Introduction
Nasopharyngeal carcinoma (NPC), which shows the
highest morbidity in otorhinolaryngeal carcinoma, is one of the
leading cause of cancer-related deaths all over the world (1). Although remarkable improvements have
been achieved in the treatment of NPC, the prognosis for the NPC
patients remains poor (2).
Identifying novel molecules that are critical for the progression
of NPC will bring new opportunity to find effective treatment
options for NPC patients (3).
DAB2IP, a Ras GTPase-activating protein (4), exerts a critical role in cancer cell
growth and metastasis as well as other aspects during tumor
progression (5). Epigenetic
silencing is the main cause of dysregulated expression of DAB2IP in
human cancers (6). The expression
of DAB2IP is silenced by promoter methylation and histone
modification in prostate cancer (7,8). In
bladder cancer, DAB2IP expression is suppressed by
posttranscriptionally regulation of miR-92b (9). Furthermore, DAB2IP is recognized as a
direct downstream target of miR-889 in esophageal squamous cell
carcinomas (10). In prostate
cancer, loss of DAB2IP facilitates the metastasis and
epithelial-mesenchymal transition (EMT) process of cancer cells
(11), and DAB2IP silencing
inhibits androgen deprivation therapy-induced apoptosis via
targeting STAT3 signaling (12).
Furthermore, DAB2IP deficiency promotes the growth of renal cell
cancer (RCC) and leads to the resistance of mTOR-targeted therapies
in metastatic RCC (13,14). DAB2IP functions as a biomarker for
prognosis prediction of colorectal cancer (CRC) and its loss
promotes EMT and stem cell-like features in CRC cells (15,16).
However, the expression status and function of DAB2IP in NPC remain
poorly known.
In this study, we found that DAB2IP was decreased in
NPC tissues and cell lines. Decreased level of DAB2IP was
correlated with malignant clinical features and poor prognosis of
NPC patients. DAB2IP restoration inhibited the growth and
metastasis of NPC cells in vitro and in vivo.
Importantly, DAB2IP exerted its tumor suppressive effects on the
growth and metastasis of NPC probably by targeting the PI3K/Akt
pathway.
Materials and methods
Cell culture and transfection
NPC cell lines including CNE-1, CNE-2, 5-8F and
6-10B as well as a human immortalized nasopharyngeal epithelial
cell line NP69 were from the Cell Bank of Chinese Academy of
Sciences (Shanghai, China) and American Type Culture Collection
(ATCC, Manassas, VA, USA). All cells were cultured in DMEM medium
(Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum
(Hyclone). Cell cultures were kept in cell incubators with
humidified atomosphere and 5% CO2 at 37°C. Retroviral
vector pMMP-DAB2IP was constructed by inserting the corresponding
cDNA into pMMP. The retroviruses were packaged and tranfected into
CRC cells as previously described (17). Akt inhibitor MK-2206 (1 µM, Selleck
Chemicals, Houston, TX, USA) was used to treat NPC cells following
the manufacturer's instructions.
Clinical samples
Clinical specimens including 68 NPC tissues and 20
non-cancerous nasopharyngeal epithelial tissues were collected at
Cangzhou Central Hospital. Twenty non-cancerous nasopharyngeal
epithelial tissues obtained at tonsillectomies were included as
normal controls. Tissue specimens were conserved in liquid
nitrogen. All these clinical tissues were pathologically confirmed
before being used for further experiments in this study. NPC
patients who previously received radiotherapy or chemotherapy were
excluded. The informed consent was obtained from each patient
involved in this study. Approvals for experiments involving the
patient samples was obtained from the Institutional Research Ethics
Committee of Cangzhou Central Hospital.
Quantitative real-time reverse
transcription-polymerase chain reaction (qRT-PCR)
The total RNA from NPC tissues and cells were
extracted with TRIzol (Invitrogen, Carlsbad, CA, USA). Reverse
transcriptional reactions and PCR were performed with the
Transcriptional First Strand cDNA Synthesis kit (Applied
Biosystems, Foster City, CA, USA) and SYBR Green PCR Master Mix
(Roche, Indianapolis, IN, USA). All primers including those for
DAB2IP and GAPDH (internal control) were from Genecopoeia
(Guangzhou, China). DAB2IP primers: 5′-TGG ACG ATG TGC TCT ATG
CC-3′ (forward), 5′-GGA TGG TGA TGG TTT GGT AG-3′ (reverse); GAPDH
primers: 5′-CAA GCT CAT TTC CTG GTA TGA C-3′ (forward) and 5′-CAG
TGA GGG TCT CTC TCT TCC T-3′ (reverse). PCR amplification protocol
was followed by 95°C (3 min) and 36 cycles of amplification cycle
[95°C (30 sec), 55°C (30 sec), and 72°C (1 min)] using the ABI
PRISM 7500 sequence detection system (Applied Biosystems). Data are
presented as relative quantification based on the calculation of
2−∆Ct. ∆Ct was derived from subtracting the Ct value of
reference cDNA from the Ct value of the cDNA of interest.
Western blot analysis
Total protein lysates (30 µg) that were extracted
from NPC tissues and cells with RIPA buffer were quantified with a
BCA protein assay kit (Pierce, Bonn, Germany) and separated in
4–20% SDS-PAGE gels. After being separated on gels, protein samples
were transferred to PVDF membranes at 4°C. The membranes were
blocked with 5–10% milk/TBST and were incubated with DAB2IP (1:500;
sc-365921, Santa Cruz Biotechnology, Santa Cruz, CA, USA), p-AKT
(1:2,000; #4060, Ser473; Cell Signaling Technology, Beverly, MA,
USA), AKT (1:1,000; #4691, Cell Signaling Technology), cyclin D1
(1:1,000; #2978, Cell Signaling Technology), MMP7 (1:800;
10374-2-AP, Proteintech, Rosemont, IL, USA) or GAPDH (1:1,000;
sc-32233, Santa Cruz Biotechnology) primary antibodies at 4°C
overnight. Then, the membranes were incubated with secondary
antibodies (1:5,000; #7074/7076, Cell Signaling Technology). The
protein signals on the membranes were detected using ECL reagents
(Santa Cruz Biotechnology) and semi-quantified by ImageJ software
(1.46; National Institutes of Health, Bethesda, MD, USA).
Proliferation assay
NPC cells that were treated with empty vector or
DAB2IP were seeded into 96-well plates (1.5×103 cells
per well). CCK-8 cell viability assay were performed as previously
reported (18). For the colony
formation assay, 2,000 NPC cells were seeded on 6-well plates, and
14 to 21 days after cell seeding, cell colonies with crystal violet
staining were counted.
Transwell assay
Transwell inserts were employed to evaluate the
migratory and invasive ability of NPC cells. NPC cells
(1×104) were resuspended in serum-free DMEM medium and
they were seeded in the upper chamber. To induce the migration and
invasion of NPC cells, the lower chambers were filled with 600 µl
DMEM supplemented with 20% FBS. Forty-eight hours after cell
seeding, NPC cells migrated or invaded through the membranes (for
invasion assay, the membrane were covered with 70 µl Matrigel) were
stained with crystal violet for cell counting under the
microscope.
Subcutaneous implantation and tail
vein injection assays
The in vivo growth ability of NPC cells was
examined using the subcutaneous implantation nude mouse model using
female BALB/c nude mice (4–5 weeks). NPC cells (1×106)
transfected with control vector or DAB2IP were subcutaneously
injected into the left flank. During 24 days, the subcutaneous
tumors were subjected to volume measurements and finally resected.
To evaluate the in vivo metastatic ability of NPC cells,
tail vein injection model was performed in the nude mice. NPC cells
(1×105) transfected with control vector or DAB2IP were
intravenous injected through the tail vein. Six weeks later, the
mice were sacrificed by cervical dislocation under anesthesia. The
lungs of nude mice were fixed with 10% formalin and
paraffin-embedded, and were then subjected to H&E staining for
potential lung metastatic lesions. All animal experiments in this
study were approved by the Animal Care Committee of Cangzhou
Central Hospital.
Statistical analysis
The data in this study are shown as mean ± SEM.
Statistical analysis including Student's t-test, Chi-square test,
ANOVA, Kaplan-Meier analysis and log-rank test were performed using
the GraphPad Prism 5 software (GraphPad Software, Inc, San Diego,
CA, USA). P-value <0.05 was considered to be statistically
significant.
Results
DAB2IP expression was decreased in NPC
tissues and cells
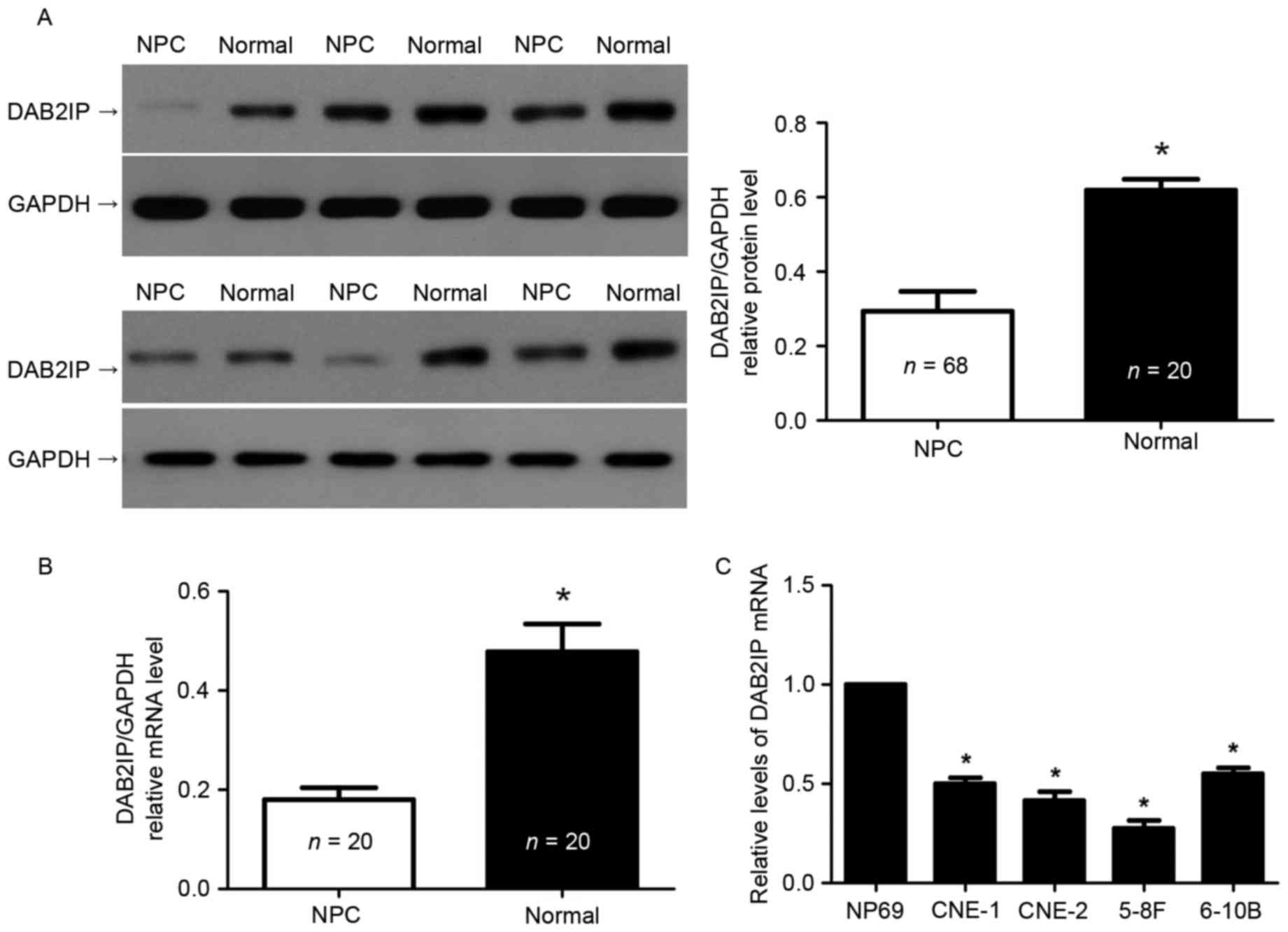
To understand the expression status of DAB2IP in
NPC, we extracted total proteins from the NPC tissues and
non-cancerous tissues. The results of immunoblotting showed that
the levels of DAB2IP protein were significantly decreased in NPC
tissues compared to non-cancerous nasopharyngeal epithelium tissues
(P<0.05, Fig. 1A). In addition,
qRT-PCR was performed to detect the expression differences of
DAB2IP mRNA in 20 paired NPC and normal tissues. DAB2IP mRNA was
also notably underexpressed in NPC specimens (P<0.05, Fig. 1B). Furthermore, we examined the
expression of DAB2IP mRNA in NPC cell lines. Compared with NP69
cells, the expressions of DAB2IP mRNA was prominently lower in all
NPC cell lines including CNE-1, CNE-2, 5-8F, and 6-10B (P<0.05,
respectively, Fig. 1C). Thus,
downregulation of DAB2IP was confirmed in NPC.
Downregulation of DAB2IP correlates
with unfavorable clinicopathological features and poor prognosis of
NPC patients
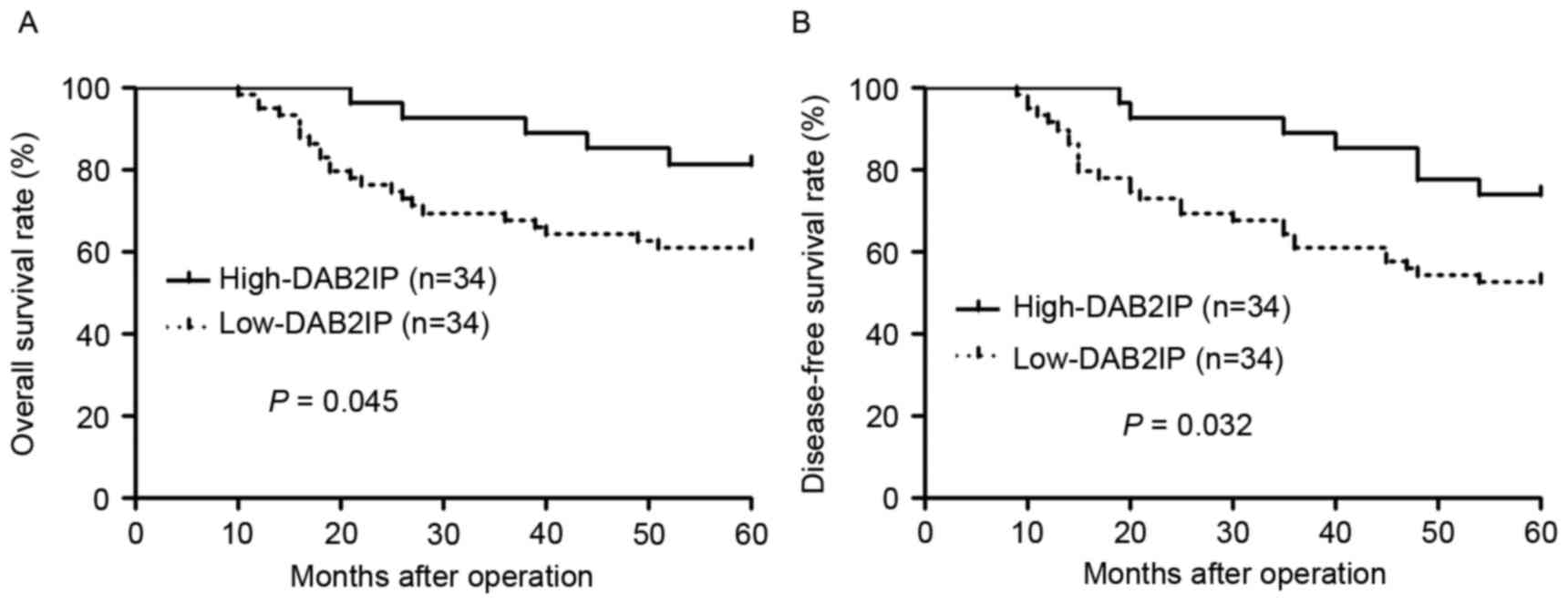
After confirming the decreased expression of DAB2IP
in NPC, we evaluated the clinical significance of DAB2IP in NPC
patients. We divided the NPC patients into two groups (DAB2IP high
or low group) based on the cutoff value which was defined as the
median value of DAB2IP protein level. As shown in Table I, DAB2IP low expression was
associated with advanced clinical stage (P=0.011) and lymph node
metastasis (P=0.006). Furthermore, DAB2IP low expressing NPC
patients showed a notable reduced 5-year overall survival and
disease-free survival (P=0.045 and P=0.032, respectively, Fig. 2). These data indicate that DAB2IP is
a promising prognostic marker for NPC patients.
 | Table I.Correlation between the
clinicopathological features and DAB2IP expression in
nasopharyngeal carcinoma. |
Table I.
Correlation between the
clinicopathological features and DAB2IP expression in
nasopharyngeal carcinoma.
|
|
| DAB2IP
expression |
|
|---|
|
|
|
|
|
|---|
| Characteristics | n | Low (n=34) | High (n=34) | P-value |
|---|
| Age (years) |
|
|
| 0.625 |
|
<50 | 38 | 20 | 18 |
|
|
≥50 | 30 | 14 | 16 |
|
| Sex |
|
|
| 0.442 |
|
Male | 45 | 21 | 24 |
|
|
Female | 23 | 13 | 10 |
|
| Clinical stage |
|
|
| 0.011a |
|
I+II | 12 | 2 | 10 |
|
|
III+IV | 56 | 32 | 24 |
|
| Lymph node
metastasis |
|
|
| 0.006a |
| No | 18 | 4 | 14 |
|
|
Yes | 50 | 30 | 20 |
|
| Distant
metastasis |
|
|
| 0.132 |
| No | 60 | 28 | 32 |
|
|
Yes | 8 | 6 | 2 |
|
DAB2IP restrains the proliferation,
migration and invasion of NPC cells
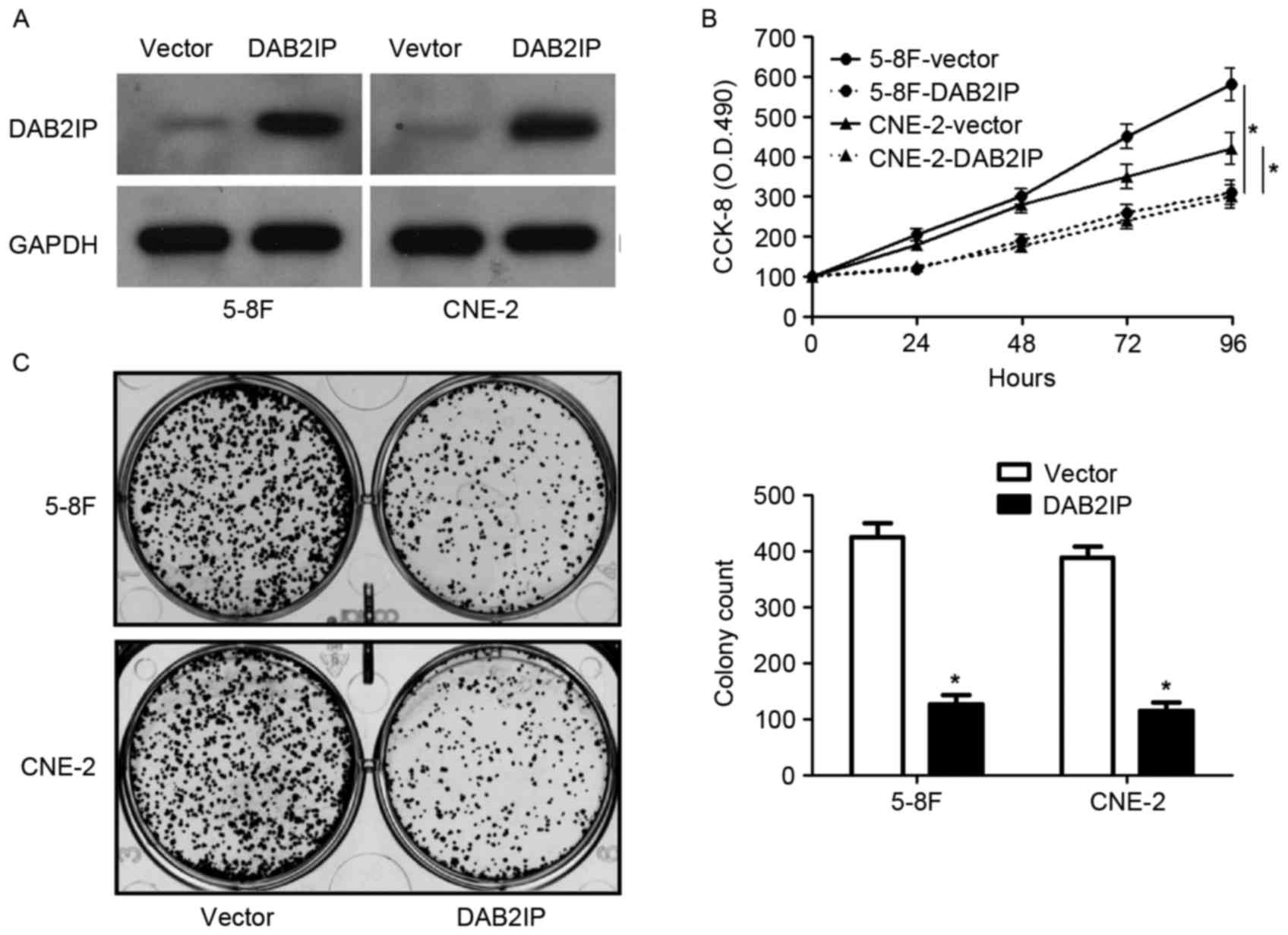
Since the levels of DAB2IP mRNA were relatively
lower in 5-8F and CNE-2 cells, DAB2IP expression was restored by
retroviruses infection in these two cells (Fig. 3A). CCK-8 assays revealed that
overexpression of DAB2IP significantly inhibited the proliferation
of both 5-8F and CNE-2 cells (P<0.05, respectively, Fig. 3B). In addition, the number of
colonies was notably reduced after DAB2IP overexpression in NPC
cells (P<0.05, respectively, Fig.
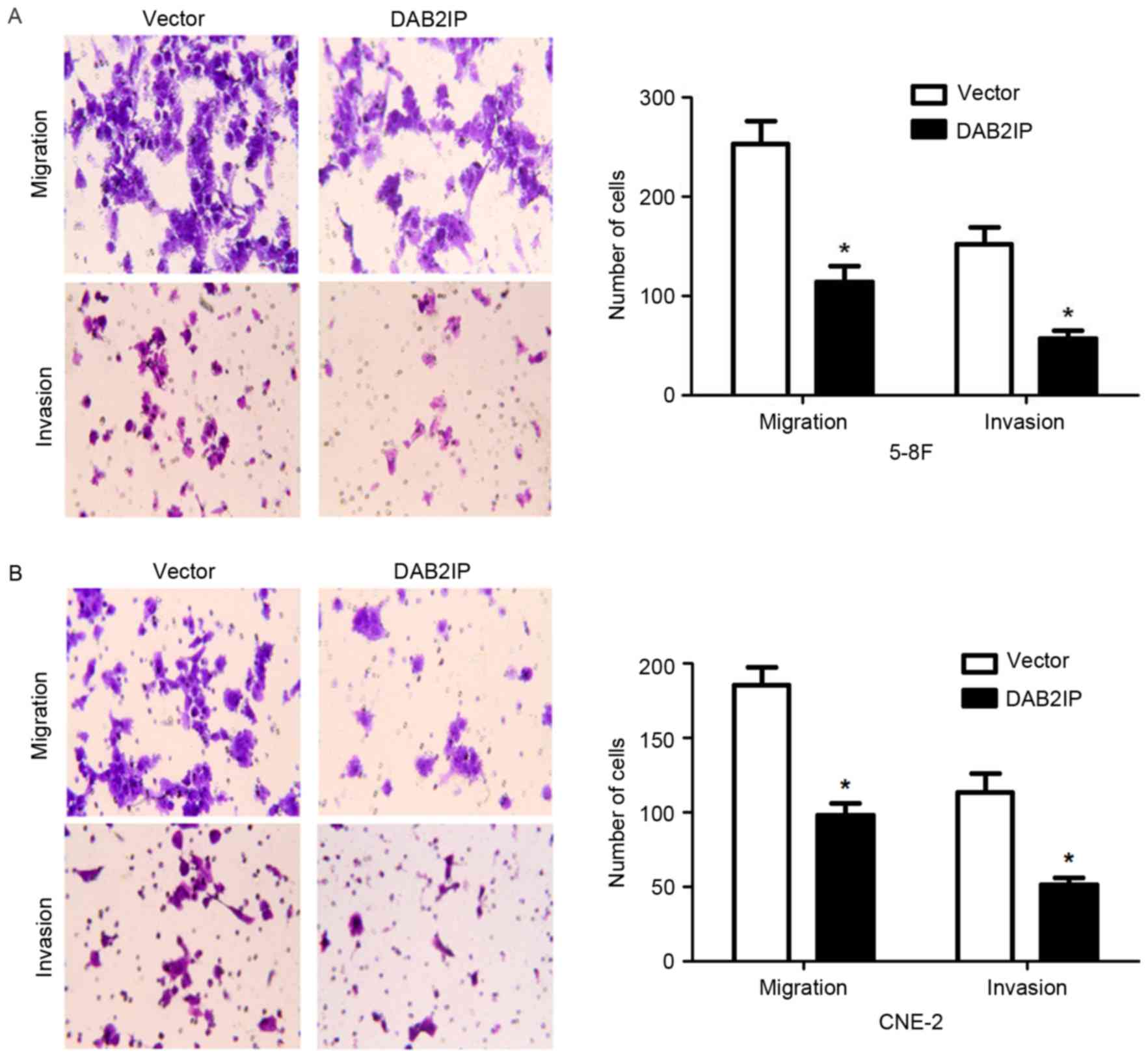
3C). Transwell assays showed that DAB2IP overexpression
obviously decreased the number of migrated and invaded cells
(P<0.05, respectively, Fig. 4).
Thus, DAB2IP suppresses NPC cell proliferation, migration and
invasion in vitro.
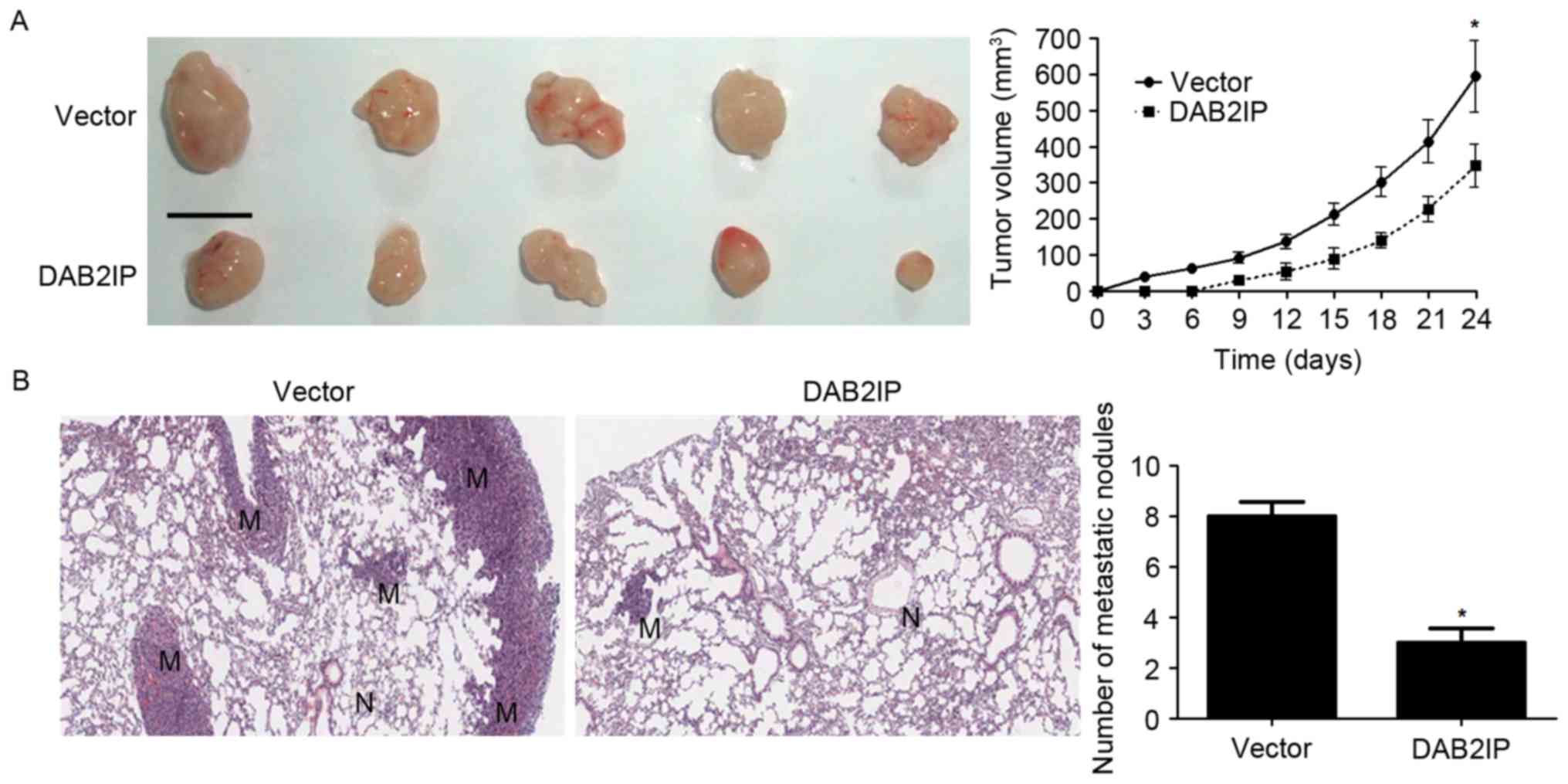
DAB2IP reduces growth and metastasis
of NPC cells in nude mice
To further confirm the functional influence of
DAB2IP on NPC cells, we performed subcutaneous tumor formation and
lung metastasis experiments to test whether DAB2IP affected growth
and metastasis of NPC cells. Overexpression of DAB2IP obviously
decreased the subcutaneous growth of NPC in nude mice as suggested
by tumor growth curves (P<0.05, Fig.
5A). In addition, lung metastasis experiments showed that
DAB2IP notably reduced the number of metastatic nodules in the
lungs of nude mice (P<0.05, Fig.
5B). Altogether, DAB2IP functions as a tumor suppressor in
NPC.
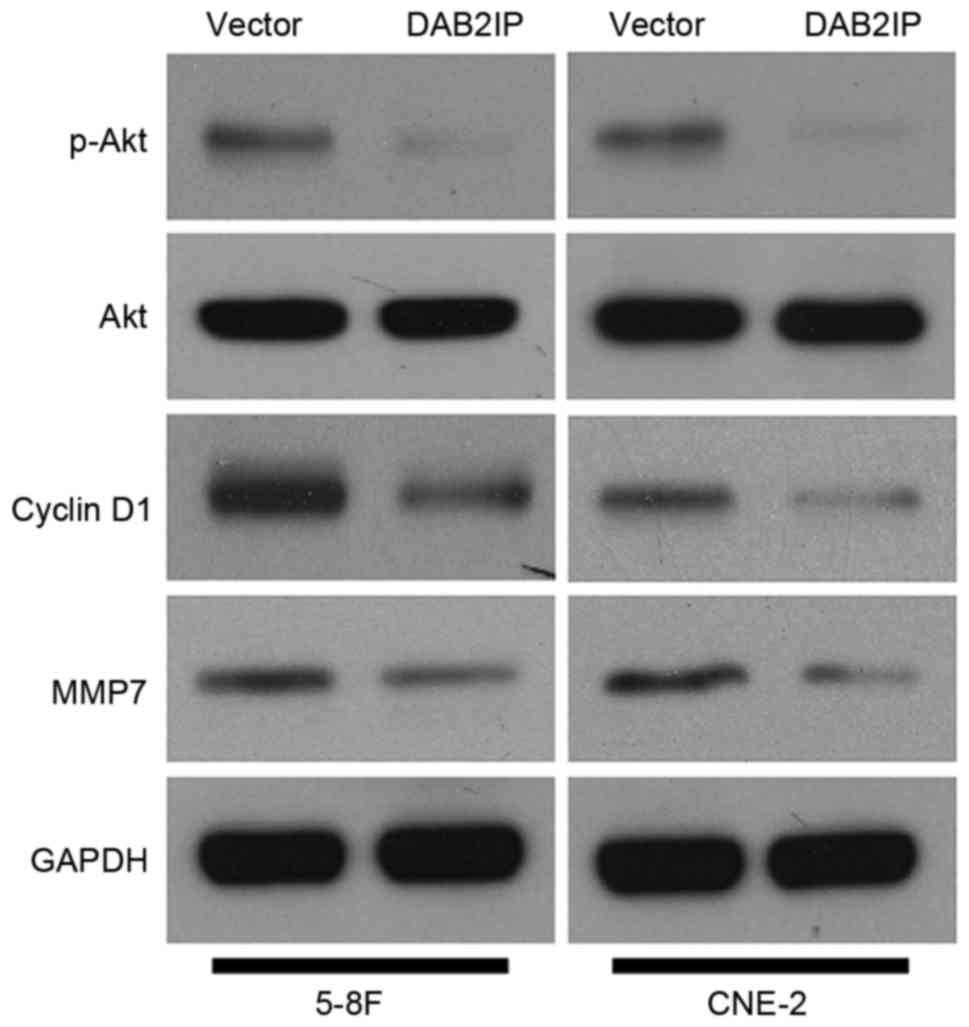
PI3K/Akt pathway is a potential
downstream target of DAB2IP in NPC cells
After determining the biological functions of DAB2IP
in NPC cells, we examined the underlying mechanisms involved in
tumor suppressive role of DAB2IP. DAB2IP has been reported to
regulate various signaling pathways including PI3K/Akt pathway in
human cancer (5). Furthermore, the
downstream targets of PI3K/Akt pathway such as cyclin D1 and MMP7
regulate proliferation, migration and invasion in NPC cells
(19,20). Subsequently, 5-8F and CNE-2 cells
that were infected with empty vector or DAB2IP retroviruses were
subjected to immunoblotting for p-Akt, Akt, cyclin D1 and MMP7. Our
results revealed that DAB2IP repressed the activation of PI3K/Akt
pathway with reduced level of phosphorylated Akt in NPC cells
(Fig. 6). Importantly, the
expressions of cyclin D1 and MMP7 was accordingly downregulated in
both 5-8F and CNE-2 cells (Fig. 6).
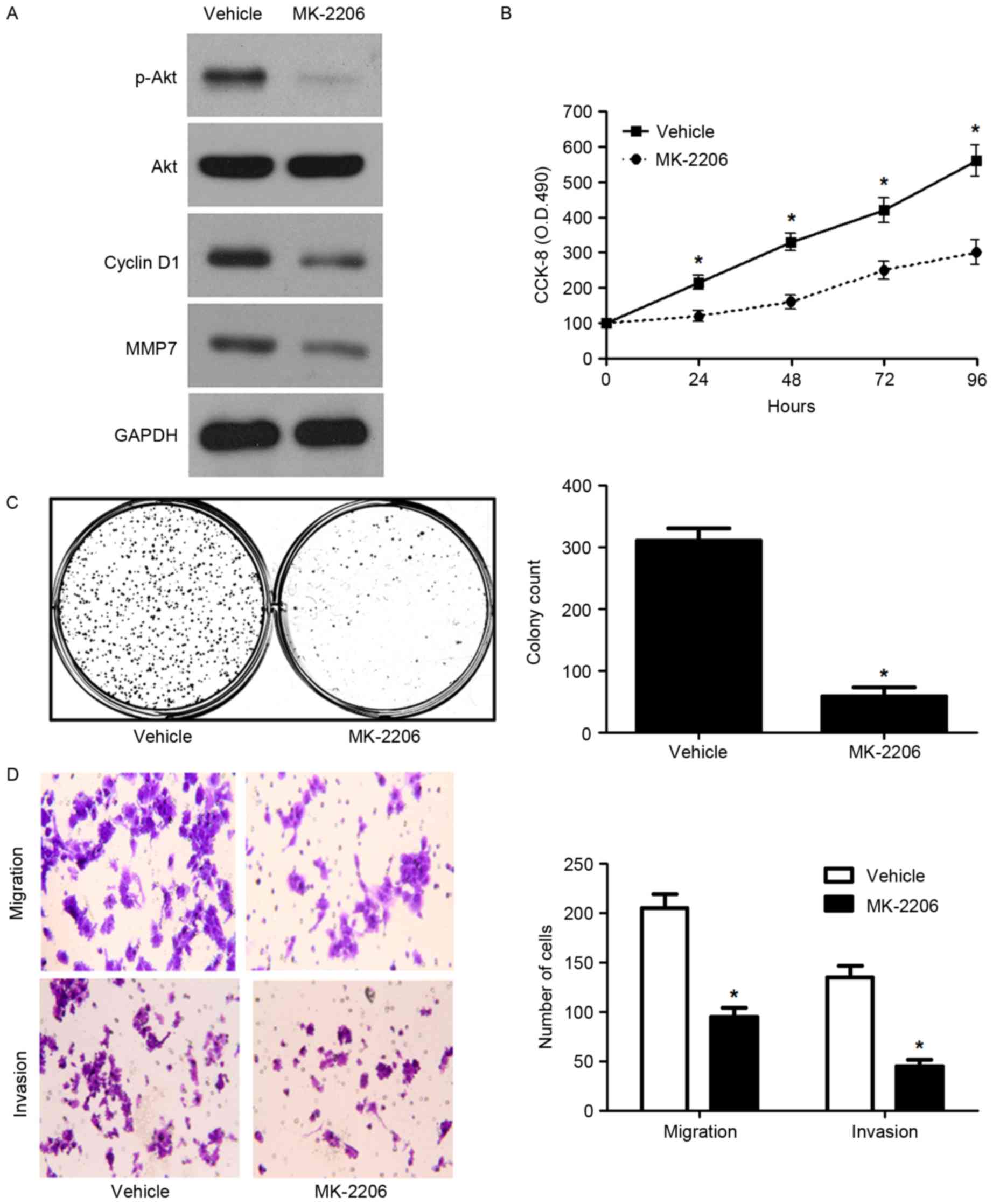
Next, 5-8F cells that were treated with MK-2206, a known Akt
inhibitor, were subjected to growth and metastasis assays. Western
blot analysis indicated that MK-2206 obviously reduced the levels
of phosphorylated Akt and its downstream proteins, cyclin D1 and
MMP7 (Fig. 7A). In addition,
MK-2206 significantly reduced the proliferation, migration and
invasion of 5-8F cells (P<0.05, respectively, Fig. 7B-D). Here, we suggest that DAB2IP
suppresses the progression of NPC possibly by targeting the
PI3K/Akt pathway.
Discussion
Loss or downregulation of tumor suppressors have
been found to be closely associated with cancer development and
progression (21,22). Among these tumor suppressors, DAB2IP
is a newly identified tumor suppressor closely related with human
cancers (5). In this study, we
confirmed for the first time that DAB2IP expression was
significantly reduced in NPC tissues and cell lines. Decreased
DAB2IP level was associated with unfavorable clinicopathological
features and poor prognosis of NPC patients. In vitro
functional assays showed that DAB2IP could restrain proliferation,
migration and invasion of NPC cells. In vivo experiments
also confirmed that DAB2IP prohibited the growth and metastasis of
NPC cells in nude mice. These data indicate that DAB2IP plays a
tumor suppressive role in NPC by inhibiting tumor growth and
metastasis.
DAB2IP was identified as a new member of the Ras
GTPase-activation protein family (5). DAB2IP regulates cell proliferation,
apoptosis, migration, invasion and survival via modulating various
signaling pathways by directly interacting with DAB2 (5). Previous studies showed DAB2IP exerts
critical roles in human cancers including prostate cancer (6,8,12), CRC
(15,16) and RCC (13,14).
PI3K/Akt pathway has been reported to play an important role in the
initiation and progression of NPC (23,24).
Notably, DAB2IP suppression of the activation of PI3K/Akt pathway
is reported in prostate cancer (25), bladder cancer (26), but not in NPC. Here, we revealed
that DAB2IP overexpression blocked that activation of PI3K/Akt
pathway with reduced level of phosphorylated Akt in NPC cells.
Accordingly, the levels of cyclin D1 (27) and MMP7 (28), downstream targets of PI3K/Akt
pathway, were downregulated by DAB2IP restoration. cyclin D1, a
regulator of cell cycle progression, promotes NPC cell
proliferation via promoting G (1)-phase transition (29). Moreover, MMP7 has been reported to
promote invasion of NPC cells (20). A previous study suggested that Wnt
signaling facilitates cell proliferation via cyclin D1 and promoted
invasion by MMP7 in NPC (20). The
expression of PI3K/Akt pathway molecules, such as p-Akt, cyclin D1
and MMP7, were upregulated in NPC tissues compared to normal
tissues as previously reported (30–32).
Furthermore, MK-2206, the Akt inhibitor (33), blocked the PI3K/Akt pathway and
suppressed the growth and metastasis of NPC cells in vitro,
which was consistent with a previous study (34). Thus, we suggest that DAB2IP
restrains growth and metastasis of NPC by inhibiting PI3K/Akt
pathway and subsequently suppressing cyclin D1 and MMP7 expression.
DAB2IP expression is frequently impaired by methylation in cancer
(35). Thus, the methylation status
of DA2BIP in NPC is worth investigation in the future.
Collectively, DAB2IP expression was decreased in NPC
tissues and cells. DAB2IP inhibited the progression of NPC by
suppressing the proliferation and metastasis of NPC cells both
in vitro and in vivo. Mechanically, PI3K/Akt was
identified to be the downstream target of DAB2IP in NPC cells.
DAB2IP potentially acts as a prognostic predictor and a drug-target
for NPC patients.
Acknowledgements
The authors thank all the patients who participated
in this study.
References
|
1
|
Ou SH, Zell JA, Ziogas A and Anton-Culver
H: Epidemiology of nasopharyngeal carcinoma in the United States:
Improved survival of Chinese patients within the keratinizing
squamous cell carcinoma histology. Ann Oncol. 18:29–35. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee AW, Ma BB, Ng WT and Chan AT:
Management of nasopharyngeal carcinoma: Current practice and future
perspective. J Clin Oncol. 33:3356–3364. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bruce JP, Yip K, Bratman SV, Ito E and Liu
FF: Nasopharyngeal cancer: Molecular landscape. J Clin Oncol.
33:3346–3355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Z, Tseng CP, Pong RC, Chen H,
McConnell JD, Navone N and Hsieh JT: The mechanism of
growth-inhibitory effect of DOC-2/DAB2 in prostate cancer.
Characterization of a novel GTPase-activating protein associated
with N-terminal domain of DOC-2/DAB2. J Biol Chem. 277:12622–12631.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu L, Xu C, Hsieh JT, Gong J and Xie D:
DAB2IP in cancer. Oncotarget. 7:3766–3776. 2016.PubMed/NCBI
|
|
6
|
Tsai YS, Lai CL, Lai CH, Chang KH, Wu K,
Tseng SF, Fazli L, Gleave M, Xiao G, Gandee L, et al: The role of
homeostatic regulation between tumor suppressor DAB2IP and
oncogenic Skp2 in prostate cancer growth. Oncotarget. 5:6425–6436.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen H, Toyooka S, Gazdar AF and Hsieh JT:
Epigenetic regulation of a novel tumor suppressor gene (hDAB2IP) in
prostate cancer cell lines. J Biol Chem. 278:3121–3130. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen H, Tu SW and Hsieh JT:
Down-regulation of human DAB2IP gene expression mediated by
polycomb Ezh2 complex and histone deacetylase in prostate cancer. J
Biol Chem. 280:22437–22444. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang J, Wang B, Hui K, Zeng J, Fan J,
Wang X, Hsieh JT, He D and Wu K: miR-92b targets DAB2IP to promote
EMT in bladder cancer migration and invasion. Oncol Rep.
36:1693–1701. 2016.PubMed/NCBI
|
|
10
|
Xu Y, He J, Wang Y, Zhu X, Pan Q, Xie Q
and Sun F: miR-889 promotes proliferation of esophageal squamous
cell carcinomas through DAB2IP. FEBS Lett. 589:1127–1135. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang B, Huang J, Zhou J, Hui K, Xu S, Fan
J, Li L, Wang X, Hsieh JT, He D, et al: DAB2IP regulates EMT and
metastasis of prostate cancer through targeting PROX1 transcription
and destabilizing HIF1α protein. Cell Signal. 28:1623–1630. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou J, Ning Z, Wang B, Yun EJ, Zhang T,
Pong RC, Fazli L, Gleave M, Zeng J, Fan J, et al: DAB2IP loss
confers the resistance of prostate cancer to androgen deprivation
therapy through activating STAT3 and inhibiting apoptosis. Cell
Death Dis. 6:e19552015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou J, Luo J, Wu K, Yun EJ, Kapur P, Pong
RC, Du Y, Wang B, Authement C, Hernandez E, et al: Loss of DAB2IP
in RCC cells enhances their growth and resistance to mTOR-targeted
therapies. Oncogene. 35:4663–4674. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yeh CR, Ou ZY, Xiao GQ, Guancial E and Yeh
S: Infiltrating T cells promote renal cell carcinoma (RCC)
progression via altering the estrogen receptor β-DAB2IP signals.
Oncotarget. 6:44346–44359. 2015.PubMed/NCBI
|
|
15
|
Min J, Liu L, Li X, Jiang J, Wang J, Zhang
B, Cao D, Yu D, Tao D, Hu J, et al: Absence of DAB2IP promotes
cancer stem cell like signatures and indicates poor survival
outcome in colorectal cancer. Sci Rep. 5:165782015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Zhu X, Hu J, He G, Li X, Wu P, Ren
X, Wang F, Liao W, Liang L, et al: The positive feedback between
Snail and DAB2IP regulates EMT invasion and metastasis in
colorectal cancer. Oncotarget. 6:27427–27439. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tu K, Yang W, Li C, Zheng X, Lu Z, Guo C,
Yao Y and Liu Q: Fbxw7 is an independent prognostic marker and
induces apoptosis and growth arrest by regulating YAP abundance in
hepatocellular carcinoma. Mol Cancer. 13:1102014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shao J, Yang X, Liu T, Zhang T, Xie QR and
Xia W: Autophagy induction by SIRT6 is involved in oxidative
stress-induced neuronal damage. Protein Cell. 7:281–290. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu M, Ye X, Deng X, Wu Y, Li X and Zhang
L: Upregulation of metastasis-associated gene 2 promotes cell
proliferation and invasion in nasopharyngeal carcinoma. Onco
Targets Ther. 9:1647–1656. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wong AM, Kong KL, Chen L, Liu M, Wong AM,
Zhu C, Tsang JW and Guan XY: Characterization of CACNA2D3 as a
putative tumor suppressor gene in the development and progression
of nasopharyngeal carcinoma. Int J Cancer. 133:2284–2295. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hazan I, Hofmann TG and Aqeilan RI: Tumor
suppressor genes within common fragile sites are active players in
the DNA damage response. PLoS Genet. 12:e10064362016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Paysan L, Piquet L, Saltel F and Moreau V:
Rnd3 in cancer: A review of the evidence for tumor promoter or
suppressor. Mol Cancer Res. 14:1033–1044. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao M, Luo R, Liu Y, Gao L, Fu Z, Fu Q,
Luo X, Chen Y, Deng X, Liang Z, et al: miR-3188 regulates
nasopharyngeal carcinoma proliferation and chemosensitivity through
a FOXO1-modulated positive feedback loop with
mTOR-p-PI3K/AKT-c-JUN. Nat Commun. 7:113092016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang CF, Yang GD, Huang TJ, Li R, Chu QQ,
Xu L, Wang MS, Cai MD, Zhong L, Wei HJ, et al: EB-virus latent
membrane protein 1 potentiates the stemness of nasopharyngeal
carcinoma via preferential activation of PI3K/AKT pathway by a
positive feedback loop. Oncogene. 35:3419–3431. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xie D, Gore C, Zhou J, Pong RC, Zhang H,
Yu L, Vessella RL, Min W and Hsieh JT: DAB2IP coordinates both
PI3K-Akt and ASK1 pathways for cell survival and apoptosis. Proc
Natl Acad Sci USA. 106:19878–19883. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shen YJ, Kong ZL, Wan FN, Wang HK, Bian
XJ, Gan HL, Wang CF and Ye DW: Downregulation of DAB2IP results in
cell proliferation and invasion and contributes to unfavorable
outcomes in bladder cancer. Cancer Sci. 105:704–712. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhuang YJ, Liao ZW, Yu HW, Song XL, Liu Y,
Shi XY, Lin XD and Zhou TC: ShRNA-mediated silencing of the
ubiquitin-specific protease 22 gene restrained cell progression and
affected the Akt pathway in nasopharyngeal carcinoma. Cancer Biol
Ther. 16:88–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu Z, Liu D, Fan C, Luan L, Zhang X and
Wang E: DIXDC1 increases the invasion and migration ability of
non-small-cell lung cancer cells via the PI3K-AKT/AP-1 pathway. Mol
Carcinog. 53:917–925. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang LY, Ho-Fun Lee V, Wong AM, Kwong DL,
Zhu YH, Dong SS, Kong KL, Chen J, Tsao SW, Guan XY, et al:
MicroRNA-144 promotes cell proliferation, migration and invasion in
nasopharyngeal carcinoma through repression of PTEN.
Carcinogenesis. 34:454–463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yip WK, Leong VC, Abdullah MA, Yusoff S
and Seow HF: Overexpression of phospho-Akt correlates with
phosphorylation of EGF receptor, FKHR and BAD in nasopharyngeal
carcinoma. Oncol Rep. 19:319–328. 2008.PubMed/NCBI
|
|
31
|
Lai JP, Tong CL, Hong C, Xiao JY, Tao ZD,
Zhang Z, Tong WM and Betz CS: Association between high initial
tissue levels of cyclin d1 and recurrence of nasopharyngeal
carcinoma. Laryngoscope. 112:402–408. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen J, Kwong DL, Zhu CL, Chen LL, Dong
SS, Zhang LY, Tian J, Qi CB, Cao TT, Wong AM, et al: RBMS3 at 3p24
inhibits nasopharyngeal carcinoma development via inhibiting cell
proliferation, angiogenesis, and inducing apoptosis. PLoS One.
7:e446362012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma BB, Lui VW, Hui CW, Lau CP, Wong CH,
Hui EP, Ng MH, Tsao SW, Li Y and Chan AT: Preclinical evaluation of
the AKT inhibitor MK-2206 in nasopharyngeal carcinoma cell lines.
Invest New Drugs. 31:567–575. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu W, Xiao L, Cao C, Hua S and Wu D: UBE2T
promotes nasopharyngeal carcinoma cell proliferation, invasion, and
metastasis by activating the AKT/GSK3β/β-catenin pathway.
Oncotarget. 7:15161–15172. 2016.PubMed/NCBI
|
|
35
|
Bellazzo A, Di Minin G and Collavin L:
Block one, unleash a hundred. Mechanisms of DAB2IP inactivation in
cancer. Cell Death Differ. 24:15–25. 2017. View Article : Google Scholar : PubMed/NCBI
|