Introduction
Glioma is a malignant brain tumor that
unsatisfactorily responds to therapy. Despite some limited advances
in surgical, radiotherapeutic and chemotherapeutic protocols, the
average life expectancy of patients with glioblastoma remains only
12-15 months (1,2).
It has been reported that cancer stem cells (CSCs),
which constitute a small proportion of tumors, play an important
role in tumor recurrence and metastasis (3,4). In
recent years, increasing evidence has shown that glioma stem cells
(GSCs) are related to radioresistance. Zhou et al (5) compared the biological characteristics
of glioma cells (GCs) and GSCs using comet tail assays, colony
formation and apoptosis percentages, among other techniques. They
found that GSCs were more resistant than GCs at the same X-ray
irradiation dosage. Various scholars believe that constitutive
activation of the DNA damage response may be a major mechanism of
GSC resistance (2).
The ataxia-telangiectasia mutated (ATM)
protein belongs to the PI3K family and functions as a proximal
component of DNA damage-induced cell cycle checkpoint pathways. The
ATM gene plays an indispensable role in the DNA
double-strand break (DSB) impairment induced by ionizing
irradiation (6,7).
Increasing studies have shown that high ATM
expression in glioma results in radioresistance (2,8–10).
GSCs are also associated to radioresistance, thus, we aimed to
determine whether ATM exhibited a high expression in GSCs and
contributed to radioresistance (11). In our previous study,
radioresistance in both GCs and GSCs was found to be linked to a
high expression of the ATM gene (5).
Numerous scholars presently have contributed insight
to radiosensitivity in glioma cells through the silencing of the
ATM gene via gene interference or drug inhibition. The usual
strategy of gene interference employs small interfering RNA (siRNA)
to deliver a silencing gene using a lentivirus system (12–14).
Pharmacological treatment primarily consists of an ATM kinase
inhibitor (KU55933 and KU60019) (8,15).
Although, the two methods have different inference paths and
efficiencies, both can suppress or silence the ATM gene.
To the best of our knowledge, no research on
silencing the ATM gene in GSCs either in vitro or
in vivo has been reported. In the present study, we mirrored
the strategy of GC sensitization to observe whether siRNA-ATM
contributed to GSC radiosensitivity. The results of the present
study may provide new clues for enhancing the effectiveness of
glioma treatment.
Materials and methods
Cell lines
Human glioma cell lines U251 and A172 were used in
the present study. The U251 cell line was gifted by the Central
Laboratory of Oncology Department of Xinqiao Hospital of The Third
Military Medical University (Chongqing, China). The A172 cell line
was purchased from the Centre of Cell Cultures of the Chinese
Academy of Medical Sciences (Shanghai, China). All cell lines were
cultured in RPMI-1640 medium supplemented with 10% fetal calf serum
(FCS) (both from Gibco, Grand Island, NY, USA), 100 U/ml
penicillin, 100 µg/ml streptomycin, 2 mM glutamine and 1 mM sodium
pyruvate.
In addition, both cell lines were then cultured in
neural stem cell medium (NSC-M) to induce GSCs. The medium was
supplemented with 20 ng/ml basic fibroblast growth factor (bFGF),
20 ng/ml epidermal growth factor (EGF) (both from PeproTech, Inc.,
Rocky Hill, NJ, USA), 10 ng/ml leukemia inhibitory factor (LIF;
Chemicon, Temecula, CA, USA), and a 1:50 dilution of B27
(Gibco-BRL, Carlsbad, CA, USA).
The GCs and GSCs were cultured in humidified
incubators at 37°C in an atmosphere with 5% CO2. The
cells were maintained by serial passaging after trypsinization with
0.1% trypsin (Gibco).
Animals
Four-week-old male BALB/c nu-nu mice weighing 20 g
each were purchased from the Shanghai Experimental Animal Center of
the Chinese Academy of Medical Sciences (Shanghai, China). They
were maintained in a pathogen-free environment at 25–27°C and
45–50% humidity, and supplied with food and water ad
libitum. All of the mice were humanely treated according to the
Institutional Policies on Human Care and Use of Laboratory Animals.
The animal experiment in the present study, was approved by the
Ethics Committee of Chongqing Cancer Institute.
Induction and identification of
GSCs
Cells were seeded into 6-well plates and cultured in
NSC-M medium that was partially changed every two days. At three to
five days of culture, large tumor balls had formed that were
considered GSCs, at which point the passages were performed weekly.
The passage procedure was the same as the general cell culture.
The tumor balls were identified as GSCs using the
double-labeling immunofluorescence technique. GSCs were collected
by centrifugation, and single-cell suspensions were plated into
24-well plates at a density of 2×105/ml after sterile
coverslips were installed under the bottom of each well. NSC-M was
supplemented into the plates and GSC tumor spheres were formed
after five days of culture. The cells were then washed three times
with phosphate-buffered saline (PBS) for 5 min each and fixed with
4% polyoxymethylene for 30 min. After three additional PBS washes,
0.5% Triton X-100 was added into the wells, followed by the
addition of 10% FBS and 50 µl of CD133 (Miltenyi Biotec, Inc.,
Auburn, CA, USA) and nestin (Abcam, Cambridge, MA, USA) antibodies
for the immunoreaction. The aforementioned medium was co-incubated
at 4°C for 12 h. After another three PBS washes, the secondary
antibodies (TRITC and FITC; R&D Systems, Inc., Minneapolis, MN,
USA) were added and incubated at 37°C for 1 h. After a final three
PBS washes, 4,6-diamidino-2-phenylindole (DAPI) (Beyotime Institute
of Biotechnology, Shanghai, China) staining reagent was added and
the immunoreactivity was observed under laser confocal microscope
(SP5; Leica, Mannheim, Germany).
Cell transfection by siRNA-ATM
lentivirus and radiation treatment in vitro
Due to economic usage of the virus drop, targeted
GSCs were created via transfection followed by induction. Two cell
lines were seeded into 6-well plates at a density of
2×105cells/ml, respectively. After 48 h of culture, 40
µl (MOI=2) of siRNA-ATMPuro or siRNA-HKPuro
lentivirus (Hanheng Bio Co. Ltd., Shanghai, China) was added to the
plates when the cells achieved ~40% confluence. Subsequently, the
medium was replaced by serum-free medium for 24 h of co-culture. An
inverted fluorescence microscope was used to observe the
transfection efficiency, and treatment with puromycin was performed
to select tolerant target cells until a stable cell line
transfected with the lentivirus was successfully established. The
GSCs of the two transfected cell lines was induced as
aforementioned.
Three groups of GSCs from each cell line were
designed in the in vitro experiments: blank control group C
(U251 and A172 cells not transfected with siRNA), negative control
group N (U251 and A172 cells transfected with
siRNA-HKPuro), and experimental group A (U251 and A172
cells transfected with siRNA-ATMPuro). The GSCs from the
three groups were irradiated using a 6-MV X Rad source (SN4474;
Varian Medical Systems, Palo Alto, CA, USA) to deliver doses of 2,
4, 6 and 8 Gy (colony forming assay) and 5 Gy (all other assays
in vitro). The dose rate was 300 cGy/min. The cells were
harvested after irradiation at different time-points: cells for
RT-qPCR and western blotting were harvested 24 h after irradiation;
cells for the comet assay were harvested after 4 h; and cells for
flow cytometric analysis were harvested after 24 h.
Animal model construction and
radiotherapy in vivo
GSCs from the two cell lines in good condition into
which siRNA-ATMPuro or siRNA-HKPuro was
transfected were collected and injected into two flanks of haunch
of the same mouse, respectively. After 2–3 weeks, the tumor volume
reached 1.5 × 1.3 × 1.1 cm, and the irradiation was performed.
The mice of each group (siRNA-ATMPuro and
siRNA-HKPuro) were divided into two subgroups
(irradiation and radiation-free) of three animals each after the
subcutaneous transplantation. The mice from the two radiation
subgroups (n=6) were administered ketamine (10 ml/kg) and fixed in
the ventricumbent position. The subcutaneous tumor was subsequently
locally irradiated under a 6-MV linear accelerator. The
radiotherapy parameters were as follows: target range, 100 cm; dose
rate, 300 MU/min; and total dose, 1,500 cGy. Three weeks later, all
of the mice were sacrificed to enable histopathological examination
of the tumors. The samples were fixed in 10% formalin for
paraffin-embedding and stained with hematoxylin and eosin for
microscopic examination.
Reverse transcription quantitative
polymerase chain reaction (RT-PCR)
Total RNA of GSCs from the two cell lines was
extracted using TRIzol reagent (Sigma-Aldrich, Milwaukee, WI, USA)
according to the manufacturer's protocol. The concentration and
purity were evaluated by an ultraviolet spectrophotometer, and the
RNA of the two cell lines was reverse transcribed into cDNA using a
reverse transcription kit (Takara, Dalian, China). A master mix (20
µl in total) was prepared on ice including 5X PrimeScript buffer (4
µl), PrimeScript RT Enemy (1 µl), 6N random 6 (1 µl), total RNA
(500 ng) and nuclease-free water (14 µl). The reaction was carried
out at 37°C for 30 min, then 85°C for 5 sec, and 4°C, thereafter.
Next, cDNA from the GSCs was subjected to RT-qPCR, which was
performed using the IQ5 PCR Instrument and the SYBR-Green Real-Time
PCR Master Mix kit (Takara). To amplify the ATM, a master mix (25
µl) was prepared on ice with 10 µl of SYBR-Green I Master Mix, 1 µl
of each primer, 5 µl of cDNA, and 8 µl of nuclease-free water. The
cDNA was initially denatured at 95°C for 30 sec, followed by 40
cycles of denaturation at 95°C for 5 sec, annealing at 60°C for 30
sec, and extension at 72°C for 40 sec. The experiments were
repeated in triplicate.
Primer sequences were designed by Software Primer
5.0, and are listed in Table I.
Actin was considered an endogenous control. A melting curve
analysis was performed to confirm the specificity of the
amplification and absence of primer dimers for each run. Relative
quantification of ATM gene expression was performed using
the 2−ΔΔCt method.
 | Table I.Sequences of the primers used for
RT-qPCR. |
Table I.
Sequences of the primers used for
RT-qPCR.
| Gene |
| Primer
sequence | Annealing
temperature (°C) | Product length
(bp) |
|---|
| Actin | F |
5′-TGACGTGGACATCCGCAAAG-3′ | 60 | 205 |
|
| R |
5′-CTGGAAGGTGGACAGCGAGG-3′ |
|
|
| ATM | F |
5′-GCACAGAAGTGCCTCCAATTC-3′ | 60 | 125 |
|
| R |
5′-ACATTCTGGCACGCTTTGG-3′ |
|
|
| P53 | F |
5′-CAGTCTACCTCCCGCCATAA-3′ | 57 | 144 |
|
| R |
5′-GTTCAAAGACCCAAAACCCA-3′ |
|
|
| PCNA | F |
5′-GGGACACTGCTGGTGGTATT-3′ | 59 | 102 |
|
| R |
5′-ACTGGTGGAGGGTAAACGGA-3′ |
|
|
| Survivin | F |
5′-TGTGATGAGGACAAAACGAAGC-3′ | 59 | 100 |
|
| R |
5′-CAGCCTGAGCAACAGAGCAA-3′ |
|
|
Western blotting
GSCs of the U251 and A172 cells were harvested on
ice following the use of a total protein extraction kit containing
a protease inhibitor. The total protein was extracted from each
cell line after homogenization, and the protein concentration was
assessed using Coomassie Brilliant Blue staining. Sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10%) was
then used to separate the proteins (50 mg of protein/sample) that
were transferred onto a polyvinylidene fluoride membrane. The
membrane was incubated overnight with the primary antibodies
diluted to 1:1,000 (5% w/v bovine serum albumin using Tris-buffered
saline/Tween-20) and then incubated with secondary antibodies
diluted to 1:3,000 (Zhongshan Biotechnology, Beijing, China).
Subsequently, the treated membrane was subjected to film
development and further analysis. The primary antibodies consisted
of anti-ATM and anti-phosph-ATM (S1981) antibodies (Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA).
Cell Counting Kit-8 (CCK-8)
After irradiation, the cell proliferation ability of
the GSCs was detected using the CCK-8 assay (Dojindo, Kumamoto,
Japan). Single-cell suspensions of GSCs from the two cell lines,
including those transfected with siRNA-ATMPuro or
siRNA-HKPuro, were plated into 96-well plates at a
concentration of 1,000 cells/well, and cultured overnight at 37°C
in NSC-M medium. The cells were then incubated with 10 µl of CCK-8
for 4 h without removal of the medium and the plates were shaken
for 15 min. Finally, the absorbance was detected within the range
of 490–630 nm with an enzyme-linked immunosorbent assay reader
(ELX800; BioTek Instruments, Inc., Winooski, VT, USA).
Cell cycle phase and cell apoptosis
analyses by flow cytometry
The percentage of cell distribution in the various
phases of the cell cycle was determined by flow cytometry. After
radiation, single-cell suspensions of U251 and A172 GSCs
(1×105 cells/ml) including transfected cells were washed
twice with PBS and fixed in 75% alcohol. After treatment with 500
µl of 1 g/l RNase at 37°C for 30 min, the cells were collected,
fixed again and then stained with propidium iodide (PI) for the
flow cytometric analysis. The percentage of cells that underwent
cell apoptosis was also detected using flow cytometry. Treated with
radiation, single-cell suspensions of GSCs (1×105
cells/ml; 100 µl) including transfected cells were added into
microcentrifuge tubes, and then mixed with 5 µl of Annexin IV-PE.
After incubation at room temperature for 15 min, the percentage of
apoptotic cells was analyzed using flow cytometry.
Clonogenic survival assay
The cell survival fraction was determined by a
standard colony-forming efficiency assay. Briefly, U251 and A172
GSCs or transfected cells were disaggregated into a single-cell
suspension and diluted to a final concentration of 2×103
cells/ml. The cells were then plated into 6-well plates, cultured
overnight at 37°C, and subjected to irradiation at doses of 2, 4, 6
and 8 Gy. After radiation treatment, all of the plates were
incubated at 37°C in a 5% CO2 atmosphere for two weeks.
The cells were washed with PBS, fixed with formalin, stained with
crystal violet (0.1% w/v), rinsed with dH2O and finally
dried. Colonies that contained >50 cells upon microscopic
examination were considered surviving colonies. The formula used
was as follows: Colony formation rate (PE) = colony number/cell
plating number × 100%; and survival fraction (SF) = number of
colonies that formed in response to a certain dose/cell plating
number × PE. After data analysis, the cell survival curve was drawn
using GraphPad Prism 5.0 software. The experiments were performed
in triplicate.
Single-cell gel electrophoresis
(neutral comet assay)
The single-cell gel electrophoresis assay was
performed using the comet assay kit (Trevigen, Gaithersburg, MD,
USA) according to the manufacturer's instructions. Briefly, 4 h
after X-ray irradiation, single-cell suspensions of U251 and A172
GSCs including transfected cells were washed with PBS and mixed
with low-melting agarose (1:10). Thereafter, the cell-agarose
mixtures were pipetted onto the comet assay slides. The cells were
lysed by incubation of the mixture at 4°C for 3 h, and the treated
cells were then electrophoresed at 4°C for 20 min. Subsequently,
the resolved samples were fixed and the DNA was visualized by
staining with 5 µg/ml GoldView (SBS Genetech, Co., Ltd., Beijing,
China). The slides were observed under a confocal laser microscope,
and digital fluorescence images were obtained to calculate the
percentage of comet tails/100 cells.
Statistical analysis
SAS 8.1 was used to analyze the experimental data,
which are presented as the mean ± SD. The RT-qPCR, CCK-8, flow
cytometry and comet tail percentage data, were analyzed using
analysis of variance (ANOVA). The data from the clonogenic survival
assay were analyzed using GraphPad Prism 5.0 software. Statistical
significance was set at a P-value of <0.05.
Results
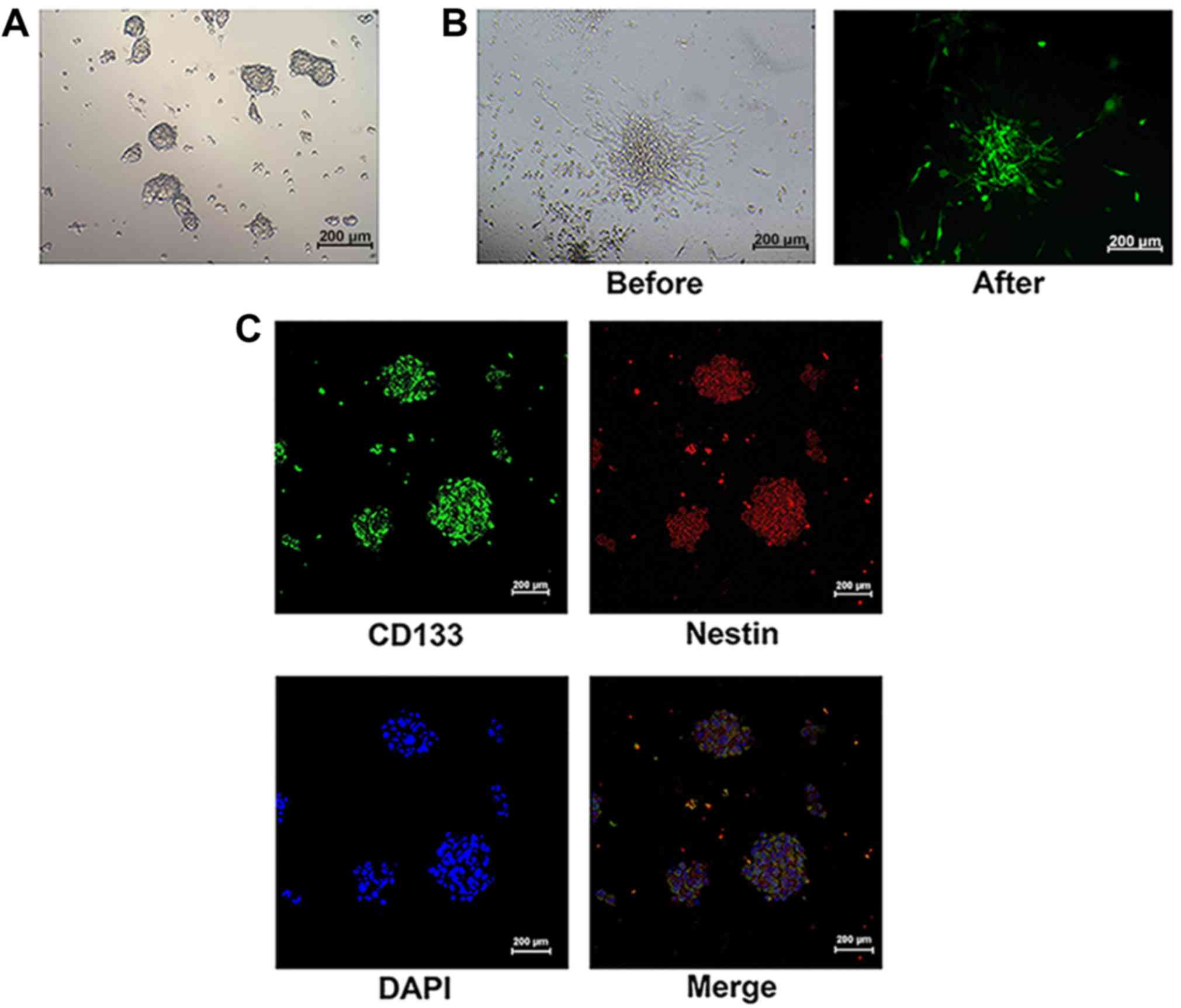
Identification of GSC
In our previous study, we successfully identified
GSCs using characteristics such as surface molecules, multipotency
and tumorigenicity (5). In the
present study, GSCs of both non-transfected and
siRNA-ATM-transfected cells were found to form neurospheres
composed of 3–5 cells and grown in bulk over ~1 week (Fig. 1A). In siRNA-ATM GSCs, the green
fluorescence protein was also observed as in GCs (Fig. 1B). After GSCs from the U251 and A172
cell lines (including transfected cells) were induced, CD133 and
nestin, surface molecules of GSC were detected in the neurospheres
(Fig. 1C).
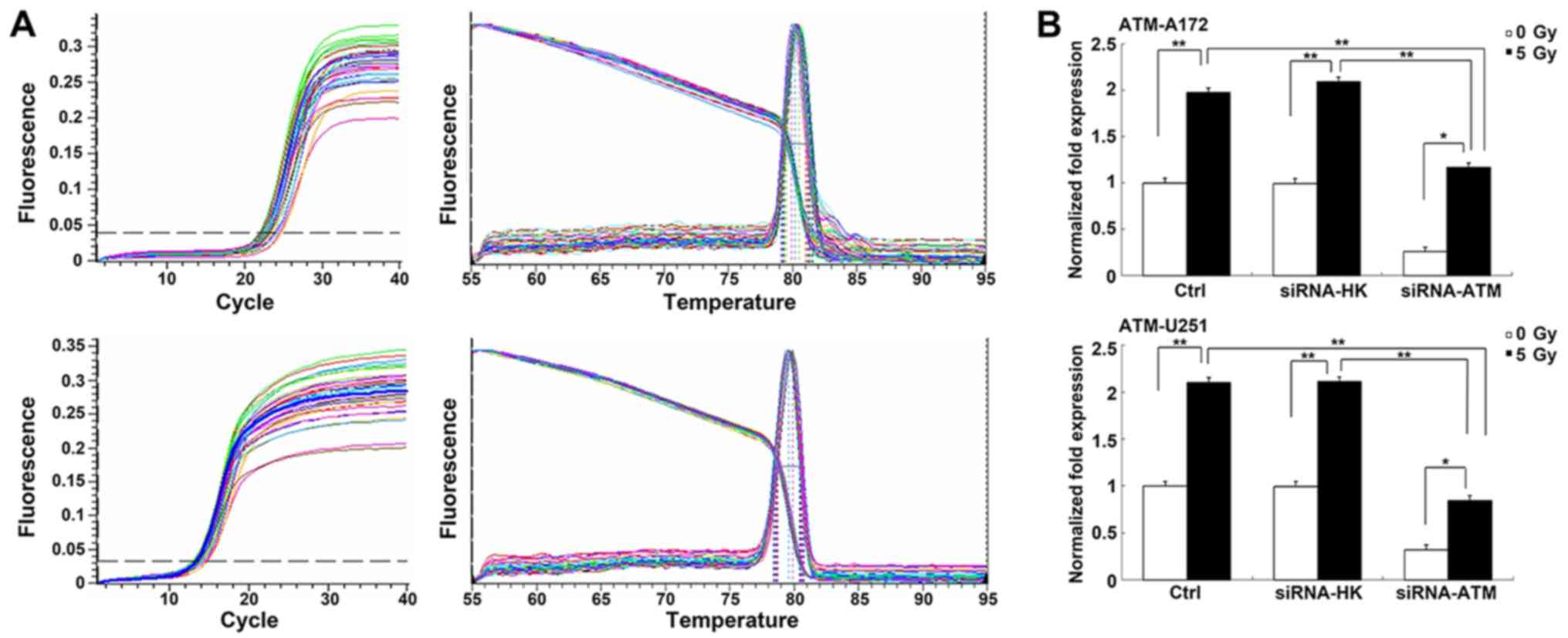
Changes in the expression of ATM after
siRNA treatment
We examined the expression of the ATM gene and
protein to assess the efficiency of siRNA-ATM in GSCs of the two
cell lines using RT-qPCR (Fig. 2A)
and western blotting. In GSCs, ATM expression was obviously
downregulated via the transfection of siRNA-ATM packaged in the
lentiviral vector. The PCR results of both U251 and A172 GSCs
revealed that the ATM expression in the C and N groups was
obviously increased post- vs. pre-irradiation (P<0.01). However,
there was little increase in the ATM expression in the A group
after irradiation (P<0.05). Moreover, ATM gene expression
was considerably lower in the A group than in the C or N groups
after irradiation (P<0.01) (Fig.
2B).
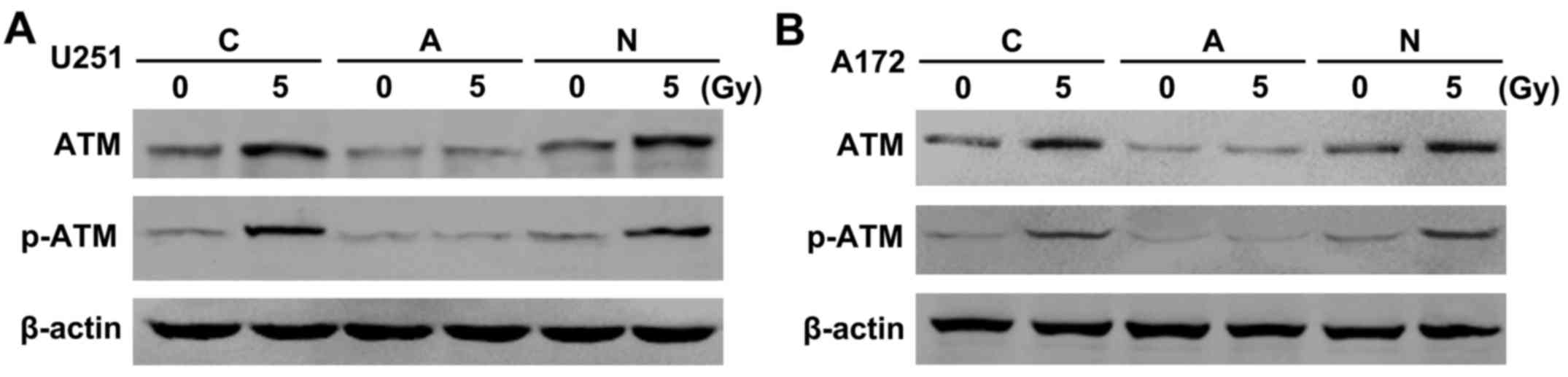
Similar results were observed from the western blot
analysis of the U251 (Fig. 3A) and
A172 GSCs (Fig. 3B). In the C and N
groups, the total ATM protein level increased only slightly after
vs. before irradiation. Surprisingly, although the expression of
the phosphorylated-ATM (p-ATM) protein was slight pre-irradiation,
it was obviously higher after irradiation (Fig. 3A and B). In the A group, the
expression of total ATM protein was similarly low pre- and
post-irradiation, but the p-ATM protein was not obviously expressed
at the same treatment condition. After irradiation, the total ATM
protein and p-ATM protein in the A group exhibited lower expression
than those in the C and N groups (Fig.
3A and B).
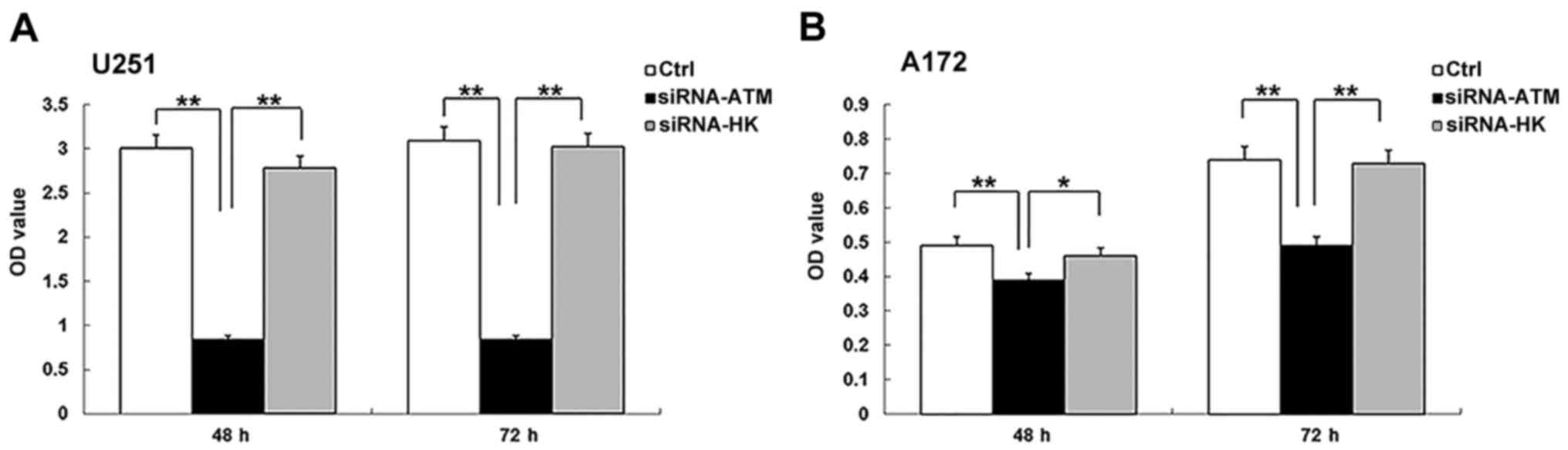
CCK-8 assay
Cell proliferation of the A group in the two GSCs
was significantly lower than those of the C and N groups at 48 and
72 h after irradiation (U251, P<0.01; A172, P<0.01, A group
vs. N group; P<0.05, A group vs. C group) (Fig. 4A and B). In the U251 GSCs, there was
no obvious increase in cell proliferation in the A group between
the two time-points. However, cell proliferation in the C and N
groups mildly increased from 48 to 72 h (P>0.05) (Fig. 4A). In the A172 GSCs, the cell
proliferation in the A group between 48 and 72 h was mildly
increased, but not significantly different. Meanwhile, there was a
significant difference in the cell proliferation number in the C
and N groups (P<0.01) (Fig.
4B).
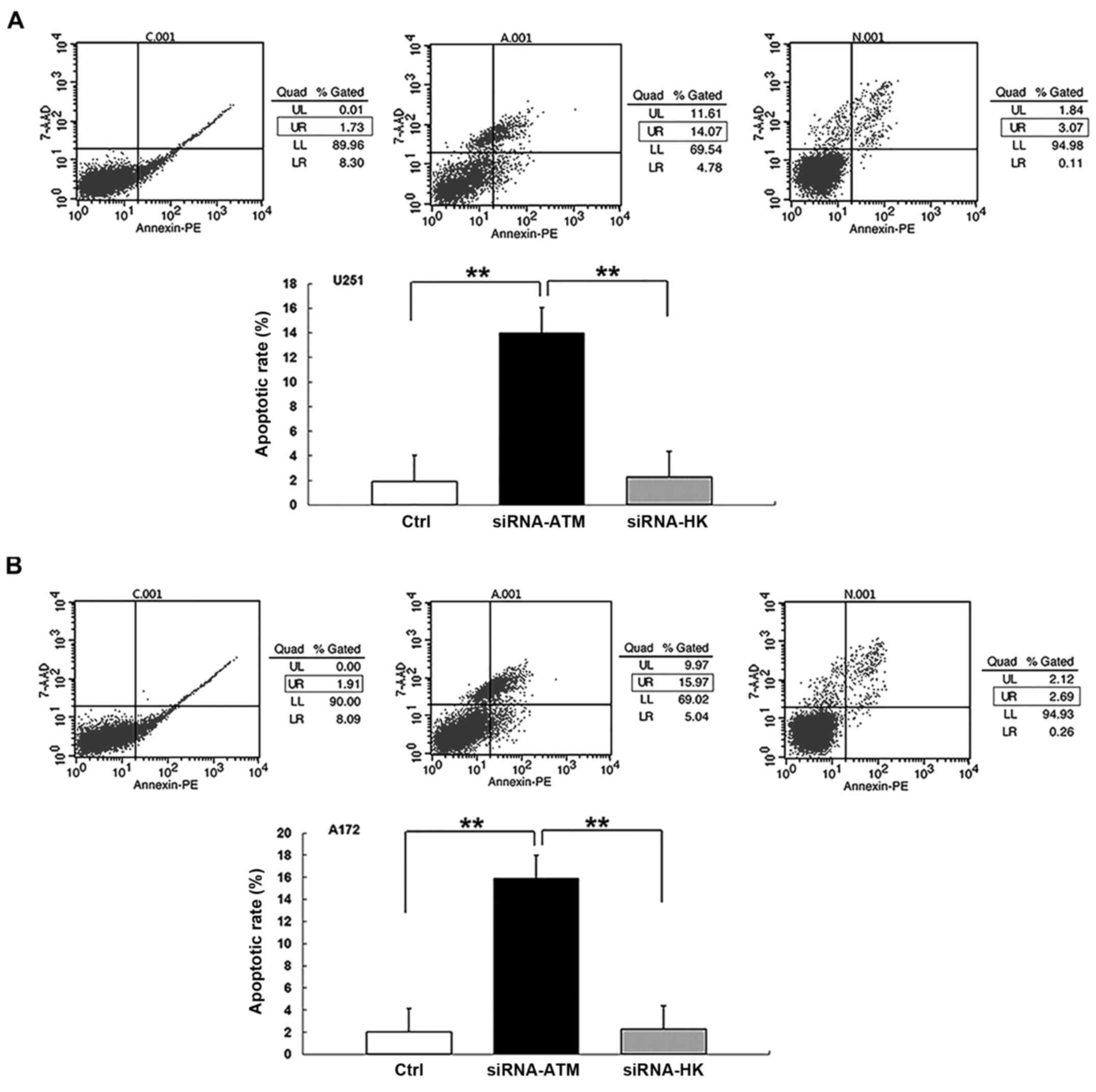
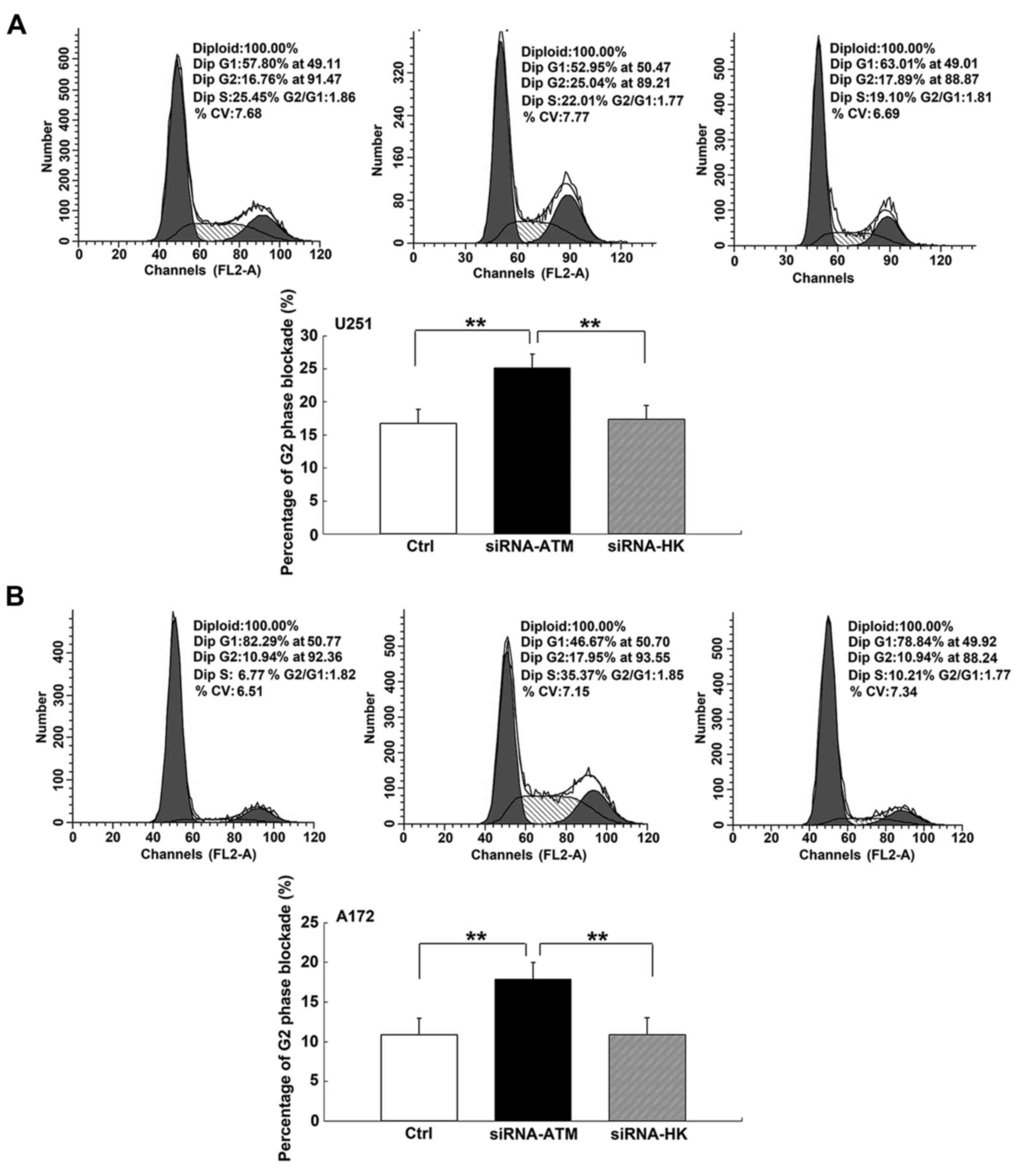
Cell apoptosis and G2 phase block by
flow cytometry
In GSCs from both cell lines, the percentage of
apoptotic cells of the latter cell cycle stages was greater in
group A than that in groups C and N (P<0.01) (Fig. 5A and B). There was no significant
difference in the apoptosis rate between the C and N groups.
Similarly, the percentage of blocked cells in the G2 phase in group
A exhibited an obvious increase compared with those of groups C and
N in U251 and A172 GSCs (P<0.01) (Fig. 6A and B).
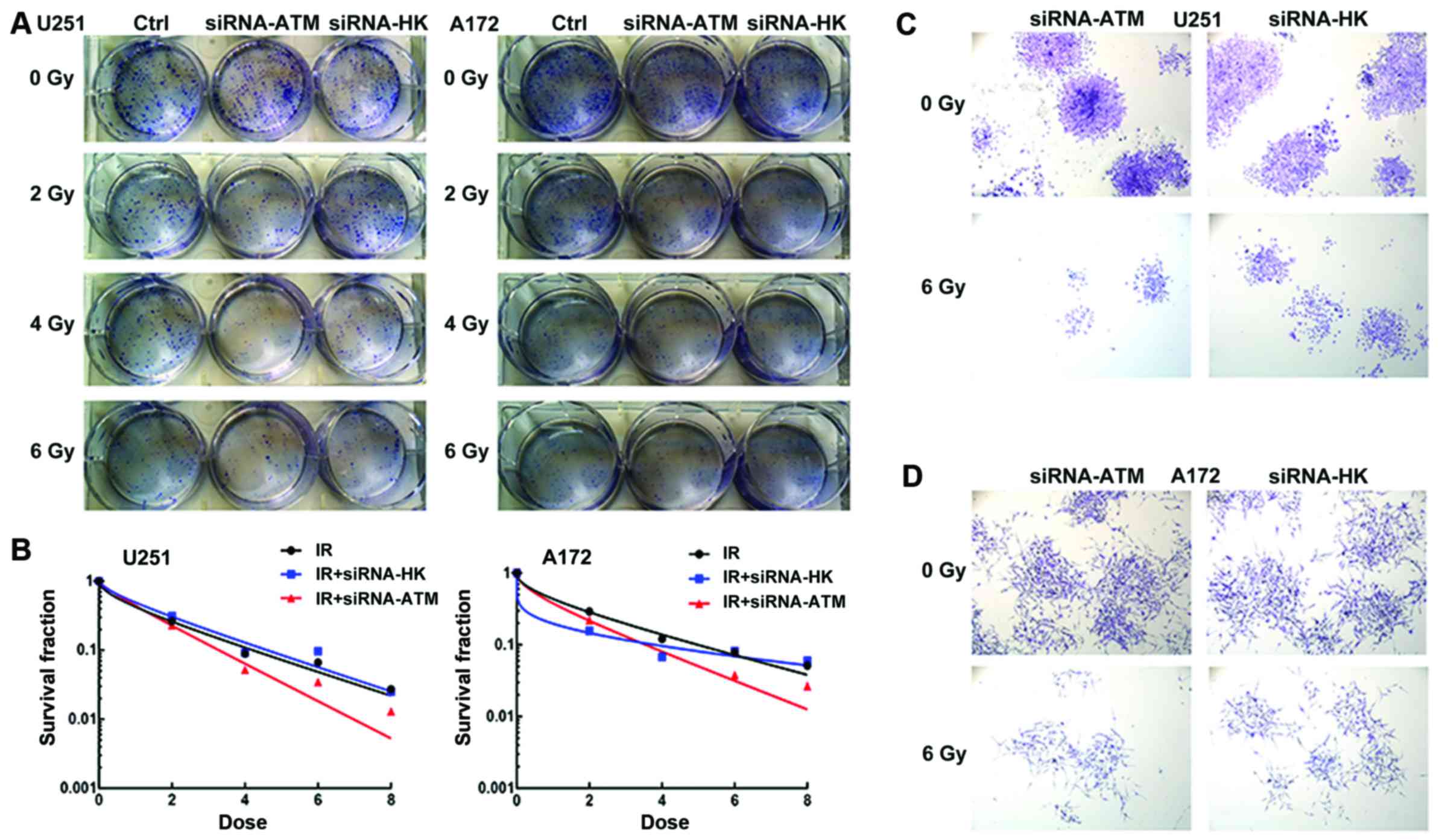
Colony-forming assay
The number of formed colonies decreased as radiation
dose increased in the three groups (C, A and N), which indicated a
dose-dependent correlation in U251 and A172 GSCs (Fig. 7A). At the same dose, the number of
colonies in the A group was less than those in the C and N groups,
while the number of colonies were similar in the C and N groups in
U251 and A172 GSCs (Fig. 7B).
Microscopic observation after crystal violet staining revealed that
the colonies formed by cells of the A group were smaller and lesser
in both GSCs (Fig. 7C and D) after
radiation with a 6 Gy dose.
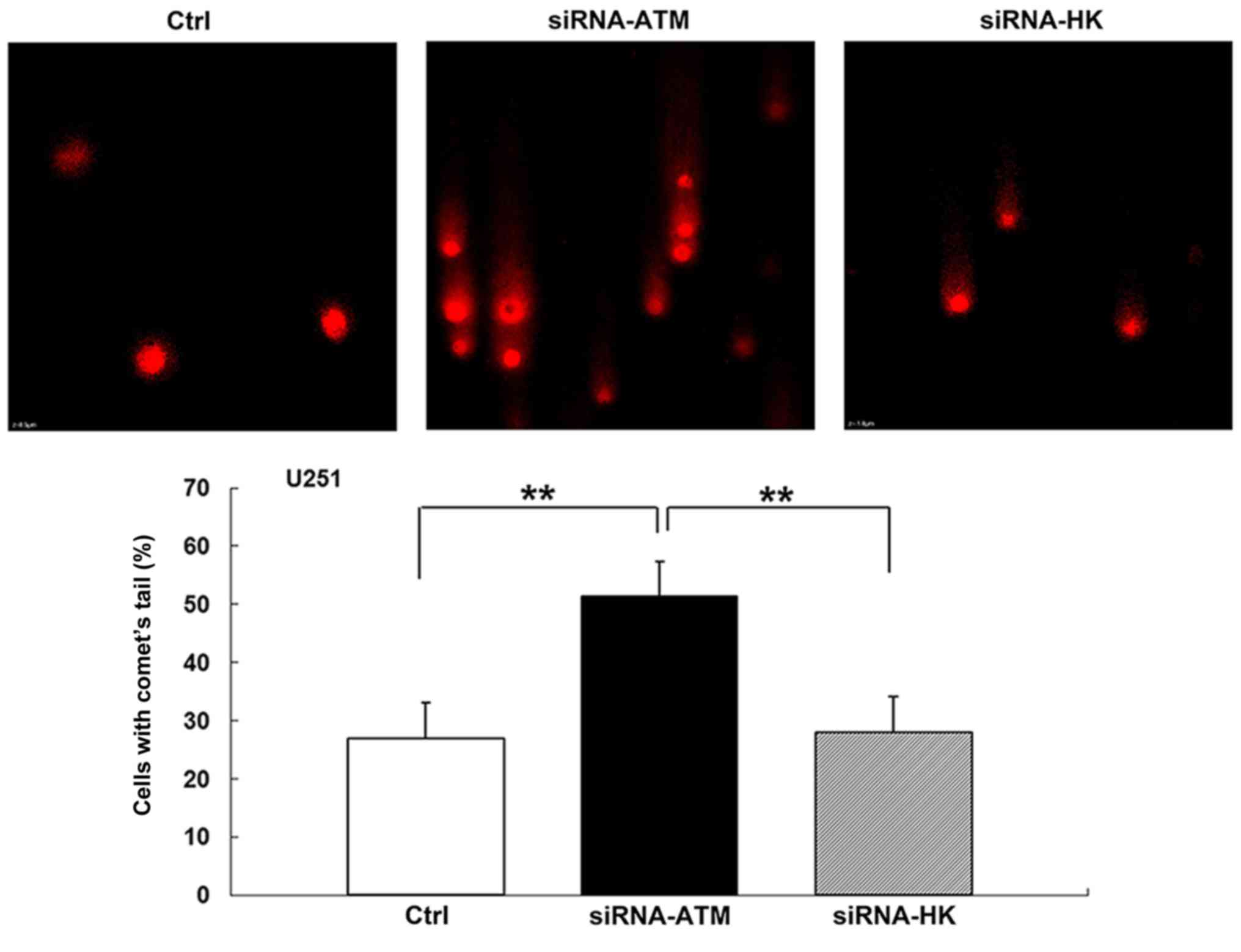
Neutral comet assay
The neutral comet assay was performed to assess the
level of double DNA damage in GSCs from both cell lines. Before
irradiation, comet tail formation was not observed in any of the
three groups. However, the percentage of comet tails in the A group
was increased after irradiation with a 5 Gy X-ray dose compared
with those in the C and N groups (P<0.01) (Fig. 8). The results in the C group were
similar to those in the N group after the radiation treatment.
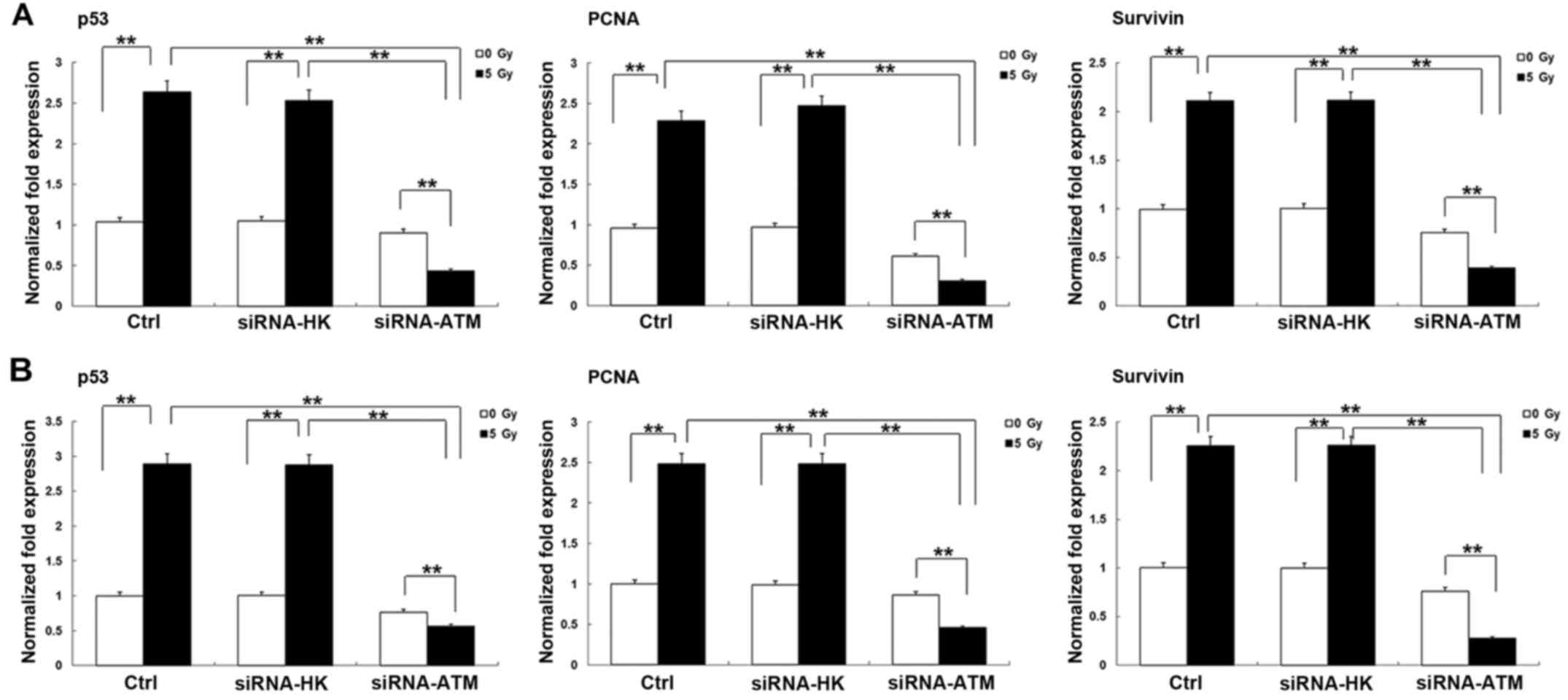
Changes in the expression of other
radiosensitivity-related genes after transfection of the siRNA-ATM
lentivirus into GSCs from the two cell lines
The expression levels of the
radiosensitivity-related genes p53, proliferating cell nuclear
antigen (PCNA), and survivin were also examined in both GSCs using
RT-qPCR. After irradiation, the expression levels of the three
genes (p53, PCNA and survivin) increased to some degree in the C
and N groups but were decreased in the A group (P<0.01). The
expression of those genes in the A group was obviously less than
those in the C and N groups after irradiation (P<0.01). The
results between the U251 GSCs (Fig.
9A) and A172 GSCs (Fig. 9B)
were similar.
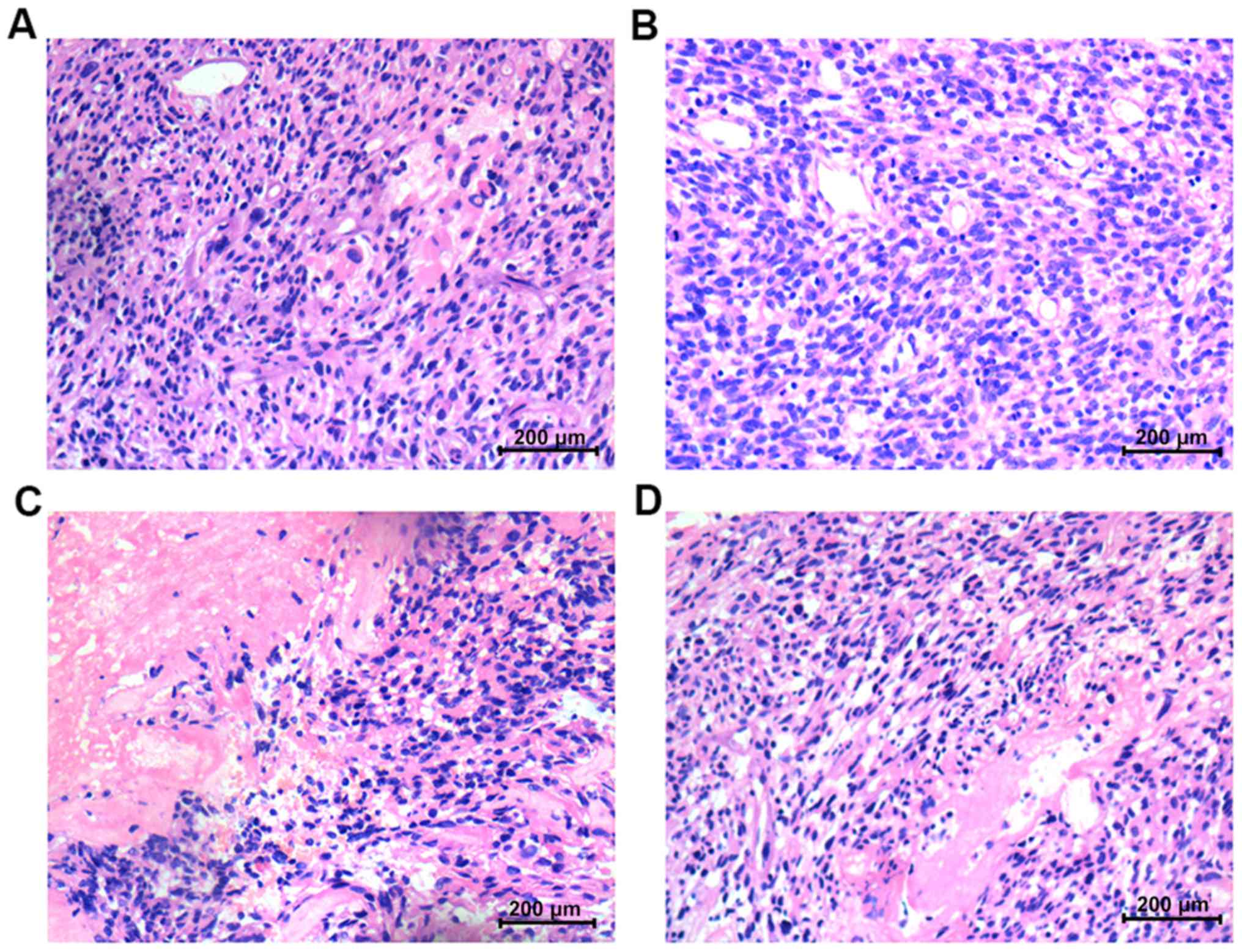
Histopathology
In the U251 and A172 animal models using GSCs, the
histopathological results differed before vs. after irradiation
therapy. Before irradiation, the histopathology of glioma tumors
was characterized by nuclear polymorphism, nuclear hyperchromatism
and considerable karyokinesis, and the histological features of
group A were similar to those of group N (Fig. 10A and B). However, after
irradiation, the necrosis and hemorrhage of the solid tumor tissue
in group A were more obvious than that in group N (Fig. 10C and D).
Discussion
In recent years, GSCs, which play an important role
in the radioresistance of glioma, have captured the attention of
many scholars in the field of oncology (15–17).
GSCs constitute a minor proportion of tumor cells that differ from
glioma cells. Between tissues of different origins, there are
marked differences in the DNA damage signaling pathways (18). It remains unclear whether the
expression and regulation of some genes in GSCs differ from those
in GCs.
In our previous study, we compared the
radiosensitization of GSCs and GCs and found that ATM exhibited a
high expression, which was linked to stronger radioresistance in
GSCs than in GCs (5). Various
studies confirmed that suppression of the ATM gene, which
exhibited a high expression after radiation in glioma, contributed
to the radiosensitivity of glioma. The current strategy mainly used
includes gene interference and pharmacological inhibition (8,15).
Both ATM inhibition methods may specifically sensitize GSCs to DSB
induced by radiation while sparing non-stem cells (15).
In the present study we employed two glioma cell
lines of different origin and further induced GSCs from those cell
lines for the subsequent experiments. Silencing of the ATM gene
contributes to changes in the downstream signaling pathway, which
thereafter affects GSC tumor behavior (19).
Genes related to radiosensitivity, including p53,
survivin and PCNA, that are downstream of ATM (20–24)
were analyzed using RT-qPCR. The results revealed that those genes
(p53, survivin and PCNA) were indeed involved in the cell repair
pathway. However, due to ATM silencing, the weakening of the repair
effect plays a role in the radiosensitivity of glioma cells. These
findings are consistent with those obtained from the CCK-8 and
colony formation assays, in which proliferation was also decreased
in response to ATM silencing.
The colony forming and comet tail assays are the
classic experiments for detecting the extent of DSB impairment.
Combining radiation with ATM-gene expression inhibition increased
the GSC damage. After irradiation, the survival fraction of the
siRNA-ATM group was decreased compared with the control groups.
Physical damage and breakage of DSB in the siRNA-ATM group
increased significantly as well. These results all indicate that
ATM gene inhibition caused GSC radiosensitivity.
The CCK-8 results revealed that the high expression
of ATM after irradiation in untreated cells was responsible for
repairing damaged cells and maintaining cell proliferation.
However, the cell repair may have been slow, resulting in decreased
cell proliferation when the ATM gene was interfered with. To
determine whether proliferation is related to time requires further
exploration. Notably, the A172 GSCs exhibited slower proliferation
than the U251 GSCs. These two cell lines are derived from different
individuals with many genetic differences, which may likely
influence the response to irradiation (1).
Cell apoptosis or the percentage of G2 phase
blockade by flow cytometry was also used to evaluate the cell
damage. We found that the apoptosis rate of the latter stages and
the percentage of G2 phase blockade in siRNA-ATM-transfected GSCs
increased after irradiation treatment. The results inferred that
inhibition of the ATM gene in GSCs decreased cell repair,
which in turn led to further cell damage.
Although, numerous studies on ATM interference have
been reported, most failed to be confirmed by in vivo
experiments (25). To the best of
our knowledge, this is the first study on siRNA-ATM transfection in
a mouse model in the field of GSC radiosensitivity. We implanted
subcutaneous tumors with U251 siRNA-ATM GSCs followed by
irradiation therapy and found obvious tumor hemorrhaging and
necrosis upon microscopic examination. The results in vivo
explained that silencing the ATM gene, which plays an
important role in cell proliferation and apoptosis, could affect
the biological behavior of glioma tumors. However, the related
mechanism of the siRNA-ATM transfection effect on tumor behavior
requires further exploration. To simulate the therapeutic effect in
this study, we intend to construct an orthotopic glioma mouse model
in the future. However, dilemmas such as the need to construct a
larger animal model and side-effects of radiation on the brain have
yet to be overcome and need to addressed in the future.
The present study, demonstrated that the
proliferation, colony formation rate and the survival fraction were
decreased the most, while the apoptotic rate, percentage of G2
phase block, and percentage of comet tail cells increased the most
after ATM gene inhibition followed by irradiation in GSCs.
The data partly revealed similarities with other scholars that have
examined glioma tumors (1,2,9). We
concluded that silencing the ATM gene in GSCs was a valuable
strategy for enhancing the therapeutic effect thus, improving
patient survival.
Acknowledgements
We thank the State Key Laboratory of Ultrasound
Engineering in Medicine Co-Founded by Chongqing and the Ministry of
Science and Technology for providing laboratory space. The present
study was supported by the National Natural Science Foundation of
China (no. 81172387), the Natural Science Foundation of Chongqing
(cstc2015jcyjBX0060), and the Post-graduate Foundation of Chongqing
(Xm2015086).
References
|
1
|
Biddlestone-Thorpe L, Sajjad M, Rosenberg
E, Beckta JM, Valerie NC, Tokarz M, Adams BR, Wagner AF, Khalil A,
Gilfor D, et al: ATM kinase inhibition preferentially sensitizes
p53-mutant glioma to ionizing radiation. Clin Cancer Res.
19:3189–3200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang SC, Wu CC, Wei YY, Hong JH and Chiang
CS: Inactivation of ataxia telangiectasia mutated gene can increase
intracellular reactive oxygen species levels and alter
radiation-induced cell death pathways in human glioma cells. Int J
Radiat Biol. 87:432–442. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lomonaco SL, Finniss S, Xiang C,
Decarvalho A, Umansky F, Kalkanis SN, Mikkelsen T and Brodie C: The
induction of autophagy by gamma-radiation contributes to the
radioresistance of glioma stem cells. Int J Cancer. 125:717–722.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hambardzumyan D, Becher OJ, Rosenblum MK,
Pandolfi PP, Manova-Todorova K and Holland EC: PI3K pathway
regulates survival of cancer stem cells residing in the
perivascular niche following radiation in medulloblastoma in vivo.
Genes Dev. 22:436–448. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou W, Sun M, Li GH, Wu YZ, Wang Y, Jin
F, Zhang YY, Yang L and Wang DL: Activation of the phosphorylation
of ATM contributes to radioresistance of glioma stem cells. Oncol
Rep. 30:1793–1801. 2013.PubMed/NCBI
|
|
6
|
Jackson SP: Sensing and repairing DNA
double-strand breaks. Carcinogenesis. 23:687–696. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Knizhnik AV, Roos WP, Nikolova T, Quiros
S, Tomaszowski KH, Christmann M and Kaina B: Survival and death
strategies in glioma cells: Autophagy, senescence and apoptosis
triggered by a single type of temozolomide-induced DNA damage. PLoS
One. 8:e556652013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nadkarni A, Shrivastav M, Mladek AC,
Schwingler PM, Grogan PT, Chen J and Sarkaria JN: ATM inhibitor
KU-55933 increases the TMZ responsiveness of only inherently TMZ
sensitive GBM cells. J Neurooncol. 110:349–357. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gil del Alcazar CR, Hardebeck MC,
Mukherjee B, Tomimatsu N, Gao X, Yan J, Xie XJ, Bachoo R, Li L,
Habib AA, et al: Inhibition of DNA double-strand break repair by
the dual PI3K/mTOR inhibitor NVP-BEZ235 as a strategy for
radiosensitization of glioblastoma. Clin Cancer Res. 20:1235–1248.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Golding SE, Rosenberg E, Adams BR,
Wignarajah S, Beckta JM, O'Connor MJ and Valerie K: Dynamic
inhibition of ATM kinase provides a strategy for glioblastoma
multiforme radiosensitization and growth control. Cell Cycle.
11:1167–1173. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Raso A, Vecchio D, Cappelli E, Ropolo M,
Poggi A, Nozza P, Biassoni R, Mascelli S, Capra V, Kalfas F, et al:
Characterization of glioma stem cells through multiple stem cell
markers and their specific sensitization to double-strand
break-inducing agents by pharmacological inhibition of ataxia
telangiectasia mutated protein. Brain Pathol. 22:677–688. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Finnegan EJ and Matzke MA: The small RNA
world. J Cell Sci. 116:4689–4693. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chuah TL, Walker DG, Wei M, Scott S and
Lavin MF: Approaches to sensitizing glioblastoma to radiotherapy:
Use of lentiviral vectors. Int J Oncol. 40:1963–1969.
2012.PubMed/NCBI
|
|
14
|
Guha C, Guha U, Tribius S, Alfieri A,
Casper D, Chakravarty P, Mellado W, Pandita TK and Vikram B:
Antisense ATM gene therapy: A strategy to increase the
radiosensitivity of human tumors. Gene Ther. 7:852–858. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vecchio D, Daga A, Carra E, Marubbi D,
Baio G, Neumaier CE, Vagge S, Corvò R, Pia Brisigotti M, Ravetti J
Louis, et al: Predictability, efficacy and safety of
radiosensitization of glioblastoma-initiating cells by the ATM
inhibitor KU-60019. Int J Cancer. 135:479–491. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Clarke MF, Dick JE, Dirks PB, Eaves CJ,
Jamieson CH, Jones DL, Visvader J, Weissman IL and Wahl GM: Cancer
stem cells - perspectives on current status and future directions:
AACR Workshop on cancer stem cells. Cancer Res. 66:9339–9344. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Golestaneh AF, Atashi A, Langroudi L,
Shafiee A, Ghaemi N and Soleimani M: miRNAs expressed differently
in cancer stem cells and cancer cells of human gastric cancer cell
line MKN-45. Cell Biochem Funct. 30:411–418. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moschos SJ, Dodd NR, Jukic DM, Fayewicz
SL, Wang X and Becker D: Suppressing the high-level expression and
function of ATM in advanced-stage melanomas does not sensitize the
cells to ionizing radiation. Cancer Biol Ther. 8:1815–1825. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Squatrito M, Brennan CW, Helmy K, Huse JT,
Petrini JH and Holland EC: Loss of ATM/Chk2/p53 pathway components
accelerates tumor development and contributes to radiation
resistance in gliomas. Cancer Cell. 18:619–629. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Burdak-Rothkamm S and Prise KM: New
molecular targets in radiotherapy: DNA damage signalling and repair
in targeted and non-targeted cells. Eur J Pharmacol. 625:151–155.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang XD, Qin ZH and Wang J: The role of
p53 in cell metabolism. Acta Pharmacol Sin. 31:1208–1212. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Poosarla C, Ramesh M, Ramesh K, Gudiseva
S, Bala S and Sundar M: Proliferating cell nuclear antigen in
premalignancy and oral squamous cell carcinoma. J Clin Diagn Res.
9:ZC39–ZC41. 2015.PubMed/NCBI
|
|
23
|
Yang M, Zhai X, Xia B, Wang Y and Lou G:
Long noncoding RNA CCHE1 promotes cervical cancer cell
proliferation via upregulating PCNA. Tumour Biol. 36:7615–7622.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tamm I, Wang Y, Sausville E, Scudiero DA,
Vigna N, Oltersdorf T and Reed JC: IAP-family protein survivin
inhibits caspase activity and apoptosis induced by Fas (CD95), Bax,
caspases, and anticancer drugs. Cancer Res. 58:5315–5320.
1998.PubMed/NCBI
|
|
25
|
Ma X, Yang L, Xiao L, Tang M, Liu L, Li Z,
Deng M, Sun L and Cao Y: Down-regulation of EBV-LMP1
radio-sensitizes nasal pharyngeal carcinoma cells via NF-κB
regulated ATM expression. PLoS One. 6:e246472011. View Article : Google Scholar : PubMed/NCBI
|