Introduction
Primary treatment for colorectal cancer (CRC) is the
surgical tumor resection, usually followed by adjuvant therapy with
chemotherapeutic agents (1). In
some cases local irradiation is associated with chemotherapy as a
preoperative treatment (2).
Chemotherapy is also the main treatment for that patients with
metastatic diseases, with the fluoropyrimidine 5-fluorouracil, the
hypomethylating agent 5-azacytidine, and the antimicrotubule taxans
such as paclitaxel and docetaxel, as the first line antineoplastic
agents for CRC patients (3).
While surgery and radiotherapy are relatively
precise and used to achieve local control, the cytotoxic
chemotherapies show a systemic effect, which is usually followed by
collateral damage to normal tissues. Indeed, conventional
chemotherapy is based on administration of the maximum tolerated
dose (MTD), which is the highest dose of a medication that induces
tolerable side effects. This schedule induces cell cycle arrest and
apoptosis not only in tumor cells, but also in non-malignant cells,
including those of the immune system. Then, collateral damage
include both myelosuppression and impaired function of dendritic
cells (DCs) and lymphocytes (4). In
contrast, the metronomic or dose-dense chemotherapy works with the
administration of 10–33% of the conventional MTD in a shorter space
of time between applications (daily or weekly). This schedule
induces weaker adverse effects, promotes antiangiogenic effects
(5,6), and activates dendritic cells while
decreasing the activation of Treg cells (7–9).
In addition, our group has previously observed an
immunomodulatory effect when chemotherapeutics are used in ultralow
non-toxic concentration (10,11).
Such immunomodulation includes upregulation of mature DC markers,
enhanced ability to stimulate allogeneic cells (10,12),
and increased synthesis of IL-1β and IL-12 (11). It was also observed that ultralow
concentration of paclitaxel regulates the differentiation of murine
conventional DCs into regulatory DCs (13), as well as prevents the generation of
tolerogenic DCs (14). We also
observed that treatment of HCT-116 tumor cells with
low-concentrations of paclitaxel, is able to induce transcriptional
changes of genes involved in their immunogenicity. These changes
include increased expression of some genes associated with antigen
presentation and tumor immunogenicity such as calmodulin,
proteasomes, heat-shock proteins and cancer antigens (12). These changes in tumor immunogenicity
affected the DC function as can be observed when tumor lysates are
loading to them and improves their ability to induce the generation
of anti-HCT-116 cytotoxic T cells (12).
Previous reports have shown that DCs can be primed
by transferring both total tumor RNA (15) and mRNA (16,17),
and we hypothesize that changes in the expression of these genes
can be transferred to immature DCs by RNA transfection, thereby
inducing them to synthesize tumor proteins or peptides. Since DCs
are able to present peptides associated with both class I and II
molecules, the transfection may improve the presentation of tumor
antigens, generating specific cytotoxic T cell clones. In this
study we tested the chemomodulatory effect of 5-fluorouracil
(5-FU), one of the most used drug for treating CRC patients. There
are no studies reporting the effects of low concentrations of 5-FU
on tumor cells. Then, HCT-116 colorectal adenocarcinoma cells were
pretreated with low concentrations of 5-FU, and their total RNA was
transfected into monocyte-derived immature DCs to sensitize them
for tumor antigens.
Our results show that transfection of DCs with total
tumor RNA improved their ability to stimulate the proliferation of
allogeneic T cells, and enhanced the generation of autologous
cytotoxic T cells. Generation of tumor-specific IFN-γ-producing
cells was also increased when T cells were co-cultured with
RNA-transfected DCs. These results lead us to conclude that
transcriptional changes induced in tumor cells by low concentration
of 5-FU can be transferred to DCs by RNA transfection, loading them
to trigger a specific immune response, reinforcing the idea that
simultaneous use of chemotherapy and immunotherapy can be an
effective anti-CRC treatment.
Materials and methods
Tumor cell lines and cultures
Human colon cancer cells HCT-116, were cultured in
RMPI-1640 medium (Cultilab, Brazil), supplemented with 10% FBS, 2
mM L-glutamine, 1 mM sodium pyruvate, 0.1 mM non-essential amino
acids, 10 mM HEPES and 0.1 mg/ml gentamicin (complete culture
medium) at 37°C under 5% CO2 tension. After 24 h of
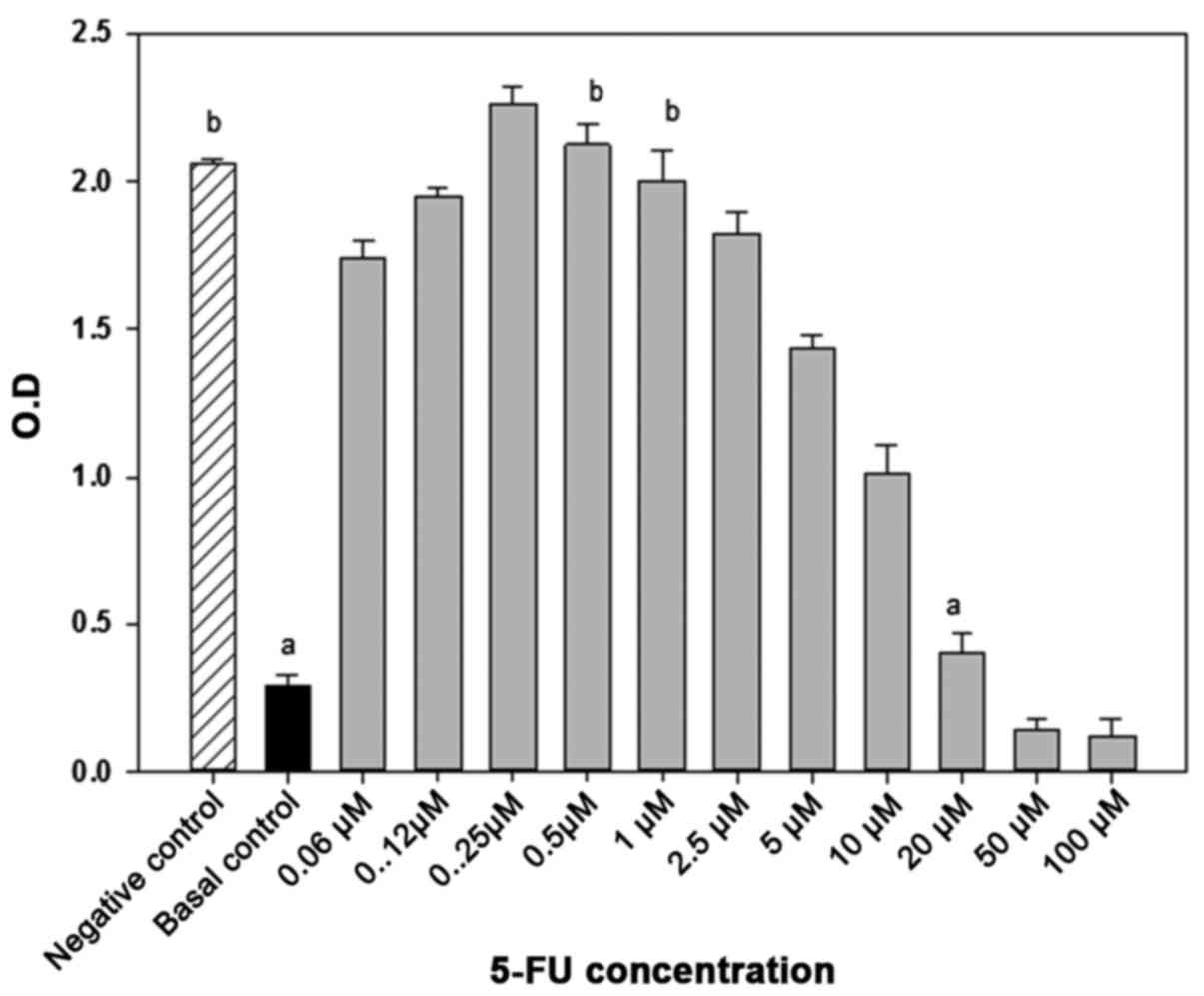
culture, cells were treated with the minimum effective (MEC = 20
µM) or non-cytotoxic (NTC= 1 µM) concentrations of 5-fluorouracil
(5-FU) (Eurofarma, São Paulo, Brazil) for 48 h, as previously
determined by MTT-based cytotoxicity assay. Briefly,
2×103 cells were distributed on 96 flat-bottomed well
culture plates and incubated with variable concentrations of 5-FU
(ranging from 0.06 to 100 µM). MEC refers to the lowest drug
concentration able to stop the tumor cell growth, while NTC refers
to a concentration that does not affect cell growth (Fig. 1).
Institutional Ethics Committee
Statement
All the procedures involving cells of healthy donors
were done according to the Declaration of Helsinki and approved by
the local Ethics Committee at the School of Medicine of Botucatu
(proc.043/2010-CEP).
Tumor cell RNA
Drug-treated tumor cells were washed with PBS, and
their total RNA was immediately extracted using RNeasy Mini kit
(Qiagen, Valencia, CA, USA). RNA purity and concentration were
checked by spectrophotometry 260/280 nm (Nanodrop Technologies,
Inc., Wilmington, DE, USA). The RNA concentration was checked by
Qubit (Thermo Fisher Scientific Inc., Waltham, MA, USA) and the
quality was evaluated by Bioanalyzer 2100 (Agilent Technologies,
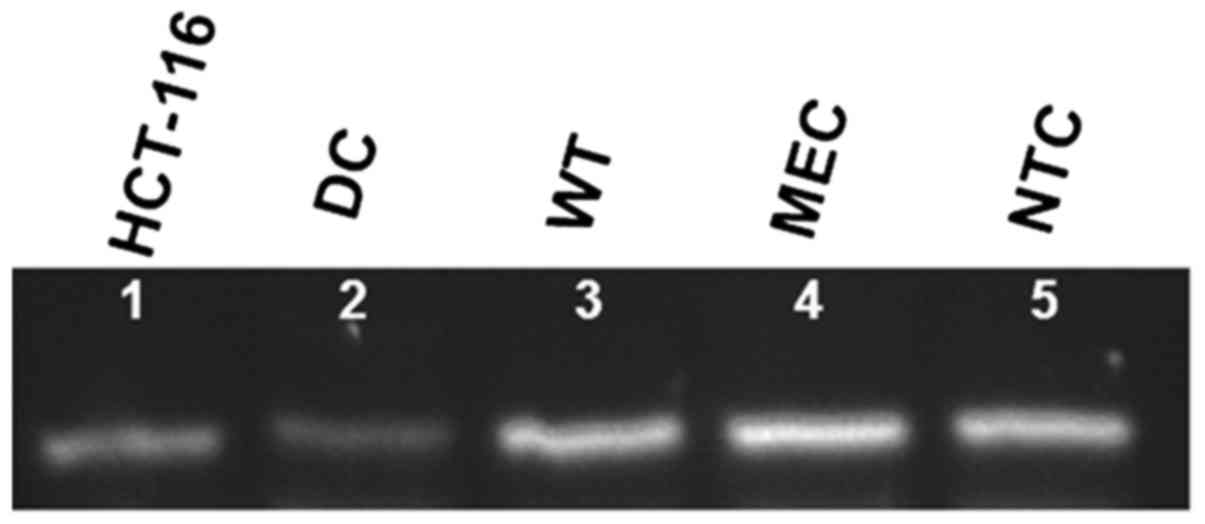
Santa Clara, CA, USA). RNA integrity was confirmed by cDNA
transcription of the EpCAM gene (Fig.
2).
Human monocyte-derived dendritic
cells
DCs were differentiated from peripheral blood
adherent mononuclear cells (PBMC) of healthy donors. PBMC were
obtained by centrifugation on Ficoll-Isopaque gradient, suspended
in AIM-V culture medium (Invitrogen, Carlsbad, CA, USA) and seeded
in 6-well culture plates (5×106/ml). After 1 h at 37°C,
non-adherent cells were removed and adherent monocytes were
cultured in complete culture medium with 80 ng/ml recombinant human
GM-CSF and IL-4 (PeproTech, Rocky Hill, NJ, USA) for 6 days
(18), when they were transfected
with total tumor RNA and kept in culture for additional 24 h.
Immature DCs were submitted to four culture conditions: DCs
(non-transfected immature DCs); WT (DCs transfected with RNA of
untreated tumor cells); MEC (DCs transfected with RNA of tumor
cells pretreated with 20 µM of 5-FU), and NTC (DCs transfected with
RNA of tumor cells pretreated with 1 µM 5-FU). All the procedures
involving cells of healthy donors were done according to the
Declaration of Helsinki and approved by the local Ethics Committee
at the School of Medicine of Botucatu (proc.043/2010-CEP).
DC transfection with tumor total
RNA
Immature DCs were seeded in 6-well plates
(2×105 cell/well) with antibiotic-free and serum-free
fresh RPMI-1640 medium, and cultured for 18 h at 37°C. Then, cells
were transfected with 5 µg of total RNA of tumor cells submitted to
the above mentioned treatments, using 7 µl of DMRIE-C reagent
(Invitrogen), following the manufacturer's instructions.
Transfected cells were maintained in culture for 24 h and harvested
for subsequent assays.
Expression of epithelial cell adhesion
molecule (EpCAM) by transfected cells
In order to confirm the effectiveness of
transcription, total RNA was extracted from transfected DC, and
cDNA was synthesized using SuperScript III Reverse Transcriptase
(Invitrogen), following the manufacturer's instructions.
Transfection of tumor RNA into DCs was evidenced by neo-expression
or increased expression of EpCAM gene by RT-PCR using GoTaq Green
Mix Promega, and EpCAM specific primers (sense: ATCGTCAATGCC
AGTGTACTTCA and antisense: TTTGCTCTTCTCCCA AGTTTTGAG).
Electrophoresis of RT-PCR products was performed in agarose gel
0.8%.
DC phenotyping
For the phenotype analysis, both transfected and
control DCs were incubated with mAbs for 30 min and washed with PBS
containing 0.1% bovine serum albumin (BSA) and 0.1% sodium azide.
DCs were labeled with mAbs for MHC-Class II-PE, CD11c-APC,
CD83-PE-Cy7, CD80-APC-H7 and CD86-FITC (BD Pharmingen, San Jose,
CA, USA). Samples were read in a FACSCanto II cytometer
(Becton-Dickinson, San Jose, CA, USA) and analyzed by the software
FlowJo, version 7.2.4.
Mixed leukocyte reaction (MLR)
Functional activity of transfected DCs was first
evaluated through their ability to stimulate the proliferation of
normal allogeneic T lymphocytes. Transfected DCs from all different
donors were co-cultured with T lymphocytes from the same healthy
donor in flat-bottomed 96-well plates in different DCs: T
proportions (1:1, 1:3, 1:10 and 1:30). The lymphocyte proliferation
was evaluated 4 days later based on the ability of living cells to
transform MTT into formazan crystals, which were further
solubilized with dimethylsulphoxide (DMSO) and measured by
spectrophotometry at 540 nm. Results were expressed as
proliferation index, calculated by the equation [(experimental OD -
lymphocytes OD)/lymphocytes OD], where lymphocytes OD refers to the
formazan reduction by only lymphocyte suspension, whereas
experimental OD refers to the measurement of lymphocytes cultured
with DCs.
Generation of cytolytic T lymphocytes
and antitumor cytotoxicity assay
For the generation of specific antitumor T cells,
transfected DCs were co-cultured with autologous T lymphocyte-rich
suspension in complete culture medium supplemented with IL-7 (5
ng/ml) and IL-2 (40 IU/ml). The culture was pulsed with IL-2 every
three days for 14 days. On day 14, the lymphocytes were harvested,
and evaluated for cytotoxic activity against HCT-116 target
cells.
Lymphocytotoxicity assay was performed with HCT-116
cells, previously cultured in a flat-bottomed 96-well plate, and
dyed with MTT solution. In vitro generated lymphocytes were
put on HCT-116 monolayers (5:1/Ly:HCT-116), and then co-cultured
for 18 h at 37°C under 5% CO2. Next, wells were
carefully washed in order to remove suspended lymphocytes and dead
cell debris. The percentage of surviving target cells was estimated
by measuring the optical density of residual formazan crystals
dissolved in DMSO.
IFN-γ and IL-10 detection
Supernatants of the lymphocytes co-cultured with
tumor cells were collected at the final lymphocyte toxicity assay
and preserved at −80°C for further analysis of in vitro
synthesis of IFN-γ and IL-10 by ELISA, according to the
manufacturer's instructions (R&D Systems, Minneapolis, MN,
USA).
Statistical analysis
Homogeneity of variance was accessed by the Bartlett
test (19), and data were analyzed
by analysis of variance (ANOVA) followed by the Tukey-Kramer test
for multiple comparisons. Differences were considered significant
when the error probability was <5% (p<0.05).
Results
Transfected DCs show higher levels of
EpCAM expression
Epithelial cell adhesion molecule (EpCAM) is a
transmembrane protein with dual function: cell adhesion and
mitogenic signaling. It is normally expressed in the basal membrane
layers and is overexpressed in various carcinomas and
tumor-initiating cells (i.e., cancer stem cells) (20). Fig.
2 illustrates the constitutive expression of EpCAM gene in
HCT-116 cells, and the increased expression in all RNA-transfected
DC, whereas non-transfected control cells show a very light or
absent expression. Since EpCAM can also be expressed by normal
cells, we considered it the housekeeping gene. The transfection
process was not able to increase its expression (data not
shown).
Effect of transfection on DC
differentiation and maturation
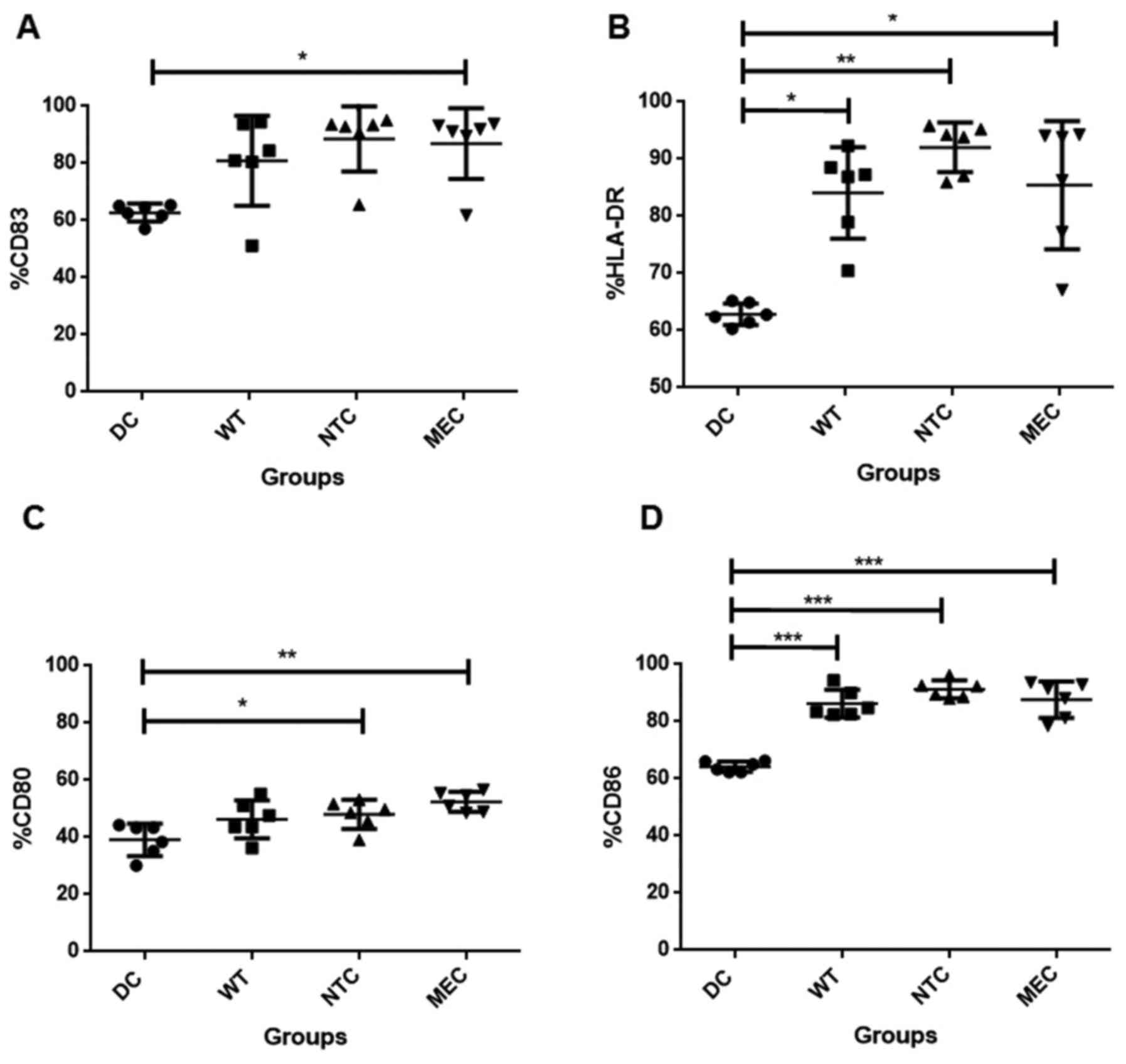
The next step was to analyse the influence of the
transfection on the expression of DC differentiation and maturation
markers by flow cytometry. Fig. 3
shows that transfection of these cells with tumor RNA increased the
frequency of CD83+, HLA-DR+, CD80+
and CD86+ (N=6). Transfection with RNA of untreated
(wild-type) HCT-116 cells was enough to slightly increase the
percentage of cells expressing these markers, as observed for CD86
(Fig. 3D). However, only those DCs
transfected with RNA of tumor cells pre-treated with 5-FU, showed
significantly higher number of cells expressing HLA-DR, CD83 and
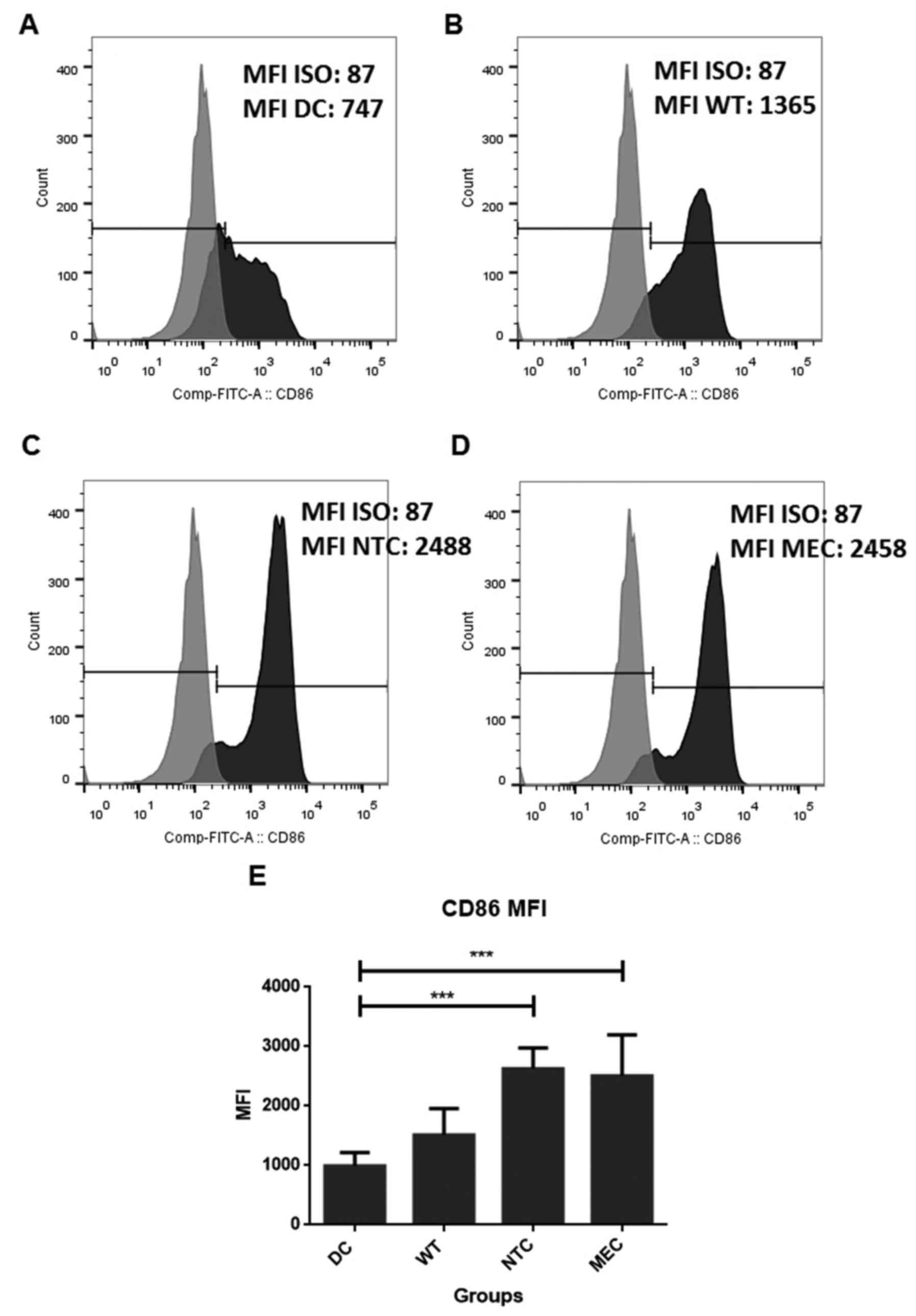
CD80. In addition, analysis of medium fluorescence intensity (MFI)
showed increased expression of CD86 molecules in cells transfected
with RNA of HCT-116 cells treated with both NTC and MEC of 5-FU (DC
= 991.8±224.0; WT = 1,511.0±446.6; CEM = 2,627.8±351.7; NTC =
2,504.2±695.6; Fig. 4).
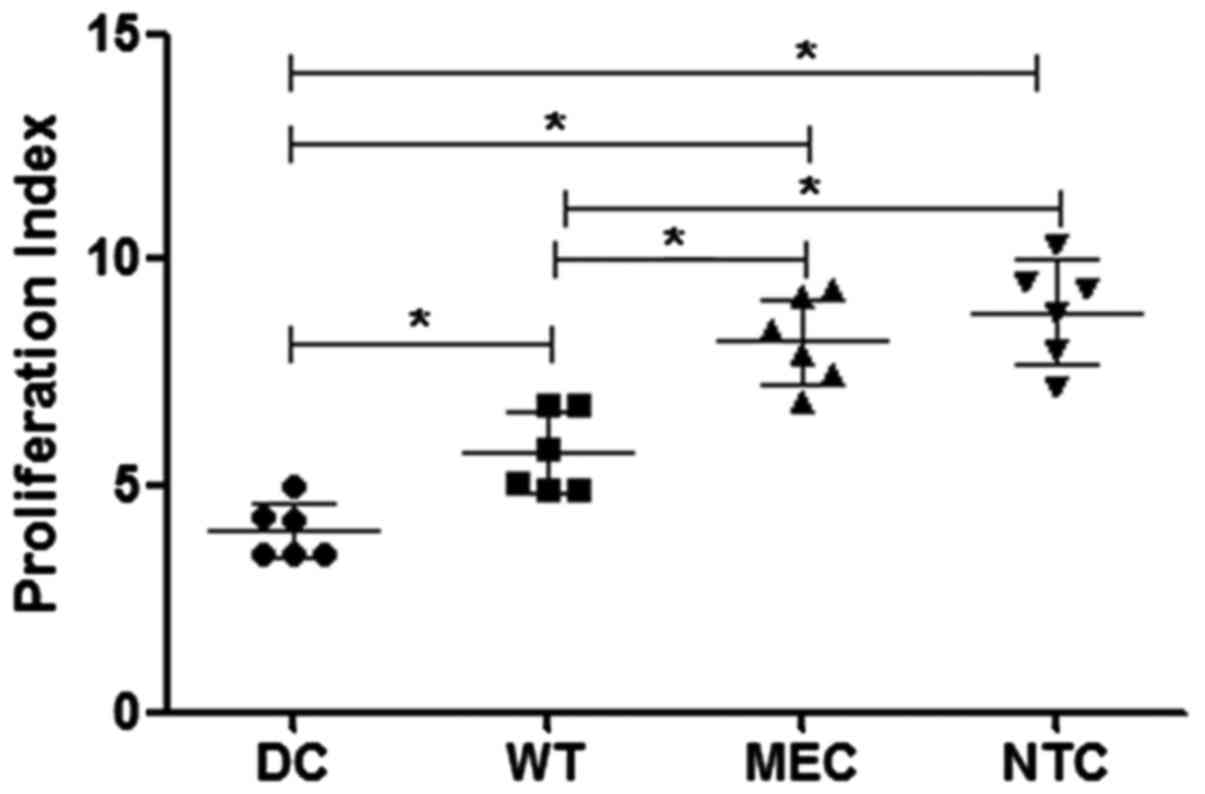
DC transfection with RNA of
5-FU-treated tumor cells increases allogeneic antigen
presentation
In order to analyse the effect of transfection on
the antigen-presentation function of DCs, we performed the MLR
assay. Fig. 5 shows that
RNA-transfected DCs induce higher levels of lymphocyte
proliferation than non-transfected cells (group DCs). Previous
exposure of tumor cells to low concentrations of 5-FU increased the
effect of RNA-transfected on DC function, in comparison with cells
transfected with tumor cell wild-type RNA (DCs = 4.01±0.62; WT =
5.74±0.91; MEC = 8.21±0.95; NCT = 8.86±1.13; expressed as
stimulation index); mean ± standard deviation of 6 independent
assays.
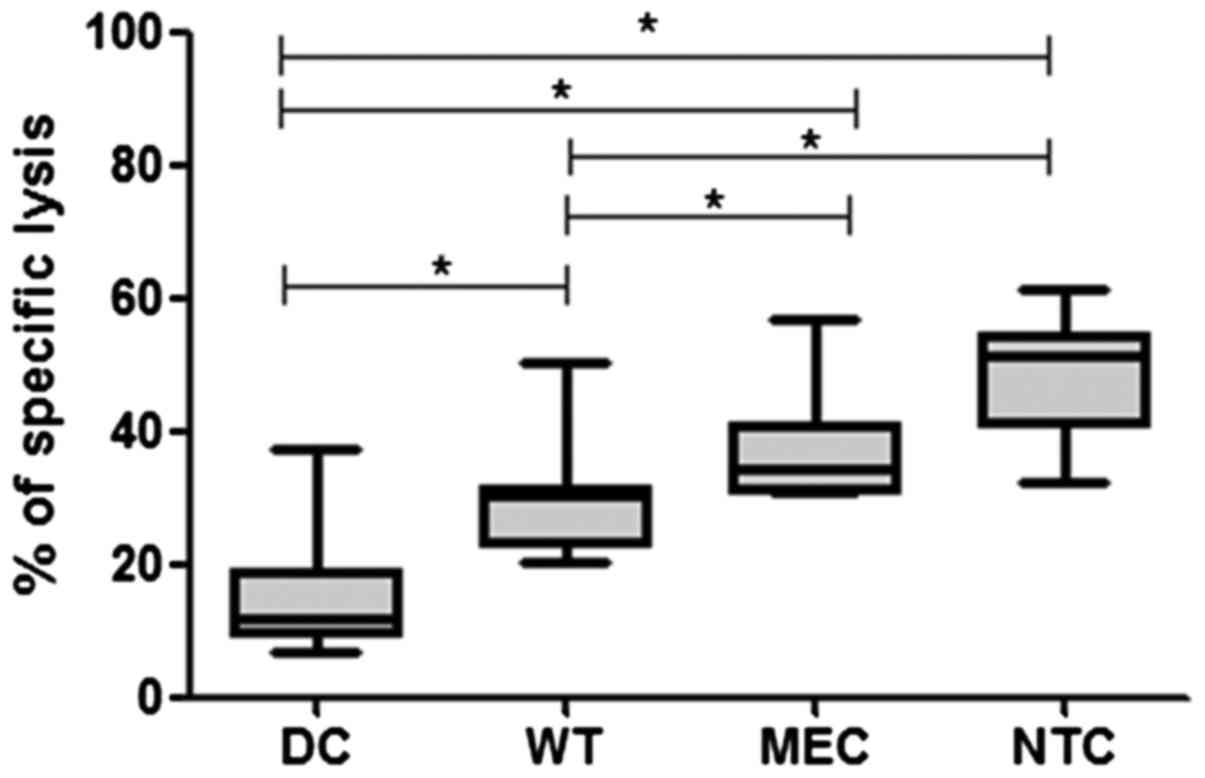
DC transfection with tumor RNA
enhances the in vitro generation of CTL
Cell death mediated by cytotoxic T lymphocytes is
considered the main mechanism for killing MHC class I+
target cells. Thus, we tested the efficiency of transfected DCs for
generating autologous tumor specific T cells. We observed that the
lymphocyte cultured with RNA-transfected DCs yielded anti-HCT-116
cytotoxic cells with higher activity than those cultured with
control DCs. The results in Fig. 6
show that DCs transfected with RNA of 5-FU-treated tumor cells
(both MEC and NTC) are able to induce higher levels of anti-HCT-116
than both DCs and WT controls (DC = 15.87±10.31; WT = 30.81±9.711;
MEC = 37.60±9.17; NCT = 47.64±9.92; expressed as percentage of
specific lysis; N=6).
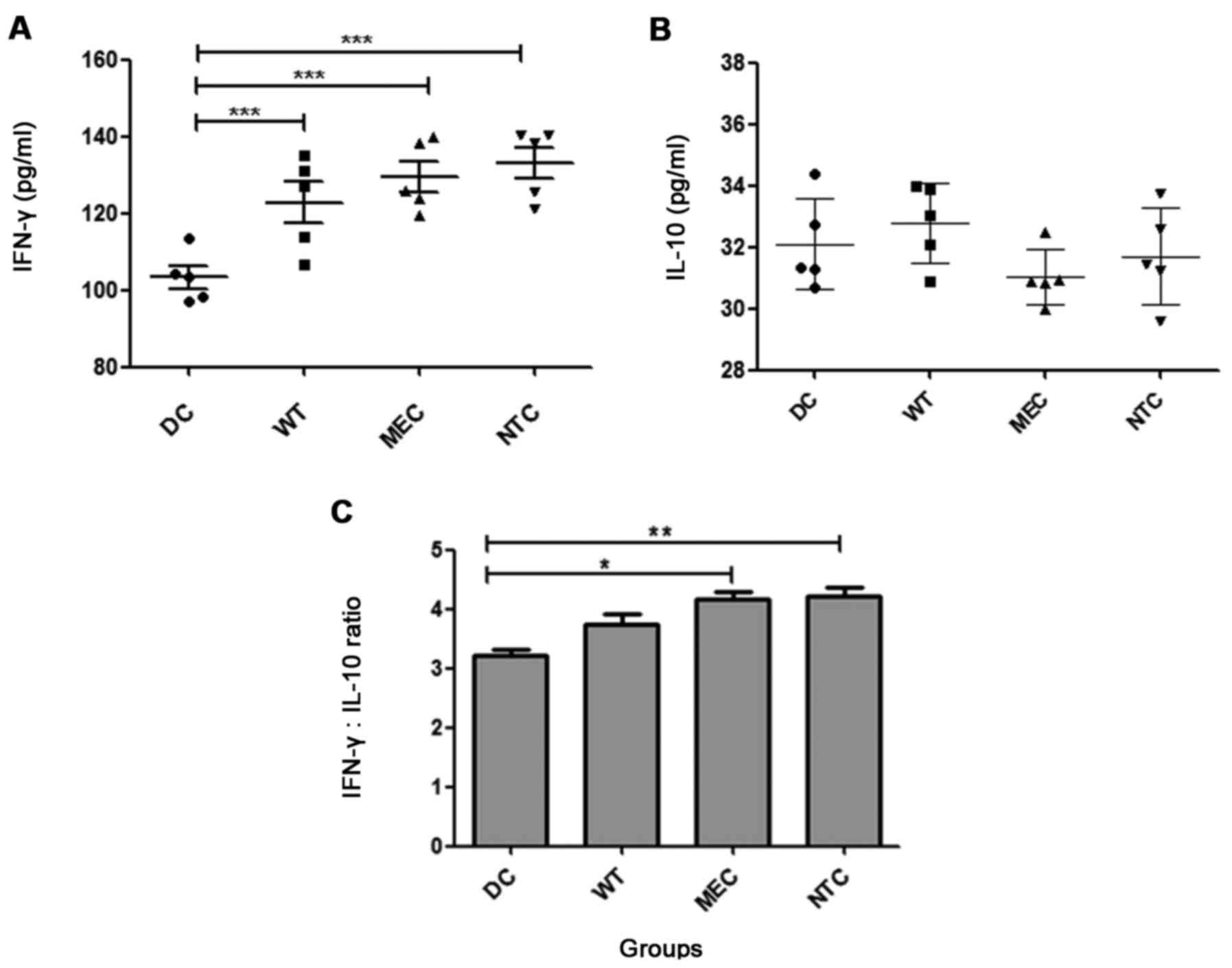
In vitro cytokine synthesis
IFN-γ is one of the main cytokines involved in the
generation of an effective antitumor immune response. Since IFN-γ
is produced by both Th1 (T helper 1) and activated CTLs, its
evaluation in the CTL-assay supernatant is a strong tool for
inferring specific responses. Fig.
7A shows that T cells generated by co-cultures with transfected
DCs produce higher IFN-γ levels than the control DCs (DCs =
103.3±6.4; WT = 122.7±12.00; MEC = 129.5±9.0; NCT = 133.1±9.1
pg/ml), however, the DC transfection did not change the ability of
these cells to induce IL-10 production (Fig. 7B). No differences were observed
between MEC, NTC and WT. In order to evaluate the relative effect
on the Th1/Th2 responsiveness profile, we calculated the
IFN-γ/IL-10 ratio, and Fig. 7C
shows that transfection of RNA from drug-treated tumor cells (MEC
and NTC groups) increased this parameter in comparison with the DC
group (DCs = 3.21±0.19; WT = 3.75±0.39; MEC = 4.17±0.24; NCT =
4.06±0.37).
Discussion
The anti-metabolic agent 5-fluorouracil acts by
interfering with DNA and RNA synthesis in both normal and tumor
cells (21). Its metabolite
fluorouridine triphosphate is extensively incorporated into RNA,
disrupting the normal processing and function of cells (22,23).
This led us to evaluate whether RNA changes induced by 5-FU can be
transferred to monocyte-derived DCs, enhancing their feasibility
for use as a therapeutic anti-CRC vaccine.
First, we chose the best strategy for RNA
transfection into DCs, comparing electroporation and liposomal
approaches. Since electroporation was very aggressive toward DCs
and induced a high level of cell mortality (data not shown), we
tested different liposome reagents, and achieved the best results
with DMRIE-C reagent (Invitrogen). In order to show the ability of
DMRIE-C to transfect total tumor RNA into DCs, we performed RT-PCR
assay using the specific primer for the epithelial cell adhesion
molecule (EpCAM) gene. EpCAM is a carcinoma-associated antigen,
expressed in gastrointestinal carcinomas and most normal epithelial
cells (20). Although some donors
we analyzed have this gene constitutively expressed
(EpCAM+), its expression increased following tumor RNA
transfection, while EpCAM-samples showed de novo expression
of this gene. This observation is in agreement with previous
reports that EpCAM is constitutively expressed on normal cells of
healthy individuals and absent in others (24), being overexpressed in tumor
cells.
In order to investigate the capacity of
RNA-transfected DCs to activate T lymphocytes we first analyzed
their ability to induce T cell alloreactivity by performing the MLR
assay. Our data show that tumor RNA is effective in stimulating DC
function, since transfected DCs more efficiently promoted
proliferation of allogeneic T lymphocytes than non-transfected
cells. Furthermore, transfection of 5-FU-treated tumor RNA (MEC and
NTC) showed a higher efficiency than RNA of untreated tumor cells
(WT) to modulate these functions. These results are in agreement
with the increase percentage of HLA-DR+,
CD83+, CD80+ and CD86+ cells among
DCs, transfected with RNA from NTC-treated or MEC-treated tumor
cells, since these molecules are markers of DC
maturation/activation and are able to enhance the formation of
immune synapses with TCR (25).
This view is reinforced by the increased expression of
co-stimulatory molecule CD86 by DCs, transfected with RNA of
5-FU-treated cells.
These results instigated us to analyze whether the
proposed DC-loading approach would improve their ability to induce
CTL generation. We observed that the DCs loaded with RNA of cells
exposed to non-toxic concentration of 5-FU was much more efficient
than not transfected control or those transfected with wild-type
tumor RNA. Therefore, our results reinforce the view that low 5-FU
concentrations are able to promote changes in tumor-cell gene
expression (12), which can be
transferred to normal DCs by RNA transfection. Such results are in
agreement with previous reports that RNA-transfected DCs can
stimulate tumor antigen-specific CTLs in different cancer systems,
such as prostate (16), cervical
(26), renal (27), and colon (28) cancers.
Another usual parameter to verify the generation of
specific antitumor response is the in vitro production of
IFN-γ by T cells, following a challenge with specific targets. Our
results show that autologous T lymphocytes generated during the
co-culture with DCs of both MEC and NTC groups showed increased
IFN-γ production, in agreement with the findings on the cytotoxic
activity of these lymphocytes. Since no changes were observed on
IL-10 production, we calculated the IFN-γ:IL-10 ratio, and the data
indicate that RNA transfection is able to drive cytokine
responsiveness to the Th1 profile, thus enhancing the establishment
of an antitumor status, as we demonstrated in gastrointestinal
cancers (29). Since stimulation of
MHC-I and -II expression is one of the main roles of IFN-γ,
increased HLA-DR expression observed in this study is also in
accordance with the data on the production of such a cytokine.
IFN-γ facilitates the interaction between DCs and tumor target
cells, and potentiates the stimulatory effect of DCs on the immune
system. In a parallel study developed by our group, we observed
that treatment of a colorectal tumor cell line with low
concentrations of paclitaxel induced the expression of HSP40, HSP70
and HSP90, whereas DCs loaded with lysates from such cells also
showed increased ability to induce allogeneic lymphoproliferative
response (unpublished data). It is possible that tumor exposure to
5-FU also increases the expression of these proteins. If so, HSPs
on target cells could preferentially bind to TLR-expressing DCs,
increasing the antigen uptake by immature DCs. This view is
reinforced by the recent observation that murine colon cancer cells
(CT-26) in vitro treated with 5-FU and/or oxaliplatin
induces secretion of HSP70 and the high-mobility group box-1
(HMGB1) (29), that seem to be
responsible for the upregulation of DC activation markers (HLA-DR,
CD80 and CD86), via interaction with TLR4. In addition, putative
expression of HSPs by tumor cells (30) could work as target for CTL (31) and NK cells (32).
Taken together, the obtained results corroborate our
previous observation that exposure of tumor cells to low
concentrations of cytotoxic agents increases their immunogenicity
(12), reinforcing our hypothesis
that changes induced by this treatment can be successfully
transferred to DCs by transfection of total tumor RNA, priming them
to trigger a specific antitumor response. Besides the number of
different protocols proposed to prime DCs, the best way to prepare
antitumor therapeutic DC-based vaccine is still unclear (33), leading us to conclude that our
protocol deserves a wider investigation in order to be improved for
clinical application, especially to reinforce the feasibility of
administration of low doses of chemotherapeutic agents in
combination with therapeutic DC vaccines. In conclusion these data
support and reinforce the idea that simultaneous use of
chemotherapy and immunotherapy can be an effective anti-CRC
treatment.
Acknowledgements
We are grateful to São Paulo Research Foundation
(FAPESP) for the grant 2009/18331-8. C.V. Almeida was a recipient
of CNPq 140541/2012-8 and Fapesp 2009/16311-0 scholarships
(non-concomitantly). G.G. Romagnoli was a recipient of
post-doctorate scholarship (2012/20494-5). J.A. Zamame and M.B.
Magalhães were recipients of CAPES (DS) and FAPESP (2009/18433-5)
scholarships. R. Kaneno was a recipient of CNPq scholarship
(303952/2010-5). This study was supported in part by grant from the
regional contribution of ‘the Programma Attuativo Regionale
(Toscana) cofinanziato dal FAS (adesso FSC) - PAR FAS
2007–2013′.
References
|
1
|
UICC (International Union Against Cancer).
TNM Classification of Malignant Tumours. 6th. Sobin LH and
Wittekind Ch: Wiley-Liss; New York, Chichester, Weinheim, Brisbane,
Singapore, Toronto: 2002
|
|
2
|
Hewett PJ, Allardyce RA, Bagshaw PF,
Frampton CM, Frizelle FA, Rieger NA, Smith JS, Solomon MJ, Stephens
JH and Stevenson AR: Short-term outcomes of the Australasian
randomized clinical study comparing laparoscopic and conventional
open surgical treatments for colon cancer: The ALCCaS trial. Ann
Surg. 248:728–738. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Valentini AM, Armentano R, Pirrelli M and
Caruso ML: Chemotherapeutic agents for colorectal cancer with a
defective mismatch repair system: The state of the art. Cancer
Treat Rev. 32:607–618. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Andre T and de Gramont A: Study Group of
Clinical Research in Radiotherapies Oncology, Oncology
Multidiciplinary Research Group: An overview of adjuvant systemic
chemotherapy for colon cancer. Clin Colorectal Cancer. 4 Suppl
1:S22–S28. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Klement G, Baruchel S, Rak J, Man S, Clark
K, Hicklin DJ, Bohlen P and Kerbel RS: Continuous low-dose therapy
with vinblastine and VEGF receptor-2 antibody induces sustained
tumor regression without overt toxicity. J Clin Invest.
105:R15–R24. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Browder T, Butterfield CE, Kräling BM, Shi
B, Marshall B, O'Reilly MS and Folkman J: Antiangiogenic scheduling
of chemotherapy improves efficacy against experimental
drug-resistant cancer. Cancer Res. 60:1878–1886. 2000.PubMed/NCBI
|
|
7
|
Apetoh L, Ghiringhelli F, Tesniere A,
Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E,
Saulnier P, et al: Toll-like receptor 4-dependent contribution of
the immune system to anticancer chemotherapy and radiotherapy. Nat
Med. 13:1050–1059. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Apetoh L, Ghiringhelli F, Tesniere A,
Criollo A, Ortiz C, Lidereau R, Mariette C, Chaput N, Mira JP,
Delaloge S, et al: The interaction between HMGB1 and TLR4 dictates
the outcome of anticancer chemotherapy and radiotherapy. Immunol
Rev. 220:47–59. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nars MS and Kaneno R: Immunomodulatory
effects of low dose chemotherapy and perspectives of its
combination with immunotherapy. Int J Cancer. 132:2471–2478. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shurin GV, Tourkova IL, Kaneno R and
Shurin MR: Chemotherapeutic agents in noncytotoxic concentrations
increase antigen presentation by dendritic cells via an
IL-12-dependent mechanism. J Immunol. 183:137–144. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
John J, Ismail M, Riley C, Askham J,
Morgan R, Melcher A and Pandha H: Differential effects of
Paclitaxel on dendritic cell function. BMC Immunol. 11:142010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaneno R, Shurin GV, Kaneno FM, Naiditch
H, Luo J and Shurin MR: Chemotherapeutic agents in low noncytotoxic
concentrations increase immunogenicity of human colon cancer cells.
Cell Oncol (Dordr). 34:97–106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhong H, Gutkin DW, Han B, Ma Y, Keskinov
AA, Shurin MR and Shurin GV: Origin and pharmacological modulation
of tumor-associated regulatory dendritic cells. Int J Cancer.
134:2633–2645. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsuhashi T, Shimizu M, Negishi Y,
Takeshita T and Takahashi H: A low, non-toxic dose of paclitaxel
can prevent dendritic cell-precursors from becoming tolerogenic
dendritic cells with impaired functions. Biomed Res. 35:369–380.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pan K, Zhao JJ, Wang H, Li JJ, Liang XT,
Sun JC, Chen YB, Ma HQ, Liu Q and Xia JC: Comparative analysis of
cytotoxic T lymphocyte response induced by dendritic cells loaded
with hepatocellular carcinoma-derived RNA or cell lysate. Int J
Biol Sci. 6:639–648. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Heiser A, Dahm P, Yancey DR, Maurice MA,
Boczkowski D, Nair SK, Gilboa E and Vieweg J: Human dendritic cells
transfected with RNA encoding prostate-specific antigen stimulate
prostate-specific CTL responses in vitro. J Immunol. 164:5508–5514.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wilgenhof S, Van Nuffel AM, Corthals J,
Heirman C, Tuyaerts S, Benteyn D, De Coninck A, Van Riet I,
Verfaillie G, Vandeloo J, et al: Therapeutic vaccination with an
autologous mRNA electroporated dendritic cell vaccine in patients
with advanced melanoma. J Immunother. 34:448–456. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaneno R, Shurin GV, Tourkova IL and
Shurin MR: Chemomodulation of human dendritic cell function by
antineoplastic agents in low noncytotoxic concentrations. J Transl
Med. 7:582009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zar JH: Biostatistical Analysis. Prentice
Hall. New Jersey: 1999.
|
|
20
|
Homo sapiens epithelial cell adhesion
molecule (EPCAM), RefSeqGene (LRG_215) on chromosome 2. NCBI
Reference Sequence: NG_012352.2. NCBI-GenBank. 2013.https://www.ncbi.nlm.nih.gov/nuccore/382544394?report=fasta&to=48866
|
|
21
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kufe DW and Major PP: 5-Fluorouracil
incorporation into human breast carcinoma RNA correlates with
cytotoxicity. J Biol Chem. 256:9802–9805. 1981.PubMed/NCBI
|
|
23
|
Glazer RI and Lloyd LS: Association of
cell lethality with incorporation of 5-fluorouracil and
5-fluorouridine into nuclear RNA in human colon carcinoma cells in
culture. Mol Pharmacol. 21:468–473. 1982.PubMed/NCBI
|
|
24
|
Anderson R, Schaible K, Heasman J and
Wylie C: Expression of the homophilic adhesion molecule, Ep-CAM, in
the mammalian germ line. J Reprod Fertil. 116:379–384. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hosseini BH, Louban I, Djandji D, Wabnitz
GH, Deeg J, Bulbuc N, Samstag Y, Gunzer M, Spatz JP and Hämmerling
GJ: Immune synapse formation determines interaction forces between
T cells and antigen-presenting cells measured by atomic force
microscopy. Proc Natl Acad Sci USA. 106:17852–17857. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thornburg C, Boczkowski D, Gilboa E and
Nair SK: Induction of cytotoxic T lymphocytes with dendritic cells
transfected with human papillomavirus E6 and E7 RNA: Implications
for cervical cancer immunotherapy. J Immunother. 23:412–418. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Heiser A, Maurice MA, Yancey DR, Coleman
DM, Dahm P and Vieweg J: Human dendritic cells transfected with
renal tumor RNA stimulate polyclonal T-cell responses against
antigens expressed by primary and metastatic tumors. Cancer Res.
61:3388–3393. 2001.PubMed/NCBI
|
|
28
|
Nair SK, Hull S, Coleman D, Gilboa E,
Lyerly HK and Morse MA: Induction of carcinoembryonic antigen
(CEA)-specific cytotoxic T-lymphocyte responses in vitro using
autologous dendritic cells loaded with CEA peptide or CEA RNA in
patients with metastatic malignancies expressing CEA. Int J Cancer.
82:121–124. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fang H, Ang B, Xu X, Huang X, Wu Y, Sun Y,
Wang W, Li N, Cao X and Wan T: TLR4 is essential for dendritic cell
activation and anti-tumor T-cell response enhancement by DAMPs
released from chemically stressed cancer cells. Cell Mol Immunol.
11:150–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Green DR, Ferguson T, Zitvogel L and
Kroemer G: Immunogenic and tolerogenic cell death. Nat Rev Immunol.
9:353–363. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Calderwood SK, Stevenson MA and Murshid A:
Heat shock proteins, autoimmunity, and cancer treatment. Autoimmune
Dis. 2012:4860692012.PubMed/NCBI
|
|
32
|
Elsner L, Flügge PF, Lozano J, Muppala V,
Eiz-Vesper B, Demiroglu SY, Malzahn D, Herrmann T, Brunner E,
Bickeböller H, et al: The endogenous danger signals HSP70 and MICA
cooperate in the activation of cytotoxic effector functions of NK
cells. J Cell Mol Med. 14:992–1002. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Romagnoli GG and Kaneno R: Dendritic cell
vaccines for cancer therapy: fundamentals and clinical trials.
Cancer Immunology: Bench to Bedside Immunotherapy of Cancer. Rezeai
N: Springer-Verlag. (Berlin, Heildelberg). 359–373. 2015.
|