Introduction
Epithelial ovarian cancer (EOC), because of the late
diagnosis and progressive development of drug resistance, remains
the most lethal gynecologic cancer (1). Despite diverse clinical treatments
that have been put into practice, EOC is still the fifth most
common cause of malignant death. Hence, elucidating the molecular
and cellular mechanisms of EOC tumorigenesis is essential for
effective early diagnosis and target-based therapies.
The tumor microenvironment plays a critical role in
cancer initiation, maintenance and progression (2,3). The
tumor microenvironment is a complex network. It contains secreted
factors, exosomes, immune cells, fibroblasts, and mesenchymal stem
cells (3). Tumor development and
progression are greatly influenced by the modulation of innate and
adaptive immune responses to cancer. Through an inflammatory
response, peripheral monocytes are recruited to the tumor (4). Then, the monocytes are reprogrammed by
molecular factors in the tumor microenvironment and obtain specific
tumor-associated macrophage (TAM) properties (5). Among the infiltrating leukocytes, TAMs
have an abundant population (6).
TAMs can regulate the tumor microenvironment via initiating
fibrosis, suppressing immune detection and promoting angiogenesis
(7). According to different
polarizations, macrophages can be separated into two phenotypic
differentiations: classically activated (M1) and alternatively
activated (M2) macrophages (8). M1
macrophages are usually considered pro-inflammatory and cytotoxic
due to producing interleukin (IL)-12, tumor necrosis factor-α and
reactive oxygen intermediates (9).
In contrast, M2 macrophages are anti-inflammatory and promote wound
healing through secreting transforming growth factor-β, IL-6 and
arginase-1 (10). M1 macrophages
are generally referred to as tumor suppressive, and M2 macrophages
are considered tumor promoting (11). TAMs are mainly constituted by M2
phenotypes (12). In various tumor
types, the density of M2 macrophages within tumors is negatively
correlated with the survival rate of patients and positively
facilitates tumorigenesis (11).
Additionally, an ovarian cancer clinical analysis also demonstrated
that a high CD163 (the M2 marker) level is associated with poor
clinical outcomes (13).
Nevertheless, the details of the mechanism by which macrophage
polarization occurs during cancer progression are poorly
understood.
Exosomes are nanometric phospholipid
bilayer-enclosed vesicles (30–100 nm) that can be secreted by
nearly all kinds of cells into the extracellular microenvironment.
Most recent cancer research has suggested that exosomes are crucial
mediators in intercellular communication during tumor progression
(14). Secreted exosomes from
cancer cells can deliver a broad set of biological contents to
nearby or distant recipient cells, including TAMs, T cells,
dendritic cells (DCs), fibroblasts and epithelial cells (15). Tumor-derived exosomes (TEXs) carry
genetic lipids, proteins, mRNAs, miRNAs, and DNA segments to
regulate cancer immunity (16). In
ovarian cancer, TEXs can inhibit dendritic cell maturation, promote
immunosuppressive myeloid-derived suppressor cell (MDSC)
differentiation and suppress tumor-reactive T-cells (17).
Hypoxia is a hallmark of solid tumors. Under hypoxic
conditions, tumor cells exhibit increased aggressiveness and
dissemination (18–20). In breast cancer, hypoxia can enhance
exosome release (21). Berchem and
colleagues found that high levels of miR-23a (compared with
normoxic tumor cells) in hypoxic tumor-derived microvesicles
inhibit natural killer (NK) cell function (22). Moreover, Ling et al
demonstrated that there are 108 miRNAs differentially expressed in
normoxic and hypoxic oral squamous cell carcinoma-derived exosomes.
Furthermore, miR-21, one of the significantly reduced miRNAs,
elicited a prometastatic phenotype (23). However, the relationship between
hypoxic TEX and TAM polarization in ovarian cancer is unclear.
Thus, we hypothesized that TEXs transported from hypoxic ovarian
cancer cells to surrounding macrophages can induce M2 phenotype
polarization via miRNAs and then enhance ovarian cancer
pathology.
Materials and methods
Ascites samples
With approval by the Ethics Committee of Shanghai
First Maternity and Infant Hospital, peritoneal fluids from three
patients with benign ovarian diseases and ascites from three
pre-therapy EOC patients were collected from Shanghai First
Maternity and Infant Hospital, Tongji University (Shanghai, China).
All the patients gave informed consent. The total exosomes in
peritoneal fluids and ascites were isolated by using ExoQuick
exosome precipitation solution reagent according to the
manufacturer's instructions (SBI System Biosciences).
Exosome extraction, labeling and
tracking
We used the total exosome isolation reagent (from
cell culture medium) (Invitrogen, CA, USA) to obtain purified
exosomes from exosome-free culture medium of SKOV3. After
centrifuging the medium at 2,500 rpm for 30 min, we retrieved the
cell culture supernatant and mixed it with the total exosome
isolation reagent. Then, after incubation at 4°C overnight,
exosomes were centrifuged at 10,000 × g for 1 h at 4°C and labeled
with D384 (Invitrogen), a phospholipid membrane dye (red stain).
Labeled exosomes were then added to DAPI-stained (blue stain)
macrophages. After incubation at 37°C for 2 h, macrophages were
observed using a Leica DM-LB confocal microscope.
Electron microscopy
Exosome pellets resuspended in PBS buffer were
dropped onto a carbon-coated copper electron microscope grid, as
described previously (24). We used
a J Tecnai G2 F20 ST transmission electron microscope to observe
the exosomes from cell culture medium, peritoneal fluids and
ascites.
Cell culture and hypoxia
treatment
The human EOC cell line SKOV3 and the monocyte cell
line U937 were obtained from Fuheng Bio (Shanghai, China). Cells
were cultured using RPMI-1640 medium (Invitrogen) with 10% FBS
(Invitrogen). The cell culture medium was ultracentrifuged at
100,000 × g for 20 h to obtain exosome-free medium. To simulate the
hypoxic growing conditions of a solid tumor, SKOV3 cells were
cultured under 1% O2 (hypoxic) conditions, balanced with
N2 in an O2/CO2 incubator (Sanyo).
The control group was treated in 20% O2 (normoxic)
conditions. U937 cells (1×106) were exposed to 100 ng/ml
PMA (Sigma-Aldrich, St. Louis, MO, USA) for 24 h to induce U937
differentiation into macrophages.
miRNA transfection
Macrophages were seeded on 60-mm dishes and were
transfected with miR-negative control and miR-940 mimic
(GenePharma, Shanghai, China) using HiperFect transfection reagent
according to the manufacturer's instructions (Qiagen GmbH, Hilden,
Germany). After 48 h, transfected macrophages were washed with PBS,
and the cell culture media of macrophages were harvested 3 days
later.
RNA preparation and qRT-PCR
Total RNAs from exosomes were extracted using
RA808A-1 (SBI System Biosciences) according to the manufacturer's
instructions, and TRIzol (Invitrogen) was used to extract cellular
RNAs. We used miScript Reverse Transcription kit to
reverse-transcribe miRNA to cDNA and analyzed quantitative PCR of
miRNAs using miScript SYBR Green PCR kit, according to the
manufacturer's instructions (Qiagen GmbH). Then, we used the
2−∆∆CT method to calculate gene expression. U6 was used
to normalize the miRNA results. The primer sequences are as
follows: miR-940, 5′-AAGGCAGGGCCCCCGCTCCCC-3′; U6,
5′-CAAGGATGACACGCAAATTCG-3′.
Western blot analysis
Total proteins lysed from exosomes and macrophages,
treated with normoxic and hypoxic exosomes (100 µg/ml) or
miR-negative control and miR-940 mimic for 48–96 h, were separated
on a 10% SDS PAGE gel. The gels were then transferred to
polyvinylidenedifluoride membranes (Millipore). After blocking with
7% non-fat milk for 2 h, membranes were incubated with rabbit
anti-CD63 (1:1,000, Santa Cruz Biotechnology), mouse anti-CD81
(1:1,000, Santa Cruz Biotechnology), rabbit anti-GAPDH (1:1,000,
Cell Signaling Technology), mouse anti-CD163 (1:200, Santa Cruz
Biotechnology) or mouse anti-CD206 (1:100, Santa Cruz
Biotechnology) at 4°C overnight. Anti-mouse or anti-rabbit IgG
(1:2,000, Cell Signaling Technology) was used as the secondary
antibody.
Migration assay
For the migration assay, 1.5×105 SKOV3
cells were seeded in the top chamber of Transwell chambers (Corning
Inc., USA) with 8-µm inserts. The cell culture media from miR-940
mimic-transfected macrophages or miR-negative control-transfected
macrophages were added in the bottom chamber. After 48–72 h of
incubation, cells that migrated through the membrane were fixed
with methanol and stained with crystal violet 1%. Cells were
counted in 4 random fields.
MTS proliferation assay
SKOV3 cells were seeded in 96-well plates
(2×103 cells/well) and cultured with 50 µl
miR-940-transfected macrophage culture medium mixed with 50 µl
ordinary medium for 48 h. Cells cultured with 50 µl miR-negative
control-transfected macrophage culture medium and 50 µl ordinary
medium were also seeded as a negative control. MTS solution reagent
(20 µl) (Promega Biosciences, CA, USA) was added to each well.
After 2 h at 37°C with 5% CO2 in incubator, cell
proliferation was measured through a 96-well plate reader.
Statistical analysis
Data are presented as the mean ± standard error of
the mean (SEM) for at least 3 independent experiments. Student's
t-test or the Mann-Whitney U test was used to analyze the
significant differences between groups. SPSS 19.0 was applied to
perform statistical analyses. p<0.05 was considered
statistically significant.
Results
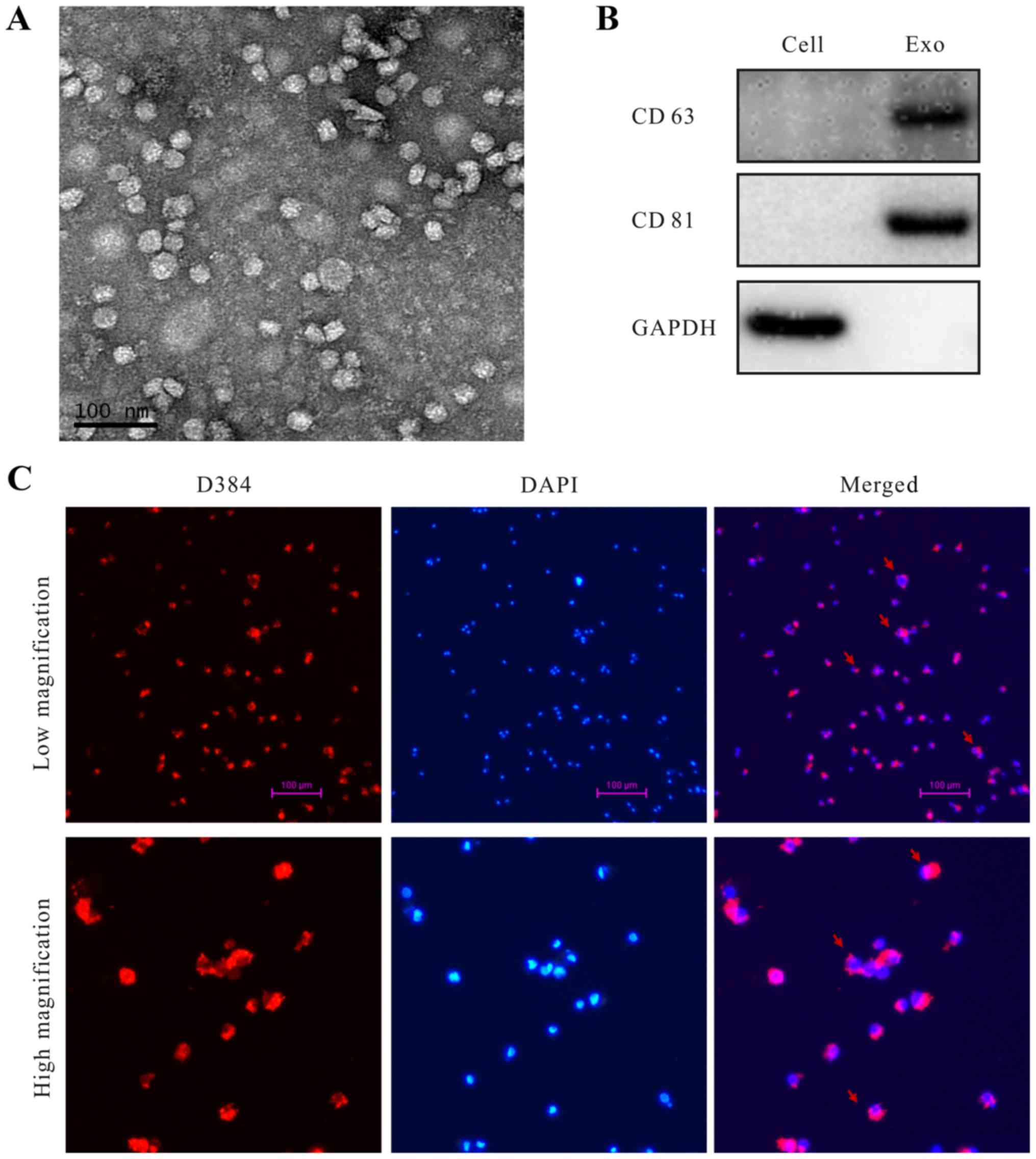
Identification of exosomes secreted by
EOC cells and exosomes taken by macrophages
Exosomes derived from SKOV3 cells in the cell
culture supernatant were initially purified by using a total
exosome isolation reagent. The extracted exosomes were examined
using transmission electron microscopy (TEM), which revealed that
the specific exosome morphology and size ranged from 30 to 80 nm in
diameter (Fig. 1A). The known
exosome markers CD63 and CD81 were further analyzed by western
blotting (Fig. 1B). Abundant
biologic information was packaged into the exosomes and
internalized by target cells (25).
We also found that the TEXs were taken up by macrophages. Exosomes
were labeled with D384 (red stain), and DAPI (blue stain) was used
to show the nuclear structure of macrophages. Fluorescence
microscopy was used to show that exosomes were located in the
cytoplasm of macrophages (Fig.
1C).
Hypoxia induces overexpression of
miR-940 both in EOC cells and EOC-derived exosomes, and exosomes
isolated from ascites of EOC patients have high expression levels
of miR-940
miRNAs in exosomes have been shown to play an
important role in tumor progression (16). The expression levels of miRNAs vary
by cell type and condition. Thus, in referring to the literature,
we found that the overexpressions of miR-940 in pancreatic
carcinoma (26) and gastric cancer
(27) were correlated with tumor
progression. However, the role of miR-940 in ovarian cancer is
unknown. We hypothesized that miR-940 may play an important role in
macrophage polarization in EOC. To confirm this hypothesis, we
first studied the relationship between miR-940 and hypoxia.
The SKOV3 cells were cultured under 20%
O2 (normoxia) or 1% O2 (hypoxia). Exosomes
were isolated, and total miRNA was extracted after 72 h. The
qRT-PCR results show that the expression of miR-940 is
significantly higher in hypoxic cells and exosomes compared with in
the normoxic group (Fig. 2A).
Additionally, we explored the degree of miR-940 expression in
exosomes isolated from ascites of EOC patients (n=3). Compared with
patients with benign ovarian diseases, miR-940 had a higher
expression level in exosomes isolated from ascites of the EOC
patients (Fig. 2B).
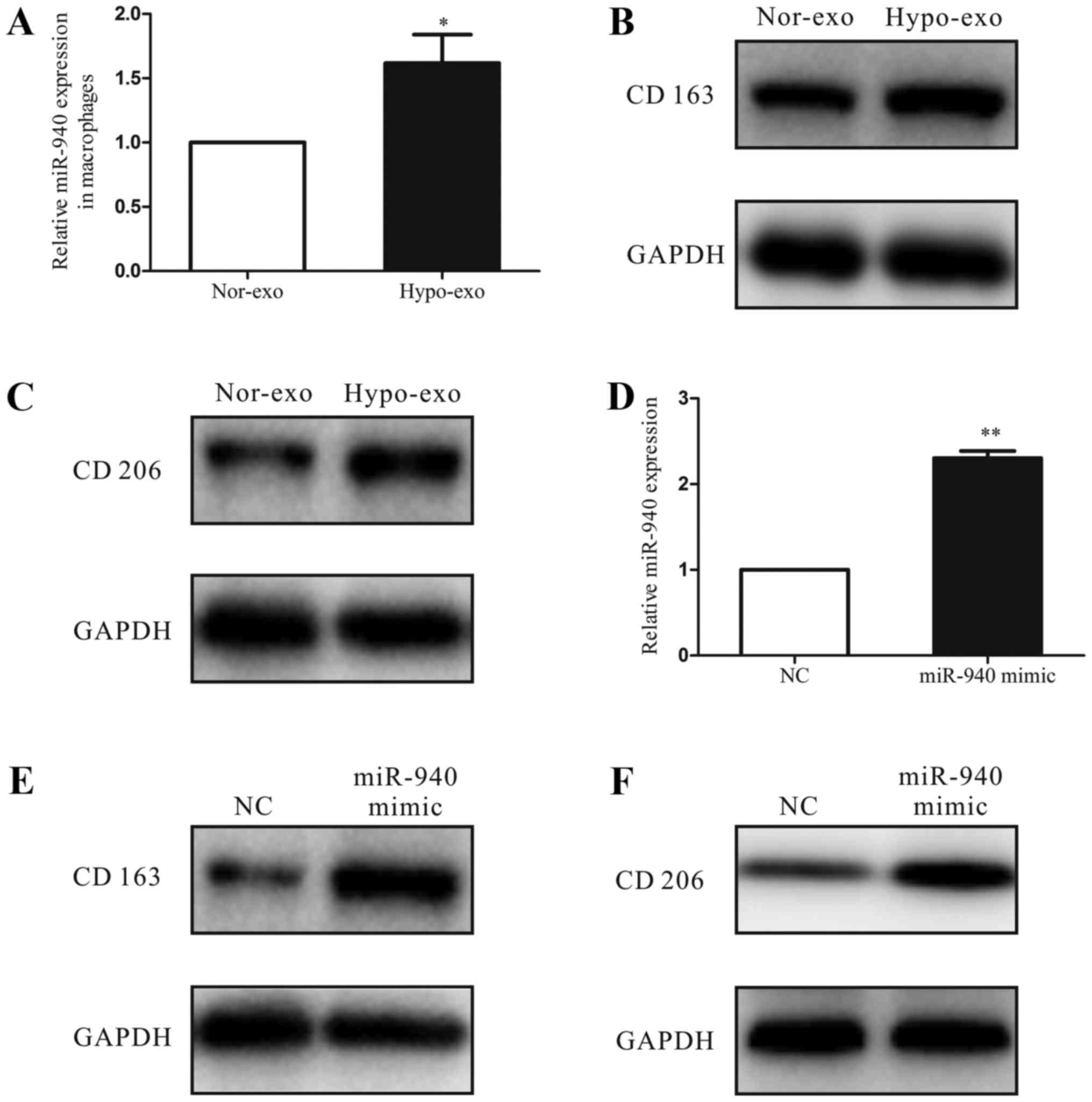
Increased miR-940 expression in
macrophages induces M2 polarization
To investigate the function of hypoxic exosomes
carrying abundant miR-940 in the tumor microenvironment, we
co-cultured the unpolarized macrophages with normoxic exosomes or
hypoxic exosomes isolated from SKOV3 cells. After 3 days, we
analyzed the miRNA expression of macrophages and found that the
miR-940 expression was much higher in the hypoxic exosome-educated
macrophages than in the normoxic group (Fig. 3A). Moreover, compared with the
normoxic exosome macrophages, the hypoxic exosome macrophages had
higher expression levels of the M2-type markers CD163 and CD206
(Fig. 3B and C). Then, to evaluate
the relationship between the M2 polarization of macrophages and the
increased miR-940 expression in hypoxic exosomes, we transfected
unpolarized macrophages with either the miR-negative control or
miR-940 mimic (Fig. 3D). After
transfection, high expression of miR-940 in macrophages enhanced
the CD163 and CD206 protein expressions remarkably (Fig. 3E and F).
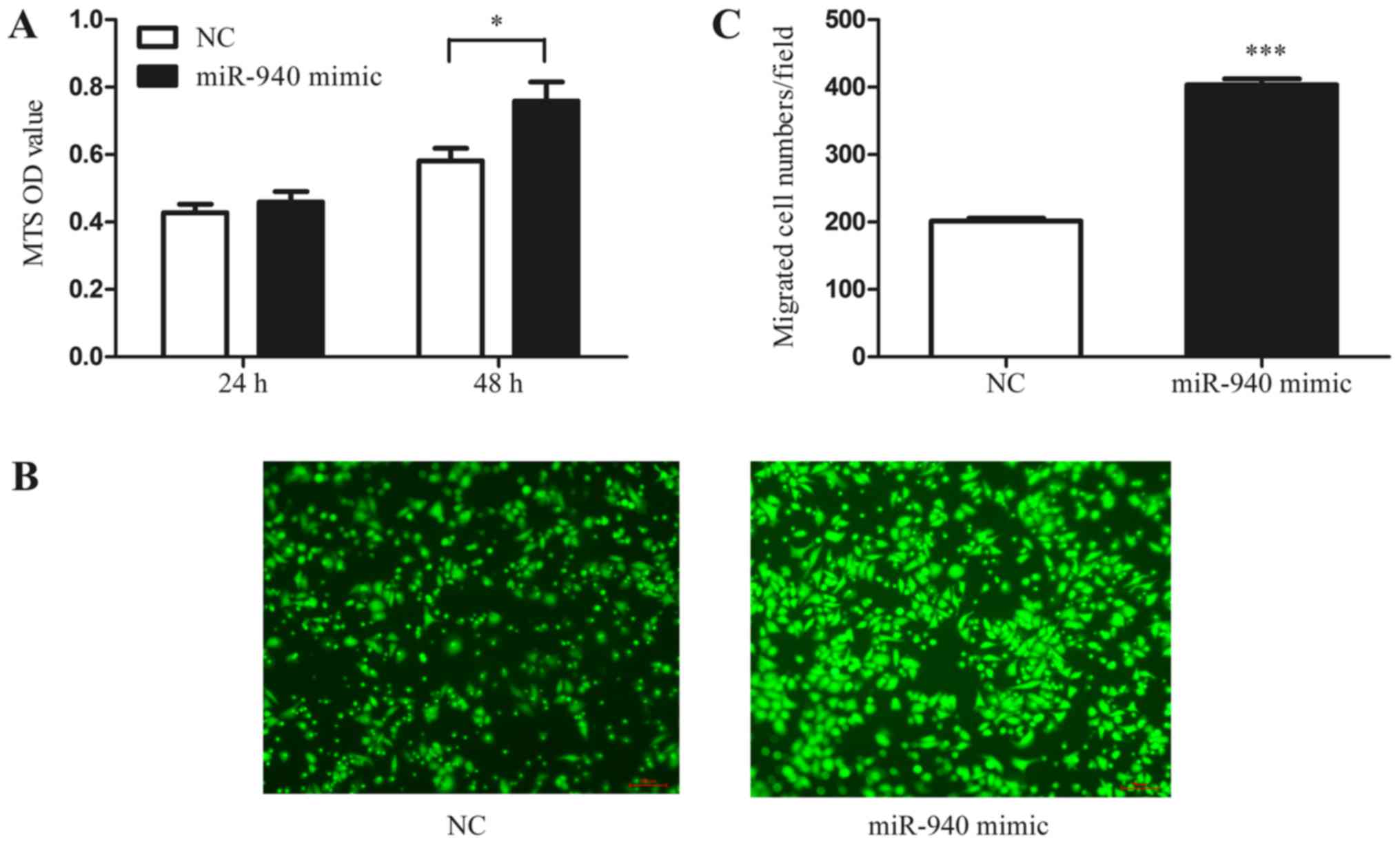
miR-940-induced M2 macrophages promote
EOC cell proliferation and migration
In addition, to further investigate whether the
miR-940-induced M2-phenotype macrophages have the characteristic
function of tumor promotion, we collected the conditioned medium of
miR-940 mimic-transfected macrophages. Normoxic SKOV3 cells were
treated with the conditioned medium, and the MTS proliferation
assay was performed. As shown in the results, the conditioned
medium from miR-940-induced M2-phenotype macrophages significantly
promoted the proliferation of SKOV3 cells (Fig. 4A). Furthermore, the Transwell assay
also indicated that miR-940-induced M2-phenotype macrophages
promote SKOV3 cell migration (Fig. 4B
and C).
Discussion
In the present study, we showed that the exosomes
secreted by EOC cells under hypoxic conditions in vitro have
a higher expression of miR-940. After co-culturing the hypoxic
exosomes with unpolarized macrophages, we found that the highly
expressed exosomal miR-940 activates macrophages to a TAM-like
phenotype. Then, we collected the conditioned medium of activated
TAM-like macrophages and demonstrated that the conditioned medium
could reversely promote EOC cell proliferation and migration. To
our knowledge, this is the first study to suggest that exosomes
function as a bridge between hypoxic EOC cells and macrophages in
the tumor microenvironment by delivering miR-940.
Tumor growth has increasingly been considered a
complex system that includes endothelial cells, fibroblasts,
myeloid-derived suppressor cells and macrophages (15). The interaction between cancer cells
and TAMs, one of the most abundant immune-related stromal cells,
plays a crucial role in cancer development (28). The exchange of contents between
cells mediated by cell-derived exosomes functions as a bridge
between cancer cells and macrophages. To demonstrate whether or not
EOC cells can induce macrophage polarization through secreting
exosomes, we initially demonstrated the specificity of isolated
exosomes (Fig. 1A and B). In
Fig. 1C, we show that the
EOC-derived exosomes could be taken by macrophages. This indicates
that, by secreting exosomes, EOC cells may participate in
regulating the biologic functions of macrophages that are recruited
to the tumor microenvironment.
Recent studies have found that hypoxia is involved
in the malignant evolution of cancers. In breast cancer, hypoxia
has been postulated to promote exosome release (21), while in ovarian cancer, hypoxia
increases tunneling nanotube formation, which stimulates drug
resistance (29). Hypoxia induces
macrophage polarization in glioblastoma and lung cancer (30,31).
However, the role of hypoxia in exosomes and TAM-like macrophage
polarization in epithelial ovarian cancer is not clear. We analyzed
the expression of miRNAs under normoxic and hypoxic conditions. We
found that SKOV3 cells under hypoxic conditions express much more
miR-940 than the cells under normoxic conditions, as well as in
exosomes (Fig. 2A). As the presence
of ascites has been directly associated with peritoneal metastasis
and poor prognosis of EOC patients (32), we also isolated exosomes from
ascites of EOC patients and demonstrated that miR-940 has a much
higher expression level compared with exosomes of peritoneal fluids
from patients with benign ovarian diseases (Fig. 2B). These results imply that the
increased expression of miR-940 in exosomes induced by hypoxia may
play an important role in EOC progression.
It has been generally recognized that exosomes
participate in tumor progression by regulating the immune function
of immune cells in the tumor microenvironment (14–17). A
recent study of melanomas found that tumor-secreted exosomes
suppress the proliferation of T cells (33). Another study on gastric cancer
suggested that cancer cell-derived exosomes activate macrophages to
increase pro-inflammatory factor expression, which promotes cancer
progression (34). Our previous
study also demonstrated that EOC-secreted exosomal miR-222-3p
induces TAM polarization (24).
However, the aberrant expression of exosomal miR-940 induced by
hypoxia during macrophage polarization is not well understood.
Thus, in order to investigate the function of hypoxic exosomes in
macrophage polarization, we co-cultured the unpolarized macrophages
with normoxic exosomes or hypoxic exosomes. We found that miR-940
is greatly increased in macrophages compared with the normoxic
group (Fig. 3A). Moreover, hypoxic
exosomes induced macrophages to express higher levels of the
M2-type markers CD163 and CD206 compared to normoxic exosomes.
These data suggested that exosomes could deliver miR-940 to
macrophages and that hypoxic exosomes could induce macrophages to
an M2-like phenotype. The overexpression of miR-940 has been found
to promote tumor progression both in pancreatic carcinoma and
gastric cancer (26,27). However, to our knowledge, the
expression level and biologic function of miR-940 in EOC have not
been reported yet. In this study, we showed that miR-940 is
aberrantly expressed in the exosomes from ascites of EOC patients.
Furthermore, induced by hypoxia, the expression of miR940 in SKOV3
cells and exosomes is increased, while miR-940 could also be
transported to macrophages. Based on these findings, we assume that
highly expressed miR-940 may play an important role in the process
of TAM-like macrophage polarization, which is enhanced by hypoxic
exosomes of SKOV3 cells. We transfected unpolarized macrophages
with an miR-negative control or miR-940 mimic. After 48 h, the high
expression of miR-940 in macrophages enhanced the CD163 and CD206
protein expression remarkably (Fig. 3E
and F). These findings suggest that overexpressed exosomal
miR-940 promotes M2 macrophage polarization.
Many studies have demonstrated that the tumor
promoting function of M2-phenotype macrophages is mediated by
soluble factors (10,11,35).
Here, to further confirmed that the miR-940-induced M2-phenotype
macrophages have a tumor promoting effect, we co-cultured normoxic
SKOV3 cells with miR-940-induced M2 macrophage conditioned medium,
and found that the proliferation and migration abilities of SKOV3
cells were increased compared with the miR-negative control
(Fig. 4).
In conclusion, these data suggest that hypoxia
increases the miR-940 levels in EOC-derived exosomes. These
miR-940-rich tumor-secreted exosomes, which are internalized by
unpolarized macrophages, drive macrophages toward a TAM-like
phenotype. These observations demonstrate that exosomes derived
from tumor cells may function as messengers that transport miR-940
between hypoxic tumor cells and macrophages, and thus remodel the
tumor immune microenvironment of EOC. These findings may provide a
theoretical foundation for a new treatment of EOC through the
modulation of TAMs.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81372787), the Shanghai
Municipal Bureau of Health (no. 20134033), the Shanghai Health
System Joint Research Project (no. 2013ZYJB0201) and the Top 100
Medical Elite in Shanghai (no. XBR2011065).
Glossary
Abbreviations
Abbreviations:
|
EOC
|
epithelial ovarian cancer
|
|
TAM
|
tumor-associated macrophage
|
|
IL
|
interleukin
|
|
TEXs
|
tumor-derived exosomes
|
|
MDSCs
|
myeloid-derived suppressor cells
|
|
SEM
|
standard error of the mean
|
|
TEM
|
transmission electron microscopy
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Casey SC, Li Y, Fan AC and Felsher DW:
Oncogene withdrawal engages the immune system to induce sustained
cancer regression. J Immunother Cancer. 2:242014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kenny PA, Lee GY and Bissell MJ: Targeting
the tumor microenvironment. Front Biosci. 12:3468–3474. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Y and Cao X: The origin and function
of tumor-associated macrophages. Cell Mol Immunol. 12:1–4. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ostuni R, Kratochvill F, Murray PJ and
Natoli G: Macrophages and cancer: From mechanisms to therapeutic
implications. Trends Immunol. 36:229–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ruffell B, Au A, Rugo HS, Esserman LJ,
Hwang ES and Coussens LM: Leukocyte composition of human breast
cancer. Proc Natl Acad Sci USA. 109:2796–2801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kerkar SP and Restifo NP: Cellular
constituents of immune escape within the tumor microenvironment.
Cancer Res. 72:3125–3130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lopes RL, Borges TJ, Zanin RF and Bonorino
C: IL-10 is required for polarization of macrophages to M2-like
phenotype by mycobacterial DnaK (heat shock protein 70). Cytokine.
85:123–129. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ribatti D: Mast cells and macrophages
exert beneficial and detrimental effects on tumor progression and
angiogenesis. Immunol Lett. 152:83–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pal R, Chakraborty B, Nath A, Singh LM,
Ali M, Rahman DS, Ghosh SK, Basu A, Bhattacharya S, Baral R, et al:
Noble metal nanoparticle-induced oxidative stress modulates tumor
associated macrophages (TAMs) from an M2 to M1 phenotype: An in
vitro approach. Int Immunopharmacol. 38:332–341. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lewis CE and Pollard JW: Distinct role of
macrophages in different tumor microenvironments. Cancer Res.
66:605–612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mantovani A and Sica A: Macrophages,
innate immunity and cancer: Balance, tolerance, and diversity. Curr
Opin Immunol. 22:231–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reinartz S, Schumann T, Finkernagel F,
Wortmann A, Jansen JM, Meissner W, Krause M, Schwörer AM, Wagner U,
Müller-Brüsselbach S, et al: Mixed-polarization phenotype of
ascites-associated macrophages in human ovarian carcinoma:
Correlation of CD163 expression, cytokine levels and early relapse.
Int J Cancer. 134:32–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baroni S, Romero-Cordoba S, Plantamura I,
Dugo M, D'Ippolito E, Cataldo A, Cosentino G, Angeloni V, Rossini
A, Daidone MG, et al: Exosome-mediated delivery of miR-9 induces
cancer-associated fibroblast-like properties in human breast
fibroblasts. Cell Death Dis. 7:e23122016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luo Z, Wang Q, Lau WB, Lau B, Xu L, Zhao
L, Yang H, Feng M, Xuan Y, Yang Y, et al: Tumor microenvironment:
The culprit for ovarian cancer metastasis? Cancer Lett.
377:174–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saleem SN and Abdel-Mageed AB:
Tumor-derived exosomes in oncogenic reprogramming and cancer
progression. Cell Mol Life Sci. 72:1–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nawaz M, Fatima F, Nazarenko I, Ekström K,
Murtaza I, Anees M, Sultan A, Neder L, Camussi G, Valadi H, et al:
Extracellular vesicles in ovarian cancer: Applications to tumor
biology, immunotherapy and biomarker discovery. Expert Rev
Proteomics. 13:395–409. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Krtolica A and Ludlow JW: Hypoxia arrests
ovarian carcinoma cell cycle progression, but invasion is
unaffected. Cancer Res. 56:1168–1173. 1996.PubMed/NCBI
|
|
19
|
Krishnamachary B, Berg-Dixon S, Kelly B,
Agani F, Feldser D, Ferreira G, Iyer N, LaRusch J, Pak B, Taghavi
P, et al: Regulation of colon carcinoma cell invasion by
hypoxia-inducible factor 1. Cancer Res. 63:1138–1143.
2003.PubMed/NCBI
|
|
20
|
Yoon SO, Shin S and Mercurio AM: Hypoxia
stimulates carcinoma invasion by stabilizing microtubules and
promoting the Rab11 trafficking of the alpha6beta4 integrin. Cancer
Res. 65:2761–2769. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
King HW, Michael MZ and Gleadle JM:
Hypoxic enhancement of exosome release by breast cancer cells. BMC
Cancer. 12:4212012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Berchem G, Noman MZ, Bosseler M, Paggetti
J, Baconnais S, Le Cam E, Nanbakhsh A, Moussay E, Mami-Chouaib F,
Janji B, et al: Hypoxic tumor-derived microvesicles negatively
regulate NK cell function by a mechanism involving TGF-β and miR23a
transfer. OncoImmunology. 5:e10629682015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li L, Li C, Wang S, Wang Z, Jiang J, Wang
W, Li X, Chen J, Liu K, Li C, et al: Exosomes derived from hypoxic
oral squamous cell carcinoma cells deliver miR-21 to normoxic cells
to elicit a prometastatic phenotype. Cancer Res. 76:1770–1780.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ying X, Wu Q, Wu X, Zhu Q and Wang X,
Jiang L, Chen X and Wang X: Epithelial ovarian cancer-secreted
exosomal miR-222-3p induces polarization of tumor-associated
macrophages. Oncotarget. 7:43076–43087. 2016.PubMed/NCBI
|
|
25
|
Montecalvo A, Larregina AT, Shufesky WJ,
Stolz DB, Sullivan ML, Karlsson JM, Baty CJ, Gibson GA, Erdos G,
Wang Z, et al: Mechanism of transfer of functional microRNAs
between mouse dendritic cells via exosomes. Blood. 119:756–766.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang HW, Liu GH, Liu YQ, Zhao HC, Yang Z,
Zhao CL, Zhang XF and Ye H: Over-expression of microRNA-940
promotes cell proliferation by targeting GSK3β and sFRP1 in human
pancreatic carcinoma. Biomed Pharmacother. 83:593–601. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu X, Kwong A, Sihoe A and Chu KM: Plasma
miR-940 may serve as a novel biomarker for gastric cancer. Tumour
Biol. 37:3589–3597. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Su S, Liu Q, Chen J, Chen J, Chen F, He C,
Huang D, Wu W, Lin L, Huang W, et al: A positive feedback loop
between mesenchymal-like cancer cells and macrophages is essential
to breast cancer metastasis. Cancer Cell. 25:605–620. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Desir S, Dickson EL, Vogel RI, Thayanithy
V, Wong P, Teoh D, Geller MA, Steer CJ, Subramanian S and Lou E:
Tunneling nanotube formation is stimulated by hypoxia in ovarian
cancer cells. Oncotarget. 7:43150–43161. 2016.PubMed/NCBI
|
|
30
|
Leblond MM, Gérault AN, Corroyer-Dulmont
A, MacKenzie ET, Petit E, Bernaudin M and Valable S: Hypoxia
induces macrophage polarization and re-education toward an M2
phenotype in U87 and U251 glioblastoma models. OncoImmunology.
5:e10564422015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang J, Cao J, Ma S, Dong R, Meng W, Ying
M, Weng Q, Chen Z, Ma J, Fang Q, et al: Tumor hypoxia enhances
non-small cell lung cancer metastasis by selectively promoting
macrophage M2 polarization through the activation of ERK signaling.
Oncotarget. 5:9664–9677. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Saini U, Naidu S, ElNaggar AC, Bid HK,
Wallbillich JJ, Bixel K, Bolyard C, Suarez AA, Kaur B, Kuppusamy P,
et al: Elevated STAT3 expression in ovarian cancer ascites promotes
invasion and metastasis: A potential therapeutic target. Oncogene.
36:168–181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu Y, Deng W, McGinley EC and DJ II
Klinke: Melanoma exosomes deliver a complex biological payload that
upregulates PTPN11 to suppress T lymphocyte function. Pigment Cell
Melanoma Res. 30:203–218. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu L, Zhang X, Zhang B, Shi H, Yuan X, Sun
Y, Pan Z, Qian H and Xu W: Exosomes derived from gastric cancer
cells activate NF-κB pathway in macrophages to promote cancer
progression. Tumour Biol. 37:12169–12180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Beatson R, Tajadura-Ortega V, Achkova D,
Picco G, Tsourouktsoglou TD, Klausing S, Hillier M, Maher J, Noll
T, Crocker PR, et al: The mucin MUC1 modulates the tumor
immunological microenvironment throughengagement of the lectin
Siglec-9. Nat Immunol. 17:1273–1281. 2016. View Article : Google Scholar : PubMed/NCBI
|