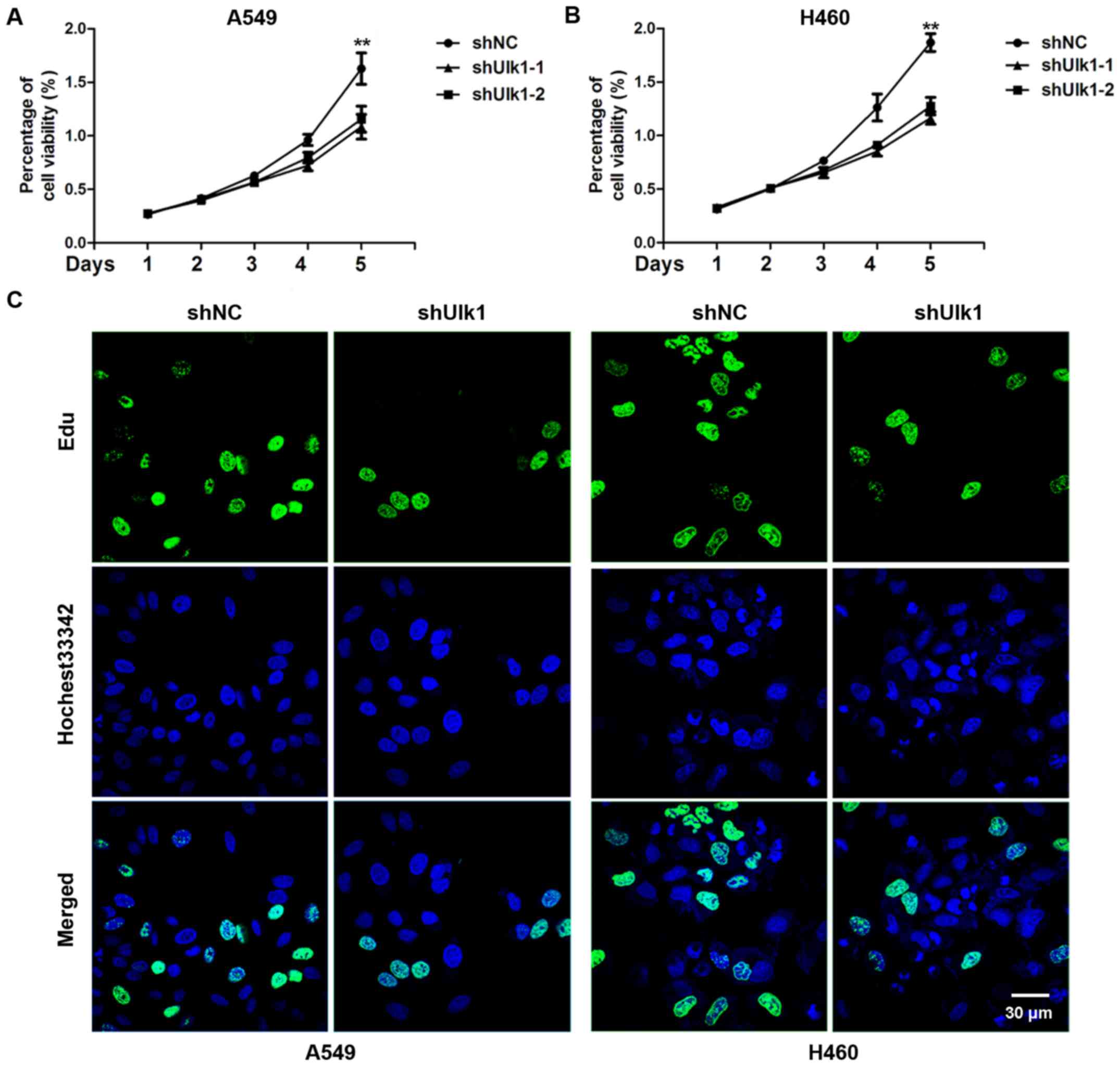
As Ulk1 is overexpressed in NSCLC, we speculated
that Ulk1 might regulate the proliferation of NSCLC cells. To
assess the effects of Ulk1 on cell proliferation, A549 and H460
cells were transfected with negative control (NC) or Ulk1-specific
shRNA plasmids, and cell proliferation was measured by CCK8 assay.
We found a significant decrease in the cell proliferation rate when
Ulk1 was downregulated (P<0.01) (Fig. 2A and B). The EdU incorporation assay
showed that the percentage of EdU-positive cells was obviously
reduced when Ulk1 was downregulated (Fig. 2C). Taken together, the results
suggest that knockdown of Ulk1 inhibits cell proliferation of NSCLC
cells.
Ulk1 confers resistance to cisplatin
in NSCLC cells
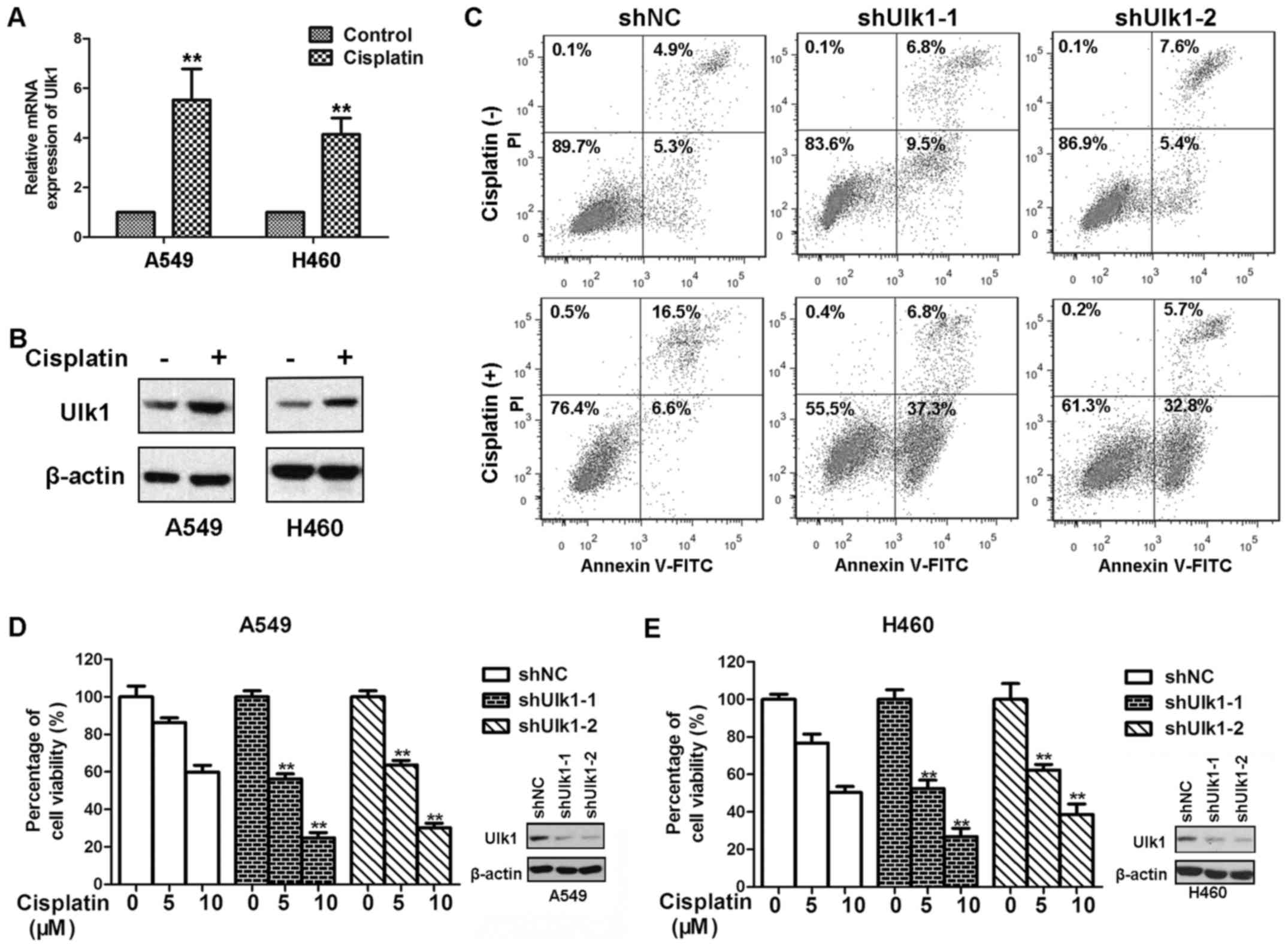
Previous studies have shown that cisplatin
upregulated both mRNA and protein levels of Ulk1 (21,22).
We speculated that cisplatin might have the same effects in Ulk1 in
NSCLC cells. In this study, we found that cisplatin treatment
upregulated the expression of mRNA and protein levels of Ulk1 in
both A549 and H460 cells (Fig. 3A and
B). Many NSCLC cells acquire resistance to the cytotoxicity
induced by cisplatin, which limits the therapeutic efficacy of
cisplatin. We wondered whether Ulk1 will contribute to resistance
to cisplatin in NSCLC cells. To confirm this hypothesis, A549 and
H460 cells transfected with NC or Ulk1-specific shRNA plasmids were
exposed to cisplatin for 72 h. As shown in Fig. 3D, and E, knockdown of Ulk1
significantly impaired the viability of NSCLC cells after cisplatin
treatment. Cisplatin causes apoptotic cell death in a
dose-dependent manner (23). We
therefore examined whether Ulk1 influences cisplatin-mediated
apoptosis in NSCLC cells. As shown in Fig. 3C, knockdown of Ulk1 remarkably
increased cisplatin-induced apoptosis. Moreover, knockdown of Ulk1
promoted apoptosis in the absence of stress. The results suggest
that knockdown of Ulk1 can sensitize NSCLC cells to cisplatin.
Inhibition of Ulk1 kinase activity by
SBI0206965 inhibits NSCLC cell proliferation and induces apoptosis
of NSCLC cells
As described above, Ulk1 is associated with the
proliferation and apoptosis of NSCLC cells. Under stress
conditions, Ulk1 is activated by AMPK-mediated phosphorylation
and/or loss of the repression of mTOR (5). The autophagy cascade can be triggered
when activated Ulk1 phosphorylates downstream targets (10,11).
Thus, the kinase activity of Ulk1 may play a vital role in
autophagy-mediated survival in NSCLC cells under stress conditions.
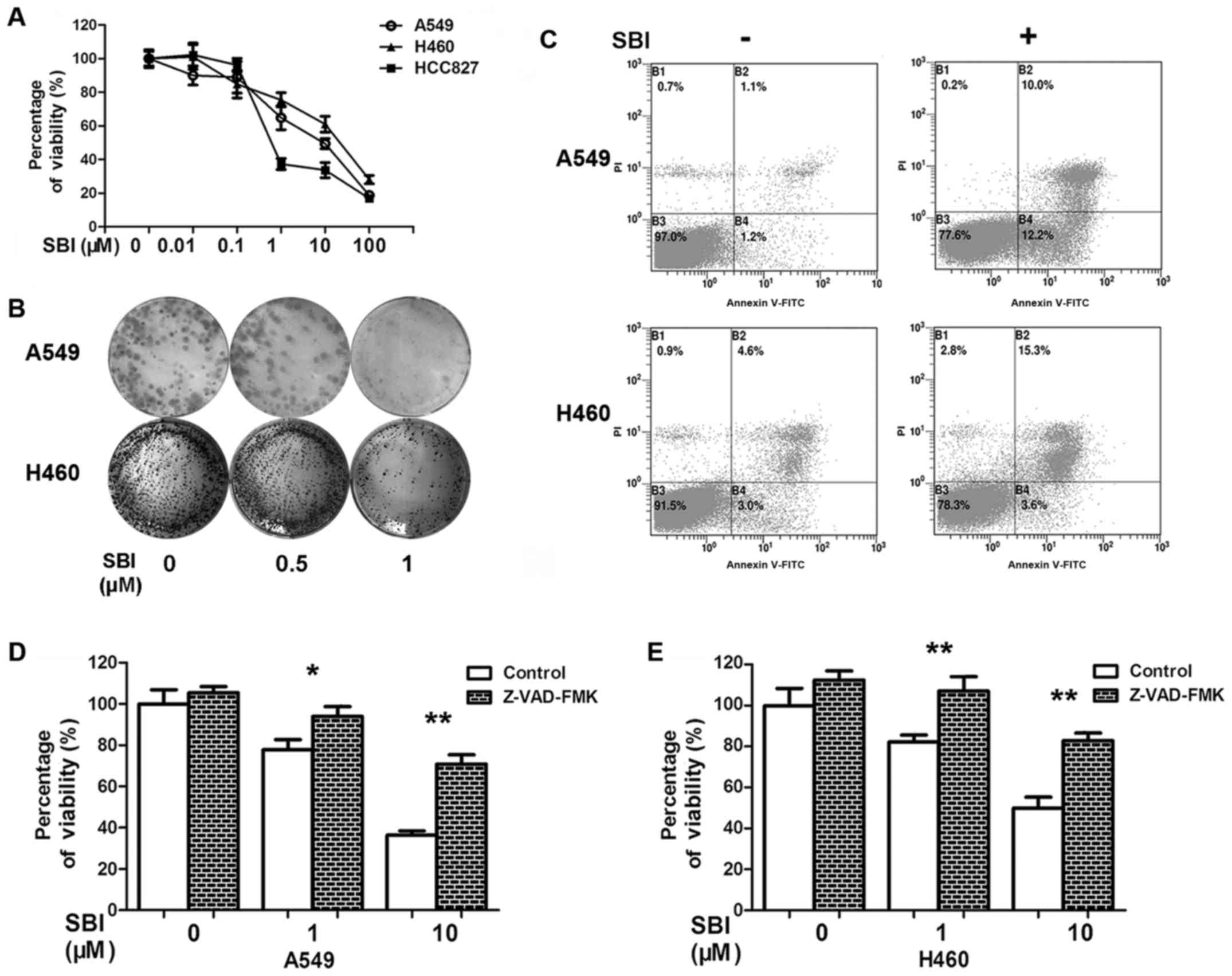
We used SBI0206965, a Ulk1-specific inhibitor, to confirm the role
of Ulk1 (5). We first assessed the
cytotoxicity of SBI0206965 in three human NSCLC cell lines: A549,
H460 and HCC827. Inhibition of Ulk1 by SBI0206965 suppressed cell
viability in a dose-dependent manner (Fig. 4A). The clonogenic survival assay
revealed that the ability to form clones was decreased in
SBI0206965 treatment groups, comparing with the control group
(Fig. 4B). SBI0206965 treatment
also induced apoptosis of NSCLC cells (Fig. 4C). Chemotherapy drugs generally kill
cancer cells by inducing apoptosis (24). To clarify whether the role of
SBI0206965 in apoptosis was responsible for the death of NSCLC
cells, we introduced the pan caspase inhibitor Z-VAD-FMK into our
study. The combination of inhibition of apoptosis by Z-VAD-FMK with
SBI0206965 remarkably improved cell viability compared with
SBI0206965 alone (Fig. 4D and E).
Taken together, the results suggest that inhibition of Ulk1 kinase
activity by SBI0206965 impairs cell proliferation and induces
apoptosis of NSCLC cells.
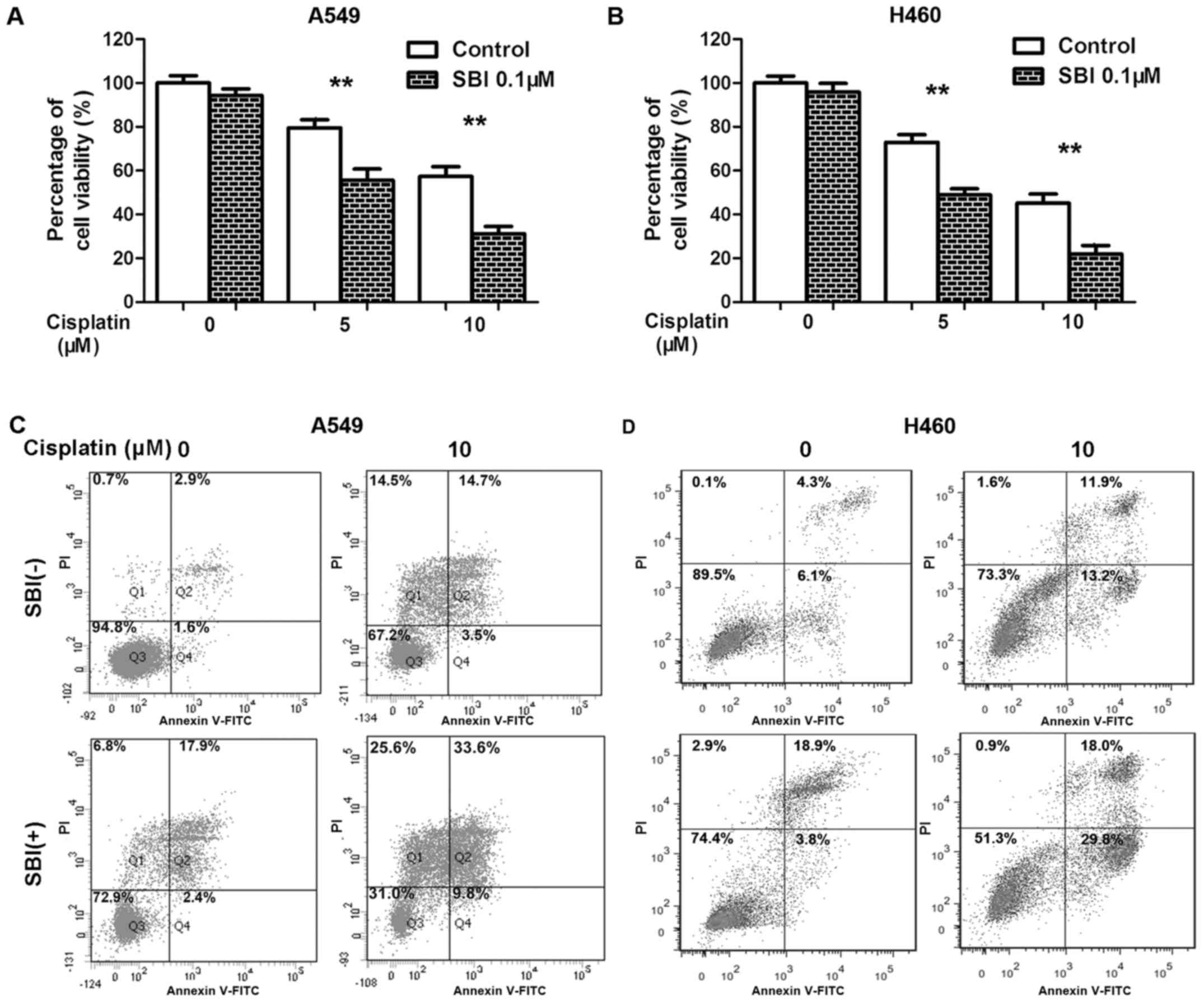
Inhibition of Ulk1 by SBI0206965
enhances the efficacy of cisplatin against NSCLC cells
Since knockdown of Ulk1 sensitized NSCLC cells to
cisplatin, we speculated that the Ulk1 inhibitor SBI0206965 might
enhance the efficacy of cisplatin against NSCLC. We therefore
treated NSCLC cells with SBI0206965 and cisplatin either alone or
in combination. In agreement with our hypothesis, targeting Ulk1
using SBI0206965 significantly impaired the viability of NSCLC
cells after cisplatin treatment compared with cisplatin treatment
without SBI0206965 (Fig. 5A and B).
As shown in Fig. 5C, the percentage
of Annexin V-positive cells was increased after co-treatment with
SBI0206965 and cisplatin compared with SBI0206965 or cisplatin
alone. Taken together, the results suggest that combination
treatment with the Ulk1 inhibitor SBI0206965 and cisplatin enhanced
cisplatin efficacy toward NSCLC cells, consistent with the results
of treating Ulk1-knockdown cells with cisplatin.
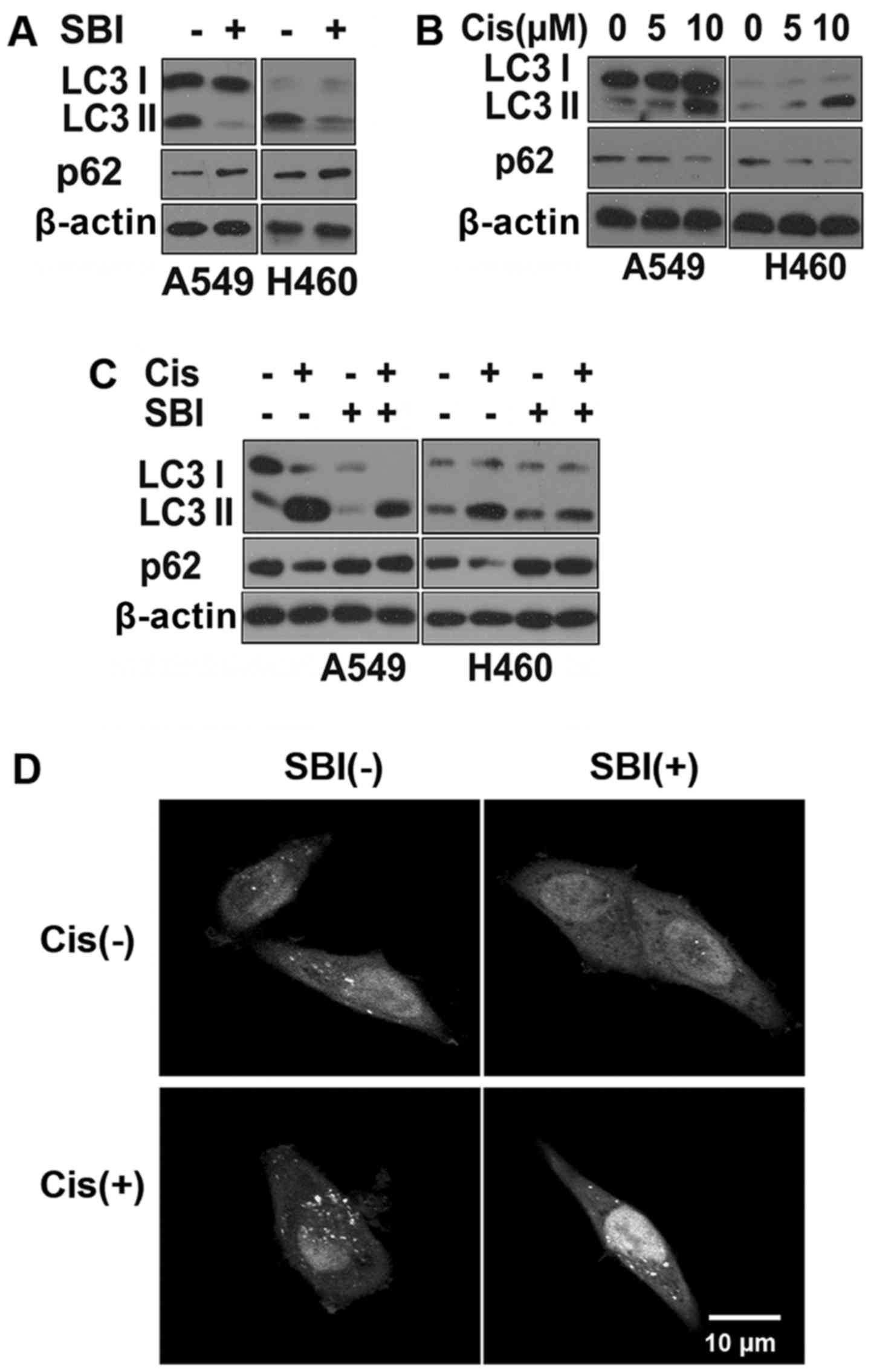
SBI0206965 sensitizes NSCLC cells to
cisplatin-induced apoptosis partly through autophagy
inhibition
Next, we wanted to clarify the mechanism by which
Ulk1 inhibition enhances cisplatin-induced death in NSCLC cells.
Ulk1 is required for early autophagosome formation, as it forms a
complex with ATG13 and RB1CC1 (10). Ulk1 also phosphorylates beclin1 and
autophagy/beclin-1 regulator 1 (AMBRA1) to trigger the autophagy
cascade (25,26). Therefore, inhibition of Ulk1 should
inhibit autophagy. As expected, SBI0206965 treatment inhibited
autophagy, as evidenced by a reduction in LC3 I conversion to LC3
II, an increase in the levels of the autophagy substrate p62 and a
decrease in GFP-LC3 dots (Fig. 6A and
D). Many tumors become dependent on autophagy for survival and
resistance to chemotherapeutic drugs, including cisplatin, by
inhibiting apoptosis (24,27). Studies have shown that inhibition of
autophagy sensitizes NSCLC cells to chemotherapy (7,8). We
confirmed that cisplatin induces autophagy in NSCLC cells (Fig. 6B and D). Co-treatment with
SBI0206965 and cisplatin blocked the autophagy process induced by
cisplatin (Fig. 6C and D). These
results indicate that SBI0206965 enhanced the efficacy against
cisplatin in NSCLC partly through inhibiting autophagy.
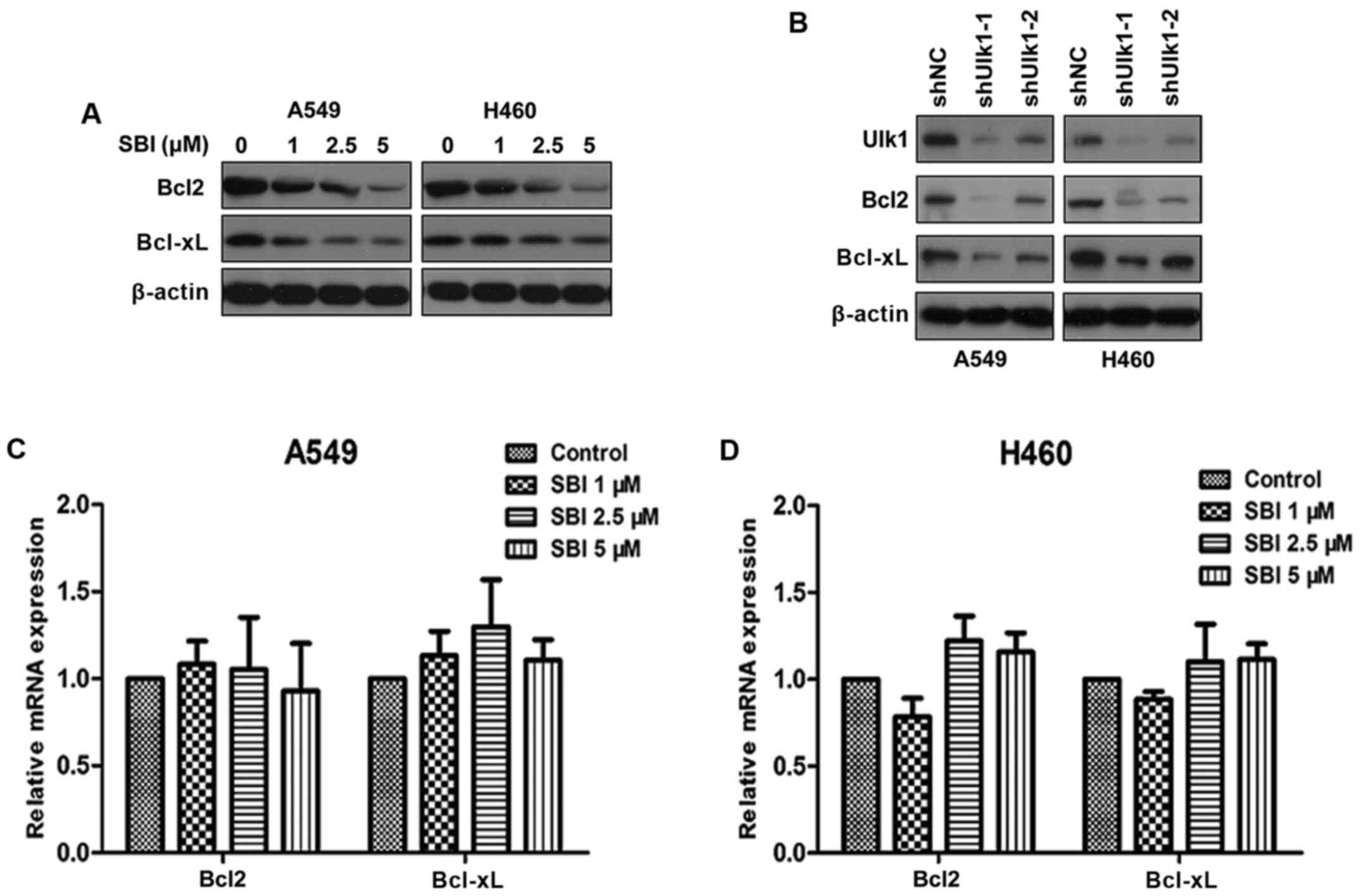
Inhibition of Ulk1 promotes apoptosis
of NSCLC cells by destabilizing Bcl2 and Bclxl
In addition to its key roles in autophagy, Ulk1 has
been shown to be involved in many signaling pathways that are
closely correlated with tumorigenesis in NSCLC (5). Our preliminary experiment demonstrated
that SBI0206965 can still inhibit cell proliferation and promote
apoptosis when autophagy is inhibited by chloroquine (data not
shown). We speculated that Ulk1 exerts oncogenic activity
independent of autophagy. As mentioned above, inhibition of Ulk1
kinase activity by SBI0206965 impairs cell proliferation mainly
through induction of apoptosis. Thus, we focused on
apoptosis-related proteins. We found that SBI0206965 treatment
decreased Bcl2 and Bclxl protein levels in a dose-dependent manner
(Fig. 7A). Moreover, knockdown of
Ulk1 showed similar results (Fig.
7B). Furthermore, we performed qRT-PCR analysis to evaluate the
mRNA levels of Bcl2 and Bclxl under SBI0206965 treatment. As shown
in Fig. 7C and D, SBI0206965 had no
obvious influence on the mRNA levels of Bcl2 and Bclxl compared
with the control groups. The results indicated that the
downregulation of Bcl2 and Bclxl by SBI0206965 treatment did not
occur at the transcription level. Taken together, SBI0206965 can
promote apoptosis of NSCLC cells independent of autophagy by
destabilizing Bcl2 and Bclxl.
Discussion
Lung cancer has become a major public health problem
worldwide and seriously affects the quality of life of affected
patients. Currently, most lung cancer patients are diagnosed when
they already have advanced-stage disease, and the 5-year survival
rate for lung cancer is only 16.8% (28). Despite potentially curative
resection treatment, recurrence and metastasis result in the death
of nearly 40% of patients with NSCLC within 5 years (29). Therefore, further studies are
required to discover and identify novel and specific biomarkers of
NSCLC to benefit patient survival and quality of life.
Autophagy is a homeostatic and evolutionarily
conserved process that degrades redundant or faulty cell
components, and has an important role in maintaining intracellular
homeostasis and survival under stressed conditions, such as energy
deprivation (6). Autophagy is
important in the normal development and cellular response to
environmental stimuli. Dysregulated autophagy is emerging as a
hallmark of malignancy (30).
Karsli-Uzunbas et al showed that autophagy may be
particularly relevant in lung cancer (4). Autophagy is a complex process that is
divided into several phases controlled by the autophagy-related
(ATG) proteins. The role of autophagy and the function of many
autophagy-related proteins in cancer have been extensively studied;
for example, Beclin1, ATG5 or ATG7 knockout cell lines or mouse
models have contributed greatly to the elucidation of autophagy in
tumorigenesis (31,32). Ulk1 plays a key role in the initial
stages of autophagy (10), but the
role of Ulk1 in cancer has remained largely unknown. Pike et
al showed that Ulk1 is required for autophagy in severe hypoxia
and that knockdown of Ulk1 causes cell death in a
caspase-3/7-independent manner (12). Several other groups also showed that
high expression of Ulk1 is associated with poor prognosis in breast
cancer, colorectal cancer, esophageal squamous cell carcinoma and
human nasopharyngeal carcinoma (12–15).
However, studies have also shown that Ulk1 inhibits cell
proliferation and induces apoptosis in other cancer cell types
(33–36). It is clear that autophagy plays
complex roles in tumorigenesis, progression and resistance to
treatment that may be context specific (30). Therefore, the function of Ulk1 in
tumorigenesis might be cancer type specific. Our study showed that
Ulk1 was upregulated in NSCLC cells and was negatively correlated
with prognosis in NSCLC, and that Ulk1 induced cell proliferation
and inhibited apoptosis in NSCLC cells. Consistent with this,
inhibition of Ulk1 by knockdown of Ulk1 or by treatment with
SBI0206965, a novel Ulk1 inhibitor, impaired proliferation and
induces cell apoptosis in NSCLC cells. Therefore, Ulk1 may
contribute to tumorigenesis and progression in NSCLC.
Autophagy initiation involves the formation of a
phagophore and Ulk1 functions as a facilitator of phagophore
formation (37). Upon stress
condition, activated AMP-activated protein kinase (AMPK) activates
Ulk1, leading to the initiation of autophagy (11). ATG4 cleaved LC3 to generate the LC3
I form. Subsequently, LC3 I is activated by Atg7 catalyzing acyl
adenylation of the C terminus, transferred to the E2-conjugating
enzyme, Atg3, and modified to a membrane-bound form, LC3 II
(38,39). LC3 protein is associated with
autophagosome development and maturation and is used to monitor
autophagic activity. In this study, we found a reduction in LC3 I
conversion to LC3 II, an increase in the level of the autophagy
substrate p62 and a decrease in the number of GFP-LC3 dots after
SBI0206965 treatment or knockdown of Ulk1. It is possible that Ulk1
promotes cell survival in NSCLC cells by inducing autophagy.
Previous studies showed an increase in the level of
Ulk1 after cisplatin treatment (21,22).
In this study we found that cisplatin upregulated the expression of
both mRNA and protein levels of Ulk1 in NSCLC cells. In addition to
traditional surgical resection and radiotherapy, chemotherapy
remains a common treatment for lung cancer. Many NSCLC cells
acquire resistance to the cytotoxicity induced by cisplatin, which
limits the therapeutic efficacy of cisplatin. Previous studies have
demonstrated that cell-protective autophagy confers resistance to
cisplatin in lung cancer cells (23,27).
Cisplatin causes apoptotic cell death in a dose-dependent manner
(23). Autophagy can promote cell
survival under stress conditions by suppressing apoptosis (24). In this study, we found that
cisplatin treatment induced autophagy, and that knockdown of Ulk1
or inhibition of Ulk1 by SBI0206965 significantly impaired the
viability of NSCLC cells and increased the apoptotic cells with
cisplatin treatment. The results indicated that Ulk1 may confer
resistance to cisplatin, and that SBI0206965 may increase the
sensitivity of cisplatin against NSCLC cells by autophagy
inhibition. These results showed an association between Ulk1 and
cisplatin resistance, and may provide a strong rationale for the
combined use of SBI0206965 and cisplatin in the clinic.
Another important phenomenon we observed was that
knockdown of Ulk1 or treatment with SBI0206965 decreased two
apoptosis-related proteins: Bcl2 and Bclxl, in NSCLC cells, without
obvious influence on the transcription levels of Bcl2 and Bclxl.
Ulk1 has been shown to be involved in many signaling pathways that
are closely correlated with tumorigenesis (5). Apoptosis and autophagy are both
closely regulated biological processes in cancer cells (40). Impaired apoptosis is both critical
in cancer development and a major barrier to effective treatment
(41). The Bcl2 family proteins
play complex roles in cell apoptosis and survival signaling
(40,42–44).
Bcl2 and Bclxl are often overexpressed in NSCLC, conferring
resistance to chemotherapy and exerting anti-apoptotic functions in
response to a wide range of apoptotic stimuli (45). Downregulation of Bcl2 and Bclxl
restores cisplatin sensitivity (46). It is possible that the inhibition of
Ulk1 may suppress NSCLC cell growth and increase the sensitivity of
cisplatin in NSCLC cells by apoptosis pathway.
There are five Ulk1 homologs in the human genome:
Ulk1, Ulk2, Ulk3, Ulk4, and serine/threonine kinase 36 (STK36)
(47). However, only Ulk1 and Ulk2
are known to have roles in regulating autophagy, and Ulk2 also has
distinct functions. It should be noted that this study examined
only the function of Ulk1 in NSCLC cells, and whether Ulk2 or other
Ulk1 homologs have the same functions as Ulk1 in lung cancer cells
requires further study. In addition, our results lack the
underlying mechanisms to explain how Ulk1 influences the protein
levels of Bcl2 and Bclxl, which needs further investigation.
In summary, we report that SBI0206965, an inhibitor
of Ulk1, exerts anti-tumor effects in NSCLC. Moreover, the effects
of SBI0206965 occur in both autophagy-dependent and
autophagy-independent manner. Therefore, Ulk1 might be a potential
target for treating NSCLC and reversing cisplatin resistance.
Acknowledgements
This study was supported by grants from the National
Natural Sciences Foundation of Hubei Province (no. 2013CFA006).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Politi K and Herbst RS: Lung cancer in the
era of precision medicine. Clin Cancer Res. 21:2213–2220. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rubinsztein DC, Gestwicki JE, Murphy LO
and Klionsky DJ: Potential therapeutic applications of autophagy.
Nat Rev Drug Discov. 6:304–312. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Karsli-Uzunbas G, Guo JY, Price S, Teng X,
Laddha SV, Khor S, Kalaany NY, Jacks T, Chan CS, Rabinowitz JD, et
al: Autophagy is required for glucose homeostasis and lung tumor
maintenance. Cancer Discov. 4:914–927. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Egan DF, Chun MG, Vamos M, Zou H, Rong J,
Miller CJ, Lou HJ, Raveendra-Panickar D, Yang CC, Sheffler DJ, et
al: Small molecule inhibition of the autophagy kinase ULK1 and
identification of ULK1 substrates. Mol Cell. 59:285–297. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo JY, Xia B and White E:
Autophagy-mediated tumor promotion. Cell. 155:1216–1219. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fung C, Chen X, Grandis JR and Duvvuri U:
EGFR tyrosine kinase inhibition induces autophagy in cancer cells.
Cancer Biol Ther. 13:1417–1424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li X, Lu Y, Pan T and Fan Z: Roles of
autophagy in cetuximab-mediated cancer therapy against EGFR.
Autophagy. 6:1066–1077. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chan EY, Kir S and Tooze SA: siRNA
screening of the kinome identifies ULK1 as a multidomain modulator
of autophagy. J Biol Chem. 282:25464–25474. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ganley IG, Lam H, Wang J, Ding X, Chen S
and Jiang X: ULK1.ATG13.FIP200 complex mediates mTOR signaling and
is essential for autophagy. J Biol Chem. 284:12297–12305. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu CC, Lin YC, Chen YH, Chen CM, Pang LY,
Chen HA, Wu PR, Lin MY, Jiang ST, Tsai TF, et al: Cul3-KLHL20
ubiquitin ligase governs the turnover of ULK1 and VPS34 complexes
to control autophagy termination. Mol Cell. 61:84–97. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pike LR, Singleton DC, Buffa F, Abramczyk
O, Phadwal K, Li JL, Simon AK, Murray JT and Harris AL:
Transcriptional up-regulation of ULK1 by ATF4 contributes to cancer
cell survival. Biochem J. 449:389–400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yun M, Bai HY, Zhang JX, Rong J, Weng HW,
Zheng ZS, Xu Y, Tong ZT, Huang XX, Liao YJ, et al: ULK1: A
promising biomarker in predicting poor prognosis and therapeutic
response in human nasopharygeal carcinoma. PLoS One.
10:e01173752015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang J, Deng R, Luo RZ, Shen GP, Cai MY,
Du ZM, Jiang S, Yang MT, Fu JH and Zhu XF: Low expression of ULK1
is associated with operable breast cancer progression and is an
adverse prognostic marker of survival for patients. Breast Cancer
Res Treat. 134:549–560. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zou Y, Chen Z, He X, He X, Wu X, Chen Y,
Wu X, Wang J and Lan P: High expression levels of unc-51-like
kinase 1 as a predictor of poor prognosis in colorectal cancer.
Oncol Lett. 10:1583–1588. 2015.PubMed/NCBI
|
|
16
|
Jiang S, Li Y, Zhu YH, Wu XQ, Tang J, Li
Z, Feng GK, Deng R, Li DD, Luo RZ, et al: Intensive expression of
UNC-51-like kinase 1 is a novel biomarker of poor prognosis in
patients with esophageal squamous cell carcinoma. Cancer Sci.
102:1568–1575. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stewart SA, Dykxhoorn DM, Palliser D,
Mizuno H, Yu EY, An DS, Sabatini DM, Chen IS, Hahn WC, Sharp PA, et
al: Lentivirus-delivered stable gene silencing by RNAi in primary
cells. RNA. 9:493–501. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fung C, Lock R, Gao S, Salas E and Debnath
J: Induction of autophagy during extracellular matrix detachment
promotes cell survival. Mol Biol Cell. 19:797–806. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Győrffy B, Surowiak P, Budczies J and
Lánczky A: Online survival analysis software to assess the
prognostic value of biomarkers using transcriptomic data in
non-small-cell lung cancer. PLoS One. 8:e822412013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sakuma Y, Yamazaki Y, Nakamura Y,
Yoshihara M, Matsukuma S, Nakayama H, Yokose T, Kameda Y, Koizume S
and Miyagi Y: WZ4002, a third-generation EGFR inhibitor, can
overcome anoikis resistance in EGFR-mutant lung adenocarcinomas
more efficiently than Src inhibitors. Lab Invest. 92:371–383. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shen M, Duan WM, Wu MY, Wang WJ, Liu L, Xu
MD, Zhu J, Li DM, Gui Q, Lian L, et al: Participation of autophagy
in the cytotoxicity against breast cancer cells by cisplatin. Oncol
Rep. 34:359–367. 2015.PubMed/NCBI
|
|
22
|
Huang Y, Guerrero-Preston R and Ratovitski
EA: Phospho-∆Np63α-dependent regulation of autophagic signaling
through transcription and micro-RNA modulation. Cell Cycle.
11:1247–1259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ren JH, He WS, Nong L, Zhu QY, Hu K, Zhang
RG, Huang LL, Zhu F and Wu G: Acquired cisplatin resistance in
human lung adenocarcinoma cells is associated with enhanced
autophagy. Cancer Biother Radiopharm. 25:75–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sui X, Chen R, Wang Z, Huang Z, Kong N,
Zhang M, Han W, Lou F, Yang J, Zhang Q, et al: Autophagy and
chemotherapy resistance: A promising therapeutic target for cancer
treatment. Cell Death Dis. 4:e8382013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Russell RC, Tian Y, Yuan H, Park HW, Chang
YY, Kim J, Kim H, Neufeld TP, Dillin A and Guan KL: ULK1 induces
autophagy by phosphorylating Beclin-1 and activating VPS34 lipid
kinase. Nat Cell Biol. 15:741–750. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Di Bartolomeo S, Corazzari M, Nazio F,
Oliverio S, Lisi G, Antonioli M, Pagliarini V, Matteoni S, Fuoco C,
Giunta L, et al: The dynamic interaction of AMBRA1 with the dynein
motor complex regulates mammalian autophagy. J Cell Biol.
191:155–168. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu JT, Li WC, Gao S, Wang F, Li XQ, Yu
HQ, Fan LL, Wei W, Wang H and Sun GP: Autophagy inhibition
overcomes the antagonistic effect between gefitinib and cisplatin
in epidermal growth factor receptor mutant non-small-cell lung
cancer cells. Clin Lung Cancer. 16:e55–e66. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ettinger DS, Wood DE, Akerley W, Bazhenova
LA, Borghaei H, Camidge DR, Cheney RT, Chirieac LR, D'Amico TA,
Demmy TL, et al: National comprehensive cancer network: Non-small
cell lung cancer, version 6.2015. J Natl Compr Canc Netw.
13:515–524. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reck M, Heigener DF, Mok T, Soria JC and
Rabe KF: Management of non-small-cell lung cancer: Recent
developments. Lancet. 382:709–719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
White E: Deconvoluting the
context-dependent role for autophagy in cancer. Nat Rev Cancer.
12:401–410. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rao S, Tortola L, Perlot T, Wirnsberger G,
Novatchkova M, Nitsch R, Sykacek P, Frank L, Schramek D, Komnenovic
V, et al: A dual role for autophagy in a murine model of lung
cancer. Nat Commun. 5:30562014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Strohecker AM, Guo JY, Karsli-Uzunbas G,
Price SM, Chen GJ, Mathew R, McMahon M and White E: Autophagy
sustains mitochondrial glutamine metabolism and growth of
BrafV600E-driven lung tumors. Cancer Discov. 3:1272–1285. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Joshi A, Iyengar R, Joo JH, Li-Harms XJ,
Wright C, Marino R, Winborn BJ, Phillips A, Temirov J, Sciarretta
S, et al: Nuclear ULK1 promotes cell death in response to oxidative
stress through PARP1. Cell Death Differ. 23:216–230. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jung CH, Seo M, Otto NM and Kim DH: ULK1
inhibits the kinase activity of mTORC1 and cell proliferation.
Autophagy. 7:1212–1221. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gao W, Shen Z, Shang L and Wang X:
Upregulation of human autophagy-initiation kinase ULK1 by tumor
suppressor p53 contributes to DNA-damage-induced cell death. Cell
Death Differ. 18:1598–1607. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mukhopadhyay S, Das DN, Panda PK, Sinha N,
Naik PP, Bissoyi A, Pramanik K and Bhutia SK: Autophagy protein
Ulk1 promotes mitochondrial apoptosis through reactive oxygen
species. Free Radic Biol Med. 89:311–321. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schaaf MB, Keulers TG, Vooijs MA and
Rouschop KM: LC3/GABARAP family proteins: Autophagy-(un)related
functions. FASEB J. 30:3961–3978. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tanida I, Ueno T and Kominami E: LC3
conjugation system in mammalian autophagy. Int J Biochem Cell Biol.
36:2503–2518. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tanida I, Tanida-Miyake E, Ueno T and
Kominami E: The human homolog of Saccharomyces cerevisiae Apg7p is
a Protein-activating enzyme for multiple substrates including human
Apg12p, GATE-16, GABARAP, and MAP-LC3. J Biol Chem. 276:1701–1706.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rikiishi H: Novel insights into the
interplay between apoptosis and autophagy. Int J Cell Biol.
2012:3176452012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Strasser A and Bouillet P: The control of
apoptosis in lymphocyte selection. Immunol Rev. 193:82–92. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cory S and Adams JM: The Bcl2 family:
Regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gil J, Ramsey D, Szmida E, Leszczynski P,
Pawlowski P, Bebenek M and Sasiadek MM: The BAX gene as a candidate
for negative autophagy-related genes regulator on mRNA levels in
colorectal cancer. Med Oncol. 34:162017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Park D, Magis AT, Li R, Owonikoko TK, Sica
GL, Sun SY, Ramalingam SS, Khuri FR, Curran WJ and Deng X: Novel
small-molecule inhibitors of Bcl-XL to treat lung cancer. Cancer
Res. 73:5485–5496. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wangpaichitr M, Wu C, You M, Kuo MT, Feun
L, Lampidis T and Savaraj N: Inhibition of mTOR restores cisplatin
sensitivity through down-regulation of growth and anti-apoptotic
proteins. Eur J Pharmacol. 591:124–127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ro SH, Jung CH, Hahn WS, Xu X, Kim YM, Yun
YS, Park JM, Kim KH, Seo M, Ha TY, et al: Distinct functions of
Ulk1 and Ulk2 in the regulation of lipid metabolism in adipocytes.
Autophagy. 9:2103–2114. 2013. View Article : Google Scholar : PubMed/NCBI
|