Introduction
Renal cell carcinoma (RCC) is the most common type
of malignant neoplasm arising from the kidney (1). RCC has the highest mortality rate of
all genitourinary cancer and its incidence has steadily risen
(2). Since RCC is resistant to
conventional chemotherapy, it commonly recurs after treatment;
thus, patients with metastatic RCC have poor prognosis (3). Typically, the drugs used to treat
metastatic RCC are targeted to vascular endothelial growth factor
(VEGF) receptors and mammalian target of rapamycin (mTOR)
signaling. However, while most patients initially respond to these
drugs, its resistance results in subsequent progression of RCC.
Thus, to address the unmet need to identify additional targets for
RCC, newly developed therapeutic agents are required for effective
treatment.
Signal transducer and activator of transcription 3
(STAT3) is a transcriptional factor that mediates the signaling
pathway of various cytokines and growth factors (4). Constitutive activation of STAT3 has
been observed in human cancer including breast, ovarian and
prostate cancer (5–7). It has been recently reported that a
high frequency of STAT3 activation is observed in RCC, particularly
in metastatic disease (8). STAT3
protein has been shown to play an important role in supporting cell
survival and proliferation (9). In
cancer cells, STAT3 becomes constitutively active through the
phosphorylation of its serine 727 (S727) and tyrosine 705 (Y705)
residues by upstream Janus-activated kinases (Jaks) or the Src
family kinases (9). Phosphorylated
STAT3 then undergoes dimerization and is translocated to the
nucleus where it binds to specific DNA response elements in the
promoters of target genes (10).
STAT3 transactivates the genes encoding various cell survival
proteins, such as cyclins, survivin, Bcl-2 and Bcl-xl (11). This evidence supports the rationale
for targeting STAT3 for suppression of tumor growth in several
cancers including RCC (12).
Apoptosis is triggered by extrinsic and/or intrinsic
pathways and is regulated by various ligands (13). The extrinsic pathway originates from
interaction between death receptors and its ligands such as Fas and
TNFα. The intrinsic pathway is closely related to the
permeabilization of the mitochondrial membrane, commanded by the
Bcl-2 superfamily (14). Reactive
oxygen species (ROS) are mainly generated by the mitochondria in
most mammalian cells. ROS can cause cellular apoptosis via both the
extrinsic cell death receptor and the intrinsic mitochondrial cell
death pathways (15). It has been
reported that elevated ROS formation and the subsequent apoptosis
induction is a novel approach to treat cancer (16).
Since evasion of apoptosis is one of the hallmarks
of cancer, it has long been attempted to develop anticancer drugs
that can selectively induce apoptosis in cancer cells (17). Various phytochemicals such as
polyphenols modulate differentiation of cells and induce apoptosis
in cancer cells (18).
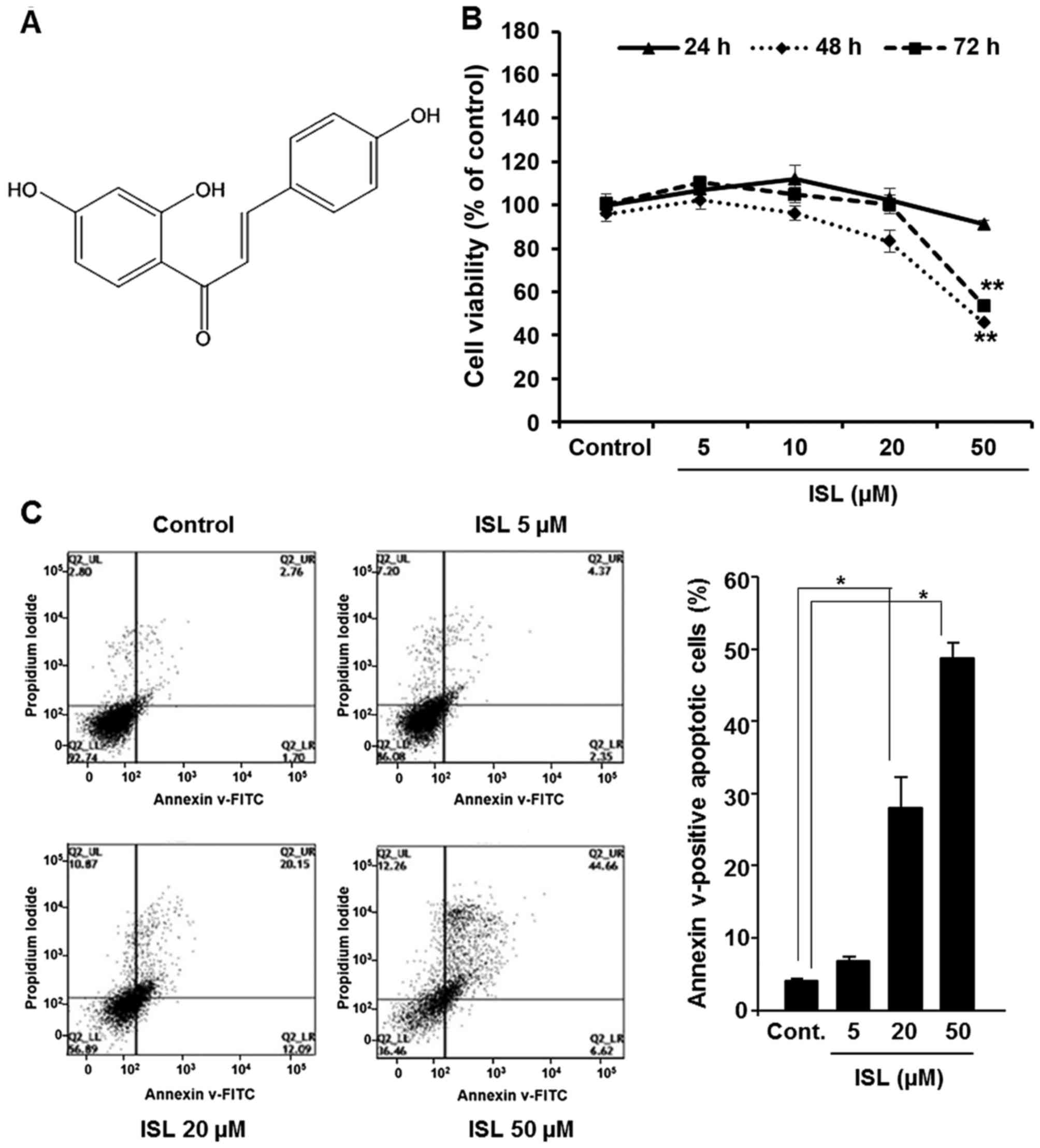
Isoliquiritigenin [2′,4′,4-trihydroxychalcone (ISL)] (Fig. 1A) is a flavonoid with chalcone
structure that has been noted in licorice and shallot, which are
generally used in traditional Chinese medicine (19). ISL shows pharmacological effects
including antioxidant, anti-inflammatory and antitumor activity
(20–22). In addition, ISL has been proved to
induce apoptosis and inhibit cell proliferation in breast, liver,
colon and cervical cancer cells (23–27).
Moreover, ISL was found to inhibit tumor growth and angiogenesis in
lung and breast cancer mouse models (22,28).
Previously, Yamazaki et al reported that ISL suppressed the
viability of murine RCC with concomitant inhibition of nitric oxide
production by lipopolysaccharide-stimulated macrophages, resulting
in decreased pulmonary metastasis (29). However, thus far, there have been no
studies regarding the effects of ISL in human RCC. Therefore, we
aimed to investigate molecular mechanisms underlying the anticancer
effects of ISL on human renal carcinoma Caki cells. The present
study revealed that ISL induced antiproliferative and apoptotic
effects in Caki cells through mitochondrial-dependent caspase
activation and interference with the STAT3 signaling pathway via
ROS generation.
Materials and methods
Materials
ISL, N-acetyl cysteine (NAC),
diphenyleneiodonium (DPI) and β-actin antibody were purchased from
Sigma-Aldrich Co. (St. Louis, MO, USA). Antibodies against cleaved
caspase-9, −7 −3 and PARP as well as Bcl-2, Bcl-xl, Bax, STAT3,
p-STAT3 (Y705), p-STAT3 (S727), Jak2, p-Jak2, cyclin D1 and cyclin
D2 were obtained from Cell Signaling Technology, Inc. (Beverly, MA,
USA). Primary antibodies against each of p53 and murine double
minute-2 (Mdm2), and horseradish peroxidase-conjugated secondary
antibodies were purchased from Santa Cruz Biotechnology (Santa
Cruz, CA, USA). 2′-7′-Dichlorofluorescein diacetate (DCF-DA) was
obtained from Invitrogen (Carlsbad, CA, USA). Hank's balanced salt
solution (HBSS) was purchased from Mediatech (Herndon, VA,
USA).
Cell culture and treatment
Caki cells were provided by Dr. T.K. Kwon (Keimyung
University, Daegu, Korea) and maintained in Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and
antibiotics (100 U/ml penicillin G and 100 µg/ml streptomycin) at
37°C in a humidified incubator containing 5% CO2 and 95%
air. The cells were plated at an appropriate density according to
each experimental scale.
Cell viability assay
Cell viability was measured by the MTT assay. Cells
(2×103) were incubated in triplicate in a 96-well plate
in the presence of ISL in a final volume of 100 µl for different
time intervals at 37°C. Thereafter, 10 µl of MTT solution (5 mg/ml)
was added to each well and incubated for 4 h. Medium was removed,
formazan was dissolved in dimethyl sulfoxide (DMSO) and absorbance
at 570 nm was measured using a microplate reader (Tecan Trading AG,
Männedorf, Switzerland). Cell viability was expressed as the
relative percentage of the control.
Annexin V staining
Annexin V staining was performed using fluorescein
isothiocyanate (FITC)-Annexin V staining kit (BD Biosciences, San
Jose, CA, USA) following the manufacturer's instructions. Briefly,
ISL-treated cells were washed with HBSS solution and resuspended in
binding buffer containing Annexin V and propidium iodide (PI).
Fluorescence intensity was measured using flow cytometry (BD
Biosciences, La Jolla, CA, USA).
Western blot analysis
Cells were lysed with RIPA buffer, and collected
protein samples were quantified using a bicinchoninic acid protein
assay kit (Pierce Biotechnology, Rockford, IL, USA). The protein
samples were electrophoresed using 8–15% sodium dodecyl
sulfate-polyacrylamide gel and immunoblot analysis was carried out
according to the protocol previously described (30). Immunoblot membranes were incubated
with SuperSignal Pico Chemiluminescent or Dura Luminol substrates
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's instruction and visualized with ImageQuant LAS 4000
(Fujifilm Life Science, Tokyo, Japan).
Measurement of ROS accumulation
Cells were treated with ISL in the presence or
absence of NAC and DPI for 24 h and then loaded with 25 µM of
DCF-DA. After incubation for 30 min at 37°C, the cells were washed
twice with HBSS solution, suspended in complete media and examined
under a fluorescence microscope to detect the intracellular
accumulation of ROS. Total cellular ROS were quantified using a
flow cytometry (BD Biosciences).
Electrophoretic mobility gel shift
assay (EMSA)
The nuclear extract was prepared from cells
incubated with or without ISL. The STAT3 oligonucleotide probe
5′-AGC TTC ATT TCC CGT AAA TCC CTA-3′ (Biomedic, Korea) was labeled
with [γ-32P]-ATP using T4 polynucleotide kinase. The
EMSA was performed according to a previously described protocol
(31).
Statistical analysis
When necessary, data are expressed as mean ± SD of
the results of at least three independent experiments, and
statistical analysis for single comparison was performed using the
Student's t-test and a p-value <0.05 was considered to indicate
a statistically significant result.
Results
ISL induces apoptosis in Caki
cells
We initially examined the effect of ISL on the
viability of Caki cells. Incubation of cells with ISL (5, 10, 20 or
50 µM) significantly reduced the cell viability (Fig. 1B). In addition, to elucidate whether
ISL-mediated cytotoxicity resulted from the induction of apoptosis,
FACS analysis was performed. Caki cells treated with ISL (5, 20 or
50 µM) for 48 h were analyzed by flow cytometry using double
staining with Annexin V and PI to quantify the population of cells
undergoing apoptosis. As shown in Fig.
1C, incubation of Caki cells with ISL for 48 h resulted in
apoptotic cell death. Quantification of apoptotic cells and
statistical analysis of ISL-induced apoptosis are presented in
Fig. 1C (right panel).
ISL induces apoptosis through the
mitochondrial pathway in Caki cells
Mitochondria are a central regulator of the
intrinsic pathway in apoptosis triggered by various stimuli
(32). The loss of mitochondrial
membrane potential leads to increased mitochondrial membrane
permeability, and then released cytochrome c, which induces
activation of the caspase cascade (32). The activation of caspases is a
critical event in the proteolytic cascade elicited by apoptotic
stimuli. We investigated the involvement of caspase activation in
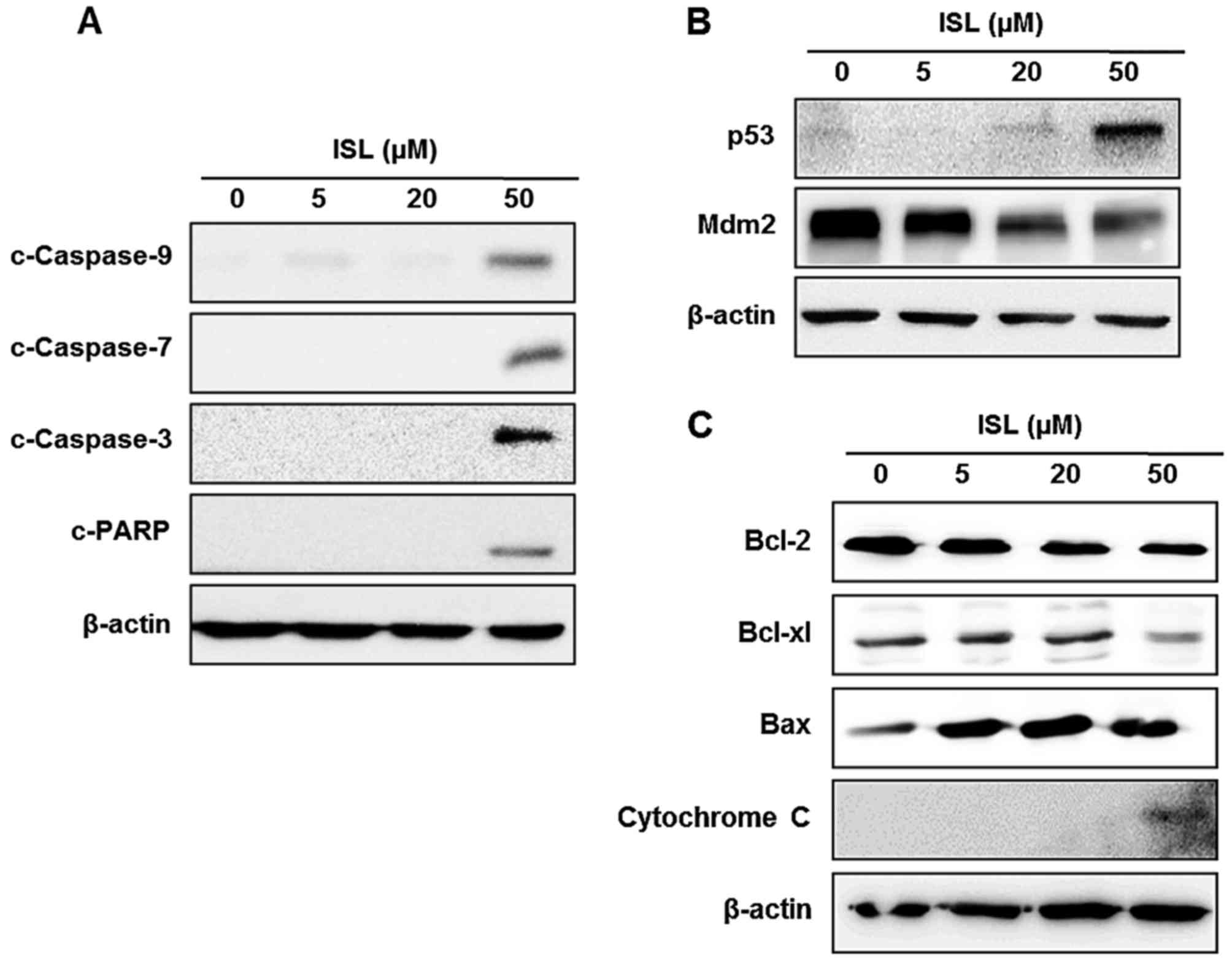
the ISL-induced apoptosis of Caki cells. Treatment of Caki cells
with ISL induced the cleavage of caspase-9, −7 and −3, and PARP
(Fig. 2A). Incubation of cells with
ISL increased the expression of p53 and diminished the expression
of its cytosolic repressor protein Mdm2 in a
concentration-dependent manner (Fig.
2B). To elucidate whether the apoptosis may be mediated by
mitochondrial alterations, we evaluated the expression of
anti-apoptotic or pro-apoptotic proteins in the ISL-treated Caki
cells. Anti-apoptotic proteins such as Bcl-2 and Bcl-xl reside in
the outer mitochondrial membrane and block the release of
cytochrome c, whereas pro-apoptotic protein Bax stimulates
its release. Since Bcl-2 family proteins regulate the mitochondrial
membrane integrity, the effect of ISL on the expression of Bcl-2
family proteins was then examined. As shown in Fig. 2C, incubation with ISL reduced the
expression of Bcl-2 and Bcl-xl, while ISL increased expression of
Bax in Caki cells. In addition, the level of cytochrome c
expression was increased by treatment of ISL in the Caki cells with
a concomitant decrease in Bcl-2 expression (Fig. 2C).
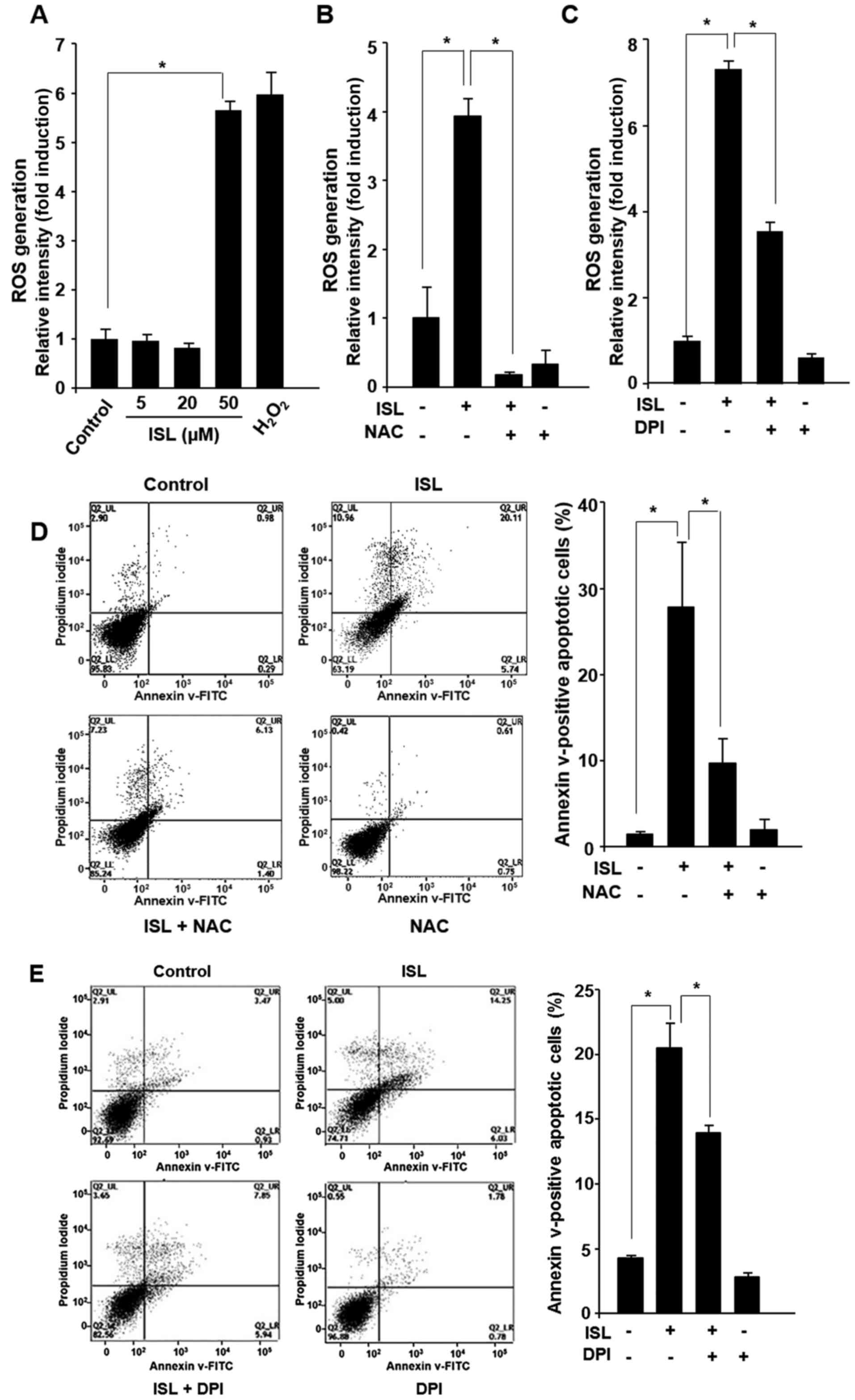
ISL induces generation of ROS in Caki
cells
Since it has been postulated that ROS production
contributes to apoptosis triggering in various cancer cell lines
(33), the effect of ISL on ROS
generation was examined. Treatment of cells with ISL (5, 20 or 50
µM) for 24 h led to ROS generation as revealed by FACS analysis
after DCF-DA staining (Fig. 3A). As
a positive control, Caki cells were incubated with hydrogen
peroxide (500 µM) under the same experimental condition. To verify
that the increase in ROS level was involved in the ISL-induced
apoptosis, we utilized the ROS scavenger NAC and specific NADPH
oxidase inhibitor DPI. It has been known that NADPH oxidases
catalyze the production of superoxide, a type of ROS. Treatment of
Caki cells with NAC and DPI abrogated the ISL-induced ROS
generation (Fig. 3B and C),
following cell death (Fig. 3D and
E). Quantification of apoptotic cell death under each
experimental condition as well as statistical analysis are
presented in the right panel of Fig. 3D
and E.
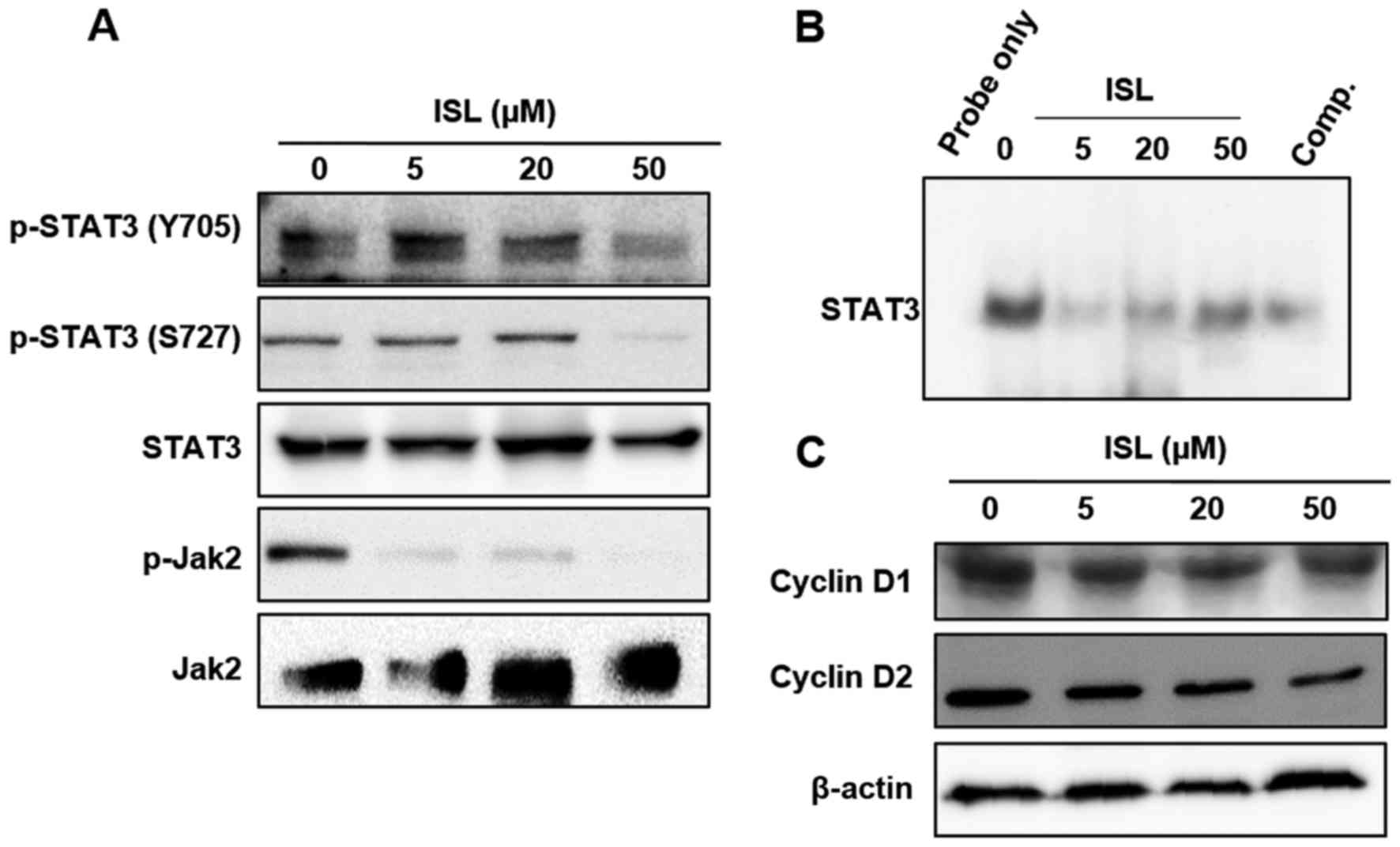
ISL-induced apoptosis in Caki cells is
mediated through inactivation of STAT3 signaling
STAT3 protein is constitutively activated in various
human cancer cells and aberrant STAT3 signaling is implicated as
playing a pivotal role in the initiation and progression of cancer
(34). It has been known that STAT3
transcriptional activity is stimulated by its simultaneous tyrosine
and serine phosphorylation via activation of Jak kinases (35). Incubation with ISL inhibited the
STAT3 DNA binding activity following inhibition of constitutive
phosphorylation of STAT3 at both Y705 and S727 residues (Fig. 4A and B). To elucidate the mechanism
of ISL-induced inactivation of STAT3, we examined the effect of ISL
on the phosphorylation of upstream kinase, Jak2, which is known to
phosphorylate STAT3. As shown in Fig.
4A, ISL attenuated the constitutive phosphorylation of Jak2 in
Caki cells. In addition, ISL attenuated the expression of STAT3
target gene products such as cyclin D1 and cyclin D2 (Fig. 4C).
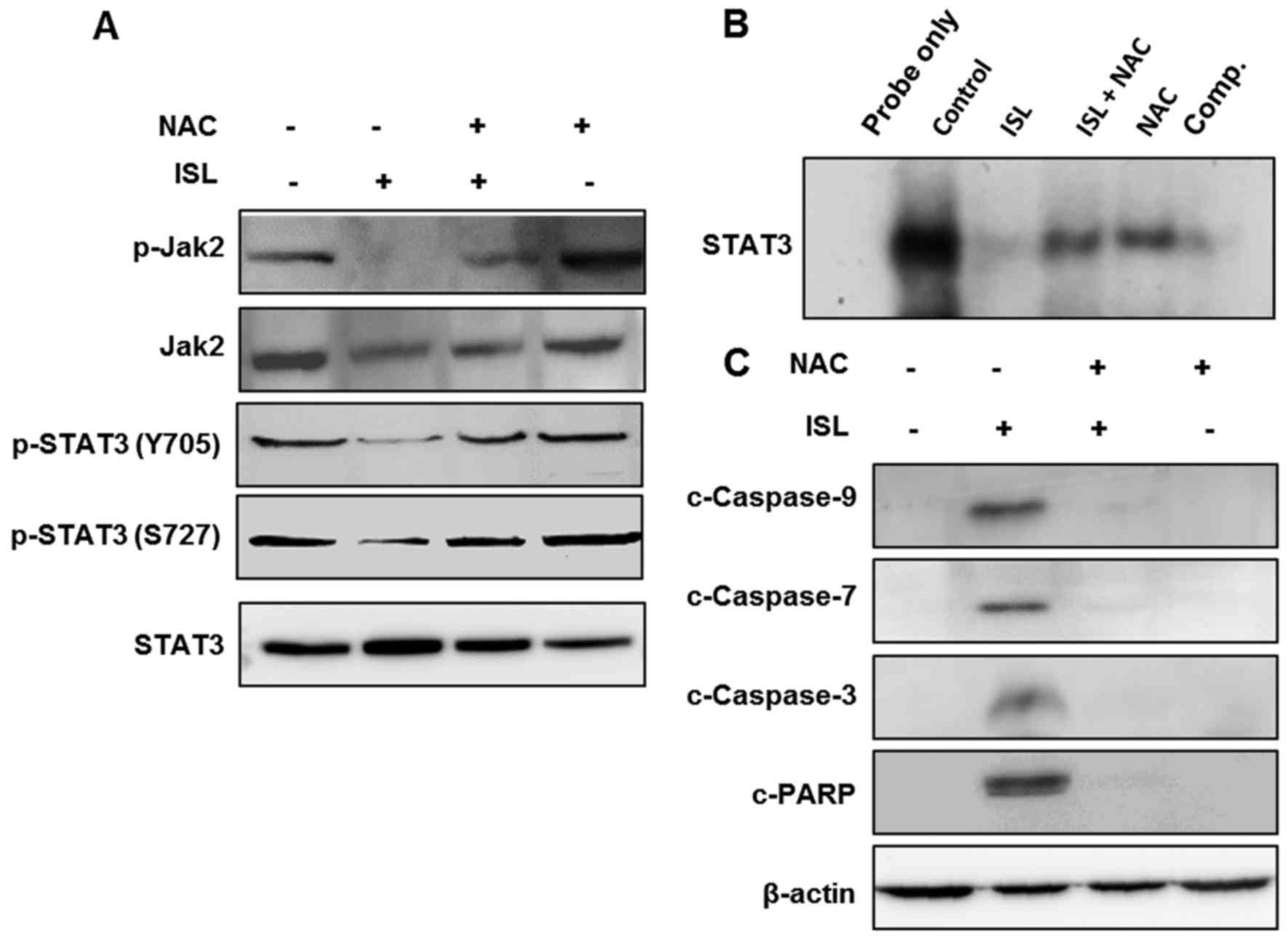
Role of ROS in ISL-induced inhibition
of STAT3 signaling and induction of apoptosis in Caki cells
We next examined the role of ISL-induced ROS
generation in the blocking of STAT3 signaling and induction of
apoptosis in Caki cells. Incubation of Caki cells with ISL in the
absence or presence of NAC revealed that inhibition of ROS
generation abrogated the inhibitory effect of ISL on the
phosphorylation of Jak2 following STAT3 activation (Fig. 5A) as well as its DNA binding
activity (Fig. 5B). Moreover,
pretreatment of NAC averted the ISL-induced cleavage of caspase-9
−7 and −3 and PARP (Fig. 5C). These
findings suggest that the ISL-induced ROS generation plays a
pivotal role in apoptosis and inactivation of STAT3 signaling in
the ISL-treated Caki cells.
Discussion
Approaches to RCC therapy have many limitations and
novel strategies are urgently required. It has been reported that
dysregulated cell growth is a hallmark of cancer development, and
naturally occurring substances derived from plant-based diet have
been suggested for used in the therapy of various types of cancers
(36). In addition, Jak/STAT3
signaling controls the ability of pre-neoplastic lesions to
transform into a malignant tumor, which suggests that intervention
of the Jak/STAT3 pathway provides the opportunities for new
approaches in cancer therapy (37).
Cumulative evidence supports activation of STAT3 as an oncogenic
pathway in many cancers including RCC (5–8). To
address the unmet need to identify therapeutic targets for RCC,
researchers have recently identified the Jak/STAT3 pathway. Our
group and other investigators have suggested that kinase inhibitors
of this pathway and phytochemicals suppressing STAT3 cause marked
tumor growth inhibition in RCC in vitro and in vivo
models (38,39).
Chalcone possesses a basic structure of two benzene
rings connected by an unsaturated carbon chain and are the
intermediate precursors for all flavonoid compounds. ISL is a
chalcone found in licorice root and other plants, and shows various
pharmacological properties including antitumor, antioxidant, and
anti-allergic activities in vitro (40–43).
ISL also contains an α,β-unsaturated carbonyl group as a Michael
acceptor site, suggesting that ISL-induced ROS is mediated through
the depletion of intracellular GSH. The electrophilic center such
as the α,β-unsaturated carbonyl group is prone to undergo Michael
addition reactions with nucleophiles, such as the free sulfhydryl
group of cysteine residues located in reduced glutathione or many
cellular proteins (44). In the
present study, we provide the novel finding that ISL attenuated the
constitutive phosphorylation, nuclear localization and DNA binding
activity of STAT3 in Caki cells. Thus, we propose that ISL may
directly bind to GSH and/or cysteine residues of STAT3.
Phosphorylation of STAT3 initiates their nuclear translocation,
followed by activated STAT3 dimers, and binds to specific promoter
regions in DNA (45). In addition,
it was reported that stattic, a small molecular inhibitor of STAT3,
prevented STAT3 dimerization and phosphorylation, and this was
accompanied by alkylation of four cysteine residues (Cys251, 259,
367 and 426) in unphosphorylated STAT3 (46). Moreover, it has been shown that
specific cysteine residues of STAT3 undergo covalent modifications
and/or oxidation, and then changes in the cellular redox status
affect the transcriptional activity of STAT3 (47). It is likely that ISL-induced ROS may
cause oxidative modification of cysteine residues of Jak2 or STAT3
proteins, thereby blunting the survival of Caki cells. Our findings
suggest that pretreatment with NAC abrogated the inhibitory effects
of ISL on the activation of Jak2/STAT3 signaling implying that ISL
can induce oxidative modification of these kinases. Similar to this
finding, natural products such as withaferin A and carnosol were
found to induce apoptosis through inhibition of Jak2/STAT3,
accompanied by generation of ROS (48,49).
The role of ROS in the apoptosis of cancer cells is
quite controversial. Although some investigators suspect that ROS
play a role in pro-tumorigenic signaling, other investigators have
reported that ROS induce apoptosis (50). It seems likely that many antitumor
agents cause oxidative stress such as ROS, which also contributes
to their anticancer effects (51).
Li et al revealed that ROS induce apoptosis through
suppression of Bcl-2 expression and an increase in the Bax protein
level in squamous cell carcinoma cells (52). The present study, also demonstrated
that upregulation of the Bax/Bcl-2 ratio may contribute to the
apoptosis in ISL-stimulated Caki cells. In addition, NAC treatment
rescued cells from ISL-induced apoptosis by blocking the cleavage
of caspases and PARP. Schumacker explained that cancer cells may be
more vulnerable to oxidative stress, since they function with a
higher level of ROS-mediated growth signaling than normal cells
(53). Therefore, ISL has important
value in the treatment of RCC, based on induction of oxidative
stress-mediated apoptotic signals.
Recent studies have confirmed that the Jak2/STAT3
pathways are implied in the regulation of apoptosis and survival of
tumor cells. STAT3 can also enhance its DNA binding activity by
activating growth factors such as IL-6 and IL-17 to regulate the
transcription of the corresponding target genes. It continuously
transduces the survival signal to tumor cells to play a role in the
promotion of tumor cell survival and inhibition of apoptosis
(54). Therefore, in the present
study, the effects of ISL on activation of STAT3 were estimated,
and inhibition of the Jak2/STAT3 pathway resulted in apoptosis in
ISL-treated Caki cells. In addition, ISL downregulated the
expression of STAT3-related anti-apoptotic and survival genes such
as Bcl-2, Bcl-xl, cyclin D1 and D2 in the Caki cells. Intracellular
kinases, such as Jak2, have been shown to phosphorylate STAT3. The
inhibitory effect of ISL on the phosphorylation of Jak2 through ROS
generation interfered with STAT3 signaling pathway in the Caki
cells. STAT3 becomes constitutively active through the
phosphorylation by Src kinase as well as Jak. Moreover, a
synergistic effect on tumor inhibition by co-treatment of Src and
STAT3 inhibitors in RCC was noted (38). In accordance with these studies, we
found that incubation of Caki cells with ISL inhibited Src
activation and pretreatment with NAC abrogated the inhibitory
effect of ISL on the phosphorylation of Src activation (data not
shown). These data imply the role of Src in STAT3 activation in
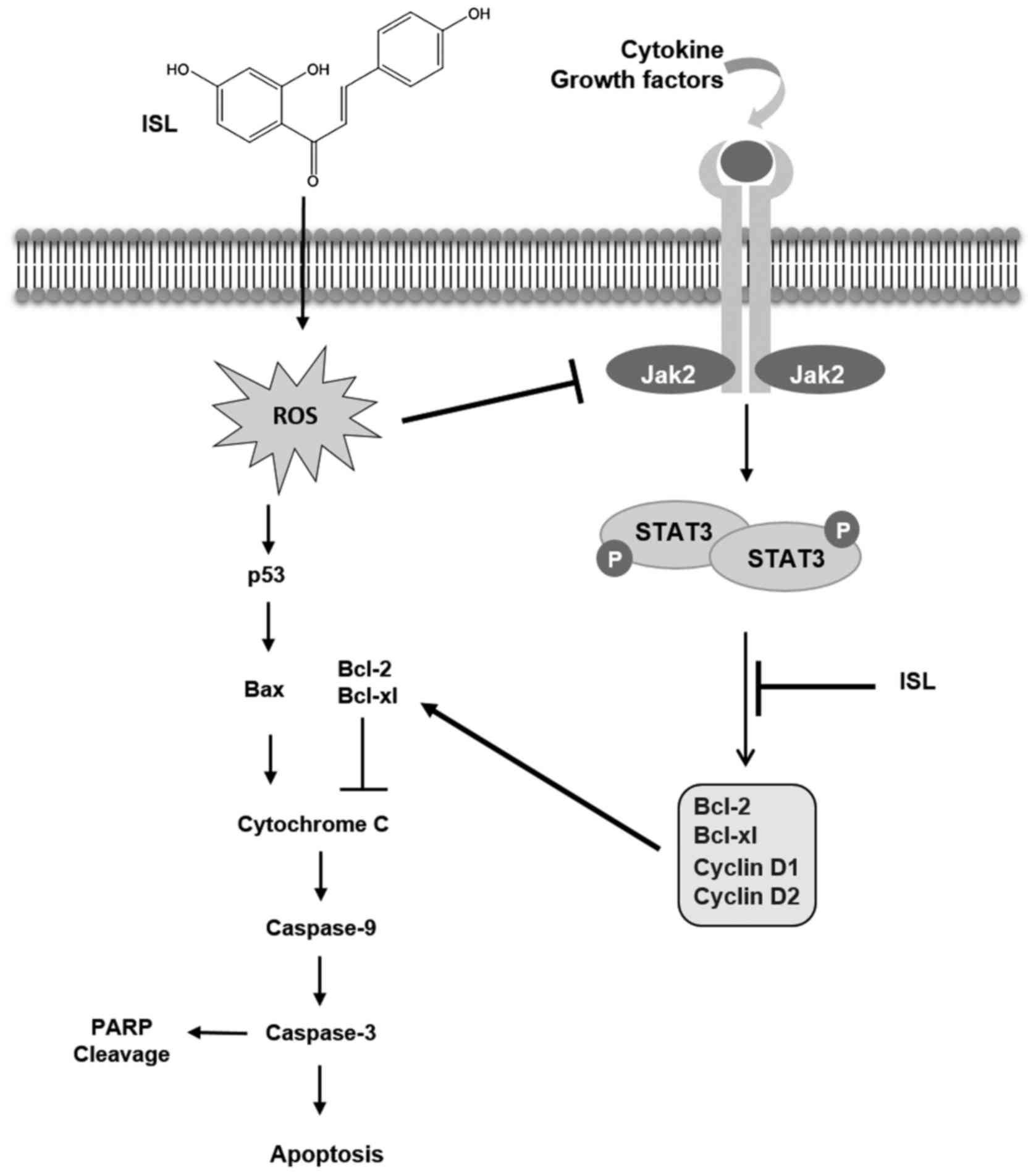
ISL-treated Caki cells. In summary, the present study demonstrated
for the first time that ISL induces apoptosis in Caki cells through
generation of ROS, which causes induction of p53 and inhibition of
the STAT3 signaling pathway (Fig.
6).
Acknowledgements
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education
(NRF-2016R1A6A1A03011325), and by the Bio and Medical Technology
Development Program of NRF funded by the Korean Government, MSIP
(2015M3A9B6073827).
References
|
1
|
Grimm MO, Wolff I, Zastrow S, Fröhner M
and Wirth M: Advances in renal cell carcinoma treatment. Ther Adv
Urol. 2:11–17. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
De P, Otterstatter MC, Semenciw R, Ellison
LF, Marrett LD and Dryer D: Trends in incidence, mortality, and
survival for kidney cancer in Canada, 1986–2007. Cancer Causes
Control. 25:1271–1281. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Motzer RJ, Michaelson MD, Redman BG, Hudes
GR, Wilding G, Figlin RA, Ginsberg MS, Kim ST, Baum CM, DePrimo SE,
et al: Activity of SU11248, a multitargeted inhibitor of vascular
endothelial growth factor receptor and platelet-derived growth
factor receptor, in patients with metastatic renal cell carcinoma.
J Clin Oncol. 24:16–24. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Darnell JE Jr: STATs and gene regulation.
Science. 277:1630–1635. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dhir R, Ni Z, Lou W, DeMiguel F, Grandis
JR and Gao AC: Stat3 activation in prostatic carcinomas. Prostate.
51:241–246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang M, Page C, Reynolds RK and Lin J:
Constitutive activation of stat 3 oncogene product in human ovarian
carcinoma cells. Gynecol Oncol. 79:67–73. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Proietti CJ, Rosemblit C, Beguelin W,
Rivas MA, Díaz Flaqué MC, Charreau EH, Schillaci R and Elizalde PV:
Activation of Stat3 by heregulin/ErbB-2 through the co-option of
progesterone receptor signaling drives breast cancer growth. Mol
Cell Biol. 29:1249–1265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo C, Yang G, Khun K, Kong X, Levy D, Lee
P and Melamed J: Activation of Stat3 in renal tumors. Am J Transl
Res. 1:283–290. 2009.PubMed/NCBI
|
|
9
|
Yu H, Lee H, Herrmann A, Buettner R and
Jove R: Revisiting STAT3 signalling in cancer: New and unexpected
biological functions. Nat Rev Cancer. 14:736–746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bowman T, Garcia R, Turkson J and Jove R:
STATs in oncogenesis. Oncogene. 19:2474–2488. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Johnston PA and Grandis JR: STAT3
signaling: Anticancer strategies and challenges. Mol Interv.
11:18–26. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carpenter RL and Lo HW: STAT3 target genes
relevant to human cancers. Cancers. 6:897–925. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Portt L, Norman G, Clapp C, Greenwood M
and Greenwood MT: Anti-apoptosis and cell survival: A review.
Biochim Biophys Acta. 1813:238–259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Riedl SJ and Salvesen GS: The apoptosome:
Signalling platform of cell death. Nat Rev Mol Cell Biol.
8:405–413. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sinha K, Das J, Pal PB and Sil PC:
Oxidative stress: The mitochondria-dependent and
mitochondria-independent pathways of apoptosis. Arch Toxicol.
87:1157–1180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cerna D, Li H, Flaherty S, Takebe N,
Coleman CN and Yoo SS: Inhibition of nicotinamide
phosphoribosyltransferase (NAMPT) activity by small molecule
GMX1778 regulates reactive oxygen species (ROS)-mediated
cytotoxicity in a p53- and nicotinic acid
phosphoribosyltransferase1 (NAPRT1)-dependent manner. J Biol Chem.
287:22408–22417. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kampa M, Nifli AP, Notas G and Castanas E:
Polyphenols and cancer cell growth. Rev Physiol Biochem Pharmacol.
159:79–113. 2007.PubMed/NCBI
|
|
19
|
Aida K, Tawata M, Shindo H, Onaya T,
Sasaki H, Yamaguchi T, Chin M and Mitsuhashi H: Isoliquiritigenin:
A new aldose reductase inhibitor from Glycyrrhizae Radix. Planta
Med. 56:254–258. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Choi YH, Bae JK, Chae HS, Choi YO, Nhoek
P, Choi JS and Chin YW: Isoliquiritigenin ameliorates dextran
sulfate sodium-induced colitis through the inhibition of MAPK
pathway. Int Immunopharmacol. 31:223–232. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gaur R, Yadav KS, Verma RK, Yadav NP and
Bhakuni RS: In vivo anti-diabetic activity of derivatives of
isoliquiritigenin and liquiritigenin. Phytomedicine. 21:415–422.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jung SK, Lee MH, Lim DY, Kim JE, Singh P,
Lee SY, Jeong CH, Lim TG, Chen H, Chi YI, et al: Isoliquiritigenin
induces apoptosis and inhibits xenograft tumor growth of human lung
cancer cells by targeting both wild type and L858R/T790M mutant
EGFR. J Biol Chem. 289:35839–35848. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Auyeung KK and Ko JK: Novel herbal
flavonoids promote apoptosis but differentially induce cell cycle
arrest in human colon cancer cell. Invest New Drugs. 28:1–13. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jung JI, Chung E, Seon MR, Shin HK, Kim
EJ, Lim SS, Chung WY, Park KK and Park JH: Isoliquiritigenin (ISL)
inhibits ErbB3 signaling in prostate cancer cells. Biofactors.
28:159–168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lai PH, Li KT, Hsu SS, Hsiao CC, Yip CW,
Ding S, Yeh LR and Pan HB: Pyogenic brain abscess: Findings from in
vivo 1.5-T and 11.7-T in vitro proton MR spectroscopy. AJNR Am J
Neuroradiol. 26:279–288. 2005.PubMed/NCBI
|
|
26
|
Li Y, Zhao H, Wang Y, Zheng H, Yu W, Chai
H, Zhang J, Falck JR, Guo AM, Yue J, et al: Isoliquiritigenin
induces growth inhibition and apoptosis through downregulating
arachidonic acid metabolic network and the deactivation of PI3K/Akt
in human breast cancer. Toxicol Appl Pharmacol. 272:37–48. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park I, Park KK, Park JH and Chung WY:
Isoliquiritigenin induces G2 and M phase arrest by inducing DNA
damage and by inhibiting the metaphase/anaphase transition. Cancer
Lett. 277:174–181. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Z, Wang N, Han S, Wang D, Mo S, Yu L,
Huang H, Tsui K, Shen J and Chen J: Dietary compound
isoliquiritigenin inhibits breast cancer neoangiogenesis via
VEGF/VEGFR-2 signaling pathway. PLoS One. 8:e685662013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamazaki S, Morita T, Endo H, Hamamoto T,
Baba M, Joichi Y, Kaneko S, Okada Y, Okuyama T, Nishino H, et al:
Isoliquiritigenin suppresses pulmonary metastasis of mouse renal
cell carcinoma. Cancer Lett. 183:23–30. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chae IG, Kim DH, Kundu J, Jeong CH, Kundu
JK and Chun KS: Generation of ROS by CAY10598 leads to inactivation
of STAT3 signaling and induction of apoptosis in human colon cancer
HCT116 cells. Free Radic Res. 48:1311–1321. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim DH, Park KW, Chae IG, Kundu J, Kim EH,
Kundu JK and Chun KS: Carnosic acid inhibits STAT3 signaling and
induces apoptosis through generation of ROS in human colon cancer
HCT116 cells. Mol Carcinog. 55:1096–1110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Indran IR, Tufo G, Pervaiz S and Brenner
C: Recent advances in apoptosis, mitochondria and drug resistance
in cancer cells. Biochim Biophys Acta. 1807:735–745. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bromberg J: Stat proteins and oncogenesis.
J Clin Invest. 109:1139–1142. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aznar S, Valerón PF, del Rincon SV, Pérez
LF, Perona R and Lacal JC: Simultaneous tyrosine and serine
phosphorylation of STAT3 transcription factor is involved in Rho A
GTPase oncogenic transformation. Mol Biol Cell. 12:3282–3294. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bishayee A and Sethi G: Bioactive natural
products in cancer prevention and therapy: Progress and promise.
Semin Cancer Biol 40–41. 1–3. 2016. View Article : Google Scholar
|
|
37
|
Arumuggam N, Bhowmick NA and Rupasinghe
HP: A Review: Phytochemicals Targeting JAK/STAT Signaling and IDO
Expression in Cancer. Phytother Res. 29:805–817. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lue HW, Cole B, Rao SA, Podolak J, Van
Gaest A, King C, Eide CA, Wilmot B, Xue C, Spellman PT, et al: Src
and STAT3 inhibitors synergize to promote tumor inhibition in renal
cell carcinoma. Oncotarget. 6:44675–44687. 2015.PubMed/NCBI
|
|
39
|
Um HJ, Min KJ, Kim DE and Kwon TK:
Withaferin A inhibits JAK/STAT3 signaling and induces apoptosis of
human renal carcinoma Caki cells. Biochem Biophys Res Commun.
427:24–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chin YW, Jung HA, Liu Y, Su BN, Castoro
JA, Keller WJ, Pereira MA and Kinghorn AD: Anti-oxidant
constituents of the roots and stolons of licorice (Glycyrrhiza
glabra). J Agric Food Chem. 55:4691–4697. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kakegawa H, Matsumoto H and Satoh T:
Inhibitory effects of some natural products on the activation of
hyaluronidase and their anti-allergic actions. Chem Pharm Bull.
40:1439–1442. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kang SW, Choi JS, Choi YJ, Bae JY, Li J,
Kim DS, Kim JL, Shin SY, Lee YJ and Kwun IS: Licorice
isoliquiritigenin dampens angiogenic activity via inhibition of
MAPK-responsive signaling pathways leading to induction of matrix
metalloproteinases. J Nutr Biochem. 21:55–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kape R, Parniske M, Brandt S and Werner D:
Isoliquiritigenin, a strong nod gene- and glyceollin
resistance-inducing flavonoid from soybean root exudate. Appl
Environ Microbiol. 58:1705–1710. 1992.PubMed/NCBI
|
|
44
|
Deponte M: Glutathione catalysis and the
reaction mechanisms of glutathione-dependent enzymes. Biochim
Biophys Acta. 1830:3217–3266. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bromberg J and Darnell JE Jr: The role of
STATs in transcriptional control and their impact on cellular
function. Oncogene. 19:2468–2473. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Heidelberger S, Zinzalla G, Antonow D,
Essex S, Basu BP, Palmer J, Husby J, Jackson PJ, Rahman KM,
Wilderspin AF, et al: Investigation of the protein alkylation sites
of the STAT3:STAT3 inhibitor Stattic by mass spectrometry. Bioorg
Med Chem Lett. 23:4719–4722. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li L, Cheung SH, Evans EL and Shaw PE:
Modulation of gene expression and tumor cell growth by redox
modification of STAT3. Cancer Res. 70:8222–8232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Park KW, Kundu J, Chae IG, Kim DH, Yu MH,
Kundu JK and Chun KS: Carnosol induces apoptosis through generation
of ROS and inactivation of STAT3 signaling in human colon cancer
HCT116 cells. Int J Oncol. 44:1309–1315. 2014.PubMed/NCBI
|
|
49
|
Sinha P and Ostrand-Rosenberg S:
Myeloid-derived suppressor cell function is reduced by Withaferin
A, a potent and abundant component of Withania somnifera root
extract. Cancer Immunol Immunother. 62:1663–1673. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sullivan LB and Chandel NS: Mitochondrial
reactive oxygen species and cancer. Cancer Metab. 2:172014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pan MH, Sin YH, Lai CS, Wang YJ, Lin JK,
Wang M and Ho CT: Induction of apoptosis by
1-(2-hydroxy-5-methylphenyl)-3-phenyl-1,3-propanedione through
reactive oxygen species production, GADD153 expression, and
caspases activation in human epidermoid carcinoma cells. J Agric
Food Chem. 53:9039–9049. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li D, Ueta E, Kimura T, Yamamoto T and
Osaki T: Reactive oxygen species (ROS) control the expression of
Bcl-2 family proteins by regulating their phosphorylation and
ubiquitination. Cancer Sci. 95:644–650. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Schumacker PT: Reactive oxygen species in
cancer cells: Live by the sword, die by the sword. Cancer Cell.
10:175–176. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jiang YQ, Zhou ZX and Ji YL: Suppression
of EGFR-STAT3 signaling inhibits tumorigenesis in a lung cancer
cell line. Int J Clin Exp Med. 7:2096–2099. 2014.PubMed/NCBI
|