Introduction
Nests of tumor cells at primary and metastatic sites
are usually infiltrated by a variety of cancer-associated
fibroblasts (CAFs), endothelial cells, tumor-infiltrating
lymphocytes (TILs), and macrophages, which collectively produce
cytokines and other mediators that have the potential to modulate
both local and systemic antitumor immune responses (1–4). These
cells may include both myeloid-derived suppressor cells (MDSCs)
and/or regulatory T cells (Tregs) (2–4), i.e.,
cells that suppress the proliferation of CD4+ helper T
(Th) cells and antigen-specific CD8+ T cells (5), thus modifying antitumor responses by
various distinct mechanisms (6,7). MDSCs
form phenotypically heterogeneous groups of cells that share the
capacity to exert a suppressor function (8,9). The
level of these cells increases in the presence of infection
(10,11) as well as in the presence of several
types of murine (4,11,12)
and human cancers (13–15). Conversely, Tregs comprise a subtype
of Th-cells marked by the expression of high levels of the
interleukin-2 receptor α chain (IL-2Rα-chain) (CD25) and forkhead
box transcription factor P3 (Foxp3) (6,16–19).
Treg levels have also been found to be increased in cancer patients
(20). Despite both these types of
cells exerting suppressive effects, at least at local tumor sites,
they may have different effects on systemic immune responses.
The lung is a major metastatic site for various
kinds of cancer, including colorectal cancer. The environment of
the pulmonary tumor is different from that of the solid primary
tumor for lymphocytes and macrophages. For instance, unlike the
hypoxic environment of solid tumors, tumors and TILs located in the
lung occur in a well-oxygenated environment. T cell-intrinsic
molecular function and proliferation is tightly controlled by
oxygen tension (21). Moreover,
effector T cell responses are regulated in the lung against
innocuous foreign antigens. The mechanism by which the spread of
the tumor to the lung influences systemic immunity remains
unknown.
In the present study, we established a syngeneic
mouse subcutaneous tumor model and experimental pulmonary tumor
model, and found that these tumors had different effects on
systemic immunity, as assessed in the spleen.
Materials and methods
Animals
Female 5-week-old BALB/c mice were obtained from
Charles River Inc. (Kanagawa, Japan) and maintained under specific
pathogen-free conditions at the Tsushima-kita Branch, Department of
Animal Resources, Advanced Research Center, Okayama University. All
animal experiments were reviewed and approved by the ethics
committee for animal experiments of Okayama University under the ID
OKU-2015229.
Monoclonal antibodies (mAbs)
Anti-mouse CD3ε (145–2C11) and CD11b (M1/70) mAbs
were purchased from BD Biosciences (San Jose, CA, USA). Anti-mouse
CD25 (PC61.5), CD16/CD32 (93), and Gr-1 (RB6-8C5) mAbs were
purchased from eBioscience (San Diego, CA, USA). Anti-mouse CD4
(RM4-5), CD8α (53-6.7), and Foxp3 (3G3) mAbs were purchased from
Tonbo Biosciences (San Diego, CA, USA).
Tumor cell cultures
The colon adenocarcinoma cell line, CT26
(N-nitro-N-methylurethane-induced tumor derived from BALB/c mouse),
was purchased from the American Type Culture Collection (Rockville,
MD, USA) and maintained in Roswell Park Memorial Institute
(RPMI)-1640 medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented
with 10% (v/v) heat-inactivated fetal bovine serum (FBS) (SAFC
Biosciences, Lenexa, KS, USA) and 1% (v/v) antibiotic-antimycotic
solution (10,000 U/ml penicillin, 10,000 µg/ml streptomycin, and 25
µg/ml amphotericin B; Life Technologies, Gaithersburg, MD,
USA).
Tumor cell transplantations and
histochemical analyses
CT26 cells (5×105 cells/200 µl/body) were
transplanted subcutaneously (s.c.) into the right flank, or
intravenously (i.v.) into a female 6-week-old mouse. A sham mouse,
as control experiments, was injected with saline alone. The mice
were euthanized on day 14 after the transplantation. Tumor volumes
were calculated as 1/2 × length × width2. Tumors were
analyzed histochemically, according to a previous study (21). Images were obtained using an Olympus
(New York, NY, USA) LX81 at 20x and processed with MetaMorph
software (Universal Imaging Corp., West Chester, PA, USA).
Splenocyte cultures and assays for
cytokine levels
Splenocytes were isolated and cultured, according to
a previous study (22). Splenocytes
(4×105 cells/well) were cultured for 48 h on flat-bottom
96-well plates (Corning Costar, Cambridge, MA, USA) coated with 5
µg/ml anti-CD3ε mAb in 200 µl RPMI-1640 medium containing 50 µM
2-mercaptoethanol (Nacalai Tesque, Inc., Kyoto, Japan), and
supplemented with 10% (v/v) FBS and 1% (v/v) antibiotic-antimycotic
solution. After cultivation, the produced IFN-γ, IL-4, IL-9, and
IL-10 levels in culture supernatants were evaluated by
cytokine-specific enzyme-linked immunosorbent assay (ELISA)
commercially available from BD Biosciences and eBioscience.
Flow cytometry (FCM)
Splenocytes (1×106) were incubated with
anti-CD16/CD32 mAb for 20 min on ice. Then, MDSCs were stained with
anti-Gr-1 and CD11b mAbs. T cells were stained with anti-CD4, CD25,
and CD8 mAbs for 30 min on ice, fixed with FACS lysing solution (BD
Biosciences) for 10 min at room temperature (RT), permeabilized
with FACS permeabilizing solution 2 (BD Biosciences) for 10 min at
RT, and then stained with anti-Foxp3 mAb for 30 min. The stained
cells were analyzed by Accuri™ (BD Biosciences) and FlowJo Software
(Treestar, Inc., San Carlos, CA, USA).
Statistical analyses
Survival curves were generated using the
Kaplan-Meier method. Statistical analyses were performed using the
Student's two-tailed t-test, the Mann-Whitney U, and the Pearson's
correlation coefficient-tests. All analyses were performed using
GraphPad Prism Software Version 6 (GraphPad Software Inc., San
Diego, CA, USA). P-values <0.05 were considered statistically
significant.
Results
Generation of murine models confined
to subcutaneous and pulmonary sites
To explore the impact of subcutaneous and pulmonary
tumors on systemic immunity with the same tumor cell line, we
created murine tumor models in which tumor cells were confined to a
single site at the subcutaneous site of injection or in which the
tumor had undergone hematogenous spread to the lungs in the absence
of growth at a subcutaneous site. To this end, we injected BALB/c
mice with 5×105 CT26 cells (a colorectal cancer cell
line) either s.c. or i.v., then examined these mice (or control
mice injected with PBS) after injection to determine the course and
extent of tumor spread. Mice administered CT26 cells by s.c.
injection were alive at day 30, whereas mice administered these
cells by i.v. injection died between 14 and 16 days (Fig. 1A and B). We therefore euthanized and
examined both s.c.- and i.v.-transplanted mice at day 14 after
inoculation. The tumor volumes of the s.c.-transplanted mice grew
to 650-2,197 mm3 by day 14, and were confined to the
original site of injection (Fig.
1E). On the other hand, a large number of pulmonary nodules
covered the lungs of i.v.-transplanted mice (Fig. 1D), and were present only in this
organ (Fig. 1C and F).
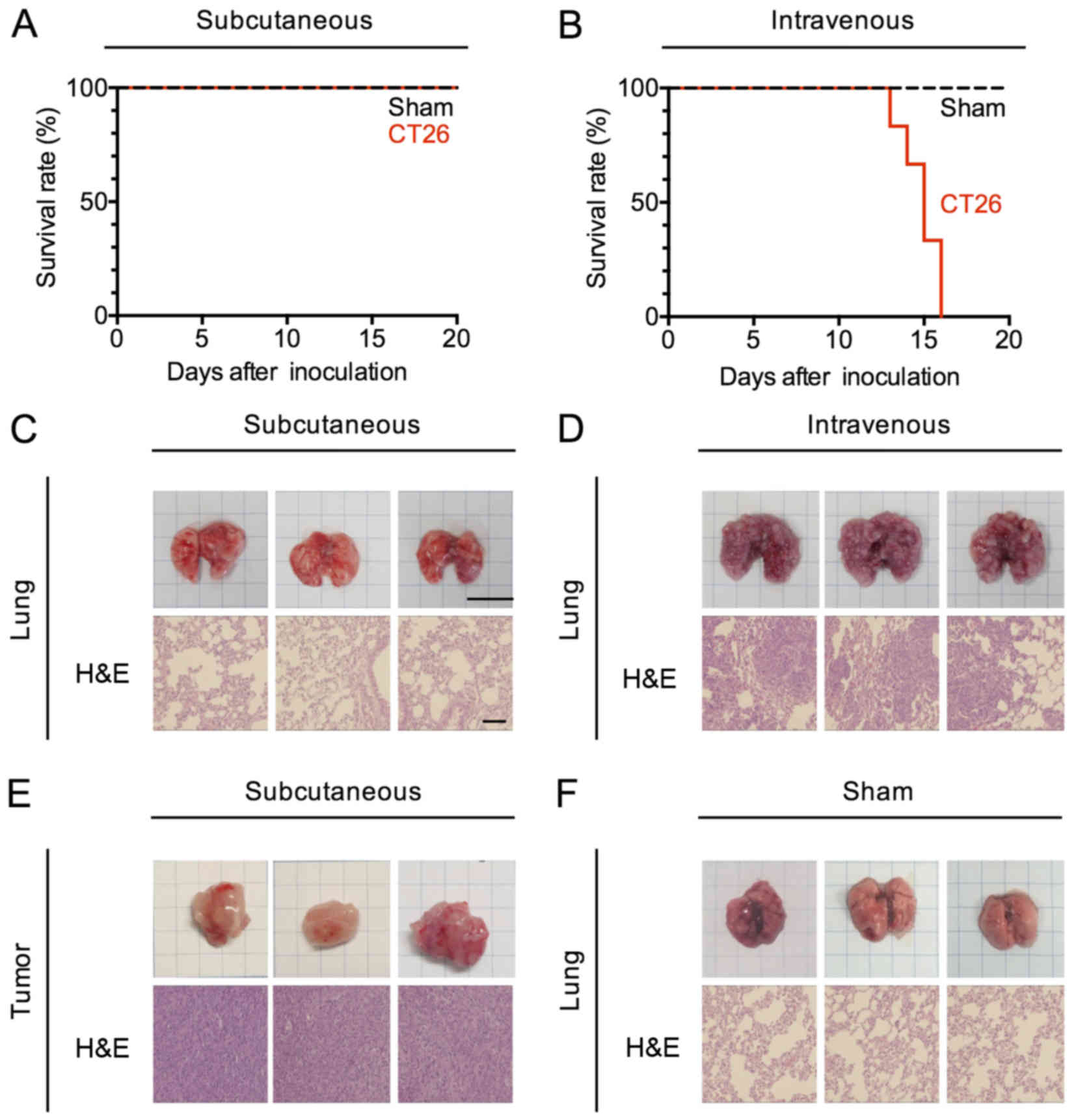 | Figure 1.Murine carcinoma models confined to
subcutaneous and pulmonary sites. (A and B) Kaplan-Meier survival
curves of CT26-transplanted mice. CT26 cells (5×105
cells/200 µl/body) were transplanted subcutaneously (s.c.) into the
right flank or intravenously (i.v.) of a female 6-week-old mouse.
Data represent three pooled experiments; sham, n=9, and s.c., n=9
(A), and sham, n=9, and i.v., n=9 (B). (C-F) Hematoxylin/eosin
(H&E) staining of subcutaneous tumors and pulmonary tumor from
the control (sham) and tumor-bearing (s.c. and i.v.) mice 14 days
after CT26 transplantation. Engraftment, bar, 10 mm; H&E
staining, bar, 100 µm. |
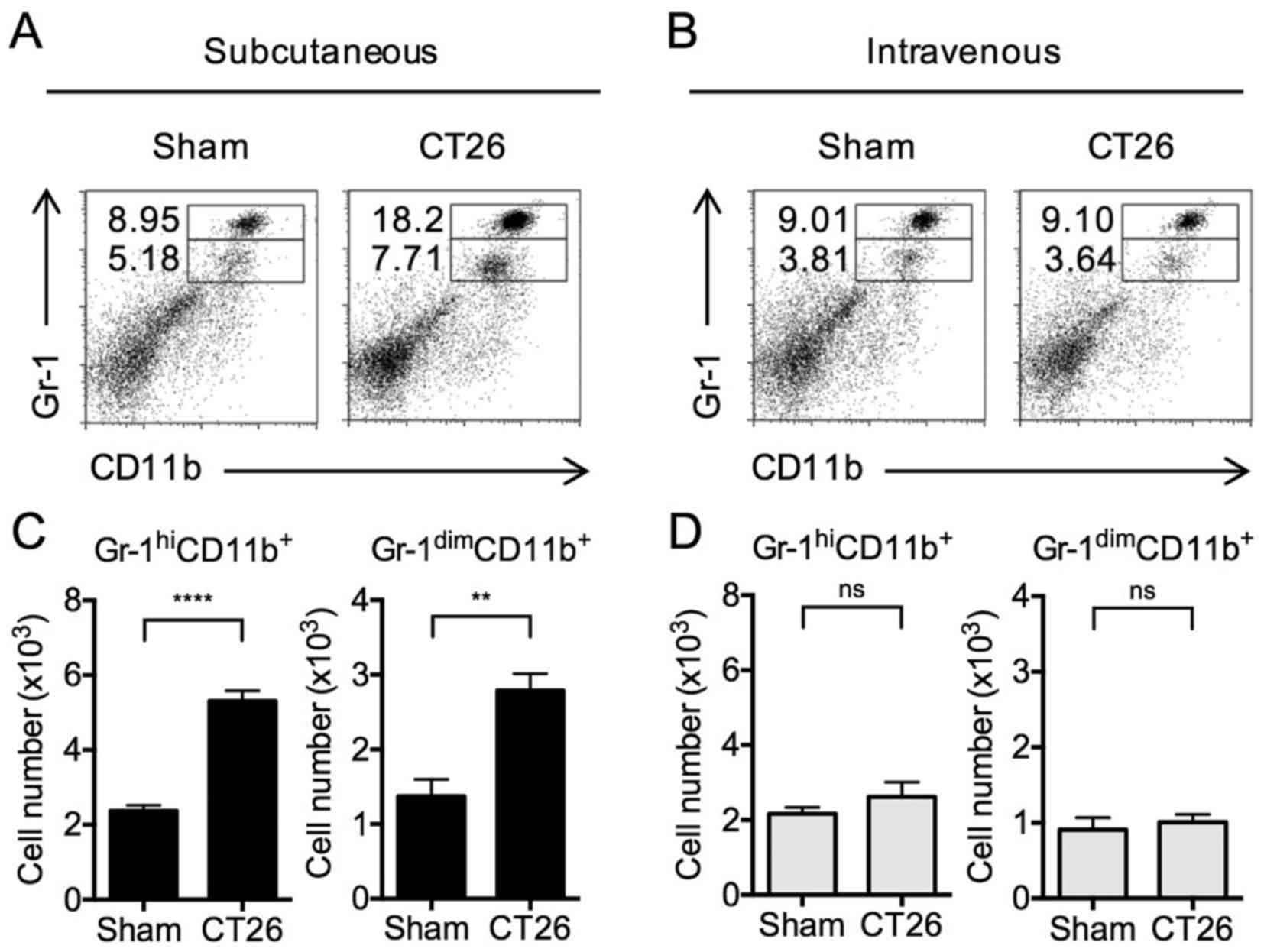
After tumor inoculation, MDSCs are
increased in s.c.-transplanted mice, but not in i.v.-transplanted
mice
Having established tumor models of tumors growing in
only subcutaneous site or pulmonary site, we then examined
regulatory cell development in the spleen in response to tumor
growth. During the initial studies, we evaluated the level of
accumulation of MDSCs in the spleens of mice comprising these two
tumor models. These cells were identified by flow cytometry, taking
advantage of their surface expression of either Gr-1hi
CD11b+ (mature myeloid cells) or Gr-1dim
CD11b+ or Gr-1intermediate CD11b+
(immature myelomonocytic cells) (8,23–25).
It should be noted that the T cell suppressive capacities of
Gr-1dim CD11b+ MDSCs are higher than that of
the Gr-1hi CD11b+ MDSCs (4,8,25);
however, Gr-1hi CD11b+ MDSCs were shown to
also acquire suppressor activities after lethal tuberculosis
infection (10).
Gr-1hi CD11b+ cells in the
spleens of s.c.-transplanted mice were significantly increased
(Fig. 2A) compared to PBS-injected
mice (i.e., sham s.c.). In addition, Gr-1dim
CD11b+ MDSCs were significantly increased (Fig. 2A). In contrast, no differences in
the levels of either type of MDSCs were observed in
i.v.-transplanted mice compared to sham-injected mice (Fig. 2B). These changes in the proportion
of MDSCs in s.c.-transplanted and i.v.-transplanted mice were
accompanied by corresponding changes in the total numbers of MDSCs
(Fig. 2C and D). The data above
clearly indicate that tumor cells existing at a subcutaneous site
versus a pulmonary site have different effects on the systemic
development of MDSCs; thus, these cells have the potential to
regulate systemic T cell function.
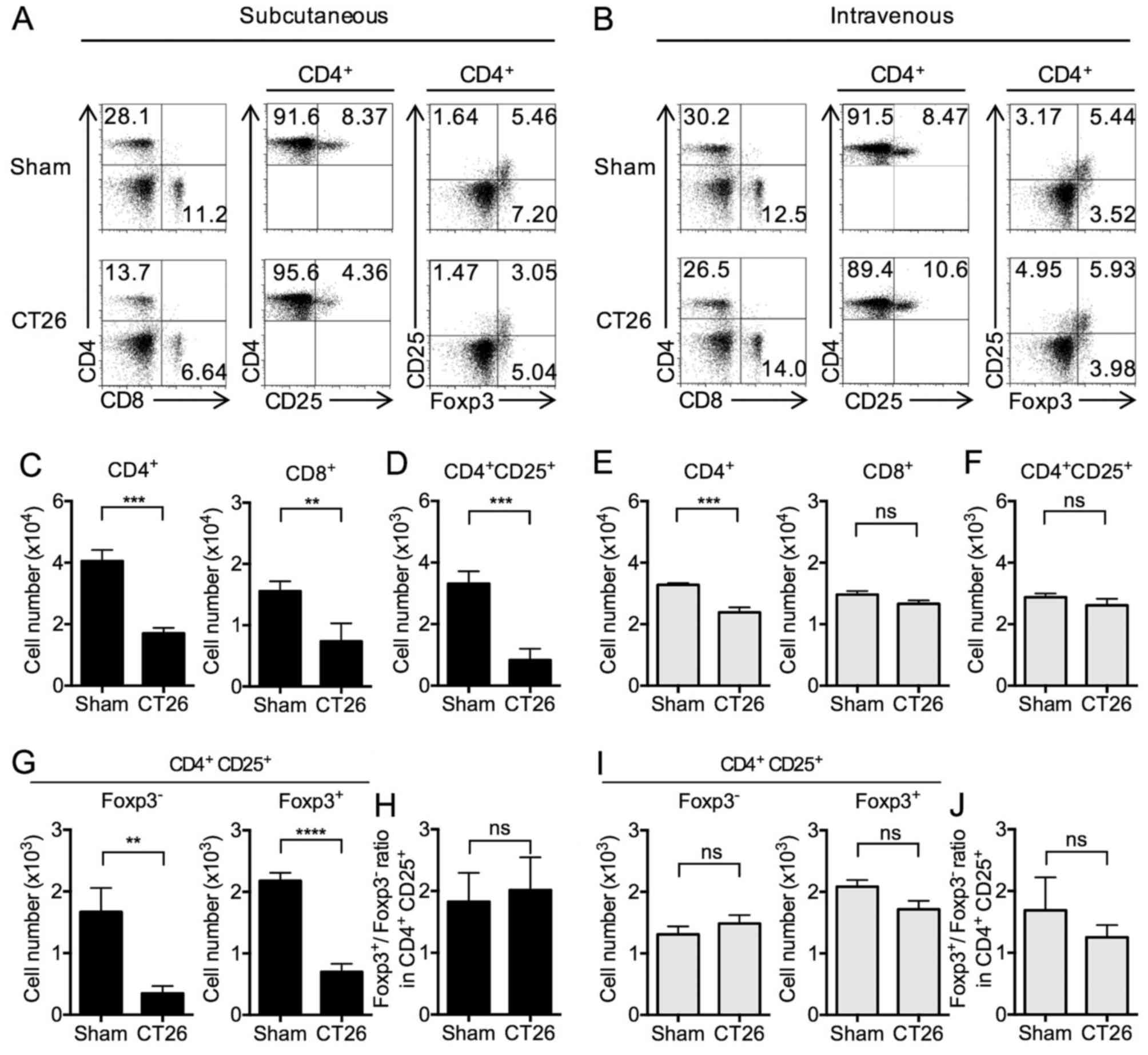
T cell subsets in s.c.- and
i.v.-transplanted CT26 tumor mice
We next turned our attention to levels (percentages)
of T cells and T cell subsets in the spleens of the s.c.- and
i.v.-transplanted mice. CD4+ T cells were significantly
decreased in both s.c.- and i.v.-transplanted mice. In addition,
CD8+ T cells were significantly decreased in
s.c.-transplanted mice, whereas a non-significant change was
observed in i.v.-transplanted mice (Fig. 3A and B). These proportion of
CD4+ and CD8+ T cells in s.c.-transplanted
mice were accompanied by corresponding changes in the total numbers
of CD4+ and CD8+ T cells (Fig. 3C and E).
In s.c.-transplanted mice, the above decrease in
levels of CD4+ T cells was accompanied by somewhat
smaller, but significant decreases in the levels of CD4+
CD25+ T cells (Fig. 3A and
D) as well as the subset of CD25+ T cells also
expressing Foxp3 (Tregs) (Fig. 3A and
G) compared to sham-inoculated mice (18,26).
In contrast, in i.v.-transplanted mice, the numbers of
CD4+ CD25+ T cells and CD4+
CD25+ Foxp3+ cells were similar compared to
sham mice (Fig. 3B, F and I). The
ratio of Foxp3+ to Foxp3− in CD4+
CD25+ cells was unchanged between the sham and
s.c.-transplanted mice (Fig. 3H)
and between the sham and i.v.-transplanted mice (Fig. 3J). Considered together, these
results showed that whereas both s.c.- and i.v.-transplanted mice
exhibit decreases in CD4+ T cell subsets, only in
s.c.-transplanted mice is this decrease also reflected in a
decrease in regulatory CD4+ CD25+
Foxp3+ T cells. In addition, these results showed that
an increase in MDSCs in the s.c.-transplanted mice is associated
with a decrease in Foxp3+ Tregs, and the lack of
increase in MDSCs in the i.v.-transplanted mice is not associated
with a change in Foxp3+ Tregs.
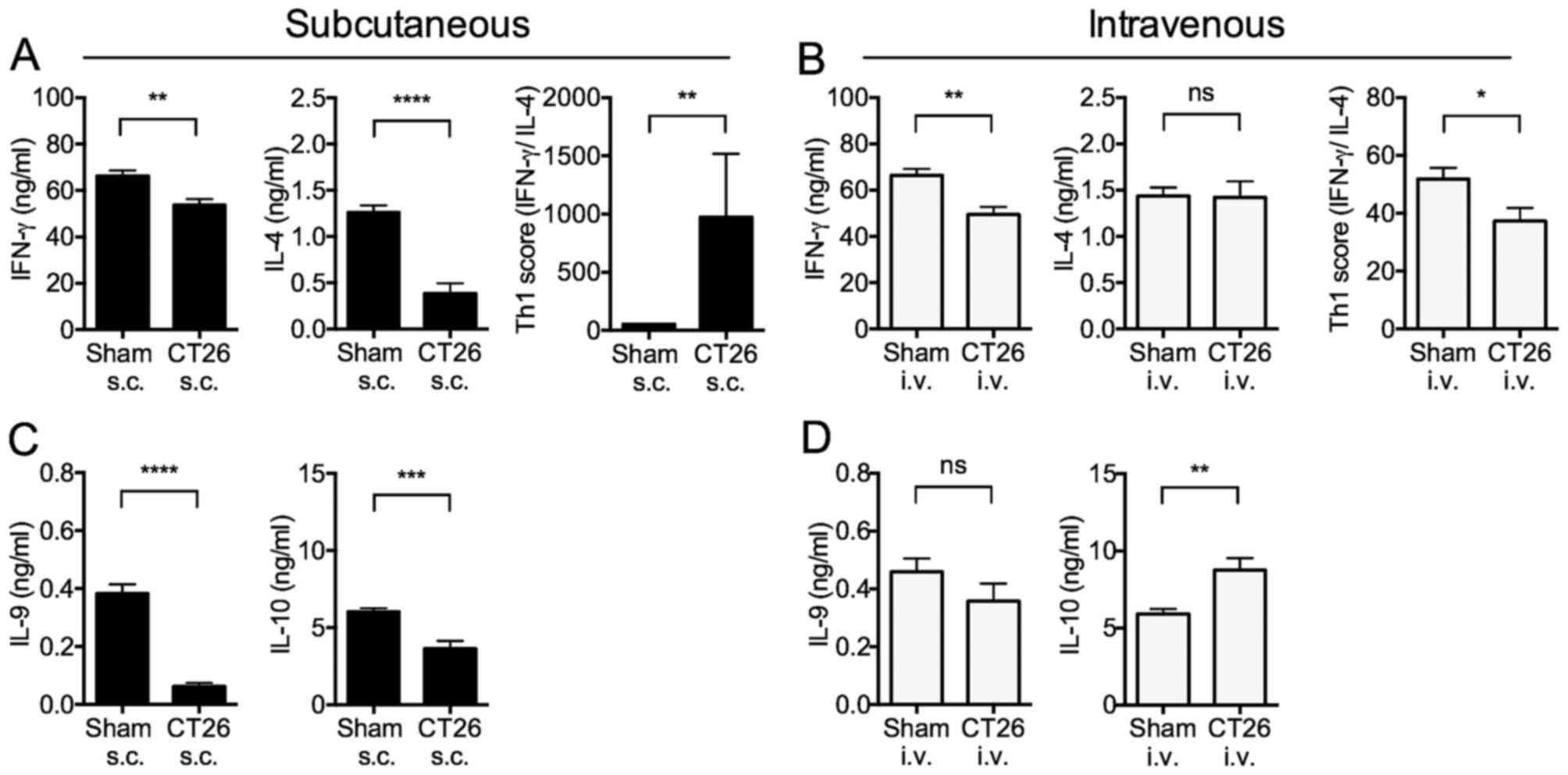
IFN-γ and IL-4 cytokine production by
splenocytes in tumor-bearing mice
Given the different regulatory cell responses of
s.c.- and i.v.-transplanted tumor-bearing mice, we examined
cytokine responses of splenocytes in the mice. We determined Th1
responses (i.e., IFN-γ production) and Th2 responses (i.e., IL-4
production) by anti-CD3ε Ab to stimulate T cells in splenocytes in
the two groups of mice.
The IFN-γ production from whole splenocytes
stimulated with immobilized anti-CD3ε antibody (Ab) was
significantly decreased in both s.c.- and i.v.-transplanted mice
(Fig. 4A and B). In contrast, IL-4
production was significantly decreased in s.c.-transplanted mice
(Fig. 4A), but not in
i.v.-transplanted mice (Fig. 4B).
These changes were reflected in the Th1 score (i.e., ratio of IFN-γ
to IL-4 production) that was significantly increased in
s.c.-transplanted mice (Fig. 4A),
and decreased in i.v.-transplanted mice (Fig. 4B). These results indicated that
s.c.-transplanted mice exhibit increased Th1 polarization, whereas
i.v.-transplanted mice exhibited marginally decreased Th1
polarization.
IL-10 and IL-9 cytokine production
from splenocytes in tumor-bearing mice
In further cytokine studies of s.c.- and
i.v.-transplanted mice, we measured splenocyte production of IL-10
and IL-9 following anti-CD3ε stimulation. IL-10 is an
anti-inflammatory cytokine with diverse effects on most
hematopoietic cell types, and inhibits activation and effector
function of T cells, monocytes, and macrophages (27,28).
IL-10 is produced mainly by Th2 T cells, although it can also be
produced by Th1 T cells under certain conditions.
In addition, IL-10 can be produced by other types of
T cells such as Tregs, Type1 regulatory T cells (Tr1), Th9 cells,
as well as by non-T cells such as MDSCs (6,18,26–28).
On the other hand, IL-9 is clearly a Th2 cytokine as its synthesis
is induced by IL-4 and transforming growth factor β (TGF-β)
(29).
IL-9 production was significantly decreased in
s.c.-transplanted mice, but unchanged in i.v.-transplanted mice
(Fig. 4C and D). Similarly, IL-10
production from whole splenocytes stimulated with immobilized
anti-CD3ε Ab was significantly decreased in s.c.-transplanted mice,
but significantly increased in i.v.-transplanted mice (Fig. 4C and D). These results are again
consistent with the view that s.c.-transplanted mice exhibit
increased Th1 polarization, whereas i.v.-transplanted mice exhibit
marginally decreased Th1 polarization.
Discussion
In the present study, we created mouse models of
tumor development, i.e., mice in which tumor cells (CT26 cells)
were limited to the site of injection and did not display
metastatic behavior (s.c.-transplanted mice) and mice in which
tumor cells were immediately subjected to hematogenous
dissemination and developed at multiple sites in the lung
(i.v.-transplanted mice). A striking difference in the immunologic
response to the two modes of tumor development in these models was
that a tumor limited to a subcutaneous site led to the appearance
of increased numbers of both Gr-1hi CD11b+
and Gr-1dim CD11b+ MDSCs in the spleen,
whereas a pulmonary tumor exhibited no such increase. At the same
time, these two modes of tumor development had an inverse effect on
CD4+ CD25+ Foxp3+ Tregs in the
spleen in that a tumor limited to a subcutaneous site exhibited
decreased numbers of Tregs, whereas a pulmonary tumor exhibited no
change in numbers of such cells. Current notions of MDSC
development suggest that the accumulation of tumor cells create a
milieu in which damage-associated molecular patterns (DAMPs) and
other tumor-derived inflammatory factors induce effector cell
production of a host of inflammatory factors. These factors
collectively induce development of MDSCs and presumably their
dissemination to distant lymphoid sites such as the spleen
(30). The studies conducted here
suggest that a tumor milieu capable of MDSC induction occurs at
subcutaneous sites of development, but not at pulmonary site. Thus,
by their differential effects on the development and dissemination
of potentially suppressive MDSCs, subcutaneous tumor development
and pulmonary tumor development might have very different effects
on systemic host immunity.
A second difference in the systemic regulatory cell
response between a tumor limited to subcutaneous site of
development and a pulmonary tumor in the models studied here was
that splenic CD4+ CD25+ Foxp3+
Treg levels were decreased in mice with the tumor limited to
subcutaneous site, whereas no change in the levels of these cells
was observed in mice with pulmonary tumor. This finding is somewhat
unexpected, given that MDSCs have been shown to induce Tregs,
possibly via their production of TGF-β and IL-10 (2). Thus, one would expect that increased
splenic MDSC levels would be accompanied by increased Treg levels.
One possible explanation of this unexpected finding is that MDSCs
have a direct suppressive effect on Treg development via the
production of arginase I, a factor that mediates T cell starvation
and inhibition of T cell proliferation of all T cells, including
Tregs (15,20,23).
This possibility is consistent with our finding that mice with
increased MDSC levels in the spleen exhibit decreases in all T
cells, including CD4+ CD25+ Foxp3−
T cells, with the latter representing activated T cells that are
not Tregs. In addition, it is consistent with our findings that
there is an inverse correlation between MDSC levels and
CD4+ CD25+ Foxp3+ T cells.
Before a spontaneous metastasis event, primary tumor
cells convert gene expression pattern to lose adhesion and
migration (31). Since we
transplanted same tumor cells, the two tumor growth models
established do not reflect primary tumors and metastatic tumors.
Nevertheless, many studies have shown anti-inflammatory Th2 shift
in breast, gastric and non-small cell lung cancer patients
(32–35), and advanced cancer patients
(36) with related cytokine
production. In addition, IL-10 levels that strongly correlate with
clinical stage and poor prognosis were elevated (37–40).
Thus, our pulmonary tumor model that also induced Th2 shift and
increase of IL-10 levels might partly be the same as stage IV
patients. On the other hand, the change of systemic immunity in
this model might possibly be caused by pulmonary embolism rather
than pulmonary tumor. Further studies are needed to compare the
change of systemic immunity in the pulmonary tumor model with
spontaneous metastasis model.
The pleiotropic cytokine IL-9 is produced by Th
cells and originally associated with the Th2 phenotype (41). IL-9 has been reported to have both a
pro-inflammatory and immunosuppressive function (42). However, IL-9 levels and function in
cancer remain poorly understood. Although the study of
tumor-bearing mice with IL-9 neutralizing antibodies showed
antitumor immune responses (42),
we found that the splenic IL-9 levels, especially in subcutaneous
tumor, were significantly lower than in the sham control (Fig. 4C), which is consistent with the
observation in colon cancer patients (43). Thus, IL-9 might not proactively
function in systemic immunities of cancer patients.
In conclusion, we established mouse models that
clearly recognize the differences between subcutaneous tumors and
pulmonary tumors. As these mouse models are able to represent well
systemic immunity in cancer patients, they might be useful for
exploitation of immunotherapy along with cancer progression.
Acknowledgements
This study was supported in part by Program to
Disseminate Tenure Tracking System, MEXT, Japan, by Okayama
Foundation for Science and Technology, by Ryobi Teien Memory
Foundation, by Sanyo Hohso Foundation, by the Grants-in-Aid for
Scientific Research from the Japan Society for the Promotion of
Science (15K11096 and 15H03002), and by the Program of the
Network-Type Joint Usage/Research Disaster Medical Science of
Hiroshima University, Nagasaki University, and Fukushima Medical
University.
Glossary
Abbreviations
Abbreviations:
|
Abs
|
antibodies
|
|
CAFs
|
cancer-associated fibroblast cells
|
|
CT26 cells
|
colon carcinoma cells
|
|
DAMPs
|
damage-associated molecular
patterns
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
FBS
|
fetal bovine serum
|
|
FCM
|
flow cytometry
|
|
Foxp3
|
forkhead box transcription factor
P3
|
|
IFN
|
interferon
|
|
IL
|
interleukin
|
|
IL-2Rα-chain
|
interleukin-2 receptor α chain
|
|
i.v.
|
intravenously
|
|
MDSCs
|
myeloid derived suppressor cells
|
|
RPMI
|
Roswell Park Memorial Institute
|
|
RT
|
room temperature
|
|
SEM
|
standard error of the mean
|
|
s.c.
|
subcutaneously
|
|
TILs
|
tumor-infiltrating lymphocytes
|
|
Th
|
helper T
|
|
TGF-β
|
transforming growth factor β
|
|
Tr1
|
type 1 regulatory T cells
|
|
Tregs
|
regulatory T cells
|
References
|
1
|
Liu Y, O'Leary CE, Wang LS, Bhatti TR, Dai
N, Kapoor V, Liu P, Mei J, Guo L, Oliver PM, et al: CD11b+Ly6G+
cells inhibit tumor growth by suppressing IL-17 production at early
stages of tumorigenesis. OncoImmunology. 5:e10611752015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ostrand-Rosenberg S and Sinha P:
Myeloid-derived suppressor cells: Linking inflammation and cancer.
J Immunol. 182:4499–4506. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mougiakakos D, Choudhury A, Lladser A,
Kiessling R and Johansson CC: Regulatory T cells in cancer. Adv
Cancer Res. 107:57–117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fichtner-Feigl S, Terabe M, Kitani A,
Young CA, Fuss I, Geissler EK, Schlitt HJ, Berzofsky JA and Strober
W: Restoration of tumor immunosurveillance via targeting of
interleukin-13 receptor-alpha 2. Cancer Res. 68:3467–3475. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nagaraj S, Schrum AG, Cho H-I, Celis E and
Gabrilovich DI: Mechanism of T cell tolerance induced by
myeloid-derived suppressor cells. J Immunol. 184:3106–3116. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alhamarneh O, Agada F, Madden L, Stafford
N and Greenman J: Serum IL10 and circulating CD4(+) CD25(high)
regulatory T cell numbers as predictors of clinical outcome and
survival in patients with head and neck squamous cell carcinoma.
Head Neck. 33:415–423. 2011.PubMed/NCBI
|
|
7
|
Shevach EM: Regulatory T cells in
autoimmmunity. Annu Rev Immunol. 18:423–449. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen S, Akbar SMF, Abe M, Hiasa Y and Onji
M: Immuno-suppressive functions of hepatic myeloid-derived
suppressor cells of normal mice and in a murine model of chronic
hepatitis B virus. Clin Exp Immunol. 166:134–142. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu J, Du W, Yan F, Wang Y, Li H, Cao S, Yu
W, Shen C, Liu J and Ren X: Myeloid-derived suppressor cells
suppress antitumor immune responses through IDO expression and
correlate with lymph node metastasis in patients with breast
cancer. J Immunol. 190:3783–3797. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsiganov EN, Verbina EM, Radaeva TV,
Sosunov VV, Kosmiadi GA, Nikitina IY and Lyadova IV: Gr-1dimCD11b+
immature myeloid-derived suppressor cells but not neutrophils are
markers of lethal tuberculosis infection in mice. J Immunol.
192:4718–4727. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Youn J-I, Nagaraj S, Collazo M and
Gabrilovich DI: Subsets of myeloid-derived suppressor cells in
tumor-bearing mice. J Immunol. 181:5791–5802. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rutkowski MR, Stephen TL, Svoronos N,
Allegrezza MJ, Tesone AJ, Perales-Puchalt A, Brencicova E,
Escovar-Fadul X, Nguyen JM, Cadungog MG, et al: Microbially driven
TLR5-dependent signaling governs distal malignant progression
through tumor-promoting inflammation. Cancer Cell. 27:27–40. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang JC, Kundra A, Andrei M, Baptiste S,
Chen C, Wong C and Sindhu H: Myeloid-derived suppressor cells in
patients with myeloproliferative neoplasm. Leuk Res. 43:39–43.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stiff A, Trikha P, Wesolowski R, Kendra K,
Hsu V, Uppati S, McMichael E, Duggan M, Campbell A, Keller K, et
al: Myeloid-derived suppressor cells express Bruton's tyrosine
kinase and can be depleted in tumor-bearing hosts by ibrutinib
treatment. Cancer Res. 76:2125–2136. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Highfill SL, Rodriguez PC, Zhou Q, Goetz
CA, Koehn BH, Veenstra R, Taylor PA, Panoskaltsis-Mortari A, Serody
JS, Munn DH, et al: Bone marrow myeloid-derived suppressor cells
(MDSCs) inhibit graft-versus-host disease (GVHD) via an
arginase-1-dependent mechanism that is up-regulated by
interleukin-13. Blood. 116:5738–5747. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pesu M, Watford WT, Wei L, Xu L, Fuss I,
Strober W, Andersson J, Shevach EM, Quezado M, Bouladoux N, et al:
T-cell-expressed proprotein convertase furin is essential for
maintenance of peripheral immune tolerance. Nature. 455:246–250.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu L, Kitani A, Stuelten C, McGrady G,
Fuss I and Strober W: Positive and negative transcriptional
regulation of the Foxp3 gene is mediated by access and binding of
the Smad3 protein to enhancer I. Immunity. 33:313–325. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alhamarneh O, Amarnath SMP, Stafford ND
and Greenman J: Regulatory T cells: What role do they play in
antitumor immunity in patients with head and neck cancer? Head
Neck. 30:251–261. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Y, Zhang P, Li J, Kulkarni AB,
Perruche S and Chen W: A critical function for TGF-beta signaling
in the development of natural CD4+CD25+Foxp3+ regulatory T cells.
Nat Immunol. 9:632–640. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gabitass RF, Annels NE, Stocken DD, Pandha
HA and Middleton GW: Elevated myeloid-derived suppressor cells in
pancreatic, esophageal and gastric cancer are an independent
prognostic factor and are associated with significant elevation of
the Th2 cytokine interleukin-13. Cancer Immunol Immunother.
60:1419–1430. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamazaki T, Masuda J, Omori T, Usui R,
Akiyama H and Maru Y: EphA1 interacts with integrin-linked kinase
and regulates cell morphology and motility. J Cell Sci.
122:243–255. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takayama E, Seki S, Ohkawa T, Ami K, Habu
Y, Yamaguchi T, Tadakuma T and Hiraide H: Mouse CD8+ CD122+ T cells
with intermediate TCR increasing with age provide a source of early
IFN-gamma production. J Immunol. 164:5652–5658. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gabrilovich DI and Nagaraj S:
Myeloid-derived suppressor cells as regulators of the immune
system. Nat Rev Immunol. 9:162–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Terabe M, Matsui S, Park J-M, Mamura M,
Noben-Trauth N, Donaldson DD, Chen W, Wahl SM, Ledbetter S, Pratt
B, et al: Transforming growth factor-beta production and myeloid
cells are an effector mechanism through which CD1d-restricted T
cells block cytotoxic T lymphocyte-mediated tumor
immunosurveillance: Abrogation prevents tumor recurrence. J Exp
Med. 198:1741–1752. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Holmgaard RB, Zamarin D, Li Y, Gasmi B,
Munn DH, Allison JP, Merghoub T and Wolchok JD: Tumor-expressed IDO
recruits and activates MDSCs in a Treg-dependent manner. Cell Rep.
13:412–424. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yao Y, Vent-Schmidt J, McGeough MD, Wong
M, Hoffman HM, Steiner TS and Levings MK: Tr1 cells, but not Foxp3+
regulatory T cells, suppress NLRP3 inflammasome activation via an
IL-10-dependent mechanism. J Immunol. 195:488–497. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Redford PS, Murray PJ and O'Garra A: The
role of IL-10 in immune regulation during M. tuberculosis
infection. Mucosal Immunol. 4:261–270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saraiva M and O'Garra A: The regulation of
IL-10 production by immune cells. Nat Rev Immunol. 10:170–181.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Beriou G, Bradshaw EM, Lozano E,
Costantino CM, Hastings WD, Orban T, Elyaman W, Khoury SJ, Kuchroo
VK, Baecher-Allan C, et al: TGF-beta induces IL-9 production from
human Th17 cells. J Immunol. 185:46–54. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao Y, Wu T, Shao S, Shi B and Zhao Y:
Phenotype, development, and biological function of myeloid-derived
suppressor cells. OncoImmunology. 5:e10049832015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Heerboth S, Housman G, Leary M, Longacre
M, Byler S, Lapinska K, Willbanks A and Sarkar S: EMT and tumor
metastasis. Clin Transl Med. 4:62015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li J, Wang Z, Mao K and Guo X: Clinical
significance of serum T helper 1/T helper 2 cytokine shift in
patients with non-small cell lung cancer. Oncol Lett. 8:1682–1686.
2014.PubMed/NCBI
|
|
33
|
Hong C-C, Yao S, McCann SE, Dolnick RY,
Wallace PK, Gong Z, Quan L, Lee KP, Evans SS, Repasky EA, et al:
Pretreatment levels of circulating Th1 and Th2 cytokines, and their
ratios, are associated with ER-negative and triple negative breast
cancers. Breast Cancer Res Treat. 139:477–488. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liang J, Li Y, Liu X, Xu X and Zhao Y:
Relationship between cytokine levels and clinical classification of
gastric cancer. Asian Pac J Cancer Prev. 12:1803–1806.
2011.PubMed/NCBI
|
|
35
|
Seledtsov VI, Goncharov AG and Seledtsova
GV: Clinically feasible approaches to potentiating cancer
cell-based immunotherapies. Hum Vaccin Immunother. 11:851–869.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sato M, Goto S, Kaneko R, Ito M, Sato S
and Takeuchi S: Impaired production of Th1 cytokines and increased
frequency of Th2 subsets in PBMC from advanced cancer patients.
Anticancer Res. 18D:3951–3955. 1998.
|
|
37
|
Szaflarska A, Szczepanik A, Siedlar M,
Czupryna A, Sierzega M, Popiela T and Zembala M: Preoperative
plasma level of IL-10 but not of proinflammatory cytokines is an
independent prognostic factor in patients with gastric cancer.
Anticancer Res. 29:5005–5012. 2009.PubMed/NCBI
|
|
38
|
Kozłowski L, Zakrzewska I, Tokajuk P and
Wojtukiewicz MZ: Concentration of interleukin-6 (IL-6),
interleukin-8 (IL-8) and interleukin-10 (IL-10) in blood serum of
breast cancer patients. Rocz Akad Med Bialymst. 48:82–84.
2003.PubMed/NCBI
|
|
39
|
Asadullah K, Sterry W and Volk HD:
Interleukin-10 therapy - review of a new approach. Pharmacol Rev.
55:241–269. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Habu Y, Seki S, Takayama E, Ohkawa T,
Koike Y, Ami K, Majima T and Hiraide H: The mechanism of a
defective IFN-γ response to bacterial toxins in an atopic
dermatitis model, NC/Nga mice, and the therapeutic effect of IFN-γ,
IL-12, or IL-18 on dermatitis. J Immunol. 166:5439–5447. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Noelle RJ and Nowak EC: Cellular sources
and immune functions of interleukin-9. Nat Rev Immunol. 10:683–687.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lu Y, Hong S, Li H, Park J, Hong B, Wang
L, Zheng Y, Liu Z, Xu J, He J, et al: Th9 cells promote antitumor
immune responses in vivo. J Clin Invest. 122:4160–4171. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huang Y, Cao Y, Zhang S and Gao F:
Association between low expression levels of interleukin-9 and
colon cancer progression. Exp Ther Med. 10:942–946. 2015.PubMed/NCBI
|